Abstract
Background:
BASILICA and LAMPOON reduce the risk of coronary and LVOT obstruction during TAVR and TMVR. Despite successful laceration, BASILICA or LAMPOON may fail to prevent obstruction caused by inadequate leaflet splay in patients having challenging anatomy such as very small valve-to-coronary distance, diffusely calcified, rigid leaflets, or undergoing TAV-in-TAV. We describe a novel technique of balloon-augmented (BA) leaflet laceration to enhance leaflet splay.
Methods:
We measured the incremental leaflet splay from BA-BASILICA in vitro. From November 2019 to March 2021, sixteen patients underwent BA-BASILICA and four BA-LAMPOON at three centers.
Results:
BA-BASILICA increased benchtop leaflet tip splay 17%, maximum splay angle 30%, and splay area 23%, resulting in a more rounded apex and larger effective area. Sixteen patients at risk for inadequate BASILICA leaflet splay, including 4 TAV-in-TAV, underwent BA-BASILICA. All had successful leaflet laceration. One had coronary obstruction requiring immediate orthotopic stenting. Two underwent elective orthotopic coronary stenting through the transcatheter valve cells for leaflet prolapse without coronary ischemia. There were no deaths during the procedure or at 30 days. Four patients at risk for inadequate anterior mitral leaflet splay underwent BA-LAMPOON. All had successful target leaflet laceration without LVOT obstruction or procedural death. One died within 30 days.
Conclusions:
Balloon-augmented leaflet laceration enhances leaflet splay in vitro and may allow TAVR and TMVR in patients otherwise ineligible for traditional BASILICA or LAMPOON due to challenging anatomy.
Keywords: Transcatheter electrosurgery, coronary artery obstruction, left ventricular outflow tract obstruction, snorkel stent, leaflet calcification, Aortic Valve Replacement/Transcatheter Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions
Graphic Abstract
Balloon-augmented BASILICA and LAMPOON Enhances Leaflet Splay
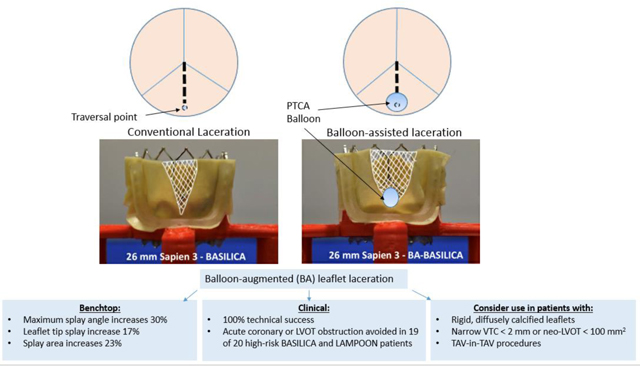
Abbreviations: PTCA – percutaneous transluminal coronary angioplasty, LVOT – left ventricular outflow obstruction, VTC – valve to coronary distance
Introduction:
Adjunctive BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction) and LAMPOON (Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction) reduce the risk of coronary and LVOT obstruction in selected patients undergoing transcatheter aortic valve replacement (TAVR) and transcatheter mitral valve replacement (TMVR), respectively.1–4
Patients with diffuse, confluent leaflet calcification suggesting rigidity, very narrow valve-to-coronary (VTC) distance or neo-LVOT dimensions may be at risk for inadequate leaflet splay with traditional BASILICA and LAMPOON, thereby increasing the risk for coronary or LVOT obstruction despite otherwise technically successful electrosurgical leaflet laceration. We hypothesized that balloon dilatation, prior to electrosurgical laceration, would improve leaflet splay and enable BASILICA-TAVR or LAMPOON-TMVR in otherwise anatomically high-risk patients. We tested the impact of balloon-augmented leaflet laceration on leaflet splay in-vitro and then performed balloon-augmented leaflet laceration (BA-BASILICA or BA-LAMPOON) in patients considered poor anatomical candidates for conventional BASILICA or LAMPOON because of rigid calcific leaflets, narrow antral dimensions, or TAV-in-TAV procedures where the cage of the indwelling THV might prevent adequate leaflet splay.5
METHODS:
The data that support findings of this study are available from the corresponding author upon reasonable request.
In Vitro Experiments:
Leaflet crossing, dilatation, and laceration:
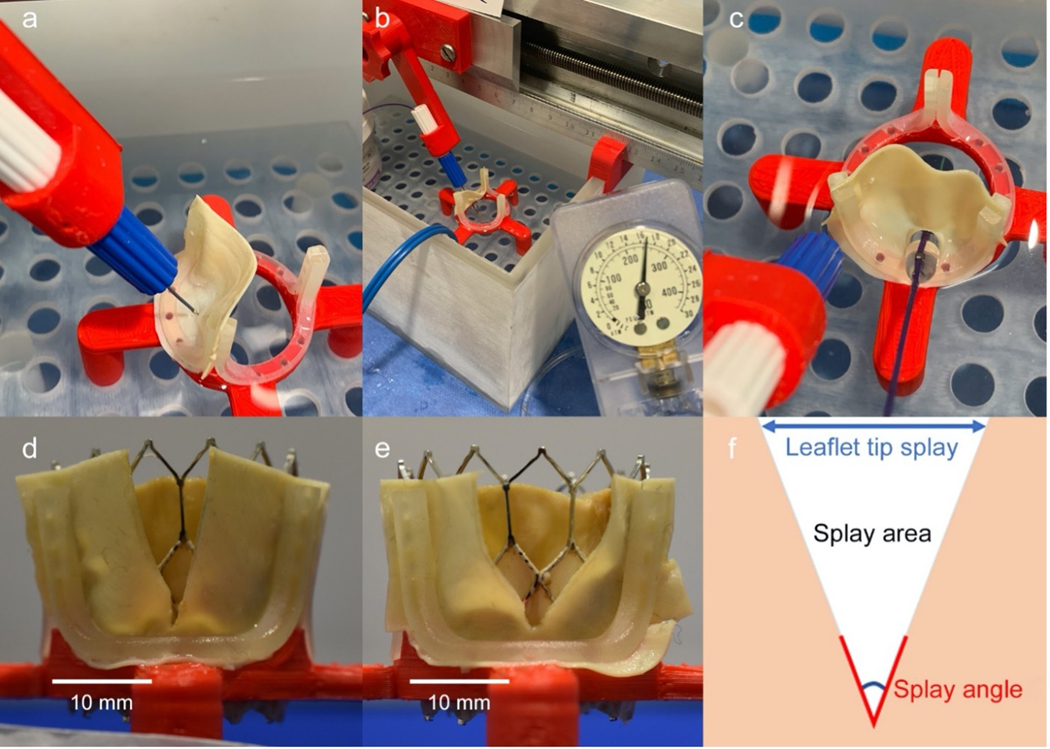
Simulated conventional BASILICA (n=5) and balloon-augmented BASILICA (BA-BASILICA) (n=5) leaflet modification procedures were performed in-vitro. A custom printed jig resembling a surgical aortic valve frame was submerged in 0.9% NaCl electrosurgical bath (Figure 1). Custom-shaped bovine pericardial leaflet templates (Peri-Guard, Synovis) were aligned with eyelets, clamped to the jig, and replaced after each experiment. Traversal points and angles were constrained by the jig for reproducibility. Leaflets were electrosurgically traversed with 0.014” AstatoXS20 (Asahi-Intecc) guidewires insulated using a microcatheter (FineCross, Terumo), energized at 30W, and lacerated with 70W pure cut energy. The leaflet crossing point was balloon dilatated after traversal but before laceration with a 4.5mm non-compliant balloon (NC Trek, Abbott) inflated to 17atm (5.07mm).
Figure 1: In Vitro BA-BASILICA Modeling.
Custom jig (a) to mount, traverse (a), optionally balloon dilatate (b, c) and lacerate pericardial “leaflets” in an electrosurgical bath and test balloon enlargement between traversal and laceration. Representative leaflet splay following BASILICA (d) and BA-BASILICA (e) laceration and subsequent implantation of a 26mm Sapien3. Schematic of a leaflet laceration (f) demonstrating splay measurements.
Simulated heart valve implantation after BA-BASILICA:
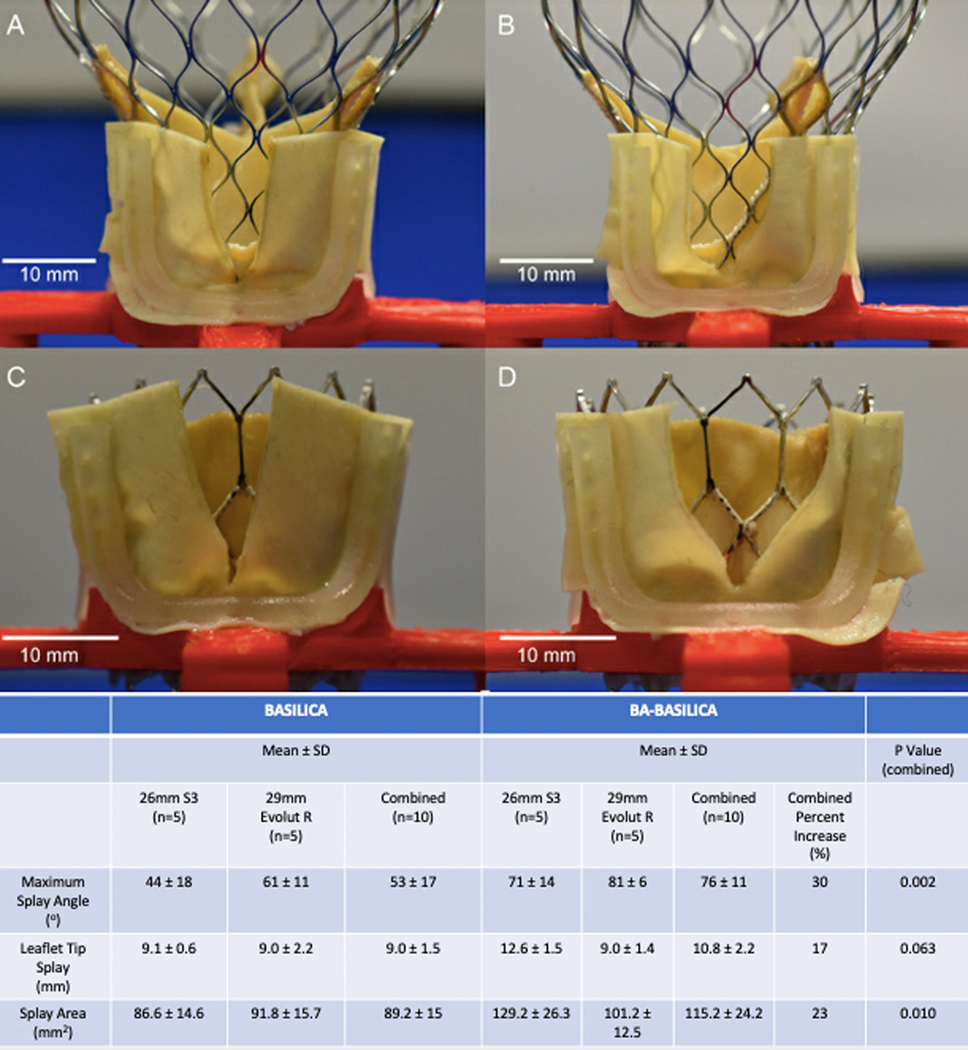
Self-expanding (29mm EvolutR, Medtronic) and balloon expandable (26mm Sapien3, Edwards) THVs were implanted in the jig to splay each lacerated leaflet. Twenty leaflet splays (BASILICA = 10, BA-BASILICA = 10) were analyzed. Splay angle was defined as the maximum angle created by lacerated/splayed leaflet edges. Leaflet tip splay was defined as the maximum distance between the two free edges of the leaflet tip. Splay area represented the total area of the resulting gap (Figure 1, Figure 2).
Figure 2: In-vitro Modeling of Leaflet Splay after Benchtop BASILICA and BA-BASILICA in Two Common TAVR Devices.
In-vitro data demonstrating increase in leaflet splay angle, tip splay distance, and leaflet splay area with BA-BASILICA. A) 29mm self-expanding THV following traditional BASILICA. B) 29mm self-expanding THV following BA-BASILICA. C) 26mm balloon expandable THV following traditional BASILICA. D) 26mm balloon expandable THV following BA-BASILICA. P value calculated using Student’s T-test.
Patients:
Between November 2019 and March 2021, sixteen BA-BASILICA and four BA-LAMPOON procedures were performed at three centers. Institutional ethics programs at all three authorized this retrospective report. End-points were classified using the Valve Academic Research Consortium-2 and Mitral Valve Academic Research Consortium definitions.6, 7
Patients were selected for BASILICA if they had: small sinuses (<30mm), low coronary heights (<10mm), small VTC distances (<4mm for bioprosthetic valves), and small VTSTJ distances (< 2mm).8, 9 A virtual valve at expected dimensions was modeled using ECG-gated contrast-enhanced cardiac CT. Patients were further selected for BA-BASILICA if they had CT diffuse bioprosthetic leaflet calcification that experience has taught us is associated with rigidity and reduced BASILICA splay, and/or ultra-narrow VTC <2mm, or TAV-in-TAV.1, 9
Patients were selected for LAMPOON when they had neo-LVOT<200mm2 measured using ECG-gated CT at the last systolic phase before aortic valve closure, along with an acceptable skirt-neo-LVOT.10 Patients were selected for BA-LAMPOON based on CT patterns suggesting rigidity risking inadequate leaflet splay, or life-threatening neo-LVOT<100mm2.
Procedure Technique:
All procedures used general anesthesia, transesophageal guidance, and cerebral embolic protection when possible.
Balloon-augmented BASILICA:
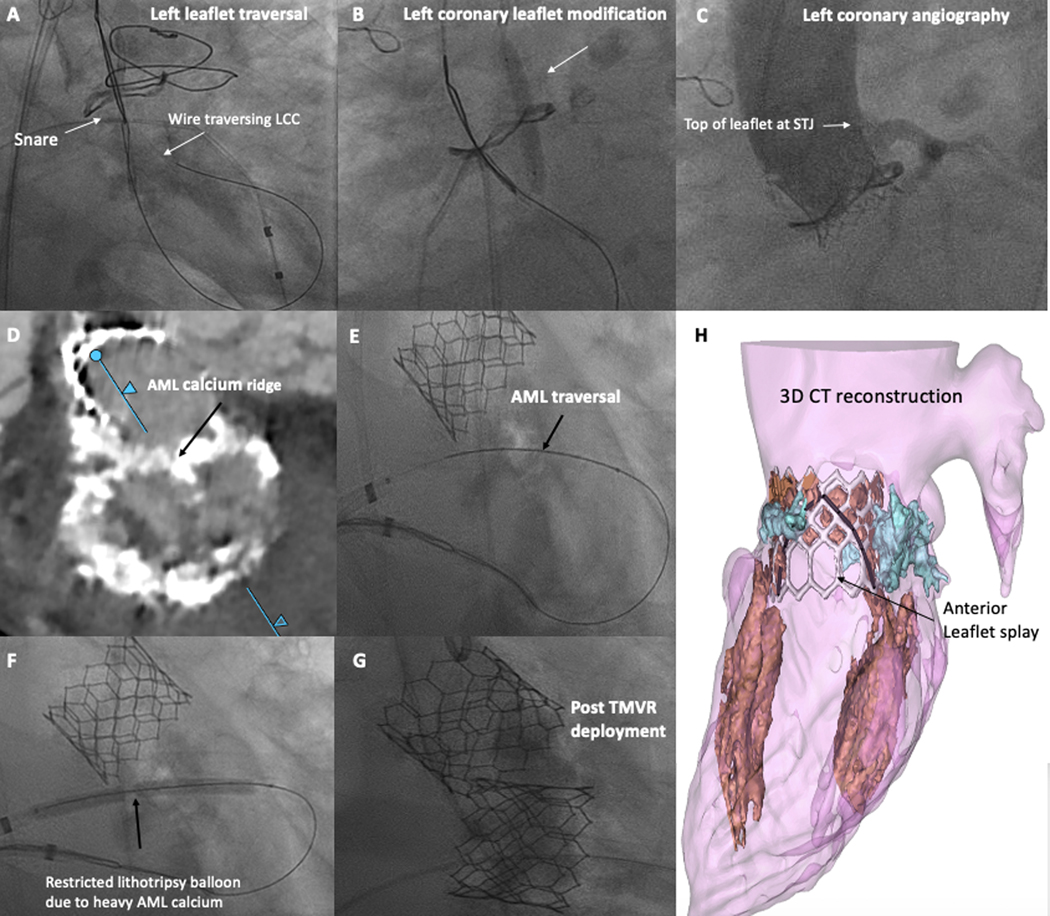
BASILICA traversal techniques have been described previously.3, 11 For BA-BASILICA, the microcatheter is temporarily removed and a 4–5mm non-compliant balloon, or 5mm lithotripsy balloon, is advanced over the traversal wire to straddle the leaflet, and inflated (Figure 3, panel B). Following successful dilatation of the traversal point, the “flying-V” lacerating surface is positioned across the leaflet alongside the reinserted microcatheter. The leaflet is lacerated in the standard fashion using 5% dextrose to displace blood and alternative current paths during brief 70W continuous duty-cycle radiofrequency ablation. Afterwards the THV is deployed as usual.
Figure 3: Demonstration of a BA-BASILICA and BA-LAMPOON Procedure.
Representative degenerated bioprosthetic aortic valve, at high risk for left coronary obstruction, who underwent BA-BASILICA-TAVR. A) Traversal of the left coronary leaflet. B) Balloon dilatation of the target leaflet after traversal, before laceration. C) Post-deployment angiogram demonstrating patent left coronary, despite leaflet visibly touching the STJ.
Representative patient with severe mitral stenosis, at high risk for LVOT obstruction, who underwent BA-LAMPOON-TMVR. D) Pre-procedural CT shows calcified basal AML. E) AML electrosurgical traversal. F) Lithotripsy pre-dilatation of the AML before laceration. G) After transcatheter mitral valve deployment. H) 3D CT reconstruction showing unobstructed cells and wide splay. (black = anterior leaflet split, white = Sapien3 valve frame, blue = annular calcification.
Abbreviations: AML – anterior mitral leaflet, LCC – left coronary cusp, LVOT – left ventricular outflow tract, STJ – sinotubular junction, TMVR – transcatheter mitral valve replacement
Balloon-augmented LAMPOON:
Antegrade LAMPOON techniques have been described previously.12 BA-LAMPOON dilatation is performed immediately before laceration using a 4–5mm balloon or lithotripsy catheter (Figure 3, panel F). The dilatation catheter is exchanged for the Piggyback microcatheter. Radiofrequency laceration is performed as above while retracting the guiding catheters into the left atrial guiding sheath. Sapien3 TMVR is then performed off-label for valve-in-ring or native valve-in-mitral annular calcification (MAC).
Statistics:
For in vitro data, continuous variables are expressed as mean ± standard deviation, and are compared using unpaired Student’s T-tests. A p value <0.05 was considered statistically significant. Statistical tests were not performed on procedural and 30-day outcomes due to the small sample size.
RESULTS:
In-Vitro Testing:
Traditional BASILICA enables triangular leaflet splay following THV implantation with the basal leaflet crossing point acting as a hinge at the apex of the triangle. Balloon dilatation of the tract prior to laceration forms a rounded apex, whereas traditional BASILICA creates a pointed apex (Figure 1). This appears to liberate the hinge by “stretching” the leaflet tissue at the apex, enhancing leaflet splay, and further enlarging the effective area for blood to flow to the coronary arteries.
In-vitro BA-BASILICA with a 4.5mm balloon increased leaflet splay area compared with BASILICA (115.2 ± 24.2mm2 vs 89.2 ± 15.0mm2, p=0.010). Similarly, leaflet tip splay (10.8 ± 2.2mm vs 9.0 ± 1.5mm, p=0.063) and maximum splay angle (76 ± 11° vs 53 ± 17°, p=0.002) were increased with BA-BASILICA (Figure 2). Results were comparable among implanted Evolut and Sapien 3 valves.
BA-BASILICA:
Sixteen patients at risk for coronary obstruction and inadequate leaflet splay with traditional BASILICA underwent BA-BASILICA. All were high or prohibitive risk for aortic valve replacement with multiple comorbidities (Table 1). TAVR was indicated for aortic stenosis in ten patients and aortic insufficiency in six. All had prior bioprosthetic valves, twelve surgical and four TAVR (Table 2). Doppio BASILICA was performed in eleven and solo BASILICA in five (Table 3). BA-BASILICA was doppio (performed on both the right and the left leaflets) for all TAV-in-TAV cases (Figure 4).13
Table 1:
Baseline Characteristics
| Baseline Characteristics | BASILICA (n=16) | LAMPOON (n=4) |
|---|---|---|
| Demographic Data | ||
|
| ||
| Age | 77.2 ± 8.1 | 75.7 ± 11.8 |
|
| ||
| Female | 11 (69%) | 3 (75%) |
|
| ||
| BSA | 1.9 ± 0.3 | 1.8 ± 0.3 |
|
| ||
| Home oxygen | 4 (25%) | 1 (25%) |
| Dialysis | 2 (13%) | 2 (50%) |
|
| ||
| Diabetes mellitus | 7 (44%) | 1 (25%) |
|
| ||
| Atrial fibrillation | 10 (63%) | 2 (50%) |
|
| ||
| Coronary artery disease | 5 (31%) | 0 |
|
| ||
| Peripheral artery disease | 4 (21%) | 0 |
| Prior stroke or TIA | 6 (38%) | 1 (25%) |
|
| ||
| Prior aortic valve replacement | 16 (100%) | 3 (75%) |
|
| ||
| NYHA Class III or IV symptoms | 16(100%) | 4 (100%) |
|
| ||
| STS predicted risk of mortality, % | 8.3 ± 4.9 | 17.3 ± 18.3 |
|
| ||
| Echocardiographic Data | ||
|
| ||
| Ejection Fraction | 55% ± 8.8 | 56% ± 8.5 |
|
| ||
| AVA, cm2 | 0.94 ± 0.6 | - |
|
| ||
| AV mean gradient, mmHg | 40 ± 17.7 | - |
|
| ||
| AV peak velocity, m/s | 4.0 ± 1.0 | - |
|
| ||
| MV mean gradient, mmHg | - | 12 ± 5.3 |
|
| ||
| MR severity ≥ moderate | - | 3 (75%) |
|
| ||
| LVOT gradient, mmHg | - | 5.3 ± 2.1 |
AV – aortic valve, AVA – aortic valve area, BSA – body surface area, LVOT – left ventricular outflow tract, MV – mitral valve, NYHA – New York Heart Association, STS – Society of Thoracic Surgeons, TIA – transient ischemic attack
Table 2:
BASILICA Valve Data
| Valve FailureMode | Prior Valve Type | New THV | Valve nominalSize | |
|---|---|---|---|---|
| Patient Number | ||||
| 1 | AS | 23 Perimount | S3 | 23 |
| 2 | AS | 21 Mitroflow | S3 | 20 |
| 3 | AS | 19 Trifecta | S3 | 20 |
| 4 | AS | 23 Trifecta | S3 | 23 |
| 5* | AI | 27 Freestyle | S3 | 23 |
| 6 | AI | 23 Trifecta | S3 | 23 |
| 7* | AI | 21 Mitroflow | S3 | 20 |
| 8 | AS | 29 Corevalve | S3 | 26 |
| 9 | AS | 29 Sapien3 | S3 | 29 |
| 10 | AS | 27 Magna | EvolutPro + | 29 |
| 11 | AI | 25 Toronto | S3 | 23 |
| 12 | AS | 29 EvolutR | S3 Ultra | 23 |
| 13 | AI | 29 Mitroflow | S3 Ultra | 26 |
| 14 | AS | 21 Magna | EvolutPro + | 23 |
| 15* | AI | 19 Mitroflow | S3 | 20 |
| 16 | AS | 26 EvolutR | S3 | 23 |
A table summarizing clinical data from all BA-BASILICA patients. Bold text denotes patients requiring coronary stenting, despite BASILICA.
denotes patients requiring coronary stenting, despite BASILICA
Abbreviations: AI – aortic insufficiency, AS – aortic stenosis, S3 – Sapien3, THV - transcatheter heart valve
Table 3:
BASILICA CT Data
| Reason for BA | Target Leaflet | Area, mm2 | Perimeter, mm | LCA Height, mm | RCA Height, mm | L Sinus Width, mm | R Sinus Width, mm | L VTC, mm | L VTSTJ, mm | R VTC, mm | R VTSTJ, mm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rigid Leaflet | Left | 328 | 64 | 5.2* | 12.3 | 28 | 28 | 4.1 | 0* | 5.4 | 5.9 |
| 2 | Rigid Leaflet | Doppio (L-BA) | 334 | 53 | 4.7* | 8.3* | 26 | 26 | 5.6 | 1.9* | 2.8* | 2 |
| 3 | Rigid Leaflet | Doppio (L-BA) | 230 | 54 | 4.2* | 8.7* | 28 | 27 | 3.9* | 2.3 | 5.1 | 1.1* |
| 4 | Rigid Leaflet | Left | 334 | 65 | 8.7* | 13.1 | 35 | 35 | 5.4 | 0* | 9.1 | 5.8 |
| 5† | Rigid Leaflet | Doppio (L-BA) | 382 | 70 | 8.7* | 8.3* | 25 | 23 | 3.5* | Above virtual valve | 0* | 0* |
| 6 | Rigid Leaflet | Doppio (L,R-BA) | 343 | 66 | 2.7* | 6.5* | 26 | 26 | 3.8* | 1.9* | 4.6 | 1.8* |
| 7† | Rigid Leaflet | Left | 230 | 54 | 7.5* | 8.8* | 25 | 23 | 3.3* | 3.6 | 2.7 (RCA CTO) | 1.9* |
| 8 | TAV-in-TAV | Doppio (L,R-BA) | 400 | 72 | 11.6 | 10.6 | 28 | 27 | 1.8* | 1.9* | 2* | 0* |
| 9 | TAV-in-TAV | Doppio (L,R-BA) | 541 | 83 | 20.7 | 23 | 34 | 34 | 3.9* | 2.8 | 0.5* | 1* |
| 10 | Small VTC | Doppio (L,R-BA) | 482 | 76 | 9.7* | 10.5 | 26 | 26 | 1.6* | 0* | 3* | 0* |
| 11 | Small VTC | Right | 357 | 67 | 11 | 8.7* | 26 | 24 | 3.3 (patent LIMA-LAD) | 3 | 1.9* | 1.3* |
| 12 | TAV-in-TAV | Doppio (L,R-BA) | 421 | 73 | 19.2 | 22.6 | 32 | 31 | 3.3* | 0* | 5.4 | 0* |
| 13 | Small VTC | Right | 483 | 78 | 15.5 | 7.5 | 30 | 30 | Above virtual valve | Above virtual valve | 2.3* | 0.5* |
| 14 | Small VTC | Doppio (R-BA) | 344 | 66 | 7.5* | 11.4 | 26 | 23 | 3.4* | 1.8* | 1.6* | 1.9* |
| 15† | Small VTC | Doppio (L-BA) | 204 | 52 | 4.6* | 6.6* | 21 | 23 | 2.2* | 0* | 3.6* | 3.1 |
| 16 | TAV-in-TAV | Doppio (L,R-BA) | 393 | 71 | 19 | 24 | 27 | 27 | 3.7* | 0* | 3.2* | 0* |
| All | 363 ± 94 | 66 ± 9 | 10 ± 5.8 | 11.9 ± 5.8 | 28 ± 3.6 | 27 ± 3.5 | 3.5 ± 1.1 | 1.4 ± 1.3 | 3.3 ± 2.2 | 1.6 ± 1.9 |
A table summarizing CT data from all BA-BASILICA patients.
denotes features considered high risk for coronary obstruction
denotes patients requiring coronary stenting, despite BASILICA.
Abbreviations: AI – aortic insufficiency, AS – aortic stenosis, BA – balloon-augmented, CTO – chronic total occlusion, L – left, LCA – left coronary height, R – right, RCA – right coronary height, S3 – Sapien3, TAVR – transcatheter aortic valve replacement, THV - transcatheter heart valve, VIV – valve in valve, VTC – valve to coronary distance, VTSTJ – valve to sinotubular junction distance
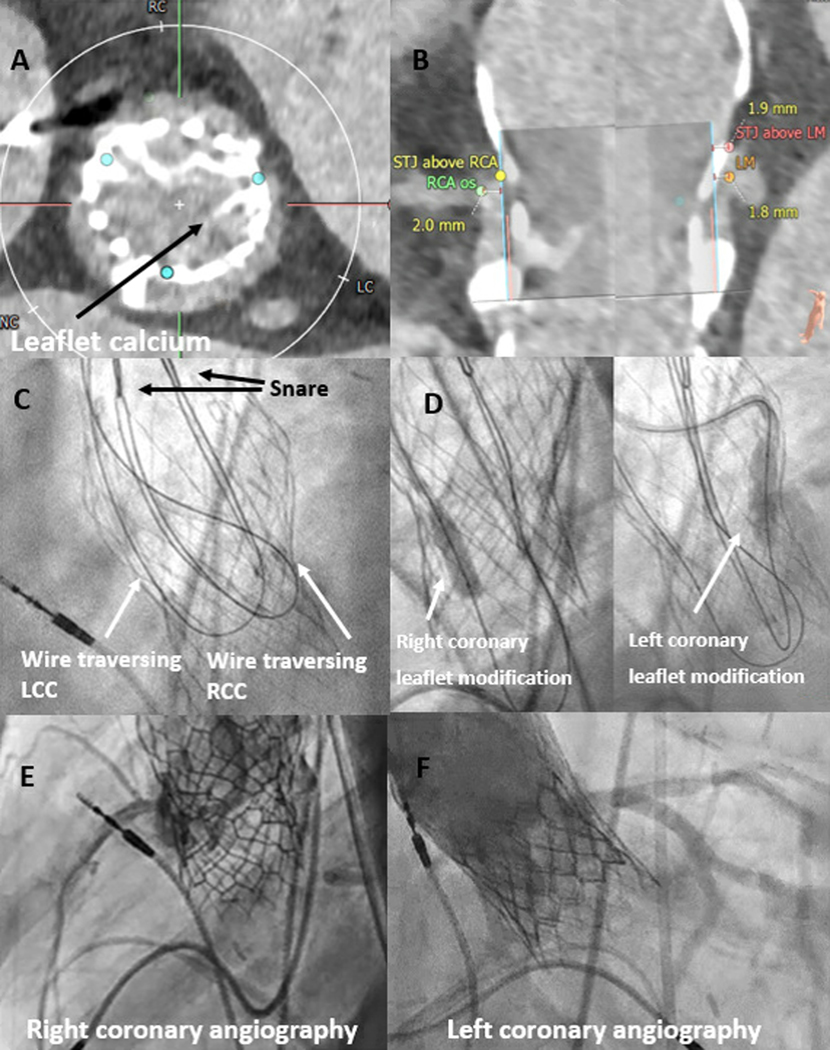
Figure 4: TAV-in-TAV Doppio BA-BASILICA.
A, B) Pre-procedural CT demonstrating calcified leaflets portending high risk for bilateral coronary obstruction. C) Standard electrosurgical traversal of left and right coronary leaflets. D) Balloon dilatation of the target leaflets. E, F) Patent left and right coronaries after TAV-in-TAV. Adapted with permission from Greenbaum 13
Abbreviations: LCC – left coronary cusp, LM – left main, RCC – right coronary cusp, RCA – right coronary artery, STJ – sinotubular junction
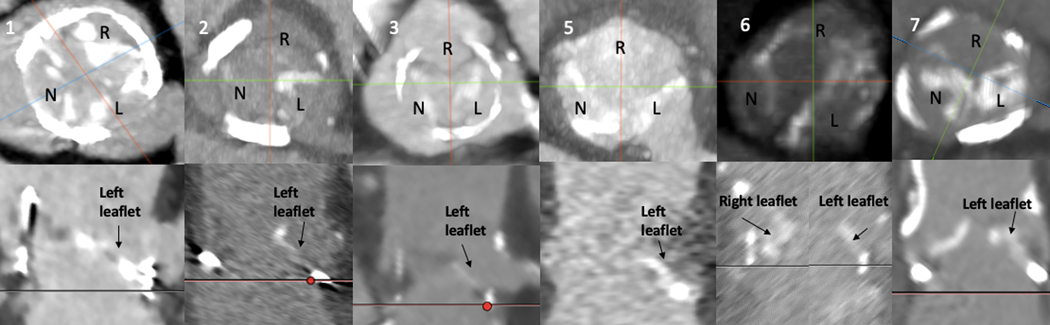
Table 3 describes the geometry of the annulus, VTC and VTSTJ distances. All risked coronary obstruction – coronary height < 10mm, sinus width < 30mm, VTC <4mm for bioprosthetic valves, VTSTJ < 2mm.8, 9, 14, 15 Seven of sixteen had patterns of diffuse CT calcification of the target leaflet that we associate with excessive leaflet rigidity and that we believe portends inadequate splay despite laceration (Figure 5). By comparison, 9 consecutive contemporaneous Emory BASILICA cases performed during the study period exhibited no significant difference in VTC/VTSTJ, but significantly less target leaflet calcification.
Figure 5: Calcium Distribution Patterns for BA-BASILICA Patients.
Patterns of heavy or diffuse calcification that might impede leaflet splay after laceration. Upper panels: Representative axial pre-procedural CT images of 6 BA-BASILICA patients with calcified target leaflets. Lower panels: Corresponding coronal images. Numbers correspond to patient number in Tables 2 and 3.
Abbreviations: L – left coronary cusp, N – non coronary cusp, R – right coronary cusp,
Table 4 describes procedural and clinical outcomes. Leaflet traversal was successful in all and lacerations confirmed midline and lengthwise in all 15 of 16 having transesophageal-echo guided procedures. Balloon leaflet dilatation did not change hemodynamics. All patients underwent successful valve-in-valve TAVR using a single THV. At 30 days, no cases required re-intervention or surgery and all patients were alive.
Table 4:
BA-BASILICA Procedural Characteristics and Outcomes
| Procedural Characteristics | N = 16 |
|---|---|
| Access | 15 (94%) Transfemoral1 (6%) Transcaval |
| Sentinel cerebral protection | 16 (100%) |
| General anesthesia | 15 (94%) |
| Procedural Outcomes | |
| Successful traversal | 16 (100%) |
| Midline/lengthwise laceration | 16 (100%) |
| Time added by BA, min | 11.8 ± 4.6 |
| New or increase pressor use following BA | 0 |
| Successful TAVR implant | 16 (100%) |
| Need for second valve | 0 |
| Need for PPM | 0 |
| Coronary obstruction | 1 (6%) – patient 15 |
| Rescue stent for leaflet prolapse | 2 (13%) – patients 5 and 7 |
| PCI or surgery at 30 days | 0 |
| Procedural Complications | |
| Stroke | 1 (6%) |
| Major vascular complication | 2 (13%) |
| Life threatening bleed | 1 (6%) |
| Echocardiographic Outcomes | |
| Ejection fraction | 57.2 ± 5.8 |
| AVA | 1.9 ± 0.7 |
| AV mean gradient, mmHg | 16 ± 5.7 |
| AV peak velocity, m/s | 2.7 ± 0.5 |
| Aortic insufficiency ≥ mild | 0 |
| Survival | |
| Survived procedure | 16 (100%) |
| Survived to 30 days | 16 (100%) |
A table summarizing procedural, echocardiographic and clinical outcomes data from all BA-BASILICA patients.
Abbreviations: AV – aortic valve, AVA – aortic valve area, BA – balloon-augmented, PCI – percutaneous coronary intervention, PPM – permanent pacemaker, TAVR – transcatheter aortic valve replacement
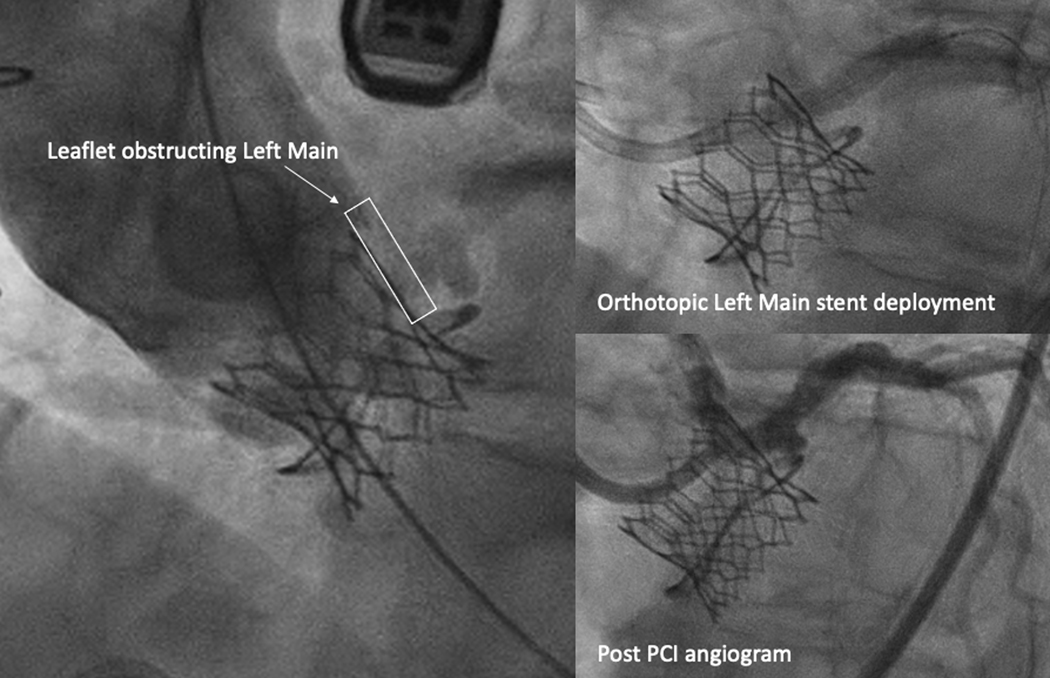
Three patients underwent coronary stenting despite BA-BASILICA. One had acute coronary obstruction causing myocardial ischemia despite BA-BASILICA (Table 3, Patient 15) requiring emergency left main PCI, as shown in Figure 6. Two exhibited leaflet prolapse into the coronary artery after BA-BASILICA, and therefore underwent non-emergency orthotopic (in the “normal” sino-coronary position through the THV struts) coronary stenting during the same procedure (Table 3, Patients 5, 7). In all three, BA-BASILICA allowed straightforward, rapid orthotopic coronary stenting through THV struts.
Figure 6: Acute Coronary Obstruction Salvaged with Orthotopic Left Main Stenting.
Left main coronary obstruction despite successful BA-BASILICA in a patient with a small Mitroflow valve and a low coronary ostium, effaced sinus, and very narrow VTC. BA-BASILICA allowed successful left main PCI in an orthotopic fashion, through the cells of the THV.
Abbreviations: PCI – percutaneous coronary intervention, THV – transcatheter heart valve, VTC – valve to coronary distance
Four procedures were TAV-in-TAV and used doppio BA-BASILICA. Despite deficient sinuses on pre-procedure CT, all four exhibited linear, mid-line splay on transesophageal echocardiography and were spared coronary obstruction or leaflet prolapse.
There was one non-disabling hemorrhagic stroke and two major vascular complications. One patient had an occluded profunda femoris artery following suture mediated closure, which was treated with balloon angioplasty restoring normal flow. The other had vascular access bleeding that required covered stent placement (Table 4).
BA-LAMPOON:
Four patients at risk for fixed LVOT obstruction and inadequate leaflet splay with traditional LAMPOON underwent BA-LAMPOON. All were high or prohibitive risk for mitral valve replacement (Table 1).
The indication for TMVR was mitral stenosis in three and mitral insufficiency in one. Three were valve-in-MAC and one was a valve-in-ring procedure (Table 5). Three of four had cerebral protection and prophylactic IABP placed before traversal of the leaflet (Table 6). Dilatation used a non-compliant coronary balloon in two and a lithotripsy balloon in two.
Table 5:
BA-LAMPOON Procedural Data
| Patient Number | All | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Reason for BA | Small neo-LVOT | Rigid leaflet +small neo-LVOT | Rigid leaflet | Rigid leaflet | |
| TMVR setting | 75% MAC, 25% Ring | Ring | MAC | MAC | MAC |
| Valve failure mode | 75% MS, 25% MR | MR | MS | MS | MS |
| Prior valve type | 75% native | 28mm Simulus | N/A | N/A | N/A |
| New THV | 100% Sapien | Sapien3 Ultra | Sapien3 | Sapien3 | Sapien3 |
| Valve nominal size | 26 | 29 | 29 | 29 | |
| CT Data | |||||
| Annular area, cm 2 | 5.1 ± 0.9 | 4 | 5.4 | 4.7 | 4.8 |
| AP distance, mm | 19.6 ± 2.7 | 18.4 | 17.1 | 19.4 | 23.3 |
| Intercommissural distance, mm | 29.4 ± 3.3 | 26.6 | 27.7 | 34 | 29.3 |
| Predicted neo-LVOT, mm2 | 132.9 ± 52.1 | 82 | 94 | 181 | 164 |
| Predicted skirt neo-LVOT, mm2 | 214.9 ± 29.9 | 199 | 188 | 256 | 217 |
A table summarizing clinical and CT data from all BA-LAMPOON patients.
Abbreviations: AP – antero-posterior, BA – balloon-augmented, LVOT – left ventricular outflow tract, MR – mitral regurgitation, MS – mitral stenosis, THV – transcatheter heart valve, TMVR – transcatheter mitral valve replacement
Table 6:
BA-Lampoon Procedural Characteristics and Outcomes
| Procedural Characteristics | N = 4 |
|---|---|
| Access | 4 (100%) Transfemoral/trans-septal |
| Sentinel cerebral protection | 3 (75%) |
| General anesthesia | 4 (100%) |
| Apical rail | 2 (50%) |
| Procedural Outcomes | |
| Successful traversal | 4 (100%) |
| Midline/lengthwise laceration | 4 (100%) |
| Time added by BA, min | 11.5 ± 4.4 |
| New or increase pressor Use | 4 (100%) |
| Prophylactic IABP | 3 (75%) |
| Successful TMVR implant | 4 (100%) |
| Need for second valve | 0 |
| Required bail-out ASA | 0 |
| LVOT obstruction | 0 |
| Pericardial effusion requiring drainage | 2 (50%) |
| Paravalvular leak > mild | 1 (25%) planned AVP during index procedure |
| MR > mild | 0 |
| Procedural Complications | |
| Stroke | 0 |
| Major vascular complication | 0 |
| Life threatening bleed | 0 |
| Echocardiographic Outcomes | |
| Ejection fraction | 53% ± 5.0 |
| MV mean gradient, mmHg | 5.3 ± 1.5 |
| MR severity > mild | 0 |
| Perivalvular MR > mild | 1 (25%) |
| LVOT gradient, mmHg | 9.8 ± 5.0 |
| LVOT gradient increase < 10 mmHg | 3 (75%) |
| AV mean gradient, mmHg | 15.3 ± 9.5 |
| Survival | |
| Survived procedure | 4 (100%) |
| Survived to 30 days | 3 (75%) |
A table summarizing procedural, echocardiographic and clinical outcomes data from all BA-LAMPOON patients.
Abbreviations: ASA – alcohol septal ablation, AV – aortic valve, ASA – alcohol septal ablation, AVP – Amplatzer vascular plug, BA – balloon-augmented, IABP – intra-aortic balloon pump, LVEF – left ventricular ejection fraction, LVOT – left ventricular outflow tract
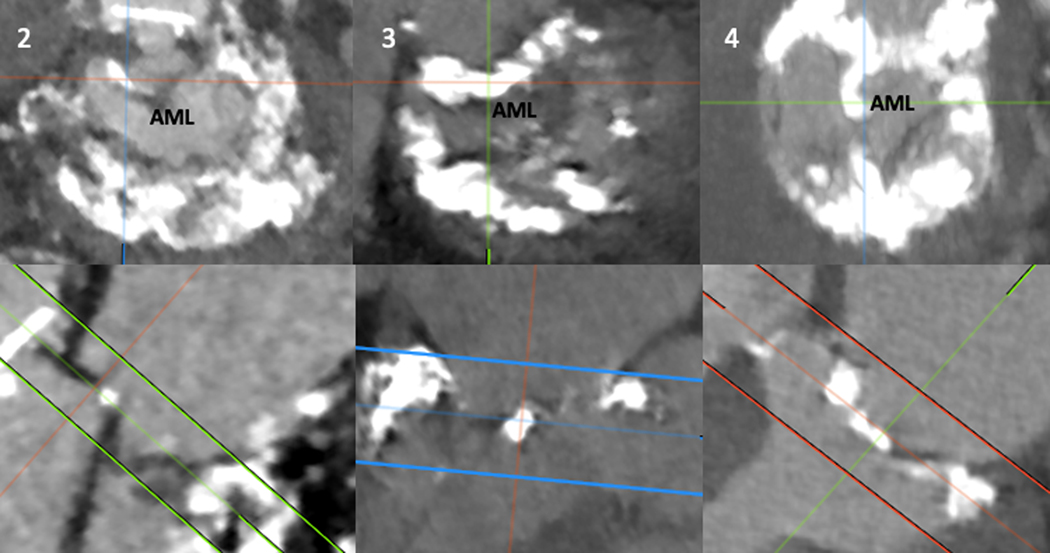
Table 5 describes the geometry of the annulus and LVOT on CT. All had a neo-LVOT of less than 200mm2. All valve-in-MAC patients had calcification of the AML that risked inadequate leaflet splay despite laceration. Patient 2 had a thick ridge of calcium extending from the base to tip of the lateral aspect of the leaflet, in addition to a small neo-LVOT. Patient 3 had extensive basal calcification extending from the annulus into the base of the AML. Patient 4 had a ridge of calcium extending from the base to tip of the mid AML (Figure 7).
Figure 7: Calcium Distribution Patterns of Valve in MAC BA-LAMPOON Patients.
Upper panels: Axial pre-procedural CT images of the annulus and AML.
Lower panels: Corresponding cross-sectional images of the annulus and AML of the same patients. Numbers correspond to patient number in Table 5.
Abbreviations: AML – anterior mitral leaflet
Table 6 describes procedural and clinical outcomes. Leaflet traversal and midline laceration were successful in all. All required increased vasopressors following leaflet laceration. The patient who did not receive prophylactic IABP developed pulmonary edema and hemodynamic compromise, which responded to therapeutic IABP. All IABPs were removed at the end of the procedure.
All patients underwent successful TMVR with implantation of a single THV and survived the immediate procedure. One patient with valve-in-MAC had a severe, anteromedial paravalvular leak following TMVR which was successfully treated with an Amplatzer vascular plug (Abbott) during the index procedure. In two patients a 6Fr transthoracic apical rail was used to assure THV coaxiality during implantation; both had a prophylactic overnight subxiphoid pericardial drain.16 None required re-intervention or surgery at 30 days. No strokes or major vascular complications occurred during follow-up.
LVOT obstruction was averted in all. The average peak echocardiographic LVOT gradient was 5.3mmHg at baseline (Table 1) and increased to 9.8mmHg at discharge (Table 6). One patient died after discharge, within thirty-days. He was offered TMVR despite end stage renal disease, advanced cirrhosis, and right heart failure/severe TR and chose hospice because of worsening liver failure
DISCUSSION:
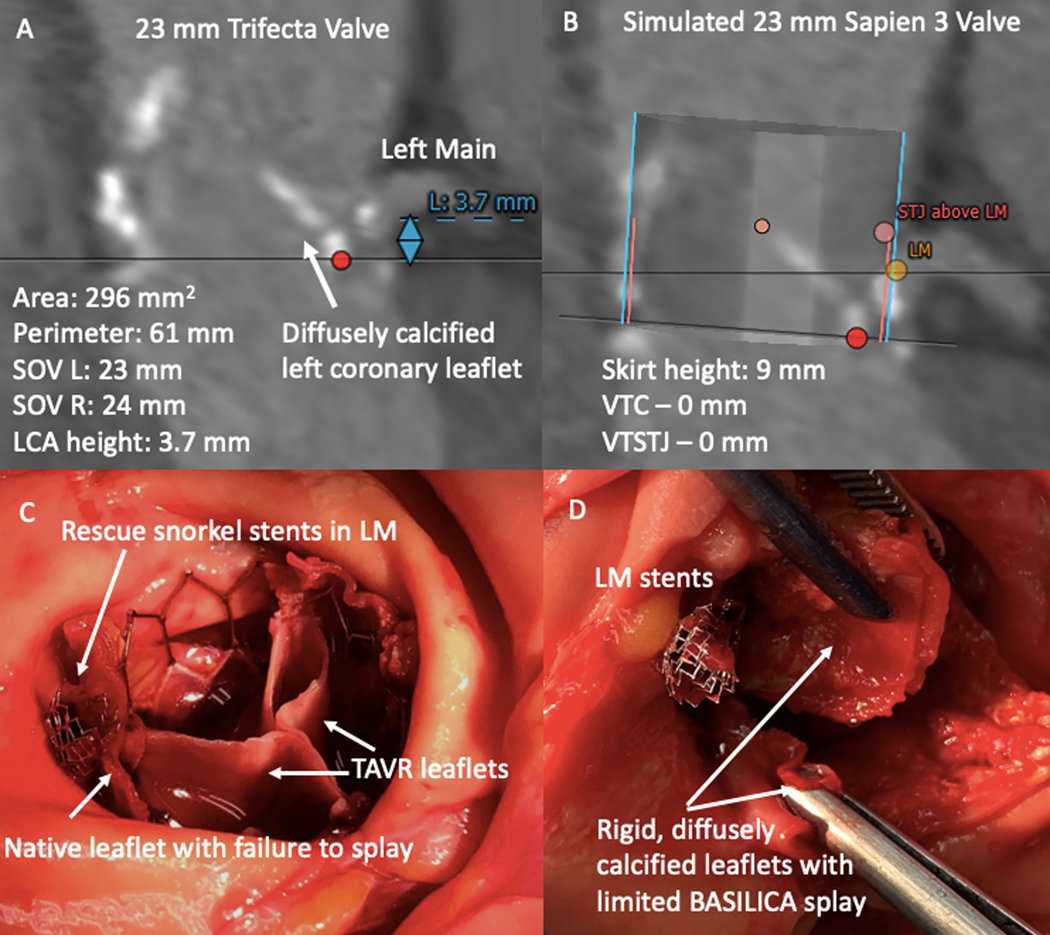
Our findings suggest that BA-leaflet modification may improve splay in selected patients undergoing BASILICA-TAVR and LAMPOON-TMVR. This project was motivated by observations from our clinical practice that certain anatomic features ― rigid, diffusely-calcified bioprosthetic leaflets; effaced sinuses with very narrow VTC; and TAV-in-TAV cages ― risk inadequate leaflet splay despite technically successful BASILICA or LAMPOON. A post-mortem examination of a patient who developed fatal coronary obstruction despite technically successful BASILICA and rescue “snorkel” stenting revealed a rigid, diffusely calcified bioprosthetic leaflet that failed to splay adequately despite the intended midline longitudinal laceration, exacerbated by THV commissural malalignment (Figure 8). Similarly, a patient with a rigid, heavily calcified AML died from acute LVOT obstruction due to inadequate leaflet splay despite successful midline electrosurgical laceration. In addition, we previously reported a rigid, surgically repaired AML which failed to splay, despite successful electrosurgical laceration in the LAMPOON IDE trial.2 We developed this technique modification to add a small incremental leaflet splay area to allow compassionate-care options for extremely high-risk patients.
Figure 8: Unsuccessful BASILICA and Fatal TAVR due to Rigid, Calcified Leaflet and Narrow VTC/VTSTJ Dimensions.
Fatal coronary obstruction despite BASILICA, which inspired development of balloon-augmented BASILICA. A) Pre-procedure CT showing a diffusely calcified left coronary leaflet, short left main, and small sinuses of Valsalva. B) Pre-procedure CT planning demonstrating high risk for coronary occlusion due to VTC and VTSTJ of 0. C) Autopsy findings with native leaflet pinned by TAVR valve, crushing rescue left main stent. D) Autopsy findings showing rigid, diffusely calcified leaflet with limited splay despite BASILICA.
Abbreviations: L – left, LM – left main, R – right, SOV – sinus of Valsalva, TAVR – transcatheter aortic valve replacement, VTC – valve to coronary distance, VTSTJ – valve to sinotubular junction distance
In the cohort reported here, balloon-expansion of the electrosurgical traversal point was feasible and added minimal complexity to the procedure. BA leaflet modification accomplished adequate leaflet splay, in all but one patient, in a group at risk for obstruction despite traditional BASILICA-TAVR or LAMPOON-TVMR. These findings are supported by in-vitro modeling, which confirmed a larger splay area when compared with standard BASILICA (Figure 2). Post TMVR 3D CT reconstruction similarly demonstrated a wide splay of the AML with full exposure of three cells of the THV valve in the LVOT (Figure 3, panel H). While BA-BASILICA failed to prevent coronary obstruction in one patient with extreme root geometry (small VTC, zero VTSTJ, low coronary height) and a prosthesis with externally-mounted leaflets, we believe that the BA-BASILICA enabled orthotopic stenting otherwise impossible because of the markedly effaced sinus of Valsalva.
Despite adequate splay of the target leaflet with BA-BASILICA, two additional patients required coronary stenting in a controlled, non-emergency fashion during the index procedure. Mobile tissue from the elongated, bioprosthetic leaflet was found to encroach on the left main coronary ostium. Following confirmatory intravascular ultrasound, we decided to perform elective left main stenting to avoid delayed left main occlusion. In retrospect, the leaflets were degenerated and excessively mobile causing baseline severe aortic insufficiency (AI) in one Freestyle xenograft and one Mitroflow pericardial valve. Importantly, BA-BASILICA allowed coronary stents to be positioned through a THV cell in an orthotopic position, rather than “snorkeled” around the THV frame at the STJ which risks delayed stent crush, fracture, and thrombosis 8, 14. While the safety and efficacy of BASILICA has been previously described, there is a paucity of data on BASILICA applied for AI.1 A recent case report describes a similar scenario of leaflet prolapse following classic BASILICA, in a patient with AI.17 Leaflet prolapse is a known consequence of spontaneous or BASILICA-induced leaflet avulsion during TAVR; we do not believe BA-BASILICA exacerbated leaflet prolapse. However we recommend vigilance during BASILICA in the setting of hypermobile bioprosthetic leaflets and AI; lacerated leaflets may prolapse into the target coronary arteries and require orthotopic stenting. Our findings highlight the need for continued refinement of leaflet modification strategies and for the development of new techniques, such as leaflet excision, for high-risk scenarios.
Despite advances in TMVR technique, thirty-day mortality for valve-in-MAC procedures remains high. 18, 19 The ViMAC arm of the recent MITRAL trial reported a 17% thirty-day mortality in a cohort selected to be free of LVOT obstruction risk.19
Balloon-augmentation per se did not add significant incremental procedural time or complexity to standalone BASILICA or LAMPOON in this small series. Leaflet modification was performed with readily available coronary or lithotripsy balloons. Lithotripsy/lithoplasty balloons did not add detectable benefit over conventional angioplasty balloons, although the two strategies were not adequately compared in this small case series.
BA-BASILICA did not cause hemodynamic instability in any patients. Conversely, all four BA-LAMPOON patients required increased catecholamines following AML laceration, compared with 20% of patients in the LAMPOON IDE trial. This may reflect the wider split achieved with BA-LAMPOON.2 We recommend prophylactic IABP to maintain hemodynamic stability despite transient acute mitral regurgitation during the interval between LAMPOON and TMVR. Indeed one patient without prophylactic IABP required IABP hemodynamic support immediately following laceration.
Lacerating heavily calcified leaflets may increase stroke risk.1, 3 As such, we used cerebral embolic protection in all cases with suitable anatomy. In this group of patients with extreme aortic and mitral leaflet calcification, there was a single non-disabling, hemorrhagic stroke occurring in the BA-BASILICA group. This patient had a pre-procedure ischemic stroke and post-TAVR had a small hemorrhagic conversion during initiation of anticoagulation. These limited data suggests that judicious use of cerebral embolic protection is warranted in BA-BASILICA and LAMPOON.
Finally, there were four TAV-in-TAV patients included in this study. Previous bench top modeling of BASILICA in newer generation TAVR valves has demonstrated less effective leaflet splay with traditional BASILICA due to leaflet camber (depth) in the latest-generation THV devices, and because the previous THV cage limits leaflet splay. This substantially increases risk of coronary obstruction despite BASILICA in certain TAV-in-TAV cases having short and narrow aortic roots.5 Coronary obstruction was successfully averted in all TAV-in-TAV patients in this series. BA-BASILICA may be especially important for TAV-in-TAV, to achieve incrementally larger splay, and where in addition commissural alignment is essential to prevent coronary obstruction.
LIMITATIONS:
Accurate leaflet splay measurements are not feasible in patients. Absent a control group, we cannot definitively conclude that balloon leaflet modification improved clinical outcomes. Because there is no suitable animal model of aortic or mitral leaflet calcification, our bench-top modeling was performed on non-calcified pericardial templates rather than transcatheter heart valves, and thus may not adequately reflect the impact of BA-laceration on calcified and rigid leaflets. We did not specifically model BA-LAMPOON in vitro and instead extended the lessons from BA-BASILICA directly. Finally, our CT-based prediction of leaflet rigidity is subjective.
CONCLUSIONS:
Balloon-augmented leaflet modification was technically feasible and successful in all patients. Balloon-augmented leaflet modification may be an effective tool for facilitating leaflet splay in challenging and high-risk anatomy and is a reasonable treatment strategy for patients previously felt to be ineligible for traditional BASILICA or LAMPOON procedures.
What is Known:
Leaflet laceration procedures (BASILICA and LAMPOON) may fail to protect against coronary and chamber obstruction in specific circumstances: very small valve-to-coronary distance (which amplifies the risk of coronary mal-alignment with the laceration), TAV-in-TAV (where prior valve frames limit leaflet splay), and diffusely-calcified and rigid leaflets (which limits splay)
What the Study Adds:
Balloon-augmented (BA-) leaflet modification is a simple procedure adjunct that appears incrementally to increase leaflet splay. Further refinement of leaflet modification strategies and the development of new techniques, such as leaflet excision, are needed.
Acknowledgments
Sources of Funding:
Supported by the Emory Structural Heart and Valve Center and by NIH Z01-HL006040.
Dr. Greenbaum is a proctor for Edwards Lifesciences and Medtronic; and has an equity interest in Transmural Systems. Dr. Greenbaum’s employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr. Khan is a proctor for Edwards Lifesciences and Medtronic. Dr. Paone is a consultant and proctor for Edwards Lifesciences. Dr. Grubb is a speaker, proctor, and principal investigator for Edwards Lifesciences; is a speaker, a proctor, and an advisory board member for Boston Scientific; and is a speaker, a proctor, a principal investigator, an advisory board member, and a national principal investigator for Medtronic. Dr. Rogers is a proctor for Edwards Lifesciences and Medtronic. Drs. Khan, Lederman, and Rogers are coinventors on patents, assigned to the National Institutes of Health, on devices for electrosurgical leaflet laceration. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular; and has an equity interest in Transmural Systems. Dr. Babaliaros’s employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr McCabe reports grant support from Abiomed and consulting at Edwards LifeSciences, Boston Scientific, Cardiovascular Systems Inc, and Teleflex.
Non-Standard Abbreviations and Acronyms:
- BASILICA
Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction
- BA-BASILICA
Balloon-augmented BASILICA
- BA-LAMPOON
Balloon-augmented LAMPOON
- LAMPOON
Intentional Percutaneous Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction
- TAV-in-TAV
Transcatheter aortic valve replacement inside existing transcatheter aortic valve replacement
- VTC
Valve-to-coronary distance
- VTSTJ
Valve-to-sinotubular junction distance
Footnotes
Conflicts of Interest/Disclosures:
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References:
- 1.Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv. 2019;12:1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J Am Coll Cardiol. 2019;73:2521–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan JM, Dvir D, Greenbaum AB, Babaliaros VC, Rogers T, Aldea G, Reisman M, Mackensen GB, Eng MHK, Paone G, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv. 2018;11:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH, O’Neill WW, Paone G, Thourani VH, Lerakis S, et al. Intentional Percutaneous Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction During Transcatheter Mitral Valve Replacement: First-in-Human Experience. JACC Cardiovasc Interv. 2017;10:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan JM, Bruce CG, Babaliaros VC, Greenbaum AB, Rogers T and Lederman RJ. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020;13:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2012;42:S45–60. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Adams DH, Abraham WT, Kappetein AP, Genereux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2. J Am Coll Cardiol. 2015;66:308–321. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro HB, Rodes-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves. Eur Heart J. 2018;39:687–695. [DOI] [PubMed] [Google Scholar]
- 9.Lederman RJ, Babaliaros VC, Rogers T, Khan JM, Kamioka N, Dvir D and Greenbaum AB. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv. 2019;12:1197–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan JM, Rogers T, Babaliaros VC, Fusari M, Greenbaum AB and Lederman RJ. Predicting Left Ventricular Outflow Tract Obstruction Despite Anterior Mitral Leaflet Resection: The “Skirt NeoLVOT”. JACC Cardiovasc Imaging. 2018;11:1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisko JC, Babaliaros VC, Lederman RJ, Khan JM, Rogers T and Greenbaum AB. Pachyderm-Shape Guiding Catheters to Simplify BASILICA Leaflet Traversal. Cardiovasc Revasc Med. 2019;20:782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisko JC, Greenbaum AB, Khan JM, Kamioka N, Gleason PT, Byku I, Condado JF, Jadue A, Paone G, Grubb KJ, et al. Antegrade Intentional Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction: A Simplified Technique From Bench to Bedside. Circ Cardiovasc Interv. 2020;13:e008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenbaum AB, Kamioka N, Vavalle JP, Lisko JC, Gleason PT, Paone G, Grubb KJ, Bruce CG, Lederman RJ and Babaliaros VC. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc Interv. 2021;14:578–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, Van Mieghem N, de Backer O, Testa L, Gatto P, Purita P, et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;71:1513–1524. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro HB, Nombela-Franco L, Urena M, Mok M, Pasian S, Doyle D, DeLarochelliere R, Cote M, Laflamme L, DeLarochelliere H, et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. 2013;6:452–61. [DOI] [PubMed] [Google Scholar]
- 16.Greenbaum AB, Lisko JC, Gleason PT, Kamioka N, Metcalf DP, Greenbaum MA, Paone G, Grubb KJ, Lederman RJ and Babaliaros VC. Annular-to-Apical “Emory Angle” to Ensure Coaxial Mitral Implantation of the SAPIEN 3 Valve. JACC Cardiovasc Interv. 2020;13:2447–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura M, Pighi M, Ribichini F and Abdel-Wahab M. Leaflet Prolapse After BASILICA and Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:e143–e145. [DOI] [PubMed] [Google Scholar]
- 18.Tiwana J, Aldea G, Levin DB, Johnson K, Don CW, Dvir D, Mackensen GB, Reisman M and McCabe JM. Contemporary Transcatheter Mitral Valve Replacement for Mitral Annular Calcification or Ring. JACC Cardiovasc Interv. 2020;13:2388–2398. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero M, Wang DD, Eleid MF, Pursnani A, Salinger M, Russell HM, Kodali SK, George I, Bapat VN, Dangas GD, et al. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC: MITRAL Trial 1-Year Outcomes. JACC Cardiovasc Interv. 2021;14:830–845. [DOI] [PubMed] [Google Scholar]