Abstract
Nearly half of women in the United States report problems with sexual function. Many healthcare providers do not ask about sexual concerns during routine clinical encounters because of personal discomfort, lack of familiarity with treatment, or the belief that they lack adequate time to address this complex issue. This may be especially true for hypoactive sexual desire disorder (HSDD), the most commonly identified sexual problem among women. HSDD is characterized by a deficiency of sexual thoughts, feelings or receptiveness to sexual stimulation that has been present for at least 6 months, causes personal distress, and is not due to another medical condition. This is an up-to-date overview of HSDD for clinicians, discussing its physiology, assessment, diagnosis, and treatment strategies. While a definitive physiology of HSDD is still unknown, multiple hormones and neurotransmitters likely participate in a Dual-Control Model to balance excitation and inhibition of sexual desire. For assessment and diagnosis, validated screening tools are discussed, and the importance of a biopsychosocial assessment is emphasized, with guidance on how this can be implemented in clinical encounters. The two recently approved medications for HSDD, flibanserin and bremelanotide, are reviewed as well as off-label treatments. Overall, HSDD represents a common yet likely underrecognized disorder that midwives and other healthcare providers who care for women across the lifespan are in a unique position to address.
Keywords: Hypoactive Sexual Desire Disorder, Sexual dysfunction, Women’s Health, libido, sexual desire
Précis:
An up-to-date overview on the evaluation and management of Hypoactive Sexual Desire Disorder.
INTRODUCTION
Women may experience problems with sexual function throughout their lifespan, however, universal assessment of sexual concerns is not generally practiced and providers do not often use standardized language or questionnaires to inquire about sexual dissatisfaction.1 Women may be unsure about where to turn for help, and may present with a sexual complaint to their gynecology provider, primary care provider, behavioral health clinician or may never bring up sexual matters during their health care visits. Although clinicians often feel inadequately trained to address sexual complaints, doing so utilizes similar principles and techniques found in everyday practice, thus, with education, most professionals have the skills necessary to assess sexual concerns and develop an evidence-based treatment plan to help women experience sexual satisfaction. The purpose of this review is to provide a general overview of one of the most prevalent female sexual health complaints, hypoactive sexual desire disorder (HSDD). Geared specifically for midwives and other healthcare providers who care for women across the lifespan, this review includes the definition and scope of HSDD, its physiology, clinical assessment and diagnosis, as well as evidence-based treatment options.
Scope of the Condition
In a large (n=31,581) 2008 survey on sexual function of women in the United States nearly half of women (43%) reported sexual concerns with the most common concern being low sexual desire.2 Women were asked about the presence of sexual problems in the domains of sexual desire, arousal, and orgasm, and distress associated with their symptoms.2 In this landmark study, Shifren et al documented a high rate of sexual problems among women, with nearly half of women reporting a concern in one or more domains studied. The most commonly reported sexual concern is low desire, which represented 64% of sexual concerns in one study.3 While low desire by itself does not necessarily indicate pathology, when severe and distressing it may constitute HSDD. The International Society for the Study of Women’s Sexual Health defines HSDD as:
Lack of motivation for sexual activity characterized by: decreased or absent spontaneous desire (ie, sexual thoughts or fantasies); decreased or absent responsive desire to erotic cues or stimulation or inability to maintain desire or interest through sexual activity; or loss of desire to initiate or participate in sexual activity including behavioral responses such as avoiding situations that could lead to sexual activity that is not secondary to sexual pain disorders, that is accompanied by clinically significant distress.4
HSDD is associated with significant psychological distress, lower quality of life, as well as psychiatric disorders such as major depressive disorder.5,6 Prior reviews on HSDD have noted variation in studies in terms of the actual prevalence of HSDD, and depending on the study this may be anywhere from 8% to 50%.4,7 HSDD occurs across the lifespan with some indication that rates vary little with age.5 As such, screening for sexual concerns, identifying modifiable risk factors, counseling women on options for treatment, and referral as needed should be a part of every well woman visit and a routine part of caring for women across the lifespan.
PHYSIOLOGY
The physiology of female sexual function is dependent on a complex biopsychosocial interplay and has been the subject of various integrative models.8,9 A full discussion of female sexual physiology is beyond the scope of this article, and has been reviewed elsewhere.10,11 Instead, the goal of this section is to provide an introduction to the physiology of sexual desire such that providers might apply it to understanding treatments and educating their patients. Most models of sexual response as well as models of reward function in neuroscience differentiate desire (wanting, motivation, libido) from arousal (physiologic changes that facilitate sexual activity, for example vaginal lubrication) and consummatory reward (feelings of pleasure and satisfaction along with orgasm).12,13 While these elements interact with one another, they likely have distinct physiology.10,14 A theme throughout this section is that while there are many potential physiological players in female sexual desire, a definitive pathophysiology of HSDD has yet to be discovered.
Excitation and inhibition of sexual desire: The Dual Control Model
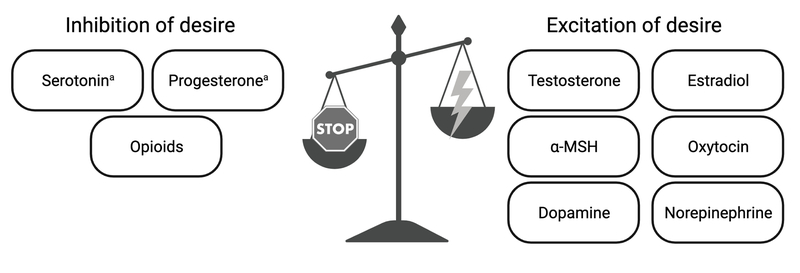
For the purposes of clinicians attempting to diagnose, treat, and counsel patients on HSDD, the action of relevant hormones and neurotransmitters in sexual desire can be simplified by incorporating them into what’s known as the Dual Control Model (Figure 1).15 This model of sexual response describes desire as the result of interaction between competing excitatory and inhibitory processes in the brain, with sexual desire occurring when excitatory processes outweigh inhibition. HSDD can result if there is dysfunction of excitatory or inhibitory signals by preventing adequate excitation or promoting excessive inhibition. Neuroimaging studies support deficient excitation in the brains of women with HSDD: there is decreased activity in brain regions responsible for sexual desire with increased activity in brain regions responsible for self-focus and moral judgement.16
Figure 1.
Various physiological substrates and their role in either exciting or inhibiting sexual desire, based on the Dual-Control Model (Bancroft et al, 200915). Deficient excitation, excessive inhibition, or a mix of both can contribute to the physiology of hypoactive sexual desire disorder. aSerotonin and progesterone have both excitatory and inhibitory properties, though are usually considered inhibitory. α-MSH (alpha-melanocyte stimulating hormone).
Steroid hormones
Hormonally, the female body produces several sex steroids that are known to influence desire. Androgens, specifically testosterone, have an excitatory influence on a woman’s sexual desire.17 Both estradiol and progesterone play a role in enhancing motivation for sexual activity18 and facilitate the release of neurotransmitters involved in sexual desire19, however progesterone is more commonly associated with inhibition of sexual desire.20 Despite the importance of sex steroids in sexual desire, endogenous steroid levels (especially androgens) have poor correlation with sexual desire.21 In addition, there is no current available evidence suggesting that women with HSDD differ from age-matched peers in terms of steroid hormone levels. Thus measuring steroid hormone levels is not routinely recommended to establish a diagnosis of HSDD.22
Excitation of sexual desire: neurotransmitters and peptides
Steroid hormones impact sexual desire at least partly through their interaction with various neurotransmitters and peptides within the brain. Neural substrates responsible for excitation of sexual desire include the neurotransmitters dopamine and norepinephrine as well as the peptides alpha-melanocyte-stimulating hormone (α -MSH) and oxytocin. Dopamine is the major neurotransmitter in the brain responsible for motivation and desire for reward, including sexual activity.23 Both testosterone and estradiol (and to some extent progesterone) facilitate dopamine release in the nucleus accumbens19,24 which is likely one of the mechanisms by which these hormones promote sexual desire. Norepinephrine, like dopamine, is considered excitatory and promotes an overall state of arousal and focused engagement.25
In addition to dopamine and norepinephrine, the two peptides, α -MSH and oxytocin, contribute to the excitatory arm of the dual-control model, which merits discussion as they can be modulated by HSDD treatments. α -MSH likely enhances sexual desire in part by increasing dopamine release.26 Oxytocin also interacts with dopamine and has been shown to promote bonding, orgasm, and sexual desire.27 Importantly, estradiol stimulates the release of α-MSH28 and oxytocin29, which has clinical relevance when treating menopausal women with HSDD, as low levels of estradiol will likely decrease oxytocin and α-MSH, and potentially contribute to symptoms of decreased desire.
Serotonin: inhibition or excitation of desire?
Serotonin, opioid peptides, glutamate and endocannabinoids have all been noted to contribute to the inhibition arm of the dual control model.30 This review focuses on serotonin given its high clinical relevance. Unlike dopamine which facilitates the drive for reward, serotonin is associated with satiety and inhibition of reward seeking.31 Enhancement of serotonin in the brain, with selective serotonin re-uptake inhibitors (SSRIs) used to treat depression and anxiety, is frequently associated with decreased sexual desire.32 However the role of serotonin and SSRIs in sexual desire is complicated by the reality that depression and anxiety on their own will inhibit sexual desire, and thus some women might find their desire normalized during successful treatment with serotonin enhancing medications, and in some cases, desire might even increase.33 In some women, distracting thoughts unrelated to sexual activity are noted as a barrier to maintaining sexual desire. Activation of serotonin type 1A receptors in the frontal regions of the brain can increase dopamine release34 which promotes focus and attention,35 potentially minimizing distraction. The use of serotonin type 1A agonists in HSDD is discussed further in the section on treatment.
Summary
The physiology of sexual desire consists of interactions between multiple hormones and neurotransmitters. HSDD is likely due to an overall decrease in sexual excitation signals, an increase in sexual inhibition signals, or some combination of the two. As such, there is no one definitive HSDD pathophysiology. Instead there are likely many pathways by which a woman can eventually experience a noted decrease brain activity in regions governing sexual desire.16
ASSESSMENT AND DIAGNOSIS
Screening for HSDD
Screening for sexual concerns such as decreased desire can be accomplished using a 4-step process that provides Permission, Limited Information, Specific Suggestions and Intensive Therapy (PLISSIT).36 The PLISSIT model has been used in various settings to guide discussions related to sexual health; further details on its use have been published by Anon (1976).36 To start the conversation, a normalizing statement followed by a question is useful and easily tailored to specific patient circumstances. For example, “Many postpartum women notice changes in sexual function, what changes have you noticed?” The question can be altered to apply to different populations (ie, menopausal women, women undergoing cancer therapies, women with diabetes, etc). Normalizing changes in sexual function gives patients permission to discuss their concerns and gives the provider permission to more fully understand the concern by seeking limited information. Appropriate follow-up questions may include those regarding the onset and frequency of decreased desire as well as the situations in which it occurs. Discussion of situational desire may include noting normal decreases in desire that accompany life circumstances such as having small children who wake frequently at night, living with chronic illness, or a partner who has sexual difficulties. It is helpful to normalize that desire varies across individuals and life stages. This presents an opportunity to introduce the concept of responsive desire: the normal occurrence of desire that follows sexual arousal which is separate from spontaneous desire.8 Basson’s model of the sexual response cycle suggests that a woman at baseline is neither motivated nor avoidant of sexual activity. With appropriate stimulation the woman will experience sexual arousal: the physical response of vaginal lubrication, vaginal heat, nipple erection, and then sexual desire occurs8. The cycle progresses to the next step of emotional and physical satisfaction, and the final part of the cycle is emotional intimacy. Sharing this model with women who have concerns about their sexual desire helps normalize responsive desire, as well as the importance of physical and emotional satisfaction with sexual activity.
If the patient reports concerns related to desire and/or arousal, sexual pain, or orgasm, the 19-item Female Sexual Function Index is an assessment tool that assists the clinician in determining which areas of sexual functioning are affected.37 Sexual pain disorders are beyond the scope of this review but must be assessed and treated prior to diagnosis and treatment of sexual desire disorder, as treating desire that results in painful sexual activity will further hamper desire. Similarly, treatment of orgasm represents another type of sexual dysfunction that is beyond the current review’s focus but must be assessed. If the patient’s sexual concerns are limited to desire, the five-item Decreased Sexual Desire Screener offers a quick assessment of symptoms 38 The first four questions of the screener assess lack of sexual desire and accompanying distress, while the fifth question assesses potential modifiable contributors that must be mitigated or addressed prior to establishing a diagnosis of HSDD. Examples of these are comorbid medical conditions, relationship challenges, and even high levels of stress or fatigue. In order to identify all potentially modifiable contributors to decreased desire, a biopsychosocial model can be applied to aid the clinician and patient to better understand the nature of their concerns and develop an evidence-based treatment plan (see Table 1). This may be completed at a subsequent visit or performed by a sexual medicine specialist.
Table 1.
Domains of the biopsychosocial assessment and examples within each domain that are targets for evaluation and/or intervention in HSDD.
| Biopsychosocial Assessment | ||||||
|---|---|---|---|---|---|---|
| Domain | Examples | |||||
| Biological | Comorbid medical conditions | Vascular and hormonal conditions | Medications | Physical limitations | Chronic pain | Sleep quality |
| Psychological | Anxiety | Depression | History of trauma | Body image perception | Substance use | Performance concerns |
| Interpersonal | Quality of current and past relationships | Partner technique | Partner communication | |||
| Sociocultural | Cultural messaging about sex and body image | Sexual knowledge | Practicalities (ie, shift work, children waking at night) | |||
Assessment of decreased sexual desire
In order to conduct a biopsychosocial assessment, the clinician takes a history to elicit the biological, psychological, and social aspects of the patient’s life that may contribute to their sexual function (Table 1). Assessment of biological factors includes identification of comorbid medical conditions including vascular and hormonal conditions (ie, type 2 diabetes, thyroid disease, menopause), medications that can have sexual side effects (ie, SSRIs, opioids) and physical factors such as sexual pain related to endometriosis, fibroids, or physical limitations. Psychological factors are evaluated such as the presence of depression, anxiety, substance use disorders, and history of physical or sexual trauma. The social/ or interpersonal context is assessed by discussing the quality of current and past relationships, partner technique, inquiring about concern for sexually transmitted infection or pregnancy. Sociocultural context is vital to assessment of the complaint of low desire and includes review of a patient’s cultural upbringing, messaging around sex and bodies, and logistical factors such as privacy (ie, young children who wake at night) and work hours.2 In the clinical practice of the authors, a printed copy of the biopsychosocial model applicable to female sexual desire is utilized in which the clinician notes the relevant items in each domain with the patient. This assists the clinician and patient to practice shared decision making in formulating a plan for addressing relevant items. At the end of the visit, the patient is given the document to take with them.
As part of the biopsychosocial assessment, screening for mental health disorders is accomplished with tools such as the GAD-7 and the PHQ-9.39,40 If the patient screens positive, a brief assessment of behavioral health concerns may be performed and referral to specialized care with a behavioral health specialist may be appropriate. Related to mental health, many women, particularly those in midlife, experience compromised sleep quality, contributing to many symptoms including decreased sexual desire.41 Addressing sleep problems through sleep hygiene or medication may positively affect sexual function.4 If the biopsychosocial assessment reveals significant relational issues, one should consider referral to couples therapy to address underlying relationship challenges and improve communication, particularly around intimacy. History of trauma is common in women with sexual concerns and these women may benefit from specialized trauma-focused therapy.42 If the biopsychosocial assessment reveals the presence of medications known to decrease desire, consultation with other members of the patient’s healthcare team can optimize or modify of such medication. In women with sexual pain due to pelvic floor dysfunction, vaginal dryness, or other causes of pain, treatment of these conditions should occur prior to addressing desire as continued pain will contribute to further decreased desire. Referral for specialist care represents the intensive therapy portion of the PLISSIT model.
Laboratory testing and physical exam
Laboratory assessment should be patient specific and may include a blood count, thyroid disease screening, vitamin D, and prolactin levels if there is suspicion for underlying disease.4 Greater consideration for laboratory assessment should be given to women with oligomenorrhea, a cancer diagnosis, other somatic symptoms, and/or multiple medical comorbidities. When women in midlife report amenorrhea for one year, the clinician may assume the patient is menopausal. Routine sex hormone assays, specifically testosterone, are not recommended in healthy-appearing women as this is not useful for establishing the diagnosis of HSDD. The clinician may consider hormone testing for patients when there is suspicion of gonadal dysfunction such as premature ovarian failure or polycystic ovarian syndrome.4
Per recent recommendations from the International Society for Women’s Sexual Health, physical exam is performed at the clinician’s discretion as current exam techniques are unlikely to reveal the cause of HSDD.4 If perimenopause or menopause is present, vulvar and vaginal exam may be useful in assessing vaginal tissue because dyspareunia related to atrophic changes and dryness is common and easily treated with local vaginal estrogen. If a pelvic examination is performed, consider assessment of pelvic floor for pain, evidence of prolapse, and clitoral adhesions. Given the high prevalence of sexual dysfunction among women with a history of trauma and the high prevalence of sexual or physical trauma among women generally, when performing any genital exam, a trauma informed approach is recommended.43
Establishing the diagnosis of HSDD
In practice, it is common that at the initial visit for a complaint of low desire, contributing modifiable risk factors are identified and treatment plan is put in to place to address them. In women who have sexual pain due to pelvic floor dysfunction, vaginal dryness, or other causes of pain, treatment of these conditions should occur prior to addressing desire, as continued pain will contribute to further decreased desire. Consultation with other members of the patient’s healthcare team may be helpful to optimize or modify medication that can contribute to low desire. When a biopsychosocial assessment suggests mental health, relationship, or other modifiable factors may be causative, consideration should be given to referral to a specialist in women’s behavioral health to address factors such as anxiety, depression, or history of trauma (intensive therapy, in the PLISSIT model). Consider referral to couples therapy to address underlying relationship challenges and to improve communication. Many patients with HSDD respond to initiation or continuation of wellness behaviors such as regular exercise, sleep hygiene, and stress management.44
If symptoms persist for 6 months once modifiable risk factors have been addressed, a diagnosis of generalized acquired HSDD is established. In this context generalized refers to the presence of symptoms in all settings, and acquired refers to the patient previously having an acceptable desire and is now experiencing a marked decrease.
TREATMENT
Treatment for HSDD is comprised of education, specific therapeutic interventions such as mindfulness therapy or cognitive behavioral therapy, and prescription medication. Education and counseling are considered first-line therapy and consist of the clinician sharing information about normal sexual function and changes in function throughout the lifespan, the impact of chronic disease on sexual desire, concepts of responsive desire, adequate sexual stimulation, and the relationship between positive or pleasurable sexual experiences and desire.4 As previously discussed, the concept of responsive desire is often a powerful revelation for many women who feel somehow broken or inadequate due to cultural messaging that sexual desire is spontaneous and ever-present. Additional education interventions may include specific suggestions (the S of the PLISSIT model) of books on the topic of sexual desire by experts in the field, information about regular use of lubricant products for every sexual encounter, and use of vibrators or other devices during partnered sexual activity to assist with arousal and orgasm. Strategies to improve the quality of sexual encounters can be introduced with discussion of how sexual habits undergo change during one’s lifetime and how one’s sexual experiences can accommodate these changes. For example, a woman who has low desire and physical limitations due to osteoarthritis of the back and knees may benefit from specific discussion about (re)building sexual encounters to accommodate her back and leg pain. Counseling on the variety of intimate activities available to her and her partner which may not include penetrative intercourse with every episode of intimacy allows an opportunity to explore sensual touch, oral sex, and/or mutual masturbation. Many people have limited language and experience discussing intimacy with their partners and may feel vulnerability or discomfort having such conversations. Clinicians can validate these feelings and offer support through examples of how to approach the conversation with partners. Some clinicians may feel more comfortable referring the patient to a behavioral health provider with expertise in sexuality.
Mindfulness-based therapies and cognitive behavioral therapy designed specifically for HSDD have demonstrated preliminary evidence for efficacy in the treatment of HSDD.45 While cognitive behavioral therapy for HSDD is effective46, the availability to women in many communities may be limited. Research continues in this area to determine if mindfulness-based cognitive therapy for sexuality that is delivered remotely via the internet is effective at improving sexual desire and decreasing distress.47 If effective, such programs could increase access to care for women living in remote or underserved areas who may not otherwise have access to specialty services.
Medications: Central Nervous System Agents
Flibanserin
In 2015, flibanserin became the first agent to gain approval from the U.S. Food and Drug Administration (FDA) for the treatment of HSDD. It is currently only approved for HSDD in premenopausal women. Flibanserin works in the brain acting as a serotonin type 1A agonist and a serotonin type 2A receptor antagonist, thus placing it in the category of a multifunctional serotonin agonist and antagonist. Animal studies have shown that flibanserin decreases serotonin but raises dopamine and norepinephrine in the prefrontal cortex.48 It is theorized that such increases in dopamine in the prefrontal cortex may facilitate greater attention and focus on sexual stimuli.7 Clinical trials with flibanserin in premenopausal women with HSDD consistently demonstrated that compared to placebo, flibanserin increased sexual desire, decreased sexual distress, and increased number of satisfying sexual encounters.49–51 The recommended dose is 100mg taken at bedtime and while maximum benefit may take several weeks to occur, it is recommended to stop treatment if no effect is noticed at eight weeks.49
Initially, there was concern over the potential for hypotension and somnolence with flibanserin especially if combined with alcohol or a CYP3A4 inhibitor. This resulted in a boxed FDA warning as well as the need for a risk evaluation and mitigation strategy (REMS) in the United States. Subsequent studies found lack of impairment by flibanserin on next-day simulated driving52 and lack of significant hypotension when moderate alcohol is consumed at least 2 hours from taking flibanserin.53,54 This resulted in the removal of the REMS, however there remains a boxed warning that patients should wait two hours after alcohol consumption to take flibanserin and that severe hypotension may occur with the use of moderate or strong CYP3A4 inhibitors such as antifungals like itraconzole.49 Overall, flibanserin has not been found to have significantly higher levels of adverse effects compared to other serotonin-active drugs like SSRIs and can be safely administered concurrently with SSRIs and dual serotonin-norepinephrine re-uptake inhibitorss.54
Although flibanserin is only FDA approved for use in premenopausal women, studies suggest that off-label use of flibanserin in postmenopausal women may be effective.55,56 Simon et al (2014) conducted a randomized double-blind placebo controlled trial (n=949) in naturally menopausal women and found statistically significant improvements in measures of desire, number of satisfying sexual events, and a decrease in distress related to sexual desire. The most common reported side effect were somnolence, headache, dizziness, and nausea.56
Bremalanotide
A newly approved pharmaceutical option for treatment of HSDD in premenopausal women is an injectable α-MSH analogue, bremelanotide. Bremelanotide enhances dopamine and oxytocin release, as well as acting directly on peripheral nerves that innervate the clitoris and vagina.57 For this reason, this medication might be useful in women with decreased or absent arousal, although this has yet to be formally assessed. Unlike flibanserin which requires daily dosing for several weeks, bremelanotide, at a dose of 1.75mg, is injected subcutaneously as needed, 45 minutes prior to anticipated sexual activity.58 In phase 3 trials, bremelanotide was found to increase sexual desire and decrease distress related to low desire to a greater extent than placebo with effect sizes similar to that seen with flibanserin.59 Nausea, flushing and headache were the most common side effects, these were rated as mild-to-moderate in severity in the majority of cases. Bremelanotide may induce transient increases in blood pressure, up to 6mmHg systolic and 3mmHg diastolic, and is thus contraindicated in those with uncontrolled hypertension or known cardiovascular disease.58,59 At time of writing, bremelanotide has yet to be studied in postmenopausal women.
Off-Label Central Nervous System Agents: Bupropion, Buspirone, Trazodone
Bupropion is approved in the United States for the treatment of major depressive disorder as well as a pharmacological aid for smoking cessation and has multiple studies supporting its efficacy as an off-label option for treatment of HSDD at doses of 150mg-450mg daily.60–62 Limited data suggests that bupropion is effective in treatment of HSDD in both pre and postmenopausal non-depressed women, with effects seen as early as two weeks.60 Bupropion can increase levels of dopamine and norepinephrine in various brain regions63, which is likely the mechanism that benefits women with HSDD. Bupropion is generally well tolerated with the most common side-effects being dry mouth, nausea, and insomnia, however it is contraindicated in those with seizure disorders and eating disorders such as bulimia.64 Given the efficacy of this medication in treatment of depression, it may be particularly effective in those for whom depression and HSDD are comorbid.
Several other central nervous system (CNS) agents deserve mention as holding promise for the treatment of HSDD, though with less established evidence. The serotonin type 1A receptor agonist buspirone approved for generalized anxiety disorder has similarities to flibanserin and has been used off-label for HSDD despite lack of published evidence.4 Buspirone has been found to decrease SSRI-induced sexual dysfunction65 and enhance the effects of testosterone in a subset of women.66 Trazodone, an older antidepressant with similar pharmacology to flibanserin, has been suggested as an underutilized agent given current evidence for pro-sexual effects,67 and in initial studies of a proprietary combination of trazodone and bupropion, it appears to be more effective than bupropion alone for HSDD in premenopausal women.68 Although the evidence for off-label agents is frequently less than those that are FDA approved, many clinicians find it helpful to have knowledge of off-label options especially when caring for women who are economically disadvantaged and may not have insurance coverage for the often cost-prohibitive FDA-approved therapies.
Medications: Hormones
Estrogen
Early studies supported a role for estrogen replacement in increasing sexual desire when used by menopausal women and systemic menopause hormone therapy remains first-line therapy in menopausal women with HSDD who are appropriate candidates.69 In addition to the role of estradiol in the central nervous system in promoting sexual desire, estradiol also has peripheral effects on vaginal atrophy and dyspareunia that may contribute to increased desire by decreasing sexual pain.18 Estrogen replacement may be particularly helpful when vasomotor symptoms associated with the menopausal transition appear to be contributing to decreased sexual desire through poor sleep quality. When considering menopause hormone therapy for any clinical indication, it is important to engage in shared decision making based on the patient’s unique medical history and risk factors for common health conditions that may be affected by initiation or continuation such as breast cancer, cardiovascular disease, and thrombus formation.70
Testosterone
Despite lack of current evidence for androgen deficiency in HSDD and lack of cut-off levels of this endogenous hormone in women with HSDD, the off-label use of testosterone to increase sexual desire in postmenopausal women is supported by evidence as well as several professional societies.17,71 Data supports the use of physiologic dosing of testosterone (ie, doses that approximate that of a premenopausal female) for treatment of HSDD in peri and postmenopausal women.17 As there are no FDA approved formulations of testosterone specifically for women, providers may consider either prescribing male-formulation products at one-tenth of the male dose or using a reputable compounding pharmacy.71 If the latter option is chosen, it is important to discuss with patients the understanding that compounded medications are unlikely to have the same safety and efficacy data as medication in a FDA approved formulation. For these reasons, every effort should be made to use FDA-approved formulas when possible.71 Testosterone therapy should be dosed such that serum levels approximate those seen in the normal range of premenopausal women to avoid masculinization effects such as deepening of voice, acne, clitoromegaly, or unwanted facial hair.71 Given that testosterone is commonly abused for its effects on athletic performance and body composition, all testosterone formulations (compounded or otherwise) are considered schedule III controlled substances in the United States, and thus providers must submit their Drug Enforcement Administration (DEA) number with each prescription. If testosterone therapy is considered, baseline and follow up monitoring of free and total testosterone levels using liquid chromatography-mass spectrometry is recommended after three months of therapy. Monitoring of lipids and ongoing assessment of cardiovascular risk is appropriate.72 If no significant improvement in symptoms is noted at three months, therapy should be discontinued. If the patient experiences improvement and desires to continue, she should be counseled that long-term safety data on testosterone therapy is lacking. Given the lack of approved formulations for testosterone preparations and the need for ongoing monitoring, consider collaboration with clinicians who are experienced in using testosterone according to current guidelines for this patient population. Contraindications to use of testosterone are similar to those with estrogen (cardiovascular disease, history of clot, stroke, venous thromboembolism, hormone receptor positive breast cancer, history of endometrial cancer), thus careful screening for appropriate patients should occur prior to initiation. Definitive data are lacking as to whether testosterone therapy affects risk for cardiovascular disease, breast cancer, and thrombotic events.73
Over-the-counter Supplements
A search on the internet easily finds that vitamin retailers abound with supplements and products that claim to enhance sexual desire. Minimal regulation of dietary supplements raises concerns about quality and safety of these products, and research is often of a lesser quality and subject to bias. However, the popularity of dietary supplements requires that health care providers have some familiarity to guide patients. Two supplements that have been studied and are frequently mentioned by women presenting for treatment merit discussion: ArginMax and Zestra. ArginMax is a non-hormonal oral supplement composed of l-arginine, ginseng, gingko, damiana, and a mixture of vitamins and minerals. Small placebo controlled studies have found that six capsules of ArginMax daily can increase sexual desire and other aspects of sexual function in premenopausal and postmenopausal women.74 However, a study of ArginMax in female cancer survivors found no improvement in sexual function in any domain, although women taking the supplement reported improvements on a measure of quality of life.75 As such, while some evidence is promising, it is far from conclusive. The active ingredient in ArginMax, l-arginine, is available on its own from several retailers, and could be effective in doses of 800mg-2.5g daily.76
Zestra is an over-the-counter topical product applied to the vulva prior to intercourse that acts to increase physical arousal in women. Two randomized, placebo-controlled studies show improvement in various domains of sexual function, including desire in women with various subtypes of female sexual dysfunction.77,78 Both these supplements seem to have minimal side-effects, although genital burning was noted in a minority of women with Zestra.77,78 While further, larger follow-up studies are needed, current research on Arginmax and Zestra does suggest that there might be a role for both oral and topical products available over-the-counter for HSDD.
CONCLUSION
HSDD is a common and distressing disorder in women for which midwives and other healthcare providers caring for women are in an appropriate position to address. Discussion may start with a simple question about concerns regarding sexual functioning and validated screening tools such as The Decreased Sexual Desire Screener can be easily used to help identify possible HSDD. Biopsychosocial assessments are essential in the evaluation of sexual complaints in order to develop a plan to address modifiable risk factors and/or determine necessary referrals to specific specialists. Interprofessional collaboration with colleagues in behavioral health who offer relationship counseling, cognitive behavioral therapy, and mindfulness-based therapies is often necessary as part of caring for women with decreased desire. The clinician also has at her disposal FDA-approved treatments for HSDD (flibanserin, bremelanotide) as well as other options including off-label use of some medications (bupropion, buspirone). In menopausal women menopause hormone therapy may be considered for treatment of HSDD, and if inadequate response to treatment, the clinician may consider the addition of physiologic doses of testosterone. For patients who prefer to try over-the-counter options, Arginmax and Zestra may offer some relief, though as with most supplements, more high-quality studies are needed. For many women, there is therapeutic value in the relationship with a provider who listens to them, validates their concerns, and is willing to work together to develop a plan of care aimed at enjoyable sexual intimacy.
Quick Points:
Hypoactive sexual desire disorder (HSDD) is the most common disorder of female sexual function
The physiology of HSDD consists of interactions between various steroid hormones, peptide hormones, and neurotransmitters
Clinicians should evaluate HSDD using a biopsychosocial assessment to determine contributing and modifiable factors
Multiple treatment strategies are available, including two recently approved drugs, flibanserin and bremelanotide
Acknowledgements:
This work was supported by funding from the Center for Women’s Health Research at the University of Colorado (AMN) and NIH grant U54AG062319 (AMN, PI Kohrt). Figures were created with biorender.com.
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interests to disclose.
REFERENCES:
- 1.Bitzer J, Giraldi A, Pfaus J. A standardized diagnostic interview for hypoactive sexual desire disorder in women: standard operating procedure (SOP Part 2). J Sex Med. 2013;10(1):50–57. [DOI] [PubMed] [Google Scholar]
- 2.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970–978. [DOI] [PubMed] [Google Scholar]
- 3.Hayes RD, Bennett CM, Fairley CK, Dennerstein L. What can Prevalence Studies Tell Us about Female Sexual Difficulty and Dysfunction? The Journal of Sexual Medicine. 2006;3(4):589–595. [DOI] [PubMed] [Google Scholar]
- 4.Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women’s Sexual Health Process of Care for Management of Hypoactive Sexual Desire Disorder in Women. Mayo Clinic Proceedings. 2018;93(4):467–487. [DOI] [PubMed] [Google Scholar]
- 5.Hayes RD, Dennerstein L, Bennett CM, Koochaki PE, Leiblum SR, Graziottin A. Relationship between hypoactive sexual desire disorder and aging. Fertility and Sterility. 2007;87(1):107–112. [DOI] [PubMed] [Google Scholar]
- 6.Clayton AH, Maserejian NN, Connor MK, Huang L, Heiman JR, Rosen RC. Depression in Premenopausal Women With HSDD: Baseline Findings From the HSDD Registry for Women. Psychosomatic Medicine. 2012;74(3):305–311. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder. Mayo Clinic Proceedings. 2017;92(1):114–128. [DOI] [PubMed] [Google Scholar]
- 8.Basson R A Model of Women’s Sexual Arousal. Journal of Sex & Marital Therapy. 2002;28(1):1–10. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan HS. Disorders of Sexual Desire and Other New Concepts and Techniques in Sex Therapy. Brunner/Mazel; 1979. [Google Scholar]
- 10.Berman JR. Physiology of female sexual function and dysfunction. International Journal of Impotence Research. 2005;17(1):S44–S51. [DOI] [PubMed] [Google Scholar]
- 11.Munarriz R, Kim NN, Goldstein I, Traish AM. Biology of female sexual function. Urologic Clinics of North America. 2002;29(3):685–693. [DOI] [PubMed] [Google Scholar]
- 12.Kingsberg SA, Clayton AH, Pfaus JG. The Female Sexual Response: Current Models, Neurobiological Underpinnings and Agents Currently Approved or Under Investigation for the Treatment of Hypoactive Sexual Desire Disorder. CNS Drugs. 2015;29(11):915–933. [DOI] [PubMed] [Google Scholar]
- 13.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. [DOI] [PubMed] [Google Scholar]
- 14.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191(3):497–506. [DOI] [PubMed] [Google Scholar]
- 15.Bancroft J, Graham CA, Janssen E, Sanders SA. The dual control model: current status and future directions. J Sex Res. 2009;46(2–3):121–142. [DOI] [PubMed] [Google Scholar]
- 16.Cacioppo S Neuroimaging of Female Sexual Desire and Hypoactive Sexual Desire Disorder. Sexual Medicine Reviews. 2017;5(4):434–444. [DOI] [PubMed] [Google Scholar]
- 17.Davis SR, Worsley R, Miller KK, Parish SJ, Santoro N. Androgens and Female Sexual Function and Dysfunction—Findings From the Fourth International Consultation of Sexual Medicine. The Journal of Sexual Medicine. 2016;13(2):168–178. [DOI] [PubMed] [Google Scholar]
- 18.Cappelletti M, Wallen K. Increasing women’s sexual desire: The comparative effectiveness of estrogens and androgens. Hormones and Behavior. 2016;78:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Hormones and Behavior. 2018;104:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roney JR, Simmons ZL. Within-cycle fluctuations in progesterone negatively predict changes in both in-pair and extra-pair desire among partnered women. Hormones and Behavior. 2016;81:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Basson R Sexual Dysfunctions in Women. Endocrinology and Metabolism Clinics of North America. 2021;50(1):125–138. [DOI] [PubMed] [Google Scholar]
- 22.Kingsberg SA, Woodard T. Female Sexual Dysfunction: Focus on Low Desire. Obstetrics & Gynecology. 2015;125(2):477–486. [DOI] [PubMed] [Google Scholar]
- 23.Alcaro A, Huber R, Panksepp J. Behavioral Functions of the Mesolimbic Dopaminergic System: an Affective Neuroethological Perspective. Brain Research Reviews. 2007;56(2):283–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souza Silva MA, Mattern C, Topic B, Buddenberg TE, Huston JP. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. European Neuropsychopharmacology. 2009;19(1):53–63. [DOI] [PubMed] [Google Scholar]
- 25.Aston-Jones G, Cohen JD. AN INTEGRATIVE THEORY OF LOCUS COERULEUS-NOREPINEPHRINE FUNCTION: Adaptive Gain and Optimal Performance. Annual Review of Neuroscience. 2005;28(1):403–450. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CA, Thody AJ, Hole DR, Grierson JP, Celis ME. Interaction of estradiol, alpha-melanocyte-stimulating hormone, and dopamine in the regulation of sexual receptivity in the female rat. Neuroendocrinology. 1991;54(1):14–22. [DOI] [PubMed] [Google Scholar]
- 27.Salonia A, Nappi RE, Pontillo M, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior. 2005;47(2):164–169. [DOI] [PubMed] [Google Scholar]
- 28.Khorram O, Bedran de Castro JC, McCann SM. The effect of the estrous cycle and estrogen on the release of immunoreactive alpha-melanocyte-stimulating hormone. Peptides. 1985;6(3):503–508. [DOI] [PubMed] [Google Scholar]
- 29.Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and Estrogen Receptor β in the Brain: An Overview. Frontiers in Endocrinology. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaus JG. REVIEWS: Pathways of Sexual Desire. The Journal of Sexual Medicine. 2009;6(6):1506–1533. [DOI] [PubMed] [Google Scholar]
- 31.Tops M, Russo S, Boksem MAS, Tucker DM. Serotonin: Modulator of a drive to withdraw. Brain Cogn. 2009. [DOI] [PubMed] [Google Scholar]
- 32.Atmaca M Selective Serotonin Reuptake Inhibitor-Induced Sexual Dysfunction: Current Management Perspectives. Neuropsychiatric Disease and Treatment. 2020;16:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altindag A, Gunes M. A case series of increased libido and spontaneous orgasm associated with venlafaxine treatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(3):895–896. [DOI] [PubMed] [Google Scholar]
- 34.Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT2A receptors in prefrontal cortex enhances dopaminergic activity. Journal of Neurochemistry. 2005;95(6):1597–1607. [DOI] [PubMed] [Google Scholar]
- 35.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188(4):567–585. [DOI] [PubMed] [Google Scholar]
- 36.Annon JS. The PLISSIT Model: A Proposed Conceptual Scheme for the Behavioral Treatment of Sexual Problems. Journal of Sex Education and Therapy. 1976;2(1):1–15. [Google Scholar]
- 37.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. [DOI] [PubMed] [Google Scholar]
- 38.Clayton AH, Goldfischer ER, Goldstein I, Derogatis L, Lewis-D’Agostino DJ, Pyke R. Validation of the decreased sexual desire screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6(3):730–738. [DOI] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 41.Lee DM, Tetley J. Sleep quality, sleep duration and sexual health among older people: Findings from the English Longitudinal Study of Ageing. Archives of Gerontology and Geriatrics. 2019;82:147–154. [DOI] [PubMed] [Google Scholar]
- 42.Pettigrew J Integrated Behavioral Health in Women’s Primary and Specialty Care. North American Society for Psychosocial OBGYN; January 2020, 2020; Webinar. [Google Scholar]
- 43.Gokhale P, Young MR, Williams MN, et al. Refining Trauma-Informed Perinatal Care for Urban Prenatal Care Patients with Multiple Lifetime Traumatic Exposures: A Qualitative Study. J Midwifery Womens Health. 2020;65(2):224–230. [DOI] [PubMed] [Google Scholar]
- 44.Finley N Lifestyle Choices Can Augment Female Sexual Well-Being. Am J Lifestyle Med. 2018;12(1):38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyke RE, Clayton AH. Psychological Treatment Trials for Hypoactive Sexual Desire Disorder: A Sexual Medicine Critique and Perspective. J Sex Med. 2015;12(12):2451–2458. [DOI] [PubMed] [Google Scholar]
- 46.Hucker A, McCabe MP. Manualized treatment programs for FSD: research challenges and recommendations. J Sex Med. 2012;9(2):350–360. [DOI] [PubMed] [Google Scholar]
- 47.Meyers M, Margraf J, Velten J. Psychological Treatment of Low Sexual Desire in Women: Protocol for a Randomized, Waitlist-Controlled Trial of Internet-Based Cognitive Behavioral and Mindfulness-Based Treatments. JMIR Res Protoc. 2020;9(9):e20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Invernizzi RW, Sacchetti G, Parini S, Acconcia S, Samanin R. Flibanserin, a potential antidepressant drug, lowers 5-HT and raises dopamine and noradrenaline in the rat prefrontal cortex dialysate: role of 5-HT1A receptors. British Journal of Pharmacology. 2003;139(7):1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addyi(TM): US Prescribing information. In: Raleigh: Sprout Pharmaceuticals; 2019. [Google Scholar]
- 50.Katz M, DeRogatis LR, Ackerman R, et al. Efficacy of Flibanserin in Women with Hypoactive Sexual Desire Disorder: Results from the BEGONIA Trial. The Journal of Sexual Medicine. 2013;10(7):1807–1815. [DOI] [PubMed] [Google Scholar]
- 51.Thorp J, Simon J, Dattani D, et al. Treatment of Hypoactive Sexual Desire Disorder in Premenopausal Women: Efficacy of Flibanserin in the DAISY Study. The Journal of Sexual Medicine. 2012;9(3):793–804. [DOI] [PubMed] [Google Scholar]
- 52.Ozkaynak M, Reeder B, Hoffecker L, Makic MB, Sousa K. Use of Electronic Health Records by Nurses for Symptom Management in Inpatient Settings: A Systematic Review. Comput Inform Nurs. 2017;35(9):465–472. [DOI] [PubMed] [Google Scholar]
- 53.Kingsberg SA, Schaffir J, Faught BM, et al. Female Sexual Health: Barriers to Optimal Outcomes and a Roadmap for Improved Patient-Clinician Communications. J Womens Health (Larchmt). 2019;28(4):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millheiser L, Clayton AH, Parish SJ, Kingsberg SA, Kim NN, Simon JA. Safety and Tolerability of Evening Ethanol Consumption and Bedtime Administration of Flibanserin in Healthy Premenopausal Female Subjects. Sexual Medicine. 2019;7(4):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portman DJ, Brown L, Yuan J, Kissling R, Kingsberg SA. Flibanserin in Postmenopausal Women With Hypoactive Sexual Desire Disorder: Results of the PLUMERIA Study. The Journal of Sexual Medicine. 2017;14(6):834–842. [DOI] [PubMed] [Google Scholar]
- 56.Simon JA, Kingsberg SA, Shumel B, Hanes V, Garcia M, Sand M. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21(6):633–640. [DOI] [PubMed] [Google Scholar]
- 57.Pfaus J, Giuliano F, Gelez H. Bremelanotide: An Overview of Preclinical CNS Effects on Female Sexual Function. The Journal of Sexual Medicine. 2007;4:269–279. [DOI] [PubMed] [Google Scholar]
- 58.Vyleesi: Highlights of Prescribing Information. In: Cranbury, NJ: Palatin Technologies, Inc.; 2021. [Google Scholar]
- 59.Simon JA, Kingsberg SA, Portman D, et al. Long-Term Safety and Efficacy of Bremelanotide for Hypoactive Sexual Desire Disorder. Obstetrics & Gynecology. 2019;134(5):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor Segraves AMHCRKJAASRBVJFCB-W R. Bupropion Sustained Release (SR) for the Treatment of Hypoactive Sexual Desire Disorder (HSDD) in Nondepressed Women. Journal of Sex & Marital Therapy. 2001;27(3):303–316. [DOI] [PubMed] [Google Scholar]
- 61.Safarinejad MR, Hosseini SY, Asgari MA, Dadkhah F, Taghva A. A randomized, double-blind, placebo-controlled study of the efficacy and safety of bupropion for treating hypoactive sexual desire disorder in ovulating women. BJU International. 2010;106(6):832–839. [DOI] [PubMed] [Google Scholar]
- 62.Segraves RT, Clayton A, Croft H, Wolf A, Warnock J. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharmacol. 2004;24(3):339–342. [DOI] [PubMed] [Google Scholar]
- 63.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Primary Care Companion to The Journal of Clinical Psychiatry. 2004;6(4):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wellbutrin XL: highlights of prescribing information. In: Steinbach, Canada: Bausch Health Companies; 2020. [Google Scholar]
- 65.Landén M, Eriksson E, Agren H, Fahlén T. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. Journal of Clinical Psychopharmacology. 1999;19(3):268–271. [DOI] [PubMed] [Google Scholar]
- 66.van Rooij K, Poels S, Bloemers J, et al. Toward Personalized Sexual Medicine (Part 3): Testosterone Combined with a Serotonin1A Receptor Agonist Increases Sexual Satisfaction in Women with HSDD and FSAD, and Dysfunctional Activation of Sexual Inhibitory Mechanisms. The Journal of Sexual Medicine. 2013;10(3):824–837. [DOI] [PubMed] [Google Scholar]
- 67.Pyke RE. Trazodone in Sexual Medicine: Underused and Overdosed? Sexual Medicine Reviews. 2020;8(2):206–216. [DOI] [PubMed] [Google Scholar]
- 68.Pyke RE, Clayton AH. Dose-Finding Study of Lorexys for Hypoactive Sexual Desire Disorder in Premenopausal Women. J Sex Med. 2019;16(12):1885–1894. [DOI] [PubMed] [Google Scholar]
- 69.Davis SR, McCloud P, Strauss BJG, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21(3):227–236. [DOI] [PubMed] [Google Scholar]
- 70.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. [DOI] [PubMed] [Google Scholar]
- 71.Davis SR, Baber R, Panay N, et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. The Journal of Clinical Endocrinology & Metabolism. 2019;104(10):4660–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women’s Sexual Health Process of Care for Management of Hypoactive Sexual Desire Disorder in Women. Mayo Clin Proc. 2018;93(4):467–487. [DOI] [PubMed] [Google Scholar]
- 73.Society TNAM. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. 2005;12(5):496–511; quiz 649. [DOI] [PubMed] [Google Scholar]
- 74.Ito TY, Polan ML, Whipple B, Trant AS. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women differing in menopausal status. J Sex Marital Ther. 2006;32(5):369–378. [DOI] [PubMed] [Google Scholar]
- 75.Greven KM, Case LD, Nycum LR, et al. Effect of ArginMax on sexual functioning and quality of life among female cancer survivors: results of the WFU CCOP Research Base Protocol 97106. J Community Support Oncol. 2015;13(3):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cieri-Hutcherson NE, Jaenecke A, Bahia A, et al. Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy (Basel). 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson DM, Steidle CP, Singh GS, Alexander JS, Weihmiller MK, Crosby MG. Randomized, placebo-controlled, double blind, crossover design trial of the efficacy and safety of Zestra for Women in women with and without female sexual arousal disorder. J Sex Marital Ther. 2003;29 Suppl 1:33–44. [DOI] [PubMed] [Google Scholar]
- 78.Ferguson DM, Hosmane B, Heiman JR. Randomized, placebo-controlled, double-blind, parallel design trial of the efficacy and safety of Zestra in women with mixed desire/interest/arousal/orgasm disorders. J Sex Marital Ther. 2010;36(1):66–86. [DOI] [PubMed] [Google Scholar]