Abstract
J Clin Hypertens (Greenwich). 2010;12:431–438. ©2010 Wiley Periodicals, Inc.
The risk of development of hypertension is greater in black people compared to white people through mechanisms that are poorly understood. Several biological and environmental factors have been proposed. Based on the role of an increased peripheral resistance in the pathogenesis of hypertension, the authors focus in this systematic review on ethnic differences in function and mechanical properties of resistance arteries in normotensive participants. PubMed was systematically searched for papers on ethnic differences in vascular function and structure. A total of 620 papers were retrieved, of which 31 papers were included in the analysis. The available data indicate that compared to normotensive whites, normotensive black people have enhanced vascular reactivity to sympathetic stimulation, attenuated responses to vasodilators, and a relatively narrow vascular lumen diameter. Of these mechanisms, the reduced vasodilation and reduced nitric oxide bioavailability in the vascular wall seem to form the most important distinction between resistance vessel properties of black and white participants.
Epidemiological surveys demonstrate that black people are at greater risk to develop hypertension than white people. 1 These ethnic differences have been attributed to an interplay of environmental and biological factors. 2 , 3 Hypertension risk seems to become more frequent with increasing urbanization, 4 apparently through exposing this population to higher levels of psychosocial stress. Enhanced sympathetic responses to environmental stress and increased vascular reactivity to sympathetic stimulation have been considered to contribute to the greater hypertension risk in black population groups. 4 , 5 , 6 , 7 In particular, greater hemodynamic responses to physiological or psychological stressors, mediated largely through an increase in peripheral vascular resistance, have been reported in normotensive black people in many studies. 4 , 5 , 7 , 8 It has been argued that black people have elevated sympathetic nervous system (SNS) activity and/or altered vascular sensitivity to vasoactive stimuli. In this review we summarize the evidence on ethnic differences in vascular reactivity and biomechanical properties of resistance arteries in normotensive black and white participants. We emphasize studies addressing sympathetic activation, vascular adrenergic sensitivity, or vascular activity related to nitric oxide (NO) in normotensive black and white participants.
Methods
Literature Search
To identify relevant articles we systematically searched PubMed for human studies (published after 1990) using keywords related to ethnicity and ethnic (black, white) differences in vascular function and structure. The final literature search was performed in June, 2009. We retrieved 620 papers. Of these, 230 papers considered a comparison of ethnicity. After applying our selection criteria below, 31 articles were included. Figure 1 shows the flow of papers and reason for exclusion of trials.
Figure 1.
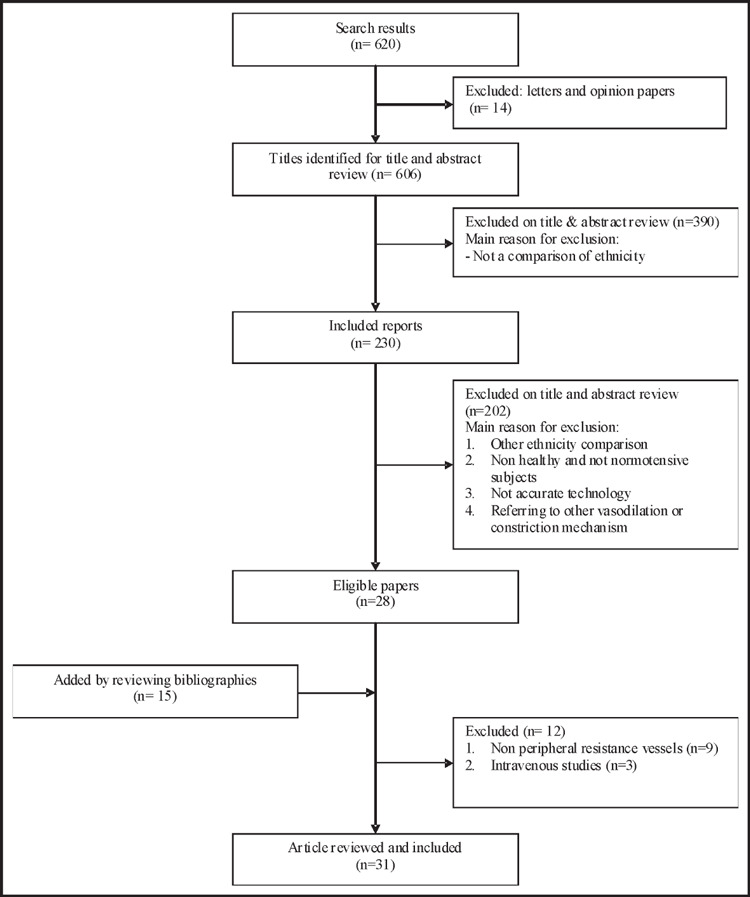
Flow chart of the selection process of articles to be included in this review.
Selection Criteria
We limited our review to the following aspects of vascular physiology in normotensive black participants compared to the normotensive white participants:
A: Functional
-
•
Differences in SNS activity and vascular reactivity in response to stress.
-
•
Differences in NO‐synthesis‐dependent or ‐independent vasodilation in resistance arteries.
B: Structural
-
•
Any differences in structure and mechanical properties of resistance vessels.
We included studies in which reactivity of systemic resistance vessels was evaluated in normotensive subjects. Since systemic SNS assessments such as blood pressure, heart rate, and plasma catecholamine are believed to be insufficiently specific for evaluation of SNS function, we focused on more specific parameters that can be obtained by local assessments. Therefore, in this review, we included papers in which reactivity of vessels was assessed by either kinetics of catecholamine tracers, or by microneurographic recording of muscle sympathetic nerve activity (MSNA). Furthermore, we included studies on vasodilation responses via the NO pathway and papers that addressed the structure of small arteries in normotensive black and white participants.
The terms “blacks” and “whites” as used in this paper are in agreement with a previous description by Myers and McClure 9 and Cooper and David, 10 namely ethnocultural groups differing in cultural, social, and psychological roots. They also differ in their biological attributes. However, as noted by Cooper and others, genetic heterogeneity exists also within these groups, especially among blacks. A biological definition of ethnicity is therefore difficult to establish. Also, the reviewed studies generally do not provide detailed genetic information, and for that reason the Myers definition is used here.
Exclusion Criteria
Opinion papers and letters were excluded. Hypertensive participants were outside the scope of this review because high blood pressure, once established, strongly affects endothelial function and vessel structure and the interest here is in resistance vessel function prior to development of hypertension. Since systemic infusion of vasoactive agents is complicated by reflex sympathetic responses, and effect of their intravenous infusion does not reflect arterial responses, we excluded vein assessment studies and studies not directly performed on forearm arterial or lower leg resistance vessels.
Results
Functional Differences
Vasoconstriction. The data on the differences in SNS activity between black and white participants are summarized in Table I. In these studies, mean blood pressures were comparable in black and white participants. Figure 2 indicates the relevant α‐adrenergic mechanisms. Stein and colleagues 11 compared α‐adrenergic‐mediated vasoconstriction and its relation to sympathetic activity in blacks and whites by application of lower body negative pressure (LBNP) and the cold pressor test. Forearm and systemic noradrenaline release spillover were measured with noradrenaline tracer kinetics. Both resting and stimulated release of noradrenaline during the cold pressor test were reported to be similar in normotensive blacks and whites, although vasoconstriction responses were increased in normotensive black participants. These authors also found that increases in sympathetic activity in response to LBNP were similar in blacks and whites, with matched hemodynamic values including higher blood pressure. Others proposed that the cold pressor test, as a powerful sympathetic stimulus, increased systemic noradrenaline spillover more than LBNP. 5
Table I.
Sympathetic Activity Determination in Normotensive Black and White Participants
| Author | Determination Method | Stimuli | Baseline Mean Arterial Pressure (mm Hg) | Sympathetic Activity | Vascular Reactivity | |||
|---|---|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |||
| Stein et al., 11 2000 | Catecholamine tracers kinetics | Cold pressor | 92.6±3 | 96.8±3 | ↑ | ↑ | ↑ | ↑↑ |
| LBNP | 91.3±3 | 96.8±3 | ↑ | ↑ | ↑ | ↑ | ||
| Calhoun and Mutinga, 13 1997 | Microneurography | Cold pressor | 93.0±3 | 92.0±2 | ↑ | ↑↑ | ↑ | ↑↑ |
| Ray and Monahan, 14 2002 | Microneurography | LBNP | 86.0a | 90.7a | ↑↑ | ↑ | ↑ | ↑↑ |
Values are expressed as mean ± standard error of the mean. ↑ represents a significant increased value after application of stressor compared to value at rest. ↑↑ represents a highly significant increased value that makes a separation between ethnic groups. aMean blood pressure (BP) is calculated from the reported diastolic and systolic values as 1/3 * systolic BP + 2/3 * diastolic BP. Abbreviation: LBNP, lower body negative blood pressure.
Figure 2.

α‐Adrenergic receptors involved in smooth muscle contraction. Indicated are the direct stimulating effects on smooth muscle cells and the negative feedback inhibition on noradrenaline (NA) release in the perivascular nerve endings. α1 indicates α‐1 adrenergic receptor; α2, α‐2 adrenergic receptor.
Data of Calhoun and colleagues 12 on MSNA, arterial blood pressure, and heart rate in normotensive black and white subjects also suggested that normotensive black subjects had increased sympathetic responses to the cold pressor test. However, these authors indicate that the sympathetic response to cold stress observed in normotensive African Americans is only greater in black participants with a positive family history of hypertension. 13
Microneurographic data by Ray and Monahan 14 suggest that normotensive blacks have a blunted sympathetic activity but elevated sympathetic vascular transduction during the LBNP test. These authors demonstrate a greater rise in forearm vascular resistance for a given increase in MSNA in black participants than in white participants. These findings are at variance with those of Calhoun and colleagues. 12 Ray and Monahan suggested that this discrepancy is caused by differences in the stressors. Baroreceptor unloading during LBNP and increased activity of cutaneous afferents during cold pressor testing could explain the difference in response. Their findings revealed similar baroreceptor unloading in whites and blacks. Why whites demonstrate a greater increase in MSNA during similar levels of LBNP than blacks remains unclear.
Regarding the sensitivity of postsynaptic α‐receptors, Lang and colleagues 15 used clonidine to measure the sensitivity of α2‐adrenoceptor‐mediated sympatho‐inhibition and the resultant hypotensive response in normotensive blacks and whites. Sympathetic activity was determined by a radiotracer method. Black participants showed a markedly blunted hypotensive response to clonidine in spite of a similar degree of central sympatho‐inhibition. This evidence suggests that elevated synaptic levels of noradrenaline do not mediate ethnic differences in the vascular responses to stress. 11 , 15 , 16 However, an increased number of post‐synaptic α‐adrenergic receptors or an increased affinity for noradrenaline by α1‐adrenoceptors may explain ethnic differences in sympathetic vascular contractility. Ethnic differences in α‐adrenergic‐mediated vasoconstrictor sensitivity were also assessed in response to noradrenaline administration. 11 To gain a more accurate estimate of α‐adrenergic sensitivity, Stein and colleagues 11 infused doses of adrenaline that were low enough to avoid systemic effects directly into the brachial artery in healthy normotensive participants. Vasoconstriction in the forearm appeared markedly increased in blacks. The response to adrenaline was not affected by the presence or absence of a family history of hypertension, and in none of the subjects was correlated with resting blood pressure. In agreement with Stein’s study, Ray and Monahan 14 demonstrated that α‐adrenergic receptor sensitivity, expressed as increase in resistance per unit increase in MSNA, is greater in young normotensive blacks as compared to matched whites.
Gene variants of α‐adrenergic receptors and potential functional effects of relevant variants have been examined in black and whites. 17 , 18 In α2‐adrenergic receptors of healthy subjects 41 polymorphisms including 24 novel variants were identified. 17 Haplotype frequencies differed between the racial groups. Also, a larger number of haplotypes in blacks reflects the greater gene diversity in this group. However, none of these polymorphisms and haplotypes showed significant association with the plasma noradrenaline concentration, blood pressure, or heart rate. Almost all were located in the 5′‐flanking region. Only black participants who were carriers of two uncommon variants had significantly higher plasma noradrenaline concentration and hence enhanced hemodynamic responses than noncarriers. Kurnik and colleagues 17 argued that these uncommon variants are associated with promoter regions regulating gene expression. As a consequence, these carriers would have a reduced receptor expression, resulting in higher baseline noradrenaline levels. However, regulatory elements within the α2‐adrenergic receptor promoter region are unknown. Thus, Kurnik and colleagues 17 suggested that common genetic variants of this receptor were not important determinants of baseline plasma noradrenaline and hemodynamic responses in healthy participants.
A study by Xie and colleagues 19 showed that the frequency of a recently identified polymorphism in the α1‐adrenoceptor, the Arg492 allele, occurs significantly more often in blacks than in whites, but this polymorphism is not associated with essential hypertension. However, it has been shown that common polymorphisms of α1‐adrenoceptors do not alter agonist‐mediated venoconstriction in man. 18 The potential role of α1‐adrenoceptors polymorphism related to ethnic differences in arterial α1‐adrenergic responses requires further investigation.
Vasodilation. Normotensive black and white people differ in the responses to both endothelial‐dependent and ‐independent agents (Table II). In these studies, mean blood pressures were comparable in black and white participants. An attenuated vasodilatation response to β‐adrenergic stimulation in black people compared to whites has been confirmed in many studies. 11 , 20 β2‐Adrenoceptors are located on both the smooth muscle cells and endothelial cells, acting through cyclic adenosine monophosphate and NO‐cyclic guanosine monophosphate signaling, respectively (Figure 3). Lang and colleagues 21 and Stein and colleagues 11 reported that vasodilation to intra‐arterial infusion of isoproterenol (a β‐receptor agonist), is blunted in black participants, through either direct or endothelium‐dependent effects. Cardillo and colleagues 20 confirmed that vasodilation to isoproterenol after L‐NMMA (NO synthesis inhibition) was still significantly lower in blacks than in whites, indicating that it is the direct, NO‐independent component of isoproterenol‐induced vasorelaxation that is attenuated in blacks. This suggests that there is an ethnic difference in the adenylyl cyclase pathway and its relevant adrenergic receptors.
Table II.
Decreased Vessel Contraction in Response to Vasodilator Stimuli in Normotensive Black and White Participants
| Author | Baseline Mean Arterial Pressure (mm Hg) | Endothelium‐Dependent Vasodilation | Endothelium‐ Independent Vasodilation | |||
|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |
| Stein et al., 11 2000 | 96.8±3 | 91.3±3 | ↓ | ↓↓ | ↓ | ↓↓ |
| Lang et al., 21 1995 | 85.0±6 | 87.0±8 | ↓ | ↓↓ | ↓ | ↓↓ |
| Cardillo et al., 28 1998 | 86.0±3 | 83.0±3 | ND | ND | ↓ | ↓↓ |
| Cardillo et al., 20 1999 | 84.0±2 | 86.0±2 | ↓ | ↓↓ | ↓ | ↓↓ |
| Stein et al., 27 1997 | 85.8±8 | 88.1±2 | ↓ | ↓↓ | ↓ | ↓↓ |
| Jones et al., 29 1999 | 80.0±6 | 83.0±6 | ↓ | ↓↓ | ↓ | ↓ |
| Gainer et al., 32 2001 | 86.6±2 | 84.1±2 | ↓ | ↓↓ | ↓ | ↓↓ |
| Rosenbaum et al., 33 2002 | 81.6±1 | 87.4±2 | ↓ | ↓↓ | ↓ | ↓ |
| Basset et al., 34 1992 | 91.7a | 87.3a | ↓ | ↓↓ | ND | ND |
| Hinderliter et al., 25 1996 | 95.0±13 | 92.0±12 | ↓ | ↓↓ | ND | ND |
| Bond and colleagues, 26 1996 | 87.7a | 90.3a | ↓ | ↓↓ | ND | ND |
| Duck and Hoffman, 30 2007 | 69.7a | 78a | ↓ | ↓↓ | ND | ND |
Values are expressed as mean ± standard error of the mean. ↓ represents a decreased value in response to vasodilator agent in comparison to value at rest. ↓↓ represents a highly significant decreased value that makes a separation between ethnic groups. aMean blood pressure is calculated from the reported diastolic and systolic values as 1/3 * systolic blood pressure + 2/3 * diastolic blood pressure. ND = Not determined.
Figure 3.
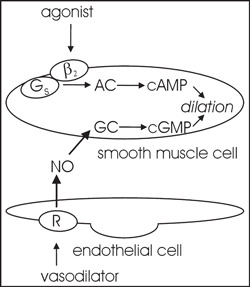
β‐adrenergic receptors in vascular smooth muscle relaxation. An adrenergic vasodilator via a β2 adrenergic receptor and G protein (Gs) stimulates adenylyl cyclase (AC) that catalyzes the formation of cyclic adenosine monophosphate (cAMP), leading to vasorelaxation. An endothelial‐dependent vasodilator (β2 adrenergic, muscarinic, other) binds to its receptor (R) on the surface of the endothelium, resulting in production of nitric oxide (NO), as well as other endothelium‐derived factors (not shown). Produced NO activates guanylyl cyclase (GC) in smooth muscle cells, leading to increases in guanosine monophosphate (cGMP) that induces blood vessel dilation.
Recently, a polymorphism of the β2‐adrenoceptor, the Arg16 allele, has been described. This β2‐adrenoceptor genotype contributed to vascular responses to isoproterenol in forearm resistance vessels in humans. However, whether this commonly occurring polymorphism is important in the genesis of hypertension remains unclear. 22 Results of a study by Xie and colleagues 23 suggest that although ethnic differences in the prevalence of this polymorphism exist, it does not affect blood pressure in black or white persons. In agreement, Candy and colleagues 24 suggested that β2‐adrenergic receptor polymorphisms are not a risk factor for hypertension. Taken together this evidence refutes the role of such polymorphisms as a potential explanation for ethnic differences in vascular responses to isoproterenol and interethnic differences in vascular responses to stressors.
Available plethysmographic data of forearm or lower leg resistance vessels demonstrate a reduced NO‐dependent vasodilator activity. 11 , 20 , 21 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 For instance, healthy black participants showed a blunted vasodilation both to intra‐arterial infusion of metacholine or acetylcholine (endothelial‐dependent vasodilators) and sodium nitroprusside (an endothelial‐independent vasodilator). Data are presented in Table II. 20 , 27 , 28 , 29 Ethnic differences in response to metacholine suggest a specific impairment of endothelial function. Yet, some studies describe a decreased vasodilation to sodium nitroprusside, pointing to a generalized alteration of resistance vascular function in healthy black people. This notion is supported by the work of Stein and colleagues 27 who confirmed that attenuated vasodilation in black participants seems to be a phenomenon that can be evoked by different receptor‐ and nonreceptor‐mediated mechanisms. Studies on small vessel reactivity using digital volume pulse photoplethysmography confirmed a considerable impairment of endothelium‐dependent vasodilation in normotensive blacks compared to whites. 34 , 35 Taken together, the majority of studies demonstrate that endothelium‐dependent mechanisms underlie the impaired vasodilator responsiveness in blacks. Such impaired endothelial responsiveness may add to the sensitivity for development of hypertension in blacks.
Ethnic divergence in sympathetic activity and vasodilator sensitivity may not only relate to genetic factors but also to lifestyle. An important aspect here is sodium intake. Table III lists the available information on sodium and potassium intake or excretion in the studies listed in I, II. As can be seen, in several of the studies, sodium intake was controlled, while in several others no ethnic differences were found in sodium or potassium excretion. In these studies at least, it seems that the differences in sympathetic activity and vasodilator responsiveness cannot be related to differences in sodium and potassium intake. This leaves the possibility that increased salt intake in urbanizing populations, for example, also affects sympathetic activity and vasodilator sensitivity.
Table III.
| Author | Sodium, Potassium Intake or Excretion |
|---|---|
| Table I | |
| Stein et al., 11 2000 | Salt‐replete diet: 150 Na+ and 70 mmol K+/d |
| Calhoun and Mutinga, 13 1997 | Urine Na+: 177 ± 11 vs 179 ± 17 mmol/d black vs white, P=NS Urine K+: 64 ± 7 vs 69 ± 7 mmol/d black vs white, P=NS |
| Ray and Monahan, 14 2002 | No data |
| Table II | |
| Stein et al., 11 2000 | Salt‐replete diet: 150 mmol Na+ and 70 mmol K+/d |
| Lang et al., 21 1995 | Salt‐replete diet: 150 mmol Na+ and 70 mmol K+/d |
| Cardillo et al., 28 1998 | No data |
| Cardillo et al., 20 1999 | No data |
| Stein et al., 27 1997 | Salt‐replete diet: 150 mmol Na+ and 70 mmol K+/d |
| Jones et al., 29 1999 | No data |
| Gainer et al., 32 2001 | Salt‐replete diet: 185 mmol Na+ and 70 mmol K+/d Urine Na+: mmol/d 172.3 ± 15.3 vs 158.2 ± 8.1 black vs white, P=NS Urine K+: mmol/d 76.7 ± 14.2 vs 70.6 ± 5 black vs white, P=NS |
| Rosenbaum et al., 33 2002 | All subjects were studied under salt‐replete conditions, as dietary Na+ intake does not affect the vasodilator response to bradykinin. (Gainer and colleagues, 32 2001) |
| Basset et al., 34 1992 | No data |
| Hinderliter et al., 25 1996 | No data (this issue is in study limitation) |
| Bond et al., 26 1996 | No data |
| Duck and Hoffman, 30 2007 | No data |
Structural Differences
Few studies consider peripheral vascular structure differences between normotensive blacks and whites. The minimum forearm vascular resistance, measured by plethysmography after forearm ischemia, revealed an ethnic difference in vessel diameter of normotensive subjects, 25 , 34 while wall thickness as measured by ultrasound was higher in blacks. Bond and colleagues 26 determined lower leg minimum vascular resistance. These authors suggest that normotensive blacks have earlier resistance vessel structural changes and narrower lumen diameter, compared with normotensive white people, regardless of a parental history of hypertension. Their finding also suggests that heredity in white more than in black populations may determine aspects of resistance artery structure such as wall thickness.
Prisant and colleagues, 36 by using a cardiovascular profiling system, showed stiffer small arteries in normotensive blacks compared with whites. They assumed that a thicker arterial wall can influence NO diffusion through the smooth muscle cell, thereby reducing vasodilatory capacity. However, this possibility seems unlikely because in patients with essential hypertension, who commonly have vascular wall hypertrophy, the vasodilation response to sodium nitroprusside is preserved, indicating that the diffusion capacity of NO is not affected by structural changes in the arterial wall. 20
Discussion
This systematic review indicates that in response to standardized stress tests, normotensive black people show an increased hemodynamic reactivity, resulting in increased blood pressure and heart rate. 4 However, studies on SNS activity in black vs white populations found heterogeneous results, with differences in size and direction of the effects. Hence the available data do not show that SNS activity is elevated in blacks. Resistance arteries of normotensive blacks did show enhanced contractility. In addition, it has been demonstrated that black people display increased sensitivity of postsynaptic α‐receptors to agonists. No major receptor gene polymorphisms were found. Therefore, the enhanced vascular tone may be related to increased responsiveness to noradrenaline. The underlying mechanism would relate to differences in post‐receptor signaling, either in the coupling of the receptor to G proteins or other events in the signaling cascade.
Since the enhanced arterial pressor response is not an appropriate predictor of future hypertension development, this review summarized approaches based on kinetics of catecholamine tracers and microneurographic studies of resistance vessels. Despite a controversial result in SNS activity, a greater cardiovascular reactivity was consistently found in all studies. The reviewed studies suggest that α1‐adrenergic receptor sensitivity is elevated in normotensive blacks compared with whites. We only selected studies using an accurate SNS activity evaluation. These studies indicate that the effector system of the G‐protein‐coupled α1‐adrenergic receptor, which includes phospholipase C and phosphatidylinositol, might contribute to ethnic differences in α1‐adrenergic sensitivity in vascular smooth muscle cells. The next question to be addressed will be whether this higher receptor sensitivity is indeed related to the development of hypertension in blacks.
The observed increase in vasoconstrictor responsiveness in black people may be due to an impaired sensitivity to dilatory influences. The available data on resistance vessels that we reviewed here point out that normotensive blacks have reduced responses to both endothelial‐dependent and ‐independent vasodilators. The reduction in endothelium‐dependent responses is related to less effective NO‐dependent mechanisms. Such racial disparities in endothelium‐dependent NO generation have been suggested to relate to cardiovascular health differences. 37 NO‐dependent endothelial dysfunction in black people may thus lead to hypertension and heart failure. Furthermore, impairment in NO production is involved in primary hypertension. 38
Taken together, the presented results show that enhanced adrenergic contraction during stress and attenuation of vasodilation mechanisms seem to act independently but can amplify each other. It should be realized that even mild elevations of vascular tone markedly increase peripheral vascular resistance, which is the hemodynamic hallmark of hypertension. Bakker and colleagues 39 have shown that resistance vessels rapidly remodel to smaller diameters when tone is elevated and episodes of vasodilation are less frequent or less intensive. This provides a link between early increased tone and later structural changes. We reviewed some studies that indeed show that normotensive blacks have a reduction in lumen diameter caused by vascular remodeling or stiffness. While more work is needed here, such structural changes in resistance vessels might be important for development and continuation of hypertension in black people.
Conclusion
We reviewed the existing evidence for ethnic differences in function and structure of resistance vessels of normotensive subjects. We conclude that, although environmental stress may play a role in differences in vascular responses between black and white people, there is also evidence of differences in contractility, vasodilation, and vessel structure that in concert are all capable of causing earlier and more severe high blood pressure in blacks compared with whites.
Disclosure: The authors declare that they have no conflict of interests.
References
- 1. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–313. [DOI] [PubMed] [Google Scholar]
- 2. Opie LH, Seedat YK. Hypertension in sub‐Saharan African populations. Circulation. 2005;23:3562–3568. [DOI] [PubMed] [Google Scholar]
- 3. Smith WR, Betancourt JR, Wynia MK, et al. Recommendations for teaching about racial and ethnic disparities in health and health care. Ann Intern Med. 2007;9:654–665. [DOI] [PubMed] [Google Scholar]
- 4. Anderson NB, McNeilly M, Myers H. Autonomic reactivity and hypertension in blacks: a review and proposed model. Ethn Dis. 1991;1(2):154–170. [PubMed] [Google Scholar]
- 5. Falkner B. The role of cardiovascular reactivity as a mediator of hypertension in African Americans. Semin Nephrol. 1996;16(2):117–125. [PubMed] [Google Scholar]
- 6. Kelsey RM, Alpert BS, Patterson SM, et al. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;19:2284–2289. [DOI] [PubMed] [Google Scholar]
- 7. Calhoun DA. Hypertension in blacks: socioeconomic stress and sympathetic nervous system activity. Am J Med Sci. 1992;304(5):306–311. [DOI] [PubMed] [Google Scholar]
- 8. Murphy JK, Alpert BS, Walker SS. Consistency of ethnic differences in children’s pressor reactivity. 1987 to 1992. Hypertension. 1994;1(suppl):I152–I155. [DOI] [PubMed] [Google Scholar]
- 9. Myers HF, McClure H. Psychosocial factors in hypertension in blacks: the case for an interactional perspective. In: Fray JCS, Douglas JG, eds. Pathophysiology of Hypertension in Blacks. Oxford, UK: Oxford University Press; 1993:90–106. [Google Scholar]
- 10. Cooper R, David R. The biological concept of race and its application to public health and epidemiology. J Health Polit Policy Law. 1986;11(1):97–116. [DOI] [PubMed] [Google Scholar]
- 11. Stein CM, Lang CC, Singh I, et al. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36(6):945–951. [DOI] [PubMed] [Google Scholar]
- 12. Calhoun DA, Mutinga ML, Collins AS, et al. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22(6):801–805. [DOI] [PubMed] [Google Scholar]
- 13. Calhoun DA, Mutinga ML. Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press. 1997;6(4):209–213. [DOI] [PubMed] [Google Scholar]
- 14. Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol. 2002;92(2):651–656. [DOI] [PubMed] [Google Scholar]
- 15. Lang CC, Stein CM, He HB et al. Blunted blood pressure response to central sympathoinhibition in normotensive blacks: increased importance of nonsympathetic factors in blood pressure maintenance in blacks. Hypertension. 1997;30(2, pt 1):157–162. [DOI] [PubMed] [Google Scholar]
- 16. Dimsdale JE, Ziegler M, Mills P, et al. Effects of salt, race, and hypertension on reactivity to stressors. Hypertension. 1990;16(5):573–580. [DOI] [PubMed] [Google Scholar]
- 17. Kurnik D, Muszkat M, Li C, et al. Variations in the alpha2A‐adrenergic receptor gene and their functional effects. Clin Pharmacol Ther. 2006;79(3):173–185. [DOI] [PubMed] [Google Scholar]
- 18. Sofowora GG, Dishy V, Landau R, et al. Alpha 1A‐adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75(6):539–545. [DOI] [PubMed] [Google Scholar]
- 19. Xie HG, Kim RB, Stein CM, et al. Alpha1A‐adrenergic receptor polymorphism: association with ethnicity but not essential hypertension. Pharmacogenetics. 1999;9(5):651–656. [PubMed] [Google Scholar]
- 20. Cardillo C, Kilcoyne CM, Cannon RO III, et al. Attenuation of cyclic nucleotide‐mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999;1:90–95. [DOI] [PubMed] [Google Scholar]
- 21. Lang CC, Stein CM, Brown RM, et al. Attenuation of isoproterenol‐mediated vasodilatation in blacks. N Engl J Med. 1995;3:155–160. [DOI] [PubMed] [Google Scholar]
- 22. Cockcroft JR, Gazis AG, Cross DJ, et al. Beta(2)‐adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36(3):371–375. [DOI] [PubMed] [Google Scholar]
- 23. Xie HG, Stein CM, Kim RB, et al. Human beta2‐adrenergic receptor polymorphisms: no association with essential hypertension in black or white Americans. Clin Pharmacol Ther. 2000;67(6):670–675. [DOI] [PubMed] [Google Scholar]
- 24. Candy G, Samani N, Norton G, et al. Association analysis of beta2 adrenoceptor polymorphisms with hypertension in a Black African population. J Hypertens. 2000;18(2):167–172. [DOI] [PubMed] [Google Scholar]
- 25. Hinderliter AL, Sager AR, Sherwood A, et al. Ethnic differences in forearm vasodilator capacity. Am J Cardiol. 1996;2:208–211. [DOI] [PubMed] [Google Scholar]
- 26. Bond V Jr, Thompson GD, Franks BD, et al. Racial differences in minimum lower leg vascular resistance in normotensive young adults with positive and negative parental histories of hypertension. J Cardiovasc Risk. 1996;3(5):423–426. [DOI] [PubMed] [Google Scholar]
- 27. Stein CM, Lang CC, Nelson R, et al. Vasodilation in black Americans: attenuated nitric oxide‐mediated responses. Clin Pharmacol Ther. 1997;62(4):436–443. [DOI] [PubMed] [Google Scholar]
- 28. Cardillo C, Kilcoyne CM, Cannon RO III, et al. Racial differences in nitric oxide‐mediated vasodilator response to mental stress in the forearm circulation. Hypertension. 1998;31(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 29. Jones DS, Andrawis NS, Abernethy DR. Impaired endothelial‐dependent forearm vascular relaxation in black Americans. Clin Pharmacol Ther. 1999;65(4):408–412. [DOI] [PubMed] [Google Scholar]
- 30. Duck MM, Hoffman RP. Impaired endothelial function in healthy African‐American adolescents compared with Caucasians. J Pediatr. 2007;150(4):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahn DF, Duffy SJ, Tomasian D, et al. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40(2):195–201. [DOI] [PubMed] [Google Scholar]
- 32. Gainer JV, Stein CM, Neal T, et al. Interactive effect of ethnicity and ACE insertion/deletion polymorphism on vascular reactivity. Hypertension. 2001;37(1):46–51. [DOI] [PubMed] [Google Scholar]
- 33. Rosenbaum DA, Pretorius M, Gainer JV, et al. Ethnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykinin. Arterioscler Thromb Vasc Biol. 2002;6:1023–1028. [DOI] [PubMed] [Google Scholar]
- 34. Bassett DR Jr, Duey WJ, Walker AJ, et al. Racial differences in maximal vasodilatory capacity of forearm resistance vessels in normotensive young adults. Am J Hypertens. 1992;5(11):781–786. [DOI] [PubMed] [Google Scholar]
- 35. Kalra L, Rambaran C, Chowienczyk P, et al. Ethnic differences in arterial responses and inflammatory markers in Afro‐Caribbean and Caucasian subjects. Arterioscler Thromb Vasc Biol. 2005;25(11):2362–2367. [DOI] [PubMed] [Google Scholar]
- 36. Prisant LM, Resnick LM, Hollenberg SM, et al. Arterial elasticity among normotensive subjects and treated and untreated hypertensive subjects: influence of race. Ethn Dis. 2002;12(1):63–68. [PubMed] [Google Scholar]
- 37. Mata‐Greenwood E, Chen DB. Racial differences in nitric oxide‐dependent vasorelaxation. Reprod Sci. 2008;15(1):9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taddei S, Virdis A, Mattei P, et al. Defective l‐arginine‐nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;6:1298–1303. [DOI] [PubMed] [Google Scholar]
- 39. Bakker EN, Van der Meulen ET, Van den Berg BM, et al. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res. 2002;39(1):12–20. [DOI] [PubMed] [Google Scholar]


