Abstract
The potential for insulin‐related weight gain in patients with type 2 diabetes presents a therapeutic dilemma and frequently leads to delays in the initiation of insulin therapy. It also poses considerable challenges when treatment is intensified. Addressing insulin‐related weight gain is highly relevant to the prevention of metabolic and cardiovascular consequences in this high‐risk population with type 2 diabetes. In addition to lifestyle changes (eg, diet and exercise) and available medical interventions to minimize the risk of weight gain with insulin treatment, familiarity with the weight gain patterns of different insulins may help deal with this problem. The use of basal insulin analogs may offer advantages over conventional human insulin preparations in terms of more physiologic time‐action profiles, reduced risk of hypoglycemia, and reduced weight gain.
Achieving and maintaining near‐normal glycemic control are therapeutic challenges for patients with type 2 diabetes, and many will eventually require exogenous insulin for adequate management. While recent advancements and discoveries in the area of insulin treatment should facilitate insulin use, insulin remains underutilized in the United States, 1 and glycemic control is not achieved in >60% of patients with type 2 diabetes. 2 One frequently encountered barrier to insulin use is patient apprehension over the possibility of insulin‐related weight gain. 1
This article highlights the necessity of starting insulin therapy early and committing to ongoing insulin treatment in many patients with type 2 diabetes, and it discusses approaches for managing insulin‐related weight gain.
Delays in Starting Insulin Therapy
Early initiation of insulin therapy and the resultant improved glycemic control in patients with type 2 diabetes not only reduces macrovascular and microvascular complications but also improves the cardiovascular profile 3 ; reduces glucotoxicity and blood glucose variability; decreases morbidity, mortality, and health care costs; and improves quality of life. 4 Ryan and colleagues 5 demonstrated that early insulin therapy also helps preserve and maintain function of pancreatic islet β‐cells in patients with type 2 diabetes.
Despite the potential benefits of early insulin initiation in patients with type 2 diabetes, delays in starting insulin are common, even when glycemic control is inadequate. 4 In a large longitudinal cohort study of 3891 patients with type 2 diabetes receiving oral antidiabetic agents, only about 42% of patients added insulin despite failures to attain or maintain hemoglobin A1c (HbA1c) levels <8%. Patients in whom HbA1c levels <8% were attained but not maintained were not more willing to initiate insulin therapy than were those in whom the goal was maintained, and more than half of these patients remained on oral agents alone for an average of nearly 3 years despite the additional glycemic burden. 6 It is not unusual for patients to have type 2 diabetes for 10 to 15 years, with significant complications, before insulin therapy is initiated. 4
Among the multiple concerns determining patients’ and health care practitioners’ reluctance to initiate and intensify insulin therapy,4, 7 the apprehension regarding possible insulin‐related weight gain frequently becomes a major factor, 8 and insulin is frequently regarded as the last resort in the treatment of type 2 diabetes. The Diabetes Attitudes, Wishes, and Needs (DAWN) study revealed that >50% of nurses and general practitioners delay the start of insulin until it becomes “unavoidable.” 7 The suboptimal glycemic control that may ensue with this practice can have devastating long‐term macrovascular and microvascular consequences, including cardiovascular disease, nephropathy, retinopathy, and neuropathy, along with the associated increases in mortality risks. 9
When instituting early intervention with insulin in overweight patients with type 2 diabetes, it is important to assess the possible presence of a metabolic syndrome and to address the manifestations of this syndrome that may relate to insulin resistance. For example, insulin resistance and hyperinsulinemia may lead to increased sympathetic activation resulting in vasoconstriction, sodium retention, increased cardiac output, and hypertension. 10 Insulin resistance may also account for substantial dyslipidemia—often known as atherogenic dyslipidemia—characterized not only by elevated low‐density lipoprotein (LDL) cholesterol levels but also by smaller and denser LDL particles, elevated triglyceride‐rich very‐low‐density lipoprotein and intermediate‐density cholesterol levels, decreased high‐density lipoprotein cholesterol values, and increased LDL oxidation. Increasing insulin levels with the addition of exogenous insulin for the control of hyperglycemia may initially appear contraindicated in patients with evidence of metabolic syndrome, so it is imperative to simultaneously address insulin resistance and the metabolic situation. 11 While discussing management options for these entities is beyond the scope of this paper, several recent publications offer detailed descriptions of these options.10, 11, 12
Postulated Causes of Insulin‐Related Weight Gain (Table)
Table.
Postulated Factors Related to Weight Gain in Insulin‐Treated Patients With Type 2 Diabetes
| Factor | Postulated Effects |
|---|---|
| Anabolic effects of insulin | Stimulation of lipogenesis in muscle fibers and adipose tissue |
| Attenuation of insulin‐evoked satiety | Enhanced hunger and increased food intake |
| Frequent hypoglycemic episodes | Defensive snacking |
| Excessive reliance on insulin to normalize glucose readings | False sense of freedom to eat |
| Genetic factors | Greater central weight gain and dyslipidemia |
| Low insulin‐like growth factor II levels prior to insulin initiation | Poor regulation of fat mass |
| Correction of glycosuria | Reduced energy loss and improved utilization of calories |
| Catch‐up process | Regain of uncontrolled diabetes‐induced weight loss |
Anabolic effects of insulin on muscle fiber and adipose tissue have long been recognized, and some of the weight gain associated with insulin can be attributed to this. 13 In addition, attenuation of insulin‐evoked responses in brain networks that control appetite and reward in insulin‐resistant patients can lead to enhanced hunger and increased food intake. 14 Mild but frequent episodes of hypoglycemia resulting in defensive snacking have long been recognized as another possible reason for weight gain in patients receiving insulin.8, 15 A false sense of freedom to eat has also been cited, as patients allow themselves to consume more food and rely on insulin to normalize glucose levels. 15
Genetic factors may also be important contributors to weight gain with insulin use. In the Diabetes Control and Complications Trial with intensive insulin therapy, adult patients (N=1168) with a family history of type 2 diabetes had greater central weight gain and dyslipidemia compared with patients with no such family history, 16 suggesting that increased weight gain with intensive therapy might be at least partly explained by genetic traits.
The correction of glycosuria reduces energy loss, improves utilization of calories, and may lead to weight gain. It has also been argued that a “catch‐up” process of regaining diabetes‐induced weight loss should be expected and that it has no long‐term consequences on cardiovascular risk or lipid profile. 17 However, most experts agree that weight gain in type 2 diabetes can have serious consequences and should be managed appropriately.15, 18 It is known that bioactive mediators released by adipose tissue trigger alterations in lipids, coagulation, and fibrinolysis that lead to endothelial dysfunction and atherosclerosis 19 as well as to a state of chronic, low‐grade inflammation with metabolic and cardiovascular consequences.19, 20
Noninsulin substances that have an insulin‐like action do not necessarily have weight‐enhancing effects. For example, baseline levels of insulin‐like growth factor II (IGF‐II) were shown to be an independent risk factor for weight gain in a 5‐year follow‐up study of 224 patients with type 2 diabetes. 21 More than 40% of participants had gained >2.0 kg at the 5‐year follow‐up. However, in the subgroup of patients with normal weight at baseline (body mass index [BMI] <26), mean IGF‐II levels were inversely related to weight gain and were significantly lower in those who gained >2.0 kg than in the patients with stable weight (454 vs 620 ng/mL; P=.01). This relationship was independent of treatment effect in patients who received insulin or sulfonylureas during the 5‐year study. Although the same pattern could not be seen in patients with higher BMIs, the study shows that baseline IGF‐II plays a role in fat‐mass regulation and that its concentration is inversely related to future weight gain in patients with normal BMIs. 21
Characteristics of Insulin‐Related Weight Gain
Risk of Weight Gain
Insulin resistance apparently originates in a tissue reaction to sustained high levels of glucose and insulin, as demonstrated by the finding that cultured adipocytes of nondiabetic persons develop insulin resistance after a few hours of such an exposure. 22 Once established, insulin resistance and compensatory hyperinsulinemia are significant risk factors for additional weight gain, as well as cardiovascular disease, in patients with type 2 diabetes. 23
The addition of exogenous insulin may cause increased weight gain in patients with insulin resistance and hyperinsulinemia. In a study that included 192 patients with type 2 diabetes, body weight increased up to 3.0% and insulin requirements increased by as much as 23% during the first year of insulin therapy (Figure 1). 23
Figure 1.
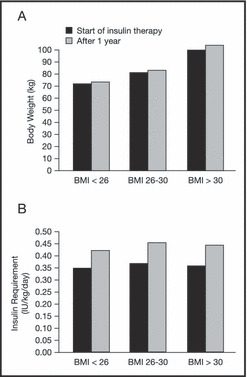
Changes in (A) body weight and (B) insulin requirements after 1 year of insulin therapy according to different baseline body mass index (BMI) levels (N=192). 23
Long‐term observations of patients receiving intensive insulin therapy have registered higher rates of weight gain. In the UK Prospective Diabetes Study Group, weight gain was significantly greater at 10 years in the intensively treated group (mean, 3.1 kg; P=.0001) than in the conventionally treated group of patients with type 2 diabetes. 24 The Diabetes Control and Complications Research Group reported a 33% increase in the mean adjusted risk of becoming overweight with intensive insulin therapy and a mean 4.6‐kg greater weight gain in 5 years in patients with type 1 diabetes receiving intensive therapy vs patients receiving conventional therapy. 25
It is worth noting that weight gain with antidiabetic agents is not limited to insulin; it is also associated with several insulin secretagogues (eg, sulfonylureas, meglitinides) and insulin sensitizers (eg, thiazolidinediones).16, 26
Recent studies have shown that glycemic variability per se contributes to weight gain. Lower plasma glucose concentrations at the end (120th minute) of an oral glucose tolerance test correlated (r = −.42; P<.0001) with long‐term weight gain in a prospective observational study involving 259 participants (25 with impaired glucose tolerance and 9 with type 2 diabetes mellitus) with weight regain following weight loss (n=44; r =−.50; P=NS). 27 While the sensitivity to glucose homeostasis varies among individuals, glycemic variability may predispose to obesity over time 28 and should be factored in when considering the addition of insulin.
Pattern of Weight Gain
Increases in body weight during the first 6 months of insulin therapy are typically related to glucose control and can be primarily attributed to the decrease of glycosuria and the process of weight “regain” previously discussed. Further into treatment, there seems to be an insulin effect that contributes to weight gain and is independent of glycemic control. Salle and associates 29 measured the body weight and composition of 32 patients with type 2 diabetes undergoing their first 12 months of insulin therapy (2 or 3 injections daily) and compared them with 32 patients who had been treated with insulin for a minimum of 1 year. Body composition was determined by simultaneous measurements of body water spaces and body density. After 6 months, glucose control had significantly improved in the newly treated group (P<.0001), as reflected by improvements in HbA1c values, whereas glucose levels remained stable in the patients already on treatment. Body weight changed significantly (P=.04) over 12 months only in the newly treated patients (+2.8 kg), essentially comprising fat‐free mass (P=.044). Weight changes correlated with HbA1c changes (P=.002) only during the initial 6 months. After 6 months, the newly treated patients continued to gain weight despite unchanged HbA1c levels, suggesting a potential anabolic role of insulin unrelated to glucose control. 29
Weight Gain With Different Insulin Types
Evidence suggests that different commercially available insulin preparations may have dissimilar effects on weight gain in patients with type 2 diabetes.
The newer basal insulin analogs, insulin detemir and insulin glargine, have a relatively flat time‐action profile and a more predictable glucose‐lowering effect when compared with neutral protamine Hagedorn (NPH). 4 Their more physiologic profiles and reduced risk of nocturnal hypoglycemia may offer benefits with regard to weight gain.
Several studies in patients with type 2 diabetes have demonstrated that insulin detemir induces less weight gain than NPH. Haak and associates 30 conducted a 26‐week multinational, open‐label, parallel‐group trial involving 505 patients with type 2 diabetes and found that patients receiving insulin detemir gained significantly less mean weight than those receiving NPH insulin (1.0 and 1.8 kg, respectively; P=.017). In another parallel‐group, multicenter, treat‐to‐target trial involving insulin‐naive patients (N=476), Hermansen and colleagues 31 documented a difference of −1.58 kg (P<.001) in baseline‐adjusted final weight at 24 weeks in favor of patients with type 2 diabetes treated with insulin detemir vs patients treated with NPH. Another study showed that the difference between insulin detemir and NPH with regard to weight gain is more noticeable when insulin detemir is administered in the evening. In this multicenter, randomized, open‐label, 3‐arm, parallel‐group trial conducted at 91 centers in Europe and the United States, which involved patients with poorly controlled type 2 diabetes (N=504), the mean weight gains at 20 weeks were 0.7 kg and 1.6 kg (P<.001) for the insulin detemir and NPH groups, respectively. 32 Finally, data pooled by Rašlová and associates 33 from 2 randomized parallel‐group trials of 22‐ and 24‐week durations involving 900 patients reaffirmed that insulin detemir may provide a clinical advantage vs NPH with regard to weight gain in insulin‐treated patients with type 2 diabetes, especially in the group of patients with a higher BMI at baseline.
One study has established that less weight gain occurs with insulin detemir than with insulin glargine. 34 Insulin detemir and insulin glargine appear to have a similar effect on glycemic control; however, there is less weight gain with insulin detemir due to mechanisms currently under investigation.35, 36
Management of Insulin‐Related Weight Gain
Weight management may be the most important therapeutic intervention for obese patients with type 2 diabetes. 18 A vicious cycle can easily be triggered in the obese patient with diabetes because of progressive β‐cell dysfunction and insulin resistance necessitating the administration of increasingly higher dosages of insulin. These higher doses can, in turn, promote more weight gain. 37
The Coronary Artery Risk Development in Young Adults (CARDIA) study found strong positive associations between fast‐food habits and weight gain/insulin resistance and suggested that frequent consumption of high‐energy foods increases the risk of obesity and type 2 diabetes. Very low–energy diets can help some patients achieve large reductions in body weight, fasting plasma glucose, serum cholesterol/triglycerides, and systolic and diastolic blood pressure (Figure 2). 38 In addition, a recent study involving patients with obesity and type 2 diabetes (n=84) has shown that a low‐carbohydrate ketogenic diet leads to greater improvements in glycemic control and more frequent medication reduction/elimination than a low–glycemic index diet. 39
Figure 2.
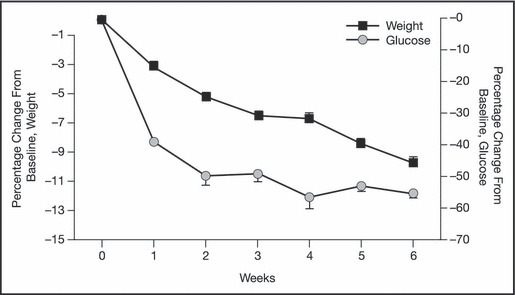
Effect of a very low–energy diet on short‐term weight loss and fasting plasma glucose in obese patients with type 2 diabetes (N=152). 18
It is not possible to recommend a specific number of calories to be eliminated from the diet in order to maintain weight neutrality when starting insulin because the number depends on each patient’s anthropometrics and metabolic characteristics, the level of commitment to an exercise program, and the type of insulin prescribed, among other factors. Guidelines for weight loss from the US National Heart, Lung and Blood Institute for overweight individuals with the cardiometabolic syndrome include a moderate reduction in calorie intake (500–1000 calories/d) if accompanied by exercise. This would translate into a calorie goal of 1200 to 1600 calories/d for most overweight patients, 40 but this estimate does not take into account the addition of insulin.
The management of insulin‐related weight gain cannot rely on diet alone. Even with specialized/comprehensive support by nutritionists and diabetes educators, erratic adherence to a prescribed diet is very commonly observed in clinical practice.
Exercise is another important component of lifestyle modification in patients with type 2 diabetes who are receiving insulin. Skeletal muscle constitutes 40% of body mass and takes up 80% of the glucose load 41; therefore, in obese patients with type 2 diabetes, insulin resistance in muscle is largely responsible for the impairment of glucose removal from the circulation. Research has shown that fatty acids derived from adipose tissue can interfere with insulin signaling in muscle, 41 and there is accumulating evidence indicating a reduction of fatty acid oxidation in the skeletal muscle of obese individuals. 42 The capacity for fatty acid oxidation can be improved with exercise. 41 A gradual and progressive exercise program is recommended for most sedentary patients with type 2 diabetes in order to minimize the occurrence of exercise‐related injuries and complications and to better adjust insulin requirements.
The use of insulin analogs can be advantageous in patients eligible for treatment with insulin, and as mentioned earlier, there are differences among the analogs with regard to weight gain. Findings from clinical trials have been supported by extensive data from a very large observational study involving 20,531 patients with diabetes from 11 countries that showed small decreases in mean body weight in patients with type 1 (0.1 kg; P<.01) and type 2 diabetes (0.4 kg; P<.0001) after 14 weeks of treatment with insulin detemir. 43 Among the 12,981 patients with type 2 diabetes included in this study, a mean body weight loss of 0.7 kg (P<.0001) was also observed after 14 weeks in a subgroup of 2377 insulin‐naive patients with type 2 diabetes and on oral antidiabetic drugs (OADs), after initiation of insulin detemir as basal therapy, with or without continuation of OADs. 44
The addition of certain OADs to the treatment of patients with type 2 diabetes can help with weight control or even facilitate weight loss. Metformin improves glycemia, decreases insulin resistance, and promotes weight loss in most patients, 37 and a recent consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes recognized the combination of insulin plus metformin as a particularly effective approach to treating hyperglycemia while limiting weight gain. 45 Incretin‐based therapies, such as glucagon‐like peptide‐1 (GLP‐1) analogs and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, are well tolerated: the GLP‐1 analogs are associated with weight loss, whereas the DPP‐4 inhibitors are weight‐neutral. These incretin‐based therapies target the islet cell dysfunction and are potentially promising new options, either as monotherapy or in combination with other antidiabetic drugs, especially in patients with early‐stage type 2 diabetes and more severe hyperglycemia.46, 47 However, insulin will still be required as the first treatment approach for patients with very high HbA1c levels.
With regard to prescription weight‐loss agents such as sibutramine and orlistat, a number of patients have tolerability problems with these medications, and long‐term weight loss benefits with these products have yet to be demonstrated. 15
Finally, several studies have shown resolution of type 2 diabetes after Roux‐en‐Y gastric bypass surgery in approximately 84% of patients, with a significant reduction in fasting plasma glucose very shortly after the procedure and concurrent reductions of C‐peptide and insulin levels. 48 However, even with bariatric surgery, significant changes in lifestyle and eating habits are required for long‐term success.
Conclusions
The management of insulin‐related weight gain in type 2 diabetes mellitus is an ongoing challenge. Discussions on the long‐term risks of postponing insulin should be an important component of patient education, along with information on the risks and patterns of weight gain. Patients with an improved understanding of the detriments of postponing insulin treatment, the causes of insulin‐related weight gain, and the patterns of weight gain with different insulins may develop a sense of being in better control of their treatment and may become more motivated and involved in the management of their diabetes therapy.
A commitment to start insulin early to address inadequate glycemic control and to continue with and intensify insulin treatment as needed (despite the possibility of weight gain) is also very critical. In the attempt to balance the benefits and adverse effects of insulin treatment, the choice of insulin and concomitant medications becomes an important management consideration. Clinical studies show that the newer insulin analogs offer advantages over conventional human insulin preparations in terms of more physiologic action profiles, reduced risk of hypoglycemia, and reduced weight gain.
Acknowledgments and disclosure:
The author thanks José Luis Traverso, MD, PhD , of Complete Medical Communications, who provided medical writing support, funded by Novo Nordisk Inc. The author actively participated in the conception and development of this manuscript, the organization and review of topics and materials, the search for and analysis of cited references, the selection of figures, and the creation of the table.
References
- 1. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18(suppl 3):S42–S49. [DOI] [PubMed] [Google Scholar]
- 2. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27(1):17–20. [DOI] [PubMed] [Google Scholar]
- 3. Nicasio J, McFarlane SI. Early insulin therapy and the risk of cardiovascular disease in type 2 diabetes. Therapy. 2005;2(5):685–688. [Google Scholar]
- 4. Meneghini L. Why and how to use insulin therapy earlier in the management of type 2 diabetes. South Med J. 2007;100(2):164–174. [DOI] [PubMed] [Google Scholar]
- 5. Ryan EA, Imes S, Wallace C. Short‐term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27(5):1028–1032. [DOI] [PubMed] [Google Scholar]
- 6. Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med. 2007; 22(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers. Results of the cross‐national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. [DOI] [PubMed] [Google Scholar]
- 8. Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ. 2006;32(6):910–917. [DOI] [PubMed] [Google Scholar]
- 9. Tibaldi J, Rakel RE. Why, when and how to initiate insulin therapy in patients with type 2 diabetes. Int J Clin Pract. 2007;61(4):633–644. [DOI] [PubMed] [Google Scholar]
- 10. Straznicky NE, Eikelis N, Lambert EA, et al. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10(6):440–447. [DOI] [PubMed] [Google Scholar]
- 11. Taylor AM. Cardiometabolic risk management in type 2 diabetes and obesity. Curr Diab Rep. 2008;8(5):345–352. [DOI] [PubMed] [Google Scholar]
- 12. Addison S, Stas S, Hayden MR, et al. Insulin resistance and blood pressure. Curr Hypertens Rep. 2008;10(4):319–325. [DOI] [PubMed] [Google Scholar]
- 13. Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2(4):282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin‐evoked responses in brain networks controlling appetite and reward in insulin resistance: The cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006; 55(11):2986–2992. [DOI] [PubMed] [Google Scholar]
- 15. Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;65(suppl):S23–S27. [DOI] [PubMed] [Google Scholar]
- 16. Purnell JQ, Dev RK, Steffes MW, et al. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes. 2003; 52(10):2623–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larger E. Weight gain and insulin treatment. Diabetes Metab. 2005;31(suppl 4, Pt 2): 4S51–4S56. [DOI] [PubMed] [Google Scholar]
- 18. Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta‐analysis of clinical studies. J Am Coll Nutr. 2003;22(5):331–339. [DOI] [PubMed] [Google Scholar]
- 19. Schernthaner GH, Schernthaner G. Insulin resistance and inflammation in the early phase of type 2 diabetes: potential for therapeutic intervention. Scand J Clin Lab Invest Suppl. 2005;240:30–40. [DOI] [PubMed] [Google Scholar]
- 20. Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006; 16(suppl 1):S35–S38. [DOI] [PubMed] [Google Scholar]
- 21. Heald AH, Karvestedt L, Anderson SG, et al. Low insulin‐like growth factor‐II levels predict weight gain in normal weight subjects with type 2 diabetes. Am J Med. 2006; 119(2):e9–e15. [DOI] [PubMed] [Google Scholar]
- 22. Renstrom F, Buren J, Svensson M, et al. Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metabolism. 2007;56(2):190–198. [DOI] [PubMed] [Google Scholar]
- 23. Biesenbach G, Raml A, Alsaraji N. Weight gain and insulin requirement in type 2 diabetic patients during the first year after initiating insulin therapy dependent on baseline BMI. Diabetes Obes Metab. 2006;8(6):669–673. [DOI] [PubMed] [Google Scholar]
- 24. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 25. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 26. Nichols GA, Gomez‐Caminero A. Weight changes following the initiation of new anti‐hyperglycaemic therapies. Diabetes Obes Metab. 2007;9(1):96–102. [DOI] [PubMed] [Google Scholar]
- 27. Boule NG, Chaput JP, Doucet E, et al. Glucose homeostasis predicts weight gain: prospective and clinical evidence. Diabetes Metab Res Rev. 2008;24(2):123–129. [DOI] [PubMed] [Google Scholar]
- 28. Chaput JP, Tremblay A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes (Lond). 2009; 33(1): 46–53. [DOI] [PubMed] [Google Scholar]
- 29. Salle A, Ryan M, Guilloteau G, et al. ‘Glucose control‐related’ and ‘non‐glucose control‐related’ effects of insulin on weight gain in newly insulin‐treated type 2 diabetic patients. Br J Nutr. 2005;94(6):931–937. [DOI] [PubMed] [Google Scholar]
- 30. Haak T, Tiengo A, Draeger E, et al. Lower within‐subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7(1):56–64. [DOI] [PubMed] [Google Scholar]
- 31. Hermansen K, Davies M, Derezinski T, et al. A 26‐week, randomized, parallel, treat‐to‐target trial comparing insulin detemir with NPH insulin as add‐on therapy to oral glucose‐lowering drugs in insulin‐naïve people with type 2 diabetes. Diabetes Care. 2006;29(6):1269–1274. [DOI] [PubMed] [Google Scholar]
- 32. Philis‐Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once‐daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006; 28(10): 1569–1581. [DOI] [PubMed] [Google Scholar]
- 33. Rašlová K, Tamer SC, Clauson P, et al. Insulin detemir results in less weight gain than NPH insulin when used in basal‐bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig. 2007;27(4):279–285. [DOI] [PubMed] [Google Scholar]
- 34. Rosenstock J, Davies M, Home PD, et al. Insulin detemir added to oral anti‐diabetic drugs in type 2 diabetes provides glycemic control comparable to insulin glargine with less weight gain. Diabetes. 2006;55(suppl): A132. [Google Scholar]
- 35. Hermansen K, Davies M. Does insulin detemir have a role in reducing risk of insulin‐associated weight gain? Diabetes Obes Metab. 2007;9(3):209–217. [DOI] [PubMed] [Google Scholar]
- 36. Fritsche A, Häring H. At last, a weight neutral insulin? Int J Obes. 2004;28(suppl 2):S41–S46. [DOI] [PubMed] [Google Scholar]
- 37. Albu J, Raja‐Khan N. The management of the obese diabetic patient. Prim Care. 2003;30(2):465–491. [DOI] [PubMed] [Google Scholar]
- 38. Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast‐food habits, weight gain, and insulin resistance (the CARDIA study): 15‐year prospective analysis. Lancet. 2005;365(9453):36–42. [DOI] [PubMed] [Google Scholar]
- 39. Westman EC, Yancy WS Jr, Mavropoulos JC, et al. The effect of a low‐carbohydrate, ketogenic diet versus a low‐glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson PW, Black HR, Fabricatore AN, et al. Challenges to the diagnosis, evaluation, treatment, and management of clustered cardiometabolic risk factors. J Cardiometab Syndr. 2008;3(3):177–182. [DOI] [PubMed] [Google Scholar]
- 41. Bonen A, Dohm GL, Van Loon LJ. Lipid metabolism, exercise and insulin action. Essays Biochem. 2006;42:47–59. [DOI] [PubMed] [Google Scholar]
- 42. Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111–R1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dornhorst A, Luddeke HJ, Sreenan S, et al. Safety and efficacy of insulin detemir in clinical practice: 14‐week follow‐up data from type 1 and type 2 diabetes patients in the PREDICTIVE European cohort. Int J Clin Pract. 2007;61(3):523–528. [DOI] [PubMed] [Google Scholar]
- 44. Dornhorst A, Luddeke HJ, Sreenan S, et al. Insulin detemir improves glycaemic control without weight gain in insulin‐naive patients with type 2 diabetes: subgroup analysis from the PREDICTIVE™ study. Int J Clin Pract. 2008;62(4):659–665. [DOI] [PubMed] [Google Scholar]
- 45. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32(1):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Gaal L, Gutkin S, Nauck M. Exploiting the antidiabetic properties of physiologic incretins to treat type 2 diabetes mellitus: exenatide or insulin for patients with inadequate glycemic control using oral antidiabetic agents? Eur J Endocrinol. 2008;158:773–784. [DOI] [PubMed] [Google Scholar]
- 47. Pratley RE, Salsali A. Inhibition of DPP‐4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007; 23(4):919–931. [DOI] [PubMed] [Google Scholar]
- 48. Cummings DE, Flum DR. Gastrointestinal surgery as a treatment for diabetes. JAMA. 2008;299(3):341–343. [DOI] [PubMed] [Google Scholar]


