Abstract
The authors report on the 44‐week open‐label extension of the 8‐week, double‐blind Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure (COACH) trial in 1684 patients. Initial therapy was amlodipine (AML) plus olmesartan medoxomil (OM) 5+40 mg/d, up‐titrated to AML+OM 10+40 mg/d plus hydrochlorothiazide (HCTZ) 12.5 mg then 25 mg if patients did not achieve blood pressure (BP) goal (<140/90 mm Hg or <130/80 mm Hg in patients with diabetes). Baseline mean BP decreased from 164/102 mm Hg to 131/82 mm Hg at end of study, with an overall 66.7% of patients, including those with diabetes, achieving BP goal. The BP goal achievement was 80% for AML+OM 5+40 mg/d, 70.6% for AML+OM 10+40 mg/d, 66.6% for AML+OM+HCTZ 10+40+12.5 mg/d, and 46.3% for AML+OM+HCTZ 10+40+25 mg/d. Study medication was safe and well tolerated. Combination antihypertensive therapy with AML+OM±HTCZ, up‐titrated as necessary, allowed a majority of patients to achieve BP goal.
Hypertension is a major risk factor for morbidity and mortality associated with stroke and cardiovascular (CV) disease. 1 , 2 Uncontrolled blood pressure (BP) (>140/90 mm Hg) has been reported in 69% of patients who experience a first heart attack and in 77% of individuals diagnosed with a first stroke and imposes high costs on the United States’ health care system. 3 One implication of this relationship is that effective BP lowering is associated with a reduction in CV and cerebrovascular events. A review of 29 randomized trials showed that lowering BP decreased the risk of major CV events and that the greater the decrease, the larger the risk reduction. 4 Further, the outcome benefit of antihypertensive treatment is directly related to BP reduction.
Guidelines produced by expert bodies such as the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension/European Society of Cardiology (ESH/ESC) aim to reduce CV disease risk by lowering BP to a target goal of <140/90 mm Hg for patients with uncomplicated disease or <130/80 mm Hg for patients with diabetes or renal impairment. 5 , 6 The guidelines recommend that in patients with stage 2 hypertension, treatment should be initiated with 2 antihypertensive agents, as these patients have a 2‐fold increase in CV disease risk compared with those whose BP is at the upper limit of the normal range. 5 , 6 , 7 Further, the guidelines recommend that initiation of treatment with 2 agents be considered for patients with stage 1 hypertension who have comorbid conditions such as diabetes, subclinical organ damage, or established CV disease.
Besides other drug combinations, coadministration of a calcium channel blocker and an angiotensin II antagonist is considered an effective and well‐tolerated therapeutic option. By including agents with complementary modes of action, this combination provides additive BP‐lowering effects while minimizing dose‐dependent drug‐related adverse events (AEs) of the individual components. 8 , 9 , 10 Examples of these drug classes, amlodipine (AML), a dihydropyridine calcium channel blocker, and olmesartan medoxomil (OM), an angiotensin II receptor blocker, have been shown to be effective in reducing BP in hypertensive patients in large‐scale clinical trials. 11 , 12
Previously, it was shown that the combination of AML plus OM is well tolerated and results in greater BP reductions in patients with mild to severe hypertension compared with the respective monotherapies. 13 At the end of this 8‐week randomized phase of the study, the patients continued a 44‐week open‐label extension (OLE), in which all patients received the combination of AML+OM, with or without hydrochlorothiazide (HCTZ), in an attempt to achieve BP goal. This paper reports on the long‐term efficacy and safety of AML+OM from the OLE, including the efficacy and safety of the triple‐therapy combination of AML+OM+ HCTZ.
Methods
Study Design
The present study is a 44‐week OLE of the 8‐week multicenter, randomized, double‐blind, placebo‐controlled, factorial‐design period of the study (Figure 1). 13 The results of the double‐blind period, including study population, inclusion and exclusion criteria, study design, efficacy and safety variables, and statistical analyses, have been published elsewhere. 13 This OLE of the study was designed to assess the long‐term efficacy and safety of AML+OM. The addition of HCTZ, as warranted, enabled assessment of triple antihypertensive therapy. A prespecified analysis of patients in the OLE stratified by age, race, and diabetes will be published elsewhere.
Figure 1.
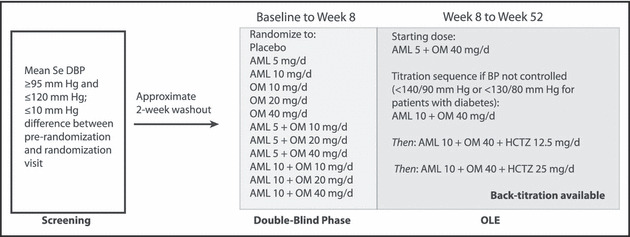
Design of the Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure (COACH) study. AML indicates amlodipine; BP, blood pressure; HCTZ, hydrochlorothiazide; OLE, open‐label extension; OM, olmesartan medoxomil; SeDBP, seated diastolic BP.
After completion of the 8‐week double‐blind portion of the study, 13 all remaining patients were switched to the open‐label combination of AML 5 mg/d + OM 40 mg/d (Figure 1). At week 52, the study was completed and the patients were discontinued from the study medication and treated per investigator’s discretion. A follow‐up visit 2 weeks later (week 54) was scheduled to assess safety. Throughout the study, patients symptomatic for hypotension or displaying intolerance to treatment were back‐titrated at the investigator’s discretion.
All medications were provided to patients free of charge and patients were compensated for costs incurred due to participation in the study, as approved by the institutional review board, but received no other compensation. Patients were instructed to take their medication at the same time each day, although treatment compliance was not recorded during the OLE portion of the study.
The study was conducted in accordance with institutional review board regulations, the Declaration of Helsinki, and good clinical practice guidelines. All patients provided written informed consent at screening. Standardization across investigator sites was maintained through establishment of a detailed clinical protocol and through monitoring for adherence to the protocol by Medpace Inc (Cincinnati, OH).
Efficacy Variables
The efficacy assessments for the OLE portion of the study included reduction in mean seated systolic BP (SeSBP) and seated diastolic BP (SeDBP) from baseline for each treatment and time point (week 8–52), the effect of all titrations upon change in SeSBP and SeDBP, and the number and percentage of patients achieving BP treatment goal (<140/90 mm Hg or <130/80 mm Hg for patients with diabetes) by treatment regimen and time point. In addition to BP goal, the cumulative achievement of various BP targets (<140/90, <130/85, <130/80, and <120/80 mm Hg) was also determined for each treatment regimen.
The effect of titration was determined by calculating the BP value at the last visit on the new dosing regimen minus the BP value at the last visit of the previous dosing regimen. BP was measured in the sitting position at all scheduled visits using a validated automatic BP monitoring device (Omron Model HEM‐705CP; Omron, Vernon Hills, IL) and recorded after a 5‐minute rest period. Three measurements were made ≥1 minute apart and the average used as the recorded BP value for that visit.
The week 52/early termination (week 52/ET) measurement was defined as BP measurement at week 52 for patients who completed treatment. For patients who terminated prior to week 52, the last measurement obtained for that patient was carried forward. 13
Safety Assessments and Evaluation of Edema
Safety was monitored by assessing the incidence of AEs at each visit from the time the informed consent form was signed until 14 days after the last intake of study medication. The occurrence and severity of edema were assessed at all scheduled clinic visits. The occurrence of edema was based on the terms edema, peripheral edema, pitting edema, generalized edema, and localized edema. Severity was assessed as no edema, mild pitting edema/slight indentation, deep pitting edema/indentations remain, and leg remains swollen. If an increase in the severity of edema occurred after entry into the OLE portion of the study, investigators were encouraged to report this as an AE.
Results
Demographics and Baseline Characteristics
Of the 4234 patients screened, 1940 were randomized to double‐blind treatment and 1684 completed double‐blind therapy and entered the OLE study. Demographics and baseline (prior to double‐blind phase) characteristics of the OLE population are shown in Table I. Approximately one third of patients (581) were not taking an antihypertensive medication at the time of screening (34.5%). A majority (1335 [79.3%]) of patients had stage 2 hypertension (SeSBP ≥160 mm Hg or SeDBP ≥100 mm Hg) at baseline.
Table I.
Demographics and Baseline Characteristics for All Patients Entering the OLE
| Characteristic a | Patients (N=1684) |
|---|---|
| Mean age, y (SD) | 54.1 (11.0) |
| Age ≥65 y, No. (%) | 331 (19.7) |
| Hispanic or Latino patients, No. (%) | 214 (12.7) |
| Black patients, No. (%) | 413 (24.5) |
| Patients with diabetes, No. (%) | 228 (13.5) |
| Men, No. (%) | 927 (55.0) |
| Not on antihypertensive therapy at screening, No. (%) | 581 (34.5) |
| Patients with stage 1 hypertension,b No. (%) | 348 (20.7) |
| Patients with stage 2 hypertension,b No. (%) | 1335 (79.3) |
| Mean body mass index, kg/m2 (SD) | 33.4 (7.1) |
| Mean SeDBP at baseline, mm Hg (SD) | 101.5 (5.0) |
| Mean SeSBP at baseline, mm Hg (SD) | 163.6 (15.7) |
| Edema grade at week 8, No. (%) | |
| No edema | 1400 (83.1) |
| Mild pitting edema, slight indentation | 228 (13.5) |
| Moderate pitting edema, slight indentation | 42 (2.5) |
| Deep pitting edema, indentation remains | 13 (0.8) |
| Deep pitting edema, leg very swollen | 1 (0.1) |
Abbreviations: JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; OLE, open‐label extension; SD, standard deviation; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. aDemographic characteristics of patients at screening. bJNC 7 definition of hypertension stages (stage 1: systolic blood pressure of 140–159 mm Hg or diastolic blood pressure of 90–99 mm Hg; stage 2: systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg).
Efficacy
In the total patient cohort, SeDBP decreased from a mean of 101.5 mm Hg at baseline to 81.9 mm Hg at week 52/ET and mean SeSBP decreased from 163.6 mm Hg at baseline to 131.2 mm Hg at week 52/ET. Five hundred and twenty‐five patients (31.2%) remained on AML 5 mg/d + OM 40 mg/d and achieved a mean SeDBP of 81.0 mm Hg from a baseline of 100.3 mm Hg and a mean SeSBP of 127.6 mm Hg from a baseline mean of 157.6 mm Hg, the lowest of all treatment regimens (Table II).
Table II.
Seated Systolic and Diastolic BP (mm Hg) at Baseline and Week 52/ET by Dosing Regimen at Week 52/ET
| regimen | AML 5 + OM 40, mg/d (n=525) | AML 10 + OM 40, mg/d (n=378) | AML 10 + OM 40 + HCTZ 12.5, mg/d (n=287) | AML 10 + OM 40 + HCTZ 25, mg/d (n=419) | Othera (n=63) | Total Cohort (N=1672) |
|---|---|---|---|---|---|---|
| Baselineb mean systolic/diastolic BP, mm Hg | 157.6/100.3 | 160.7/101.1 | 165.5/102.2 | 172.9/103.2 | 160.7/100.3 | 163.6/101.5 |
| Week 52/ET mean systolic/diastolic BP, mm Hg | 127.6/81.0 | 130.9/82.4 | 130.7/81.0 | 136.8/83.4 | 126.2/79.4 | 131.2/81.9 |
| Mean change from baseline, mm Hg | 30.0/19.3 | 29.8/18.7 | 34.8/21.2 | 36.1/19.8 | 34.5/20.9 | 32.4/19.6 |
Abbreviations: AML, amlodipine; BP, blood pressure; ET, early termination; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil. aOther includes the following nonstandard treatment regimens as the final dosage: OM 40 mg/d, HCTZ 25 mg/d, AML 5 mg/d, AML 10 mg/d + OM 20 mg/d, AML 2.5 mg/d + OM 20 mg/d, AML 5 mg/d + OM 20 mg/d, AML 5 mg/d + OM 20 mg/d + HCTZ 12.5 mg/d, AML 10 mg/d + OM 20 mg/d + HCTZ 12.5 mg/d, AML 2.5 mg/d + OM 40 mg/d + HCTZ 12.5 mg/d, AML 5 mg/d + OM 40 mg/d + HCTZ 12.5 mg/d, and AML 5 mg/d + OM 40 mg/d + HCTZ 25 mg/d. bBaseline BP refers to BP at the start of the randomized phase of the study and was defined as the average of the visit values from the randomization visit and the visit before the randomization visit.
Increasing the dose of AML from 5 mg/d to 10 mg/d in combination with OM 40 mg/d (n=1096) produced further decreases in mean SeDBP of 4.8±7.9 (standard deviation [SD]) mm Hg and mean SeSBP of 7.3±12.8 (SD) mm Hg. Addition of HCTZ 12.5 mg/d to the AML 10 mg/d + OM 40 mg/d combination (n=693) further decreased mean SeDBP by 4.5±8.3 (SD) mm Hg and mean SeSBP by 7.7±14.0 (SD) mm Hg. Doubling the HCTZ dose from 12.5 to 25 mg/d (n=418) decreased mean SeDBP and mean SeSBP by an additional 6.0±8.6 and 9.9±15.0 (SD) mm Hg, respectively. Patients who received the triple‐therapy regimen including HCTZ 25 mg had the greatest mean SeSBP reduction (36.1 mm Hg, from 172.9 mm Hg at baseline to 136.8 mm Hg at week 52/ET) (Table II).
Across all treatment regimens, 66.7% of patients achieved BP goal (<140/90 mm Hg or <130/80 mm Hg in patients with diabetes) by week 52/ET (Figure 2). At week 52/ET, the BP goal was achieved by 80% of patients still treated with AML 5 mg/d + OM 40 mg/d, 70.6% of patients still treated with AML 10 mg/d + OM 40 mg/d, 66.6% of patients treated with AML 10 mg/d + OM 40 mg/d + HCTZ 12.5 mg/d, and 46.3% of patients treated with AML 10 mg/d + OM 40 mg/d + HCTZ 25 mg/d.
Figure 2.
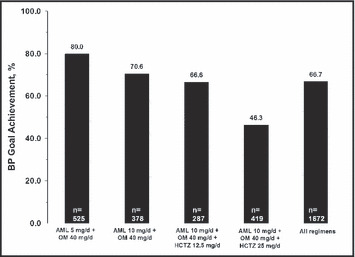
Achievement of blood pressure (BP) goal by treatment regimen. Values represent the percentage of patients achieving the BP treatment goal at the week 52/early termination (ET) time point and “n” represents the number of patients on that regimen at week 52/ET. “All regimens” cohort includes 63 patients who received non–protocol‐specified treatments defined as “other.” The BP goal was <140/90 mm Hg or <130/80 mm Hg for patients with diabetes. AML indicates amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil.
Since clinicians were at liberty to adjust dosing in order to optimize the therapeutic effect and maximize tolerability, patients moved in and out of particular dose regimens during the course of the study. Thus, the number of patients for a given regimen at week 52/ET may be lower than the total number of patients exposed to that regimen through the duration of the study. When goal achievement was calculated using the total number of patients exposed to a specific dose regimen, the highest rate was observed for patients who received AML 10 mg/d + OM 40 mg/d + HCTZ 25 mg/d (67.7%, data not shown).
The effects of titration were also examined with regard to BP targets of <140/90, <130/85, <130/80, and <120/80 mm Hg (Figure 3). The addition of HCTZ 25 mg enabled more patients to achieve BP targets of <140/90 mm Hg (77.7%), <130/85 mm Hg (47.5%), and <130/80 mm Hg (36.4%) (Figure 3) compared with the other treatment regimens, even though these patients had the highest baseline BP.
Figure 3.
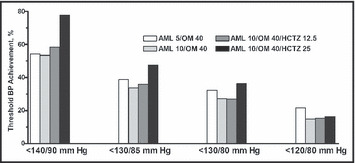
Achievement of blood pressure (BP) targets by treatment regimen. Data represent all patients who were exposed to a particular treatment regimen throughout the duration of the open‐label extension, regardless of whether they remained on the regimen or were titrated to another. All drug concentrations are mg/d. AML indicates amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil.
Safety
The total mean duration of drug exposure for all patients, regardless of treatment regimen, was 278.6±76.7 days. Overall, no major safety issues emerged with long‐term therapy. The frequency of treatment‐emergent AEs (TEAEs) ranged from 37.0% to 56.4%, and drug‐related TEAEs (DR‐TEAEs) ranged from 13.2% to 20.2% across the 4 treatment regimens (Table III). Most TEAEs were mild in severity. The frequency of edema (including new‐onset and worsening severity) ranged from 8.9% in patients treated with AML 5 mg/d + OM 40 mg/d to 14.5% in patients treated with AML 10 mg/d + OM 40 mg/d + HCTZ 25 mg/d. Other TEAEs experienced by ≥3% of patients included upper respiratory tract infection (6.5%), nasopharyngitis (5.2%), extremity pain (4.1%), sinusitis (3.6%), arthralgia (3.3%), and back pain (3.1%). There was one death (gunshot wound to the head), which was not considered to be drug‐related.
Table III.
No. (%) of Patients Having Treatment‐Emergent Adverse Events (TEAEs) and Drug‐Related TEAEs (DR‐TEAEs)
| AML 5 + OM 40, mg/d (n=1679) | AML 10 + OM 40, mg/d (n=1124) | AML 10 + OM 40 + HCTZ 12.5, mg/d (n=736) | AML 10 + OM 40 + HCTZ 25, mg/d (n=440) | |
|---|---|---|---|---|
| Any TEAE, No. (%) | 622 (37.0) | 455 (40.5) | 312 (42.4) | 248 (56.4) |
| Any discontinuations due to TEAE, No. (%) | 28 (1.7) | 17 (1.5) | 11 (1.5) | 11 (2.5) |
| Any DR‐TEAE, No. (%) | 221 (13.2) | 195 (17.3) | 124 (16.8) | 89 (20.2) |
| Serious DR‐TEAE, No. (%) | 1 (0.1) | 0 | 0 | 0 |
| Discontinuations due to DR‐TEAE, No. (%) | 16 (1.0) | 11 (1.0) | 3 (0.4) | 4 (0.9) |
| Specific DR‐TEAEs | ||||
| Edema,a No. (%) | 118 (7.0) | 125 (11.1) | 67 (9.1) | 47 (10.7) |
| Dizziness, No. (%) | 24 (1.4) | 19 (1.7) | 13 (1.8) | 9 (2.0) |
| Headache, No. (%) | 15 (0.9) | 10 (0.9) | 5 (0.7) | 5 (1.1) |
| Hypotension,b No. (%) | 11 (0.7) | 7 (0.6) | 10 (1.4) | 3 (0.7) |
| Cough, No. (%) | 2 (0.1) | 2 (0.2) | 1 (0.1) | 2 (0.5) |
Abbreviations: AML, amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil. aIncludes preferred terms of edema, edema peripheral, and pitting edema. bIncludes preferred terms of hypotension, orthostatic hypotension, blood pressure decreased, and diastolic blood pressure decreased.
Overall, the most common DR‐TEAE in the total population was edema, which ranged from 7.0% in patients taking AML 5 mg/d + OM 40 mg/d to 11.1% in patients taking AML 10 mg/d + OM 40 mg/d. Other DR‐TEAEs experienced by ≥1% of patients included dizziness (3.9%), headache (2.0%), hypotension (1.8%), and fatigue (1.6%). The incidence of cough, another TEAE of interest for this drug class, was 0.4% for DR‐TEAE. There was one DR‐TEAE that was considered serious (noncardiac chest pain).
Discussion
This OLE study showed that the coadministration of AML+OM (plus HCTZ, as needed) is efficacious and safe during long‐term administration, decreases BP, and enables most patients with hypertension to achieve BP goal. In general, the higher the baseline BP, the more likely a patient was to be titrated to a more intense treatment regimen, reflecting that the study design separated patients into final treatment regimens based on antihypertensive responsiveness and/or severity of hypertension. These results are in concordance with previous trials that have assessed the BP‐lowering efficacy of olmesartan in combination with other antihypertensive agents, including study protocols that used titration‐to‐goal schedules. 14 , 15
All combination treatment regimens enabled BP reductions at week 52/ET. In patients who remained on AML 5 mg/d + OM 40 mg/d mean seated BP was reduced by 30.0/19.3 mm Hg. For patients titrated to more intensive antihypertensive regimens, mean changes at week 52/ET ranged from 29.8 mm Hg to 36.1 mm Hg for SeSBP and 18.7 mm Hg to 21.2 mm Hg for SeDBP. The reduction from baseline in SeSBP was greatest in the patients that were titrated to AML 10 mg/d + OM 40 mg/d + HCTZ 25 mg/d (36.1 mm Hg). These patients had the highest baseline mean BP (172.9/103.2 mm Hg) and 46.3% of these patients achieved their BP treatment goal. Thus, the addition and up‐titration of HCTZ produced further BP decreases and allowed more patients to achieve their BP goal. HCTZ was safe and well tolerated in patients already receiving a combination of AML and OM.
Advantages of combination antihypertensive therapy include more rapid achievement of BP goals, additive effects of drugs, decreased dosages of constituent drugs, avoidance of physician and patient frustration with antihypertensive regimen adjustments, and increased patient adherence due to the availability of fixed low‐dose combination formulations. 6 , 9 Among factors contributing to inadequate control of BP, physician‐related issues include excessive reliance on monotherapy and reluctance to increase drug dosage if the initial dose fails to achieve BP goal (clinical inertia) or to add additional antihypertensive agents as necessary. Patients may be unwilling to take multiple concomitant therapies due to the number of pills, dosing complexity, and side effects. 16 , 17 , 18 , 19
This study modeled the dosing flexibility available to physicians in clinical practice. 20 , 21 This included alternative treatment regimens and the possibility of back‐titration, which could be instituted at the discretion of physicians involved in this study and resulted in a number of cases in unexpected alternative treatment regimens. Doubling the dosage of one of the component therapies or adding a new drug to the combination regimen afforded additional 5‐ to 6‐mm Hg DBP and 7‐ to 10‐mm Hg SBP reductions.
At the end of the study (week 52/ET), 525 (31.2%) patients remained on the initial dosage of AML 5 mg/d + OM 40 mg/d and 80.0% of these patients achieved their BP goal of <140/90 mm Hg (or <130/80 mm Hg for patients with diabetes). However, although the study design required investigators to titrate to the next dose level in patients with BP above the treatment goal, 20% of the patients who remained on AML 5 mg/d + OM 40 mg/d had not achieved their BP goal and were candidates for titration therapy to higher dose levels. At each titration step in the protocol, a larger proportion of patients on each treatment regimen met the criteria for up‐titration but failed to receive the next protocol‐defined dose combination.
The lack of titration to the next dose level in these patients may reflect physician inertia, which may account for as much as 20% of the failure to achieve BP control in the clinical practice setting 22 , 23 and has also been reported in clinical trials. 24 Physician inertia in the practice setting may be due to several reasons, including uncertainty about choosing appropriate combinations of antihypertensive medications (particularly multiple medications to be administered as separate tablets/capsules), questions about patient adherence, AEs, and uncertainty concerning the patient’s true BP value and the cost‐effectiveness of various therapeutic regimens. 21 , 25 , 26 Concerns about cost of medication and questions about appropriate combinations of medications do not apply to this study because medicines were supplied free of charge and the regimens specified by the protocol had been shown to be effective in the randomized double‐blind phase of the study.
Fixed‐dose combinations of antihypertensive agents have been associated with improved patient adherence, few tolerability or safety issues, and improved cost‐effectiveness compared with combination therapy comprised of monotherapy components. 8 , 21 Indeed, the highest BP control rates in any multinational trial have been reported in the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) study, where single‐tablet combinations were administered. 27 Control rates were 75.4% for AML plus an angiotensin‐converting enzyme inhibitor and 72.4% for an angiotensin‐converting enzyme inhibitor plus HCTZ.
The safety, effectiveness, and tolerability of the treatment regimen of the OLE study were similar to the randomized phase of the study and to other studies of OM and AML, either as monotherapy or in combination with a diuretic. 11 , 12 , 13 The frequencies of AEs were consistent with the expected safety profiles of these agents as monotherapies and the tolerability of the AML+OM combinations is reflected by the low dropout rate due to DR‐TEAEs (2.0%). In the OLE portion of the study the incidence of edema in patients receiving AML+OM was lower than the incidence reported for AML monotherapy in the 8‐week double‐blind portion of the study. 13
The OLE study shows that a combination of AML+OM, with or without HCTZ, appears effective in producing sustained BP control in patients with hypertension. The results reported here support the use of even a triple fixed‐dose combination, including AML+OM+HCTZ. A randomized, double‐blind, parallel‐group study evaluating the efficacy and tolerability of coadministration of these agents is currently underway (NCT00649389).
Acknowledgments and disclosures: This study was supported by Daiichi Sankyo, Inc. We thank Jennifer M. Kulak, PhD, and Christopher J. Jones, PhD, for providing editorial assistance in the preparation of this manuscript. Steven G. Chrysant, MD, received research grants from Daiichi Sankyo, Inc. Novartis, Boehringer Ingelheim, Merck, Bristol‐Myers Squibb, and Takeda Pharmaceuticals and serves as a consultant to Daiichi Sankyo, Inc., Novartis, and Boehringer Ingelheim. Suzanne Oparil, MD, is the recipient of grants‐in‐aid from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Forest Laboratories, GlaxoSmithKline, Novartis, Merck & Co, Pfizer, and Daiichi Sankyo, Inc. Suzanne Oparil, MD, serves as a consultant for Bristol‐Myers Squibb, Daiichi Sankyo, Inc, Novartis, sanofi‐aventis, and The Salt Institute. Michael Melino, PhD, Sulekha Karki, BAMS, James Lee, PhD, and Reinilde Heyrman, MD, are all employees of Daiichi Sankyo, Inc.
References
- 1. Bestehorn K, Wahle K, Kirch W. Stroke risk screening of adults with hypertension: prospective cross‐sectional study in primary care. Clin Drug Investig. 2008;28(5):281–289. [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. [DOI] [PubMed] [Google Scholar]
- 4. Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. [DOI] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. [DOI] [PubMed] [Google Scholar]
- 7. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 8. Bakris GL. Combined therapy with a calcium channel blocker and an angiotensin II type 1 receptor blocker. J Clin Hypertens (Greenwich). 2008;10(1 Suppl 1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chrysant SG. Using fixed‐dose combination therapies to achieve blood pressure goals. Clin Drug Investig. 2008;28(11):713–734. [DOI] [PubMed] [Google Scholar]
- 10. Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein BJ, Vogel K, Palmer BF. Dihydropyridine calcium channel antagonists in the management of hypertension. Drugs. 2007;67(9):1309–1327. [DOI] [PubMed] [Google Scholar]
- 12. Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs. 2008;68(9):1239–1272. [DOI] [PubMed] [Google Scholar]
- 13. Chrysant S, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double‐blind, placebo‐controlled, 8‐week factorial efficacy and safety study. Clin Ther. 2008;30(4):587–604. [DOI] [PubMed] [Google Scholar]
- 14. Barrios V, Boccanelli A, Ewald S, et al. Efficacy and tolerability of olmesartan medoxomil in patients with mild to moderate essential hypertension: the OLMEBEST Study. Clin Drug Investig. 2007;27(8):545–558. [DOI] [PubMed] [Google Scholar]
- 15. Izzo JL Jr, Neutel JM, Silfani T, et al. Efficacy and safety of treating stage 2 systolic hypertension with olmesartan and olmesartan/HCTZ: results of an open‐label titration study. J Clin Hypertens (Greenwich). 2007;9(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19(11):1190–1196. [DOI] [PubMed] [Google Scholar]
- 17. Elliott W. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich). 2008;10(1 Suppl 1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gregoire J, Moisan J, Guibert R, et al. Predictors of self‐reported noncompliance with antihypertensive drug treatment: a prospective cohort study. Can J Cardiol. 2006;22(4):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens (Greenwich). 2003;5(5):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackshear JL, Schwartz GL. Step care therapy for hypertension in diabetic patients. Mayo Clin Proc. 2001;76(12):1266–1274. [DOI] [PubMed] [Google Scholar]
- 21. Ofili EO. Dispelling the myth of “aggressive” antihypertensive therapy. J Clin Hypertens (Greenwich). 2006;8(1 Suppl 1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47(3):345–351. [DOI] [PubMed] [Google Scholar]
- 23. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834. [DOI] [PubMed] [Google Scholar]
- 24. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4(6):393–404. [DOI] [PubMed] [Google Scholar]
- 25. Kerr EA, Zikmund‐Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148(10):717–727. [DOI] [PubMed] [Google Scholar]
- 26. Phillips LS, Twombly JG. It’s time to overcome clinical inertia. Ann Intern Med. 2008;148(10):783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359(23):2417–2428. [DOI] [PubMed] [Google Scholar]


