Abstract
A European multicenter study of immunoblotting for the serodiagnosis of Lyme borreliosis showed considerable variation in results obtained from tests with a panel of 227 serum samples. Six laboratories used different immunoblot methods, and a wide range of bands was detected in all the assays. Multivariable logistic regression analysis of data from individual laboratories was used to determine the most discriminatory bands for reliable detection of antibodies to Borrelia burgdorferi sensu lato. These bands were used to construct individual interpretation rules for the immunoblots used in the six laboratories. Further analysis identified a subset of eight bands, which were important in all the laboratories, although with variations in significance. Possible European rules, all closely related, were formulated from these bands, although there was no single rule that gave high levels of sensitivity and specificity for all the laboratories. This is a reflection of the wide range of methodologies used, especially the use of different species and strains of B. burgdorferi sensu lato. The panel of European rules provides a framework for immunoblot interpretation which may be adapted in relation to the characteristics of Lyme borreliosis in local areas.
The clinical diagnosis of Lyme borreliosis (LB) can be difficult because symptoms, other than a typical erythema migrans (EM) of early infection, may be of a nonspecific nature. In addition, the interpretation of laboratory diagnostic test results has been problematic because of wide variation in the sensitivities and specificities of the tests used.
Immunoblotting is both sensitive and specific, has been in wide use in diagnostic laboratories, and in the United States has been recommended as a confirmatory test for the serodiagnosis of Lyme disease (5). However, in Europe an extensive range of blotting methodologies is in use (antigens prepared from different genospecies of Borrelia burgdorferi sensu lato, different polyacrylamide gel electrophoresis [PAGE] and immunoblotting protocols), and although recommendations on the interpretation of band patterns have been published in Europe and the United States (5, 8, 10, 11, 15, 17, 18, 23, 25, 28, 34), no consensus exists.
As part of the European Union Concerted Action on Lyme Borreliosis (EUCALB) program from 1994 to 1996, clinicians and scientists in several European countries participated in a multicenter immunoblotting study. The aims of the study were to identify criteria for the interpretation of immunoblots for individual participating laboratories, to assess the sensitivity and specificity of European immunoblot methods for the diagnosis of LB, and to determine, if possible, a set of criteria for the interpretation of immunoblots which could be used in diagnostic laboratories across Europe.
(Preliminary accounts of this work were presented at the Fifth International Potsdam Symposium on Tick-Borne Diseases, Berlin, Germany, 26–27 February 1999, and at the Eighth International Conference on Lyme Borreliosis and Other Emerging Tick-Borne Diseases, Munich, Germany, 20–24 June 1999.)
MATERIALS AND METHODS
Collection and validation of serum samples.
Sera from patients with typical LB were contributed to the study by expert clinicians in eight countries: Austria, Germany, Hungary, Italy, Portugal, Sweden, Switzerland, and the United Kingdom (Table 1). Questionnaires that supplied clinical details were returned with the sera and were used to classify samples into two groups: those from patients with EM (n = 45) or those from patients with other manifestations of LB with or without EM (LB; n = 52). The cases were not culture confirmed but satisfied the EUCALB European case definition (29) for EM and other manifestations of LB (neuroborreliosis, arthritis, lymphocytoma, and acrodermatitis chronica atrophicans). Potentially cross-reacting sera (CR; n = 40) included sera from patients with Epstein-Barr virus infection and sera from patients with other spirochetal infections such as syphilis and leptospirosis. Since the study was designed to identify important immunoblotting bands for the diagnosis of LB in Europe and was not designed for epidemiological purposes, sera from healthy individuals (the negative control group [NE]; n = 90) were mainly contributed from individuals from a country with a low incidence of LB. In total, 227 serum samples were collected. Control sera positive for immunoglobulin G (IgG) and IgM antibodies were supplied by a reference laboratory in Germany. In order to help with the identification of any samples with discrepant results, the serum panel was tested for antibody to B. burgdorferi sensu lato by enzyme immunoassay (EIA) at a reference laboratory in Sweden by using commercial kits specific for IgG and IgM (Dako).
TABLE 1.
Sources of sera submitted to the study
| Country | No. of serum samples from the following study groups:
|
||||
|---|---|---|---|---|---|
| EM | LB | CR | NE | Total | |
| Austria | 10 | 15 | 0 | 0 | 25 |
| Germany | 3 | 7 | 8 | 5 | 23 |
| Hungary | 18 | 16 | 5 | 1 | 40 |
| Italy | 5 | 5 | 10 | 5 | 25 |
| Portugal | 0 | 2 | 9 | 0 | 11 |
| Russia | 0 | 0 | 1 | 1 | 2 |
| Sweden | 4 | 3 | 5 | 4 | 16 |
| Switzerland | 2 | 1 | 1 | 2 | 6 |
| United Kingdom | 3 | 3 | 1 | 72 | 79 |
| All countries | 45 | 52 | 40 | 90 | 227 |
Participating laboratories.
Six European laboratories (in Germany, Italy, Spain, Switzerland, The Netherlands, and the United Kingdom) with extensive experience in the use of immunoblotting for the diagnosis of LB participated in the study. The laboratories were randomly coded A to F.
Laboratory methods.
A wide range of immunoblotting methodologies was in use in the participating laboratories, with variations in the choice of the B. burgdorferi sensu lato genospecies for use as antigen (Table 2) and the use of different protocols for PAGE, protein transfer, blocking of nonspecific binding sites, and processing of patients' samples. In order to assist with subsequent band identification, all laboratories submitted unstained strips of B. burgdorferi sensu lato antigen to a reference laboratory in Germany for calibration with a panel of monoclonal antibodies (15) prior to testing the panel of 227 serum samples. The contributed sera were sent to a reference laboratory in the United Kingdom, where they were aliquoted and distributed by courier to the participating laboratories. All samples were immunoblotted for IgM and IgG antibody by the participants' own methodology, and all observed bands were recorded on a strength scale of 1 to 4. Participants were not required to interpret their blots.
TABLE 2.
Genospecies and strains of B. burgdorferi sensu lato used for immunoblotting in participating laboratories
| Laboratory | Genospecies | Strain | Immunoglobulin class |
|---|---|---|---|
| A | B. afzelii | ACA1 | IgM, IgG |
| B | B. burgdorferi sensu stricto | ESP1 | IgM, IgG |
| C | B. afzelii | A395 | IgM |
| B. burgdorferi sensu stricto | B31 | IgG | |
| D | B. afzelii | PKo | IgM, IgG |
| B. garinii | PBi | IgM, IgG | |
| E | B. afzelii | VS461 | IgM, IgG |
| F | B. garinii | Ne83 | IgM, IgG |
Statistical analysis.
Frequency plots were used to visualize IgG and IgM bands in all sample groups reported from all laboratories and also to determine the feasibility of analyzing data for EM and LB cases together. This approach had two advantages: the possibility of simpler rules when the bands from the IgG- and IgM-specific assays were combined and also the requirement for a greater number of bands for a positive blot result without a loss of sensitivity. Backward stepwise logistic regression analysis based on Mallow's Cp statistic (31) was used to determine which bands best independently discriminated between sera with B. burgdorferi sensu lato antibody (EM and LB) and those without (CR and NE). Rules were constructed from the subset of independently significant bands, and their sensitivities and specificities were assessed. The “best” rules were found under the constraint of requiring values for sensitivity and specificity close to 70 and 90%, respectively (in practice, laboratories in different countries may use other values, depending on the LB incidence). To check that the levels of sensitivity and specificity obtained were not excessively biased as a result of testing of the rules with the data used to construct them, a separate analysis was performed in which rules constructed with one-half of the data were used to test the other half. These results are not given but suggested that any bias was small. The statistical analysis was performed by using the package S-PLUS.
RESULTS
Calibration of antigen strips by monoclonal antibody testing.
The antigen strips from all laboratories reacted with monoclonal antibodies specific for seven bands (p83/100, p41, and p39, OspA, p30, OspC, and p19). However, for OspC, B. afzelii antigen from laboratory E reacted only with rabbit immune serum against recombinant antigen (Table 3).
TABLE 3.
Monoclonal antibody characterization of B. burgdorferi sensu lato strains used for immunoblotting
| Laboratory | Strain | Genospecies | Reactivity of the following monoclonal antibody (identified antigen):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L100 22G3 (p83/100) | L41 1C11 (p41) | L39 B1 (p39) | L32 1F11 (OspA) | L30 1B10 (p30) | L22 1F8 (OspC) | L22 2B8 (OspC) | Anti-rOspCa (OspC) | L19 A11 (p19) | |||
| A | ACA1 | B. afzelii | ++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ |
| B | ESP1 | B. burgdorferi sensu stricto | ++ | +++ | +++ | +++ | ++ | − | +++ | +++ | +++ |
| C | B31 | B. burgdorferi sensu stricto | − | + | + | +++ | + | − | + | + | +++ |
| C | A395 | B. afzelii | + | + | ++ | +++ | + | + | − | ++ | +++ |
| D | PKo | B. afzelii | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ |
| D | PBi | B. garinii | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ |
| E | VS461 | B. afzelii | ++ | +++ | ++ | +++ | Trace | Trace | Trace | ++ | ++ |
| F | Ne83 | B. garinii | +++ | +++ | + | ++ | + | − | ++ | +++ | ++ |
Rabbit immune serum against recombinant OspC derived from B. afzelii strain PKo (31).
Immunoblotting results.
A wide range of band positions from 12 to 100 kDa was reported by all laboratories. All recognized IgG bands at p83/100, p60, p58, p41, and p39 and OspA, and most reported OspC and p17 bands. All reported IgM bands at 41 and 39 kDa but IgM OspC bands were reported by only five of the six laboratories. In two laboratories a few bands that could not be precisely identified were reported as having a small range of molecular masses.
Constructing interpretation rules for individual laboratories.
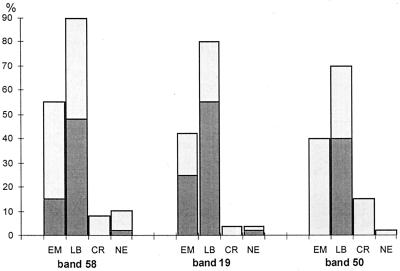
The frequency plots (see Fig. 1) showed that it was feasible to combine bands from both groups of patients (EM and LB) and both immunoglobulin classes for the purposes of analysis. Backward stepwise logistic regression analysis of bands was used to formulate decision rules. An example rule for IgG blots in one laboratory with a rule of three bands (p58, p19, and p50) is given in Table 4.
FIG. 1.
Example frequency plot for IgG bands in one laboratory.  , strong bands;
, strong bands;  , weak bands.
, weak bands.
TABLE 4.
Example of a three-band rule (bands p58, p19, and weak p50) applied to IgG blots in one laboratorya
| No. of bands present | % Positive blots in the following study group:
|
|||
|---|---|---|---|---|
| EM | LB | CR | NE | |
| 0 | 25 | 8 | 82 | 87 |
| 1 | 32 | 11 | 13 | 12 |
| 2 | 15 | 19 | 5 | 1 |
| 3 | 28 | 62 | 0 | 0 |
If none of the three bands is present, the serum sample is more likely to be from a patient without LB than from a patient with LB but may be from a patient with EM. If one band is present, the sample is more likely to be from a patient with early LB. If two bands are present, the sample is much more likely to be from a patient with LB but may be from a patient with early or late infection. If all three bands are present the sample is almost certainly from a patient with LB and is more likely to be from a patient with a later LB infection than from a patient with EM.
The effects of all proposed rules on sensitivity and specificity were tested, and an example of the effects of three IgG rules for the blot used by one laboratory is shown in Table 5: rule 1 is the least stringent and gives the highest sensitivity; rule 3 is the most stringent and gives the highest specificity. This process was repeated for all laboratories, and a summary of the 19 most discriminatory bands is shown in Table 6. For IgM blots, OspC was an important band for the majority of laboratories. Important IgG bands showed more variability, reflecting the range of blotting methodologies and strains used; however, some consistencies were apparent. Band p58 appeared important in all laboratories using B. afzelii as antigen (except laboratory C, which uses B. afzelii for IgM only), and four of six laboratories found OspC important for IgG. From these bands and others, individual “best” rules (as defined in the Statistical Analysis section in Materials and Methods) were formulated for the six laboratories.
TABLE 5.
Example effect of three different IgG rules on percent sensitivity (patients with EM and LB) and specificity (CR and NE groups)
| Rule | Band(s) required | % Sensitivity for patients with EM or LB | % Specificity
|
||
|---|---|---|---|---|---|
| CR | NE | CR and NE | |||
| 1 | p58 or p19 | 93 | 93 | 89 | 90 |
| 2 | p58 only | 91 | 93 | 90 | 91 |
| 3 | Two bands from p58, p19, p50, and p56 | 89 | 88 | 99 | 95 |
TABLE 6.
Summary of the most discriminatory immunoblot bands identified in participating laboratories
| Laboratory | Genospecies | Strain | Discriminatory bandsa
|
|
|---|---|---|---|---|
| IgG | IgM | |||
| A | B. afzelii | ACA1 | p58, p19, p50, p56 | OspC, p41 |
| B | B. burgdorferi sensu stricto | ESP1 | p39, OspC, p21, p17 | OspC, p41 |
| C | B. burgdorferi sensu stricto | B31 | p34, p41, p21 | |
| B. afzelii | A395 | OspC | ||
| D | B. afzelii | PKo | p58, p43, OspC, p14, p41 | OspC |
| B. garinii | PBi | p100, p30, OspC, p41, p21 | OspC | |
| E | B. afzelii | VS461 | p93, p60, p58, p41, p39, p28 | p60, p41 |
| F | B. garinii | Ne83 | p54/55, p43, p41, OspC | p41, OspC |
Listed in order of significance.
EIA results.
The Dako EIA results for IgG and IgM were calculated together (for comparison with the performance of the immunoblot assays) and overall gave 84% sensitivity and 87% specificity. There was complete concordance between the EIA and all seven blot assays, using the best rules for individual laboratories, for 128 samples from the panel of 227 serum samples. Among the samples that showed some discrepancy, the IgG EIA was positive for 11 samples (NE, n = 3; CR, n = 8) found to be negative by all the blot assays. The IgM EIA was negative for 14 of 18 serum samples from patients with EM that were found to be positive by more than two blot assays, and the IgG EIA was negative for 2 serum samples from patients with later Lyme disease that were positive by at least five of seven blot assays. Two serum samples from patients with EM were found to be negative by EIA and all the blot assays. Three samples that were probably misclassified included one from a patient with Lyme arthritis found to be negative on all the blots and by EIA, one submitted from a healthy individual but found to be IgG positive by all the blot assays and by EIA, and one from a patient with arthralgia submitted as a potential cross-reactor but found to be positive by the IgG EIA and six of seven blots.
European rules.
Multivariable logistic regression was used to assess the importance of the commonly reported bands in each laboratory. After discarding bands which were of no significance in any immunoblot, there remained a subset of eight bands with various levels of significance in each laboratory (Table 7). The most important of these were OspC and p41 for IgM blots and p83/100 and p58 for IgG. A set of seven, closely related, possible European rules were constructed from the subset bands (Table 8), and these rules were applied to the data from each laboratory in order to determine whether any one rule could generate satisfactory specificities and sensitivities for all laboratories (Table 9). Table 9 illustrates that the application of these closely related rules generates similar specificities and sensitivities in some laboratories. No single rule gave high sensitivities and specificities in all laboratories, and on that basis, rules 1 and 3 were discarded as not being useful. Of the remaining five rules, the best one varied by laboratory, although at least one rule in each laboratory gave sensitivities and specificities that were almost as good as those that resulted from the use of individual laboratories' rules (Table 10).
TABLE 7.
Significance of a subset of eight discriminatory immunoblot bands in multivariable analysis
| Band | Significance for the following laboratoriesa:
|
||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D (B. afzelii) | D (B. garinii) | E | F | |
| p83/100 | **** | * | **** | *** | ** | ||
| p58 | **** | ** | *** | * | **** | ||
| p41 | ** | ** | * | * | *** | ||
| p39 | **** | * | * | ** | ** | ||
| OspC | ** | ** | |||||
| p17 | * | * | **** | ** | |||
| IgM p41 | **** | ** | *** | **** | *** | ||
| IgM OspC | **** | *** | **** | **** | **** | NA | ** |
Symbols and abbreviations: ∗, P value, <0.2; ∗∗, P value <0.05; ∗∗∗, P value <0.01; ∗∗∗∗, P value <0.001; NA; not applicable. P values were determined by chi-square test (one degree of freedom). OspC bands were not observed in laboratory E.
TABLE 8.
Possible European rules formulated from eight immunoblot bands important in all the participating laboratories
| Rule no. | No. of bands required | Antigensa |
|---|---|---|
| R1 | 2 or 3 | p41 (M), OspC (M), p83/100, p58 |
| R2 | 2 or 3 | p41 (M), OspC (M), p83/100, p58, p39 |
| R3 | 2 or 3 | p41 (M), OspC (M), p83/100, p39, p41 |
| R4 | 2 or 3 | p41 (M), OspC (M), p83/100, p39, p17 |
| R5 | 2 or 3 | p41 (M), OspC (M), p83/100, p39, p41, p17 |
| R6 | 2 or 3 | p41 (M), OspC (M), p83/100, p39, p17, OspC |
| R7 | 2, 3, or 4 | p41 (M), OspC (M), p83/100, p39, p41, p17, OspC |
M, IgM.
TABLE 9.
Illustrative effect of European immunoblot interpretation rules on percent sensitivity and specificitya
| Ruleb | No. of bands | Average
|
Laboratory A
|
Laboratory B
|
Laboratory C
|
Laboratory D (B. afzelii)
|
Laboratory D (B. garinii)
|
Laboratory E (no IgM OspC)
|
Laboratory F
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Sensc | % Specd | % Sens | % Spec | % Sens | % Spec | % Sens | % Spec | % Sens | % Spec | % Sens | % Spec | % Sens | % Spec | % Sens | % Spec | ||
| 1 | 2 | 68 | 91 | 85 | 92 | 59 | 95 | 73 | 78 | 55 | 99 | 53 | 99 | 81 | 91 | 73 | 80 |
| 3 | 29 | 100 | 45 | 100 | 25 | 100 | 33 | 99 | 19 | 100 | 11 | 100 | 34 | 99 | 38 | 99 | |
| 2 | 2 | 75 | 89 | 85 | 92 | 81 | 94 | 73 | 78 | 61 | 98 | 57 | 99 | 88 | 85 | 82 | 79 |
| 3 | 49 | 98 | 54 | 99 | 53 | 99 | 41 | 99 | 43 | 99 | 37 | 100 | 64 | 97 | 50 | 97 | |
| 3 | 2 | 87 | 72 | 92 | 73 | 87 | 88 | 96 | 44 | 75 | 92 | 72 | 89 | 95 | 70 | 95 | 47 |
| 3 | 69 | 92 | 76 | 95 | 66 | 98 | 71 | 82 | 57 | 99 | 50 | 99 | 84 | 91 | 81 | 80 | |
| 4 | 2 | 77 | 88 | 86 | 89 | 81 | 94 | 74 | 76 | 67 | 97 | 57 | 99 | 89 | 84 | 82 | 79 |
| 3 | 56 | 98 | 66 | 99 | 56 | 99 | 47 | 99 | 54 | 99 | 40 | 100 | 67 | 97 | 56 | 97 | |
| 5 | 2 | 87 | 72 | 93 | 71 | 88 | 88 | 96 | 46 | 80 | 92 | 74 | 89 | 95 | 69 | 95 | 47 |
| 3 | 71 | 92 | 80 | 94 | 67 | 98 | 73 | 81 | 62 | 98 | 51 | 99 | 85 | 91 | 81 | 80 | |
| 6 | 2 | 79 | 86 | 86 | 87 | 85 | 92 | 78 | 76 | 68 | 97 | 57 | 99 | 89 | 84 | 88 | 67 |
| 3 | 62 | 96 | 73 | 95 | 60 | 99 | 56 | 99 | 56 | 99 | 42 | 100 | 68 | 96 | 78 | 87 | |
| 7 | 2 | 90 | 70 | 93 | 69 | 91 | 86 | 96 | 44 | 83 | 92 | 74 | 89 | 95 | 69 | 97 | 39 |
| 3 | 74 | 90 | 82 | 92 | 69 | 98 | 77 | 81 | 62 | 98 | 57 | 99 | 85 | 91 | 88 | 69 | |
| 4 | 58 | 97 | 69 | 97 | 49 | 99 | 55 | 99 | 54 | 99 | 38 | 100 | 66 | 97 | 77 | 87 | |
Examples of the “best” qualifying rules (70% sensitivity and 90% specificity) for each laboratory are given in boldface italic type.
Rules 1 and 3 were eliminated because they did not provide an adequate combination of sensitivity and specificity for all laboratories.
Sens, sensitivity was calculated by combining data for IgG and IgM.
Spec, specificity was calculated by combining data for potential cross-reactor and healthy individuals' sera.
TABLE 10.
Comparison of “best” European immunoblot interpretation rule with best rule for individual laboratories
| Laboratory | Strain | European rule no. | European rule | % Sens/% spec for European rulea | Individual laboratory rule | % Sens/% spec for individual laboratory rulea |
|---|---|---|---|---|---|---|
| A | ACA1 | 5 | 3 bands from p41 (M)b, OspC (M), p83/100, p39, p41, p17 | 80/94 | 19 alone or 2 bands from p58, OspC (M), p41 (M) | 80/92 |
| B | ESP1 | 2 | 2 bands from p41 (M), OspC (M), p83/100, p58, p39 | 81/94 | 2 bands from p39, OspC, OspC (M), p21 | 85/98 |
| C | A395 | 6 | 3 bands fsrom p41 (M), OspC (M), p83/100, p39, p17, OspC | 56/99 | 2 bands from p34, OspC(M), p21, p41 | 73/97 |
| D | PKo | 7 | 2 bands from p41 (M), OspC (M), p83/100, p39, p41, p17, OspC | 83/92 | p41 and p43, or p58, or p14, or OspC(M) | 83/95 |
| D | PBi | 5 | 2 bands from p41 (M), OspC (M), p83/100, p39, p41, p17 | 74/89 | p58, or OspC (M), or p21, or p30 | 79/93 |
| E | VS461 | 4 | 3 bands from p41 (M), OspC (M), p83/100, p39, p17 | 67/97 | 3 bands from p58, p39, p28, p83/100, p41 (M), p60 (M) | 78/96 |
| F | Ne83 | 6 | 3 bands from p41 (M), OspC (M), p83/100, p39, p17, OspC | 78/87 | 3 bands from p43, p54/55, p41, OspC (M), p41 (M) | 80/96 |
Sens, sensitivity; spec, specificity. Sensitivity calculated by combining data for patient with EM and LB. Specificity was calculated by combining data for potential cross-reactor and healthy individuals' sera.
M, IgM.
DISCUSSION
The use of EIA and immunoblotting in a two-step testing strategy has gained wide acceptance, and the greater specificity of immunoblotting, also shown by the present study, has led to the view that it may be used as a confirmatory test. However, immunoblotting still has many problems, including the rationale and predictive value of tests, which have prompted recent reevaluations of their use (1, 3, 7, 26, 30). Several studies have reported that the use of different species and strains of B. burgdorferi sensu lato as antigen leads to inconsistency in blotting results because of variations in the expression of immunogenic proteins (4, 6, 15, 22, 23, 27, 33). This aspect of serodiagnosis of LB in Europe has led to the publication of several different recommendations for blot interpretation. Further difficulties result from the subjectivity of interpreting band strength, from problems with band resolution and identification, and from differences in the immune response to the various clinical presentations of LB (2, 9, 10, 13, 20, 25, 32).
Although recently published interpretation criteria have recognized the amount of possible variation in European immunoblotting assays, the studies were conducted at individual laboratories (17, 23, 27). This contrasts with the present study, which included several laboratories in different parts of Europe and which aimed to provide accessible interpretation criteria for wider application. In order to reduce subjectivity and variation in this study, the antigens used in the immunoblots were well characterized in advance with monoclonal antibodies. However, monoclonal antibodies were not available for all bands recorded, and it is probable that discrepancies have arisen in identifying some bands, particularly those of lower molecular masses, such as p17 and p18. In an analysis such bands would appear to be less significant than they really are.
The study identified the important immunoblot bands detectable throughout Europe and made it possible to formulate the best interpretation rules for the blots used by individual laboratories, based on this panel of samples collected from different parts of Europe. However, it should be emphasized that laboratories' own (more complex) rules in routine use have been tailored to sera from local populations and would be expected to perform better than either of the two groups of rules (European and the individual laboratories' rules) that emerged from this study. Two laboratories, laboratories C and F, reported many weak bands in the negative and cross-reactor groups and, because of the way in which the results were analyzed, tended to give lower specificities than the other laboratories (Table 9). If proposed rules required strong bands in these positions, then specificity would increase and sensitivity would decrease. Alternatively, a greater number of positive (weak or strong) bands could be required. Test sensitivity may therefore be a factor, in addition to subjectivity.
No useful single European rule resulted from the study, but finally, five very similar rules that gave acceptable sensitivities and specificities were formulated from a subset of eight bands of common importance. These European rules are not intended for the interpretation of any single immunoblot but could be used by diagnostic laboratories as a guide for the one-off formulation of working rules suited to their methodology and local populations. For example, laboratories in countries with a low prevalence of LB may prefer to use a rule that gives a higher specificity at the expense of some sensitivity. A laboratory's eventual selection of one of these relatively simple European rules would require comparison with existing working rules, if any.
The present study was primarily a reporting exercise to determine whether standardized interpretation criteria could be used for immunoblots for the diagnosis of LB in Europe, despite existing variation in immunoblotting methods. True standardization of an immunoblotting method for the diagnosis of LB would require agreement on the strains used for antigen preparation and on the protocol. This approach is unlikely to be useful in Europe because LB is not the same in all geographic areas due to different local prevalences of species and strains of B. burgdorferi sensu lato and also to heterogeneity within those strains. For these reasons, published recommendations for the interpretation of blots have not always been applicable to populations in geographic areas other than where they were developed (4, 22, 24, 27). The different genospecies are major sources of immunoblot variation, and laboratories developing diagnostic immunoblots using their local isolates should confirm expression of important immunogenic proteins by testing with monoclonal antibodies. Commercial companies should be aware that diagnostic criteria for immunoblots must be developed for the strain used in the test and must be based on a clinically defined panel of sera. In addition, proper identification of diagnostic bands must be given. Furthermore, it is important that commercial companies recognize that rules devised for diagnostic kits, both EIA cutoffs and immunoblot interpretation, may not be applicable to LB in different geographical areas.
More defined immunoblots based on recombinant proteins have been evaluated (12, 14, 16, 19, 21, 32). However, some technical problems are associated with these assays, and not all diagnostically important bands are available as recombinant proteins.
In view of the many sources of variation, it is suggested that immunoblotting in Europe be regarded as an additional test with an increased emphasis on specificity, which supports the clinical diagnosis rather than confirms it. It is also evident that a European quality assurance scheme for diagnostic laboratories would be desirable.
ACKNOWLEDGMENTS
This work was supported by EUCALB (BioMed 1 program, contract BMH-CT93-1183). SmithKline Beecham kindly funded a final meeting of the study participants.
The help of the clinicians who contributed sera and clinical information to the study is gratefully acknowledged.
REFERENCES
- 1.American College of Physicians. Guidelines for laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1106–1108. doi: 10.7326/0003-4819-127-12-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Assous M V, Postic D, Paul G, Névot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 3.Brown S L, Hansen S L, Langone J J. Role of serology in the diagnosis of Lyme disease. JAMA. 1999;282:62–66. doi: 10.1001/jama.282.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Bunikis J, Olsén B, Westman G, Bergström S. Variable serum immunoglobulin responses against different Borrelia burgdorferi sensu lato species in a population at risk for and patients with Lyme disease. J Clin Microbiol. 1995;33:1473–1478. doi: 10.1128/jcm.33.6.1473-1478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morbid Mortal Weekly Rep. 1995;44:590. [PubMed] [Google Scholar]
- 6.Cinco M, Murgia R, Ruscio M, Andriolo B. IgM and IgG significant reactivity to Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii among Italian patients affected by Lyme arthritis or neuroborreliosis. FEMS Immunol Med Microbiol. 1996;14:159–166. doi: 10.1111/j.1574-695X.1996.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 7.Coyle P K. Advances and pitfalls in the diagnosis of Lyme disease. FEMS Immunol Med Microbiol. 1997;19:103–109. doi: 10.1111/j.1574-695X.1997.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 8.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 9.Dressler F, Ackerman R, Steere A. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis. 1994;169:313–318. doi: 10.1093/infdis/169.2.313. [DOI] [PubMed] [Google Scholar]
- 10.Dunand V A, Bretz A-G, Suard A, Pratz G, Dayer E, Petér O. Acrodermatitis chronica atrophicans and serologic confirmation of infection due to Borrelia afzelii and/or Borrelia garinii by immunoblot. Clin Microbiol Infect. 1998;4:159–163. doi: 10.1111/j.1469-0691.1998.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for the serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fawcett P T, Rosé C D, Gibney K M, Doughty R A. Comparison of immunodot and Western blot assays for diagnosing Lyme borreliosis. Clin Diagn Lab Immunol. 1998;5:503–506. doi: 10.1128/cdli.5.4.503-506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flisiak R, Wierzbicka I, Prokopowicz D. Western blot banding pattern in early Lyme borreliosis among patients from an endemic region of northeastern Poland. Rocz Akad Med Bialymst. 1998;43:210–220. [PubMed] [Google Scholar]
- 14.Gilmore R D, Murphree R L, James A M, Sullivan S A, Johnson B J. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J Clin Microbiol. 1999;37:548–552. doi: 10.1128/jcm.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser U, Lehnert G, Lobentanzer R, Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997;35:1433–1444. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser U, Lehnert G, Wilske B. Diagnostic value of proteins of three Borrelia species (Borrelia burgdorferi sensu lato) and implications for development and use of recombinant antigens for serodiagnosis of Lyme borreliosis in Europe. Clin Diagn Lab Immunol. 1998;5:456–462. doi: 10.1128/cdli.5.4.456-462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J Clin Microbiol. 1999;37:2241–2247. doi: 10.1128/jcm.37.7.2241-2247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton E, Devoti J, Sood S. Recommendation to include OspA and OspB in the new immunoblotting criteria for serodiagnosis of Lyme disease. J Clin Microbiol. 1996;34:1353–1354. doi: 10.1128/jcm.34.6.1353-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honegr K, Hulinská D, Havlasova J, Dostal V. Importance of the immunoblot test in the diagnosis of Lyme borreliosis. Epidemiol Mikrobiol Immunol. 1997;46:149–154. [PubMed] [Google Scholar]
- 20.Hulinská D. Diagnosis of Lyme borreliosis with Western blotting. Epidemiol Mikrobiol Immunol. 1997;46:3–8. [PubMed] [Google Scholar]
- 21.Jauris-Heipke S, Roessle B, Wanner G, Habermann C, Roessler D, Fingerle V, Lehnert G, Loebentanzer R, Pradel I, Hillenbrand B, Schulte-Spechtel U, Wilske B. Osp17, a novel immunodominant outer surface protein of B. afzelii: recombinant expression in Escherichia coli and its use as a diagnostic antigen for serodiagnosis of Lyme borreliosis. Med Microbiol Immunol. 1999;187:213–219. doi: 10.1007/s004300050095. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson I, von Rosen I A. Serum antibodies against Borrelia afzelii, Borrelia burgdorferi sensu stricto and the 41-kilodalton flagellin in patients from a Lyme borreliosis endemic area: analysis by EIA and immunoblot. APMIS. 1996;104:907–914. doi: 10.1111/j.1699-0463.1996.tb04957.x. [DOI] [PubMed] [Google Scholar]
- 23.Norman G, Antig J M, Bigaignon G, Hogrefe W R. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii Western blots (immunoblots) J Clin Microbiol. 1996;43:1732–1738. doi: 10.1128/jcm.34.7.1732-1738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunez M E, Gutierrez F J, Guerrero F A M, Piedrola A G, Maroto V C. Comparative study of Western blot interpretation criteria for Lyme disease serological diagnosis in our environment. Ann Med Int. 1998;15:8–12. [PubMed] [Google Scholar]
- 25.Petér O, Bretz A-G, Postic D, Dayer E. Association of distinct species of Borrelia burgdorferi sensu lato with neuroborreliosis in Switzerland. Clin Microbiol Infect. 1997;3:423–431. doi: 10.1111/j.1469-0691.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 26.Porwancher R. A reanalysis of IgM Western blot criteria for the diagnosis of early Lyme disease. J Infect Dis. 1999;179:1021–1024. doi: 10.1086/314651. [DOI] [PubMed] [Google Scholar]
- 27.Ryffel K, Péter O, Binet L, Dayer E. Interpretation of immunoblots for Lyme borreliosis using a semiquantitative approach. Clin Microbiol Infect. 1998;4:205–212. doi: 10.1111/j.1469-0691.1998.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 28.Sood S K, Zemel L S, Ilowite N T. Interpretation of immunoblot in pediatric Lyme arthritis. J Rheumatol. 1995;22:758–761. [PubMed] [Google Scholar]
- 29.Stanek G, O'Connell S, Cimmino M, Arberer E, Kristoferitsch W, Granström M, Guy E, Gray J. European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–747. [PubMed] [Google Scholar]
- 30.Trevejo R T, Krause P J, Sikand V K, Schriefer M E, Ryan T, Lepore T, Porter W, Dennis D T. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J Infect Dis. 1999;179:931–938. doi: 10.1086/314663. [DOI] [PubMed] [Google Scholar]
- 31.Venables W N, Ripley B D. Modern applied statistics with S-plus. New York, N.Y: Springer-Verlag; 1994. [Google Scholar]
- 32.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister H W, Preac-Mursic V, Soutschek E, Weber K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med Microbiol Immunol (Berlin) 1993;182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 33.Wilske B, Hauser U, Lehnert G, Jauris-Heipke S. Genospecies and their influence on immunoblot results. Wien Klin Wochenschr. 1998;110:882–885. [PubMed] [Google Scholar]
- 34.Zöller L, Burkhard S, Schäfer H. Validity of immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991;29:174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]