Abstract
Chronic kidney disease (CKD) increases the risk of cardiovascular events and is often associated with the nondipping pattern of blood pressure (BP). We evaluated ambulatory BP, CKD, and the incidence of cardiovascular events in 811 older hypertensive patients. CKD and the dipping pattern increased the risk of cardiovascular events independent of the 24‐hour systolic BP level (CKD: hazard ratio [HR], 2.37; 95% confidence interval [CI], 1.24–4.54; nondippers: HR, 2.16; 95% CI, 1.19–3.91; extreme dippers: HR, 2.38; 95% CI, 1.17–4.83). However, after adjustment for covariates that included CKD, the risk in nondippers was insignificant (HR, 1.83; 95% CI, 0.998–3.34; P=.051), while the risk in extreme dippers remained (HR, 2.59; 95% CI, 1.26–5.32; P=.009) (CKD: HR, 1.81; 95% CI, 0.93–3.54; P=.081). Patients with CKD have an increased risk of cardiovascular events. CKD and other cardiovascular risk factors may account for some of the increased risk in nondippers, but it does not explain the higher risk in extreme dippers.
Patients with chronic kidney disease (CKD) have an increased risk of cardiovascular events. 1 The severity of CKD can be evaluated using the estimated glomerular filtration rate (eGFR) derived from the Cockcroft‐Gault equation 2 or the Modification of Diet in Renal Disease (MDRD) equation. 3 In clinical practice guidelines for CKD from the National Kidney Foundation and the American Heart Association, 4 , 5 an eGFR <60 mL/min/1.73 m2 is defined as CKD stages 3 to 5 and has been selected as the cutoff for the definition of CKD, regardless of age.
It is reported that impaired kidney function can cause nondipping, which is a blunted nocturnal dip in blood pressure (BP) (sleep/awake systolic BP ratio <0.1) detected by ambulatory BP monitoring (ABPM). 6 , 7 , 8 Patients with CKD are typically nondippers. 9 Nondipping is also reported to be associated with sodium or salt sensitivity, 10 , 11 while sodium restriction 12 and diuretic use 13 turn nondippers into dippers. In a general population, nondippers have been reported to have a 2.16 times higher risk of cardiovascular mortality than dippers. 14 An increased risk of nondipping has been detected even in patients with a normal 24‐hour BP value in a general population. 14 In addition, in elderly hypertensive patients, risers (nocturnal systolic BP decline <0%) and extreme dippers (nocturnal systolic BP decline >20%) had 1.93 and 2.37 times higher risks for stroke than dippers, respectively. 15 However, no previous reports have shown whether nondippers’ increased risk of cardiovascular events is associated with CKD in hypertensive patients.
There were three purposes to this study: (1) to compare cardiovascular risks between elderly Japanese hypertensive nondippers with and without CKD, (2) to determine whether the increased risk in nondippers might be mediated by CKD, and (3) to evaluate the risk of CKD in patients with extreme dipping.
Methods
Participants
This study was based on 811 older patients (older than 50 years) with diagnosed essential hypertension who were participants in the Jichi Medical School ABPM Study Wave 1. The diagnosis of hypertension was based on clinic BP readings (≥140/90 mm Hg). Patients with white‐coat hypertension identified after ABPM were also included in the present study. Characteristics of the patients in the present study were reported previously. 16 The results of the Jichi Medical School ABPM study have also been reported elsewhere. 17 , 18 The present paper is a retrospective analysis of the prospective study. The patients analyzed in the present report represent 99% of the 821 patients who were initially enrolled from 6 participating institutes (3 clinics, 2 hospitals, and 1 outpatient clinic of the university hospital) between January 1, 1992, and January 1, 1998. No patient had taken any antihypertensive medications for at least 14 days before the ABPM study, but 51% had a history of taking antihypertensive medication. We excluded patients with apparent renal failure (serum creatinine level ≥176 mmol/L [2.0 mg/dL]) or hepatic damage; with present illnesses; or with a history of coronary artery disease, stroke (including transient ischemic attack [TIA]), congestive heart failure, or arrhythmia at baseline. The study was approved by the institutional review board of Jichi Medical University School of Medicine, Japan. Informed consent to participate was obtained from all patients.
Twenty‐Four–Hour ABPM
Noninvasive ABPM was performed on a weekday with one of three automatic ABPM devices (ABPM‐630, Nippon Colin Co., Aichi, Japan; TM‐2421 or TM‐2425, A&D Co., Tokyo, Japan) that recorded BP (by the oscillometric method) and heart rate every 30 minutes for 24 hours. We excluded patients from whom we obtained valid BP readings in <80% of either awake or asleep attempts and those who reported in our post‐ABPM questionnaire that wearing the ABPM severely disturbed their sleep. Sleep and waking time was defined by diaries of the patients. Sleep BP was defined as the average of BP readings from the time the patient went to bed until the time he or she got out of bed, and awake BP was defined as the average of BP values recorded during the rest of the day. Nondippers, dippers, and extreme dippers were defined as those with sleep/awake systolic BP rates >0.9, 0.8–0.9, and <0.8, respectively. Morning BP was defined as the average of 4 readings (2 hours) after waking.
Laboratory Data
Blood samples were drawn from the cubital vein in the fasting state within 2 months of ABPM. Serum creatinine (Scr) levels were measured using the enzyme method in a single laboratory (SRL Inc., Tokyo, Japan) from 1992 to 1998, and the serum samples were frozen at −80°C until measurement. The eGFR was calculated afterward using the MDRD study equation modified for Japanese patients 19 :
CKD was defined as the eGFR <60 mL/min/1.73 m2. The eGFR was also calculated using the Cockcroft‐Gault equation 2 :
Follow‐Up and Events
After the patients entered the study, their medical records were periodically reviewed for drug therapy and occurrence of cardiovascular events; the follow‐up evaluations were performed during a 20‐month period from 1996 to 1998. The mean follow‐up period was 41±14 months, with a range of 1 to 68 months. If a patient stopped coming to the clinic, we conducted a telephone interview. Cardiovascular events were defined as cardiac or clinical stroke events. Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined type of stroke but excluded TIA (transient neurologic deficits that disappeared within 24 hours after onset). Cardiac events included fatal and nonfatal acute myocardial infarction, unexplained sudden death within 6 hours of the abrupt onset of symptoms, and coronary revascularization. We did not include heart failure in cardiac events. Physicians who were caring for the patients at the time of cardiovascular events diagnosed the occurrence of cardiovascular events. These events were accepted if they were documented in the medical records or confirmed by a general practitioner. We excluded 15 possible TIAs from the stroke events. Of the total of 821 eligible patients at baseline, follow‐up was performed in 811 (98.8%); the data analysis was restricted to these patients.
Statistical Analysis
Data are expressed as the mean±SD or as a percentage. Relationship between eGFR and sleep/awake systolic BP ratio was performed using Pearson’s correlation. One‐way ANOVA was performed to detect differences among the mean values of groups, and the chi‐square test was used to detect differences among the prevalence rates of groups such as nondippers, dippers, and extreme dippers with or without CKD. One‐way ANOVA was also performed to detect differences of the mean values between patients with or without CKD and cardiovascular events, and the chi‐square test was used to detect differences of the prevalence rates. Unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for cardiovascular events among nondippers, dippers, and extreme dippers, with or without CKD, were calculated using Cox regression analysis. The adjusted HRs of dipping status were calculated in three models (we adjusted for 24‐hour systolic BP in model 1, for 24‐hour systolic BP and presence of CKD or eGFR in model 2, and for age, sex, body mass index, smoking, diabetes, hyperlipidemia, antihypertensive medication use, and presence of CKD or eGFR in model 3). For patients who experienced multiple nonfatal cardiovascular events during the follow‐up period, the analysis included only the first event. These statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL). Differences with P<.05 (two‐tailed) were considered statistically significant.
Results
Baseline Characteristics
The average age was 72.9±9.8 years; 38.3% of the patients were male. The eGFR by the MDRD equation, which ranged from 19 to 122 (mean, 54±13) mL/min/1.73 m2, was negatively and significantly correlated with the sleep/awake systolic BP ratio (r=−0.086, P=.014). The prevalence of CKD according to dipping status at baseline was as follows: nondippers, 70.3%; dippers, 64.7%; and extreme dippers, 61.8% (overall, P<.001). Patients with diabetes had a higher percentage of nondipping than those without diabetes (42.4% vs 36.7%), but the relationship between presence of diabetes and dipping status was not statistically significant (P=.53).
The baseline characteristics of nondippers, dippers, and extreme dippers without and with CKD are shown in Table I. Both dipping status and the presence of CKD were closely related to 24‐hour systolic BP level and age. Body mass index was lower in the nondippers, and there was no significant difference in the presence of diabetes among the groups.
Table I.
Characteristics of Patients in Different Dipping Status
| Extreme Dippers | Dippers | Nondippers | Overall P Value | ||||
|---|---|---|---|---|---|---|---|
| CKD (−) | CKD (+) | CKD (−) | CKD (+) | CKD (−) | CKD (+) | ||
| n=63 | n=102 | n=121 | n=222 | n=90 | n=213 | ||
| Age, y | 67.4±8.3 | 71.8±9.6 | 68.5±8.6 | 73.8±10.3 | 70.8±9.0 | 75.2±9.6 | <.001 |
| Male, % | 39.7 | 35.3 | 45.5 | 33.3 | 51.1 | 35.2 | .03 |
| Body mass index, kg/m2 | 24.6±3.3 | 24.2±3.8 | 23.9±3.0 | 24.4±3.4 | 23.5±3.7 | 23.2±3.7 | .005 |
| Current smoking, % | 9.5 | 25.5 | 18.2 | 17.6 | 25.6 | 23.5 | .07 |
| Diabetes, % | 7.9 | 12.7 | 10.7 | 11.7 | 14.4 | 13.6 | .82 |
| Hyperlipidemia, % | 28.6 | 18.6 | 19.0 | 17.6 | 14.4 | 18.3 | .38 |
| Antihypertensive medication, % | 50.8 | 50.0 | 61.2 | 50.5 | 53.3 | 51.2 | .48 |
| Clinic SBP, mm Hg | 163±17 | 164±19 | 165±17 | 163±18 | 164±16 | 166±18 | .79 |
| Clinic DBP, mm Hg | 92±12 | 91±14 | 92±13 | 91±14 | 89±13 | 89±16 | .33 |
| Clinic PR, /min | 78±12 | 77±12 | 76±11 | 77±11 | 75±12 | 78±13 | .44 |
| 24‐Hour SBP, mm Hg | 135±12 | 134±15 | 140±16 | 137±16 | 139±17 | 141±18 | .006 |
| 24‐Hour DBP, mm Hg | 77±7 | 77±9 | 80±11 | 77±10 | 78±11 | 79±10 | .04 |
| 24‐Hour PR, /min | 72±8 | 71±8 | 71±7 | 70±7 | 70±8 | 71±8 | .37 |
| Awake SBP, mm Hg | 150±12 | 149±17 | 148±17 | 145±18 | 140±18 | 142±18 | <.001 |
| Awake DBP, mm Hg | 86±7 | 84±10 | 84±11 | 81±11 | 79±11 | 79±10 | <.001 |
| Awake PR, /min | 80±9 | 78±10 | 77±8 | 76±8 | 75±9 | 76±8 | .001 |
| Sleep SBP, mm Hg | 113±11 | 113±13 | 126±15 | 124±15 | 135±18 | 138±18 | <.001 |
| Sleep DBP, mm Hg | 65±7 | 65±9 | 73±10 | 70±9 | 76±11 | 77±11 | <.001 |
| Sleep PR, /min | 61±9 | 61±9 | 61±8 | 60±7 | 61±8 | 62±8 | .40 |
| Morning SBP, mm Hg | 145±19 | 144±20 | 146±20 | 145±20 | 144±19 | 147±21 | .84 |
| Morning DBP, mm Hg | 84±10 | 83±12 | 86±13 | 82±12 | 82±13 | 83±12 | .11 |
| Morning PR, /min | 78±11 | 75±12 | 76±11 | 75±11 | 76±12 | 76±10 | .36 |
| ECG‐LVH, % | 9.5 | 14.7 | 14.0 | 15.3 | 11.0 | 20.7 | .18 |
| Hematocrit, % | 40±3 | 40±4 | 40±4 | 40±5 | 41±6 | 39±6 | .03 |
| Serum creatinine, mg/dL | 0.72±0.12 | 0.99±0.24 | 0.73±0.11 | 0.99±0.23 | 0.75±0.11 | 0.98±0.18 | <.001 |
| eGFR by Cockcroft‐Gault, mL/min | 77±19 | 51±13 | 73±19 | 49±15 | 68±18 | 46±14 | <.001 |
| Cockcroft‐Gault eGFR <60 mL/min, % | 7.9 | 77.5 | 24.0 | 73.4 | 42.2 | 86.4 | <.001 |
| eGFR by MDRD equation, mL/min/1.73 m2 | 70±13 | 48±8 | 69±10 | 47±8 | 68±8 | 47±8 | <.001 |
Data are mean±SD or percentage. Abbreviations: CKD, chronic kidney disease; DBP, diastolic blood pressure; ECG‐LVH, left ventricular hypertrophy diagnosed by electrocardiography; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; PR, pulse rate; SBP, systolic blood pressure. Overall P values were calculated by ANOVA or chi‐square test.
Follow‐Up
At the time of the final follow‐up, 426 (53%) of the total of 811 patients were receiving antihypertensive medication (diuretics, α‐ or β‐blockers, calcium antagonists, or angiotensin‐converting enzyme inhibitors). The characteristics of the patients with and without CKD and cardiovascular events are shown in Table II. In the patients with CKD, patients with cardiovascular events were older, and more were male and current smokers. Systolic BP levels and prevalence of nondippers and extreme dippers were higher than in those without cardiovascular events.
Table II.
Characteristics of Patients With and Without Chronic Kidney Disease (CKD) and Cardiovascular Events (N=811)
| CKD (−) | CKD (+) | P Value | |||
|---|---|---|---|---|---|
| CV event (−) | CV event (+) | CV event (−) | CV event (+) | ||
| n=263 | n=11 | n=482 | n=55 | ||
| Age, y | 68.8±8.8 | 73.6±5.2 | 73.3±10.1 | 79.5±7.0 | <.001 |
| Male, % | 46.0 | 45.5 | 32.6 | 50.9 | .001 |
| Body mass index, kg/m2 | 24.0±3.4 | 22.2±2.6 | 24.0±3.6 | 22.8±4.0 | .032 |
| Current smoking, % | 18.3 | 27.3 | 18.7 | 45.5 | <.001 |
| Diabetes, % | 11.0 | 18.2 | 12.0 | 18.2 | .47 |
| Hyperlipidemia, % | 19.4 | 27.3 | 18.3 | 16.4 | .83 |
| Antihypertensive medication, % | 56.7 | 45.5 | 50.8 | 49.1 | .42 |
| Fasting glucose, mg/dL | 93.9±25.4 | 93.4±25.0 | 95.0±25.2 | 97.8±24.4 | .75 |
| Total cholesterol, mg/dL | 200.7±35.7 | 208.5±42.1 | 200.2±34.1 | 189.9±33.2 | .14 |
| Triglycerides, mg/dL | 144.4±81.7 | 98.2±42.5 | 143.5±79.1 | 129.4±64.1 | .16 |
| Hematocrit, % | 40±5 | 39±3 | 39±5 | 39±7 | .11 |
| Serum creatinine, mg/dL | 0.73±0.11 | 0.71±0.12 | 0.98±0.21 | 1.02±0.20 | <.001 |
| Clinic SBP, mm Hg | 163±16 | 171±21 | 164±18 | 170±18 | .03 |
| Clinic DBP, mm Hg | 91±13 | 97±13 | 90±15 | 92±14 | .35 |
| Clinic PR, /min | 76±12 | 79±13 | 77±12 | 78±15 | .46 |
| 24‐Hour SBP, mm Hg | 138±16 | 146±18 | 137±16 | 148±16 | <.001 |
| 24‐Hour DBP, mm Hg | 79±10 | 82±11 | 77±10 | 82±9 | .004 |
| 24‐Hour PR, /min | 71±8 | 73±10 | 71±7 | 72±9 | .59 |
| Awake SBP, mm Hg | 145±17 | 154±18 | 144±18 | 153±16 | .001 |
| Awake DBP, mm Hg | 83±11 | 87±11 | 81±10 | 84±10 | .01 |
| Awake PR, mm Hg | 77±9 | 79±11 | 76±8 | 77±10 | .55 |
| Sleep SBP, mm Hg | 125±17 | 134±21 | 126±18 | 138±20 | <.001 |
| Sleep DBP, mm Hg | 72±11 | 76±13 | 71±10 | 76±12 | .01 |
| Sleep PR, /min | 61±8 | 64±11 | 61±8 | 62±8 | .33 |
| Morning SBP, mm Hg | 145±19 | 154±23 | 144±20 | 161±19 | <.001 |
| Morning DBP, mm Hg | 84±12 | 90±15 | 82±12 | 88±12 | <.001 |
| Morning PR, /min | 76±12 | 79±11 | 75±11 | 75±12 | .51 |
| ECG‐LVH, % | 11.0 | 36.4 | 13.9 | 47.3 | <.001 |
| Dipping status | |||||
| Extreme dippers, % | 22.8 | 27.3 | 18.7 | 21.8 | .032 |
| Nondippers, % | 32.3 | 45.5 | 38.0 | 54.5 | |
| Cockcroft‐Gault eGFR, mL/min | 73±19 | 65±15 | 49±15 | 41±12 | <.001 |
| Cockcroft‐Gault eGFR <60 mL/min, % | 25.5 | 45.5 | 77.6 | 94.5 | <.001 |
| eGFR by MDRD equation, mL/min/1.73 m2 | 69±10 | 71±18 | 47±8 | 47±8 | <.001 |
Data are mean±SD or percentage. Abbreviations: DBP, diastolic blood pressure; ECG‐LVH, left ventricular hypertrophy diagnosed by electrocardiography; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; PR, pulse rate; SBP, systolic blood pressure. P values were calculated using ANOVA or chi‐square test.
Incidence of Cardiovascular Events
During the 41‐month follow‐up period (2799 person‐years), 66 patients had cardiovascular events (23.6 events/1000 person‐years), including 59 events of clinical stroke (38 ischemic strokes, 9 hemorrhagic strokes, and 12 unknown subtype) and 11 events of myocardial infarction. Among the patients with cardiovascular events, 4 patients had both clinical stroke events and myocardial infarction during the follow‐up period.
When we analyzed patients according to dipping status and presence of CKD, the crude incidence of cardiovascular events in nondippers with CKD was higher (41.1 events/1000 person‐years) than that in extreme dippers with CKD (34.8 events/1000 person‐years) and higher than that in dippers with CKD (Figure 1).
Figure 1.
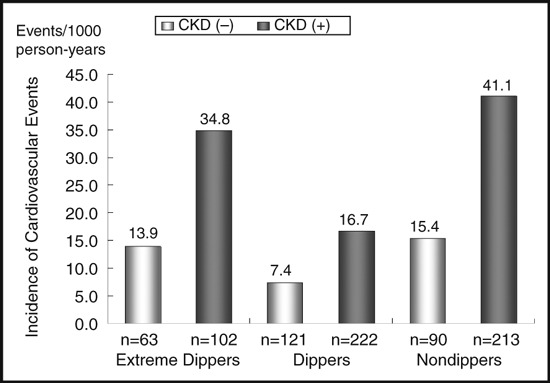
Crude incidence of cardiovascular events by dipping status with or without chronic kidney disease (CKD).
Cutoffs for eGFR for Prediction of Cardiovascular Events
Because it remained unknown whether the limit of 60 mL/min for eGFR was appropriate in elderly Japanese patients, we evaluated the risk of cardiovascular events as a function of eGFR. In univariate Cox regression analysis, a higher eGFR (as a continuous variable) was associated with a lower incidence of cardiovascular events (10 mL/min/1.73 m2 higher: HR, 0.81; 95% CI, 0.66–0.98; P=.030). As expected, patients with an eGFR <60 mL/min/1.73 m2 (as a categorical variable) had a significantly higher risk of cardiovascular events (HR, 2.50; 95% CI, 1.31–4.77; P=.006) than those with a normal eGFR. When we used other cutoffs (40 and 50 mL/min/1.73 m2) for the definition of CKD, the patients with CKD did not have the significant risk.
Risks of Cardiovascular Events of Dipping Status and Presence of CKD
In multivariate Cox regression analysis, nondippers and extreme dippers had an increased risk of cardiovascular events even after adjustment for 24‐hour systolic BP level (Table III, model 1). When eGFR (as a continuous variable) was put into model 1 for adjustment, dipping status (nondippers or extreme dippers) was an independent risk factor of cardiovascular events (Table III, model 2), but eGFR was not. After adjustment for the confounding factors such as age, sex, body mass index, current smoking, diabetes, hyperlipidemia, antihypertensive medication use, and eGFR, which were added into model 2 (Table III, model 3), the significance of extreme dippers and nondippers remained (extreme dipper: HR, 2.58; 95% CI, 1.26–5.29; P=.010; nondipper: HR, 1.83; 95% CI, 1.00–3.35; P=.0497). The other significant risk factors in model 3 adjusted for eGFR were age (10 years older: HR, 1.77; 95% CI, 1.32–2.37; P<.001), current smoking (HR, 2.24; 95% CI, 1.28–3.93; P=.005), and 24‐hour systolic BP (10‐mm Hg increase: HR, 1.36; 95% CI, 1.18–1.57; P<.001).
Table III.
Cox Regression Analysis for Cardiovascular Events in Dipping Status and eGFR or Presence of CKD (N=811)
| Model 1 (Adjusted for 24‐Hour SBP) | Model 2 (Adjusted for eGFR or CKD) | Model 3 (Adjusted for All Factors) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Risk of dipping status and eGFR | |||||||||
| Dipping status | |||||||||
| Extreme dipper | 2.31 | 1.14–4.07 | .02 | 2.33 | 1.15–4.73 | .019 | 2.58 | 1.26–5.29 | .010 |
| Dipper | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| Nondipper | 2.25 | 1.24–4.07 | .008 | 2.20 | 1.21–3.98 | .010 | 1.83 | 1.00–3.35 | .0497 |
| 24‐Hour SBP, 10 mm Hg higher | 1.41 | 1.24–1.61 | <.001 | 1.40 | 1.23–1.59 | <.001 | 1.39 | 1.20–1.61 | <.001 |
| eGFR, 10 mL/min/1.73 m2 higher | – | – | 0.83 | 0.68–1.01 | .064 | 0.89 | 0.73–1.09 | .28 | |
| Risk of dipping status and presence of CKD | |||||||||
| Dipping status | |||||||||
| Extreme dipper | 2.31 | 1.14–4.07 | .02 | 2.38 | 1.17–4.83 | .017 | 2.59 | 1.26–5.32 | .009 |
| Dipper | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| Nondipper | 2.25 | 1.24–4.07 | .008 | 2.16 | 1.19–3.91 | .011 | 1.83 | 0.998–3.34 | .051 |
| 24‐Hour SBP, 10 mm Hg | 1.41 | 1.24–1.61 | <.001 | 1.40 | 1.23–1.59 | <.001 | 1.39 | 1.20–1.60 | <.001 |
| CKD | – | – | 2.37 | 1.24–4.54 | .009 | 1.81 | 0.93–3.54 | .081 | |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration ratio by Modification of Diet in Renal Disease equation; HR, hazard ratio; SBP, systolic blood pressure. HR, 95% CI, and P values were calculated by Cox regression analysis. Model 1 is a multivariate analysis adjusted for 24‐hour SBP level. Model 2 is an analysis adjusted for eGFR (as a continuous variable) or presence of CKD (as a dichotomous variable) in addition to model 1. Model 3 is an analysis adjusted for known covariates such as age, sex, body mass index, smoking, diabetes, hyperlipidemia, and antihypertensive medication in addition to model 2.
When we adjusted for the presence of CKD (instead of eGFR) in the multivariate Cox regression analyses (Table III), the risks of nondipper status were not significant in the two models used: (1) dipping status adjusted for cardiovascular risk factors and presence or absence of CKD (Table III, model 3) and (2) dipping status adjusted for cardiovascular risk factors other than CKD (ie, age, sex, body mass index, current smoking, diabetes, hyperlipidemia, 24‐hour systolic BP, and antihypertensive medication use) (nondipper: HR, 1.82; 95% CI, 0.99–3.32; P=.053; extreme dipper: HR, 2.59; 95% CI, 1.26–5.30; P=.009). The other significant risk factors in model 3 adjusted for presence of CKD were age (10 years older: HR, 1.77; 95% CI, 1.32–2.38; P<.001), current smoking (HR, 2.18; 95% CI, 1.25–3.80; P=.006), and 24‐hour systolic BP (10‐mm Hg increase: HR, 1.39; 95% CI, 1.20–1.60; P<.001).
Discussion
The main findings of this study are that nondipping was not independently associated with a risk of future cardiovascular events when the presence of CKD and other cardiovascular risk factors were taken into account. Extreme dipping, however, was independently associated with the risk of future cardiovascular disease events, even after adjustment for CKD and other factors.
We found that the presence of CKD increased the risk of cardiovascular events and that it may be one of the factors that contribute to the risk described previously for nondippers. However, the pathophysiologic mechanisms by which CKD increases risk of cardiovascular events remain unclear. The cardiovascular risk of CKD was significant only when we evaluated the presence of CKD as a dichotomous variable, not when we evaluated eGFR as a continuous variable.
Nondipping status per se may be an early marker of decreased renal function before the development of definable renal dysfunction in elderly hypertensive patients. Decreased eGFR causes delayed sodium excretion that persists during the nighttime periods, 6 and nondipping status has been noted to precede deterioration in renal function. 20 It is reported that nocturnal hypertension sustained into the morning hours is related to morning hypertension and cardiovascular events. 21 In this study, however, there were no significant differences in morning systolic BP among the patients classified by dipping status and the presence or absence of CKD as defined (Table I).
In the present study of elderly Japanese hypertensive patients, the presence of CKD was one of the contributors to the poor prognosis in nondippers. Agarwal and Andersen 22 , 23 reported that in patients with CKD (53% of whom had hypertension), nondippers had an increased risk of events, but the significance disappeared after adjustment for covariates related to CKD. Although the patients in the present study (elderly hypertensives) and Agarwal and Andersen’s study (CKD) were different, the results suggest that the risk of nondippers might be largely explained by CKD and other confounding factors.
On the other hand, Bjorklund and associates 24 reported in a Swedish study (mean age 70 years, all men) that nondipping is a marker of risk in diabetic persons, but in the nondiabetic majority of the study population nondipping was not associated with target organ damage. In the present study, there were fewer indicators among the nondippers with CKD than the nondippers without CKD, although diabetic nephropathy is one of the causes of CKD. Factors related to nondipping may be different between elderly Japanese hypertensive patients and some other populations because body mass index and presence of diabetes were not significant risk factors for cardiovascular events in this study, although it has been reported that insulin resistance and central obesity enhance sodium sensitivity and cause nondipping. 25 , 26 The results of both Bjorklund and colleagues’ data and the present report indicate that the higher risk in nondippers might be explained by background factors that that of cause nondipping.
Aging is one of the risk factors for both cardiovascular events and nondipping. In patients with CKD, Minutolo and coworkers 9 reported that aging (not eGFR) was the most important risk predictor of nondipping. In the present study, the cardiovascular risk of nondipping and CKD were not eliminated after adjustment for age (data not shown). In addition, the cardiovascular risks of nondipping remained significant after adjusted for confounding factors including eGFR and age. The MDRD equation includes age as a parameter for calculating eGFR. Therefore, the risk of CKD includes the risk of aging, although we separately adjusted for age in the Cox regression analyses. Most cardiovascular events in this study were stroke; the incidence of stroke is more strongly affected by age than that of coronary heart disease is.
The sleep apnea syndrome is another risk for nondipping and cardiovascular events. In the present study, the increased cardiovascular risk of nondippers still had borderline significance, even after adjustment for confounding factors (Table III, model 3), and might be attributable to sleep apnea. We previously reported that nondipping was associated with increased low‐grade inflammation, a cardiovascular risk marker, in patients with sleep apnea, but not in those without. 27
It is reported that there are racial differences in the prevalence of nondipping, and blacks tend to demonstrate more nondipping because of decreased daytime sodium excretion. 28 It is difficult to clarify racial differences of nondipping and cardiovascular events from data in this study.
The cardiovascular event risk of extreme dippers was enhanced after adjustment for CKD and other risk factors. This suggests that the presence of CKD in extreme dippers (although uncommon) may add to the increased risk associated with extreme dipping on its own. The pathophysiologic mechanisms that give extreme dippers this increased risk may be different from those of CKD. Maeda and associates 29 reported that both extreme dipping and morning BP surge were related to increased reactive oxygen species formation by mononuclear cells.
Study Limitations
Nondippers have various backgrounds, and we need to assess the cardiovascular events between dipping status and other factors that may cause nondipper status (including sleep apnea syndrome), especially in younger populations. The other limitations of this study are the limited demographics of the study population (age and race/ethnicity), exclusion of patients with baseline Scr level >2.0 mg/dL, and the retrospective nature of the study.
Conclusions
Patients with CKD have an increased risk of cardiovascular events. The presence of CKD may account for some of the increased risk seen in nondippers in elderly Japanese hypertensives, but in contrast, the presence of CKD does not explain the increased risk that is also seen in extreme dippers.
Acknowledgments
Acknowledgments and disclosures: This study was partly supported by a grant‐in‐aid from the Foundation for the Development of the Community (KK), a research grant for cardiovascular medicine (14‐6) from the Ministry of Health, Labor and Welfare (KK), and a research grant (C‐2) from the Ministry of Education, Science and Culture (KK), Japan. All the authors declare that they participated in this study, and that they have seen and approved the final version. They also declare that they have no conflict of interest in connection with this paper. No part of this manuscript has been submitted or published elsewhere.
The first author is supported in part by a grant from the Mitsubishi Tanabe Pharma Research Foundation.
References
- 1. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Letter: creatinine clearance and age. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 3. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 4. National Kidney Foudation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 5. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 6. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. [DOI] [PubMed] [Google Scholar]
- 7. Fukuda M, Munemura M, Usami T, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–625. [DOI] [PubMed] [Google Scholar]
- 8. Portaluppi F. Loss of nocturnal blood pressure fall in patients with renal impairment. Arch Intern Med. 2006;166:2158–2159. [DOI] [PubMed] [Google Scholar]
- 9. Minutolo R, Borrelli S, Chiodini P, et al. Effects of age on hypertensive status in patients with chronic kidney disease. J Hypertens. 2007;25:2325–2333. [DOI] [PubMed] [Google Scholar]
- 10. Uzu T, Fujii T, Nishimura M, et al. Determinants of circadian blood pressure rhythm in essential hypertension. Am J Hypertens. 1999;12:35–39. [DOI] [PubMed] [Google Scholar]
- 11. Higashi Y, Oshima T, Ozono R, et al. Nocturnal decline in blood pressure is attenuated by NaCl Loading in salt‐sensitive patients with essential hypertension: noninvasive 24‐hour ambulatory blood pressure monitoring. Hypertension. 1997;30:163–167. [DOI] [PubMed] [Google Scholar]
- 12. Uzu T, Ishikawa K, Fujii T, et al. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. [DOI] [PubMed] [Google Scholar]
- 13. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 14. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 15. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 16. Kario K, Ishikawa J, Eguchi K, et al. Sleep pulse pressure and awake mean pressure as independent predictors for stroke in older hypertensive patients. Am J Hypertens. 2004;17:439–445. [DOI] [PubMed] [Google Scholar]
- 17. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 18. Kario K, Shimada K, Schwartz JE, et al. Silent and clinically overt stroke in older Japanese subjects with white‐coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. [DOI] [PubMed] [Google Scholar]
- 19. Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. [DOI] [PubMed] [Google Scholar]
- 20. Davidson MB, Hix JK, Vidt DG, et al. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846–852. [DOI] [PubMed] [Google Scholar]
- 21. Kario K. Time for focus on morning hypertension: pitfall of current antihypertensive medication. Am J Hypertens. 2005;18:149–151. [DOI] [PubMed] [Google Scholar]
- 22. Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol. 2006;26:503–510. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. [DOI] [PubMed] [Google Scholar]
- 24. Bjorklund K, Lind L, Andren B, et al. The majority of nondipping men do not have increased cardiovascular risk: a population‐based study. J Hypertens. 2002;20:1501–1506. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki M, Kimura Y, Tsushima M, et al. Association of insulin resistance with salt sensitivity and nocturnal fall of blood pressure. Hypertension. 2000;35:864–868. [DOI] [PubMed] [Google Scholar]
- 26. Uzu T, Kimura G, Yamauchi A, et al. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens. 2006;24:1627–1632. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa J, Hoshide S, Eguchi K, et al. Increased low‐grade inflammation and plasminogen‐activator inhibitor‐1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bankir L, Bochud M, Maillard M, et al. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. [DOI] [PubMed] [Google Scholar]
- 29. Maeda K, Yasunari K, Watanabe T, et al. Oxidative stress by peripheral blood mononuclear cells is increased in hypertensives with an extreme‐dipper pattern and/or morning surge in blood pressure. Hypertens Res. 2005;28:755–761. [DOI] [PubMed] [Google Scholar]


