Abstract
The outpatient treatment of select emergency department patients with acute pulmonary embolism (PE) or deep vein thrombosis (DVT) has been shown to be safe, cost effective and associated with high patient satisfaction. Despite this, outpatient PE and DVT treatment remains uncommon. To address this, the American College of Emergency Physicians assembled a multidisciplinary team of content experts to provide evidence‐based recommendations and practical advice to help clinicians safely treat patients with low‐risk PE and DVT without hospitalization. The emergency clinician must stratify the patient's risk of clinical decompensation due to their PE or DVT as well as their risk of bleeding due to anticoagulation. The clinician must also select and start an anticoagulant and ensure that the patient has access to the medication in a timely manner. Reliable follow‐up is critical, and the patient must also be educated about signs or symptoms that should prompt a return to the emergency department. To facilitate access to these recommendations, the consensus panel also created 2 web‐based “point‐of‐care tools.”
Keywords: deep vein thrombosis, outpatient, pulmonary embolism, treatment, venous thromboembolism
1. INTRODUCTION
Approximately 1 million patients are diagnosed with deep vein thrombosis (DVT) or pulmonary embolism (PE) every year in the United States, and most of these diagnoses are made in emergency departments (EDs). 1 , 2 , 3 , 4 , 5 Historically, >90% of patients with PE and >50% of patients with DVT have been hospitalized for anticoagulation and monitoring. 6 However, in recent years, the availability of direct‐acting oral anticoagulants (DOACs) and the ability to identify patients at low risk of short‐term clinical deterioration have reduced the need for hospitalization. 7 , 8 , 9 , 10
The outpatient treatment of low‐risk PE was first suggested in the early 2000s. 11 , 12 , 13 Subsequent clinical trials 14 , 15 , 16 and observational studies 17 , 18 , 19 , 20 confirmed the safety of this approach, demonstrating that outpatient and inpatient PE treatment were associated with similarly low rates of mortality, recurrent venous thromboembolism (VTE), and bleeding. More than 98% of patients treated as outpatients have an uncomplicated course. 21 Outpatient treatment is also associated with high patient satisfaction 17 , 18 , 22 , 23 , 24 , 25 , 26 at lower cost than inpatient hospitalization. 27 , 28 However, the definition of “outpatient treatment” varies widely in the published literature, including discharge from the ED or up to 48 hours on an inpatient floor. 29 In this article, we focused on care provided by emergency physicians, so we limit our definition of “outpatient treatment” to patients discharged from the ED or an ED observation unit.
Despite the potential benefits, outpatient PE and DVT treatment remains uncommon. 30 This may be because of incomplete knowledge on the part of emergency clinicians, local practice culture, and logistical and operational concerns about risk stratification, medication access, and follow‐up. 23 , 31 To safely and effectively treat a patient with PE and DVT in the outpatient setting, several steps are required. The emergency clinician must stratify the patient's risk of clinical decompensation due to their PE or DVT as well as their risk of bleeding due to anticoagulation. The clinician must also select and start an anticoagulant and ensure that the patient has access to the medication in a timely manner. Reliable follow‐up is critical, and the patient must also be educated about signs or symptoms that should prompt a return to the ED. Although these are standard steps for the discharge of any ED patient, emergency clinicians may find them particularly daunting for patients with PE or DVT. 23
To address these clinical needs, we assembled a multidisciplinary team of content experts to provide evidence‐based recommendations and practical advice to help clinicians safely treat patients with low‐risk PE and DVT without hospitalization. The results of this effort are described here as a series of steps (Figure 1.)
FIGURE 1.
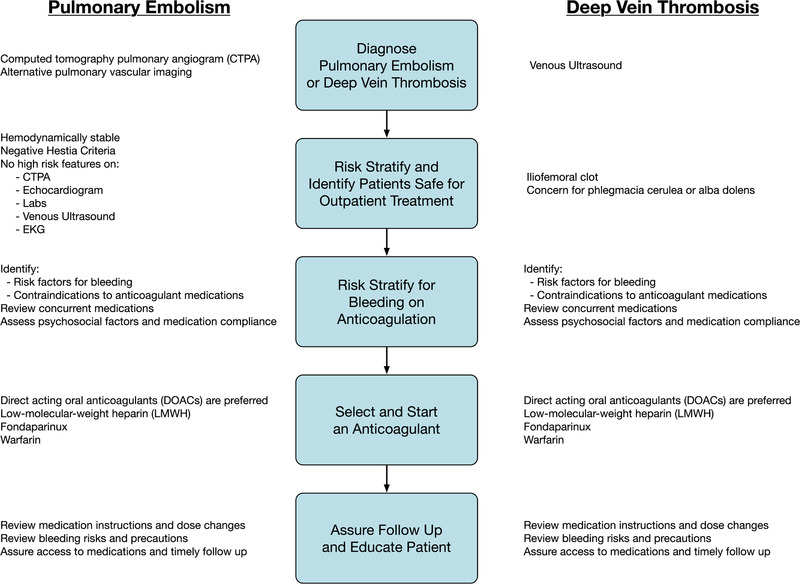
Steps for successful outpatient pulmonary embolism (PE) and deep vein thrombosis (DVT) treatment
2. METHODS
2.1. Goal of the project
In 2020, the American College of Emergency Physicians (ACEP), supported by an unrestricted grant from Janssen Pharmaceuticals, convened a multidisciplinary expert panel to produce consensus recommendations for the outpatient treatment of ED patients with low‐risk acute PE or DVT.
2.2. Establishment of the panel
Panel participants included 5 board‐certified emergency physicians, 1 board‐certified internal medicine physician, and 1 board‐certified hematologist, all with national subject matter expertise in the diagnosis, treatment, and outpatient management of acute PE and DVT. One panelist practices in a community hospital and another in a Veterans Administration hospital. Four of 7 panelists are women and 2 are of Latinx ethnicity. Panelists represented diverse geographic regions across the United States. Some members had significant experience with policy formation and protocol derivation. All members received a nominal stipend for their participation and time. During 5 sessions over 3 months’ time, panel members reviewed the relevant literature and engaged in iterative discussions using a modified‐Delphi approach to reach consensus on the best‐practice recommendations presented in the app and this article.
2.2.1. Step 1: Diagnose PE or DVT
The outpatient VTE treatment tool starts after the emergency clinician has diagnosed a patient with PE or DVT. By far, the most common imaging test used in the ED to diagnose PE is computed tomography pulmonary angiography (CTPA). Ventilation‐perfusion lung scanning (V/Q) can also be used, and in some centers, catheter pulmonary angiography or pulmonary magnetic resonance (MR) angiography may be available. A diagnosis of PE can also be made in a patient with an identified DVT and the presence of symptoms indicative of PE, but the absence of CT imaging may preclude complete risk stratification needed to identify those eligible for discharge home. A diagnosis of DVT is typically made with venous ultrasonography. Both emergency‐clinician‐performed point‐of‐care ultrasound and radiology‐performed "formal" ultrasound are acceptable. 32 , 33 Occasionally, CT or MR venography may be used to diagnose DVT. 34
2.2.2. Step 2: Risk‐stratify a patient with acute PE or DVT to determine eligibility for outpatient treatment
Once the clinician has confirmed the diagnosis of PE or DVT, the next step is risk stratifying the patient to determine whether home treatment is appropriate. The initial disposition decision (ie, home vs. hospital) for an ED patient diagnosed with acute PE should be based on the risk the PE poses to the patient, suited to their health care needs, and tailored to their clinical characteristics and psychosocial situation. Therefore, the first step in our PE clinical decision framework is the risk stratification of the patient and their PE.
Several validated prognostic tools are available to help identify which ED patients are at low risk for short‐term complications, and, as such, may be eligible for discharge home. 35 Among the PE risk stratification tools, the most validated and commonly used are the Hestia clinical decision rule, 17 , 36 , 37 the PE Severity Index (PESI) 14 , 18 , 38 , 39 and the simplified PESI (sPESI). 40 When compared head to head, the Hestia clinical decision rule and sPESI have been shown to be similarly safe and effective, identifying over one third of acute PE patients for outpatient treatment with reassuring outcomes. 41 , 42 However, the PESI and sPESI estimate 30‐day all‐cause mortality, an outcome that may not be as germane to ED disposition decisions as shorter‐term outcomes. Furthermore, validation studies of the PESI and sPESI excluded patients with certain clinical and comorbid conditions before applying the risk score. 14 , 39 , 43 The Hestia clinical decision rule, on the other hand, was specifically designed to identify patients who can safely be treated as outpatients and are, therefore, comprised only of contraindications to outpatient treatment (Table 1). 36 Application of the Hestia clinical decision rule still requires clinical judgment, as several factors lack specificity and no tool can account for every situation. Accordingly, the panel chose to recommend risk stratification with the Hestia clinical decision rule, while acknowledging that tools like the PESI and sPESI can be useful.
TABLE 1.
Hestia clinical decision rule for excluding patients with acute pulmonary embolism from outpatient treatment
| Criteria | Description |
| Prearrival anticoagulation | PE diagnosed while already on anticoagulation |
| Hemodynamics | SBP < 100 mmHg + pulse > 100 beats per minute or unstable by clinical judgment or requiring critical care |
| O2 saturation | >24 hours of O2 supply needed to maintain O2 saturation >90% |
| Treatment | Requiring thrombolysis or embolectomy for reasons other than hemodynamic instability |
| Pain | Severe pain needing intravenous pain medication >24 hours |
| Comorbid conditions | |
| Bleeding or risk thereof | Active bleeding or high risk of bleeding: gastrointestinal bleeding or surgery ≤2 weeks ago, stroke ≤1 month ago, bleeding disorder or platelet count <75 × 10⁹/L, uncontrolled hypertension (SBP >180 mmHg or DBP >110 mmHg), or by clinician judgment |
| Renal function | Creatinine clearance <30 mL/min (according to the Cockroft–Gault formula) |
| Liver function | Severe liver impairment by physician judgment |
| Pregnancy | Pregnant |
| Heparin intolerance | Documented history of heparin‐induced thrombocytopenia |
| Extenuating factors | Medical or social reason for admission >24 hours (infection, malignancy, no support system) |
Abbreviations: DBP, diastolic blood pressure; PE, pulmonary embolism; SBP, systolic blood pressure.
The 11 Hestia criteria were originally framed as questions; if any are answered in the affirmative, outpatient treatment is contraindicated. 36
2.3. Psychosocial criteria for outpatient treatment of a patient with acute PE or DVT
Once a patient is identified as having a low‐risk PE that could be managed as an outpatient, the emergency clinician must assess whether the patient's psychosocial situation is suitable for discharge. The American College of Chest Physicians concisely addresses the psychosocial factors that inform site‐of‐care decision‐making by expecting outpatients “to be compliant with treatment.” 44 Impediments to treatment compliance that may contraindicate discharge include, but are not limited to, homelessness, untreated substance use disorder, dysregulated psychiatric disease, inability to follow up (eg., transportation challenges, patient unreliability), and inability to obtain medications (eg., no health insurance).
2.4. Clinical criteria for outpatient treatment of a patient with acute PE
An ED patient with acute PE may not be eligible for immediate discharge if they have high‐risk clinical features, some of which are enumerated in, and some of which extend beyond, the Hestia clinical decision rule (Table 2). A patient with high‐risk features has a higher likelihood of short‐term complications and may benefit from continued observation with close monitoring, further prognostic evaluation, or advanced therapy. The panel concluded that, to be eligible for discharge, a patient must not have any high‐risk features.
TABLE 2.
Clinical criteria for discharging home emergency department patients with acute pulmonary embolism based on absence of high‐risk features
| High‐risk features in patients with acute pulmonary embolism | Description |
| No evidence of hemodynamic instability | During prehospital and emergency department course, include syncope and presyncope a |
| Negative Hestia clinical decision rule | |
| No high‐risk features on computed tomography pulmonary angiography, if performed | Right ventricle (RV) diameter to left ventricle (LV) diameter ratio >1.0 a |
| Main pulmonary artery or saddle PE | |
| Clot visualized in the heart | |
| No high‐risk features on echocardiogram (bedside or formal), if performed | Right ventricular hypokinesis |
| Right ventricular dilatation | |
| (RV:LV ratio >1.0) | |
| Bowing of the intraventricular septum | |
| (ie., D‐sign) | |
| Clot visualized in the heart | |
| No high‐risk features on laboratory testing | Troponin elevation |
| No high‐risk features on lower extremity compression ultrasound (bedside or formal), if performed | Deep vein thrombosis in iliofemoral vein |
| Evidence of phlegmasia cerulea or alba dolens | |
| No high‐risk features on 12‐lead electrocardiogram a | New right heart strain pattern, including right bundle branch block, deep T‐wave inversions in anterior precordial leads, or S1Q3T3 pattern |
| New‐onset atrial fibrillation or flutter |
If a high‐risk feature is identified, consider echocardiography to evaluate for right heart dysfunction.
Clinicians should assess the patient for high‐risk features, which may include clinical (hemodynamic), imaging, laboratory, electrocardiographic, and ultrasound findings. The most serious high‐risk feature is hemodynamic instability, especially hypotension. Attention to hypotension is critical, even if it was transient or observed only prior to ED arrival. We include syncope and presyncope as evidence of transient hypotension. 45 Most PE diagnoses are made on CTPA, which may demonstrate several high‐risk features: clot in the most proximal pulmonary vasculature, clot in the heart (ie., clot‐in‐transit), and right ventricular dilatation. 46 , 47 The prognostic value of proximal clot location has been demonstrated 48 but is not firmly established. 49 However, the panel recognized that physicians are less likely to discharge home patients with proximal clot, even when adjusting for other known risk factors. 39 , 50 CTPA is highly sensitive (88%) for right ventricular dysfunction but poorly specific (39%) and generally not as accurate as echocardiography in the evaluation of the right ventricle. 51 , 52 , 53 , 54 If CTPA demonstrates right ventricular dilatation, echocardiography may be indicated. Echocardiography is also better suited to evaluate clot in transit, another high‐risk imaging feature. 55 , 56 , 57 , 58 High‐risk laboratory values include an elevated troponin. 59 , 60 Conversely, a negative high‐sensitivity troponin can support discharge to home, as it has a high negative predictive value for adverse events in patients with acute PE. 61 The presence of concurrent DVT, especially in an iliofemoral vein, also portends higher risk. 9 , 62 Findings on 12‐lead ECG that suggest right ventricular strain also have prognostic value in acute PE 63 (Table 2) and should prompt echocardiographic evaluation to evaluate for evidence of right ventricular dysfunction. Although not every patient with acute PE being considered for discharge requires echocardiography or venous ultrasonography, such imaging should be considered in patients with findings suggestive of right heart strain (eg., on electrocardiogram) or concurrent symptoms of DVT, respectively.
2.5. Clinical criteria for outpatient treatment of a patient with acute DVT
Many ED patients with acute DVT are eligible for home treatment, including those with upper extremity DVT. 64 , 65 , 66 Psychosocial features conducive to outpatient treatment should be in place, as described. High‐risk clinical features that would preclude immediate outpatient treatment include iliofemoral DVT 67 and evidence of phlegmasia cerulea or alba dolens. 68 , 69
2.5.1. Step 3: Risk stratify for bleeding on anticoagulation
Anticoagulation is the mainstay of therapy for a patient with acute PE or DVT. Initiating anticoagulation early after a PE or DVT diagnosis has been associated with a reduction in PE‐related mortality. 70 However, the benefits of anticoagulation need to be weighed against the risk of bleeding for each patient. An ED patient at high risk of bleeding is not a candidate for discharge to home and should be admitted to the hospital for close observation and monitoring. Similarly, a patient with a contraindication to anticoagulation should be admitted for close monitoring. The emergency clinicians’ history and physical examination should focus on findings that suggest an increased risk of bleeding. Although bleeding risk scores such as VTE‐BLEED, HAS‐BLED, and HEMORR2HAGES are available, 71 , 72 , 73 these were designed to gauge the long‐term risk of bleeding for patients requiring chronic anticoagulation and, therefore, are unlikely to be helpful in determining which PE and DVT patients are safe for discharge home from the ED.
The panel considered high bleeding risk to be a contraindication to outpatient PE or DVT treatment. High bleeding risk includes active bleeding or any history of critical‐organ bleeding (eg., intracranial, intraspinal, intraocular, retroperitoneal, pericardial, intramuscular with compartment syndrome, pulmonary or airway bleeding). Additionally, a patient who has had recent major surgery, major trauma, or stroke is also at high risk of bleeding. Malignancy is a risk factor for the development of PE and DVT, but a patient with a malignancy in a critical site such as intracranial, spinal, ocular, oropharyngeal, or retroperitoneal is also at high risk of bleeding. Thrombocytopenia (ie, a platelet count <75 × 109 L−1) is associated with elevated bleeding risk. 74 , 75 The best management of a patient who requires anticoagulation during periods of severe thrombocytopenia, especially someone for whom the thrombocytopenia will last longer than a few days, such as a patient with cancer undergoing chemotherapy, is uncertain. Both full‐dose anticoagulation with transfusion support and dose‐modified anticoagulation have been proposed. 76 , 77 , 78 , 79 , 80 Importantly, thrombocytopenia is not protective against recurrent VTE. 74 Admitting a thrombocytopenic patient to the hospital will help the medical team elucidate the reason for the patient's thrombocytopenia and decide on the best treatment strategy. A patient with cirrhosis or severe alcohol use disorder may be at higher risk of bleeding. Severe liver dysfunction is associated with coagulopathy, which increases the risk of anticoagulant medications and makes monitoring their effect challenging. Lastly, the risk of falling and injury should be considered, as trauma‐related bleeding is often more serious in an anticoagulated patient. Decisions regarding whether anticoagulation should be used, and which anticoagulant is best, will depend on each patient's unique situation.
Exploring the specific characteristics and risks for each patient will allow the clinician to decide on the optimal treatment approach. It is essential for a patient who is at high risk of bleeding to understand the risks and benefits of anticoagulation. For patients at high risk of bleeding, clinicians should use shared decision making to generate an individualized treatment plan.
2.5.2. Step 4: Select and start an anticoagulant
There are several choices for anticoagulation in PE and DVT patients. These include DOACs, parenteral agents like heparin and low molecular weight heparin (LMWH), and vitamin K antagonists (VKA) like warfarin. In a patient without contraindications, the panel recommends anticoagulation with a DOAC. 44 , 81 This is consistent with current guidelines and based on large randomized controlled trials comparing DOACs to standard treatment (usually a LMWH bridge to long‐term warfarin therapy). 82 , 83 , 84 , 85 , 86
There are currently 4 DOACs approved for the treatment of PE and DVT. Compared to warfarin, these medications affect only one part of the coagulation cascade, have a rapid onset of action, fixed dosing, and few drug and dietary interactions. Several studies have compared DOACs to standard therapy, 82 , 83 , 84 , 85 , 86 but no large study has compared individual DOACs to one another. Apixaban and rivaroxaban can be used as initial therapy and do not require initial parental anticoagulation, whereas dabigatran and edoxaban require a parenteral agent for the first 5–10 days prior to initiation. In terms of parental agents, LMWH or fondaparinux are the 2 choices for initial treatment before starting dabigatran or edoxaban.
Although DOACs are preferred for most patients, certain patient populations may not be good candidates for these medications. The clinical decision frameworks, therefore, enumerate patient populations in whom DOAC therapy should be avoided in favor of other anticoagulation strategies (eg., LMWH with or without warfarin). In general, DOACs are not recommended in patients with severe renal impairment as few such patients were included in trials. Similarly, few patients with severe obesity were included in DOAC clinical trials and thus DOACs should be avoided in those who weigh >120 kg or have a body mass index >40. In these patients, intravenous heparin and warfarin, which can be more easily monitored, may be necessary. Patients with liver impairment (Child‐Pugh Class B and C) should also avoid DOACs. For patients with active cancer, apixaban, edoxaban, and rivaroxaban appear to provide similar protection against recurrent VTE as standard therapy (LMWH). 84 However, the literature regarding bleeding risk associated with DOAC therapy in patients with cancer is mixed. Some studies have found DOACs to be associated with an increased rate of major bleeding, especially among patients with gastrointestinal malignancies, 87 , 88 whereas other studies have found no increase in the rate of major bleeding in cancer patients. 89 , 90 The panel felt that, overall, DOAC therapy is reasonable for most patients with cancer, though some patients, such as those with gastrointestinal malignancies, may be better managed with LMWH. LMWH is also the recommended treatment for VTE in pregnancy. 91
For those patients in whom a DOAC is contraindicated, a subcutaneous LMWH such as enoxaparin or dalteparin may be used. In those that may bridge to an oral agent, warfarin may be started concurrently, and therapeutic levels need to be monitored. There are relatively few contraindications to LMWH. These include a history of heparin‐inducted thrombocytopenia (HIT), heparin‐induced thrombocytopenia with thrombosis (HITT), and other sensitivity to heparin or pork products. LMWH dosages need to be adjusted for renal function. Patients who cannot perform daily subcutaneous injections are also not candidates for LMWH. Warfarin has a narrow threshold of toxicity and levels are antagonized by dietary factors high in vitamin K. Warfarin is contraindicated during pregnancy and with coagulation defects such as decompensated liver disease. Furthermore, warfarin should not be used as the initial therapy in patients with HIT or HITT.
2.5.3. Step 5: Ensure follow‐up and provide education to the patient
Safe outpatient anticoagulation therapy starts by ensuring that a patient has a good home support system, the ability to fill prescriptions (eg., financial, transportation), the ability to follow up in an outpatient clinic, and no history of or concern for non‐adherence to treatment. One survey of emergency physicians found that lack of clear follow‐up was the single biggest barrier to outpatient PE treatment, 23 and successful outpatient PE treatment programs described in the literature ensured follow‐up with an appropriate clinician after ED discharge. 19 , 26 , 92 A patient who is unlikely to follow up after a PE or DVT is not a good candidate for ED discharge.
At the time of discharge from the ED, it is essential that the patient receives written and verbal education about their anticoagulation therapy, including potential side effects like bleeding, drug‐drug interactions, and drug‐food interactions. For example, the patient should take rivaroxaban with food, and a patient taking warfarin should avoid foods rich in vitamin K. The patient should also be informed of the importance of adherence to treatment, how to identify signs and symptoms of new or worsening PE or DVT, instructions of when to contact their primary‐care clinician or return to the ED, and detailed information about their follow‐up plan. When possible, the panel felt that follow‐up should occur during the first week after discharge. 93 , 94 Patients should also be instructed to inform all of their healthcare practitioner that they are now taking an anticoagulant and to seek guidance from their primary care clinician if their anticoagulant needs to be held for any medical, surgical, or dental procedures.
Patient follow‐up is imperative to ensure treatment compliance, evaluate any ongoing or new symptoms, and give the patient the opportunity to ask questions about their disease and its treatment. 95 The timing of follow‐up may vary depending on the treatment (DOAC vs. LMWH/VKA) as well as the needs and resources of the institution. If discharged on a DOAC, the patient should have timely follow‐up with their primary care clinician or specialist (eg, a hematologist). Apixaban and rivaroxaban require a dose change after 7 and 21 days of initial therapy, respectively. Whenever possible, the panel suggested that follow‐up occur before the dose change (eg, 7–10 days), to minimize the chance of medication errors occurring during this critical transition. A patient who is initially treated with a parenteral anticoagulant for whom dabigatran or edoxaban has been prescribed should schedule a follow‐up appointment at the time of DOAC initiation (eg, after 5 days of treatment with the parenteral anticoagulant). If discharged on a VKA, the patient should have timely follow‐up with a primary care clinician or an appropriate specialist as well as an appointment at a reasonable interval (typically, 2–3 days after discharge) to have their prothrombin time and international normalized ratio checked.
During off hours, it may be challenging to coordinate the follow‐up care necessary and, therefore, clinicians may need to admit the patient to an observation unit to allow time to address medication and follow‐up issues. If available, case management can help with setting up a follow‐up appointment and help address insurance and financial issues. 19
An important part of the outpatient treatment of DVT and PE is educating the patient about issues they might have while on anticoagulation. It is important to provide counseling on predictable effects like bleeding and bruising. Informing patients on how to reduce risk of bleeding by using a soft toothbrush, shaving with an electric razor, using caution when handling sharp objects, using appropriate safety equipment, avoiding aspirin and other antiplatelet agents, and avoiding contact sports or activities that carry a high risk of falling or injury. The patient should be advised to call their primary‐care clinician for relatively minor bleeding such as heavy menstrual periods, epistaxis, oral bleeding, or hematuria. The patient should be instructed to return to the ED immediately if they vomit blood, cough up blood, develop bleeding from their rectum, sudden severe back pain, severe epistaxis that does not stop quickly despite pressure, if they fall or suffer an injury to the head, or develop a severe or usual headache or neurologic symptoms like slurred speech, arm weakness, or facial drooping.
Instructing patients about the signs and symptoms of worsening or recurrent DVT and PE is equally important. The patient should be advised to call their primary‐care clinician or specialist if they develop pain, swelling and redness in their leg or arm. The patient should be advised to return to ED if they develop worsening or new chest pain, shortness of breath, fast heartbeat, sweating, lightheadedness, or fainting.
Lastly, a patient may also need to return to the ED if they run out of their anticoagulant medication and cannot get their prescription refilled through their primary‐care clinician or anticoagulant clinic.
2.6. Pearls and pitfalls
The items included as pearls and pitfalls were identified concurrently with the construction of the preceding sections and fall into the following categories: (1) patient‐focused items identified as important, but not included in other sections; (2) items identified by the team as requiring additional emphasis; and (3) items that address expected barriers to successful transition of care and outpatient treatment of a patient with low‐risk PE or DVT. They reflect the collective clinical experience of the panel with respect to the outpatient treatment of low‐risk PE and DVT, and include the following:
Systematic, interdepartmental protocols that support outpatient treatment will increase the likelihood of sustained success. Multidisciplinary engagement in the development and running of an outpatient PE and DVT treatment program can facilitate the safe and reliable transition of care to the outpatient setting.
Reliable access to anticoagulant medications is essential for outpatient treatment.
Giving the first dose of anticoagulant in the ED is recommended and provides the patient with time to fill their outpatient prescriptions.
Programs that provide medications to a patient before they leave the ED (sometimes called “med to bed” programs) can ensure that a patient has access to the prescribed anticoagulant upon discharge. Although this recommendation was derived based primarily on the collective experience of the panel, there is published support for this approach. 96
Follow‐up must be coordinated before the patient is discharged.
2.7. Web‐based resource for the application of the clinical decision framework
To facilitate access to the clinical decision framework described here, the panel assisted in the development of 2 applications, available online as part of ACEP's suite of Point‐of‐Care (POC) Tools: https://www.acep.org/patient‐care/point‐of‐care‐tools/. The PE tool is available at PE: https://www.acep.org/patient‐care/low‐risk‐pe/ and the DVT tool is available at DVT: https://www.acep.org/patient‐care/low‐risk‐dvt/.
The panel considered several factors when formulating the clinical decision framework that informed the POC Tools. First, ACEP's POC Tools are intended for use by practicing emergency clinicians with a range of experience and training, both in general and with managing PE and DVT. Further, they are designed for application across the spectrum of emergency care settings within the United States. They are not designed to be comprehensive resources for current knowledge about PE and DVT, but rather brief, pragmatic references for use in the clinical setting. Each section of the POC Tools can be accessed independently, though the POC Tool can also be navigated from start to finish, guiding the emergency clinician through all aspects of outpatient PE and DVT management.
3. CONCLUSIONS
The outpatient treatment of DVT and PE is safe and may be applicable to as many as one third of ED patients diagnosed with PE and two thirds of ED patients diagnosed with DVT. 19 , 21 , 37 , 39 In order to aid emergency clinicians in managing low‐risk patients with PE and DVT without hospitalization, an expert panel convened, provided recommendations, and aided in the creation of 2 new ACEP POC Tools. These clinical decision frameworks can help emergency physicians risk stratify a patient's PE or DVT as well as their bleeding risk, select an anticoagulant, and arrange timely follow‐up. They also help emergency clinicians determine when admission is appropriate. Given current issues related to ED and hospital overcrowding, the ability to safely discharge patients can improve patient care, reduce costs, and improve the patient care experience. ACEP's POC Tools have the potential to help thousands of ED patients receive safe, timely outpatient PE and DVT care.
CONFLICTS OF INTEREST
CK: Grants from Janssen, Diagnostica Stago, Siemens Healthcare Diagnostics, Grifols; Consulting: Boston Scientific. DRV: Grants from Janssen. AMM: Grants from Janssen. RPR: Grants from Janssen and BMS; Consulting/Advisory Board: Janssen, BMS, Dova, Inari. AMC: grants from Janssen, Diagnostica Stago, Siemens (to institution). JH‐N: Grants from Janssen, National Institutes of Health/National Heart, Lung, and Blood Institute. K12HL 133310‐01. SJW: Grants from Janssen.
ACKNOWLEDGEMENTS
The creation of the point‐of‐care tools was funded by a grant to the American College of Emergency Physicians from Janssen Pharmaceuticals.
Kabrhel C, Vinson DR, Mitchell AM, et al. A clinical decision framework to guide the outpatient treatment of emergency department patients diagnosed with acute pulmonary embolism or deep vein thrombosis: Results from a multidisciplinary consensus panel. JACEP Open. 2021;2:e12588. 10.1002/emp2.12588
Supervising Editor: Henry Wang, MD, MS.
REFERENCES
- 1. Barnes GD, Gafoor S, Wakefield T, Upchurch GR Jr, Henke P, Froehlich JB. National trends in venous disease. J Vasc Surg. 2010;51(6):1467‐1473. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith SB, Geske JB, Kathuria P, et al. Analysis of national trends in admissions for pulmonary embolism. Chest. 2016;150(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jimenez S, Ruiz‐Artacho P, Merlo M, et al. Risk profile, management, and outcomes of patients with venous thromboembolism attended in Spanish emergency departments: the ESPHERIA registry. Medicine (Baltimore). 2017;96(48):e8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30‐month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384‐393. [DOI] [PubMed] [Google Scholar]
- 6. Singer AJ, Thode HC Jr, WFt Peacock. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: a US perspective. Clin Exp Emerg Med. 2016;3(3):126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low‐risk patients with pulmonary embolism. J Intern Med. 2007;261(6):597‐604. [DOI] [PubMed] [Google Scholar]
- 8. Becattini C, Agnelli G. Risk stratification and management of acute pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2016;2016(1):404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kabrhel C, Okechukwu I, Hariharan P, et al. Factors associated with clinical deterioration shortly after PE. Thorax. 2014;69(9):835‐842. [DOI] [PubMed] [Google Scholar]
- 10. Weeda ER, Kohn CG, Peacock WF, et al. External validation of the hestia criteria for identifying acute pulmonary embolism patients at low risk of early mortality. Clin Appl Thromb Hemost. 2017;23(7):769‐774. [DOI] [PubMed] [Google Scholar]
- 11. Kovacs MJ, Anderson D, Morrow B, Gray L, Touchie D, Wells PS. Outpatient treatment of pulmonary embolism with dalteparin. Thromb Haemost. 2000;83(2):209‐211. [PubMed] [Google Scholar]
- 12. Wells PS. Outpatient treatment of patients with deep‐vein thrombosis or pulmonary embolism. Curr Opin Pulm Med. 2001;7(5):360‐364. [DOI] [PubMed] [Google Scholar]
- 13. Wells PS, Buller HR. Outpatient treatment of patients with pulmonary embolism. Semin Vasc Med. 2001;1(2):229‐234. [DOI] [PubMed] [Google Scholar]
- 14. Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open‐label, randomised, non‐inferiority trial. Lancet. 2011;378(9785):41‐48. [DOI] [PubMed] [Google Scholar]
- 15. Singer AJ, Xiang J, Kabrhel C, et al. Multicenter Trial of Rivaroxaban for Early Discharge of Pulmonary Embolism From the Emergency Department (MERCURY PE): rationale and design. Acad Emerg Med. 2016;23(11):1280‐1286. [DOI] [PubMed] [Google Scholar]
- 16. Kline JA, Adler DH, Alanis N, et al. Monotherapy anticoagulation to expedite home treatment of patients diagnosed with venous thromboembolism in the emergency department: a pragmatic effectiveness trial. Circ Cardiovasc Qual Outcomes. 2021;14(7):e007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barco S, Schmidtmann I, Ageno W, et al. Survival and quality of life after early discharge in low‐risk pulmonary embolism. Eur Respir J. 2021;57(2). [DOI] [PubMed] [Google Scholar]
- 18. Bledsoe JR, Woller SC, Stevens SM, et al. Management of low‐risk pulmonary embolism patients without hospitalization: the low‐risk pulmonary embolism prospective management study. Chest. 2018;154(2):249‐256. [DOI] [PubMed] [Google Scholar]
- 19. Kabrhel C, Rosovsky R, Baugh C, et al. Multicenter implementation of a novel management protocol increases the outpatient treatment of pulmonary embolism and deep vein thrombosis. Acad Emerg Med. 2019;26(6):657‐669. [DOI] [PubMed] [Google Scholar]
- 20. Vinson DR, Ballard DW, Huang J, et al. Outpatient management of emergency department patients with acute pulmonary embolism: variation, patient characteristics, and outcomes. Ann Emerg Med. 2018;72(1):62‐72. e63. [DOI] [PubMed] [Google Scholar]
- 21. van der Wall SJ, Hendriks SV, Huisman MV, Klok FA. Home treatment of acute pulmonary embolism: state of the art in 2018. Curr Opin Pulm Med. 2018;24(5):425‐431. [DOI] [PubMed] [Google Scholar]
- 22. Agterof MJ, Schutgens RE, Snijder RJ, et al. Out of hospital treatment of acute pulmonary embolism in patients with a low NT‐proBNP level. J Thromb Haemost. 2010;8(6):1235‐1241. [DOI] [PubMed] [Google Scholar]
- 23. Kline JA, Kahler ZP, Beam DM. Outpatient treatment of low‐risk venous thromboembolism with monotherapy oral anticoagulation: patient quality of life outcomes and clinician acceptance. Patient Prefer Adher. 2016;10:561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik AH, Aronow WS. Safety, efficacy, length of stay and patient satisfaction with outpatient management of low‐risk pulmonary embolism patients—A meta‐analysis. Arch Med Sci. 2021;17(1):245‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Othieno R, Okpo E, Forster R. Home versus in‐patient treatment for deep vein thrombosis. Cochrane Database Syst Rev. 2018;1:Cd003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon LE, Iskin HR, Vemula R, et al. Emergency department patient satisfaction with treatment of low‐risk pulmonary embolism. West J Emerg Med. 2018;19(6):938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghazvinian R, Elf J, Lofvendahl S, Holst J, Gottsater A. Outpatient treatment in low‐risk pulmonary embolism patients receiving direct acting oral anticoagulants is associated with cost savings. Clin Appl Thromb Hemost. 2020;26:1076029620937352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bledsoe JR, Woller SC, Stevens SM, et al. Cost‐effectiveness of managing low‐risk pulmonary embolism patients without hospitalization. The low‐risk pulmonary embolism prospective management study. Am J Emerg Med. 2021;41:80‐83. [DOI] [PubMed] [Google Scholar]
- 29. Shan JID, Bath H, Johnson DJ, Julien D, Vinson DR. Outpatient management of pulmonary embolism defined in the primary literature: a narrative review. Perm J. 2021;25:20.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westafer LM, Shieh MS, Pekow PS, Stefan MS, Lindenauer PK. Outpatient management of patients following diagnosis of acute pulmonary embolism. Acad Emerg Med. 2021;28(3):336‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vinson DR, Mark DG, Ballard DW. Overcoming barriers to outpatient management of emergency department patients with acute pulmonary embolism. Acad Emerg Med. 2021;28(3):377‐378. [DOI] [PubMed] [Google Scholar]
- 32. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56(6):601‐610. [DOI] [PubMed] [Google Scholar]
- 33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician‐performed ultrasonography in the diagnosis of deep‐vein thrombosis: a systematic review and meta‐analysis. Thromb Haemost. 2013;109(1):137‐145. [DOI] [PubMed] [Google Scholar]
- 34. Karande GY, Hedgire SS, Sanchez Y, et al. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(6):493‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elias A, Mallett S, Daoud‐Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta‐analysis. BMJ Open. 2016;6(4):e010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zondag W, Mos IC, Creemers‐Schild D, et al. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9(8):1500‐1507. [DOI] [PubMed] [Google Scholar]
- 37. Peacock FW, Coleman CI, Diercks DB, et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018;25(9):995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinson DR, Mark DG, Chettipally UK, et al. Increasing safe outpatient management of emergency department patients with pulmonary embolism: a controlled pragmatic trial. Ann Intern Med. 2018;169(12):855‐865. [DOI] [PubMed] [Google Scholar]
- 40. Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383‐1389. [DOI] [PubMed] [Google Scholar]
- 41. Roy PM, Penaloza A, Hugli O, et al. Triaging acute pulmonary embolism for home treatment by Hestia or simplified PESI criteria: the HOME‐PE randomized trial. Eur Heart J. 2021;42(33):3146‐3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konstantinides SV. Home treatment of pulmonary embolism: are all the questions answered now after the HOME‐PE trial?. Cardiovasc Res. 2020;116(13):e179‐e181. [DOI] [PubMed] [Google Scholar]
- 43. Roy PM. Hestia rule versus simplified PESI for home treatment of patients with acute pulmonary embolism: a multinational randomised clinical trial (HOME‐PE). Eur Heart J. 2021. press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315‐352. [DOI] [PubMed] [Google Scholar]
- 45. Vinson DR, Engelhart DC, Bahl D, et al. Presyncope is associated with intensive care unit admission in emergency department patients with acute pulmonary embolism. West J Emerg Med. 2020;21(3):703‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Becattini C, Agnelli G, Vedovati MC, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J. 2011;32(13):1657‐1663. [DOI] [PubMed] [Google Scholar]
- 47. Meinel FG, Nance JW Jr, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta‐analysis. Am J Med. 2015;128(7):747‐759. e742. [DOI] [PubMed] [Google Scholar]
- 48. Hariharan P, Dudzinski DM, Rosovsky R, et al. Relation among clot burden, right‐sided heart strain, and adverse events after acute pulmonary embolism. Am J Cardiol. 2016;118(10):1568‐1573. [DOI] [PubMed] [Google Scholar]
- 49. Gouin B, Blondon M, Jimenez D, et al. Clinical prognosis of nonmassive central and noncentral pulmonary embolism: a registry‐based cohort study. Chest. 2017;151(4):829‐837. [DOI] [PubMed] [Google Scholar]
- 50. Méan M, Tritschler T, Limacher A, et al. Association between computed tomography obstruction index and mortality in elderly patients with acute pulmonary embolism: a prospective validation study. PLoS One. 2017;12(6):e0179224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pruszczyk P, Goliszek S, Lichodziejewska B, et al. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc Imaging. 2014;7(6):553‐560. [DOI] [PubMed] [Google Scholar]
- 52. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta‐analysis. BMC Cardiovasc Disord. 2014;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dudzinski DM, Hariharan P, Parry BA, Chang Y, Kabrhel C. Assessment of right ventricular strain by computed tomography versus echocardiography in acute pulmonary embolism. Acad Emerg Med. 2017;24(3):337‐343. [DOI] [PubMed] [Google Scholar]
- 54. Trujillo‐Santos J, den Exter PL, Gomez V, et al. Computed tomography‐assessed right ventricular dysfunction and risk stratification of patients with acute non‐massive pulmonary embolism: systematic review and meta‐analysis. J Thromb Haemost. 2013;11(10):1823‐1832. [DOI] [PubMed] [Google Scholar]
- 55. Garvey S, Dudzinski DM, Giordano N, Torrey J, Zheng H, Kabrhel C. Pulmonary embolism with clot in transit: an analysis of risk factors and outcomes. Thromb Res. 2020;187:139‐147. [DOI] [PubMed] [Google Scholar]
- 56. Barrios D, Rosa‐Salazar V, Jiménez D, et al. Right heart thrombi in pulmonary embolism. Eur Respir J. 2016;48(5):1377‐1385. [DOI] [PubMed] [Google Scholar]
- 57. Barrios D, Rosa‐Salazar V, Morillo R, et al. Prognostic significance of right heart thrombi in patients with acute symptomatic pulmonary embolism: systematic review and meta‐analysis. Chest. 2017;151(2):409‐416. [DOI] [PubMed] [Google Scholar]
- 58. Koć M, Kostrubiec M, Elikowski W, et al. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016;47(3):869‐875. [DOI] [PubMed] [Google Scholar]
- 59. El‐Menyar A, Sathian B, Al‐Thani H. Elevated serum cardiac troponin and mortality in acute pulmonary embolism: systematic review and meta‐analysis. Respir Med. 2019;157:26‐35. [DOI] [PubMed] [Google Scholar]
- 60. Becattini C, Maraziti G, Vinson DR, et al. Right ventricle assessment in patients with pulmonary embolism at low risk for death based on clinical models: an individual patient data meta‐analysis. Eur Heart J. 2021;42(33):3190‐3199. [DOI] [PubMed] [Google Scholar]
- 61. Lankeit M, Friesen D, Aschoff J, et al. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31(15):1836‐1844. [DOI] [PubMed] [Google Scholar]
- 62. Jimenez D, Aujesky D, Diaz G, et al. Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010;181(9):983‐991. [DOI] [PubMed] [Google Scholar]
- 63. Digby GC, Kukla P, Zhan ZQ, et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: a consensus paper. Ann Noninvasive Electrocardiol. 2015;20(3):207‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693‐4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39(47):4208‐4218. [DOI] [PubMed] [Google Scholar]
- 66. Rosa‐Salazar V, Trujillo‐Santos J, Díaz Peromingo JA, et al. A prognostic score to identify low‐risk outpatients with acute deep vein thrombosis in the upper extremity. J Thromb Haemost. 2015;13(7):1274‐1278. [DOI] [PubMed] [Google Scholar]
- 67. Liu D, Peterson E, Dooner J, et al. Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guideline. CMAJ. 2015;187(17):1288‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 69. Said A, Sahlieh A, Sayed L. A comparative analysis of the efficacy and safety of therapeutic interventions in phlegmasia cerulea dolens. Phlebology. 2021;36(5):392‐400. [DOI] [PubMed] [Google Scholar]
- 70. Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713‐719. [DOI] [PubMed] [Google Scholar]
- 72. Klok FA, Barco S, Konstantinides SV. External validation of the VTE‐BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb Haemost. 2017;117(6):1164‐1170. [DOI] [PubMed] [Google Scholar]
- 73. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093‐1100. [DOI] [PubMed] [Google Scholar]
- 74. Di Micco P, Ruiz‐Gimenez N, Nieto JA, et al. Platelet count and outcome in patients with acute venous thromboembolism. Thromb Haemost. 2013;110(5):1025‐1034. [DOI] [PubMed] [Google Scholar]
- 75. Nieto JA, Solano R, Ruiz‐Ribo MD, et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2010;8(6):1216‐1222. [DOI] [PubMed] [Google Scholar]
- 76. Lubberts B, Paulino Pereira NR, Kabrhel C, Kuter DJ, DiGiovanni CW. What is the effect of venous thromboembolism and related complications on patient reported health‐related quality of life? A meta‐analysis. Thromb Haemost. 2016;116(3):417‐431. [DOI] [PubMed] [Google Scholar]
- 77. Samuelson Bannow BR, Lee AYY, Khorana AA, et al. Management of anticoagulation for cancer‐associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2(4):664‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer‐associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(6):1246‐1249. [DOI] [PubMed] [Google Scholar]
- 79. Streiff MB, Holmstrom B, Angelini D, et al. NCCN guidelines insights: cancer‐associated venous thromboembolic disease, version 2.2018. J Natl Compr Canc Netw. 2018;16(11):1289‐1303. [DOI] [PubMed] [Google Scholar]
- 80. Tufano A, Guida A, Di Minno MN, Prisco D, Cerbone AM, Di Minno G. Prevention of venous thromboembolism in medical patients with thrombocytopenia or with platelet dysfunction: a review of the literature. Semin Thromb Hemost. 2011;37(3):267‐274. [DOI] [PubMed] [Google Scholar]
- 81. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Respir J. 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
- 82. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799‐808. [DOI] [PubMed] [Google Scholar]
- 83. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499‐2510. [DOI] [PubMed] [Google Scholar]
- 84. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378(7):615‐624. [DOI] [PubMed] [Google Scholar]
- 85. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus Warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342‐2352. [DOI] [PubMed] [Google Scholar]
- 86. Edoxaban versus Warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406‐1415. [DOI] [PubMed] [Google Scholar]
- 87. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2017;378(7):615‐624. [DOI] [PubMed] [Google Scholar]
- 88. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36(20):2017‐2023. [DOI] [PubMed] [Google Scholar]
- 89. McBane RD 2nd, Wysokinski WE, Le‐Rademacher JG, et al. Apixaban and dalteparin in active malignancy‐associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411‐421. [DOI] [PubMed] [Google Scholar]
- 90. Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599‐1607. [DOI] [PubMed] [Google Scholar]
- 91. Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317‐3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kabrhel C, Rosovsky R, Baugh C, et al. The creation and implementation of an outpatient pulmonary embolism treatment protocol. Hosp Pract. 2017;45(3):123‐129. (1995). [DOI] [PubMed] [Google Scholar]
- 93. Peacock WF, Singer AJ. Reducing the hospital burden associated with the treatment of pulmonary embolism. J Thromb Haemost. 2019;17(5):720‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vinson DR, Ballard DW, Huang J, et al. Timing of discharge follow‐up for acute pulmonary embolism: retrospective cohort study. West J Emerg Med. 2015;16(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Condliffe R. Pathways for outpatient management of venous thromboembolism in a UK centre. Thromb J. 2016;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hayes BD, Zaharna L, Winters ME, Feemster AA, Browne BJ, Hirshon JM. To‐Go medications for decreasing ED return visits. Am J Emerg Med. 2012;30(9):2011‐2014. [DOI] [PubMed] [Google Scholar]


