Abstract
J Clin Hypertens (Greenwich). 2010;12:414–421. ©2010 Wiley Periodicals, Inc.
This was a systematic assessment of the efficacy and safety of telmisartan and valsartan for the management of blood pressure (BP) in patients with essential hypertension. The authors reviewed randomized controlled trials (RCTs) and quasi‐RCTs comparing telmisartan and valsartan for the management of essential hypertension in which the participants were followed for at least 6 weeks. When a metaanalysis was possible, included studies were analyzed by Review Manager 5.0 provided by Cochrane Collaboration Group. Statistics were calculated as weight mean differences (WMDs) and relative risk with a random‐effect model. A total of 6 RCTs with 3762 patients were included in this metaanalysis. When the authors combined data from all treatment categories as a group, no difference was found between telmisartan and valsartan in reduction of systolic BP and diastolic BP. In subgroup analysis, telmisartan showed a significant benefit on lowering systolic BP and diastolic BP (WMD, −2.88; 95% confidence interval [CI], −5.03 to −0.73; P<0.01; and WMD,−1.73; 95% CI, −2.47 to −0.98; P<0.01; respectively). It was found that telmisartan had a higher rate of management of clinical BP compared with valsartan (relative risk, 1.09; 95% CI, 1.00–1.19; P=0.05). No difference was found in the incidence of adverse events. Telmisartan’s BP‐lowering capabilities were comparable to those of valsartan in monotherapy. When combined with hydrochlorothiazide, telmisartan was more effective than valsartan. Telmisartan had the same safety in the treatment of essential hypertensive patients in comparison with valsartan.
Insufficient suppression of the renin‐angiotensin‐aldosterone system has been implicated in the development of high arterial pressure. 1 The angiotensin‐converting enzyme inhibitors (ACEIs) such as enalapril and ramipril show organic protection in the management of blood pressure (BP). The renin‐angiotensin receptor blockers (ARBs) are a relatively new class of antihypertensive medications that selectively and specifically affect the action of angiotensin II. ARBs are becoming increasingly popular because they are effective and well tolerated. ARBs have different molecular structures which lead to different pharmacological function, respectively. For example telmisartan has the longest half‐life of any ARB, providing 24‐hour coverage of BP control from a single daily dose. The half‐life of valsartan is only 7 hours. Valsartan has the highest affinity ratio for angiotensin type 1 vs angiotensin type 2 receptor in the ARB family. The affinity ratio of valsartan is about 20,000‐fold, while it is only 3000‐fold in telmisartan. 2 , 3 , 4 Based on such findings, it is therefore relevant to compare telmisartan with valsartan.
Valsartan is an orally active, specific, and selective ARB. With its trough:peak ratio greater than 75%, valsartan can provide effective BP control throughout a whole day with a single morning dose. Its BP lowering action is within 2 hours and its peak effect occurrs within 4∼6 hours. 5 On the other hand, telmisartan, a nonpeptide ARB, is recommended for use in a once‐daily regimen, supported by its peak plasma levels about 1 hour after oral drug administration and a terminal elimination half‐life of approximately 24 hours. 6 The treatment efficacy of telmisartan and valsartan have been previously compared in several randomized clinical trials. However, there is rarely a systemic evaluation of these 2 drugs in BP control. In this study, we gathered the clinical controlled trials which related to telmisartan and valsartan for the management essential hypertension and systematically assessed their capability in lowering BP and other benefits.
Methods
Trial Inclusion Criteria
Data from reports of randomized controlled trials (RCTs) published as full articles addressing the effect of telmisartan vs valsartan for the management of essential hypertension were included. The following items should be considered regarding the inclusion criteria: the enrollment patients were ≥18 years old. The average seated diastolic BP (DBP) was ≥95 mm Hg, but <110 mm Hg during 2 consecutive weeks. The participants should be confirmed with no coronary disease, stroke, congestive heart failure, secondary hypertension, poorly controlled diabetes mellitus, or chronic kidney failure. The participants should not receive any ACEIs or ARBs within 2 weeks. The participants should have a planned follow‐up of at least 6 weeks.
Search Techniques
We searched the Cochrane Central Register of Controlled Trials (Cochrane Library Issue 1, 2009), MEDLINE and Pre‐MEDLINE ( 1966–May 2009), EMBASE ( 1980–May 2009), review articles, prospective trial registers, relevant trials, abstracts from hypertension meetings, and RCTs and quasi‐RCTs comparing any benefits of telmisartan and valsartan for the treatment of essential hypertension. The following search words were used: telmisartan, valsartan, randomized controlled trials, essential hypertension, blood pressure, and angiotensin receptor blockers. Reference lists of retrieved reports were checked.
Data Collection
Included trials were evaluated independently by 2 reviewers. For studies that could possibly have been RCTs or in the case of disagreement between the 2 reviewers, the full article was obtained. In turn, the same reviewers, who were not blinded to authorship or journal, compiled an ad hoc questionnaire and independently revised these articles. The questionnaires were then cross‐checked and disagreement was resolved by consensus or with a third reviewer. The following general descriptive information was extracted from each trial: number, age, gender, and race of participants; hypertension duration; baseline trough seated systolic BP (SBP)and DBP; number of participants randomly assigned to each intervention; duration of follow‐up; number of drop outs or withdrawals due to adverse events and discontent; change from baseline of seated SBP and DBP; response rate based on achievement of target BP; and the incidence of adverse events. Trials were inspected for 3 principle aspects of the randomization process: (1) generation of allocation sequence; (2) allocation concealment; and (3) blinding. In generation of allocation sequence, the approaches were considered as computer, random number table, shuffled cards, or tossed coins. Allocation concealment strategies were considered as central randomization, numbered or coded containers, and sequentially numbered, opaque, sealed envelopes. Whether or not the analysis was by intention‐to‐treat (ITT) and the completeness of follow‐up were also assessed. The following Cochrane quality checklist was compiled: (1) allocation concealment, adequate/unclear/inadequate; (2) blinding: blinding of investigators (yes/no/not stated), blinding of participants (yes/no/not stated), blinding of outcome assessors (yes/no/not stated), blinding of data analysis (yes/no/not stated); (3) ITT: (A) yes; specifically reported by investigators that ITT analysis was undertaken and this was confirmed on study assessment; (B) yes; not stated, but confirmed on study assessment; (C) no; not reported and lack of ITT analysis confirmed on study assessment; (D) no; stated, but not confirmed on study assessment; and (E) not stated; unable to be determined on study assessment.
Statistical Analysis
Data were quantitatively combined by 2 independent reviewers through a metaanalysis undertaken using Review Manager 5.0 provided by Cochrane Collaboration Group. The calculated relative risk (RR) with 95% confidence interval (CI) for dichotomous data and the weight mean difference (WMD) for continuous data were determined. A random effect model was used for analyses of both dichotomous and continuous data. Additionally, robustness of the model chosen was evaluated using the fixed effect model. When analyzing unfavorable events, an RR <1 favors the experimental treatment, whereas an RR >1 favors the control treatment. In the case of favorable events, an RR <1 favors the control treatment, whereas an RR >1 favors the experimental treatment. Statistical heterogeneity across studies was explored using the χ2 test for heterogeneity. Because this was a low‐power test, α level was set at 0.10. Two main sources of heterogeneity across trials were considered a priori: trial quality and characteristics of study participants.
Results
Description of Studies
Overall 6 RCTs published as full articles were identified and included in this review. The total number of patients randomized in the included trials was 3762. Of these studies, 4 studies provided data on effectiveness and safety for the comparison between telmisartan and valsartan in monotherapy. 7 , 8 , 9 , 10 Two studies compared these 2 agents in combination with hydrochlorothiazide 12.5 mg or 25 mg. 11 , 12 Three trials reported blinding of both investigators and participants. 9 , 10 , 12 The remaining 3 trials were open‐label studies and 2 of them were endpoint blind to both assessors and patients. 7 , 11 ITT analysis was confirmed in 4 trials 9 , 10 , 11 , 12 whereas it was not clear in the remaining two trials. 7 , 8 Information regarding completeness of follow‐up was reported or deducible in 5 trials, ranging from 88.5% to 100%. Main trial and patient characteristics of the RCTs are listed in Table I. The quality of RCTs included in the metaanalysis are described in Table II. We also used a funnel plot to visualize 4 endpoints: SBP control, DBP control, target BP achievement, and adverse events (Figure 1).
Table I.
Characteristics of Included Studies
| Investigator | Pattern of Study | Participants | Intervention | Follow‐Up | Drop Out |
|---|---|---|---|---|---|
| Bakris 2002 7 | Prospective, randomized, open‐label, blinded‐end study | 426 Patients | Telmisartan 80 mg or valsartan 80 mg | 8 Weeks | 2 Patients |
| Cavlo 2004 8 | Prospective, randomized, open‐label, parallel‐group study | 70 Patients | Telmisartan 80 mg or valsartan 160 mg | 12 Weeks | 0 Patients |
| White 2004 9 | Multicenter, double blind, double dummy, randomized, parallel group, forced titration study | 490 Patients | Telmisartan 40 mg or valsartan 80 mg, then titrated to 80 mg or 160 mg, respectively | 8 Weeks | 36 Patients |
| Sharma 2007 10 | Prospective, randomized, open‐label, blinded‐endpoint, multicenter study | 840 Patients | Telmisartan 80 mg or valsartan 160 mg, add hydrochlorothiazide 12.5 mg | 10 Weeks | 97 Patients |
| White 2008 11 | Multicenter, double blind, double dummy, randomized, parallel group study | 1051 Patients | Telmisartan 80 mg or valsartan 160 mg, add hydrochlorothiazide 25 mg | 6 Weeks | 112 Patients |
| Galle 2008 12 | Multicenter, double blind, double dummy, randomized, parallel group, forced titration study | 885 Patients | Telmisartan 40 mg or valsartan 80 mg, then titrated to 80 mg or 160 mg, respectively | 52 Weeks | 31 Patients |
Table II.
Quality Assessment of Included Studies
| Investigators | Allocation Concealment | Blinding of Investigators | Blinding of Participants | Blinding of Outcome Assessors | Blinding of Data Analysis | ITT Analysis |
|---|---|---|---|---|---|---|
| Bakris 2002 7 | Adequate | No | No | Yes | Not stated | c |
| White 2004 9 | Not stated | Yes | Yes | Not stated | Not stated | a |
| Cavlo 2004 8 | Adequate | No | No | Not stated | Not stated | c |
| Sharma 2007 10 | Adequate | No | No | Yes | Not stated | b |
| White 2008 11 | Adequate | Yes | Yes | Not stated | Not stated | b |
| Galle 2008 12 | Adequate | Yes | Yes | Not stated | Not stated | b |
Abbreviation: ITT, intention to treat.
aYes, specifically reported by investigators that ITT analysis was undertaken, and this was confirmed on study assessment. bYes, not stated, but confirmed on study assessment. cNot stated, unable to be determined on study assessment.
Figure 1.
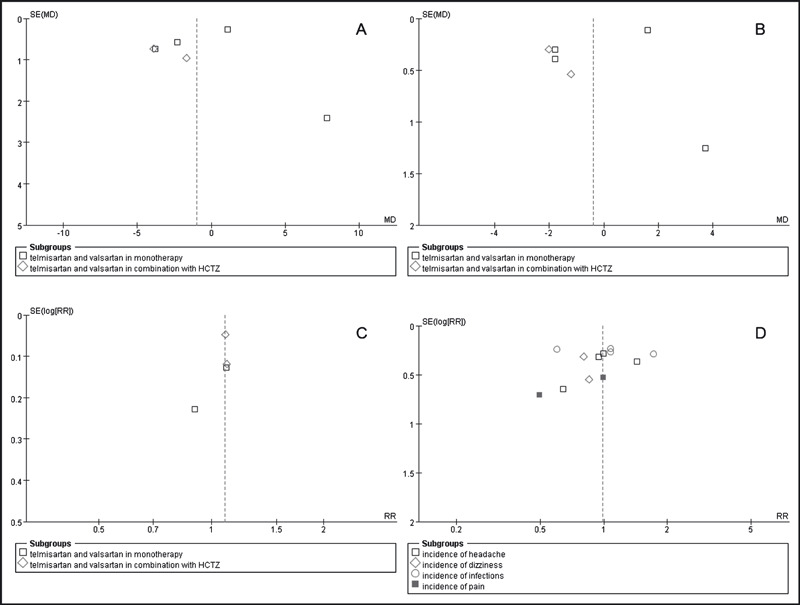
Funnel plot of end points. (A) Systolic blood pressure control. (B) Diastolic blood pressure control. (C) Target blood pressure achievement. (D) Adverse events. HCTZ indicates hydrochlorothiazide; MD, mean difference; RR, relative risk; SE, standard error.
Efficacy of Telmisartan Vs Valsartan in BP Control
We analyzed the efficacy of telmisartan and valsartan in BP control. A total of 6 RCTs with 3762 patients were included in this metaanalysis. When we combined the data of all treatment categories as a group, no difference was found between telmisartan and valsartan in reduction of SBP and DBP (WMD, −1.01; 95% CI, −3.38∼1.36; P=0.40; Figure 2 and WMD, −0.39; 95% CI, −2.24∼1.45; P=0.68; Figure 3, respectively). However, combined with hydrochlorothiazide in subgroup analysis, telmisartan showed a significant benefit on lowering SBP and DBP compared with valsartan (WMD, −2.88; 95% CI, −5.03∼−0.73; P<0.01; Figure 2 and WMD, −1.73; 95% CI, −2.47∼−0.98; P<0.01; Figure 3, respectively).
Figure 2.
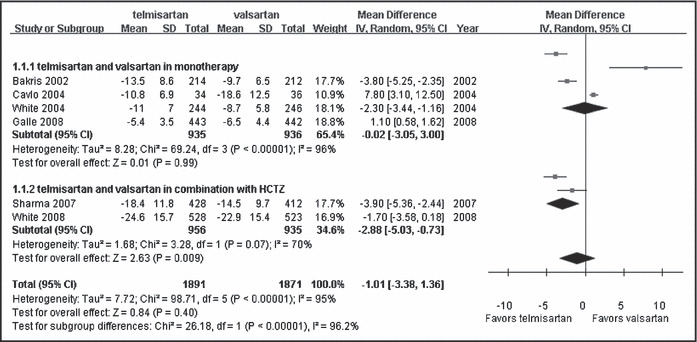
Comparison of telmisartan vs valsartan on systolic blood pressure control. CI indicates confidence interval; df, degrees of freedom; HCTZ, hydrochlorothiazide; IV, intravenous; SD, standard deviation.
Figure 3.
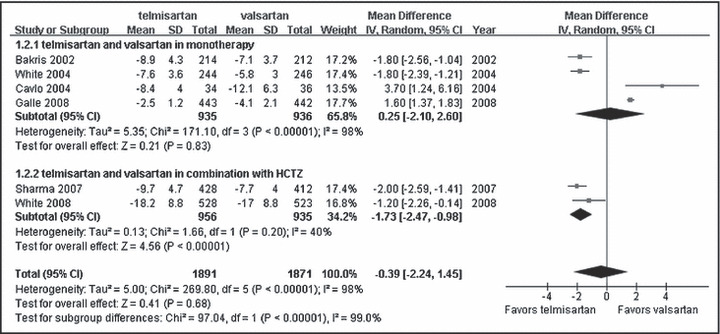
Comparison of telmisartan vs valsartan on diastolic blood pressure control. CI indicates confidence interval; df, degrees of freedom; HCTZ, hydrochlorothiazide; IV, intravenous; SD, standard deviation.
No beneficial effect was found on achievement of the target BP by ARB agent monotherapy (RR, 1.05; 95% CI, 0.84∼1.30; P=0.69; Figure 4). Telmisartan combined with hydrochlorothiazide provided significantly greater power vs valsartan combined with hydrochlorothiazide on target BP achievement (RR, 1.09; 95% CI, 1.00∼1.19; P=0.05; Figure 4).
Figure 4.
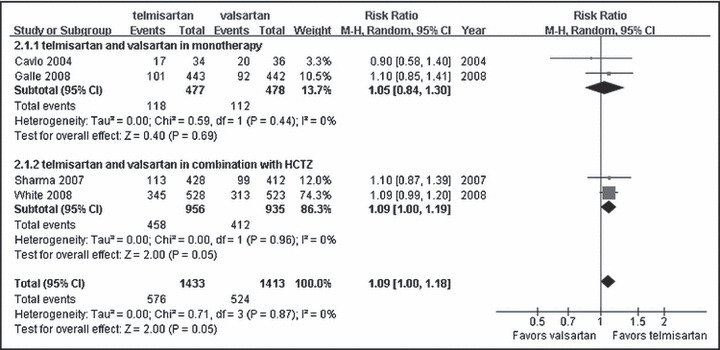
Comparison of telmisartan vs valsartan on target blood pressure achievement. CI indicates confidence interval; df, degrees of freedom; HCTZ, hydrochlorothiazide; M‐H, Mantel‐Haenszel test.
Safety of Telmisartan Vs Valsartan in BP Control
We also compared the common adverse events which related to telmisartan and valsartan treatment. Unfortunately not all trials included in the systemic review provided the particular descriptions of adverse events. The most common adverse events related to treatment were headache, dizziness, infections, and pain. The incidence of these adverse events was comparable between telmisartan and valsartan, so no differences were found in headache (RR, 1.03; 95% CI, 0.73∼1.45; P=0.88), dizziness (RR, 0.98; 95% CI, 0.44∼2.21; P=0.97), infections (RR, 1.03; 95% CI, 0.67∼1.56; P=0.90), and pain (RR, 0.77; 95% CI, 0.34∼1.77; P=0.54) (Figure 5).
Figure 5.
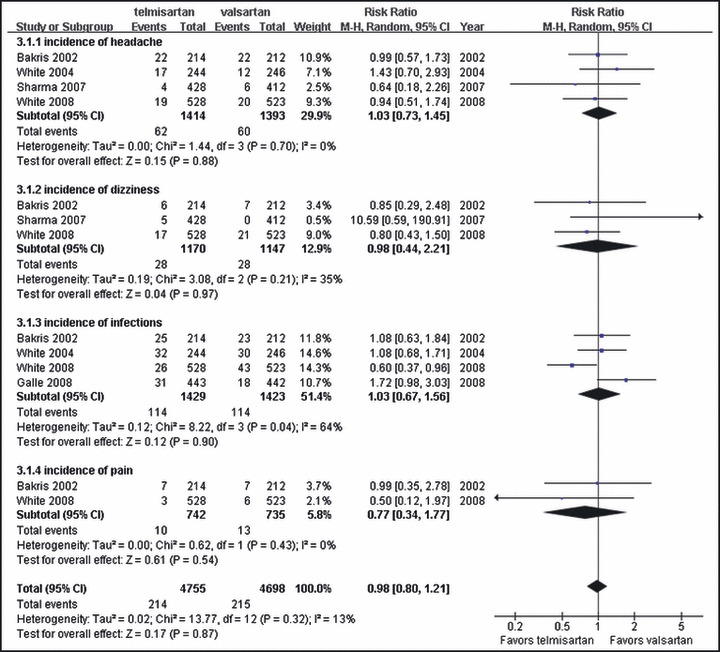
Comparison of telmisartan vs valsartan on common adverse events. CI indicates confidence interval, df, degrees of freedom; M‐H, Mantel‐Haenszel test.
Discussion
ARBs, which did not produce a dry cough, unlike ACEIs, were increasingly being used to treat hypertension. The first member of the ARB family was losartan, while valsartan, candesartan, irbesartan, and telmisartan came in turn. In this review we focused on telmisartan and valsartan and systematically compared their capability of management of BP. It was found that telmisartan 80 mg had a comparable magnitude of lowering BP with valsartan 160 mg in monotherapy. An explanation of this phenomenon is perhaps linked to the inherent pharmacokinetic profile of telmisartan. When telmisartan was administrated orally, its peak plasma level arrived within 1 hour and its terminal elimination half‐life was approximately 24 hours, which was the longest half‐life of agents in the ARB family. 6 On the other hand, telmisartan, a nonpeptide ARB, had the highest trough:peak ratio (the average BP reduction during the last 2 hours of the dosing interval compared to the average of the maximal reduction in BP over 2 consecutive hours) which was nearly 100%. The same ratio of valsartan was about 75%. These pharmacokinetic advantages in telmisartan gave it a better BP lowering effect in hypertensive patients. Although no difference was found in reduction of BP between telmisartan and valsartan in monotherapy, several studies suggest that telmisartan could be better at lowering BP in the early morning period than valsartan. 7 , 9
Our study further confirmed the superiority of telmisartan vs valsartan when both were used in fixed‐dose combination with HCTZ. Although physicians were likely to use an up‐titration approach to patient management, there was evidence to suggest that a fixed‐dose combination was beneficial in hypertensive patients, and might help to overcome the barrier of poor patient compliance as a result of polypharmacy or lack of efficacy. Utilization of a higher dose of HCTZ in combination with ARBs demonstrated greater blood pressure reduction with telmisartan compared with valsartan. Although there was no specific explanation for why and how telmisartan could be more powerful than valsartan when combined with HCTZ, it was clear that greater reduction of BP would give us more benefits. In an analysis involving one million adults in 60 prospective studies, the relationship between the reduction in BP and cardiovascular morbidity and mortality events supported that a 2‐mm Hg reduction in SBP would provide about 10% lower stroke mortality and 7% lower mortality from ischemic heart disease or other vascular death without a BP threshold down to the 115/75 mm Hg level. 13 In another study, it was suggested that a 1‐mm Hg DBP reduction would be associated with a 5% reduction in the risk of coronary heart disease and an 8% reduction in the risk of stroke. 14 These findings might provide a useful strategy in clinical hypertension management.
Our systematic review found only 3 prospective studies that minutely evaluated adverse events of these 2 agents. Interestingly, the proportion of reported adverse events in the whole study process was fairly high. The incidence of reported adverse events ranged from about 30% to 70%. But these reported adverse events were generally mild or moderate, and were tolerated by the majority of participants. The common adverse events were headache, dizziness, pain, and infections. Serious adverse events in the 2 groups were very lower and comparable. This information indicated that telmisartan and valsartan were safe agents when administrated in therapeutic doses.
There were several limitations to this metaanalysis such as high heterogeneity, different patient populations, lack of detailed data, all of which limited the conclusions that could be drawn from this analysis. In our systematic review, although we undertook the subgroup analyses, the high heterogeneity was still found. We had to use the random effect model to make a more conservative evaluation. In general, larger sample studies can have lesser bias than smaller sample studies. When higher heterogeneity of studies existed, a fixed effect model would not be used in the metaanalysis and the bias from smaller sample studies would be potentially amplified.
In conclusion, the efficacy and safety of once‐daily 80 mg telmisartan was comparable with 160 mg valsartan in monotherapy. Combined with HCTZ, telmisartan was more effective than valsartan in reduction of BP in essential hypertensive patients.
Acknowledgments
Acknowledgement: The authors express their sincere appreciation to Dr Yuqiou Zhang and Dr Quanping Yang for their helpful contributions, and to Dr Zhonghui Jia and Junya Jia for editing the manuscript.
References
- 1. Rahmouni K, Correia ML, Haynes WG, et al. Obesity‐associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- 2. Burnier M, Maillard M. The comparative pharmacology of angiotensin II receptor antagonists. Blood Press Suppl 2001;10:6–10. [PubMed] [Google Scholar]
- 3. Kakuta H, Sudoh K, Sasmata M, et al. Telmisartan has the strongest binding affinity to angiotensin II type I receptor: comparison with other angiotensin II type I receptor blockers. Int J Clin Pharmacol Res 2005;25:41–46. [PubMed] [Google Scholar]
- 4. Wienen W, Entzeroth M, Van Meel JCA, et al. A review on telmisartan: a novel, long‐acting angiotensin II receptor antagonist. Cardiovasc Drug Rev. 2002;18:127–156. [Google Scholar]
- 5. Lasko BH, Laplante A, Hebert D, et al. Canadian valsartan study in patients with mild‐to‐moderate hypertension. Blood Press Monit. 2001;6:91–99. [DOI] [PubMed] [Google Scholar]
- 6. McClennan KJ, Markham A. Telmisartan. Drugs. 1998;56:1039–1044. [DOI] [PubMed] [Google Scholar]
- 7. Bakris G. Comparison of telmisartan vs. valsartan in the treatment of mild to moderate hypertension using ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich). 2002;4(4 suppl 1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calvo C, Hermida RC, Ayala DE, et al. Effects of telmisartan 80 mg and valsartan 160 mg on ambulatory blood pressure in patients with essential hypertension. J Hypertens 2004;22:837–846. [DOI] [PubMed] [Google Scholar]
- 9. White WB, Lacourciere Y, Davidai G. Effects of the angiotensin II receptor telmisartan versus valsartan on the circadian variation of blood pressure: impact on the early morning period. Am J Hypertens 2004;17:347–353. [DOI] [PubMed] [Google Scholar]
- 10. Galle J, Schwedhelm E, Pinnetti S, et al. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant 2008;23:3174–3183. [DOI] [PubMed] [Google Scholar]
- 11. Sharma AM, Davidson J, Koval S, et al. Telmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH study. Cariovasc Diabetol. 2007;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White WB, Murwin D, Chrysant SG, et al. Effects of the angiotensin II receptor blockers telmisartan versus valsartan in combination with hydrochlorothiazide: a large, confirmatory trial. Blood Press Monit. 2008;13:21–27. [DOI] [PubMed] [Google Scholar]
- 13. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual BP to vascular mortality: a meta‐analysis of individual data for one million adults in 60 prospective studies. Lancet 2002;14:1903–1913. [DOI] [PubMed] [Google Scholar]
- 14. Cook NR, Cohen J, Hebert PR, et al. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 1995;155:701–709. [PubMed] [Google Scholar]


