Abstract
Antibiotic treatment in early life influences gastrointestinal (GI) microbial composition and function. In humans, the resultant intestinal dysbiosis is associated with an increased risk for certain diseases later in life. The objective of this study was to determine the temporal effects of antibiotic treatment on the GI microbiome of young cats. Fecal samples were collected from cats randomly allocated to receive either amoxicillin/clavulanic acid (20 mg/kg q12h) for 20 days (AMC group; 15 cats) or doxycycline (10 mg/kg q24h) for 28 days (DOX group;15 cats) as part of the standard treatment of upper respiratory tract infection. In addition, feces were collected from healthy control cats (CON group;15 cats). All cats were approximately two months of age at enrolment. Samples were collected on days 0 (baseline), 20 or 28 (AMC and DOX, respectively; last day of treatment), 60, 120, and 300. DNA was extracted and sequencing of the 16S rRNA gene and qPCR assays were performed. Fecal microbial composition was different on the last day of treatment for AMC cats, and 1 month after the end of antibiotic treatment for DOX cats, compared to CON cats. Species richness was significantly greater in DOX cats compared to CON cats on the last day of treatment. Abundance of Enterobacteriales was increased, and that of Erysipelotrichi was decreased in cats of the AMC group on the last day of treatment compared to CON cats. The abundance of the phylum Proteobacteria was increased in cats of the DOX group on days 60 and 120 compared to cats of the CON group. Only minor differences in abundances between the treatment groups and the control group were present on day 300. Both antibiotics appear to delay the developmental progression of the microbiome, and this effect is more profound during treatment with amoxicillin/clavulanic acid and one month after treatment with doxycycline. Future studies are required to determine if these changes influence microbiome function and whether they have possible effects on disease susceptibility in cats.
Introduction
Antibiotic discovery represents one of the most important achievements in the history of medicine [1]. However, overuse of antibiotics compromises their health benefits because of the development and dissemination of antibiotic resistant genes and their impact on the gastrointestinal microbiome [2,3]. The extent that the microbiome is affected by antibiotics has become apparent after the application of “omics” approaches in research that allow the assessment of whole microbial communities and their functions [4]. The GI microbiome is a community of microorganisms and has been called “a hidden organ” [5]. This community of microorganisms is responsible for maintaining colonization resistance and produces substances with an impact on the host’s metabolism, immune system development and response, and appears to participate in the communication among different organs as well as in the manifestation and progression of diseases [6–14].The term GI dysbiosis is used to describe the compositional and functional alterations of the GI microbiome in response to exogenous factors and/or the health status of the host [15]. Antibiotic-induced dysbiosis is characterized by a decrease in bacteria beneficial for the host (“health-associated bacteria”), allowing overgrowth of potentially pathogenic bacteria, and a shift in microbially derived metabolic products [15,16]. Antibiotic-induced microbial shifts can persist long term, and the abundances of some bacterial taxa might never return to their initial state. Other members of the microbiome, including the mycobiome and the virome are also affected by antibiotics, highlighting a global imbalance among members of microorganisms not directly inhibited by antibiotics [17]. Antibiotic-induced dysbiosis depends on the spectrum of antibacterial activity, type, duration, dosage, and route of administration in addition to individual host characteristics [18,19].
The GI microbiome appears to be more susceptible to antibiotics when administered early in life [20]. During that period, maturation of the immune system takes place concurrently with microbiome maturation. Antibiotics result in exposure of the host to a reduced number of microbes in the gut, which also results in changes in the microbially produced metabolites that the host is exposed to [21]. A depletion of bacteria known to ferment fiber and carbohydrates for short chain fatty acids (SCFAs) (Faecalibacterium spp., Turicibacter spp., Blautia spp., Bacteroides spp.), or convert primary into secondary bile acids (Clostridium hiranonis) is a common effect of antibiotic therapy in dogs [8,22–24]. In addition, bacteria that have been associated with GI diseases (Proteobacteria spp., Fusobacterium spp., Escherichia coli) or lactid-acid-producing bacteria (Streptococcus spp.) have been found upregulated after antibiotic therapy in dogs and cats [8,23–28]. In humans, antibiotics administered early in life appear to delay the developmental progression of the microbiome into an adult-like state [29,30]. Previous studies have shown that children exposed to antibiotics were more likely to develop inflammatory bowel disease [31,32], obesity [33,34], or asthma [35,36] during childhood. Currently, limited data are available for cats. In one-study, all cats previously treated with amoxicillin/clavulanic acid and pradofloxacin developed diarrhea after experimental infection with enteropathogenic E.coli in contrast to non-treated cats, none of which developed clinical signs [37]. This study highlights that similarly to humans, antibiotic-induced dysbiosis likely reduces colonization resistance in cats.
Feline upper respiratory tract disease (URTD) is a condition of mainly viral etiologies and clinical signs can vary in severity. Acute URTD can manifest with conjunctivitis, ocular or nasal discharge, and sneezing. In some cases, signs of other system involvement or systemic disease might occur [38]. Published guidelines suggest the use of either amoxicillin (with or without clavulanic acid) or doxycycline as a first line of treatment for the acute form of URTD [39]. Amoxicillin is a semisynthetic penicillin that is active against some non-beta-lactamase producing gram-positive bacteria and few gram-negative bacteria. The addition of a beta lactamase inhibitor, such as clavulanic acid, increases the spectrum of activity of amoxicillin [40]. From a microbiome perspective, amoxicillin, with or without clavulanic acid, commonly increases fecal abundances of Proteobacteria members and decreases Firmicutes and Actinobacteria members in humans and gnotobiotic animals [41–44]. Discordant findings have been reported in members of the phylum Bacteroides with some studies reporting an increase [20,41,43] and others a decrease [44–46] in members of this phylum. Some studies suggest that amoxicillin-induced microbial shifts are only temporary and subside within 2 to 4 weeks after its withdrawal [42]. Other studies suggest long-term changes including a persistent reduction in Lachnospiraceae 6 months after amoxicillin discontinuation [44]. Doxycycline belongs to tetracyclines, a class of bacteriostatic antibiotics with broad spectrum activity against bacteria, rickettsiae, and protozoal organisms. [47]. Doxycycline administration in humans and rodents has been associated with reduced fecal abundance of the phylum Firmicutes and an increased or reduced abundance of the phylum Bacteroidetes [48–50]. In mice treated with doxycycline, microbial shifts were still detected 1 month after doxycycline withdrawal [49] yet no published studies present results for a longer follow-up period.
Previous molecular studies investigating the effects of antibiotics on the feline GI microbiome have enrolled healthy cats under laboratory-controlled conditions. In these studies, cats were adults, but belonged to various age groups and were fed the same diet for the duration of each study [25,26,51]. In humans, antibiotics with an anaerobic spectrum of activity, such as amoxicillin and clindamycin, seem to have a more profound and prolonged effect on the gut microbiome, given that 95% of the GI bacteria are anaerobic [52]. In cats, administration of clindamycin affected the microbiome and metabolome long-term, with a persistent reduction in the families Prevotellaceae, Veillonelaceae, Enterobacteriaceae, and Porphyromonadaceae as well as deoxycholic acid, a secondary bile acid, for at least 2 years after withdrawal of the antibiotic [25]. In another study in cats, amoxicillin-clavulanic acid caused an increase in Enterobacteriaceae and Enterococcus spp. and these changes were still detected 7 days after withdrawal of the antibiotic [51]. No studies to date have investigated the effect of doxycycline and antibiotic treatment in general on the gastrointestinal microbiome of young cats until they reach maturity.
The aim of this study was to describe and compare the fecal microbiome of cats receiving amoxicillin/clavulanic acid or doxycycline and control cats not receiving antibiotics and follow them over a period of 10 months. A second goal was to describe the normal age-related changes of the feline microbiome during development.
Materials and methods
Study population
The protocol was reviewed and approved by the Animal Ethics Committee of the University of Thessaly, Greece (AUP number: 54/13.2.2018). A total of 72 eight-week-old rescue domestic shorthair (DSH) cats were enrolled in the study. Forty-four out of 72 cats were diagnosed with upper respiratory tract disease (URTD) before inclusion into the study. Diagnosis was based on a typical clinical presentation, including conjunctivitis, blepharospasm, ocular or nasal discharges, nasal congestion, sneezing, and coughing. The cats were treated with antibiotics (see Treatment) as part of the standard treatment for this condition. In addition, 26 clinically healthy cats or cats with very mild URTD that did not require antibiotic treatment were enrolled as controls.
Cats were either housed in foster homes or in individual cages at the Clinic of Medicine at the Faculty of Veterinary Science of the University of Thessaly. All cats were eventually adopted into private homes by the end of the study and owners signed an informed owner consent form. Upon initial enrollment, cats were kept under observation for a few days in case they developed clinical signs of GI disease. A physical examination was performed and antiparasitic treatment (Broadline, Boehringer Ingelheim) was administered to each cat before inclusion into the study. Data including sex, body weight, body condition score (BCS), presence of diarrhea and vomiting, temperature, and heart rate were recorded (S1 Table). Evaluation of BCS and fecal score (FS) was based on previously published scoring systems [53,54]. Concurrent health conditions were recorded, and cats were excluded if these were severe enough to require hospitalization. All cats were on the same diet (GEMON Cat Breeder Kitten) for the duration of the study, to ensure that diet did not influence results. No more than two related cats were included in the same treatment group to ensure that relatedness did not bias results. All cats were vaccinated according to recent vaccination guidelines [55] and clinical data were collected throughout the study period (S1 Table).
Treatments
Cats with URTD were randomly allocated to receive either amoxicillin/clavulanate at 20 mg/kg q 12 h for 20 days (n = 23, AMC group) or doxycycline at 10 mg/kg q 24 h for 28 days (n = 21, DOX group). These antibiotics were chosen because they are first line treatments for URTD in cats [39]. In addition, 26 clinically healthy cats were enrolled as controls and did not receive any antibiotics during the study period (n = 26, CON group).
Sample collection and follow-up period
Fecal samples were collected from each cat on days: 0 (all groups; one day after initial presentation and antiparasitic treatment), 20 (AMC group; last day of antibiotic treatment for AMC group), 28 (DOX and CON groups, last day of antibiotic treatment for DOX group), 60 (all groups), 120 (all groups), and 300 (all groups). Naturally voided fecal samples were collected from the litter box and placed into Eppendorf tubes. For cats that were adopted, owners were instructed to collect fecal samples from the litter box, freeze them over night and either bring them to the clinic or ship them packed with icepacks by overnight courier. In case other animals were living in the same household, owners were asked to properly isolate their cat before sample collection. Upon receipt, samples were immediately stored at -80°C pending analysis. On each sampling day, cats underwent a physical examination and the same data as for initial presentation were collected for all cats.
DNA extraction
Genomic DNA was extracted from 100 mg of each fecal sample using a MoBio PowerSoil® DNA isolation kit (Mo Bio Laboratories, USA) according to the manufacturer’s instructions.
16S rRNA sequencing
Illumina sequencing of the bacterial 16S rRNA genes was performed using primers 515F (5’-GTGYCAGCMGCCGCGGTAA) [56] to 806RB (5’-GGACTACNVGGGTWTCTAAT) [57] at the MR DNA laboratory (Shallowater, TX).
Sequences were processed and analyzed using a Quantitative Insights Into Microbial Ecology 2 (QIIME 2) [58] v 2018.6 pipeline. Briefly, the sequences were demultiplexed and the amplicon sequence variant (ASV) table was created using DADA2 [59]. Prior to downstream analysis, sequences assigned as chloroplast, mitochondria, and low quality and unassigned ASVs in the dataset were removed. A filter was used to remove rare taxa, defined as taxa that were not present in at least 50% of samples of at least one group or time point. All samples were rarefied to even sequencing depth, based on the lowest read depth of samples, to 8,275 sequences per sample. The raw sequences were uploaded to NCBI Sequence Read Archive under project number SRP16253.
Alpha diversity was measured with the Chao1 (non-parametric estimator of the number of rare or infrequent ASVs), Shannon diversity (evenness estimator; takes into account the proportion of each species) and observed ASVs (richness estimator) metrics within QIIME2. Beta diversity was evaluated with the weighted and unweighted phylogeny-based UniFrac [60] distance metric and visualized using Principal Coordinate Analysis (PCoA) plots, generated within QIIME2.
Quantitative PCR (qPCR)
The qPCRs assays for total bacteria, Faecalibacterium spp., Turicibacter spp., Streptococcus spp., Escherichia coli, Blautia spp., Fusobacterium spp., Clostridium hiranonis, and Bifidobacterium spp. were performed using previously published primers and probes, cycling and annealing conditions [61,62]. Extracted DNA was also used as the template for PCR amplification in a thermocycler (Bio-rad Gradient Thermal Cycler, Bio-rad Laboratories Inc., Hercules, California) of an approximately 184 bp of the Bacteroides spp. 16SrRNA with group-specific bacterial primers Bacteroides gen 16SF (TCGGCTAACTCCGTGCCAGC) and Bacteroides gen 16SF (ACACCACGAATTCCGCCCACC). Samples were amplified using the following protocol: initial denaturation at 95°C for 2 min; 40 cycles of denaturation at 95°C for 5 s, and annealing at 52°C for 4 s.
Statistical analysis
Statistical analyses were performed using statistical software packages (SPSS version 23.0; and Prism version 9.0, GraphPad Software). For clinical data, a Kolmogorov-Smirnov test was used to assess the normality assumption. Clinical data did not pass normality testing, and therefore Kruskal-Wallis tests were used for among group comparisons while Friedman tests were used for within group comparisons. Pairwise comparisons were performed using Dunn’s post hoc tests to determine which group categories were significantly different from each other as well as which time points were significantly different.
To determine differences in microbiome composition among and within the study groups, the analysis of similarities (ANOSIM) was performed using the statistical software package PRIMER 7 (PRIMER-E Ltd., Lutton, UK), and PERMANOVA was performed on QIIME2, based on the unweighted and weighted UniFrac distance matrices. Differences in alpha diversity indices were evaluated using Kruskal-Wallis tests for baseline values and linear mixed model followed by within group post hoc comparisons using Wilcoxon signed-rank tests and Bonferroni correction of p values. Differences in the abundances of bacterial taxa among and within groups were determined using Kruskal-Wallis tests for baseline values and linear mixed models. Data were rank transformed prior to fitting linear mixed models. Microbial compositions were initially screened for differences among groups with p values adjusted for multiple hypothesis testing using the Benjamini and Hochberg false discovery rate (FDR) and overall significance set at p < 0.05. For comparisons that were significant after FDR adjustment, a linear mixed model was fit including time, group, and the interaction between time and group as fixed effects and cat as a random effect. Multiple pairwise post hoc comparisons were adjusted using Bonferroni correction.
Results
Clinical data
Twenty-seven cats were excluded from the study because of owner non-compliance (7/72), death (9/72; 2 due to accidents, 1 due to feline infectious peritonitis, 1 due to heart failure, while 5 had unknown cause of death), a required second course of antibiotics (5/72), antifungal treatments (3/72), or escape from home (3/72). Fifteen cats in each treatment group (45 cats total) completed the study. These included 25 males and 20 females. Microbiome analysis and clinical data assessment were only performed for the cats that completed the study (S1 Table).
On day 0, cats of the AMC group had significantly lower body weights (BW) (median 0.61 kg, range 0.37–0.95 kg) compared to CON cats (median 0.74 kg, range 0.52–1.4 kg) (p = 0.026; Table 1). No other BW or BCS differences were identified among groups. On day 0, cats belonging to the DOX group had a significantly higher fecal score (FS) (median 4/7, range 2/7-7/7), i.e., had more commonly abnormal fecal consistency, compared to CON cats (median 2/7, range 1/7-6/7) (p = 0.045). On days 20/28 and 60, AMC cats had a significantly higher FS (day 20, median 4/7, range 1/7-6/7; day 60, median 3/7, range 1/7-6/7) compared to CON cats (days 28 and 60, median 2/7, range 1/7-3/7) (p <0.05).
Table 1. Clinical characteristics of cats included in the study.
| Body weight (kg) | |||||||
| AMC | DOX | CON | P value | ||||
| Median | Range | Median | Range | Median | Range | ||
| Day 0 | 0.61 | 0.37–0.95 | 0.68 | 0.39–1.20 | 0.74 | 0.52–1.40 | 0.026 |
| Day 20/28 | 1.00 | 0.80–1.56 | 1.18 | 0.87–1.73 | 1.26 | 0.77–1.60 | 0.217 |
| Day 60 | 1.70 | 1.36–1.80 | 1.70 | 1.25–2.00 | 1.92 | 1.15–2.50 | 0.120 |
| Day 120 | 2.50 | 1.98–3.79 | 2.62 | 1.99–3.05 | 2.80 | 1.69–3.80 | 0.746 |
| Day 300 | 4.10 | 2.70–5.75 | 4.19 | 3.33–5.80 | 4.00 | 2.30–6.00 | 0.797 |
| Body condition score (1 to 9) | |||||||
| AMC | DOX | CON | P value | ||||
| Median | Range | Median | Range | Median | Range | ||
| Day 0 | 4 | 2–6 | 4 | 3–5 | 4 | 3–5 | 0.107 |
| Day 20/28 | 4 | 4–5 | 4 | 4–5 | 4 | 4–5 | 0.717 |
| Day 60 | 4 | 4–6 | 4 | 4–5 | 4 | 3–5 | 0.651 |
| Day 120 | 4 | 4–6 | 4 | 4–6 | 4 | 4–6 | 0.935 |
| Day 300 | 4 | 3–7 | 5 | 4–6 | 5 | 3–6 | 0.281 |
| Fecal score (1 to 7) | |||||||
| AMC | DOX | CON | P value | ||||
| Median | Range | Median | Range | Median | Range | ||
| Day 0 | 3 | 2–6 | 4 | 2–7 | 2 | 1–6 | 0.045 |
| Day 20/28 | 4 | 1–6 | 3 | 2–5 | 2 | 1–3 | <0.001 |
| Day 60 | 3 | 1–6 | 3 | 1–5 | 2 | 1–3 | 0.035 |
| Day 120 | 2 | 1–5 | 2 | 1–5 | 2 | 1–4 | 0.221 |
| Day 300 | 2 | 1–5 | 2 | 1–3 | 2 | 1–3 | 0.195 |
AMC, cats treated with amoxicillin/clavulanic acid for 20 days; DOX, cats treated with doxycycline for 28 days; CON, healthy cats that did not receive antibiotics. Bolded p-values indicate a statistically significant difference between groups.
1. Effect of aging on the microbiome of untreated cats
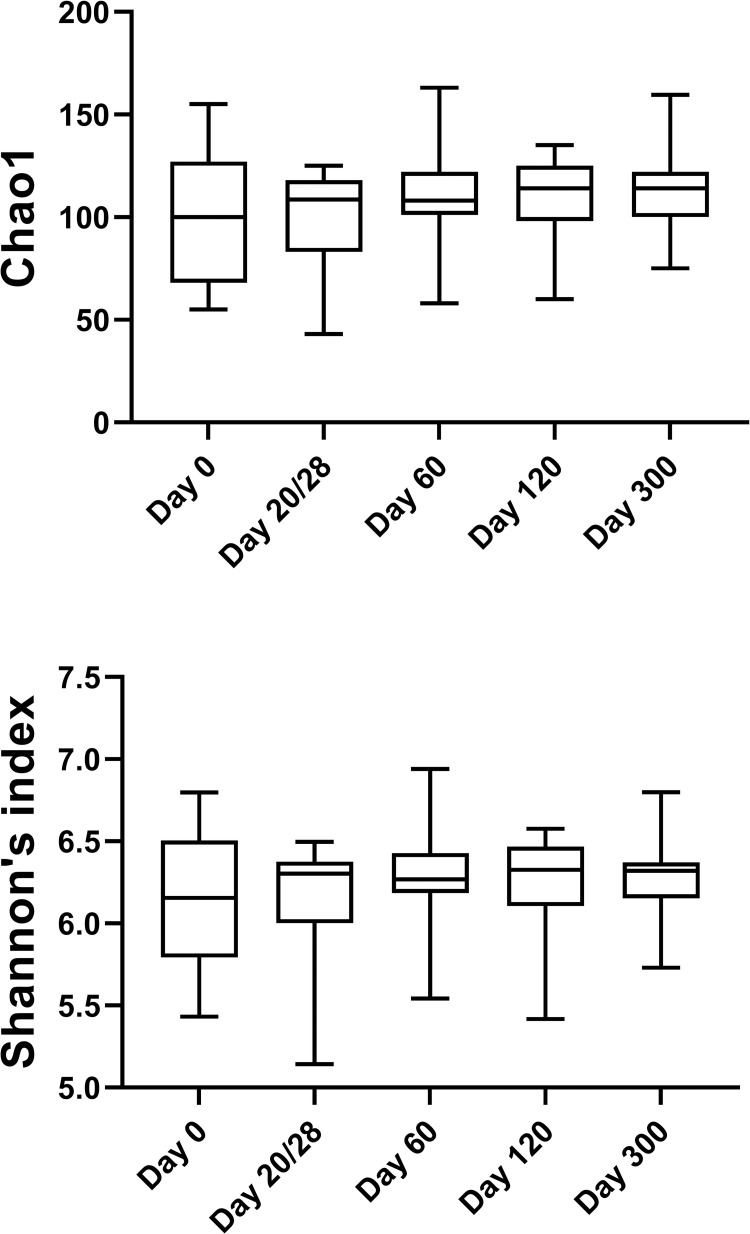
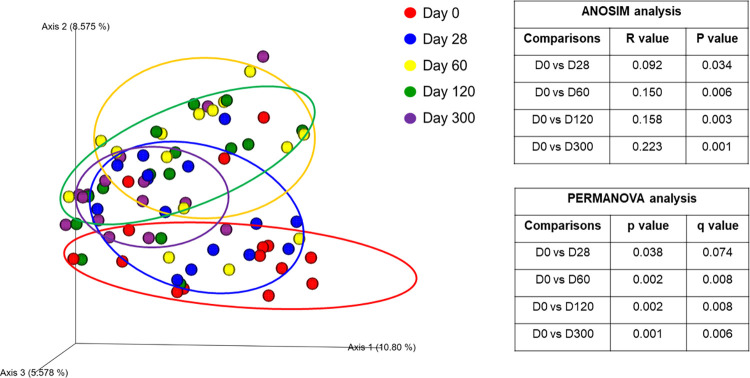
1.A) Sequence analysis—alpha and beta diversity. High interindividual variations in bacterial abundances were observed in all groups on day 0 and within the CON group significant changes occurred over time. These changes were attributed to the process of microbial maturation, therefore results from this group are discussed separately. In total, the sequence analysis of the 225 fecal samples yielded 1,861,875 quality sequences. There were no significant differences in any of the species richness and evenness indices over time in control cats (Fig 1, S2 Table). However, the phylogenetic community structure clustered differently over time (p < 0.05) and was increasingly more distinct as cats were getting older based on the increasing ANOSIM effect size of unweighted and weighted UniFrac distances (Fig 2, S3 Table). PERMANOVA analysis also showed a distinct microbial composition over time in cats of the CON group (Fig 2, S4 Table).
Fig 1. Alpha diversity differences in control cats over time.
Means with minimums and maximums values are displayed at each sampling day.
Fig 2. Principal Coordinate Analysis of unweighted UniFrac distances of 16S rRNA genes representing the differences in microbial community composition within the control group on day 0 (red circles), day 28 (blue circles), 60 (yellow circles), 120 (green circles), and 300 (purple circles).
R values and P values calculated with ANOSIM and PERMANOVA p and q values are displayed.
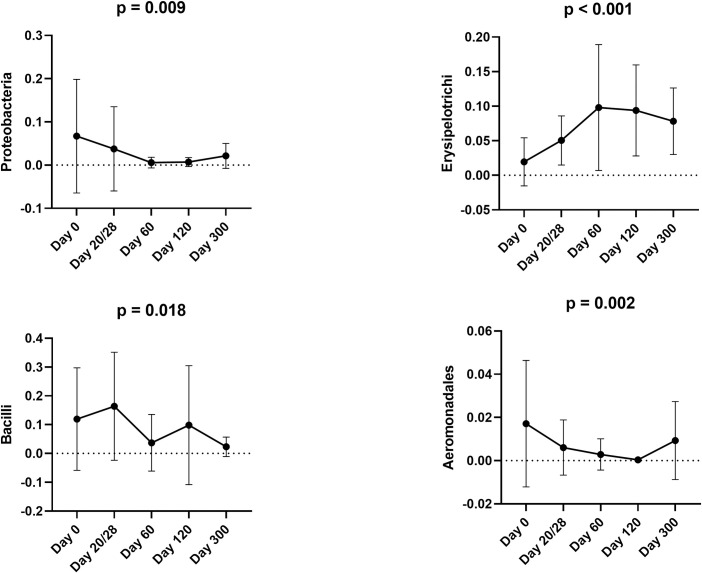
1.B) Sequence analysis–abundance of individual bacterial taxa. At 2 months of age (day 0) the most prevalent phylum (regardless of the group) was Firmicutes (63.5%), followed by Actinobacteria (13.9%), Bacteroidetes (11.6%), Proteobacteria (6.0%), and Fusobacteria (4.9%). The abundance of Proteobacteria was significantly reduced to less than 1% (p = 0.009) by 4 months of age in the control cats (Fig 3). S5 Table contains summary statistics for all taxonomic classifications (i.e., phylum, class, order, family, genus, and species).
Fig 3. Bacterial groups that significantly changed over time within the control group based on sequence analysis.
Means and standard deviations are displayed.
Clostridia, Clostridiales, and Lachnospiraceae, were the most prevalent class, order, and family, respectively, present in fecal samples from control cats during their first year of age. In addition, Blautia spp., Collinsella spp., Lactobacillus spp., Bifidobacterium spp., Bacteroides spp., and unclassified Lachnospiraceae constituted the predominant genera.
The majority of differences in the abundances of bacteria within the control cats occurred between 2 and 6 months of age. The abundance of Gammaproteobacteria significantly decreased from 5.5% at 2 months to 3.2% at 3 months of age (p = 0.007) and that of Enterobacteriales from 3.7% to less than 0.5% (p = 0.009) during the same period. The abundance of Erysipelotrichi increased from 1.9% at 2 months to 5% at 3 months of age (p = 0.030) (Fig 3). The abundance of Bacilli reduced from 16.4% at 3 months to 3.7% at 4 months of age (p = 0.018). The only changes observed after 6 months of age included an increase in the abundance of Aeromonadales (p = 0.002) (Fig 3).
1.C) Quantitative polymerase chain reaction (qPCR) for selected bacterial groups. In the CON group, E.coli decreased (p < 0.001), and Faecalibacterium spp. increased (p = 0.032) from 2 to 3 months of age (Fig 4). No other significant differences were identified in bacteria quantified with PCR in CON cats. S6 Table contains a summary of all bacterial taxa analyzed by qPCR.
Fig 4. Bacterial groups that significantly changed over time within the control group based on qPCR analysis.
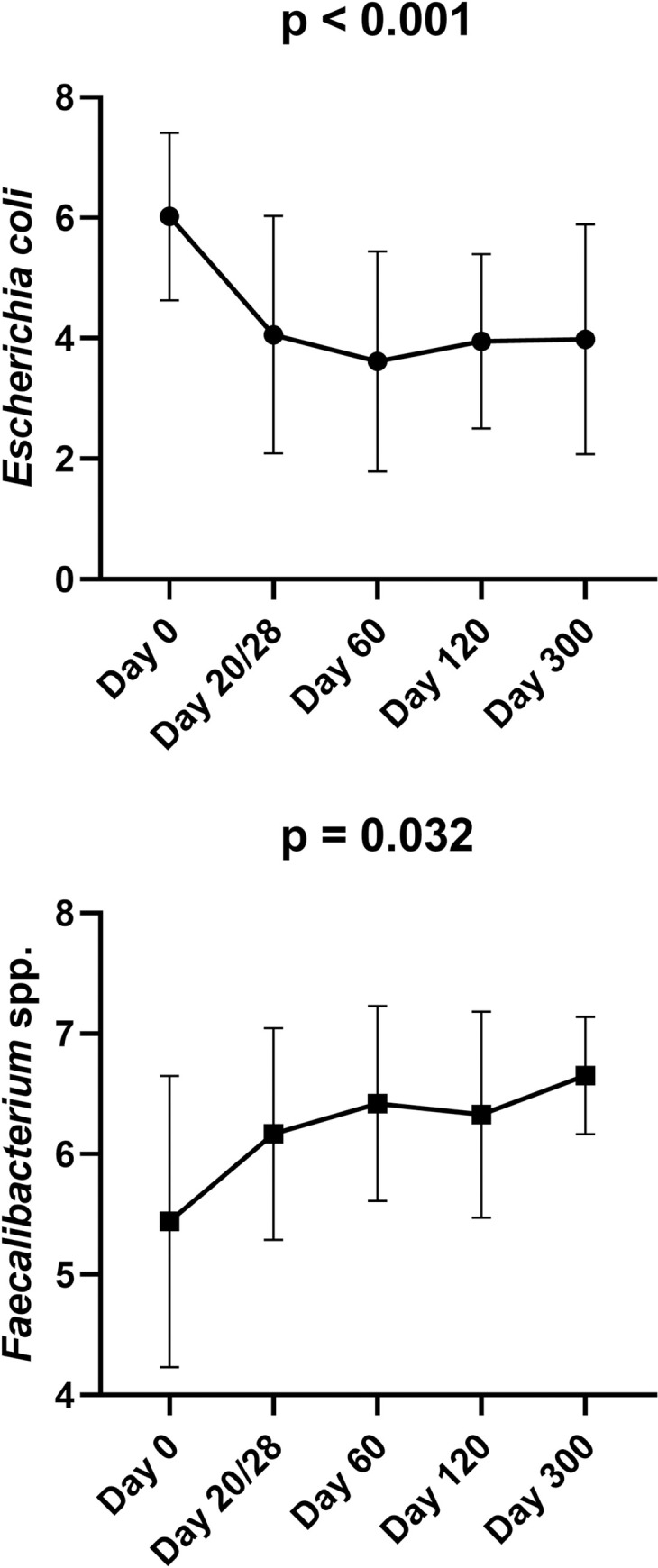
Means and standard deviations are displayed.
2. Effect of antibiotics on the GI microbiome
Visualization of the data suggested a high interindividual variation of bacterial abundances in all groups on day 0. The alpha diversity indices (S2 Table), the ANOSIM and PERMANOVA of unweighted and weighted UniFrac distances (S1 Fig, S3 and S4 Tables), and bacterial abundances (S5 and S6 Tables) did not differ significantly among groups on day 0.
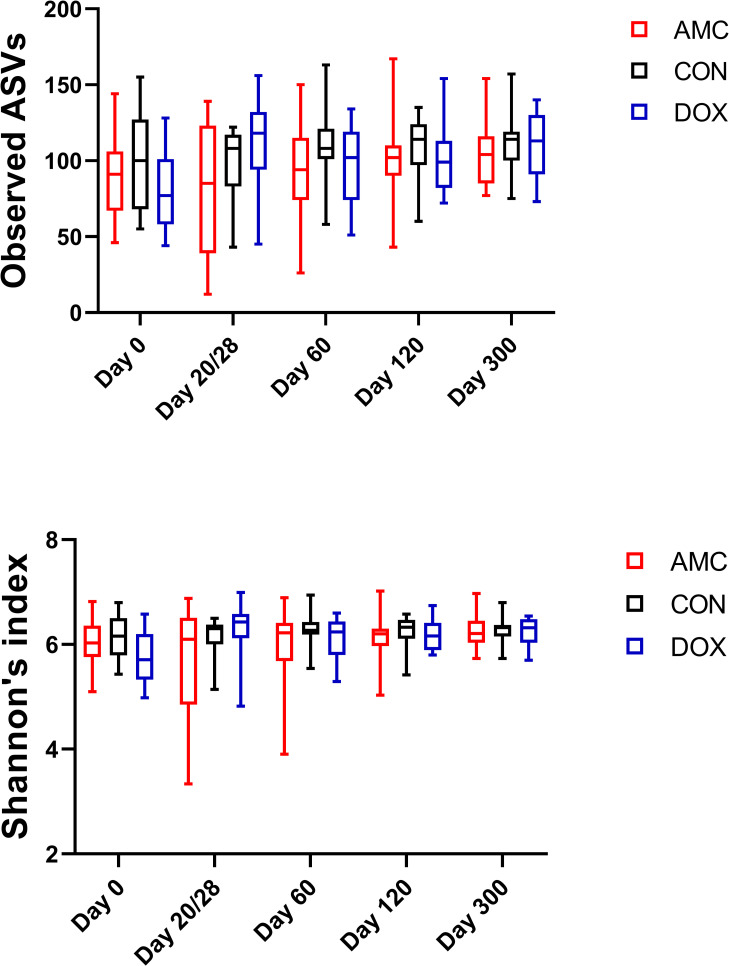
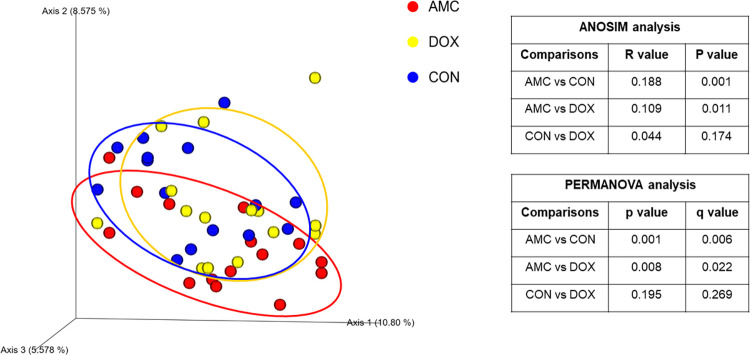
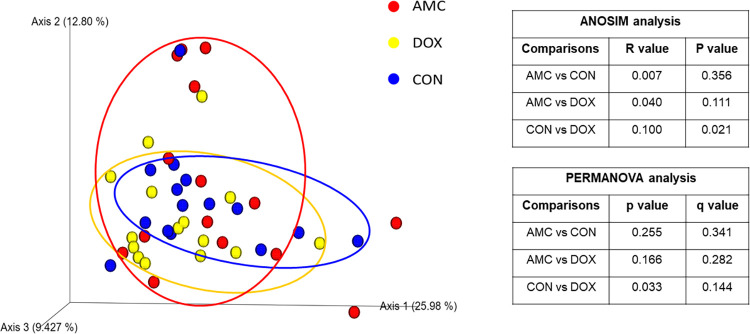
2.1.Amoxicillin/Clavulanic acid group. 2.1.A) Sequence analysis—alpha and beta diversity. The AMC group had reduced evenness on the last day of treatment (day 20) compared to DOX and CON groups, but this decrease did not reach statistical significance (Shannon index, p = 0.061) (Fig 5, S2 Table). Alpha diversity indices did not vary over time in AMC cats (S2 Table). The microbial community composition on the last day of treatment (day 20) was different for AMC cats compared to both DOX (ANOSIM R = 0.109, p = 0.011; PERMANOVA p = 0.008, q = 0.022) and CON (ANOSIM R = 0.188, p = 0.001; PERMANOVA p = 0.001, q = 0.006) cats based on unweighted analysis (Fig 6, S3 and S4 Tables). On days 60 and 300, there was a less distinct clustering of the microbiome in AMC cats compared to CON cats (based on decreasing ANOSIM effect size) as demonstrated by unweighted (day 60, ANOSIM R = 0.056, p = 0.075; PERMANOVA p = 0.086, q = 0.143 and day 300, ANOSIM R = 0.077, p = 0.058; PERMANOVA p = 0.056, q = 0.100) and weighted distances (day 300, ANOSIM R = 0.057, p = 0.074; PERMANOVA p = 0.127, q = 0.238), but this difference did not reach statistical significance (S1 Fig).
Fig 5. Alpha diversity differences between cats treated with amoxicillin/clavulanic acid (black), cats treated with doxycycline (blue), and healthy control cats (red).
Means and standard deviations within each group are displayed.
Fig 6. Principal Coordinate analysis (PCoA) plot of unweighted Unifrac distance in cats treated with amoxicillin clavulanic acid (red = AMC), cats treated with doxycycline (yellow = DOX), and control cats (blue = CON) on day 20/28.
R values and P values calculated with ANOSIM and PERMANOVA p and q values are displayed.
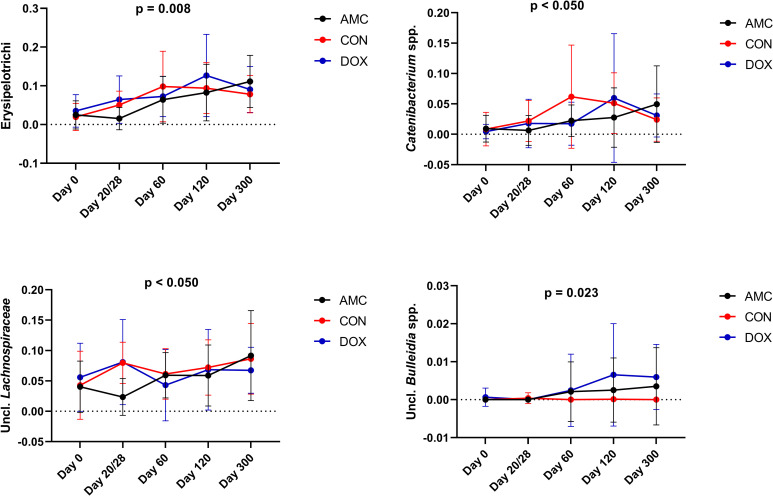
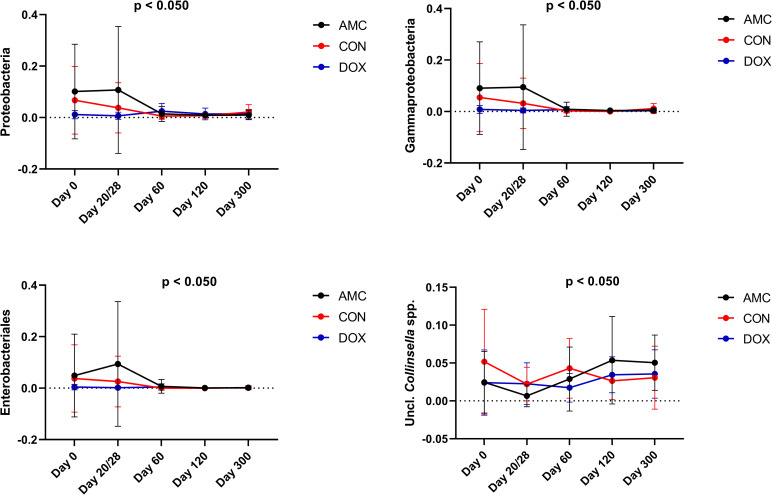
2.1.B) Sequence analysis—abundance of individual bacterial taxa. Amoxicillin/clavulanic acid had a significant impact on the GI microbiome. In fact, the normal age-related changes of the microbiome observed in CON cats were not observed in this group. Erysipelotrichi (p = 0.008), Catenibacterium spp. (p = 0.045), and unclassified Lachnospiraceae (p = 0.002) were detected in significantly lower abundances, whereas Enterobacteriales (p = 0.010) was found in significantly higher abundances in feces from AMC cats compared to CON cats on the last day of treatment (day 20/28) (Figs 6 and 7). Three (day 120) and 9 months (day 300) after amoxicillin/clavulanic acid discontinuation, AMC cats harbored significantly higher abundances of unclassified Collinsella spp. compared to CON cats (Fig 8).
Fig 7. Bacterial groups that showed a significantly decreased abundance after antibiotic treatment (AMC and DOX group) compared to the control group (CON group).
Means and standard deviations within each group are displayed.
Fig 8. Bacterial groups that showed a significantly increased abundance after antibiotic treatment (AMC and DOX groups) compared to the control group (CON group).
Means and standard deviations within each group are displayed.
Most of the differences in bacterial abundances between AMC and CON groups were found during treatment, (from 2 to 3 months of age), while after that period only minor changes were observed. In AMC cats, Gammaproteobacteria abundances remained the same during treatment (i.e., from 2 to 3 months) representing approximately 9% of total sequences, while in CON cats they decreased during the same period, representing 3% of total sequences (p = 0.009). At 1 month after antibiotic withdrawal (4 months of age), Gammaproteobacteria decreased to <1% in AMC cats (p = 0.030), reaching similar levels to those in CON cats at this age (Fig 8). Erysipelotrichi abundances represented 2.5% of the total sequences in AMC cats before treatment, and decreased to less than 2% after treatment, while in CON cats, Erysipelotrichi abundances increased at this age. On day 60, both groups harbored similar abundances of this bacterium (Fig 7).
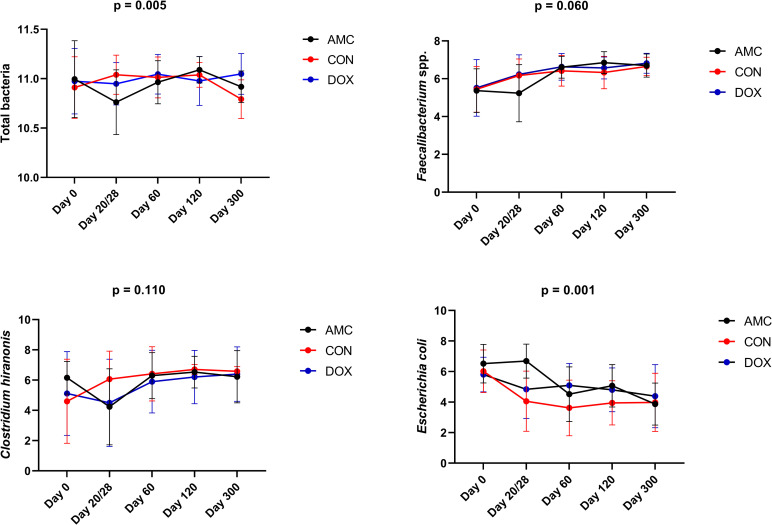
2.1.C) qPCR for selected bacterial groups. On the last day of treatment, lower total bacterial counts (p = 0.003) and higher abundances of E. coli (p = 0.002) were detected in the feces of AMC cats compared to CON cats (Fig 9).
Fig 9. Fecal abundances of selected bacterial taxa among cats treated with amoxicillin/clavulanic acid (AMC), cats treated with doxycycline (DOX), and healthy cats (CON) analyzed with qPCR.
Means and standard deviations within each group are displayed.
Bacterial abundances in the AMC group demonstrated a different pattern compared to the CON group. In the AMC group, E. coli abundances did not change between 2 to 3 months of age (i.e., during treatment), and then significantly decreased at 4 months of age (p = 0.012) (Fig 9).
2.2. Doxycycline group. 2.2.A) Sequence analysis–alpha and beta diversity. DOX cats had a significantly higher species richness (observed ASVs, p = 0.025; Chao1, p = 0.029) (Fig 5, S2 Table) on the last day of treatment and a different clustering of the microbiome 1 month after treatment (day 60) compared to CON cats (ANOSIM R = 0.100, p = 0.021; PERMANOVA p = 0.033, q = 0.144) (Fig 10, S3 and S4 Tables). Species richness and evenness indices were also significantly higher within the DOX cats on day 300 compared to day 0 (observed ASVs; Chao1; Shannon p = 0.010).
Fig 10. Principal Coordinate analysis (PCoA) plot of weighted Unifrac distances in cats treated with amoxicillin/clavulanic acid (red = AMC), cats treated with doxycycline (yellow = DOX), and control cats (blue = CON) on day 60.
R values and P values calculated with ANOSIM and PERMANOVA p and q values are displayed.
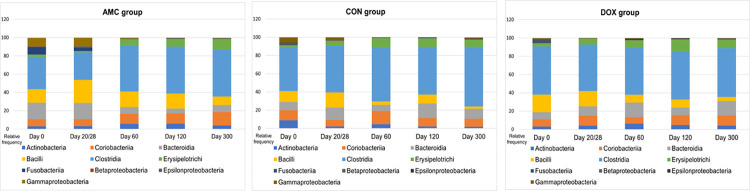
2.2.B) Sequence analysis–abundance of individual bacterial taxa. Doxycycline caused pronounced changes in the abundances of bacterial communities, but its effects appeared 1 month after its discontinuation. Catenibacterim spp., and unclassified Lachnospiraceae spp. (both p = 0.039) were detected at significantly lower abundances whereas Proteobacteria (p = 0.001) and Enterobacteriales (p = 0.018) at significantly higher abundances in the feces of DOX cats compared to CON cats on day 60 (Figs 7 and 8). The increase in the abundance of Proteobacteria persisted for 3 months after antibiotic withdrawal (p = 0.026). In addition, at 3 and 9 months after antibiotic withdrawal, the abundance of unclassified Collinsella spp. was significantly higher in cats of the DOX group compared to cats of the CON group (p = 0.025) (Fig 8). Unclassified Bulleidia spp. were detected at higher abundances (p = 0.023) in DOX cats 9 months after its discontinuation (Fig 7). Fig 11 shows a percentage plot of bacterial abundances at a class level among groups.
Fig 11. Relative abundance of bacterial taxa at a class level among groups.
2.2.C) qPCR for selected bacterial groups. On day 60, higher E. coli abundances (p = 0.035) were found in DOX cats compared to CON cats (Fig 9).
Discussion
Our goals were to describe the effects of treatment with amoxicillin/clavulanic acid or doxycycline on the GI microbiome of young cats and the microbial recovery after antibiotic exposure early in life. Our study showed substantial changes in the GI microbiome from 2 months until one year of age in cats, with antibiotics having a differential impact on the developing GI microbiome. Amoxicillin/clavulanic acid caused pronounced effects during treatment while the effects of doxycycline appeared 1 month after its withdrawal. Both antibiotics mainly affected members of Firmicutes and Proteobacteria and resulted in a delay in the developmental progression of the microbiome compared to the pattern of microbial changes observed over time in cats not treated with antibiotics.
Importantly, a high interindividual variation in bacterial abundances was observed in cats at 2 months of age (before exposure to antibiotics). In humans and dogs, during the phase of microbiota maturation, high-interindividual differences in bacterial abundances occur [63–65], therefore the large variation observed in our study likely represents an immature microbiome in cats at 2 months of age. In addition, the largest shifts in the GI microbiota in the control cats occurred during the age of 2 to 6-months suggesting that the normal GI microbiome evolves in kittens and reaches maturity around the age of 6 months. Although conflicting evidence exists about whether the microbiome reaches an adult-like state at the end of the weaning period in dogs and cats [8,66,67], in a previous canine study, 2-month-old puppies still harbored a significantly different microbiome compared to adult dogs [63].
In adult humans, the abundances of approximately 70% of the GI bacterial members are relatively stable for at least 12 months [8]. Therefore, in contrast to adult cats, the duration of antibiotic effects on the developing GI microbiome could only be investigated by evaluating a control group to adjust for age-related changes. The fact that there was a large variation in microbial community composition at baseline among cats likely led to unique responses to antibiotics. The microbiome is considered as unique as an individual’s fingerprint [68], and during the maturation period, unpredictable shifts could occur that have not been adequately described in cats. Despite the high variability, the core bacterial taxa in cats of our study were Firmicutes and Actinobacteria from 2 months until 1 year of age. This is in agreement with previous studies investigating the effects of dietary nutrient composition [66,69,70], sex, and sexual status [67] on the fecal microbiome of young cats.
Current knowledge suggests that the first microbes colonizing the GI tract are mainly facultative anaerobic bacteria that reduce oxygen concentrations in the gut and allow for successful colonization of the obligative anaerobic bacteria [71]. The phylum Proteobacteria, which is comprised by facultative and obligative anaerobic bacteria, is among the first colonizers of the GI tract in humans [71,72]. At the weaning period and after the introduction of a solid diet (i.e., around 5–6 months of age), the abundance of Proteobacteria gradually decreases [73]. Our finding of an age-dependent decrease in bacterial taxa belonging to Proteobacteria (i.e., Enterobacteriales, Escherichia coli) observed between 2 to 4 months of age in control cats in this study is in agreement with these data in humans. In addition, a concurrent increase in the abundance of taxa belonging to Firmicutes (i.e., Erysipelotrichales) occurred in the same group during the same period, which has also been reported by another study in cats of a similar age and reflects the introduction of dietary macronutrients that are utilized by these bacteria [69].
Treatment with amoxicillin/clavulanic acid led to a reduced species richness and evenness, although this varied among cats and it did not reach statistical significance, while doxycycline let to a significant increase in species richness. Antibiotics, including amoxicillin and doxycycline are most commonly reported to either decrease [41,43,50,74,75] or have no effect on species richness [76]. Only a few studies have reported an increase in species richness indices [46,77]. In adult laboratory cats, amoxicillin/clavulanic acid administration for 7 days reduced the number of different species observed and this effect persisted for 7 days after discontinuation of the antibiotic [51]. In our study, species richness indices were indistinguishable from untreated cats by 1 month after discontinuation of amoxicillin/clavulanic acid Doxycycline had no effect on bacterial abundances and community composition at the last day of the treatment period (day 28). Alternatively, the lack of an effect of doxycycline on bacterial genera that would be expected to decrease as shown in control cats, could be responsible for the observed increased species richness in doxycycline-treated cats. The bloom of these genera might be attributed either to resistance to tetracyclines or to the concurrent decrease of some bacteria that produce antimicrobial peptides thus allowing members of these genera to remain at increased levels [78].
Microbial community composition was distinct in cats treated with amoxicillin/clavulanic acid and indistinguishable in cats treated with doxycycline compared to control cats on the last day of treatment. Interestingly, the effect of doxycycline was not evident until 1 month after drug discontinuation. Similar results have been described in a single study in mice, where the most profound changes in microbial community composition started 1 month after doxycycline discontinuation [49]. In addition, in our study, a trend for significant differences in microbial community composition were observed in amoxicillin/clavulanic acid-treated cats 3 and 9 months after antibiotic withdrawal. Contradictory findings exist in the literature with humans, laboratory animals and in vitro studies reporting high interindividual effects [79], no effects [44,80], only short-term effects [41,46], or both short- and long-term effects on microbial composition [20,45,75] after administration of amoxicillin with or without clavulanic acid. In a study in rats, a 7-day course of amoxicillin during the weaning period caused transient alterations in microbial composition that resolved by 20 days after its discontinuation [43]. In another study in infants, a 5- to 8-day course of amoxicillin caused long-term changes in microbial composition that persisted for 6 months after treatment withdrawal [20].
While the total abundance of the phylum Firmicutes was not significantly altered, certain bacterial members of this phylum showed significant shifts in response to antibiotics. Amoxicillin/clavulanic acid and doxycycline administration caused a transient decrease of the abundance of the order Erysipelotrichales and its sub-groups Erysipelotrichaceae and Catenibacterium spp. The family Erysipelotrichaceae contains bile salt hydrolase (BSH) genes, and this enzyme is responsible for the deconjugation of primary bile acids [81,82]. Thus, the decrease observed could potentially lead to increased concentrations of deconjugated primary bile acids in the gut. In addition to potential bile acid dysmetabolism in cats treated with antibiotics, one of the main converters of primary bile acids into secondary bile acids in dogs and cats is Clostridium hiranonis, which showed a decreased abundance in response to both antibiotics in our study, although this change did not reach statistical significance for either treatment [83]. Families belonging to Clostridiales were affected by antibiotics with a significant decrease in unclassified Lachnospiraceae. The family Lachnospiraceae was the predominant family present at all time points in all groups. Members of this family ferment carbohydrates leading to the production of butyrate [84]. Butyrate is one of the main short chain fatty acids (SCFAs) in the gut and has anti-inflammatory properties, is a major energy source for colonocytes, and its absence causes autophagy of epithelial intestinal cells in germ-free mice [85,86]. As a result, SCFAs might be another main metabolic class influenced by antibiotic treatment. The mechanisms by which antibiotics affect the abundance of bacteria as well as the impact of this reduction on microbial metabolites could be unraveled by applying other “omics” approaches including resistome and metabolomic analysis in future studies.
Among Actinobacteria, the abundance of unclassified Collinsella spp. was higher in both antibiotic-treated groups than in controls at 3 months after discontinuation of treatment. This effect persisted in the amoxicillin-clavulanic acid group for 9 months. Early colonization with Collinsella spp. within the first 6 months of life is associated with increased adiposity in humans, [73] and also increased Collinsella spp. abundances have been reported in cats with diarrhea [87,88].
Based both on sequencing and qPCR analysis, bacterial taxa belonging to Proteobacteria (Gammaproteobacteria, order Enterobacteriales, family Enterobacteriaceae, Escherichia coli) were found at significantly higher abundances on the last day of treatment (20 days) for amoxicillin/clavulanic acid and at 3 months after discontinuation of doxycycline before decreasing to similar abundances to that of control cats. The family Enterobacteriaceae is the most common microbial member that increases in abundance after antibiotic treatment in humans regardless of the antibiotic class [89]. In dogs, metronidazole [27] and amoxicillin [24], but not tylosin [23,28], are reported to increase the abundance of Enterobacteriaceae. In cats, this effect has been observed for amoxicillin [51] and clindamycin [25,26] with the latter leading to a 2-months persistent increase in Enterobacteriaceae [26]. The phylum Proteobacteria encompasses some of the most well-known pathogens [72] and members of this phylum are commonly increased in dogs [90–95] and cats with GI disease [87,88,96–98], as well as during consumption of high-protein, canned and raw diets [66,69,70,99]. Both antibiotic treated groups had higher fecal scores during treatment compared to healthy cats, therefore episodes of diarrhea may be associated with increased abundances of Proteobacteria members.
Previous studies in humans have shown that antibiotics delay the developmental progression of the microbiome into an adult-like state [29,30]. In agreement with these findings and compared to untreated cats of our study, a delay in maturation was observed in both antibiotic-treated groups. This delay was characterized by reduced abundances of taxa belonging to Firmicutes and increased abundances of taxa belonging to Proteobacteria. The most profound delay occurred between 2 to 3 months of age in the amoxicillin/clavulanic acid-treated cats and between 3 to 6 months of age in the doxycycline-treated cats.
Antibiotic treatment prenatally or during early life in humans has been associated with weight gain and obesity [33,34,100]. Both penicillins and tetracyclines have been used in livestock for facilitation of weight gain and growth [48,101]. Facilitation of weight gain has also been observed in severely malnourished children after receiving antibiotics [35]. In our study, no differences in weight gain and body condition scores were observed in any group, though a larger sample size might be required for investigating such effects.
Our study had some limitations. A larger number of animals could have helped in minimizing the interindividual differences observed in microbiome composition. However, previous studies investigating the effects of antibiotics have enrolled similar numbers of animals. All cats were stray at study initiation; thus, their exact date of birth was unknown and slight differences in the enrollment age might have influenced the microbiota composition. Differences in ambient could have also interfered with our results [102]. Some cats were malnourished, and malnourishment has been associated with a persistently immature microbiome in children [103]. In addition, some cats were found at a very young age and required formula feeding, which in children is also reported to impact microbiome colonization compared to breastfeeding [104]. The maternal diet of cats also has an impact on the microbiome of the offspring until its 17th week of age [105] and in our study the maternal dietary status was unknown. Although the above factors have been investigated in humans, no studies regarding their impact on the feline microbiota exist. Finally, cats treated with doxycycline had a significantly higher fecal scores at baseline, which might also have influenced the abundance of some bacterial taxa.
Conclusion
Overall, our results indicate that the GI microbiome of cats changes after 2 months of age and reaches an adult-like state around 6 months of age. Amoxicillin/clavulanic acid and doxycycline treatment early in life significantly affected the developing microbiome richness and composition in cats. The abundance of members of Firmicutes decreased and that of members of Proteobacteria increased after 20 days of amoxicillin/clavulanic acid treatment and 1 month after a 28-day course of doxycycline. Only minor changes were observed 9 months after amoxicillin/clavulanic acid or doxycycline discontinuation with an increase in the abundance of unclassified Collinsella spp. and unclassified Bulleidia spp., respectively. Our results suggest that doxycycline had a delayed impact whereas amoxicillin/clavulanic acid had a more immediate impact on bacterial community composition and only minor changes persisted 9 months after discontinuation of either antibiotic. Future studies utilizing additional approaches to gain a better understanding of the microbial functional changes caused by antibiotics would be useful.
Supporting information
A) Principal Coordinate Analysis of unweighted UniFrac distances of 16S rRNA genes representing the difference in microbial communities among cats treated with amoxicillin clavulanic acid (blue circles), cats treated with doxycycline (yellow circles), and healthy control cats (red circles) on days 20/28 (last day of treatment), 60, 120, and 300. B) Principal Coordinate Analysis of weighted UniFrac distances of 16S rRNA genes representing the difference in microbial communities among cats treated with amoxicillin clavulanic acid (blue circles), cats treated with doxycycline (yellow circles), and healthy control cats (red circles) on days 20/28 (last day of treatment), 60, 120, and 300.
(DOCX)
Rarefaction curves for A) Chao1, B) Observed ASVs, and C) Shannon Index.
(DOCX)
(XLSX)
(XLSX)
CON, healthy cats that did not receive antibiotics; AMC, cats treated with amoxicillin/clavulanic acid for 20 days; DOX, cats treated with doxycycline for 28 days.
(XLSX)
CON, healthy cats that did not receive antibiotics; AMC, cats treated with amoxicillin/clavulanic acid for 20 days; DOX, cats treated with doxycycline for 28 days.
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
Preliminary results were presented at the: a) 29th European College of Veterinary Internal Medicine Congress, Milan, Italy, September 19th to 21st 2019; b) the 38th Forum of the American College of Veterinary Internal Medicine (ACVIM), June 10th to13th 2020; and c) the 39th Forum of the American College of Veterinary Internal Medicine (ACVIM), June 9th to12th 2021.
The authors would like to thank Gerolymatos International S.A. for providing products for antiparasitic treatment (Broadline) and vaccines (Purevax RCPh, Purevax Rabies) for the cats in this study.
Data Availability
The raw sequences files are uploaded to NCBI Sequence Read Archive (project number SRP16253). The remaining relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was funded by the Miller Trust Award of the Winn Feline Foundation (ΜΤ18-003; JSS, PGX, JMS, EMS). The URL of the funder’s website is https://everycat.org/. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mohr KI. History of antibiotics research. Curr Top Microbiol Immunol. 2016;398:237–72. doi: 10.1007/82_2016_499 [DOI] [PubMed] [Google Scholar]
- 2.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–8. doi: 10.1172/JCI72333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agyepong N, Govinden U, Owusu-Ofori A, Essack SY. Multidrug-resistant gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control. 2018;7:37. doi: 10.1186/s13756-018-0324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessandri G, Milani C, Mancabelli L, Longhi G, Anzalone R, Lugli GA, et al. Deciphering the Bifidobacterial populations within the canine and feline gut microbiota. Appl Environ Microbiol. 2020;86(7). doi: 10.1128/AEM.02875-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–93. doi: 10.1038/sj.embor.7400731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyu Y, Su C, Verbrugghe A, Van de Wiele T, Martos Martinez-Caja A, Hesta M. Past, present, and future of gastrointestinal microbiota research in cats. Front Microbiol. 2020;11:1661. doi: 10.3389/fmicb.2020.01661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilla R, Suchodolski JS. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front Vet Sci. 2019;6:498. doi: 10.3389/fvets.2019.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tizard IR, Jones SW. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2018;48(2):307–22. doi: 10.1016/j.cvsm.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Kaur H, Bose C, Mande SS. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front Neurosci. 2019;13:1365. doi: 10.3389/fnins.2019.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinson LF. Establishment of the early-life microbiome: a DOHaD perspective. J Dev Orig Health Dis. 2020;11(3):201–10. doi: 10.1017/S2040174419000588 [DOI] [PubMed] [Google Scholar]
- 12.Candon S, Perez-Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One. 2015;10(5):e0125448. doi: 10.1371/journal.pone.0125448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 14.Soontararak S, Chow L, Johnson V, Coy J, Webb C, Wennogle S, et al. Humoral immune responses against gut bacteria in dogs with inflammatory bowel disease. PLoS One. 2019;14(8):e0220522. doi: 10.1371/journal.pone.0220522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–32. doi: 10.1038/nri.2017.7 [DOI] [PubMed] [Google Scholar]
- 16.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17(5):553–64. doi: 10.1016/j.chom.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrer M, Mendez-Garcia C, Rojo D, Barbas C, Moya A. Antibiotic use and microbiome function. Biochem Pharmacol. 2017;134:114–26. doi: 10.1016/j.bcp.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Neuman H, Forsythe P, Uzan A, Avni O, Koren O. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev. 2018;42(4):489–99. doi: 10.1093/femsre/fuy018 [DOI] [PubMed] [Google Scholar]
- 19.Koo H, Hakim JA, Crossman DK, Kumar R, Lefkowitz EJ, Morrow CD. Individualized recovery of gut microbial strains post antibiotics. NPJ Biofilms Microbiomes. 2019;5:30. doi: 10.1038/s41522-019-0103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korpela K, Salonen A, Saxen H, Nikkonen A, Peltola V, Jaakkola T, et al. Antibiotics in early life associate with specific gut microbiota signatures in a prospective longitudinal infant cohort. Pediatr Res. 2020;88(3):438–43. doi: 10.1038/s41390-020-0761-5 [DOI] [PubMed] [Google Scholar]
- 21.Hornef MW, Torow N. ’Layered immunity’ and the ’neonatal window of opportunity’—timed succession of non-redundant phases to establish mucosal host-microbial homeostasis after birth. Immunology. 2020;159(1):15–25. doi: 10.1111/imm.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilla R, Gaschen FP, Barr JW, Olson E, Honneffer J, Guard BC, et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J Vet Intern Med. 2020. doi: 10.1111/jvim.15871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchester AC, Webb CB, Blake AB, Sarwar F, Lidbury JA, Steiner JM, et al. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J Vet Intern Med. 2019;33(6):2605–17. doi: 10.1111/jvim.15635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronvold AM L ’Abee-Lund TM, Sorum H, Skancke E, Yannarell AC, Mackie RI. Changes in fecal microbiota of healthy dogs administered amoxicillin. FEMS Microbiol Ecol. 2010;71(2):313–26. doi: 10.1111/j.1574-6941.2009.00808.x [DOI] [PubMed] [Google Scholar]
- 25.Whittemore JC, Stokes JE, Laia NL, Price JM, Suchodolski JS. Short and long-term effects of a synbiotic on clinical signs, the fecal microbiome, and metabolomic profiles in healthy research cats receiving clindamycin: a randomized, controlled trial. PeerJ. 2018;6:e5130. doi: 10.7717/peerj.5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittemore JC, Stokes JE, Price JM, Suchodolski JS. Effects of a synbiotic on the fecal microbiome and metabolomic profiles of healthy research cats administered clindamycin: a randomized, controlled trial. Gut Microbes. 2019;10(4):521–39. doi: 10.1080/19490976.2018.1560754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi H, Maeda S, Ohno K, Horigome A, Odamaki T, Tsujimoto H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS One. 2014;9(9):e107909. doi: 10.1371/journal.pone.0107909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suchodolski JS, Dowd SE, Westermarck E, Steiner JM, Wolcott RD, Spillmann T, et al. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rDNA sequencing. BMC Microbiology. 2009;2(9):210. doi: 10.1186/1471-2180-9-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009;56(1):80–7. doi: 10.1111/j.1574-695X.2009.00553.x [DOI] [PubMed] [Google Scholar]
- 30.Gasparrini AJ, Wang B, Sun X, Kennedy EA, Hernandez-Leyva A, Ndao IM, et al. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat Microbiol. 2019;4(12):2285–97. doi: 10.1038/s41564-019-0550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortqvist AK, Lundholm C, Halfvarson J, Ludvigsson JF, Almqvist C. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut. 2019;68(2):218–25. doi: 10.1136/gutjnl-2017-314352 [DOI] [PubMed] [Google Scholar]
- 32.Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109(11):1728–38. doi: 10.1038/ajg.2014.246 [DOI] [PubMed] [Google Scholar]
- 33.Shao X, Ding X, Wang B, Li L, An X, Yao Q, et al. Antibiotic exposure in early life increases risk of childhood obesity: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2017;8:170. doi: 10.3389/fendo.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aversa Z, Atkinson EJ, Schafer MJ, Theiler RN, Rocca WA, Blaser MJ, et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clinic Proceedings. 2020:96(1);66–77. doi: 10.1016/j.mayocp.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8(11):1094–105. doi: 10.1016/S2213-2600(20)30052-7 [DOI] [PubMed] [Google Scholar]
- 37.Watson VE, Jacob ME, Bruno-Barcena JM, Amirsultan S, Stauffer SH, Piqueras VO, et al. Influence of the intestinal microbiota on disease susceptibility in kittens with experimentally-induced carriage of atypical enteropathogenic Escherichia coli. Vet Microbiol. 2019;231:197–206. doi: 10.1016/j.vetmic.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sykes JE. Pediatric feline upper respiratory disease. Vet Clin North Am Small Anim Pract. 2014;44(2):331–42. doi: 10.1016/j.cvsm.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 39.Lappin MR, Blondeau J, Boothe D, Breitschwerdt EB, Guardabassi L, Lloyd DH, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279–94. doi: 10.1111/jvim.14627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White AR, Stokes DH, Slocombe B, Sutherland R. Bactericidal effects of amoxycillin/clavulanic acid and ticarcillin/clavulanic acid in in-vitro kinetic models. J Antimicrob Chemother. 1985;15 Suppl A:227–32. doi: 10.1093/jac/15.suppl_a.227 [DOI] [PubMed] [Google Scholar]
- 41.Graversen KB, Bahl MI, Larsen JM, Ballegaard AR, Licht TR, Bogh KL. Short-term amoxicillin-induced perturbation of the gut microbiota promotes acute intestinal immune regulation in brown Norway rats. Front Microbiol. 2020;11:496. doi: 10.3389/fmicb.2020.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elvers KT, Wilson VJ, Hammond A, Duncan L, Huntley AL, Hay AD, et al. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10(9):e035677. doi: 10.1136/bmjopen-2019-035677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galla S, Chakraborty S, Cheng X, Yeo JY, Mell B, Chiu N, et al. Exposure to amoxicillin in early life is associated with changes in gut microbiota and reduction in blood pressure: Findings from a study on rat dams and offspring. J Am Heart Assoc. 2020;9(2):e014373. doi: 10.1161/JAHA.119.014373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abeles SR, Jones MB, Santiago-Rodriguez TM, Ly M, Klitgord N, Yooseph S, et al. Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome. 2016;4(1):39. doi: 10.1186/s40168-016-0187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabbani TA, Pallav K, Dowd SE, Villafuerte-Galvez J, Vanga RR, Castillo NE, et al. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8(1):17–32. doi: 10.1080/19490976.2016.1267890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Wang Q, Lin H, Das R, Wang S, Qi H, et al. Amoxicillin increased functional pathway genes and beta-lactam resistance genes by pathogens bloomed in intestinal microbiota using a simulator of the human intestinal microbial ecosystem. Front Microbiol. 2020;11:1213. doi: 10.3389/fmicb.2020.01213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: an in vitro study. Mediators Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angelakis E, Million M, Kankoe S, Lagier JC, Armougom F, Giorgi R, et al. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob Agents Chemother. 2014;58(6):3342–7. doi: 10.1128/AAC.02437-14 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Becker E, Schmidt TSB, Bengs S, Poveda L, Opitz L, Atrott K, et al. Effects of oral antibiotics and isotretinoin on the murine gut microbiota. Int J Antimicrob Agents. 2017;50(3):342–51. doi: 10.1016/j.ijantimicag.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 50.Boynton FDD, Ericsson AC, Uchihashi M, Dunbar ML, Wilkinson JE. Doxycycline induces dysbiosis in female C57BL/6NCrl mice. BMC Res Notes. 2017;10(1):644. doi: 10.1186/s13104-017-2960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres-Henderson C, Summers S, Suchodolski J, Lappin MR. Effect of Enterococcus Faecium strain SF68 on gastrointestinal signs and fecal microbiome in cats administered amoxicillin-clavulanate. Top Companion Anim Med. 2017;32(3):104–8. doi: 10.1053/j.tcam.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 52.Abu-Sbeih H, Herrera LN, Tang T, Altan M, Chaftari AP, Okhuysen PC, et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J Immunother Cancer. 2019;7(1):242. doi: 10.1186/s40425-019-0714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Development Laflamme D. and validation of a body condition score system for cats: a clinical tool. Feline practice. 1997;25(5/6):13–8. [Google Scholar]
- 54.Laflamme DP, Xu H, Cupp CJ, Kerr WW, Ramadan Z, Long GM. Evaluation of canned therapeutic diets for the management of cats with naturally occurring chronic diarrhea. J Feline Med Surg. 2012;14(10):669–77. doi: 10.1177/1098612X12446906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone AE, Brummet GO, Carozza EM, Kass PH, Petersen EP, Sykes J, et al. 2020 AAHA/AAFP Feline Vaccination Guidelines. J Feline Med Surg. 2020;22(9):813–30. doi: 10.1177/1098612X20941784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18(5):1403–14. doi: 10.1111/1462-2920.13023 [DOI] [PubMed] [Google Scholar]
- 57.Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology. 2015;75(2):129–37. doi: 10.3354/ame01753 [DOI] [Google Scholar]
- 58.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–35. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritchie LE, Burke KF, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Characterization of fecal microbiota in cats using universal 16S rRNA gene and group-specific primers for Lactobacillus and Bifidobacterium spp. Vet Microbiol. 2010;144(1–2):140–6. doi: 10.1016/j.vetmic.2009.12.045 [DOI] [PubMed] [Google Scholar]
- 62.AlShawaqfeh MK, Wajid B, Minamoto Y, Markel M, Lidbury JA, Steiner JM, et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol Ecol. 2017;93(11). doi: 10.1093/femsec/fix136 [DOI] [PubMed] [Google Scholar]
- 63.Guard BC, Mila H, Steiner JM, Mariani C, Suchodolski JS, Chastant-Maillard S. Characterization of the fecal microbiome during neonatal and early pediatric development in puppies. PLoS One. 2017;12(4):e0175718. doi: 10.1371/journal.pone.0175718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blake AB, Cigarroa A, Klein HL, Khattab MR, Keating T, Van De Coevering P, et al. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J Vet Intern Med. 2020. doi: 10.1111/jvim.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tauchi H, Yahagi K, Yamauchi T, Hara T, Yamaoka R, Tsukuda N, et al. Gut microbiota development of preterm infants hospitalised in intensive care units. Benef Microbes. 2019;10(6):641–51. doi: 10.3920/BM2019.0003 [DOI] [PubMed] [Google Scholar]
- 66.Deusch O, O’Flynn C, Colyer A, Morris P, Allaway D, Jones PG, et al. Deep Illumina-based shotgun sequencing reveals dietary effects on the structure and function of the fecal microbiome of growing kittens. PLoS One. 2014;9(7):e101021. doi: 10.1371/journal.pone.0101021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deusch O, O’Flynn C, Colyer A, Swanson KS, Allaway D, Morris P. A longitudinal study of the feline faecal microbiome identifies changes into early adulthood irrespective of sexual development. PLoS One. 2015;10(12):e0144881. doi: 10.1371/journal.pone.0144881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tierney BT, Yang Z, Luber JM, Beaudin M, Wibowo MC, Baek C, et al. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe. 2019;26(2):283–95 e8. doi: 10.1016/j.chom.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooda S, Vester Boler BM, Kerr KR, Dowd SE, Swanson KS. The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br J Nutr. 2013;109(9):1637–46. doi: 10.1017/S0007114512003479 [DOI] [PubMed] [Google Scholar]
- 70.Bermingham EN, Young W, Kittelmann S, Kerr KR, Swanson KS, Roy NC, et al. Dietary format alters fecal bacterial populations in the domestic cat (Felis catus). Microbiologyopen. 2013;2(1):173–81. doi: 10.1002/mbo3.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4). doi: 10.1128/MMBR.00036-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon CD, Young W, Maclean PH, Cookson AL, Bermingham EN. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiologyopen. 2018;7(5):e00677. doi: 10.1002/mbo3.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Bruck WM, Berger B, et al. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. mBio. 2015;6(1). doi: 10.1128/mBio.02419-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Sun Y, Wang R, Zhang J. Gut microbiota-mediated drug-drug interaction between amoxicillin and aspirin. Sci Rep. 2019;9(1):16194. doi: 10.1038/s41598-019-52632-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulder M, Radjabzadeh D, Kiefte-de Jong JC, Uitterlinden AG, Kraaij R, Stricker BH, et al. Long-term effects of antimicrobial drugs on the composition of the human gut microbiota. Gut Microbes. 2020;12(1):1795492. doi: 10.1080/19490976.2020.1791677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU, et al. Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. mBio. 2015;6(6):e01693–15. doi: 10.1128/mBio.01693-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung JY, Ahn Y, Khare S, Gokulan K, Pineiro SA, Cerniglia CE. An in vitro study to assess the impact of tetracycline on the human intestinal microbiome. Anaerobe. 2018;49:85–94. doi: 10.1016/j.anaerobe.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 78.Gendrin M, Yerbanga RS, Ouedraogo JB, Lefevre T, Cohuet A, Christophides GK. Differential effects of azithromycin, doxycycline, and cotrimoxazole in ingested blood on the vectorial capacity of malaria mosquitoes. Open Forum Infect Dis. 2016;3(2):ofw074. doi: 10.1093/ofid/ofw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi: 10.1038/ncomms8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacPherson CW, Mathieu O, Tremblay J, Champagne J, Nantel A, Girard SA, et al. Gut bacterial microbiota and its resistome rapidly recover to basal state levels after short-term amoxicillin-clavulanic acid treatment in healthy adults. Sci Rep. 2018;8(1):11192. doi: 10.1038/s41598-018-29229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaakoush NO. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;5:84. doi: 10.3389/fcimb.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baars A, Oosting A, Knol J, Garssen J, van Bergenhenegouwen J. The gut microbiota as a therapeutic target in IBD and metabolic disease: a role for the bile acid receptors FXR and TGR5. Microorganisms. 2015;3(4):641–66. doi: 10.3390/microorganisms3040641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Q, Larouche-Lebel E, Loughran KA, Huh TP, Suchodolski JS, Oyama MA. Gut dysbiosis and its associations with gut microbiota-derived metabolites in dogs with myxomatous mitral valve disease. mSystems. 2021;6(2). doi: 10.1128/mSystems.00111-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12). doi: 10.3390/ijms18122645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. doi: 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramadan Z, Xu H, Laflamme D, Czarnecki-Maulden G, Li QJ, Labuda J, et al. Fecal microbiota of cats with naturally occurring chronic diarrhea assessed using 16S rRNA gene 454-pyrosequencing before and after dietary treatment. J Vet Intern Med. 2014;28(1):59–65. doi: 10.1111/jvim.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suchodolski JS, Foster ML, Sohail MU, Leutenegger C, Queen EV, Steiner JM, et al. The fecal microbiome in cats with diarrhea. PLoS One. 2015;10(5):e0127378. doi: 10.1371/journal.pone.0127378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–8. doi: 10.1159/000443360 [DOI] [PubMed] [Google Scholar]
- 90.Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. Fems Microbiol Ecol. 2008;66:579–89. doi: 10.1111/j.1574-6941.2008.00556.x [DOI] [PubMed] [Google Scholar]
- 91.Suchodolski JS, Xenoulis PG, Paddock CG, Steiner JM, Jergens AE. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet Microbiol. 2010;142(3–4):394–400. doi: 10.1016/j.vetmic.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 92.Suchodolski JS, Dowd SE, Wilke V, Steiner JM, Jergens AE. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 2012;7(6):e39333. doi: 10.1371/journal.pone.0039333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minamoto Y, Otoni CC, Steelman SM, Buyukleblebici O, Steiner JM, Jergens AE, et al. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015;6(1):33–47. doi: 10.1080/19490976.2014.997612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bresciani F, Minamoto Y, Suchodolski JS, Galiazzo G, Vecchiato CG, Pinna C, et al. Effect of an extruded animal protein-free diet on fecal microbiota of dogs with food-responsive enteropathy. J Vet Intern Med. 2018;32(6):1903–10. doi: 10.1111/jvim.15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalenyak K, Isaiah A, Heilmann RM, Suchodolski JS, Burgener IA. Comparison of the intestinal mucosal microbiota in dogs diagnosed with idiopathic inflammatory bowel disease and dogs with food-responsive diarrhea before and after treatment. FEMS Microbiol Ecol. 2018;94(2). doi: 10.1093/femsec/fix173 [DOI] [PubMed] [Google Scholar]
- 96.Inness VL, McCartney AL, Khoo C, Gross KL, Gibson GR. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to Desulfovibrio spp. J Anim Physiol Anim Nutr. 2007;91(1–2):48–53. doi: 10.1111/j.1439-0396.2006.00640.x [DOI] [PubMed] [Google Scholar]
- 97.Janeczko S, Atwater D, Bogel E, Greiter-Wilke A, Gerold A, Baumgart M, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol. 2008;128(1–2):178–93. doi: 10.1016/j.vetmic.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 98.Hoehne SN, McDonough SP, Rishniw M, Simpson KW. Identification of mucosa-invading and intravascular bacteria in feline small intestinal lymphoma. Vet Pathol. 2017;54(2):234–41. doi: 10.1177/0300985816664792 [DOI] [PubMed] [Google Scholar]
- 99.Schmidt M, Unterer S, Suchodolski JS, Honneffer JB, Guard BC, Lidbury JA, et al. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS One. 2018;13(8):e0201279. doi: 10.1371/journal.pone.0201279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jess T, Morgen CS, Harpsoe MC, Sorensen TIA, Ajslev TA, Antvorskov JC, et al. Antibiotic use during pregnancy and childhood overweight: A population-based nationwide cohort study. Sci Rep. 2019;9(1):11528. doi: 10.1038/s41598-019-48065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. 2017;106:162–70. doi: 10.1016/j.micpath.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 102.Yang Y, Li X, Cao Z, Qiao Y, Lin Q, Liu J, et al. Effects of different ambient temperatures on caecal microbial composition in broilers. Pol J Microbiol. 2021;70(1):33–43. doi: 10.33073/pjm-2021-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. doi: 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–17. doi: 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Young W, Moon CD, Thomas DG, Cave NJ, Bermingham EN. Pre- and post-weaning diet alters the faecal metagenome in the cat with differences in vitamin and carbohydrate metabolism gene abundances. Sci Rep. 2016;6:34668. doi: 10.1038/srep34668 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Principal Coordinate Analysis of unweighted UniFrac distances of 16S rRNA genes representing the difference in microbial communities among cats treated with amoxicillin clavulanic acid (blue circles), cats treated with doxycycline (yellow circles), and healthy control cats (red circles) on days 20/28 (last day of treatment), 60, 120, and 300. B) Principal Coordinate Analysis of weighted UniFrac distances of 16S rRNA genes representing the difference in microbial communities among cats treated with amoxicillin clavulanic acid (blue circles), cats treated with doxycycline (yellow circles), and healthy control cats (red circles) on days 20/28 (last day of treatment), 60, 120, and 300.
(DOCX)
Rarefaction curves for A) Chao1, B) Observed ASVs, and C) Shannon Index.
(DOCX)
(XLSX)
(XLSX)
CON, healthy cats that did not receive antibiotics; AMC, cats treated with amoxicillin/clavulanic acid for 20 days; DOX, cats treated with doxycycline for 28 days.
(XLSX)
CON, healthy cats that did not receive antibiotics; AMC, cats treated with amoxicillin/clavulanic acid for 20 days; DOX, cats treated with doxycycline for 28 days.
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The raw sequences files are uploaded to NCBI Sequence Read Archive (project number SRP16253). The remaining relevant data are within the manuscript and its Supporting Information files.