Abstract
The COVID-19 pandemic is having a major impact on public health worldwide and there is an urgent need for the creation of an armamentarium of effective therapeutics, including vaccines, biologics, and small molecule therapeutics, to combat SARS-CoV-2 and emerging variants. Inspection of the virus life cycle reveals multiple viral and host-based choke points that can be exploited to combat the virus. SARS-CoV-2 3C-like protease (3CLpro), an enzyme essential for viral replication, is an attractive target for therapeutic intervention and the design of inhibitors of the protease may lead to the emergence of effective SARS-CoV-2-specific antivirals. We describe herein the results of our studies related to the application of X-ray crystallography, the Thorpe-Ingold effect, deuteration, and stereochemistry in the design of highly potent and non-toxic inhibitors of SARS-CoV-2 3CLpro.
Graphical Abstract:

INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiological agent of coronavirus disease (COVID-19).1 The severity of the ongoing pandemic is having a major impact on public health worldwide, and is further exacerbated by the emergence of more virulent strains.2–3 Intense worldwide efforts to combat the virus have led to the successful development of FDA-approved vaccines, and an array of potential therapeutics, such as monoclonal antibodies, repurposed drugs and others, are currently being evaluated in clinical trials, or are at various stages of clinical development.4–5
The SARS-CoV-2 life cycle encompasses multiple virus and host-based druggable targets that can be exploited, including for example inhibitors that block virus entry and fusion, and replication inhibitors targeting the 3C-like protease (3CLpro) and papain-like protease and the RNA-dependent RNA polymerase, among others. Attractive host-based targets include the proteases transmembrane serine protease 2 (TMPRSS2), cathepsin L, and furin. Thus, the development of small molecule therapeutics that target host or viral targets essential for viral replication is a potentially fruitful avenue of investigation.6–9
The SARS-CoV-2 genome contains two open reading frames (ORF1a and ORF1b). Translation of the genomic mRNA of ORF1a yields a polyprotein (pp1a), while a second polyprotein (pp1ab) is produced by a ribosomal frameshift that joins ORF1a together with ORF1b. The two polyproteins are processed by two cysteine proteases, a 3C-like protease (3CLpro), at eleven distinct cleavage sites and a papain-like protease (PLpro) at three distinct cleavage sites, resulting in 16 mature nonstructural proteins. The two proteases are essential for viral replication, making SARS-CoV-2 3CLpro an attractive target for therapeutic intervention.9–16
SARS-CoV-2 3CLpro is a homodimer with a catalytic Cys-His dyad (Cys145-His41) and an extended binding cleft. The protease displays a strong preference for a -Y-Z-Leu-Gln-X sequence, corresponding to the residues -P4-P3-P2-P1-P1’-,17 where X is a small amino acid (Ser, Ala, Gly), Y is small hydrophobic amino acid, and Z is solvent exposed and can tolerate polar or nonpolar amino acid chains.18
Our foray in this area has focused on the structure-guided design of inhibitors of SARS-CoV-2 and MERS-CoV 3CLpro,19–21 as well as feline infectious peritonitis virus (FIPV) protease inhibitors.22–23 We recently described the structure-guided design of a dipeptidyl series of MERS-CoV and SARS-CoV-2 3CLpro inhibitors incorporating in their structure a piperidine20 or cyclohexyl19 moiety capable of engaging in favorable binding interactions with the S4 pocket. We furthermore demonstrated that members of the cyclohexyl series of compounds improve survival in a mouse model of MERS-CoV infection.19 In this report, we established a cell-based assay to screen inhibitors against SARS-CoV-2 3CLpro, which is safe (BSL2) and fast (takes less than 24 h). Furthermore, we report the results of structure-guided studies intended to interrogate the effects of stereochemistry, conformation, and structure, including the systematic introduction of fluorine (F-walk)24–25 around the structure of GC37621–23 and the synthesis of deuterated inhibitors,26–28 to modulate pharmacological activity, pharmacokinetic properties, and oral bioavailability.
RESULTS AND DISCUSSION
Chemistry
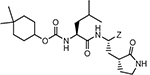
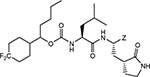
The synthesis of compounds 1–24b/c entailed the use of a structurally diverse set of precursor alcohols (Table 1), some of which were commercially available. Alcohols 12–16 were readily synthesized from 4,4-difluorocyclohexane carboxylic acid via reduction to the corresponding alcohol by treatment with carbonyl diimidazole and sodium borohydride,29 followed by oxidation with Dess-Martin periodinane reagent to yield the aldehyde. Subsequent treatment with an array of Grignard reagents generated alcohols 12, 14–16 (Scheme 1/panel A). Alcohol 13 was synthesized by reacting the methyl ester of 4,4-difluorocyclohexane carboxylic acid with excess methyl magnesium iodide, followed by acidic work up (Scheme 1/panel A). Deuterated alcohols 9, 11, 20, and 22 were obtained by treatment of the precursor carboxylic acid with carbonyl diimidazole followed by the addition of sodium borodeuteride. All trans-substituted alcohols were synthesized by reducing the precursor 4-substituted cyclohexanone with sodium borohydride/CeCl3.30
Table 1.
Alcohol inputs

|
Scheme 1.
A Synthesis of precursor alcohols 12-16
B Synthesis of inhibitors 1-24b-c
C Synthesis of amino alcohol A
Compounds 1–24b/c were readily obtained by reacting each precursor alcohol with disuccinimidyl carbonate,31 followed by coupling with amino alcohol A. The resulting product was treated with Dess-Martin periodinane to yield aldehydes 1–24b which were converted to the corresponding bisulfite adducts 1–24c upon treatment with sodium bisulfite (Scheme 1/panel B).32 An alternative synthesis was used in the case of compounds 6–8, 10–16, 23,24 which involved the reaction of the precursor alcohol with (L) leucine methyl ester isocyanate, as described in detail previously.33 The synthesis of precursor amino alcohol A was readily accomplished by coupling (L) Z-Leu with a glutamine surrogate, followed by sequential reduction with LiBH4 and removal of the protective group (H2/Pd) (Scheme 1/panel C).
Biochemical Studies
Enzyme assays.
The inhibitory activity of compounds 1–24b/c against SARS-CoV-2 3CLpro in biochemical assays was determined as described in the experimental section. The IC50 values against SARS-CoV-2 and MERS-CoV-2 in the enzyme assays are summarized in Table 2 and they are the average of at least two determinations. Most of the compounds potently inhibited SARS-CoV-2 3CLpro and displayed IC50 values that ranged between 0.13 to 1.25 μM. Compounds 15b and 15c were the most effective against SARS-CoV-2 3CLpro with IC50 values 0.13 and 0.17 μM, respectively. The inhibitory activity of a select number of compounds against MERS-CoV 3CLpro was also investigated. The compounds were found to be 3–5-fold more potent against MERS-CoV-3CLpro, with IC50 values in the 40 to 150 nM range (Table 2). Interestingly, compounds 15b and 15c were the most effective against MERS-CoV-3CLpro as well with IC50 values 0.04 and 0.05 μM, respectively. The broad spectrum of inhibitory activity displayed by these compounds enhances their therapeutic potential.
Table 2.
IC50 values of compounds 1-24b-c against SARS-CoV-2 3CLpro, IC50 values of selected compounds against MERS-CoV 3CLpro, EC50 values against SARS-CoV-2 3CLpro and CC50 values of selected compounds.
| Compound Code | Structure | Z | IC50 (μM) | EC50 (μM) SARS-CoV-2 |
CC50 (μM) | ||
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | MERS-CoV | ||||||
| 1b |
|
-CHO | 0.25±0.05 | 0.12±0.02 | 3.48±0.05 | > 100 | |
| 1c | -CH(OH)SO3Na | 0.24±0.01 | 0.12±0.04 | 1.67±0.45 | > 100 | ||
| 2b |

|
-CHO | 0.24±0.01 | Not Determined | |||
| 2c | -CH(OH)SO3Na | 0.24±0.02 | |||||
| 3b |

|
-CHO | 0.25±0.02 | ||||
| 3c | -CH(OH)SO3Na | 0.19±0.02 | |||||
| 4b |

|
-CHO | 0.17±0.01 | 0.12±0.01 | 4.84±0.64 | 55±5 | |
| 4c * | -CH(OH)SO3Na | 0.18±0.01 | 0.15±0.01 | 4.23±1.54 | 58±8 | ||
| 5b |

|
-CHO | 0.22±0.03 | Not Determined | |||
| 5c | -CH(OH)SO3Na | 0.23±0.04 | |||||
| 6b |

|
-CHO | 0.25±0.07 | ||||
| 6c | -CH(OH)SO3Na | 0.19±0.03 | |||||
| 7b |

|
-CHO | 0.23±0.04 | ||||
| 7c | -CH(OH)SO3Na | 0.20±0.03 | |||||
| 8b |

|
-CHO | 0.20±0.03 | 0.06±0.01 | 1.03±0.49 | 43±9 | |
| 8c | -CH(OH)SO3Na | 0.20±0.01 | 0.11±0.01 | 4.43±0.35 | 44±4 | ||
| 9b |

|
-CHO | 0.33±0.11 | Not Determined | |||
| 9c | -CH(OH)SO3Na | 0.33±0.01 | |||||
| 10b # |

|
-CHO | 0.48±0.08 | 0.08±0.01 | Not Determined | ||
| 10c # | -CH(OH)SO3Na | 0.45±0.10 | 0.10±0.01 | ||||
| 11b |

|
-CHO | 0.24±0.06 | Not Determined | |||
| 11c | -CH(OH)SO3Na | 0.21±0.13 | |||||
| 12b |

|
-CHO | 0.26±0.07 | ||||
| 12c | -CH(OH)SO3Na | 0.26±0.08 | |||||
| 13b |

|
-CHO | 1.20±0.57 | ||||
| 13c | -CH(OH)SO3Na | 1.25±0.49 | |||||
| 14b |

|
-CHO | 0.31±0.11 | 0.10±0.02 | 2.95±0.94 | > 100 | |
| 14c | -CH(OH)SO3Na | 0.29±0.09 | 0.14±0.03 | 3.50±1.20 | > 100 | ||
| 15b |

|
-CHO | 0.13±0.04 | 0.04±0.01 | 1.03±0.47 | > 100 | |
| 15c * | -CH(OH)SO3Na | 0.17±0.01 | 0.05±0.01 | 1.45±0.42 | > 100 | ||
| 16b |
|
-CHO | 0.19±0.04 | Not Determined | |||
| 16c | -CH(OH)SO3Na | 0.27±0.03 | |||||
| 17b |

|
-CHO | 0.34±0.06 | Not Determined | |||
| 17c | -CH(OH)SO3Na | 0.38±0.04 | |||||
| 18b |

|
-CHO | 0.35±0.04 | 0.07±0.01 | 5.28±2.62 | > 100 | |
| 18c | -CH(OH)SO3Na | 0.36±0.06 | 0.07±0.01 | 3.40±0.59 | > 100 | ||
| 19b |

|
-CHO | 0.39±0.04 | Not Determined | |||
| 19c | -CH(OH)SO3Na | 0.40±0.03 | |||||
| 20b |

|
-CHO | 0.39±0.06 | ||||
| 20c | -CH(OH)SO3Na | 0.32±0.06 | |||||
| 21b |

|
-CHO | 0.33±0.01 | 0.05±0.01 | 3.27±1.03 | > 100 | |
| 21c | -CH(OH)SO3Na | 0.36±0.01 | 0.08±0.01 | 4.84±0.64 | > 100 | ||
| 22b |

|
-CHO | 0.47±0.23 | Not Determined | |||
| 22c | -CH(OH)SO3Na | 0.60±0.21 | |||||
| 23b |

|
-CHO | 0.33±0.25 | 0.08±0.01 | 2.39±1.51 | > 100 | |
| 23c | -CH(OH)SO3Na | 0.21±0.04 | 0.13±0.01 | 3.72±1.60 | > 100 | ||
| 24b |

|
-CHO | 0.50±0.16 | Not Determined | |||
| 24c | -CH(OH)SO3Na | 0.31±0.01 | |||||
IC50s taken from Rathnayake et al.10
EC50: 0.85±0.1 and 0.70±0.08 μM for 4c and 15c, respectively, from live SARS-CoV-2 in Vero E6 cells.
Establishment of the cell-based assay for SARS-CoV-2 3CLpro inhibitors.
We have previously reported EC50 values determined by incubating SARS-CoV-2 3CLpro inhibitors and Vero E6 cells that were inoculated with SARS-CoV-2 at 50–100 plaque forming units/well.19,33 This cell-based assay requires a BSL3 facility and takes at least 2–3 days. As an alternative method, we report herein a relatively fast and safe cell-based assay system to screen SARS-CoV-2 3CLpro inhibitors using two-plasmids. A similar cell-based assay has been reported;34 however, in contrast, the present system utilizes the replication units of porcine respiratory and reproductive syndrome virus (PRRSV)35 to express SARS-CoV-2 3CLpro. In this system plasmid 1, pR-SARS-CoV-2 3CLpro, was used to express SARS-CoV-2 3CLpro whereas plasmid 2, pGlo-VRLQS, was used to express luciferase-VRLQS in HEK293T cells (Figure 1/ panel A). The expressed inactive luciferase is activated by the catalytic mechanism of SARS-CoV-2 3CLpro in HEK293T cells. Hence, the inhibition of SARS-CoV-2 3CLpro was measured as a function of firefly luciferase activity (Figure 1/ panel B). The plasmid 2 also contains intact Renilla luciferase gene as an expression control. As a negative control, inactive form of the 3CLpro was introduced to pR-SARS-CoV-2 3CLpro by the mutagenesis, and resulting plasmid was designated as pR-SARS-CoV-2 3CLpro C145A. The mock-transfection was also used a negative control. The expression of coronavirus 3CLpro was reported to induce cytotoxicity in the transfected cells.36,37 The level of cytotoxicity in our assay system was about 7%, and the ratio of firefly to Renilla luminescence was adjusted to reduce variability due to cytotoxicity and transfection efficiency. The EC50 values of a select number of compounds, including 1b/1c, 4b/4c, 8b/8c, 14b/14c, 15b/15c, 18b/18c, 21b/21c, and 23b/23c, were determined (Table 2). Inhibition curves by each compound were consistent with a dose-dependent mode and R2 > 0.8 (Figure 1/ panel C). The IC50 and EC50 values of 15b and 15c were the lowest among tested compounds listed in Table 2.
Figure 1.
Generation of a cell-based assay for screening SARS-CoV-2 3CLpro inhibitors in HEK293T cells. Panel A. Plasmid 1; pR-SA2–3CLpro, encodes SARS-CoV-2 3CLpro from the PRRSV reverse genetics system. The gene of SARS-CoV-2 3CLpro is inserted between ORF1b and 2a of PRRSV genome. Plasmid 2; pGlo-VRLQS encodes firefly luciferase with coronavirus 3CLpro recognition sequences VRLQS. Active luciferase is generated by the cleavage with CoV 3CLpro. Panel B. Semi-confluent HEK293T cells were transfected with two plasmids, and after overnight, various concentrations of each compound are applied to the cells. The inhibition of SARS-CoV-2 3CLpro is determined by measuring luciferase activity. Panel C. Inhibition curves of selected compounds, 1c, 4c, 8c, 14c, 15c, 18c, 21c, and 23c using the cell-based assay with pR-SA2–3CLpro and pGlo-VRLQS.
For comparative purposes, we determined EC50 values using live SARS-CoV-2 in Vero E6 cells and the established two-plasmid system of two compounds (4c, 15c) from this work, and a previously published 3CLpro inhibitor, GC376. Compounds 4c and 15c were selected on the basis of having the lowest IC50 values in this series. The EC50 of GC376 was found to be 0.23±0.01 μM in live SARS-CoV-2 in Vero E6 cells.33 In the two-plasmid system, the EC50 of GC376 was determined to be 3.15 ± 0.67 μM (14-fold higher), and the R2 values of the inhibition curves were > 0.9. When the antiviral effects of compounds 4c and 15c were examined from live SARS-CoV-2 in Vero E6 cells, the EC50 values were 0.85±0.1 and 0.70±0.08 μM, respectively (Table 2). The results show that while compounds in this series are cell permeable, the EC50 values were higher from the two-plasmid system (2-fold for 15c and 5-fold for 4c) than those by live SARS-CoV-2 in Vero E6 cells. The higher EC50s may be due to various reasons including different cell types, presence of transfection reagent, incubation time and overexpression of the 3CLpro and luciferase in HEK293T cells due to inhibition of cytotoxic 3CLpro in the two-plasmid system.36,37 Most of the examined compounds showed minimal toxicity up to 100 μM, however, the CC50 values for 4b/4c and 8b/8c were in the 40–60 μM range (Table 2). Although the EC50 values obtained from the two-plasmid system are higher than those obtained from live SARS-CoV-2 in Vero E6 cells, considering the feasibility of conducting the experiments under BSL2 laboratory conditions and the relatively short amount of time required (24h), the cell based two-plasmid method could be a useful initial screening tool for 3CLpro inhibitors against SARS-CoV-2.
X-Ray Crystallographic Studies
A series of high resolution cocrystal structures were determined to elucidate the interaction of the inhibitors with the active site of SARS-CoV-2 3CLpro. Specifically, we sought to confirm the mechanism of action, identify the structural determinants associated with the binding of the inhibitors to the active site of the protease and ultimately harness the accumulated structural information and insights gained to further optimize pharmacological activity and PK parameters. Three groups of inhibitor types were analyzed with respect to their structural elements that interact within the S4 subsite environment, which are 1) non-polar substituents (Table 2, entry 1–9), 2) 4,4-difluorocyclohexyl groups that are connected to a stereocenter (Table 2, entry 10–16) and, 3) fluorinated aryl compounds based on the structure of GC376 (Table 2, entry 17–22).
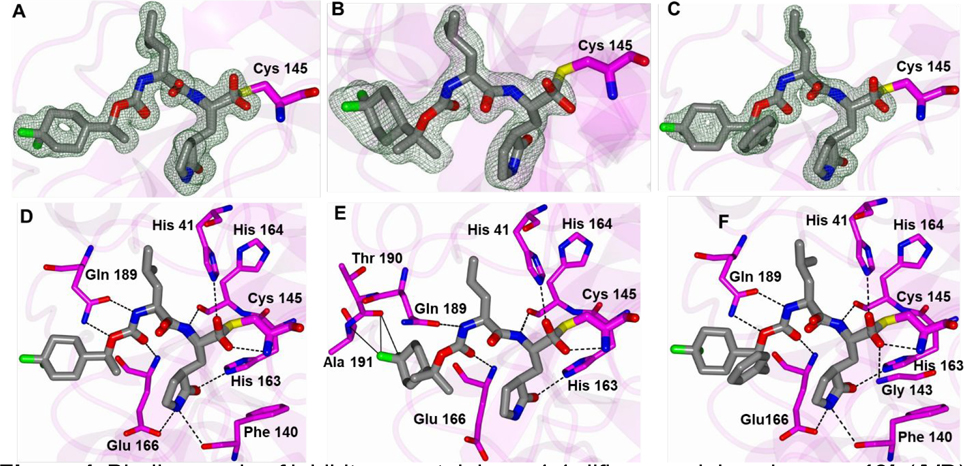
For all structures described below, the active sites contained prominent difference electron density consistent with inhibitors covalently bound to Cys 145. Additionally, the electron density was consistent with both the R and S enantiomers at the stereocenter formed by covalent attachment of the Sγ atom of Cys 145 and were therefore, modeled as each enantiomer with 0.5 occupancy. The γ-lactam ring of the inhibitor forms direct hydrogen bonds with Glu166 and His163, and Glu166 and Gln189 form additional H-bonds with the C=O and NH of the carbamate moiety in the inhibitor. The inhibitor engages in hydrophobic interactions with the leucine side chain, which is snugly accommodated in the S2 pocket. The cocrystal structure confirms that the reaction of Cys145 with the aldehyde warhead results in the formation of a tetrahedral hemithioacetal that is stabilized by a H-bond to His164.
Non-polar substituents.
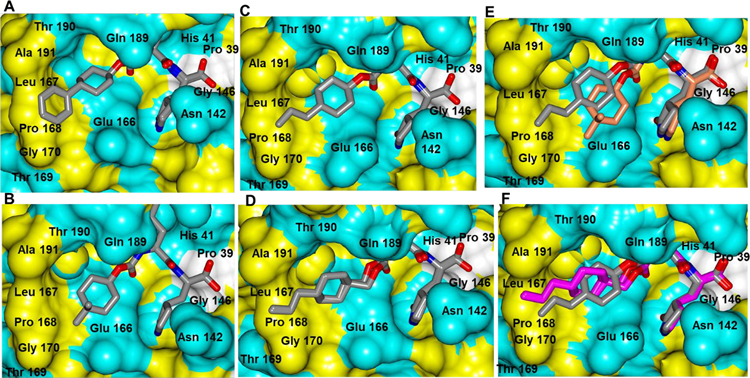
The structures of 5c, 1c, 3c and 8b displayed well-defined electron density and similar hydrogen bond interactions as shown in Figure 2. For all structures, the non-polar groups are mainly positioned within the S4 subsite near a hydrophobic ridge formed by residues Leu 167, Pro 168, Gly 170 and Ala 191 (Figure 3). However, the dimethyl cyclohexyl ring in 1c is too short to fully engage the hydrophobic ridge in the S4 subsite (Figure 3B). The addition of an n- propyl group in 3c permits further engagement with the hydrophobic cleft and the extra carbon atom in 8b allows the propyl group to extend even further (Figures 3C and 3D). Superposition of 3c and 1c (Figure 3E) shows that the 4,4-dimethylcyclohexyl ring is moved slightly out of the S4 subsite relative to the n-propyl group in 3c. Additionally, superposition of 3c and 8b revealed quite similar binding modes although the n-propyl group of 8b is positioned deeper within the S4 subsite (Figure 3F). Overall, the similar binding modes and attendant high potency of the inhibitors are reflected in their low IC50 values and similar potencies (Table 2, compounds 1–5b/c). With respect to compound 8, it was envisaged that the corresponding deuterated compound 9, found to be nearly equipotent to non-deuterated compound 8 (Table 2), would likely display improved PK properties due to its enhanced in vivo stability arising from the greater strength of the C-D bond and the resulting deuterium kinetic isotope effect.26–28
Figure 2.
Binding mode of inhibitors containing non-polar substituents. 5c (A/E), 1c (B/F), 3c (C/G) and 8b (D/H) with SARS-CoV-2 3CLpro. Fo-Fc Polder omit map (A-D) contoured at 3σ. Hydrogen bond interactions (E-H) are drawn as dashed lines. PDB IDs: 5c (7LZZ), 1c (7LZX), 3c (7LZY), 8b (7LZT).
Figure 3.
Surface representation showing the orientation of non-polar groups near the S4 subsite of SARS-CoV-2 3CLpro with neighboring residues colored yellow (nonpolar), cyan (polar), and white (weakly polar). 5c (A), 1c (B), 3c (C), 8b (D). Superposition of 3c (gray) and 1c (coral) (E). Superposition of 3c (gray) and 8b (magenta) (F). PDB IDs: 5c (7LZZ), 1c (7LZX), 3c (7LZY), 8b (7LZT).
4,4-Difluorocyclohexyl compounds.
In previous studies related to norovirus 3CLpro inhibitors, the strategic introduction of a gem-dimethyl group into the inhibitor structure resulted in enhanced potency by restricting rotation around the nearby single bonds and lowering the entropic penalty associated with binding.38 Thus, we sought to capitalize on this by synthesizing gem-dimethyl-substituted compound 13c and, additionally, achieve the same end by introducing a stereocenter (12c). The structures of 12b, 13c and 14c with SARS-CoV 3CLpro displayed well-defined electron density and the typically observed hydrogen bond interactions (Figure 4). The 4,4-difluorocyclohexyl rings for all structures are positioned near the hydrophobic cleft in the S4 subsite as shown in Figure 5 A–C. Superposition of these structures revealed a nearly identical binding mode for 12b and 13c in which the 4,4-difluorocyclohexyl groups are positioned in the same region within the S4 subsite (Figure 5D). For 14c, the benzyl ring is oriented in a wide cleft formed by Asn 142 and Gln 189. However, the 4,4-difluorocyclohexyl ring of 13c contacts residues Thr 190 and Ala 191 (3.0–3.2 Å) and forms new hydrogen bond interactions with the backbone oxygen and nitrogen atoms respectively (Figure 4E). This positions the 4,4-difluorocyclohexyl ring of 13c deeper into the S4 pocket and results in a conformational change in the loop spanning Gln 189 to Gly 195 and Glu 166 to Gly 170, in order to accommodate the new interactions and avoid steric clash. This results in the loss of the typical hydrogen bond between the side chain of Glu 166 and the glutamine surrogate of 13c (Figures 4E and 5D) which may explain why the IC50 of 13c is ~4-fold higher than those of 12b and 14c.
Figure 4.
Binding mode of inhibitors containing a 4,4-difluorocyclohexyl group. 12b (A/D), 13c (B/E) and 14c (C/F) with SARS-CoV-2 3CLpro. Fo-Fc Polder omit map (A-C) contoured at 3σ. Hydrogen bond interactions (D-F) are drawn as dashed lines. PDB IDs: 12b (7LZU), 13c (7M00), 14c (7M01).
Figure 5.
Surface representation showing the orientation of the 4,4-difluorocyclohexyl groups near the S4 subsite of SARS-CoV-2 3CLpro with neighboring residues colored yellow (nonpolar), cyan (polar), and white (weakly polar). 12b (A), 13c (B) and 14c (C). Superposition of 12b (gold), 13c (coral) and 14c (gray) (D). The loop between Gln 189 and Gly 195 is colored cyan for 13c and magenta for 12b/14c. Hydrogen bond interactions with Glu 166 are indicated by the dashed lines. PDB IDs: 12b (7LZU), 13c (7M00), 14c (7M01).
Fluorinated Aryl Compounds.
Positional analogue scanning is a widely used strategy for optimizing binding affinity, selectivity, and physicochemical properties of lead compounds containing aromatic or heteroaromatic rings.24 For instance, the introduction of fluorine (F-walk)25 or nitrogen (N-walk)39 is an effective means for multiparameter optimization by leveraging the beneficial impact of fluorine (or nitrogen) and minor structural changes. In an effort to determine the effect of fluorine on the binding mode in the S4 subsite of GC376, the structures of the fluorinated benzyl compounds 17c, 18c, 19b, 20b (deuterated analog of 19b) and 21c were determined with SARS-CoV-2 3CLpro. The inhibitor o-fluorobenzyl (17c) and m-fluorobenzyl (18c) compounds displayed well-defined electron density and similar hydrogen bond interactions as shown in Figure 6. Interestingly, the o-fluorobenzyl ring of 17c adopts a conformation in which the fluorine atom is directed away from Thr 190 and is instead positioned 3.38 Å from the backbone oxygen atom of Glu 166 (Figure 6C). Conversely, the fluorine atom in 18c is positioned between Thr 190/Ala 191 in the S4 pocket and is 3.10 Å from the backbone nitrogen atom of Ala 191 (Figure 6D). The orientations of the fluorine atoms in 17c and 18c relative to the hydrophobic ridge in the S4 pocket is shown in Figures 6E–F.
Figure 6.
Binding mode of inhibitors containing a fluorinated aromatic group. 17c (A/C), 18c (B/D) with SARS-CoV-2 3CLpro. Fo-Fc Polder omit map (A-B) contoured at 3σ. Hydrogen bond interactions (C-D) are drawn as dashed lines. The 3.38 Å contact between the F-atom of 17c and the backbone O-atom of Glu 166 is drawn as a solid line in panel C. Surface representation of 17c (E) and 18c (F) showing the orientation of the 4,4-difluorocyclohexyl groups near the S4 subsite of SARS-CoV-2 3CLpro with neighboring residues colored yellow (nonpolar), cyan (polar), and white (weakly polar). PDB IDs: 17c (7M02), 18c (7M03).
The compounds that contain a p-fluorobenzyl group 19b and its deuterated analog 20b not surprisingly adopt very similar binding modes and hydrogen bond interactions as shown in Figures S1 and S2. Interestingly, the inhibitor adopts two conformations in which the p-fluorobenzyl ring is projected away from the S4 subsite in subunit B and is positioned in the S4 pocket in subunit A. However, the electron density for the p-fluorobenzyl ring is somewhat weaker in subunit A, which suggests that the pose in subunit B is likely the predominant conformation. This may due to the fact that the fluorine atom does not form any contacts with polar atoms in the S4 subsite and results in a conformation in which the aryl ring is positioned out of the pocket which is the same conformation observed for the parent compound GC376.
The perfluorinated compound 21c also displayed well-defined difference electron density consistent with the aryl ring in one conformation (Figure 7A). Interestingly, one of the o-fluorine atoms interacts with the backbone oxygen of Glu 166 (3.08 Å) which is shorter than that observed for 17c described above (3.38 Å). The other o-fluorine atom is positioned 2.92 Å from the backbone N-atom of Thr 190 and 3.12 Å from the side chain N-atom of Gln 189 (Figure 7B). Similarly, the m-fluorine atom is positioned near the backbone nitrogen atom of Ala 191 (3.40 Å) which is longer than the distance observed for 18c (3.10 Å). The pentafluorobenzyl ring is positioned on top of the hydrophobic cleft within the S4-subsite (Figure 7C), unlike GC376 where the phenyl ring undergoes a hydrophobic collapse with the γ-lactam ring and the inhibitor assumes a “paper clip” shape.
Figure 7.
Binding mode of 21c containing a perfluorinated aromatic group. Fo-Fc Polder omit map contoured at 3σ (A). Hydrogen bond interactions are drawn as dashed lines. Close contacts to the perfluorinated ring that are longer than typical polar contact distances are drawn as solid lines (B). Surface representation showing the orientation of 21c near the S4 subsite of SARS-CoV-2 3CLpro with neighboring residues colored yellow (nonpolar), cyan (polar), and white (weakly polar) (C). PDB ID: 21c (7M04).
Finally, GC376 variants 23b/c and 24b/c were synthesized and screened as mixtures of epimers. The aldehyde and bisulfite adduct compounds 23b/c were found to potently inhibit 3CLpro (IC50 0.15 and 0.18 μM, respectively), and these were 27-fold and 19-fold more potent than the corresponding 24b/c aldehyde and bisulfite adducts, respectively. These findings provide tentative validation of the design regarding the use of a chiral center to attain directional control and augment binding interactions.
CONCLUSIONS
Effective management of SARS-CoV-2, the causative agent of the COVID-19 pandemic, would require the availability of safe and effective vaccines (already realized), as well as the availability of small-molecule therapeutics and prophylactics that target viral and host-based druggable targets. SARS-CoV-2 3CLpro is an attractive target for the development of COVID-19 therapeutics because of its vital role in viral replication. An array of approaches was utilized to optimize potency and physicochemical parameters, including conformational and stereochemical control via the introduction of a gem-dimethyl group (Thorpe-Ingold effect) or stereocenter, deuteration, and fluorine, into the inhibitors. Virtually all inhibitors were found to display sub-micromolar potency against SARS-CoV-2 and MERS-CoV 3CLpro, and the inhibitory activities were confirmed in a newly established fast and safe cell-based assay. Furthermore, several deuterated inhibitors which are likely to exhibit improved pharmacokinetics were found to be equipotent with the corresponding non-deuterated inhibitors. The fluorine-walk approach was applied to explore bioisosteric replacements for the phenyl ring in GC376 by replacing one or more hydrogen atoms. The effects of these modifications included unanticipated binding modes of the F-substituted phenyl ring and modestly-enhanced potency. The introduction of multiple fluorine atoms resulted in an orientation that allowed the fluorine atoms to engage in H-bonding with residues in the S4 pocket, although with sub-optimal bond angles. High resolution cocrystal structures with an array of inhibitors unraveled the mechanism of action and provided valuable insights regarding the binding of the inhibitors to the active site and the identity of the structural determinants involved in binding. The 4,4-difluorocyclohexane methyl moiety connected to the benzylic carbon, coupled with the directional control imparted by the chiral center, resulted in a near optimal fit in this series (Table 2, entry 15). Collectively, the results of the studies described herein are significant and timely and provide an effective launching pad for conducting further pre-clinical studies.
EXPERIMENTAL SECTION
General
Reagents and dry solvents were purchased from various chemical suppliers (Sigma-Aldrich, Acros Organics, Chem-Impex, TCI America, Oakwood chemical, APExBIO, Cambridge Isotopes, Alpha Aesar, Fisher and Advanced Chemblocks) and were used as obtained. Silica gel (230–450 mesh) used for flash chromatography was purchased from Sorbent Technologies (Atlanta, GA). Thin layer chromatography was performed using Analtech silica gel plates. Visualization was accomplished using UV light and/or iodine. NMR spectra were recorded in CDCl3 or DMSO-d6 using Varian XL-400 spectrometer. The purity of most of the aldehyde inhibitors was found to be ≥95%, determined by absolute qNMR analysis using a Bruker AV III 500 equipped with a CPDUL CRYOprobe and CASE autosampler at the KU NMR lab.54 Dimethyl sulfone TraceCERT® was used as the internal calibrant. High resolution mass spectrometry (HRMS) was performed at the Wichita State University Mass Spectrometry lab using Orbitrap Velos Pro mass spectrometer (ThermoFisher, Waltham, MA) equipped with an electrospray ion source.
Synthesis of compounds
Preparation of compounds 1–5a, 9a, 17–22a. General procedure.
To a solution of alcohol (1 eq) (Table 1) in anhydrous acetonitrile (10 mL/g alcohol) was added N,N’-disuccinimidyl carbonate (1.2 eq) and TEA (3.0 eq) and the reaction mixture was stirred for 4h at room temperature. The solvent was removed in vacuo and the residue was dissolved in ethyl acetate (40 mL/g alcohol). The organic phase was washed with saturated aqueous NaHCO3 (2 × 20 mL/ g alcohol), followed by brine (20 mL/ g alcohol). The organic layers were combined and dried over anhydrous Na2SO4, filtered and concentrated in vacuo to yield the mixed carbonate which was used in the next step without further purification.
To a solution of Leu-Gln surrogate amino alcohol (1.0 eq) in dry methylene chloride (10 mL/g of amino alcohol) was added TEA (1.5 eq) and the reaction mixture was stirred for 20 min at room temperature (solution 1). In a separate flask, the mixed carbonate was dissolved in dry methylene chloride (10 mL/g of carbonate) (solution 2). Solution 1 was added to solution 2 and the reaction mixture was stirred 3h at room temperature. Methylene chloride was added to the organic phase (40 mL/g of carbonate) and then washed with saturated aqueous NaHCO3 (2 × 20 mL/ g alcohol), followed by brine (20 mL/ g alcohol). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The resultant crude product was purified by flash chromatography (hexane/ethyl acetate) to yield dipeptidyl alcohol a as a white solid.
4,4-Dimethylcyclohexyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (1a).
Yield (36%), 1H NMR (400 MHz, DMSO-d6) δ 7.57 (d, J = 8.9 Hz, 1H), 7.51 (s, 1H), 7.07 (d, J = 8.3 Hz, 1H), 4.67 – 4.63 (m, 1H), 4.47 – 4.40 (m, 1H), 3.98 – 3.88 (m, 1H), 3.78 – 3.74 (m, 1H), 3.36 – 3.28 (m, 1H), 3.27 – 3.17 (m, 1H), 3.13 (t, J = 8.9 Hz, 1H), 3.09 – 2.98 (m, 1H), 2.26 – 2.16 (m, 1H), 2.15 – 2.10 (m, 1H), 1.83 – 1.72 (m, 1H), 1.68 – 1.63 (m, 2H), 1.62 – 1.50 (m, 2H), 1.48 – 1.30 (m, 6H), 1.25 – 1.13 (m, 3H), 0.92 – 0.81 (m, 12H).
(1r,4S)-4-iso-Propylcyclohexyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (2a).
Yield (35%), 1H NMR (400 MHz, DMSO-d6) δ 7.57 (d, J = 8.9 Hz, 1H), 7.51 (s, 1H), 7.07 (d, J = 8.2 Hz, 1H), 4.65 (t, J = 5.5 Hz, 1H), 4.34 (td, J = 11.0, 5.5 Hz, 1H), 3.97 – 3.88 (m, 1H), 3.79 – 3.74 (m, 1H), 3.38 – 3.28 (m, 1H), 3.27 – 3.18 (m, 1H), 3.14 (t, J = 9.0 Hz, 1H), 3.05 (q, J = 8.5 Hz, 1H), 2.29 – 2.20 (m, 1H), 2.18 – 2.07 (m, 1H), 1.91 (d, J = 7.9 Hz, 2H), 1.84 – 1.72 (m, 1H), 1.71 – 1.66 (m, 2H), 1.62 – 1.50 (m, 2H), 1.48 – 1.30 (m, 4H), 1.26 – 1.13 (m, 2H), 1.01 (s, 3H), 0.93 – 0.80 (m, 12H).
(1s,4S)-4-propylcyclohexyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (3a).
Yield (38%), 1H NMR (400 MHz, DMSO-d6) δ 7.57 (d, J = 9.0 Hz, 1H), 7.51 (s, 1H), 7.07 (d, J = 8.2 Hz, 1H), 4.65 (s, 1H), 4.36 (td, J = 11.0, 5.5 Hz, 1H), 3.98 – 3.88 (m, 1H), 3.78 – 3.74 (m, 1H), 3.36 – 3.29 (m, 1H), 3.28 – 3.18 (m, 1H), 3.14 (t, J = 8.9 Hz, 1H), 3.10 – 2.99 (m, 1H), 2.30 – 2.19 (m, 1H), 2.17 – 2.07 (m, 1H), 1.93 – 1.84 (m, 2H), 1.77 – 1.67 (m, 3H), 1.62 – 1.48 (m, 2H), 1.48 – 1.30 (m, 3H), 1.33 – 1.21 (m, 3H), 1.21 – 1.08 (m, 4H), 0.97 – 0.90 (m, 2H), 0.90 – 0.81 (m, 9H).
(1s,4S)-4-butylcyclohexyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (4a).
Yield (35%), 1H NMR (400 MHz, DMSO-d6) δ 7.57 (d, J = 9.0 Hz, 1H), 7.51 (s, 1H), 7.07 (d, J = 8.2 Hz, 1H), 4.65 (t, J = 5.5 Hz, 1H), 4.36 (tt, J = 10.9, 4.2 Hz, 1H), 3.98 – 3.88 (m, 1H), 3.79 – 3.74 (m, 1H), 3.38 – 3.28 (m, 1H), 3.28 – 3.18 (m, 1H), 3.14 (t, J = 8.9 Hz, 1H), 3.10 – 2.99 (m, 1H), 2.30 – 2.18 (m, 1H), 2.18 – 2.07 (m, 1H), 1.93 – 1.84 (m, 2H), 1.79 – 1.65 (m, 3H), 1.62 – 1.50 (m, 2H), 1.48 – 1.30 (m, 3H), 1.29 – 1.14 (m, 9H), 0.97 – 0.90 (m, 2H), 0.90 – 0.81 (m, 9H).
(1r,4S)-4-phenylcyclohexyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (5a).
Yield (51%), 1H NMR (400 MHz, DMSO-d6) δ 7.59 (d, J = 9.0 Hz, 1H), 7.51 (s, 1H), 7.32 – 7.10 (m, 6H), 4.66 (t, J = 5.5 Hz, 1H), 4.52 (ddd, J = 15.2, 10.8, 4.3 Hz, 1H), 3.99 – 3.90 (m, 1H), 3.80 – 3.75 (m, 1H), 3.41 – 3.28 (m, 1H), 3.28 – 3.18 (m, 1H), 3.14 (t, J = 8.9 Hz, 1H), 3.10 – 3.00 (m, 1H), 2.29 – 2.20 (m, 1H), 2.20 – 2.09 (m, 1H), 2.03 – 1.98 (m, 3H), 1.81 (d, J = 13.0 Hz, 3H), 1.69 – 1.50 (m, 4H), 1.49 – 1.31 (m, 5H), 0.86 (dd, J = 8.8, 6.5 Hz, 6H).
(4-(Trifluoromethyl) cyclohexyl) methyl ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (6a).
Yield (83%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.16 (d, J = 8.3 Hz, 1H), 4.66 (s, 1H), 3.99 – 3.89 (m, 1H), 3.83 – 3.71 (m, 2H), 3.27 – 3.19 (m, 2H), 3.15 (t, 2H), 3.10 – 3.02 (m, 1H), 2.26 – 2.08 (m, 3H), 1.91 – 1.73 (m, 4H), 1.64 – 1.52 (m, 4H), 1.49 – 1.31 (m, 1H), 1.30 – 1.15 (m, 1H), 1.08 – 0.94 (m, 4H), 0.90 – 0.81 (m, 6H).
((1r,4S)-4-(Trifluoromethyl) cyclohexyl) methyl ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (7a).
Yield (80%). 1H NMR (400 MHz, DMSO-d6) δ 7.61 (d, J = 9.0 Hz, 1H), 7.53 (s, 1H), 7.19 (d, J = 8.2 Hz, 1H), 4.67 (s, 2H), 4.07 – 3.86 (m, 2H), 3.76 (s, 1H), 3.26 – 3.18 (m, 1H), 3.17 – 3.11 (m, 2H), 3.09 – 3.01 (m, 2H), 2.33 – 2.19 (m, 3H), 2.18 – 2.09 (m, 1H), 1.89 – 1.85 (m, 2H), 1.84 – 1.72 (m, 2H), 1.64 – 1.32 (m, 9H), 0.90 – 0.81 (m, 6H).
((1r,4R)-4-propylcyclohexyl) methyl ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (8a).
Yield (86%). 1H NMR (400 MHz, DMSO-d6) δ 7.62 – 7.57 (m, 1H), 7.52 (s, 1H), 7.12 (d, J = 8.3 Hz, 1H), 4.65 (s, 1H), 3.99 – 3.89 (m, 1H), 3.82 – 3.68 (m, 3H), 3.37 – 3.19 (m, 2H), 3.18 – 3.02 (m, 2H), 2.26 – 2.07 (m, 4H), 1.76 – 1.65 (m, 4H), 1.62 – 1.51 (m, 8H), 1.49 – 1.32 (m, 2H), 1.32 – 1.24 (m, 2H), 1.21 – 1.10 (m, 2H), 0.99 – 0.78 (m, 9H).
((1s,4S)-4-propylcyclohexyl) methyl-d2 ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (9a).
Yield (43%). 1H NMR (400 MHz, DMSO-d6) δ 7.59 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.12 (d, J = 8.3 Hz, 1H), 4.65 (s, 1H), 3.98 – 3.89 (m, 1H), 3.80 – 3.70 (m, 1H), 3.27 – 3.10 (m, 2H), 3.10 – 3.00 (m, 2H), 2.28 – 2.06 (m, 4H), 1.70 (s, 4H), 1.63 – 1.51 (m, 2H), 1.49 – 1.34 (m, 8H), 1.32 – 1.23 (m, 2H), 1.20 – 1.10 (m, 2H), 0.90 – 0.81 (m, 9H).
(4,4-Difluorocyclohexyl)methyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (10a).
Yield (90%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.20 (d, J = 8.3 Hz, 1H), 4.66 (s, 1H), 3.95 (td, J = 8.9, 5.4 Hz, 1H), 3.90 – 3.69 (m, 3H), 3.23 (d, J = 5.7 Hz, 1H), 3.18 – 3.00 (m, 2H), 2.28 – 2.07 (m, 2H), 2.06 – 1.93 (m, 2H), 1.90 – 1.64 (m, 6H), 1.56 (dq, J = 11.9, 8.8 Hz, 2H), 1.51 – 1.30 (m, 3H), 1.30 – 1.12 (m, 2H), 0.86 (dd, J = 10.5, 6.6 Hz, 6H).
(4,4-Difluorocyclohexyl) methyl-d2 ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (11a).
Yield (81%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.20 (d, J = 8.2 Hz, 1H), 3.99 – 3.90 (m, 1H), 3.77 (s, 1H), 3.37 – 3.19 (m, 2H), 3.18 – 3.00 (m, 2H), 2.27 – 2.07 (m, 2H), 2.03 – 1.94 (m, 2H), 1.87 – 1.63 (m, 6H), 1.63 – 1.49 (m, 2H), 1.47 – 1.29 (m, 3H), 1.29 – 1.13 (m, 2H), 0.90 – 0.81 (m, 6H).
1-(4,4-Difluorocyclohexyl)ethyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (12a).
Yield (76%). 1H NMR (400 MHz, DMSO-d6) δ 7.61 – 7.50 (m, 2H), 7.11 (d, J = 8.2 Hz, 1H), 4.66 (t, J = 5.3 Hz, 1H), 4.59 – 4.50 (m, 1H), 3.98 – 3.90 (m, 1H), 3.79 – 3.75 (m, 1H), 3.39 – 3.29 (m, 1H), 3.28 – 3.18 (m, 1H), 3.18 – 3.10 (m, 1H), 3.10 – 2.99 (m, 1H), 2.27 – 2.18 (m, 1H), 2.16 – 2.09 (m, 1H), 2.02 – 1.97 (m, 2H), 1.83 – 1.63 (m, 5H), 1.63 – 1.51 (m, 3H), 1.47 – 1.31 (m, 3H), 1.27 – 1.22 (m, 2H), 1.12 (dd, 2H), 0.90 – 0.81 (m, 6H).
2-(4,4-Difluorocyclohexyl)propan-2-yl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (13a).
Yield (72%). 1H NMR (400 MHz, DMSO6) δ 7.59 – 7.47 (m, 2H), 6.94 (d, J = 8.2 Hz, 1H), 4.66 (t, J = 5.5 Hz, 1H), 3.93 – 3.83 (m, 1H), 3.79 – 3.75 (m, 1H), 3.42 – 3.29 (m, 1H), 3.27 – 3.18 (m, 1H), 3.14 (t, J = 8.9 Hz, 1H), 3.10 – 2.99 (m, 1H), 2.27 – 2.09 (m, 2H), 2.06 – 1.91 (m, 3H), 1.85 – 1.61 (m, 5H), 1.61 – 1.51 (m, 2H), 1.46 – 1.34 (m, 3H), 1.33 (s, 6H), 1.31 – 1.18 (m, 2H), 0.90 – 0.81 (m, 6H).
1-(4,4-Difluorocyclohexyl)-2-phenylethyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (14a).
Yield (79%). 1H NMR (400 MHz, DMSO-d6) δ 7.61 – 7.54 (m, 1H), 7.52 (s, 1H), 7.11 (d, J = 8.2 Hz, 1H), 4.66 (t, J = 5.3 Hz, 1H), 4.59 – 4.50 (m, 1H), 3.98 – 3.90 (m, 1H), 3.79 – 3.75 (m, 1H), 3.39 – 3.29 (m, 1H), 3.28 – 3.18 (m, 1H), 3.18 – 3.10 (m, 1H), 3.10 – 2.99 (m, 1H), 2.27 – 2.18 (m, 1H), 2.16 – 2.09 (m, 1H), 2.02 – 1.97 (m, 2H), 1.83 – 1.63 (m, 5H), 1.63 – 1.51 (m, 3H), 1.47 – 1.31 (m, 3H), 1.27 – 1.22 (m, 2H), 1.12 (dd, 3H), 0.90 – 0.81 (m, 6H).
(4,4-Difluorocyclohexyl)(phenyl)methyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (15a).
Yield (86%). 1H NMR (400 MHz, DMSO-d6) δ 7.64 – 7.50 (m, 2H), 7.39 – 7.23 (m, 6H), 4.68 – 4.64 (m, 1H), 4.00 – 3.89 (m, 1H), 3.87 – 3.73 (m, 2H), 3.39 – 3.29 (m, 1H), 3.27 – 3.20 (m, 1H), 3.19 – 3.10 (m, 1H), 3.10 – 3.02 (m, 1H), 2.25 – 2.09 (m, 2H), 2.06 – 1.92 (m, 2H), 1.87 – 1.66 (m, 5H), 1.65 – 1.51 (m, 2H), 1.49 – 1.32 (m, 4H), 1.32 – 1.18 (m, 2H), 0.93 – 0.71 (m, 6H).
1-(4,4-Difluorocyclohexyl)pentyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (16a).
Yield (91%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 9.0 Hz, 1H), 7.52 (s, 1H), 7.20 (d, J = 8.3 Hz, 1H), 4.66 (t, J = 5.6 Hz, 1H), 4.00 – 3.90 (m, 1H), 3.90 – 3.77 (m, 1H), 3.77 – 3.74 (m, 1H), 3.40 – 3.31 (m, 1H), 3.29 – 3.19 (m, 1H), 3.14 (t, J = 9.0 Hz, 1H), 3.11 – 3.00 (m, 1H), 2.25 – 2.15 (m, 1H), 2.15 – 2.09 (m, 1H), 2.02 – 1.97 (m, 3H), 1.88 – 1.80 (m, 1H), 1.79 – 1.65 (m, 8H), 1.63 – 1.51 (m, 2H), 1.49 – 1.31 (m, 4H), 1.26 – 1.18 (m, 3H), 0.93 – 0.81 (m, 9H).
2-Fluorobenzyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (17a).
Yield (33%). 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 7.2 Hz, 1H), 7.42 – 7.34 (m, 1H), 7.34 – 7.27 (m, 1H), 7.12 (td, J = 7.5, 1.2 Hz, 1H), 7.09 – 7.00 (m, 1H), 6.23 (s, 1H), 5.56 (d, J = 8.3 Hz, 1H), 5.19 – 5.14 (m, 2H), 4.27 – 4.22 (m, 1H), 4.01 – 3.96 (m, 1H), 3.77 – 3.46 (m, 2H), 3.37 – 3.24 (m, 2H), 2.46 – 2.33 (m, 2H), 2.11 – 1.87 (m, 2H), 1.85 – 1.74 (m, 1H), 1.74 – 1.58 (m, 2H), 1.57 – 1.46 (m, 1H), 0.94 (d, J = 5.8 Hz, 6H).
3-Fluorobenzyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (18a).
Yield (41%). 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 7.2 Hz, 1H), 7.35 – 7.28 (m, 1H), 7.12 – 7.02 (m, 2H), 7.02 – 6.94 (m, 1H), 6.41 (s, 1H), 5.70 (d, J = 8.3 Hz, 1H), 5.16 – 5.00 (m, 2H), 4.29 – 4.22 (m, 1H), 4.02 – 3.93 (m, 1H), 3.74 – 3.53 (m, 2H), 3.36 – 3.22 (m, 2H), 2.45 – 2.30 (m, 2H), 2.07 – 1.95 (m, 1H), 1.79 (td, J = 9.4, 2.8 Hz, 1H), 1.73 – 1.43 (m, 4H), 0.94 (d, J = 6.1 Hz, 6H).
4-Fluorobenzyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (19a).
Yield (37%). 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 7.4 Hz, 1H), 7.37 – 7.28 (m, 2H), 7.08 – 6.97 (m, 2H), 6.37 (s, 1H), 5.56 (d, J = 8.1 Hz, 1H), 5.12 – 5.02 (m, 2H), 4.31 – 4.13 (m, 1H), 4.05 – 3.90 (m, 1H), 3.66 – 3.53 (m, 2H), 3.39 – 3.25 (m, 2H), 2.46 – 2.31 (m, 2H), 2.05 – 1.93 (m, 1H), 1.88 – 1.76 (m, 1H), 1.74 – 1.57 (m, 3H), 1.57 – 1.45 (m, 1H), 0.93 (d, J = 6.1 Hz, 6H).
(4-Fluorophenyl)methyl-d2 ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (20a).
Yield (37%). 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 7.3 Hz, 1H), 7.36 – 7.29 (m, 2H), 7.07 – 6.98 (m, 2H), 6.32 (s, 1H), 5.55 (d, J = 8.3 Hz, 1H), 4.27 – 4.20 (m, 1H), 4.00 – 3.96 (m, 1H), 3.66 – 3.53 (m, 2H), 3.37 – 3.23 (m, 2H), 2.48 – 2.32 (m, 2H), 2.03 – 1.93 (m, 1H), 1.87 – 1.76 (m, 1H), 1.73 – 1.57 (m, 3H), 1.55 – 1.45 (m, 1H), 0.93 (d, J = 6.1 Hz, 6H).
(Perfluorophenyl)methyl ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (21a).
Yield (21%). 1H NMR (400 MHz, DMSO-d6) δ 7.66 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.46 (d, J = 8.0 Hz, 1H), 5.18 – 5.07 (m, 2H), 4.64 (s, 1H), 3.99 – 3.90 (m, 1H), 3.75 (s, 1H), 3.25 – 3.02 (m, 4H), 2.27 – 2.16 (m, 1H), 2.15 – 2.05 (m, 2H), 1.81 – 1.71 (m, 1H), 1.62 – 1.50 (m, 2H), 1.47 – 1.31 (m, 2H), 0.90 – 0.78 (m, 6H).
(Perfluorophenyl)methyl-d2 ((2S)-1-(((2S)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl) carbamate (22a).
Yield (11%). 1H NMR (400 MHz, DMSO-d6) δ 7.65 (d, J = 8.9 Hz, 1H), 7.52 (s, 1H), 7.46 (d, J = 8.0 Hz, 1H), 4.64 (s, 1H), 3.99 – 3.90 (m, 1H), 3.75 (s, 1H), 3.25 – 3.02 (m, 4H), 2.26 – 2.16 (m, 1H), 2.15 – 2.05 (m, 2H), 1.81 – 1.71 (m, 1H), 1.60 – 1.49 (m, 2H), 1.46 – 1.31 (m, 2H), 0.90 – 0.77 (m, 6H).
1-Phenylbutyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (23a).
Yield (60%). 1H NMR (400 MHz, CDCl3) δ 7.59 (dd, J = 79.1, 7.3 Hz, 1H), 7.36 – 7.21 (m, 5H), 6.24 (d, J = 37.3 Hz, 1H), 5.60 (t, J = 7.1, 7.1 Hz, 1H), 5.41 (dd, J = 23.4, 8.0 Hz, 1H), 4.21 – 4.11 (m, 1H), 4.04 – 3.89 (m, 1H), 3.69 – 3.47 (m, 2H), 3.36 – 3.19 (m, 2H), 2.53 – 2.17 (m, 2H), 2.03 – 1.80 (m, 3H), 1.79 – 1.42 (m, 3H), 1.41 – 1.21 (m, 2H), 0.98 – 0.83 (m, 11H).
1,2-Diphenylethyl ((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)carbamate (24a).
Yield (83%). 1H NMR (400 MHz, CDCl3) δ 7.58 (dd, J = 49.0, 7.4 Hz, 1H), 7.33 – 7.16 (m, 8H), 7.11 – 7.02 (m, 2H), 6.09 (d, J = 26.6 Hz, 1H), 5.88 – 5.75 (m, 1H), 5.42 – 5.32 (m, 1H), 4.17 – 4.07 (m, 1H), 4.04 – 3.87 (m, 1H), 3.68 – 3.43 (m, 2H), 3.33 – 2.98 (m, 4H), 2.52 – 2.21 (m, 2H), 2.02 – 1.39 (m, 6H), 0.94 – 0.79 (m, 6H).
Preparation of compounds 1–24b. General procedure.
To a solution of dipeptidyl alcohol a (1 eq) in anhydrous dichloromethane (300 mL/g dipeptidyl alcohol) kept at 0–5 °C under a N2 atmosphere was added Dess-Martin periodinane reagent (3.0 eq) and the reaction mixture was stirred for 3 h at 15–20 °C. The organic phase was washed with 10% aq Na2S2O3 (2 × 100 mL/g dipeptidyl alcohol), followed by saturated aqueous NaHCO3 (2 × 100 mL/ g dipeptidyl alcohol), distilled water (2 × 100 mL/ g dipeptidyl alcohol), and brine (100 mL/ g dipeptidyl alcohol). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The resulting crude product was purified by flash chromatography (hexane/ethyl acetate) to yield aldehyde b as a white solid.
4,4-Dimethylcyclohexyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (1b).
Yield (80%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.39 (d, J = 7.7 Hz, 1H), 7.62 (s, 1H), 7.19 (d, J = 8.0 Hz, 1H), 4.49 – 4.40 (m, 1H), 4.24 – 4.14 (m, 1H), 4.07 – 3.97 (m, 1H), 3.16 (t, J = 9.2 Hz, 1H), 3.12 – 3.00 (m, 1H), 2.31 – 2.22 (m, 1H), 2.19 – 2.09 (m, 1H), 1.95 – 1.83 (m, 1H), 1.74 – 1.55 (m, 5H), 1.54 – 1.31 (m, 6H), 1.25 – 1.15 (m, 2H), 0.92 – 0.81 (m, 12H). HRMS m/z: [M+Na]+ Calculated for C22H37N3NaO5 446.2631; Found 446.2612.
(1r,4S)-4-isopropylcyclohexyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (2b).
Yield (78%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.40 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.19 (d, J = 8.0 Hz, 1H), 4.42 – 4.30 (m, 1H), 4.19 (ddd, J = 11.4, 7.6, 4.2 Hz, 1H), 4.07 – 3.96 (m, 1H), 3.28 – 3.01 (m, 2H), 2.35 – 2.19 (m, 1H), 2.19 – 2.08 (m, 1H), 1.95 – 1.84 (m, 3H), 1.73 – 1.57 (m, 5H), 1.53 – 1.36 (m, 3H), 1.27 – 1.20 (m, 2H), 1.02 (s, 3H), 0.97 – 0.80 (m, 12H). HRMS m/z: [M+Na]+ Calculated for C23H39N3NaO5 460.2788; Found 460.2777.
(1s,4S)-4-propylcyclohexyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (3b).
Yield (73%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.40 (d, J = 7.7 Hz, 1H), 7.63 (s, 1H), 7.19 (d, J = 7.9 Hz, 1H), 4.37 (td, J = 11.0, 5.5 Hz, 1H), 4.24 – 4.12 (m, 1H), 4.07 – 3.97 (m, 1H), 3.21 – 3.01 (m, 2H), 2.35 – 2.22 (m, 1H), 2.19 – 2.08 (m, 1H), 1.95 – 1.85 (m, 3H), 1.76 – 1.55 (m, 5H), 1.54 – 1.37 (m, 2H), 1.35 – 1.19 (m, 4H), 1.19 – 1.09 (m, 3H), 1.01 – 0.91 (m, 2H), 0.91 – 0.81 (m, 9H). HRMS m/z: [M+H]+ Calculated for C23H40N3O5 438.2968; Found 438.2952.
(1s,4S)-4-Butylcyclohexyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (4b).
Yield (65%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.40 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.19 (d, J = 7.9 Hz, 1H), 4.37 (td, J = 11.0, 5.3 Hz, 1H), 4.19 (ddd, J = 11.5, 7.7, 4.1 Hz, 1H), 4.02 (q, J = 8.5 Hz, 1H), 3.17 (t, J = 9.1 Hz, 1H), 3.13 – 3.01 (m, 1H), 2.32 – 2.23 (m, 1H), 2.20 – 2.08 (m, 1H), 1.96 – 1.84 (m, 3H), 1.76 – 1.72 (m, 3H), 1.72 – 1.55 (m, 3H), 1.53 – 1.37 (m, 2H), 1.29 – 1.13 (m, 8H), 1.01 – 0.91 (m, 2H), 0.91 – 0.80 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H41N3NaO5 474.2944; Found 474.2931.
(1r,4S)-4-Phenylcyclohexyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (5b).
Yield (63%). 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.43 (d, J = 7.7 Hz, 1H), 7.63 (s, 1H), 7.32 – 7.10 (m, 6H), 4.53 (td, J = 10.9, 5.4 Hz, 1H), 4.20 (ddd, J = 11.4, 7.5, 4.0 Hz, 1H), 4.07 – 3.92 (m, 1H), 3.29 – 3.02 (m, 2H), 2.36 – 2.23 (m, 1H), 2.21 – 2.10 (m, 1H), 2.07 – 1.96 (m, 2H), 1.96 – 1.85 (m, 1H), 1.81 (d, J = 12.8 Hz, 2H), 1.70 – 1.56 (m, 6H), 1.56 – 1.42 (m, 4H), 0.89 (dd, J = 9.5, 6.6 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C26H37N3NaO5 494.2631; Found 494.2608.
(4-(Trifluoromethyl) cyclohexyl) methyl ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (6b).
Yield (88%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.43 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.29 (d, J = 8.0 Hz, 1H), 4.22 – 4.15 (m, 1H), 4.07 – 3.99 (m, 1H), 3.78 (d, J = 2.0 Hz, 2H), 3.20 – 3.05 (m, 3H), 2.31 – 2.08 (m, 3H), 1.95 – 1.76 (m, 4H), 1.70 – 1.40 (m, 5H), 1.29 – 1.16 (m, 1H), 1.02 (q, J = 13.0 Hz, 4H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+H]+ Calculated for C22H35F3N3O5 478.2529; Found 478.2522. HRMS m/z: [M+Na]+ Calculated for C22H34F3N3NaO5 500.2349; Found 500.2328.
((1r,4S)-4-(Trifluoromethyl) cyclohexyl) methyl ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (7b).
Yield (91%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.43 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.24 – 4.14 (m, 1H), 4.08 – 3.99 (m, 1H), 3.98 – 3.92 (m, 2H), 3.21 – 3.04 (m, 3H), 2.33 – 2.20 (m, 3H), 2.19 – 2.08 (m, 1H), 1.92 – 1.83 (m, 4H), 1.69 – 1.38 (m, 9H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+H]+ Calculated for C22H35F3N3O5 478.2529; Found 478.2512. HRMS m/z: [M+Na]+ Calculated for C22H34F3N3NaO5 500.2349; Found 500.2320.
((1r,4R)-4-Propylcyclohexyl) methyl ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (8b).
Yield (42%). 1H NMR (400 MHz, DMSO-6) δ 9.40 (s, 1H), 8.42 (d, J = 7.7 Hz, 1H), 7.63 (s, 1H), 7.25 (d, J = 8.0 Hz, 1H), 4.22 – 4.15 (m, 1H), 4.05 – 4.01 (m, 1H), 3.78 – 3.74 (m, 2H), 3.20 – 3.05 (m, 2H), 2.19 – 2.10 (m, 3H), 1.94 – 1.85 (m, 3H), 1.75 – 1.60 (m, 10H), 1.54 – 1.40 (m, 2H), 1.33 – 1.23 (m, 2H), 1.17 – 1.10 (m, 2H), 0.97 – 0.81 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H41N3NaO5 474.2944; Found 474.2013.
((1s,4S)-4-Propylcyclohexyl) methyl-d2 ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (9b).
Yield (96%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.42 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.25 (d, J = 8.0 Hz, 1H), 4.24 – 4.14 (m, 1H), 4.08 – 3.97 (m, 1H), 3.21 – 3.03 (m, 4H), 2.31 – 2.10 (m, 4H), 1.75 – 1.67 (m, 4H), 1.55 – 1.35 (m, 8H), 1.35 – 1.22 (m, 2H), 1.18 – 1.10 (m, 2H), 1.00 – 0.76 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H39D2N3NaO5 476.3070; Found 476.3051.
(4,4-Difluorocyclohexyl)methyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (10b).
Yield (51%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.44 (d, J = 7.5 Hz, 1H), 7.95 (s, 1H), 7.36 – 7.28 (m, 1H), 4.19 (ddd, J = 11.4, 7.6, 4.2 Hz, 1H), 4.03 (td, J = 8.8, 6.2 Hz, 1H), 3.84 (d, J = 6.1 Hz, 2H), 3.24 – 3.02 (m, 2H), 2.37 – 2.08 (m, 2H), 2.08 – 1.94 (m, 2H), 1.94 – 1.80 (m, 1H), 1.80 – 1.55 (m, 7H), 1.55 – 1.33 (m, 2H), 1.33 – 1.11 (m, 3H), 0.97 – 0.79 (m, 6H). HRMS m/z: [M+H]+ Calculated for C21H34F2N3O5 446.2467; Found 446.2452, m/z: [M+Na]+ Calculated for C21H33F2N3O5Na 468.2286; Found 468.2281.
(4,4-Difluorocyclohexyl) methyl-d2 ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (11b).
Yield (63%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.44 (d, J = 7.5 Hz, 1H), 7.63 (s, 1H), 7.32 (d, J = 8.1 Hz, 1H), 4.23 – 4.15 (m, 1H), 4.07 – 3.99 (m, 1H), 3.21 – 3.05 (m, 2H), 2.32 – 2.20 (m, 1H), 2.19 – 2.10 (m, 2H), 2.05 – 1.95 (m, 2H), 1.79 – 1.58 (m, 8H), 1.55 – 1.40 (m, 2H), 1.30 – 1.15 (m, 2H), 0.92 – 0.83 (m, 6H). HRMS m/z: [M+H]+ Calculated for C21H32D2F2N3O5 448.2592; Found 448.2576. HRMS m/z: [M+Na]+ Calculated for C21H31D2F2N3NaO5 470.2412; Found 470.2412.
1-(4,4-Difluorocyclohexyl)ethyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (12b).
Yield (70%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.46 – 8.36 (m, 1H), 7.63 (s, 1H), 7.23 (d, J = 8.2 Hz, 1H), 4.59 – 4.52 (m, 1H), 4.23 – 4.18 (m, 1H), 4.06 – 3.98 (m, 1H), 3.21 – 3.03 (m, 2H), 2.32 – 2.22 (m, 1H), 2.18 – 2.09 (m, 1H), 2.06 – 1.95 (m, 3H), 1.95 – 1.76 (m, 2H), 1.72 – 1.59 (m, 5H), 1.55 – 1.40 (m, 2H), 1.31 – 1.14 (m, 3H), 1.12 (d, J = 6.3 Hz, 3H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H35F2N3NaO5 482.2443; Found 482.2433.
2-(4,4-Difluorocyclohexyl)propan-2-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (13b).
Yield (42%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.35 (d, J = 7.7 Hz, 1H), 7.70 – 7.60 (m, 1H), 7.06 (d, J = 8.1 Hz, 1H), 4.02 – 3.89 (m, 2H), 3.21 – 3.00 (m, 2H), 2.31 – 2.11 (m, 1H), 2.07 – 1.83 (m, 4H), 1.82 – 1.74 (m, 4H), 1.69 – 1.58 (m, 4H), 1.56 – 1.36 (m, 3H), 1.34 (s, 6H), 1.30 – 1.23 (m, 1H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H38F2N3O5 474.2779; Found 474.2771.
1-(4,4-Difluorocyclohexyl)-2-phenylethyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (14b).
Yield (80%). 1H NMR (400 MHz, DMSO-d6) δ 9.39 (s, 1H), 8.38 – 8.30 (m, 1H), 7.62 (s, 1H), 7.26 – 7.15 (m, 5H), 4.77 – 4.73 (m, 1H), 4.22 – 4.18 (m, 1H), 3.93 – 3.87 (m, 1H), 3.18 – 2.99 (m, 2H), 2.92 – 2.83 (m, 1H), 2.78 – 2.68 (m, 1H), 2.32 – 2.18 (m, 1H), 2.16 – 2.07 (m, 1H), 2.07 – 1.94 (m, 2H), 1.91 – 1.80 (m, 3H), 1.79 – 1.68 (m, 1H), 1.67 – 1.41 (m, 7H), 1.41 – 1.27 (m, 3H), 0.92 – 0.80 (m, 4H), 0.75 (dd, J = 11.6, 6.5 Hz, 2H). HRMS m/z: [M+Na]+ Calculated for C28H39F2N3NaO5 558.2756; Found 558.2740.
(4,4-Difluorocyclohexyl)(phenyl)methyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (15b).
Yield (63%). 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.48 – 8.35 (m, 1H), 7.65 (d, J = 5.4 Hz, 1H), 7.52 – 7.43 (m, 1H), 7.39 – 7.20 (m, 5H), 4.22 – 4.17 (m, 1H), 4.00 – 3.96 (m, 1H), 3.85 (d, J = 6.0 Hz, 1H), 3.23 – 3.05 (m, 2H), 2.32 – 2.22 (m, 1H), 2.17 – 2.13 (m, 1H), 2.05 – 1.87 (m, 3H), 1.84 – 1.72 (m, 2H), 1.70 – 1.59 (m, 4H), 1.55 – 1.38 (m, 3H), 1.33 – 1.16 (m, 3H), 0.97 – 0.74 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C27H37F2N3NaO5 544.2599; Found 544.2586.
1-(4,4-Difluorocyclohexyl)pentyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (16b).
Yield (88%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.43 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.32 (d, J = 8.0 Hz, 1H), 4.24 – 4.14 (m, 1H), 4.05 – 4.00 (m, 1H), 3.87 – 3.81 (m, 1H), 3.21 – 3.04 (m, 2H), 2.30 – 2.22 (m, 1H), 2.19 – 2.08 (m, 1H), 2.06 – 1.94 (m, 3H), 1.94 – 1.80 (m, 3H), 1.79 – 1.71 (m, 5H), 1.69 – 1.58 (m, 4H), 1.55 – 1.41 (m, 3H), 1.26 – 1.18 (m, 3H), 0.92 – 0.81 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C25H41F2N3NaO5 524.2912; Found 524.2888.
2-Fluorobenzyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (17b).
Yield (68%). 1H NMR (400 MHz, CDCl3) δ 9.48 (s, 1H), 8.35 (d, J = 5.9 Hz, 1H), 7.39 (t, J = 7.6 Hz, 1H), 7.35 – 7.27 (m, 1H), 7.18 – 7.00 (m, 2H), 6.02 (s, 1H), 5.38 (d, J = 8.5 Hz, 1H), 5.22 – 5.16 (m, 2H), 4.44 – 4.26 (m, 2H), 3.39 – 3.27 (m, 2H), 2.49 – 2.30 (m, 2H), 2.01 – 1.92 (m, 2H), 1.91 – 1.80 (m, 1H), 1.81 – 1.45 (m, 3H), 0.97 (d, J = 5.8 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C21H28FN3NaO5: 444.1911, Found: 444.1907.
3-Fluorobenzyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (18b).
Yield (68%). 1H NMR (400 MHz, CDCl3) δ 9.48 (s, 1H), 8.45 (d, J = 5.7 Hz, 1H), 7.35 – 7.24 (m, 1H), 7.13 – 7.05 (m, 2H), 7.05 – 6.94 (m, 1H), 6.18 (s, 1H), 5.48 (d, J = 8.6 Hz, 1H), 5.16 – 5.03 (m, 2H), 4.41 – 4.26 (m, 2H), 3.41 – 3.27 (m, 2H), 2.56 – 2.29 (m, 2H), 2.00 – 1.79 (m, 2H), 1.77 – 1.64 (m, 3H), 1.60 – 1.51 (m, 1H), 0.97 (d, J = 6.0 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C21H28FN3NaO5: 444.1911, Found: 444.1911.
4-Fluorobenzyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (19b).
Yield (60%). 1H NMR (400 MHz, CDCl3) δ 9.48 (s, 1H), 8.36 (d, J = 5.7 Hz, 1H), 7.38 – 7.29 (m, 2H), 7.09 – 6.98 (m, 2H), 6.00 (s, 1H), 5.36 (d, J = 8.6 Hz, 1H), 5.11 – 5.04 (m, 2H), 4.35 – 4.28 (m, 2H), 3.41 – 3.28 (m, 2H), 2.50 – 2.31 (m, 2H), 1.97 – 1.93 (m, 1H), 1.90 – 1.76 (m, 1H), 1.74 – 1.65 (m, 3H), 1.59 – 1.51 (m, 1H), 0.96 (d, J = 6.0 Hz, 6H). HRMS m/z: [M+H]+ Calculated for C21H29FN3O5: 422.2091, Found: 422.2085. HRMS m/z: [M+Na]+ Calculated for C21H28FN3NaO5: 444.1911, Found: 444.1903.
(4-Fluorophenyl)methyl-d2 ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (20b).
Yield (75%). 1H NMR (400 MHz, CDCl3) δ 9.48 (s, 1H), 8.36 (d, J = 5.7 Hz, 1H), 7.37 – 7.28 (m, 2H), 7.08 – 6.98 (m, 2H), 6.06 (s, 1H), 5.38 (d, J = 8.6 Hz, 1H), 4.39 – 4.14 (m, 2H), 3.41 – 3.30 (m, 2H), 2.48 – 2.29 (m, 2H), 2.01 – 1.92 (m, 1H), 1.92 – 1.80 (m, 1H), 1.80 – 1.62 (m, 3H), 1.59 – 1.46 (m, 1H), 0.96 (d, J = 5.9 Hz, 6H). HRMS m/z: [M+H]+ Calculated for C21H27D2FN3O5: 424.2217, Found: 424.2210. HRMS m/z: [M+Na]+ Calculated for C21H26D2FN3NaO5: 446.2037, Found: 446.2027.
(Perfluorophenyl)methyl ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (21b).
Yield (86%). 1H NMR (400 MHz, DMSO-d6) δ 9.39 (s, 1H), 8.47 (d, J = 7.5 Hz, 1H), 7.63 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 5.14 (s, 2H), 4.23 – 4.14 (m, 1H), 4.07 – 3.98 (m, 1H), 3.22 – 3.03 (m, 2H), 2.33 – 2.20 (m, 1H), 2.17 – 2.06 (m, 2H), 1.93 – 1.83 (m, 1H), 1.70 – 1.56 (m, 2H), 1.51 – 1.39 (m, 2H), 0.91 – 0.80 (m, 6H). HRMS m/z: [M+H]+ Calculated for C21H25F5N3O5 494.1714; Found 494.1715. HRMS m/z: [M+Na]+ Calculated for C21H24F5N3NaO5 516.1534; Found 516.1520.
(Perfluorophenyl)methyl-d2 ((2S)-4-methyl-1-oxo-1-(((2S)-1-oxo-3-(2-oxopyrrolidin-3-yl) propan-2-yl) amino) pentan-2-yl) carbamate (22b).
Yield (83%). 1H NMR (400 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.46 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.58 (d, J = 7.8 Hz, 1H), 4.22 – 4.14 (m, 1H), 4.06 – 3.99 (m, 1H), 3.20 – 3.05 (m, 2H), 2.27 (d, J = 7.9 Hz, 1H), 2.16 – 2.07 (m, 2H), 1.92 – 1.83 (m, 1H), 1.68 – 1.58 (m, 2H), 1.50 – 1.41 (m, 2H), 0.90 – 0.80 (m, 6H). HRMS m/z: [M+H]+ Calculated for C21H23D2F5N3O5 496.1840; Found 496.1837. HRMS m/z: [M+Na]+ Calculated for C21H22D2F5N3NaO5 518.1660; Found 518.1646.
1-Phenylbutyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (23b).
Yield (71%). 1H NMR (400 MHz, CDCl3) δ 9.44 (d, J = 36.5 Hz, 1H), 8.21 (dd, J = 43.6, 6.1 Hz, 1H), 7.41 – 7.17 (m, 5H), 6.28 (d, J = 30.3 Hz, 1H), 5.68 – 5.54 (m, 1H), 5.36 (dd, J = 26.5, 8.5 Hz, 1H), 4.40 – 4.19 (m, 2H), 3.41 – 3.15 (m, 2H), 2.56 – 2.14 (m, 2H), 2.01 – 1.81 (m, 3H), 1.77 – 1.62 (m, 3H), 1.59 – 1.44 (m, 1H), 1.42 – 1.25 (m, 1H), 1.00 – 0.77 (m, 11H). HRMS m/z: [M+Na]+ Calculated for C24H35N3NaO5 468.2475; Found 468.2463.
1,2-Diphenylethyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (24b).
Yield (82%). 1H NMR (400 MHz, DMSO-d6) δ 9.35 (dd, J = 24.5, 7.6 Hz, 1H), 8.48 – 8.32 (m, 1H), 7.61 (d, J = 21.6 Hz, 1H), 7.53 – 7.42 (m, 1H), 7.36 – 7.09 (m, 10H), 5.83 – 5.71 (m, 1H), 4.24 – 4.08 (m, 1H), 4.06 – 3.89 (m, 1H), 3.24 – 2.86 (m, 4H), 2.34 – 2.05 (m, 3H), 1.94 – 1.78 (m, 1H), 1.67 – 1.28 (m, 4H), 0.90 – 0.68 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C28H35N3NaO5 516.2475; Found 516.2462.
Preparation of compounds 1–24c. General procedure.
To a solution of dipeptidyl aldehyde b (1 eq) in ethyl acetate (10 mL/g of dipeptidyl aldehyde) was added absolute ethanol (5 mL/g of dipeptidyl aldehyde) with stirring, followed by a solution of sodium bisulfite (1 eq) in water (1 mL/ g of dipeptidyl aldehyde). The reaction mixture was stirred for 3 h at 50 °C. The reaction mixture was allowed to cool to room temperature and then vacuum filtered. The solid was thoroughly washed with absolute ethanol and the filtrate was dried over anhydrous sodium sulfate, filtered, and concentrated to yield a white solid. The white solid was stirred with dry ethyl ether (3 × 10 mL/ g of dipeptidyl aldehyde), followed by careful removal of the solvent using a pipette and dried using a vacuum pump for 2 h to yield dipeptidyl bisulfite adduct c as a white solid.
Sodium (2S)-2-((S)-2-((((4,4-dimethylcyclohexyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (1c).
Yield (39%). 1H NMR (400 MHz, DMSO-d6) δ 7.50 (dd, J = 13.4, 9.1 Hz, 1H), 7.44 (s, 1H), 7.21 – 7.11 (m, 1H), 5.45 – 5.27 (m, 1H), 4.53 – 4.39 (m, 1H), 4.01 – 3.92 (m, 1H), 3.95 – 3.78 (m, 1H), 3.14 – 3.08 (m, 1H), 3.06 – 3.01 (m, 1H), 2.19 – 2.05 (m, 1H), 2.04 – 1.86 (m, 1H), 1.70 – 1.65 (m, 3H), 1.62 – 1.52 (m, 3H), 1.52 – 1.31 (m, 6H), 1.28 – 1.09 (m, 2H), 0.92 – 0.80 (m, 12H). HRMS m/z: [M+Na]+ Calculated for C22H38N3Na2O8S 550.2175; Found 550.2154.
Sodium (2S)-1-hydroxy-2-((S)-2-(((((1r,4S)-4-isopropylcyclohexyl)oxy)carbonyl)amino)-4-methylpentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (2c).
Yield (36%). 1H NMR (400 MHz, DMSO-d6) δ 7.54 – 7.42 (m, 2H), 7.23 – 7.11 (m, 1H), 5.45 – 5.28 (m, 1H), 4.41 – 4.30 (m, 1H), 4.00 – 3.89 (m, 1H), 3.89 – 3.78 (m, 1H), 3.17 – 3.09 (m, 1H), 3.08 – 2.97 (m, 1H), 2.14 – 2.04 (m, 2H), 2.01 – 1.84 (m, 3H), 1.73 – 1.65 (m, 2H), 1.62 – 1.51 (m, 3H), 1.48 – 1.34 (m, 3H), 1.30 – 1.14 (m, 2H), 1.01 (s, 3H), 0.89 – 0.80 (m, 12H). HRMS m/z: [M+Na]+ Calculated for C23H40N3Na2O8S 564.2332; Found 564.2450.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-(((((1s,4S)-4-propylcyclohexyl)oxy)carbonyl)amino)pentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (3c).
Yield (40%). 1H NMR (400 MHz, DMSO-d6) δ 7.54 – 7.39 (m, 2H), 7.23 – 7.10 (m, 1H), 5.45 – 5.28 (m, 1H), 4.42 – 4.32 (m, 1H), 4.03 – 3.88 (m, 1H), 3.89 – 3.78 (m, 1H), 3.18 – 3.08 (m, 1H), 3.07 – 2.97 (m, 1H), 2.19 – 2.03 (m, 2H), 1.97 – 1.80 (m, 3H), 1.71 (d, J = 13.2 Hz, 2H), 1.59 – 1.56 (m, 3H), 1.48 – 1.37 (m, 2H), 1.34 – 1.02 (m, 7H), 0.97 – 0.89 (m, 2H), 0.92 – 0.80 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C23H40N3Na2O8S 564.2332; Found 564.2311.
Sodium (2S)-2-((S)-2-(((((1s,4S)-4-butylcyclohexyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (4c).
Yield (33%). 1H NMR (400 MHz, DMSO-d6) δ 7.55 – 7.42 (m, 2H), 7.22 – 7.12 (m, 1H), 5.48 – 5.30 (m, 1H), 4.42 – 4.32 (m, 1H), 3.97 – 3.89 (m, 1H), 3.88 – 3.79 (m, 1H), 3.17 – 3.08 (m, 1H), 3.07 – 2.98 (m, 1H), 2.19 – 2.02 (m, 2H), 1.99 – 1.83 (m, 3H), 1.72 (d, J = 13.3 Hz, 3H), 1.60 – 1.55 (m, 3H), 1.48 – 1.37 (m, 2H), 1.27 – 1.17 (m, 6H), 1.17 – 1.13 (m, 3H), 0.97 – 0.92 (m, 1H), 0.90 – 0.80 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H42N3Na2O8S 578.2488; Found 578.2473.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-(((((1r,4S)-4-phenylcyclohexyl)oxy)carbonyl)amino)pentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (5c).
Yield (35%). 1H NMR (400 MHz, DMSO-d6) δ 7.57 – 7.47 (m, 1H), 7.44 (s, 1H), 7.32 – 7.10 (m, 6H), 5.52 – 5.22 (m, 1H), 4.55 – 4.50 (m, 1H), 4.06 – 3.89 (m, 1H), 3.89 – 3.77 (m, 1H), 3.15 – 3.10 (m, 1H), 3.09 – 3.00 (m, 1H), 2.14 – 2.09 (m, 2H), 2.05 – 1.97 (m, 2H), 1.81 (d, J = 13.1 Hz, 3H), 1.62 – 1.52 (m, 5H), 1.50 – 1.39 (m, 4H), 1.14 – 1.03 (m, 1H), 0.93 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H38N3Na2O8S 598.2175; Found 598.2152.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((4-(trifluoromethyl) cyclohexyl) methoxy) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (6c).
Yield (81%). 1H NMR (400 MHz, DMSO-d6) δ 7.62 – 7.49 (m, 1H), 7.46 (s, 1H), 7.25 – 7.13 (m, 1H), 5.50 (d, J = 6.3 Hz, 1H), 5.34 (d, J = 6.0 Hz, 1H), 4.41 – 4.33 (m, 1H), 4.27 – 4.18 (m, 1H), 3.81 – 3.70 (m, 2H), 3.13 (s, 2H), 3.08 – 2.99 (m, 1H), 2.20 – 2.06 (m, 3H), 1.91 – 1.76 (m, 4H), 1.63 – 1.57 (m, 4H), 1.49 – 1.37 (m, 1H), 1.27 – 1.16 (m, 1H), 1.13 – 0.92 (m, 4H), 0.93 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H35F3N3Na2O8S 604.1893; Found 604.1871.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-(((((1r,4S)-4-(trifluoromethyl) cyclohexyl) methoxy) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (7c).
Yield (76%). 1H NMR (400 MHz, DMSO-d6) δ 7.58 – 7.50 (m, 1H), 7.46 (d, J = 9.6 Hz, 1H), 7.25 – 7.16 (m, 1H), 5.41 (d, J = 6.3 Hz, 1H), 5.28 (d, J = 5.9 Hz, 1H), 4.45 – 4.32 (m, 1H), 4.00 – 3.90 (m, 2H), 3.50 – 3.25 (m, 1H), 3.08 – 3.01 (m, 2H), 2.31 – 2.26 (m, 3H), 2.15 – 2.08 (m, 1H), 1.90 – 1.85 (m, 4H), 1.67 – 1.33 (m, 10H), 0.91 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H35F3N3Na2O8S 604.1893; Found 604.1862.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-(((((1r,4R)-4-propylcyclohexyl) methoxy) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (8c).
Yield (82%). 1H NMR (400 MHz, DMSO-d6) δ 7.53 – 7.40 (m, 1H), 7.20 – 7.09 (m, 1H), 6.10 – 6.04 (m, 1H), 5.41 (d, J = 6.2 Hz, 1H), 5.29 (d, J = 6.0 Hz, 1H), 4.43 – 4.32 (m, 1H), 4.00 – 3.89 (m, 1H), 3.74 (s, 2H), 3.16 – 3.01 (m, 2H), 2.15 – 2.05 (m, 6H), 1.71 (d, J = 11.4 Hz, 10H), 1.53 – 1.36 (m, 2H), 1.35 – 1.22 (m, 2H), 1.19 – 1.03 (m, 2H), 0.98 – 0.77 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H42N3Na2O8S 578.2488; Found 578.2460.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-(((((1s,4S)-4-propylcyclohexyl) methoxy-d2) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (9c).
Yield (77%). 1H NMR (400 MHz, DMSO-d6) δ 7.57 – 7.42 (m, 1H), 7.27 – 7.09 (m, 1H), 6.07 (d, J = 10.0 Hz, 1H), 5.40 (d, J = 6.4 Hz, 1H), 5.29 (d, J = 6.0 Hz, 1H), 3.99 – 3.89 (m, 1H), 3.78 – 3.69 (m, 1H), 3.17 – 2.98 (m, 4H), 2.20 – 2.06 (m, 4H), 1.71 (d, J = 11.6 Hz, 4H), 1.49 – 1.35 (m, 8H), 1.35 – 1.22 (m, 2H), 1.18 – 1.09 (m, 2H), 0.90 – 0.80 (m, 9H). HRMS m/z: [M+Na]+ Calculated for C24H40D2N3Na2O8S 580.2614; Found 580.2582.
Sodium (2S)-2-((S)-2-((((4,4-difluorocyclohexyl)methoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (10c).
Yield (50.5%). 1H NMR (400 MHz, DMSO-d6) δ 7.57 (t, J = 8.9 Hz, 1H), 7.45 (s, 1H), 7.38 – 7.17 (m, 1H), 4.29 – 4.10 (m, 1H), 4.05 – 3.67 (m, 4H), 3.09 (dt, J = 29.8, 8.8 Hz, 2H), 2.33 – 2.05 (m, 2H), 2.05 – 1.88 (m, 4H), 1.88 – 1.64 (m, 5H), 1.64 – 1.48 (m, 2H), 1.43 (q, J = 7.3 Hz, 2H), 1.30 – 1.11 (m, 2H), 1.04 – 0.78 (m, 6H).
Sodium (2S)-2-((S)-2-((((4,4-difluorocyclohexyl) methoxy-d2) carbonyl) amino)-4-methylpentanamido)-1-hydroxy-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (11c).
Yield (81%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 – 7.50 (m, 1H), 7.45 (s, 1H), 7.29 – 7.18 (m, 1H), 5.41 (d, J = 6.3 Hz, 1H), 5.24 (d, J = 6.0 Hz, 1H), 4.37 – 4.32 (m, 1H), 3.98 – 3.89 (m, 1H), 3.15 – 3.02 (m, 2H), 2.14 – 2.05 (m, 3H), 2.01 – 1.96 (m, 1H), 1.84 – 1.70 (m, 8H), 1.62 – 1.53 (m, 4H), 1.45 – 1.38 (m, 1H), 0.90 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H32D2F2N3Na2O8S 574.1956; Found 574.1931.
Sodium (2S)-2-((2S)-2-(((1-(4,4-difluorocyclohexyl)ethoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (12c).
Yield (48%). 1H NMR (400 MHz, DMSO-d6) δ 7.52 (d, J = 9.9 Hz, 1H), 7.43 (s, 1H), 7.28 – 7.13 (m, 1H), 5.36 – 5.17 (m, 1H), 4.58 – 4.51 (m, 1H), 3.96 – 3.91 (m, 1H), 3.83 – 3.78 (m, 1H), 3.18 – 3.09 (m, 1H), 3.06 – 3.01 (m, 1H), 2.17 – 1.88 (m, 3H), 1.87 – 1.77 (m, 4H), 1.74 – 1.66 (m, 2H), 1.65 – 1.51 (m, 3H), 1.48 – 1.35 (m, 2H), 1.30 – 1.16 (m, 3H), 1.16 – 1.04 (m, 3H), 0.89 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H36F2N3Na2O8S 586.1987; Found 586.1978.
Sodium (2S)-2-((S)-2-((((2-(4,4-difluorocyclohexyl)propan-2-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (13c).
Yield (39%). 1H NMR (400 MHz, DMSO-d6) δ 7.49 (d, J = 10.6 Hz, 1H), 7.43 (s, 1H), 7.12 – 6.95 (m, 1H), 5.47 – 5.26 (m, 1H), 4.06 – 3.71 (m, 2H), 3.17 – 3.08 (m, 1H), 3.08 – 2.97 (m, 1H), 2.14 – 1.93 (m, 6H), 1.86 – 1.65 (m, 5H), 1.64 – 1.49 (m, 2H), 1.44 – 1.36 (m, 2H), 1.33 (s, 6H), 1.28 – 1.23 (m, 2H), 0.84 (ddd, J = 11.6, 6.5, 3.0 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C23H38F2N3Na2O8S 600.2143; Found 600.2131.
Sodium (2S)-2-((2S)-2-(((1-(4,4-difluorocyclohexyl)-2-phenylethoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (14c).
Yield (41%). 1H NMR (400 MHz, DMSO-d6) δ 7.53 – 7.47 (m, 1H), 7.43 (s, 1H), 7.39 – 7.33 (m, 1H), 7.28 – 7.15 (m, 5H), 5.38 – 5.15 (m, 1H), 4.75 – 4.71 (m, 1H), 3.96 – 3.91 (m, 1H), 3.87 – 3.68 (m, 1H), 3.13 – 3.09 (m, 2H), 3.07 – 2.96 (m, 1H), 2.90 – 2.82 (m, 1H), 2.00 (s, 4H), 1.92 – 1.80 (m, 3H), 1.76 (s, 3H), 1.59 – 1.51 (m, 4H), 1.49 – 1.33 (m, 3H), 0.89 – 0.79 (m, 4H), 0.79 – 0.67 (m, 2H). HRMS m/z: [M+Na]+ Calculated for C28H40F2N3NaO8S 662.2300; Found 662.2288.
Sodium (2S)-2-((2S)-2-((((4,4-difluorocyclohexyl)(phenyl)methoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (15c).
Yield (66%). 1H NMR (400 MHz, DMSO-d6) δ 7.64 – 7.50 (m, 1H), 7.49 – 7.44 (m, 1H), 7.42 – 7.19 (m, 6H), 5.48 – 5.35 (m, 1H), 4.01 – 3.75 (m, 3H), 3.18 – 3.10 (m, 1H), 3.08 – 3.01 (m, 1H), 2.22 – 2.06 (m, 1H), 2.06 – 1.91 (m, 3H), 1.87 – 1.68 (m, 4H), 1.61 – 1.54 (m, 3H), 1.49 – 1.40 (m, 3H), 1.35 – 1.14 (m, 3H), 0.93 – 0.71 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C27H38F2N3Na2O8S 648.2143; Found 648.2121.
Sodium (2S)-2-((2S)-2-((((1-(4,4-difluorocyclohexyl)pentyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (16c).
Yield (58%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 – 7.51 (m, 1H), 7.44 (s, 1H), 7.38 – 7.23 (m, 1H), 5.46 – 5.21 (m, 1H), 3.98 – 3.74 (m, 3H), 3.14 – 3.07 (m, 1H), 3.06 – 2.98 (m, 1H), 2.16 – 2.04 (m, 2H), 2.04 – 1.92 (m, 4H), 1.86 – 1.66 (m, 8H), 1.64 – 1.52 (m, 3H), 1.49 – 1.36 (m, 3H), 1.21 – 1.17 (m, 3H), 0.83 (ddd, J = 11.9, 6.5, 3.1 Hz, 9H). HRMS m/z: [M+Na]+ Calculated for C25H42F2N3Na2O8S 628.2456; Found 628.2419.
Sodium (2S)-2-((S)-2-((((2-fluorobenzyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (17c).
Yield (71%). 1H NMR (400 MHz, DMSO-d6) δ 7.77 – 7.70 (m, 1H), 7.70 – 7.59 (m, 1H), 7.59 – 7.33 (m, 3H), 7.26 – 7.12 (m, 2H), 5.65 (d, J = 78.6 Hz, 1H), 5.16 – 5.01 (m, 2H), 4.11 – 3.84 (m, 2H), 3.17 – 2.97 (m, 2H), 2.39 – 2.07 (m, 2H), 2.07 – 1.85 (m, 1H), 1.70 – 1.51 (m, 3H), 1.51 – 1.33 (m, 2H), 0.92 – 0.77 (m, 6H). HRMS m/z: [M]− Calculated for C21H29FN3O8S: 502.1659, Found: 502.1650. HRMS m/z: [M+Na]+ Calculated for C21H29FN3Na2O8S: 548.1455, Found: 548.1446.
Sodium (2S)-2-((S)-2-((((3-fluorobenzyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (18c).
Yield (89%). 1H NMR (400 MHz, DMSO-d6) δ 7.75 (d, J = 9.0 Hz, 1H), 7.64 (d, J = 9.3 Hz, 1H), 7.57 (d, J = 7.9 Hz, 1H), 7.54 – 7.35 (m, 2H), 7.25 – 7.08 (m, 2H), 5.75 – 5.46 (m, 1H), 5.15 – 4.98 (m, 2H), 4.13 – 3.87 (m, 2H), 3.17 – 2.89 (m, 2H), 2.23 – 2.04 (m, 2H), 2.04 – 1.91 (m, 1H), 1.89 – 1.74 (m, 1H), 1.70 – 1.31 (m, 4H), 0.91 – 0.77 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H29FN3Na2O8S: 548.1455, Found: 548.1450. HRMS m/z: [M]− Calculated for C21H29FN3O8S: 502.1659, Found: 502.1655.
Sodium (2S)-2-((S)-2-((((4-fluorobenzyl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (19c).
Yield (68%). 1H NMR (400 MHz, DMSO-d6) δ 7.70 – 7.57 (m, 1H), 7.57 – 7.49 (m, 1H), 7.48 – 7.37 (m, 3H), 7.23 – 7.14 (m, 2H), 5.43 (d, J = 85.6 Hz, 1H), 5.09 – 4.92 (m, 2H), 4.12 – 3.81 (m, 2H), 3.14 – 2.94 (m, 2H), 2.23 – 1.91 (m, 2H), 1.62 – 1.50 (m, 4H), 1.50 – 1.38 (m, 2H), 0.90 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H29FN3Na2O8S: 548.1455, Found: 548.1448. HRMS m/z: [M]− Calculated for C21H29FN3O8S: 502.1659, Found: 502.1645.
Sodium (2S)-2-((S)-2-((((4-fluorophenyl)methoxy-d2)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (20c).
Yield (90%). 1H NMR (400 MHz, DMSO-d6) δ 7.68 (d, J = 9.2 Hz, 1H), 7.63 (d, J = 9.2 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.48 – 7.35 (m, 2H), 7.23 – 7.13 (m, 2H), 5.52 (dd, J = 85.5, 6.2 Hz, 1H), 4.04 – 3.84 (m, 2H), 3.18 – 2.97 (m, 2H), 2.27 – 1.90 (m, 2H), 1.63 – 1.50 (m, 3H), 1.53 – 1.38 (m, 3H), 0.92 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H27D2FN3Na2O8S: 550.1581, Found: 550.1573. HRMS m/z: [M+Na]+ Calculated for C21H27D2FN3O8S: 504.1785, Found: 504.1769.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((perfluorophenyl) methoxy) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (21c).
Yield (81%). 1H NMR (400 MHz, DMSO-d6) δ 7.68 – 7.42 (m, 2H), 6.06 (s, 1H), 5.41 (d, J = 6.3 Hz, 1H), 5.24 (d, J = 5.9 Hz, 1H), 5.13 (s, 2H), 4.36 (d, J = 7.2 Hz, 1H), 4.01 – 3.89 (m, 1H), 3.48 – 3.41 (m, 3H), 2.20 – 2.04 (m, 3H), 1.63 – 1.33 (m, 4H), 0.89 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H25F5N3Na2O8S 620.1078; Found 620.1069.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((perfluorophenyl)methoxy-d2) carbonyl) amino) pentanamido)-3-(2-oxopyrrolidin-3-yl) propane-1-sulfonate (22c).
Yield (89%). 1H NMR (400 MHz, DMSO-d6) δ 7.67 (d, J = 9.1 Hz, 1H), 7.58 (s, 1H), 7.50 (d, J = 8.8 Hz, 1H), 5.12 (d, J = 12.4 Hz, 1H), 4.42 – 4.36 (m, 1H), 3.99 – 3.90 (m, 2H), 3.16 – 3.00 (m, 2H), 2.20 – 2.05 (m, 3H), 1.77 (s, 1H), 1.62 – 1.50 (m, 2H), 1.47 – 1.37 (m, 2H), 0.88 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H23D2F5N3Na2O8S 622.1204; Found 622.1193.
Sodium(2S)-1-hydroxy-2-((2S)-4-methyl-2-(((1-phenylbutoxy)carbonyl)amino)pentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (23c).
Yield (63%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 9.8 Hz, 1H), 7.47 (d, J = 5.0 Hz, 1H), 7.41 (d, J = 7.4 Hz, 1H), 7.38 – 7.22 (m, 5H), 5.54 (t, J = 6.6, 6.6 Hz, 1H), 5.37 – 5.25 (m, 1H), 4.12 – 3.78 (m, 2H), 3.17 – 2.95 (m, 2H), 2.31 – 1.88 (m, 3H), 1.85 – 1.38 (m, 4H), 1.36 – 1.18 (m, 2H), 0.93 – 0.82 (m, 10H), 0.79 – 0.70 (m, 2H). HRMS m/z: [M+Na]+ Calculated for C24H36N3Na2O8S 572.2019; Found 572.2005.
Sodium (2S)-2-((2S)-2-(((1,2-diphenylethoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (24c).
Yield (69%). 1H NMR (400 MHz, DMSO-d6) δ 7.69 – 7.36 (m, 2H), 7.36 – 7.07 (m, 11H), 5.82 – 5.71 (m, 1H), 5.59 – 5.32 (m, 1H), 4.15 – 3.81 (m, 2H), 3.18 – 2.76 (m, 4H), 2.32 – 1.72 (m, 4H), 1.71 – 1.30 (m, 4H), 0.90 – 0.60 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C28H36N3Na2O8S 620.2019; Found 620.2007.
Biochemical Studies
Enzyme assays and inhibition studies.
Cloning and expression of the 3CLpro of SARS-CoV-2 and FRET enzyme assays. The codon-optimized cDNA of full length of 3CLpro of SARS-CoV-2 (GenBank number MN908947.3) fused with sequences encoding 6 histidine at the N-terminal was synthesized by Integrated DNA (Coralville, IA). The synthesized gene was subcloned into the pET-28a(+) vector. The expression and purification of SARS-CoV-2 3CLpro were conducted following a standard procedure described previously.19 Briefly, a stock solution of an inhibitor was prepared in DMSO and diluted in assay buffer comprised of 20 mM HEPES buffer, pH 8, containing NaCl (200 mM), EDTA (0.4 mM), glycerol (60%), and 6 mM dithiothreitol (DTT). The SARS-CoV-2 3CLpro was mixed with serial dilutions of compound or with DMSO in 25 μL of assay buffer and incubated at 37°C for 1 h, followed by the addition of 25 μL of assay buffer containing substrate (FAM-SAVLQ/SG-QXL®520, AnaSpec, Fremont, CA). The substrate was derived from the cleavage sites on the viral polyproteins of SARS-CoV. Fluorescence readings were obtained using an excitation wavelength of 480 nm and an emission wavelength of 520 nm on a fluorescence microplate reader (FLx800; Biotec, Winoosk, VT) 1 h following the addition of substrate. Relative fluorescence units (RFU) were determined by subtracting background values (substrate-containing well without protease) from the raw fluorescence values, as described previously. The dose-dependent FRET inhibition curves were fitted with a variable slope by using GraphPad Prism software (GraphPad, La Jolla, CA) in order to determine the IC50 values of the compounds. The expression and purification of the 3CLpro of MERS-CoV, as well as the FRET enzyme assays were performed as described previously.19–21,33
Cell based assay to screen SARS-CoV-2 3CLpro inhibitors.
Two plasmids, pR-SARS-CoV-2 3CLpro and pGlo-VRLQS were used for the system (Figure 1/panel A). First, the open reading frame of SARS-CoV-2 3CLpro was cloned to the reverse genetics system of PRRSV with GFP35 and designated as pR-SARS-CoV-2 3CLpro. The GFP gene was replaced with SARS-CoV-2 3CLpro gene with AflII and MluI enzyme sites. As a negative control, inactive form of the 3CLpro was introduced to pR-SARS-CoV-2 3CLpro by the mutagenesis, and resulting plasmid was designated as pR-SARS-CoV-2 3CLpro C145A. Second plasmid was utilized with the pGloSensor caspase 3/7 biosensor (Promega, Madison, WI) which contains a caspase-3/7 cleavage site engineered in the Firefly luciferase gene. This plasmid also contains intact Renilla luciferase gene as an expression control. After transfection of pGloSensor caspase 3/7 to cells, the Firefly luciferase is expressed as an inactive form, and in the presence of caspase 3/7, it is activated after the cleavage. The caspase-3/7 cleavage site in this plasmid was replaced with CoV 3CLpro recognition sequences, VRLQS, designated as pGlo-VRLQS.34 As a result, the expressed inactive luciferase is activated by the cleavage with CoV 3CLpro in the cells (Figure 1/panel A). HEK293T cells were used for transfection and the compound screening (Figure 1/panel B). One-day old HEK293T cells in 48-well plates were co-transfected with two plasmids, pR-SARS-CoV-2 3CLpro and pGlo-VRLQS, or pR-SARS-CoV-2 3CLpro C145A (serves as a negative control) and pGlo-VRLQS. Following morning, medium containing Mock-DMSO or serial concentrations of each compound were replaced to the transfected cells and incubate at 37 C for 6 h. Cell lysates were prepared for testing the levels of Firefly and Renilla luciferases (Dual Luciferase assay kit, Promega) in a luminometer (Promega). The expression levels of Firefly luciferase were normalized with Renilla luciferase levels. The transfection of pR-SARS-CoV-2 3CLpro C145A and pGlo-VRLQS resulted in minimal levels of Firefly luciferase, and this was applied to adjust all Firefly expression levels. The inhibition curve (Figure 1/panel C) for each compound was prepared, and the 50% effective concentration (EC50) values were determined by GraphPad Prism software using a variable slope (GraphPad, La Jolla, CA). Compounds 4c and 15c were selected and examined for the antiviral effects with live SARS-CoV-2 in Vero E6 cells as described before33, and the EC50 was calculated by the same method described above. A known SARS-CoV-2 3CLpro inhibitor; GC376 was used as the positive control in each experiment. When GC376 and the selected compounds were examined if they inhibited the replication of PRRSV, none of them resulted in the reduction of viral replication.
Nonspecific cytotoxic effects/In vitro cytotoxicity.
HEK293T cells grown in 96-well plates were incubated with various concentrations (1 to 100 μM) of each compound for 72 h. Cell cytotoxicity was measured by a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega), and the CC50 values were calculated using a variable slope by GraphPad Prism software.
X-ray Crystallographic Studies
Crystallization and Data Collection.
Purified SARS-CoV-2 3CLpro19 in 100mM NaCl, 20mM Tris pH 8.0 was concentrated to 9.6 mg/mL (0.28 mM) for crystallization screening. All crystallization experiments were setup using an NT8 drop-setting robot (Formulatrix Inc.) and UVXPO MRC (Molecular Dimensions) sitting drop vapor diffusion plates at 18 °C. 100 nL of protein and 100 nL crystallization solution were dispensed and equilibrated against 50 uL of the latter. A stock solution of 100 mM compound was prepared in DMSO and the SARS-CoV-2 3CLpro:compound complex was prepared by mixing 1 μL of the ligand (2 mM) with 49 μL (0.28 mM) of SARS2 3CLpro and incubating on ice for 1 hour. Crystals were obtained in 1–2 days from various conditions for the following complexes. 8b: Proplex HT screen (Molecular Dimensions) condition F7 (0.5 M ammonium sulfate, 100 mM MES pH 6.5). 12b, 13c, 14c and 21c: Index HT screen (Hampton Research) condition D10 (20% (w/v) PEG 5000 MME, 100 mM Bis-Tris pH 6.5). 19b and 20b: Proplex HT screen (Rigaku Reagents) condition C5 (20% (w/v) PEG 4000, 100 mM Tris pH 8.0). 1c: Index HT screen (Hampton Research) condition F2 (20% (w/v) PEG 2000 MME, 100 mM Tris pH 8.5, 200 mM Trimethylamine N-oxide dihydrate). 3c: Index HT screen (Hampton Research) condition F5 (17% (w/v) PEG 10,000, 100 mM Bis-Tris pH 5.5, 100 mM ammonium acetate). 5c: Index HT screen (Hampton Research) condition F1 (10% (w/v) PEG 3350, 100 mM Hepes pH 7.5, 200 L-proline). 17c and 18c: Index HT screen (Rigaku Reagents) condition H11 (30% (w/v) PEG 2000 MME, 100 mM potassium thiocyanate). Samples were transferred to a fresh drop composed of 80% crystallization solution and 20% (v/v) PEG 200 and stored in liquid nitrogen. Crystals of SARS-CoV-2 3CLpro with 8b were transferred to a cryoprotectant solution containing 80% crystallant and 20% (v/v) glycerol prior to freezing. X-ray diffraction data were collected at the Advanced Photon Source beamline except for the SARS-CoV-2 3CLpro complex with 14c which were collected at the National Synchrotron Light Source II (NSLS-II) AMX beamline 17-ID-1. All diffraction data were collected using a Dectris Eiger2 X 9M pixel array detector.
Structure Solution and Refinement.
Intensities were integrated using XDS40–41 via Autoproc42 and the Laue class analysis and data scaling were performed with Aimless43. Structure solution was conducted by molecular replacement with Phaser44 using a previously determined structure of SARS2 3CLpro (PDB 6XMK19) as the search model. Structure refinement and manual model building were conducted with Phenix45 and Coot46 respectively. Disordered side chains were truncated to the point for which electron density could be observed. Structure validation was conducted with Molprobity47 and figures were prepared using the CCP4MG48 package. Crystallographic data are provided in Table S1.49–53
Supplementary Material
ACNKOWLEDGEMENTS
This research was supported in part by grants from the National Institutes of Health (NIH) (R01 AI109039 to K.O.C). Use of the University of Kansas Protein Structure Laboratory was supported by a grant from the National Institute of General Medical Sciences (P30GM110761) of the NIH. Support for the NMR instrumentation was provided by NIH Shared Instrumentation Grant # S10RR024664 and NSF Major Research Instrumentation Award # 1625923. Use of the IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02–06CH11357. This research used the AMX beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The Center for BioMolecular Structure (CBMS) is primarily supported by the National Institutes of Health, National Institute of General Medical Sciences (NIGMS) through a Center Core P30 Grant (P30GM133893), and by the DOE Office of Biological and Environmental Research (KP1605010).
Abbreviations used
- CC50
50% cytotoxic concentration in cell-based assays
- CDI
carbonyl diimidazole
- CPE
cytopathic effects
- DMSO
dimethyl sulfoxide
- DMP
Dess-Martin periodinane
- DSC
N,N’-disuccinimidyl carbonate
- DTT
dithiothreitol
- EC50
the 50% effective concentration in cell culture
- GESAMT
general efficient structural alignment of macromolecular targets
- IC50
the 50% inhibitory concentration in the enzyme assay
- MME
monomethyl ether
- MNV
murine norovirus
- MOI
multiplicity of infection
- ORF
open reading frame
- PK
pharmacokinetics
- RMSD
root mean square deviation
- TCID50
the 50% tissue culture infectious dose
- TEA
Triethyl amine
- XDS
X-ray detector software
Footnotes
The authors declare no competing financial interests.
Accession Codes
Coordinates and structure factors for the following SARS2 3CLpro complexes with inhibitors were deposited to the Worldwide Protein Databank (wwPDB) with the accession codes: 8b (7LZT), 12b (7LZU), 19b (7LZV), 20b (7LZW), 1c (7LZX), 3c (7LZY), 5c (7LZZ), 13c (7M00), 14c (7M01), 17c (7M02), 18c (7M03) and 21c (7M04). Authors will release the atomic coordinates upon article publication.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supplemental information - Binding mode of 19b and 20b with SARS-CoV-2 3CLpro associated with subunit A and subunit B, surface representation showing the orientation of 19b on the SARS-CoV-2 3CLpro surface, crystallographic data for SARS-CoV-2 3CLpro inhibitor complexes, and absolute qNMR data. (.docx)
Molecular formula strings – SMILES codes (.csv)
References
- 1.Wu F; Zhao S; Yu B; Chen Y-M; Wang W; Song Z-G; Hu Y; Tao Z-W; Tian J-H; Pei Y-Y; Yuan M-L; Zhang Y-L; Dai F-H; Liu Y; Wang Q-M; Zheng J-J; Xu L; Holmes EC; Zhang Y-Z A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontanet A; Autran B; Lina B; Kieny MP; Abdool Karim SS; Sridhar D SARS-CoV-2 variants and ending the COVID-19 pandemic. The Lancet 2021, 397, 952–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurkovetskiy L; Wang X; Pascal KE; Tomkins-Tinch C; Nyalile TP; Wang Y; Baum A; Diehl WE; Dauphin A; Carbone C; Veinotte K; Egri SB; Schaffner SF; Lemieux J; Munro JB; Rafique A; Barve A; Sabeti PC; Kyratsous CA; Dudkina NV; Shen K; Luban J Structural and functional analysis of the D614G SARS-CoV-2 spike protein. CellPress 2020, 183, 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D; Koganti R; Lambe UP; Yadavalli T; Nandi SS; Shukla D Vaccines and therapies in development for SARS-CoV-2 infections. J. Clin. Med. 2020, 9, 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C; Li W; Drabek D; Okba NMA; Haperen RV; Osterhaus ADME; Kuppeveld FJMV; Haagmans BL; Grosveld F; Bosch B-J A human monoclonal antibody blocking SARS-CoV-2. Nature Comm. 2020, 11 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh AK; Brindisi M; Shahabi D; Chapman ME; Mesecar AD Drug development and medicinal chemistry efforts toward SARS-Coronavirus and Covid-19 therapeutics. Chem. Med. Chem. 2020, 15, 907–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil C; Ginex T; Maestro I; Nozal V; Barrado-Gil L; Cuesta-Geijo MA; Urquiza J; Ramírez D; Alonso C; Campillo NE, Martinez A, COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020, 63, 12359–12386. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A; Tiwari S; Deb MK; MARTY JL, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A global pandemic and treatments strategies. Intl J. Antimicrob. Agents 2020, 56, 106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannalire R; Cerchia C; Beccari AR; Di Leva FS; Summa V, Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: State of the art and future opportunities. J. Med. Chem. [online early access]. DOI: 10.1021/acs.jmedchem.0c01140. Published online: Nov 13, 2020. 10.1021/acs.jmedchem.0c01140 (accessed Nov 13, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullrich S; Nitsche C, The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020, 30, 127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konwar M; Sarma D, Advances in developing small molecule SARS 3CLpro inhibitors as potential remedy for Corona Virus infection. Tetrahedron 2021, 77, 131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J; Hu L; Huang X; Wang C; Zhang Z; Wang Y; Zhang D; Ye W, Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: Insights from structures of protease and inhibitors. Intl J. Antimicrob. Agents 2020, 56(2), 106055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z; Du X; Xu Y; Deng Y; Liu M; Zhao Y; Zhang B; Li X; Zhang L; Peng C, Duan Y; Yu J; Wang L; Yang K; Liu F; Jiang R; Yang X; You T; Liu X; Yang X; Bai F; Liu H; Liu X; Guddat LW; Xu W; Xiao G; Qin C; Shi Z; Jiang H; Rao Z; Yang H, Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L; Lin D; Sun X; Curth U; Drosten C; Sauerhering L; Becker S; Rox K; Hilgenfeld R, Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai W; Zhang B; Jiang X-M; Su H; Li J; Zhao Y; Xie X; Jin Z; Peng J; Liu F, Li C; Li Y; Bai F; Wang H; Cheng X; Cen X; Hu S; Yang X; Wang J,; Liu X.; Xiao G; Jiang H; Rao Z; Zhang L-K; Xu Y; Yang H; Liu H Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao J; Li Y-S; Zeng R; Liu F-L; Luo R-H; Huang C; Wang Y-F; Zhang J; Quan B; Shen C; Mao X,; Liu X; Sun W; Yang W; Ni X; Wang K; Xu L; Duan Z-L; Zou Q-C; Zhang H-L; Qu W; Long Y-H-P; Li M-H; Yang R-C; Liu X; You J; Zhou Y; Yao R; Li W-P; Liu J-M; Chen; Liu Y; Lin G-F; Yang X; Zou J; Li L; Hu Y; Lu G-W; Li W-M; Wei Y-Q; Zheng Y-T; Lei J; Yang S, SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechter I, Reprint of “on the size of the active site in proteases. I. Papain”. Biochem. Biophys. Res. Com. 2012, 425, 497–502. The nomenclature used is that of Schechter, I. and Berger, A., where the residues on the N-terminus side of the peptide bond that is cleaved are designated as P1-Pn and those on the C-terminus side are designated P1-P1’. The corresponding active site subsites are designated S1-Sn and S1-Sn’. S1 is the primary substrate specificity subsite and P1-P1’ is the scissile bond. [DOI] [PubMed] [Google Scholar]
- 18.Kreutzer AG; Krumberger M; Parrocha CMT; Morris MA; Guaglianone G; Nowick JS Structure-based design of a cyclic peptide inhibitor of the SARS-CoV-2 Main protease. bioRixiv [online early access]. DOI: 10.1101/2020.08.03.234872. Published online: Aug 03, 2020. (accessed Aug 03, 2020). [DOI] [Google Scholar]
- 19.Rathnayake AD; Zheng J; Kim Y; Perera KD; Mackin S; Meyerholz DK; Kashipathy MM; Battaile KP; Lovell S; Perlman S, 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV–infected mice. Science Transl. Med. 2020, 12 :eabc5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galasiti Kankanamalage AC; Kim Y; Damalanka VC; Rathnayake AD; Fehr AR; Mehzabeen N; Battaille KP; Lovell S; Lushington GH; Perlman S; Chang KO; Groutas WC Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur. J. Med. Chem. 2018, 150, 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y; Lovell S; Tiew K-C; Mandadapu SR; Alliston KR; Battaile KP; Groutas WC; Chang K-O, Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 2012, 86, 11754–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y; Liu H; Galasiti Kankanamalage AC; Weerasekara S; Hua DH; Groutas WC; Chang K-O; Pedersen NC, Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathogens 2016, 12, e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen NC; Kim Y; Liu H; Galasiti Kankanamalage AC; Eckstrand C; Groutas WC; Bannasch M; Meadows JM; Chang K-O, Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennington LD; Aquila BM; Choi Y; Valiulin RA; Muegge I Positional analog scanning: an effective strategy for multiparameter optimization in drug design. J. Med. Chem. 2020, 63, 8956–8976. [DOI] [PubMed] [Google Scholar]
- 25.Vulpetti A; Hommel U; Landrum G; Lewis R; Dalvit C Design and NMR-based screening of LEF, a library of chemical fragments with different local environments of fluorine. J. Am. Chem. Soc. 2009, 131, 12949–12959. [DOI] [PubMed] [Google Scholar]
- 26.Pirali T; Serafini M; Cargnin S; Genazzani AA, Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019, 62, 5276–5297. [DOI] [PubMed] [Google Scholar]
- 27.Timmins GS Deuterated drugs: updates and obviousness analysis. Expert Opin. Ther. Pat. 2017, 27, 1353–1361. [DOI] [PubMed] [Google Scholar]
- 28.Liu JF A decade of deuteration in medicinal chemistry. Ann. Rep. Med. Chem. 2017, 50, 519–542. [Google Scholar]
- 29.Kim H-O; Kahn M The synthesis of aminoazole analogs of lysine and arginine: the Mitsunobu reaction with lysinol and arginol. Synlett. 1999, 8, 1239–1240. [Google Scholar]
- 30.Maity P; Gujjar M; Vellingiri R; Lakshminarasimhan T; DelMonte AJ; Young IS; Eastgate MD; Vaidyanathan R Cerium(III) chloride-mediated stereoselective reduction of a 4-substituted cyclohexanone using NaBH4. Org. Process Res. Dev. 2019, 23, 2754–2757. [Google Scholar]
- 31.Ghosh AK; Duong TT; McKee SP; Thompson WJ, N, N’-Disuccinimidyl carbonate: A useful reagent for alkoxycarbonylation of amines. Tetrahedron Lett. 1992, 33, 2781–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kjell DP; Slattery BJ; Semo MJ, A novel, nonaqueous method for regeneration of aldehydes from bisulfite adducts. J. Org. Chem. 1999, 64, 5722–5724. [DOI] [PubMed] [Google Scholar]
- 33.Dampalla CS; Zheng J; Perera KD; Wong L-YR; Meyerholz DK; Nguyen HN; Kashipathy MM; Battaile KP; Lovell S; Kim Y; Perlman S; Groutas WC; Chang K-O Post-infection treatment with a protease inhibitor increases survival of mice with a fatal SARS-CoV-2 infection. Proc Natl Acad Sci U S A, 2021,118, e2101555118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien A; Chen D-Y; Hackbart M; Close BJ; O'Brien TE; Saeed M; Baker SC; Detecting SARS-CoV-2 3CLpro expression and activity using a polyclonal antiserum and a luciferase-based biosensor. Virology, 2021, 556: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson SR; Li Y; Patton JB; Langenhorst RJ; Sun Z; Jiang Z; Christopher-Hennings J; Nelson EA; Knudsen D; Fang Y; Chang K-O Interleukin-1β expression by a recombinant PRRSV enhanced viral specific host immunity. Virus Research. 2012, 163, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao W; Cho C-CD; Geng ZZ; Ma XR; Allen R; Shaabani N; Vatansever EC; Alugubelli YR; Ellenburg WH; Yang K. s.; Qiao Y; Ji H; Xu S; Liu WR Cellular activity of SARS-CoV-2 main protease inhibitors reveal their unique characteristics. bioRxiv [online early access]. DOI: 10.1101/2021.06.08.447613. Published online: June 9, 2021. 10.1101/2021.06.08.447613 (accessed Aug 3, 2021). [DOI] [Google Scholar]
- 37.Resnick SJ; Iketani S; Hong SJ; Zask A; Liu H; Kim S; Melore S; Nair MS; Huang Y; Tay NES; Rovis T; Yang HW; Stockwell BR.; Ho DD; Chavez A A simplified cell-based assay to identify coronavirus 3CL protease inhibitors. bioRxiv [online early access]. DOI: 10.1101/2020.08.29.272864. Published online: Aug 29, 2020. 10.1101/2020.08.29.272864 (accessed Aug 3, 2021). [DOI] [Google Scholar]
- 38.Talele TT Natural products-inspired use of the gem-dimethyl group in medicinal chemistry. J. Med. Chem. 2018, 61, 2166–2210. [DOI] [PubMed] [Google Scholar]
- 39.Pennington LD; Moustakas DT The necessary nitrogen atom: a versatile high-impact design element for multiparameter optimization. J. Med. Chem. 2017, 60, 3552–3579. [DOI] [PubMed] [Google Scholar]
- 40.Kabsch W, Automatic indexing of rotation diffraction patterns. J. Applied Crystallogr. 1988, 21, 67–72. [Google Scholar]
- 41.Kabsch W, XDS. Acta Crystallogr. D 2010, 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vonrhein C; Flensburg C; Keller P; Sharff A; Smart O; Paciorek W; Womack T; Bricogne G, Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. Section D: Biolog. Crystallogr. 2011, 67, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans PR, An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. Section D: Biolog. Crystallogr. 2011, 67, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ, Phaser crystallographic software. J. Applied Crystallogr. 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams PD; Afonine PV; Bunkóczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung L-W; Kapral GJ; Grosse-Kunstleve RW, PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley P; Lohkamp B; Scott WG; Cowtan K, Features and development of Coot. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen VB; Arendall WB; Headd JJ; Keedy DA; Immormino RM; Kapral GJ; Murray LW; Richardson JS; Richardson DC, MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potterton L; McNicholas S; Krissinel E; Gruber J; Cowtan K; Emsley P; Murshudov GN; Cohen S; Perrakis A; Noble M, Developments in the CCP4 molecular-graphics project. Acta Crystallogr. Section D: Biolog. Crystallogr. 2004, 60, 2288–2294. [DOI] [PubMed] [Google Scholar]
- 49.Evans P Scaling and assessment of data quality, Acta Crystallogr. Sect D: Biol. Crystallogr. 2006, 62, 72–82. [DOI] [PubMed] [Google Scholar]
- 50.Diederichs K; Karplus PA Improved R-factors for diffraction data analysis in macromolecular crystallography, Nat Struct Biol. 1997, 4, 269–275. [DOI] [PubMed] [Google Scholar]
- 51.Weiss MS Global indicators of X-ray data quality, J. Appl. Crystallogr. 2001, 34 130–135. [Google Scholar]
- 52.Karplus PA; Diederichs K Linking crystallographic model and data quality, Science 2012, 336, 1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans P Biochemistry. Resolving some old problems in protein crystallography, Science 2012, 336, 986–987. [DOI] [PubMed] [Google Scholar]
- 54.The purity of some of the aldehyde inhibitors ranged between 89 – 94% due to the facile epimerazation of the aldehyde α-carbon upon storage (see supporting informatioon).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.