Abstract
Background
Depression occurs frequently in individuals with coronary artery disease (CAD) and is associated with a poor prognosis.
Objectives
To determine the effects of psychological and pharmacological interventions for depression in CAD patients with comorbid depression.
Search methods
We searched the CENTRAL, MEDLINE, Embase, PsycINFO, and CINAHL databases up to August 2020. We also searched three clinical trials registers in September 2021. We examined reference lists of included randomised controlled trials (RCTs) and contacted primary authors. We applied no language restrictions.
Selection criteria
We included RCTs investigating psychological and pharmacological interventions for depression in adults with CAD and comorbid depression. Our primary outcomes included depression, mortality, and cardiac events. Secondary outcomes were healthcare costs and utilisation, health‐related quality of life, cardiovascular vital signs, biomarkers of platelet activation, electrocardiogram wave parameters, non‐cardiac adverse events, and pharmacological side effects.
Data collection and analysis
Two review authors independently examined the identified papers for inclusion and extracted data from the included studies. We performed random‐effects model meta‐analyses to compute overall estimates of treatment outcomes.
Main results
Thirty‐seven trials fulfilled our inclusion criteria. Psychological interventions may result in a reduction in end‐of‐treatment depression symptoms compared to controls (standardised mean difference (SMD) −0.55, 95% confidence interval (CI) −0.92 to −0.19, I2 = 88%; low certainty evidence; 10 trials; n = 1226). No effect was evident on medium‐term depression symptoms one to six months after the end of treatment (SMD −0.20, 95% CI −0.42 to 0.01, I2 = 69%; 7 trials; n = 2654). The evidence for long‐term depression symptoms and depression response was sparse for this comparison. There is low certainty evidence that psychological interventions may result in little to no difference in end‐of‐treatment depression remission (odds ratio (OR) 2.02, 95% CI 0.78 to 5.19, I2 = 87%; low certainty evidence; 3 trials; n = 862). Based on one to two trials per outcome, no beneficial effects on mortality and cardiac events of psychological interventions versus control were consistently found. The evidence was very uncertain for end‐of‐treatment effects on all‐cause mortality, and data were not reported for end‐of‐treatment cardiovascular mortality and occurrence of myocardial infarction for this comparison.
In the trials examining a head‐to‐head comparison of varying psychological interventions or clinical management, the evidence regarding the effect on end‐of‐treatment depression symptoms is very uncertain for: cognitive behavioural therapy compared to supportive stress management; behaviour therapy compared to person‐centred therapy; cognitive behavioural therapy and well‐being therapy compared to clinical management. There is low certainty evidence from one trial that cognitive behavioural therapy may result in little to no difference in end‐of‐treatment depression remission compared to supportive stress management (OR 1.81, 95% CI 0.73 to 4.50; low certainty evidence; n = 83). Based on one to two trials per outcome, no beneficial effects on depression remission, depression response, mortality rates, and cardiac events were consistently found in head‐to‐head comparisons between psychological interventions or clinical management.
The review suggests that pharmacological intervention may have a large effect on end‐of‐treatment depression symptoms (SMD −0.83, 95% CI −1.33 to −0.32, I2 = 90%; low certainty evidence; 8 trials; n = 750). Pharmacological interventions probably result in a moderate to large increase in depression remission (OR 2.06, 95% CI 1.47 to 2.89, I2 = 0%; moderate certainty evidence; 4 trials; n = 646). We found an effect favouring pharmacological intervention versus placebo on depression response at the end of treatment, though strength of evidence was not rated (OR 2.73, 95% CI 1.65 to 4.54, I2 = 62%; 5 trials; n = 891). Based on one to four trials per outcome, no beneficial effects regarding mortality and cardiac events were consistently found for pharmacological versus placebo trials, and the evidence was very uncertain for end‐of‐treatment effects on all‐cause mortality and myocardial infarction.
In the trials examining a head‐to‐head comparison of varying pharmacological agents, the evidence was very uncertain for end‐of‐treatment effects on depression symptoms. The evidence regarding the effects of different pharmacological agents on depression symptoms at end of treatment is very uncertain for: simvastatin versus atorvastatin; paroxetine versus fluoxetine; and escitalopram versus Bu Xin Qi.
No trials were eligible for the comparison of a psychological intervention with a pharmacological intervention.
Authors' conclusions
In individuals with CAD and depression, there is low certainty evidence that psychological intervention may result in a reduction in depression symptoms at the end of treatment. There was also low certainty evidence that pharmacological interventions may result in a large reduction of depression symptoms at the end of treatment. Moderate certainty evidence suggests that pharmacological intervention probably results in a moderate to large increase in depression remission at the end of treatment. Evidence on maintenance effects and the durability of these short‐term findings is still missing. The evidence for our primary and secondary outcomes, apart from depression symptoms at end of treatment, is still sparse due to the low number of trials per outcome and the heterogeneity of examined populations and interventions. As psychological and pharmacological interventions can seemingly have a large to only a small or no effect on depression, there is a need for research focusing on extracting those approaches able to substantially improve depression in individuals with CAD and depression.
Plain language summary
Treatments for depression in individuals with coronary artery disease
This review examined clinical trials on psychological treatments and antidepressant drugs in individuals with coronary artery disease and depression. The objective was to determine the effects of these treatments on depression, mortality, cardiac events such as another heart attack, or heart surgery.
We identified 37 trials as relevant for the review. Fifteen trials investigated psychological treatments, and 21 trials investigated pharmacological interventions including antidepressant drugs.
Generally, psychological treatments compared to controls, and antidepressant drugs compared to placebo (inactive drug), may result in a reduction in depression symptoms at the end of treatment; however, the evidence is generally of low certainty. The evidence is very uncertain as to whether psychological treatments compared to control and antidepressant drugs compared to placebo reduce mortality and cardiovascular events.
The evidence is current to August 2020.
Summary of findings
Background
Coronary artery disease (CAD) is amongst the leading causes of death for both men and women in middle‐ and high‐ income countries (Roth 2017). A strong association between CAD and comorbid depression has been consistently reported (Baune 2012; Kendler 2009; Scherrer 2003; Schulman‐Marcus 2016; Stenman 2014), which is similar to the association observed in other chronic disease populations (Chen 2019; Härter 2007; Matte 2016; Mezuk 2015; Petrak 2015). Results from the World Mental Health Surveys, Ormel 2007, indicate a twofold increased risk of depression for individuals with heart disease compared to those without heart disease and conversely, an increased risk of developing incident heart disease in individuals with depression compared to those without depression (Scott 2013). Prevalence rates of major depression in CAD populations, including those undergoing coronary revascularisation procedures, range from 15% to 20% (Nieuwsma 2017; Thombs 2008; Tully 2012), and are thus disproportionate to that observed in the general community (Kessler 2010).
The increased prevalence rates raise the issue of the impact of comorbid depression on the lives of these individuals and the healthcare system. Several original studies and systematic reviews document a significant prognostic association between comorbid depression and increased mortality, morbidity, and healthcare costs, as well as diminished quality of life and adherence to treatment regimen (Abberger 2017; Barth 2004; Baumeister 2011c; Frasure‐Smith 2003a; Frasure‐Smith 2008; Lichtman 2014; Nicholson 2006).
Description of the condition
Coronary artery disease is one of the most common forms of heart disease. One of the main underlying problems in cardiovascular disease is atherosclerosis, a process that obstructs blood vessels with deposits of fat, cholesterol, and other substances (WHO 1992). It is most serious when it restricts the blood supply to the heart itself (myocardial ischaemia). Clinical manifestations of CAD are acute coronary syndrome comprising myocardial infarction (MI) and unstable angina (Antman 2004), as well as stable angina pectoris (Fox 2006). MI refers to what is commonly known as a 'heart attack'. It occurs when prolonged myocardial ischaemia leads to myocardial cell death (necrosis) (Alpert 2000).
Depression is an emotional state characterised by strong feelings of sadness, worthlessness and guilt, withdrawal from others, sleeplessness, and loss of appetite, sexual desire, and interest in usual activities (Davison 2003), occurring in several subtypes (Baune 2012). Two key diagnostic criteria for major depression are depressed mood and loss of pleasure or interest in activities (anhedonia; APA 2013). Depressive disorders can be reliably diagnosed through structured clinical interviews. The severity of depressive symptoms is usually assessed by patient‐ or clinician‐administered rating scales that have undergone psychometric validation. Cut‐off scores have been validated for these scales that correspond to the likelihood of an indication of depression (Sadock 2009). Recommendations for the assessment of depression in individuals with cardiovascular disease are available (Davidson 2006; Lichtman 2014; Nieuwsma 2017; Thombs 2008).
Description of the intervention
Psychological interventions comprise cognitive behavioural therapy (CBT), psychodynamic psychotherapy, interpersonal therapy (IPT), other approaches such as problem‐solving therapy, non‐directive or supportive therapy and counselling as well as single techniques of these interventions (Davison 2003). Other interventions comprise acceptance and commitment therapy, mindfulness‐based cognitive therapy and mindfulness‐based stress reduction, emotion‐focused therapy, and metacognitive therapy (Australian Psychological Society 2018). The mode of delivery comprises individual, group, or family (including couple) therapy carried out by a healthcare professional.
A network meta‐analysis comparing seven psychotherapeutic approaches concluded that most approaches were equally effective, with IPT being more effective than supportive therapy (Cohen's d = −0.30, 95% confidence interval (CI) −0.54 to −0.05) (Barth 2013). However, this conclusion needs to be considered preliminary, as single trials lack statistical power, and meta‐analyses are limited by the heterogeneous methodological standards of primary studies (Cuijpers 2016). This might be even more true regarding psychological depression interventions for individuals with CAD, given the diversity of psychological interventions offered, from nurse‐led and low‐intensive, two‐session interventions to regular psychotherapies with at least 12 to 16 therapy sessions, offered at varying time points post‐cardiac event (Baumeister 2011c; Baumeister 2012b; Doyle 2021).
Antidepressant drugs are commonly used treatments in people with depression. In general, the available medications do not differ in their overall efficacy and effectiveness, but differ substantially with regard to short‐ and long‐term side effects (NICE 2009; Sadock 2009). Antidepressant treatment selection depends on the type of depressive disorder and the presence of comorbid somatic or mental disorders. The main pharmacological classes of antidepressant medications are selective serotonin reuptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs). For CAD patients with moderate, severe, or recurrent depression, SSRIs are viewed as safe and effective pharmacological agents (Lichtman 2008). In contrast, TCAs and MAOIs are contraindicated in CAD patients because of their cardiac side effects such as prolongation of the QT interval on electrocardiogram (Lichtman 2008). Other potential pharmacological interventions include repurposing vascular drugs intended to lower cholesterol or blood pressure for the treatment of depression (Cipriani 2016; Taragano 2005). In addition, interventions may explore diet and supplements such as n‐3 polyunsaturated fatty acids, also known as omega‐3 oils (Appleton 2015).
A systematic review experimentally comparing psychological or psychotherapy and pharmacological approaches indicated that overall, psychological and pharmacological interventions are equally effective for treating depression, with pharmacotherapy seemingly being superior in dysthymia (Hedges' g = 0.3) as well as compared to non‐directive counselling (Hedges' g = 0.33), and psychotherapy being superior to tricyclic antidepressants (Hedges' g = 0.21) (Cuijpers 2013). Combining both pharmacotherapy and psychotherapy is superior to pharmacotherapy alone at six months or longer postrandomisation (odds ratio (OR) 2.93). However, and conversely, psychotherapy alone compared to combined therapy resulted in equal depression effects at six months follow‐up and longer (Karyotaki 2016).
How the intervention might work
Many biological and behavioural mechanisms linking CAD and depression have been proposed (Carney 2017; Härter 2007a; Joynt 2003; Musselman 1998; Skala 2006), comprising pathophysiological pathways such as decreased heart rate variability, platelet activation, and endothelial dysfunction in depressed CAD patients (Antman 2004). Furthermore, an accumulation of behavioural (smoking, physical inactivity, and imbalanced diet) and medical risk factors (hypertension, diabetes, and obesity) in depressed patients might affect the development and course of CAD (Joynt 2003; Whooley 2008). Psychosocial stress constitutes a risk factor for both CAD and depression (Joynt 2003).
A review concluded that pharmacological interventions for depression might influence physiological pathways linking depression and CAD (Carney 2017). Psychological treatments may also affect physiological processes, but the interrelations between behavioural and physiological mechanisms remain less clear (Carney 2017). Psychological interventions might improve not only depression outcomes in CAD patients with comorbid depressive disorder, but also medical outcome parameters, by encouraging behaviour changes towards a healthier lifestyle in these patients (Firth 2019; Richards 2017).
Why it is important to do this review
Due to high prevalence rates and the impact of comorbid depression on both medical and psychosocial outcomes, there is a need for effective depression treatments in CAD. In various systematic reviews, psychological and psychopharmacological interventions have proven to be effective interventions for the treatment of major depression (Cuijpers 2008a; Cuijpers 2008b; Cuijpers 2013; Karyotaki 2016; NICE 2009; Sadock 2009). However, the evidence on the effectiveness of psychological and pharmacological depression interventions for people with CAD and depression is far less conclusive (Baumeister 2011c), and prone to bias in the literature (Doyle 2021), which argues for an update of our Cochrane Review. Several clinical guidelines recommend depression intervention in CAD populations whilst noting the limited efficacy in preventing major adverse cardiac events, based on few trials to date (Hillis 2011; Lichtman 2014).
Another Cochrane Review examined the effects of non‐specific psychological interventions in CAD patients and found small to moderate reductions in depression, anxiety, and stress symptoms as well as a 22% reduction in MI compared to usual care (Richards 2017). However, the review did not study the effects of depression‐specific treatment in the population of CAD patients with a comorbid depressive disorder or depression symptoms. Furthermore, the review included non‐specific psychological interventions and interventions delivered in combination with cardiac rehabilitation, whereas the focus of our review is on depression‐specific psychological or pharmacological interventions explicitly used for treating depression in populations with depression. Some randomised controlled trials may be included in both reviews, but the research questions remain different owing to the focus of our review on the effects of depression treatments in depressed CAD patients.
The current review will permit the drawing of conclusions on the effects of depression treatment in CAD patients with comorbid depressive disorders. Depending on the number of primary studies, conclusions may be drawn concerning differential effects of type of intervention on depression and mortality or cardiovascular events, as well as on participant quality of life (QoL), thus providing a basis for treatment recommendations. Furthermore, follow‐up data may be examined concerning the healthcare costs of the interventions. Sources of heterogeneity in the results of the primary studies can be explored and could help provide suggestions for the design of future studies.
Objectives
To determine the effects of psychological and pharmacological interventions for depression in CAD patients with comorbid depression.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) of any length of treatment and any length of follow‐up. Both individually and cluster‐randomised clinical trials were eligible. We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
Adults (18 years or older) with CAD (International Statistical Classification of Diseases and Related Health Problems (ICD‐10): I20‐I25, WHO 1992, or later versions of the ICD) and comorbid depressive disorder (ICD‐10: F32/33/34.1 (WHO 1992); Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM‐III‐R): 296.xx; 300.4, APA 1987, or later versions of diagnostic classification systems; including subthreshold conditions) assessed by standardised interviews, self‐reports, medical records, or physicians' diagnosis. Studies comprised of individuals with non‐CAD conditions were ineligible. Inclusion of primary studies was not further limited to specific clinical subgroups in order to increase the generalisability of the results of the review.
With regard to comorbid depression, studies comprising mixed study samples (e.g. both depressed CAD patients and CAD patients with low social support (ENRICHD 2003)) were included in the review.
Types of interventions
Psychological interventions comprise CBT, psychodynamic psychotherapy, IPT, non‐directive or supportive therapy and counselling (Davison 2003), acceptance and commitment therapy, mindfulness‐based cognitive therapy, mindfulness‐based stress reduction, emotion‐focused therapy, and metacognitive therapy (Australian Psychological Society 2018). In the first instance, we pooled all psychological interventions together, conducted analyses of heterogeneity, and took this into consideration when adjudicating the strength of evidence. The mode of delivery was defined as individual, group, or family (including couple) therapy carried out in whole or in part by a healthcare professional. The comparison group was defined consistent with a similar review of type 1 diabetes interventions (Winkley 2020): 'no intervention', 'usual care', 'wait‐list control', 'attention‐control' or 'clinical management' (CM).
With regard to differential or incremental effects of different treatment approaches, we also considered trials with a control group receiving pharmacological treatment or another psychological treatment (Comparison 2 and Comparison 4). In accordance with the previous review (Baumeister 2011c), we grouped separately studies using CM as a comparator intervention or other psychological intervention. The rationale for this was that CM, which consists of information about depression and depression treatment, provides a more concerted approach to depression management than does usual care (CREATE 2007), with CM delivered by health professionals and often for equal intensity as an intervention. By contrast, usual care commonly involves no depression treatment at all, even when incentives are provided (Jani 2013; Rollman 2009). In head‐to‐head comparison trials of psychological interventions or CM, we abstained from pooling across different treatments, consistent with the original review (Baumeister 2011c), owing to the heterogeneity in clinical interventions and their heterogenous comparators.
Pharmacological interventions included all antidepressant medications and other drug therapies used explicitly for treating depressive disorders (Sadock 2009). The control group was placebo. In the first instance, we pooled all pharmacological interventions and conducted analyses of heterogeneity. We included pharmacological treatments compared to other pharmacological medications, as well as add‐on therapies or augmentation strategies, or by comparison to psychological interventions, to determine differential or incremental effects. In accordance with the previous review (Baumeister 2011c), we grouped separately head‐to‐head comparison trials of pharmacological interventions. We abstained from pooling across different studies owing to the heterogeneity in clinical interventions and their heterogenous comparators.
Types of outcome measures
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review. Where a published report did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether the outcomes were measured but not reported. Relevant trials that measured these outcomes but did not report the data at all, or not in a useable format, were included in the review as part of the narrative.
We assessed outcomes at three follow‐up periods, consistent with the previous review by Baumeister 2011c:
short term (at the end of treatment), which was the primary time point of clinical interest for the review;
medium term (one to six months after the end of treatment);
long term (more than six months after the end of treatment).
Multiple observations in primary studies were allocated to separate analyses by different time frames, which reflect short‐, medium‐, and long‐term follow‐up. The rationale for subdividing outcomes by time was to assess the durability of interventions, given that evidence was sparse for longer‐term outcomes in the previous review (Baumeister 2011c).
Primary outcomes
Depression (measured either dimensionally or categorically) following the intervention, as assessed by validated self‐report questionnaires or standardised interviews. Depression may be quantified categorically as 'remitted' or 'response', the latter defined as a 50% or more reduction in severity from baseline.
All‐cause mortality.
Cardiovascular mortality.
-
Non‐fatal cardiac events according to standardised criteria (e.g. WHO 1992 or subsequent iterations):
myocardial infarction (MI);
angina;
heart failure;
arrhythmia;
stroke;
Coronary revascularisation for CAD: coronary artery bypass graft (CABG) and/or percutaneous coronary intervention (PCI) or angioplasty.
We analysed the primary outcomes separately and abstained from pooling a composite outcome, with two exceptions: 1) acute coronary syndromes (inclusive of ST and non‐ST elevated MI, and/or unstable angina) were collapsed into MI (for one study, U‐CARE 2018); and 2) coronary revascularisation for CAD was inclusive of CABG and/or PCI or angioplasty. Here we grouped coronary revascularisation for CAD under cardiac events, as opposed to healthcare utilisation, in line with common definitions of major adverse cardiac events (Bosco 2021).
Secondary outcomes
-
Healthcare costs or resource utilisation, including:
hospitalisations;
emergency department visits;
length of stay.
Health‐related quality of life.
-
Cardiovascular vital signs:
systolic blood pressure (BP) measured in mmHg;
diastolic BP measured in mmHg;
heart rate measured in beats per minute (bpm).
-
Biomarkers of platelet activation:
β‐thromboglobulin (βTG);
platelet factor 4 (PF4);
P‐selectin;
platelet/endothelial cell adhesion molecule‐1 (PECAM‐1);
thromboxane B2 (TxB2).
-
Electrocardiogram (ECG) wave recording in milliseconds:
PR interval;
QRS interval;
QT interval;
QTc interval.
Non‐cardiac adverse events (psychiatric admission, suicide, worsening depression). In pharmacological interventions side effects were also assessed.
Analysis of the secondary outcomes cardiovascular vital signs and biomarkers of platelet activation was considered in the previous review but not reported (Baumeister 2011c). We also added important adverse effects (ECG wave recording, non‐cardiac adverse events, and pharmacological side effects) to this updated review, which we define as post hoc outcomes. We analysed the secondary outcomes separately and abstained from pooling any composite outcomes apart from pharmacological side effects, which we considered as a composite of any quantified side effect by self‐report scale, checklist, or adverse outcome.
Search methods for identification of studies
Electronic searches
We searched the following databases for RCTs of treatment of depressive disorders in CAD patients on 3 August 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, Issue 8 of 12, 2020);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 31 July 2020);
Embase (Ovid, 1980 to 2020 Week 31);
PsycINFO (Ovid, 1806 to July Week 4 2020);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO, 1937 to 3 August 2020);
Database of Abstracts of Reviews of Effects (DARE) and NHS Economic Evaluation Database (EED) (Cochrane Library, Issue 2 of 4, 2015);
Health Technology Assessment Database (HTA) (Cochrane Library Issue 4 of 4, 2016).
The Cochrane sensitivity‐maximising RCT filter was used for MEDLINE, and for Embase, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions were applied (Lefebvre 2011). Adaptations of these RCT filters were applied to the other databases, except CENTRAL. See Appendix 1 for details of the 2009 search strategies and Appendix 2 for the updated 2020 search strategies. No language restrictions were applied.
Searching other resources
We searched the World Heath Organization International Clinical Trials Registry Platform (https://trialsearch.who.int/), ISRCTN registry (http://isrctn.org/), and ClinicalTrials.gov (clinicaltrials.gov) on 2 September 2021 (Appendix 2). We also examined the reference lists of all included trials to identify other potentially relevant studies. We contacted corresponding authors of the included trials to ask about other RCTs, published or unpublished, which might be relevant to the review. We handsearched the list of included and excluded studies in the Cochrane Review by Richards 2017 and the network meta‐analysis by Doyle 2021.
Data collection and analysis
Selection of studies
Two review authors independently in pairs selected studies for inclusion (original review: NH, HB; update: SYA, EJLL, EB, NB). We examined a list of titles and abstracts; if title and abstract contained sufficient information to determine exclusion, the article was rejected. We retrieved the full papers of all remaining articles, which two review authors independently reviewed. In addition, any other potentially relevant articles identified by checking the reference lists or personal communications were also reviewed. We kept a record of all rejected papers and the reasons for rejection. We used this information to construct a PRISMA flow diagram (Figure 1), and reported the reasons for exclusion of excluded studies in the Characteristics of excluded studies table. Important parts of foreign language papers of included studies (i.e. not English, German, or Chinese) were translated into English. If the two review authors disagreed about the inclusion of an article, a third review author (original review: JB; update: PJT) was asked to review the article. Any disagreements were resolved by consensus discussion.
1.
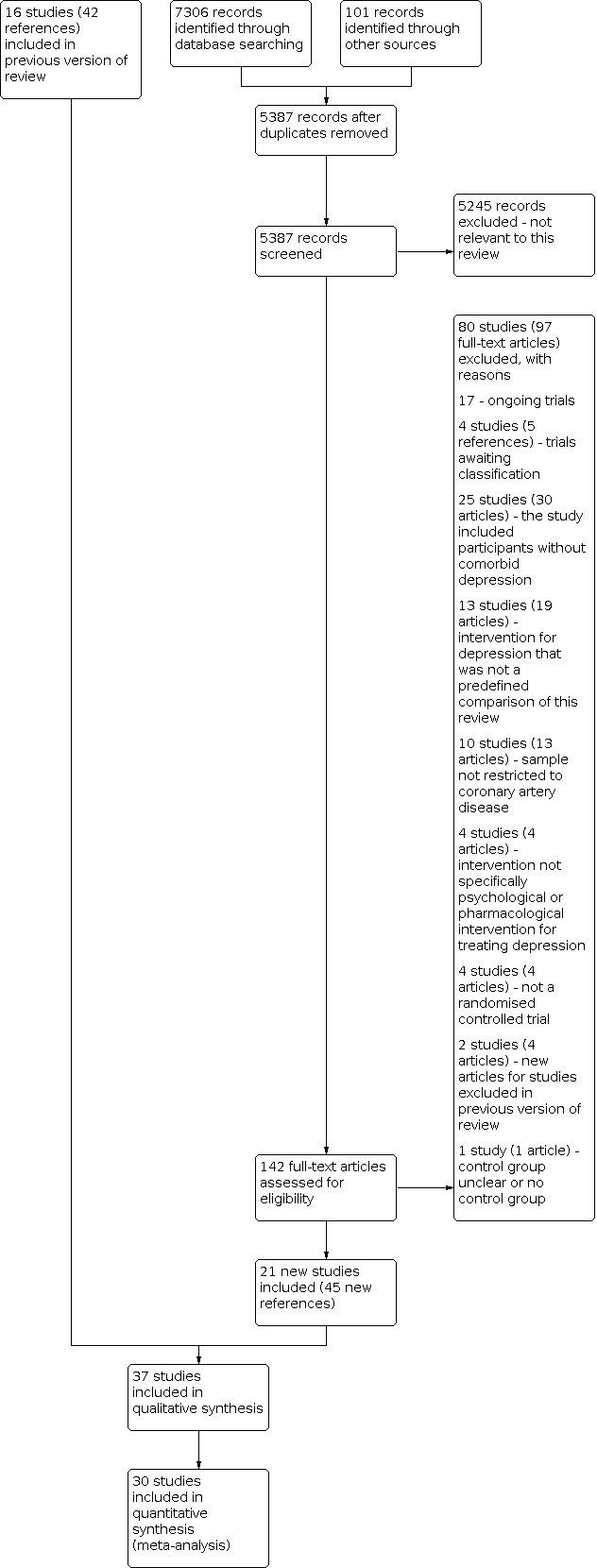
Summary of the 2020 literature search update and study selection.
Data extraction and management
Two review authors (original review: HB, NH; update: PJT, SYA, EJLL, NB, EB) independently in pairs extracted data from the full copies of primary studies using a data extraction form. We extracted study characteristics including participants (sample size at baseline and follow‐up, type of CAD, gender, age), type of depression (major depression, minor depression or dysthymic disorder), assessment method (standardised diagnostic interview, self‐report questionnaire, medical record or physician’s diagnosis), cut‐off used to indicate depression on self‐report questionnaire, type of intervention (type of psychological treatment versus type of pharmacological treatment), comparison group (usual care, other control, another psychological treatment or pharmacological treatment), length of follow‐up, descriptive statistics of primary and secondary outcomes, effect sizes and confidence intervals.
Assessment of risk of bias in included studies
Two review authors (original review HB, NH; update: PJT, SYA, EJLL, EB, NB) independently in pairs assessed risk of bias in the included studies using Cochrane's tool for assessing risk of bias (Higgins 2011). We described sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. With regard to psychological interventions, blinding of healthcare providers or participants to the treatment is not feasible. In pharmacological trials blinding is possible for participants, personnel, and outcome assessors, and was evaluated accordingly. We considered a trial as having an overall high risk of bias when four domains out of six were assessed as high or unclear for: allocation (sequence generation and concealment), blinding (participants, personnel, and outcome assessors), incomplete outcome data, and selective reporting. In the event that we identified an other source of bias at high risk, this also contributed to the overall adjudication of a trial at high risk of bias.
Measures of treatment effect
Continuous outcomes measured using different scales necessitated the standardisation of the results of the studies to a uniform scale. We computed standardised mean differences (SMD) with 95% confidence intervals (CIs) for continuous outcomes measured using different scales. As a first preference we analysed the mean scores of final assessment, followed by mean change scores from baseline to final assessment if only these scores were available. If no measures of variability were provided in the study reports, we used exact P values as well as t‐statistic or Chi2 statistic to compute an SMD.
For dichotomous variables, we computed odds ratios (OR) with 95% CI. For continuous primary and secondary outcomes assessed by the same method (i.e. Hamilton Depression Rating Scale (HAM‐D), BP, heart rate, ECG parameters), we used a mean difference (non‐standardised).
Several strategies have been proposed to help readers interpret results presented as SMDs (e.g. re‐expressing SMDs using Cohen's rules of thumb for effect sizes (Cohen 1988), re‐expressing SMDs by transformation to OR, re‐expressing SMDs using a familiar instrument, reporting the ratio of the means, or expressing as minimal important difference units; see also Section 15.5.3 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). However, all of these strategies have substantial disadvantages and introduce imprecision. For example, re‐expressing SMDs by means of familiar instruments does not account for between‐study heterogeneity. An SMD of a specific magnitude translates into different scores (e.g. on the Beck Depression Inventory (BDI)) depending on, for example, the baseline severity of depression. Conclusions based on this strategy might thus be substantially misleading. We decided to use the rule of thumb proposed by Cohen 1988 and suggested by the Cochrane Handbook to re‐express SMDs (Higgins 2011). Based on the assumptions of Cohen 1988, SMDs around 0.2 must be regarded as small, 0.5 as moderate, and 0.8 as large. As previously mentioned, this strategy also comprises substantial disadvantages, as a small, moderate, or large effect size depends on the specific outcome and the assessment instrument being used. Moreover, patient importance of a finding is context‐dependent and not amenable to generic statements (Higgins 2011). When interpreting the results (Baumeister 2012b), readers should keep this limitation of the rules of thumb in mind (Cohen 1988).
Unit of analysis issues
The unit of analysis in the primary studies was the participant, which is randomised to either the treatment or control group. The number of observations thus matches the number of units that are randomised. In instances where observational units were correlated (e.g. by cluster), we planned to reduce the sample to an 'effective sample size', dividing the original sample size by the ‘design effect’ (Higgins 2021).
Dealing with missing data
We requested missing information from published RCTs from the corresponding authors or obtained it from trial data repositories. Of 18 authors contacted for missing data, five replied, and three were able to provide at least some of the requested data. No imputation methods were used due to the small amount of trials per outcome.
Assessment of heterogeneity
We tested for statistically significant heterogeneity using the Q‐statistics with a 95% CI. We computed the I2 to examine the extent of heterogeneity. Meta‐analytically pooled effect estimates should be interpreted in accordance with any substantial clinical or methodological or statistical heterogeneity. We planned to specifically examine heterogeneity with the I2 statistic quantifying inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis. Interpretation of heterogeneity would include the magnitude and direction of effects, the strength of evidence for heterogeneity (e.g. P value from the Chi2 test), and the I2 statistic where:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity (Higgins 2021).
A meta‐regression was considered to explore potential sources of heterogeneity but was not performed owing to the small amount of trials per outcome.
Assessment of reporting biases
We did not create funnel plots to investigate reporting bias due to the limited number of trials per outcome (Higgins 2021). To examine outcome reporting bias, we analysed discrepancies in reported outcomes between published protocols and original papers. Where no protocol was available, we contacted the corresponding trial authors for published or unpublished protocols.
Data synthesis
We performed random‐effects meta‐analyses to compute overall estimates of treatment outcomes based on the assumption of high clinical and methodological heterogeneity between RCTs. Both SMD and OR effect sizes were pooled using the inverse‐variance method, which is best suited to random‐effects meta‐analysis (Higgins 2011). The effect sizes of the primary studies are presented in forest plots. In the case of considerable methodological heterogeneity owing to different intervention types and their heterogenous comparators, we abstained from meta‐analytical pooling of trial results (Comparison 2: psychological versus psychological/CM; Comparison 4: pharmacological versus pharmacological). Where no dichotomous events occurred in both arms of a trial, we described the finding narratively in the text and in the summary of findings tables.
In the case of multiple assessment tools used for the same outcome, we followed a hierarchical approach to decide which assessment to use in the meta‐analyses. Clinician‐rated assessments were given priority over patient self‐report questionnaires. In the case of assessment tools on the same hierarchical level, we chose the most frequently used assessment tool across the included studies, followed by the measure with the least missing data (per‐protocol), followed by random selection of one of the assessment tools.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to examine the impact of sex (men versus women), CAD subtype, time of onset of depression (pre‐existing versus new‐onset depression), CAD severity, and risk of bias of included studies on the results, but did not conduct them due to the sparseness of trial data. We will reconsider these subgroup analyses in future updates of the review.
Sensitivity analysis
Because pooling results across different types of psychological interventions may level out specific treatment effects and be potentially misleading (Baumeister 2011c), we conducted sensitivity analysis on depression symptoms at end of treatment to update the results of Baumeister 2011c for Comparison 1: psychological versus control and Comparison 3: pharmacological versus placebo. For Comparison 1, we performed sensitivity analysis in CBT‐only trials, and similarly in Comparison 3 conducted sensitivity analysis restricted to serotonergic antidepressant interventions. Specifically, the sensitivity analysis included SSRIs and mirtazepine, which can be classed as a noradrenergic and specific serotonergic antidepressant and tetracyclic analogue (see Types of interventions) (de Boer 1995). We also performed sensitivity analyses according to depressive disorders and secondly by depression‐only trials (e.g. excluding mixed depression/anxiety studies).
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence for the primary outcomes (short‐term) using the GRADE approach (GRADEpro GDT), which takes into consideration risk of bias (see Assessment of risk of bias in included studies), consistency of effect, imprecision, indirectness, and publication bias (Schünemann 2019). We constructed a summary of findings table for the primary outcomes (short‐term, end of treatment) for five outcomes: depression symptoms, depression remission, all‐cause mortality, cardiovascular mortality, and occurrence of MI. We made comments narratively to qualitatively describe the certainty of the evidence for the five end‐of‐treatment outcomes per comparison.
Results
Description of studies
See: PRISMA flow chart (Figure 1), Characteristics of included studies; Table 5; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
1. Overview of study population.
| Study ID | Intervention | [n]screened | [n] randomised | [n] ITT | [n] finishing study | [%] of randomised participants finishing study | Comments |
| Abbasi 2015 | Intervention 1 (I1): simvastatin Intervention 2 (I2): atorvastatin |
Total: 206 | I1: 29 I2: 29 Total: 58 |
I1: NR I2: NR Total: NR (per‐protocol) |
I1: 23 I2: 23 Total: 46 |
I1: 79.3% I2: 79.3% Total: 79.3% |
|
| ANDROS 2015 | Intervention (I): sertraline Control (C): placebo |
Total: ? | I: ? C: ? Total: 2 |
I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
Comment: trial terminated early, no results posted |
| Barth 2005 | Intervention (I): resource‐orientated psychotherapy Control (C): usual care |
Total: 1709 | I: 27 C: 32 Total: 59 |
I: 27 C: 32 Total: 59 (per‐protocol) |
I: 27 C: 28 Total: 55 |
I: 100% C: 87.5% Total: 93.2% |
|
| Brown 1993 | Intervention 1 (I1): behaviour therapy Intervention 2 (I2): person‐centred therapy |
Total: 107 | I1: NR I2: NR Total: 54 |
I1: NR I2: NR Total: NR (per‐protocol) |
I1: 20 I2: 20 Total: 40 |
I1: ? I2: ? Total: 74.1% |
Comment: dropout reported in text, no flow chart |
| CREATE 2007 | Intervention 1 (I1): interpersonal psychotherapy, citalopram, clinical management Intervention 2 (I2): citalopram, clinical management Control 1 (C1): interpersonal psychotherapy, placebo, clinical management Control 2 (C2): placebo, clinical management |
Total: 1897 | I1: 67 I2: 75 C1: 75 C2: 67 Total: 284 |
I1: 67 I2: 75 C1: 75 C2: 67 Total: 284 |
I1: 59 I2: 72 C1: 59 C2: 47 Total: 237 |
I1: 88.1% I2: 96.0% C1: 78.7% C2: 70.1% Total: 83.5% |
Comment: 2 x 2 factorial trial; only I2 and C2 data are eligible for this review |
| Carney 2009 | Intervention 1 (I1): sertraline plus omega‐3 Intervention 2 (I2): sertraline plus placebo |
Total: 941 | I1: 62 I2: 60 Total: 122 |
I1: 62 I2: 60 Total: 122 |
I1: 59 I2: 56 Total: 115 |
I1: 95.2% I2: 93.3% Total: 94.3% |
|
| Dao 2011 | Intervention (I): cognitive‐ behavioural therapy C: usual care |
Total: 513 | I: 50 C: 50 Total: 100 |
I: NR C: NR Total: NR (per‐protocol) |
I: 48 C: 48: Total: 96 |
I: 96% C: 96% Total: 96% |
|
| Divsalar 2018 | Intervention 1 (I1): sertraline plus red yeast rice Intervention 2 (I2): sertraline plus placebo |
Total: 101 | I1: 28 I2: 28 Total: 56 |
I1: NR I2: NR Total: NR |
I1: 25 I2: 25 Total: 50 |
I1: 89.3% I2: 89.3% Total: 89.3% |
|
| Doering 2007 | Intervention (I): cognitive‐ behavioural therapy Control (C): usual care |
Total: 117 | I: NR C: NR Total: NR |
I: NR C: NR Total: NR (per‐protocol) |
I: 7 C: 8 Total: 15 |
I: ? C: ? Total: ? |
Comment: reasons for dropout not stated, no flow chart Comment: nested trial within observational study (non‐depressed cohort) |
| EsDEPACS 2014 | Intervention (I): escitalopram Control (C): placebo |
Total: 4809 | I:149 C: 151 Total: 300 |
I: 108 C: 109 Total: 217 (per‐protocol) |
I: 78 C: 79 Total: 157 |
I: 52.3% C: 52.3% Total: 52.3% |
Comment: nested trial within observational study (depressed cohort receiving usual care) |
| ENRICHD 2003 | Intervention (I): cognitive‐ behavioural therapy Control (C): usual care |
Total: 33780 | I: 1238 C: 1243 Total: 2481 |
I: 1238 C: 1243 Total: 2481 |
I: 983 C: 985 Total: 1968 |
I: 79.4% C: 79.2% Total: 79.3% |
|
| Fang 2003 | Intervention (I): health education and psychological intervention Control (C): usual care |
Total: ? | I: 27 C: 30 Total: 57 |
I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
Comment: translated paper |
| Freedland 2009 | Intervention 1 (I1): cognitive‐ behavioural therapy Intervention 2 (I2): supportive stress management Control (C): usual care |
Total: 2955 | I1: 41 I2: 42 C1: 40 Total: 123 |
I1: 41 I2: 42 C1: 40 Total: 123 |
I1: 40 I2: 33 C1: ? Total: ? |
I1: 98% I2: 79% C1: ? Total: ? |
|
| Freeman 1986 | Intervention (I): alprazolam Control (C): placebo |
Total: 459 | I: 54 C: 53 Total: 107 |
I: NR C: NR Total: NR (per‐protocol) |
I: 32 C: 28 Total: 60 |
I: 59.3% C: 52.8% Total: 56.1% |
Comment: no flow chart |
| Kennedy 2005 | Intervention (I): escitalopram Control (C): placebo |
Total: NR | I: 9 C: 10 Total: 19 |
I: NR C: NR Total: NR (per‐protocol) |
I: 2 C: 2 Total: 4 |
I: 22.2% C: 20.0% Total: 21.1% |
Comment: trial terminated early, redacted results posted |
| Li 2005 | Intervention (I): St John's wort extract Control (C): placebo |
Total: ? | I: ? C: ? Total: 87 |
I: ? C: ? Total: ? |
I: 43 C: 39 Total: 82 |
I: ? C: ? Total: 94.3% |
Comment: translated paper |
| Liu 1999 | Intervention (I): fluoxetine Control (C): placebo |
Total: ? | I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
I: 31 C: 37 Total: ? |
I: ? C: ? Total: ? |
Comment: translated paper |
| Liu 2016 | Intervention 1 (I1): sertraline and Shugan Jieyu Intervention 2 (I2): sertraline and placebo |
Total: 3907 | I1: 76 I2: 73 Total: 149 |
I1: 76 I2: 73 Total: 149 |
I1: 48 I2: 46 Total: 94 |
I1: 63.2% I2: 63.0% Total: 63.1% |
Comment: no flow chart, reasons for dropout reported in text |
| MIND‐IT 2007 | Intervention (I): mirtazapine Control (C): placebo |
Total: 2177 | I: 47 C: 44 Total: 91 |
I: 47 C: 44 Total: 91 |
I: 22 C: 18 Total: 40 |
I: 46.8% C: 40.9% Total: 44.0% |
Comment: nested trial within observational study (depressed cohort receiving usual care) |
| Ma 2019 | Intervention (I): Xinkeshu Control (C): placebo |
Total: 312 | I: 30 C: 30 Total: 60 |
I: NR C: NR Total: NR |
I: 28 C: 27 Total: 55 |
I: 93.3% C: 90% Total: 91.7% |
|
| McFarlane 2001 | Intervention (I): sertraline Control (C): placebo |
Total: 238 | I: 18 C: 20 Total: 38 |
I: NR C: NR Total: NR (per‐protocol) |
I: 12 C: 15 Total: 27 |
I: 66.7% C: 75.0% Total: 71.1% |
Comment: no flow chart, reasons for dropout reported in text |
| McLaughlin 2005 | Intervention (I1): telephone counselling Control (C): usual care |
Total: 700 | I: 53 C: 47 Total: 100 |
I: NR C: NR Total: NR (per‐protocol) |
I: 45 C: 34 Total: 79 |
I: 84.9% C: 72.3% Total: 79% |
|
| MoodCare 2011 | Intervention (I): cognitive‐ behavioural therapy Control (C): usual care | Total: 3071 | I: 61 C: 60 Total: 121 |
I: NR C: NR Total: NR |
I: 53 C: 53 Total: 106 |
I: 86.9% C: 88.3% Total: 87.6% |
|
| Pizzi 2009 | Intervention (I): sertraline Control (C): placebo |
Total: 630 | I: 50 C: 50 Total: 100 |
I: NR C: NR Total: NR (per‐protocol) |
I: 47 C: 48 Total: 95 |
I: 94% C: 96% Total: 95% |
|
| Roose 1998 | Intervention 1 (I1): paroxetine Intervention 2 (I2): nortriptyline |
Total: NR | I1: 41 I2: 40 Total: 81 |
I1: 41 I2: 40 Total: 81 |
I1: 37 I2: 30 Total: 67 |
I1: 90.2% I2: 75.0% Total: 82.7% |
Comment: no flow chart, reasons for dropout reported in text |
| SADHART 2002 | Intervention (I): sertraline Control (C): placebo |
Total: 11546 | I: 186 C: 183 Total: 369 |
I: 186 C: 183 Total: 169 |
I: 133 C: 137 Total: 270 |
I: 71.5% C: 74.9% Total: 73.1% |
|
| SPIRR‐CAD 2011 | Intervention (I): stepwise psychotherapy intervention Control (C): usual care |
Total: 21780 | I: 285 C: 285 Total: 570 |
I: 284 C: 284 Total: 568 |
I: 110 C: 194 Total: 304 |
I: 38.6% C: 68.1% Total: 53.3% |
|
| Shahmansouri 2014 | Intervention 1 (I1): fluoxetine Intervention 2 (I2): Crocus sativus L. (saffron) |
Total: 75 | I1: 22 I2: 22 Total: 44 |
I1: NR I2: NR Total: NR (per‐protocol) |
I1: 20 I2: 20 Total: 40 |
I1: 90.9% I2: 90.9% Total: 90.9% |
|
| Strik 2000 | Intervention (I): fluoxetine Control (C): placebo |
Total: 556 | I: 27 C: 27 Total: 54 |
I: 27 C: 27 Total: 54 |
I: 22 C: 18 Total: 40 |
I: 81.5% C: 66.7% Total: 74.1% |
|
| Tian 2016 | Intervention 1 (I1): paroxetine Intervention 2 (I2): fluoxetine |
Total: ? | I1: 23 I2: 23 Total: 46 |
I1: 23 I2: 23 Total: 46 (per‐protocol) |
I1: 23 I2: 23 Total: 46 |
I1: 100% I2: 100% Total: 100% |
Comment: no flow chart was reported. It is unclear whether 16 participants who did not finish the study were from I1 or I2 groups, or non‐treatment or non‐depressed groups. |
| TREATED‐ACS 2020 | Intervention 1 (I1): cognitive‐behavioural therapy and well‐being therapy Intervention 2 (I2): clinical management |
Total: 740 | I1: 50 I2: 50 Total: 100 |
I1: 50 I2: 50 Total: 100 |
I1: 42 I2: 40 Total: 82 |
I1: 84% I2: 80% Total: 82% |
|
| U‐CARE 2018 | Intervention (I): internet cognitive‐behavioural therapy Control (C): usual care |
Total: 3928 | I: 117 C: 122 Total: 239 |
I: 117 C: 122 Total: 239 |
I: 96 C: 115 Total: 211 |
I: 82.1% C: 94.3% Total: 88.3% |
|
| UPBEAT 2012 | Intervention 1 (I1): sertraline Intervention 2 (I2): exercise Control (C): placebo |
Total: 1680 | I1: 40 I2: 37 C: 24 Total: 101 |
I1: NR I2: NR C: NR Total: NR |
I1: 36 I2: 36 C: 23 Total: 95 |
I1: 90% I2: 97.3% C: 95.8% Total: 94.1% |
Comment: only I1 sertraline and C placebo are eligible for this review |
| Wang 2020 | Intervention 1 (I1): escitalopram Intervention 2 (I2): Bu Xin Qi decoction |
Total: 300 | I1: 140 I2: 140 Total: 280 |
I1: NR I2: NR Total: NR (per‐protocol) |
I1: 113 I2: 115 Total: 228 |
I1: 80.7% I2: 82.1% Total: 81.4% |
Comment: reasons for dropout not stated in flow chart |
| WIDeCAD 2017 | Intervention (I): internet cognitive‐behavioural therapy Control (C): wait‐list control |
Total: 72 | I: 18 C: 16 Total: 34 |
I: 18 C: 16 Total: 34 |
I: 13 C: 13 Total: 26 |
I: 72.2% C: 81.3% Total: 76.5% |
|
| Yang 2019 | Intervention (I): intensive telephone‐based care Control (C): usual care |
Total: 354 | I: 112 C: 112 Total: 224 |
I: NR C: NR Total: NR |
I: 107 C: 105 Total: 212 |
I: 95.5% C: 93.8% Total: 94.6% |
|
| Zarea 2014 | Intervention (I): Peplau's therapeutic communication model Control (C): usual care |
Total: ? | I: ? C: ? Total: ? |
I: ? C: ? Total: ? (per‐protocol) |
I: 37 C: 37 Total: 74 |
I: ? C: ? Total: ? |
Comment: total sample estimated from degrees of freedom in Table 3 |
ITT = intention‐to‐treat; NR = not reported; ? = unclear
Results of the search
The database and trial registry search resulted in 7407 references (101 from trial registries), 5387 of which were unique references. We excluded 5245 articles at the title/abstract level, and 80 studies (97 articles) after full‐text review, most commonly because the study did not investigate participants without comorbid depression (25 studies, 30 articles), the intervention for depression was not a predefined comparison of this review (13 studies, 19 articles), the sample was not restricted to CAD (10 studies, 13 articles), the intervention not specifically psychological or pharmacological intervention for treating depression (4 studies, 4 articles), the study was not an RCT (4 studies, 4 articles), the control group was unclear or there was no control group (1 study, 1 reference), or new citations to studies already excluded in the previous review (2 studies, 4 references). We also identified 17 ongoing studies (17 references) and 4 trials awaiting classification (5 references). Twenty‐one new studies were found to be eligible in this updated review (45 new references) and included in the narrative review or synthesis. See the study flow chart for details of the study selection process (Figure 1).
Included studies
Thirty‐seven trials fulfilled the inclusion criteria of the review (Abbasi 2015; ANDROS 2015; Barth 2005; Brown 1993; Carney 2009; CREATE 2007; Dao 2011; Divsalar 2018; Doering 2007; ENRICHD 2003; EsDEPACS 2014; Fang 2003; Freedland 2009; Freeman 1986; Kennedy 2005; Li 2005; Liu 1999; Liu 2016; Ma 2019; McFarlane 2001; McLaughlin 2005; MIND‐IT 2007; MoodCare 2011; Pizzi 2009; Roose 1998; SADHART 2002; Shahmansouri 2014; SPIRR‐CAD 2011; Strik 2000; Tian 2016; TREATED‐ACS 2020; U‐CARE 2018; UPBEAT 2012; Wang 2020; WIDeCAD 2017; Yang 2019; Zarea 2014).
Fifteen trials investigated psychological interventions, which comprised CBT (Dao 2011; Doering 2007; ENRICHD 2003; MoodCare 2011; U‐CARE 2018; WIDeCAD 2017), resource‐orientated psychotherapy (Barth 2005), telephone counselling (McLaughlin 2005; Yang 2019), individual and group psychotherapy (SPIRR‐CAD 2011), therapeutic communication sessions (Zarea 2014), and an intervention comprising health education and various psychological treatments (Fang 2003). One three‐arm trial examined CBT, supportive stress management, and usual care (Freedland 2009). One trial examined eight sessions of CBT in combination with four sessions of well‐being therapy versus CM (TREATED‐ACS 2020). One trial examined behaviour therapy versus person‐centred therapy (Brown 1993). Two psychological therapy trials delivered the CBT intervention entirely online with therapist or eCoach support (U‐CARE 2018; WIDeCAD 2017); all others delivered the intervention face‐to‐face.
Twenty‐one trials investigated the effects of pharmacological depression treatments with sertraline (ANDROS 2015; McFarlane 2001; Pizzi 2009; SADHART 2002; UPBEAT 2012), mirtazapine (MIND‐IT 2007), fluoxetine (Liu 1999; Strik 2000), escitalopram (EsDEPACS 2014; Kennedy 2005), paroxetine and nortriptyline (Roose 1998), paroxetine and fluoxetine (Tian 2016), alprazolam (Freeman 1986), sertraline plus omega‐3 (Carney 2009), sertraline plus red yeast rice (Divsalar 2018), St John's wort (Li 2005), simvastatin compared to atorvastatin (Abbasi 2015), Xinkeshu (Ma 2019), saffron compared to fluoxetine (Shahmansouri 2014), Shugan Jieyu compared to sertraline (Liu 2016), Bu Xin Qi compared to escitalopram (Wang 2020).
One trial had a 2 x 2 factorial design (CREATE 2007). In accordance with our inclusion criteria, we restricted analyses to the citalopram and CM versus placebo and CM arms of the trial. The IPT plus citalopram plus CM, and IPT plus placebo plus CM arms of the trial were ineligible and are not described further.
The trial size in psychological intervention studies ranged from 15 participants in Doering 2007 to 2481 participants in ENRICHD 2003. In the pharmacological intervention studies, the trial size ranged from 2 participants in ANDROS 2015 to 369 participants in SADHART 2002.
The mean age of participants ranged from 52.6 in Shahmansouri 2014 to 64.0 years in UPBEAT 2012. The percentage of female participants ranged from 10% in Brown 1993 to 56.8% in Shahmansouri 2014. One study was restricted to female participants only (Doering 2007).
Ten studies originated from the USA (Brown 1993; Carney 2009; Dao 2011; Doering 2007; ENRICHD 2003; Freedland 2009; Freeman 1986; McLaughlin 2005; Roose 1998; UPBEAT 2012), eight from China (Fang 2003; Li 2005; Liu 1999; Liu 2016; Ma 2019; Tian 2016; Wang 2020; Yang 2019), four from Iran (Abbasi 2015; Divsalar 2018; Shahmansouri 2014; Zarea 2014), three from Germany (Barth 2005; SPIRR‐CAD 2011; WIDeCAD 2017), two from Canada (CREATE 2007; McFarlane 2001), two from the Netherlands (MIND‐IT 2007; Strik 2000), two from Italy (Pizzi 2009; TREATED‐ACS 2020), one from Australia (MoodCare 2011), one from Korea (EsDEPACS 2014), one from France (ANDROS 2015), and one from Sweden (U‐CARE 2018). Two studies were performed across multiple sites in different countries, taking place in the USA, Europe, Canada, and Australia in SADHART 2002 and Denmark, Estonia, and Norway in Kennedy 2005.
Sixteen studies investigated individuals with MI or acute coronary syndromes (ANDROS 2015; ENRICHD 2003; EsDEPACS 2014; Fang 2003; Kennedy 2005; Liu 1999; Liu 2016; McFarlane 2001; McLaughlin 2005; MIND‐IT 2007; MoodCare 2011; SADHART 2002; Strik 2000; Tian 2016; TREATED‐ACS 2020; U‐CARE 2018). Twelve trials studied diverse CAD populations comprising MI, angina pectoris, and patients undergoing cardiac procedures (Barth 2005; Brown 1993; Carney 2009; CREATE 2007; Ma 2019; Pizzi 2009; Roose 1998; SPIRR‐CAD 2011; UPBEAT 2012; Wang 2020; WIDeCAD 2017; Yang 2019). Seven trials investigated patients awaiting or after CABG (Abbasi 2015; Dao 2011; Doering 2007; Freedland 2009; Freeman 1986; Li 2005; Zarea 2014), and two trials investigated patients after PCI (Divsalar 2018; Shahmansouri 2014).
We also identified 17 ongoing trials (Ahmadi 2018; Ardakani 2020; COMBAT‐DS 2021; eMindYourHeart 2021; Firouzjaei 2017; Geng 2018; Hamzehpour 2020; Irfan 2020; Jazayeri 2017; Luberto 2021; Ma 2014; Mohammadian 2018; Moudi 2016; Qiaoning 2019; Sourizahi 2017; Wang 2015; Yang 2020). Three studies are awaiting classification, two of which were identified as conference abstracts in our search of the databases, without any contact information available (Ahangarezaiezadeh 2017; Cai 2012; Gu 2017).
Excluded studies
A total of 96 studies (118 articles) that appeared to be relevant to the review were excluded after careful examination of eligibility criteria (see Characteristics of excluded studies for reasons for exclusion). Sixteen studies (21 references) were excluded in the previous review reported by Baumeister 2011c, and 80 studies (97 references) were excluded from the 2020 updated literature search.
Risk of bias in included studies
Risk of bias in the included studies varied across studies (see Figure 2; Figure 3). The information available after translating parts of three trials published in Chinese was insufficient to determine risk of bias in these studies (Fang 2003; Li 2005; Liu 1999). We assessed risk of bias for the two trials that were terminated early by the investigators based on information reported in the clinical trial registries (ANDROS 2015; Kennedy 2005).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twelve trials used an appropriately generated and adequately concealed randomisation procedure (Abbasi 2015; Barth 2005; Carney 2009; CREATE 2007; Divsalar 2018; ENRICHD 2003; EsDEPACS 2014; Freedland 2009; MoodCare 2011; Shahmansouri 2014; SPIRR‐CAD 2011; UPBEAT 2012). The generation of the randomisation sequence appeared to be appropriate in eight trials; however, they did not sufficiently describe the concealment of the allocation, Dao 2011; Liu 2016; MIND‐IT 2007; Roose 1998; U‐CARE 2018; WIDeCAD 2017; Yang 2019, or failed to conceal the allocation adequately (McLaughlin 2005). Two trials used an inappropriate randomisation procedure and provided insufficient information on concealment (Ma 2019; Zarea 2014). One trial described a sufficient sequence generation but was an open‐label trial (Wang 2020). Details regarding sequence generation and allocation concealment were unclear for the remaining 14 trials (ANDROS 2015; Brown 1993; Doering 2007; Fang 2003; Freeman 1986; Kennedy 2005; Li 2005; Liu 1999; McFarlane 2001; Pizzi 2009; SADHART 2002; Strik 2000; Tian 2016; TREATED‐ACS 2020).
Blinding
No trial of psychological interventions utilised an attention‐control design, thus we judged participants in all psychological intervention trials as unblinded to treatment allocation. The outcome assessor was blinded in seven psychological intervention trials (Barth 2005; Doering 2007; Freedland 2009; MoodCare 2011; SPIRR‐CAD 2011; TREATED‐ACS 2020; Yang 2019). Seven trials did not report sufficient details regarding blinding to make a judgement of low or high risk (Brown 1993; Dao 2011; ENRICHD 2003; Fang 2003; U‐CARE 2018; WIDeCAD 2017; Zarea 2014). We assessed one psychological trial as high risk and unblinded, as the outcome was assessed using patient self‐report without sufficient information regarding blinding (McLaughlin 2005).
In six pharmacological trials blinding was adequately realised and described (Abbasi 2015; CREATE 2007; Divsalar 2018; EsDEPACS 2014; Shahmansouri 2014; UPBEAT 2012). Four pharmacological trials reported using a double‐blind method but did not describe who was blinded (MIND‐IT 2007; Roose 1998; SADHART 2002; Strik 2000). One trial was described as open‐label trial and hence unblinded (Wang 2020). The remaining 11 trials did not report sufficient information regarding blinding of staff, participants, and outcome assessors (ANDROS 2015; Carney 2009; Freeman 1986; Kennedy 2005; Li 2005; Liu 1999; Liu 2016; Ma 2019; McFarlane 2001; Pizzi 2009; Tian 2016).
Incomplete outcome data
Fourteen trials provided intention‐to‐treat (ITT) analyses for primary outcomes (Carney 2009; CREATE 2007; Divsalar 2018; Freedland 2009; MIND‐IT 2007; MoodCare 2011; Roose 1998; SADHART 2002; SPIRR‐CAD 2011; Strik 2000; TREATED‐ACS 2020; UPBEAT 2012; WIDeCAD 2017; Yang 2019). One trial reported both ITT and per‐protocol analyses simultaneously (SPIRR‐CAD 2011). Depression outcomes were analysed per‐protocol in two trials that reported cardiovascular mortality and cardiac events as ITT (ENRICHD 2003; U‐CARE 2018). Conversely, Strik 2000 reported ITT analyses for depression outcomes and per‐protocol analyses for cardiac events, cardiovascular vital signs, and ECG waves. Fourteen trials reported per‐protocol analyses (Abbasi 2015; Barth 2005; Brown 1993; Doering 2007; EsDEPACS 2014; Freeman 1986; Kennedy 2005; Ma 2019; McFarlane 2001; McLaughlin 2005; Pizzi 2009; Shahmansouri 2014; Tian 2016; Wang 2020). The remaining seven studies provided insufficient information to make a determination (ANDROS 2015; Dao 2011; Fang 2003; Li 2005; Liu 1999; Liu 2016; Zarea 2014).
Selective reporting
We judged eight studies as free of selective reporting based on the comparison of outcomes reported in published study protocols, methods sections, and original papers (CREATE 2007; EsDEPACS 2014; Freedland 2009; MoodCare 2011; Shahmansouri 2014; TREATED‐ACS 2020; U‐CARE 2018; WIDeCAD 2017). Three trials have as yet not reported the results of all the outcomes mentioned in published protocols (Carney 2009; MIND‐IT 2007; SPIRR‐CAD 2011). We assessed four trials as high risk of bias due to incomplete or inadequate outcome reporting (ENRICHD 2003; Kennedy 2005; Ma 2019; Strik 2000). Furthermore, in UPBEAT 2012, we rated selective reporting as unclear risk bias, as measures of variance (standard deviation (SD) or standard error (SE)) were not reported, and P values were reported for active treatment (sertraline group, exercise group), thereby combining two separate interventions. No published or unpublished trial protocols were available other than trial registries for the remaining 21 trials (Abbasi 2015; ANDROS 2015; Barth 2005; Brown 1993; Dao 2011; Divsalar 2018; Doering 2007; Fang 2003; Freeman 1986; Li 2005; Liu 1999; Liu 2016; McFarlane 2001; McLaughlin 2005; Pizzi 2009; Roose 1998; SADHART 2002; Tian 2016; Wang 2020; Yang 2019; Zarea 2014), thus it remains unclear whether or not there is a risk of selective reporting in these trials.
Other potential sources of bias
We judged eight studies as free of other sources of bias (Abbasi 2015; CREATE 2007; EsDEPACS 2014; Freedland 2009; Pizzi 2009; Shahmansouri 2014; Strik 2000; UPBEAT 2012). The risk of other sources of bias remains unclear for the three trials translated from Chinese (Fang 2003; Li 2005; Liu 1999). Ten psychotherapy studies may exhibit performance bias because the adherence of therapists in the treatment group was unclear (Barth 2005; Brown 1993; Doering 2007; McLaughlin 2005; TREATED‐ACS 2020; U‐CARE 2018; WIDeCAD 2017; Yang 2019; Zarea 2014), or was undertaken differently from the protocol (MoodCare 2011). In two trials there was evidence of statistically significant differences between groups at baseline on depression (Brown 1993; MIND‐IT 2007), or differences at baseline were not established (ENRICHD 2003; Wang 2020). In McFarlane 2001 (p 619 and p 620) and McLaughlin 2005 (discrepancy between text and figure of depression score on Hospital Anxiety and Depression Scale (HADS)), results were inconsistently reported. In three trials (Carney 2009; SPIRR‐CAD 2011; U‐CARE 2018), there was a change in the inclusion criteria.
In two trials there was evidence of high risk of bias (selection bias), with eligible participants not recruited (Divsalar 2018; WIDeCAD 2017). There was evidence of high risk of other bias in two internet CBT interventions that were terminated early by investigators with ITT results reported (U‐CARE 2018; WIDeCAD 2017). Similarly, a high risk of other bias was evident in two drug trials terminated early with results not reported (ANDROS 2015), or partially reported per protocol in redacted form (Kennedy 2005). A high risk of other bias was evident in two trials that either did not register the trial (Tian 2016), or did so retrospectively after recruitment had commenced (Ma 2019). A high risk of other bias was evident in two drug trials that included pharmaceutical company employees in the trial design, conduct, analysis, and reporting of results (Roose 1998; SADHART 2002). A high risk of other bias was evident in Liu 2016, where the primary results were reported mid‐treatment.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Summary of findings table ‐ Psychological treatment compared to control for depression in patients with coronary artery disease.
| Psychological treatment compared to control for depression in patients with coronary artery disease | ||||||
| Patient or population: health problem or population Setting: cardiology in‐ and outpatient Intervention: Psychological treatment Comparison: Control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Control | Risk with Psychological treatment | |||||
| Depression symptoms ‐ short‐term assessed with: objective and self‐reported measures of depression symptoms, higher scores indicate more severe symptoms | ‐ | SMD 0.55 SD lower (0.92 lower to 0.19 lower) | ‐ | 1226 (10 RCTs) | ⊕⊕⊝⊝ Lowa,b | There is low certainty evidence that psychological treatment may result in a moderate reduction in depression symptoms at the end of treatment. |
| Depression remission ‐ short term assessed with: below cut‐points on objective and self‐report measures of depression | 319 per 1000 | 486 per 1000 (267 to 708) | OR 2.02 (0.78 to 5.19) | 862 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c | There is low certainty evidence that psychological treatment may result in no difference in depression remission at the end of treatment. |
| All‐cause mortality ‐ short‐term assessed with: mortality records | 25 per 1000 | 8 per 1000 (1 to 50) | OR 0.31 (0.05 to 2.02) | 324 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,e | The evidence is very uncertain about the effect of psychological treatment on all‐cause mortality at the end of treatment. |
| Cardiovascular mortality ‐ long‐term assessed with: cause of death according to standardised criteria on mortality records | 85 per 1000 | 72 per 1000 (54 to 93) | OR 0.83 (0.62 to 1.10) | 2720 (2 RCTs) | ‐ | No data for cardiovascular mortality at end of treatment in trials comparing psychological interventions versus usual care |
| Myocardial infarction ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for occurrence of myocardial infarction at end of treatment in trials comparing psychological interventions versus usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_427596582080189491. | ||||||
a Risk of bias rated down one level ‐ trials that contributed to this outcome were rated as unclear risk of bias b Inconsistency rated down one level ‐ though confidence intervals generally overlapped, there was considerable unexplained statistical heterogeneity c Imprecision rated down one level ‐ confidence intervals encompass an adverse effect to beneficial effect d Risk of bias rated down two levels ‐ most trials that contributed to this outcome were rated as high or unclear risk of bias e Imprecision rated down two levels ‐ sparse events and wide confidence intervals encompass an adverse effect to beneficial effect
Summary of findings 2. Summary of findings table ‐ Psychological treatment 1 compared to psychological treatment 2 for depression in patients with coronary artery disease.
| Psychological treatment 1 compared to psychological treatment 2 for depression in patients with coronary artery disease | ||||||
| Patient or population: health problem or population Setting: cardiology outpatient settings Intervention: Psychological Treatment 1 Comparison: Psychological Treatment 2 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Psychological Treatment 2 | Risk with Psychological Treatment 1 | |||||
| Depression symptoms ‐ short term (end of treatment) assessed with: objective and self‐reported measures of depression symptoms; higher scores indicate more severe symptoms | Not pooled | Not pooled | Not pooled | 219 (3 RCTs) | ‐ | No meta‐analysis performed due to clinical heterogeneity. The evidence is very uncertain as to whether different psychological interventions may result in a reduction in depression symptoms at the end of treatment for: cognitive‐behavioural therapy compared to supportive stress management (Freedland 2009); behaviour therapy compared to person‐centred therapy (Brown 1993); cognitive‐behavioural therapy and well‐being therapy compared to clinical management (TREATED‐ACS 2020). |
| Depression remission ‐ short term (end of treatment) assessed with: below cut‐off on Hamilton Rating Scale for Depression | 571 per 1000 | 707 per 1000 (493 to 857) | OR 1.81 (0.73 to 4.50) | 83 (1 RCT) | ⊕⊕⊝⊝ Lowa | There is low certainty evidence from one trial that cognitive‐behavioural therapy may result in no difference in depression remission at the end of treatment compared to supportive stress management (Freedland 2009). |
| All‐cause mortality ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for all‐cause mortality at end of treatment in trials comparing psychological intervention versus another psychological intervention/clinical management |
| Cardiovascular mortality ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for cardiovascular mortality at end of treatment in trials comparing psychological intervention versus another psychological intervention/clinical management |
| Myocardial infarction ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for the occurrence of myocardial infarction at end of treatment in trials comparing psychological intervention versus another psychological intervention/clinical management |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_427665509108780589. | ||||||
a Imprecision rated down two levels ‐ wide confidence intervals from one trial encompass an adverse effect to beneficial effect
Summary of findings 3. Summary of findings table ‐ Pharmacological treatment compared to placebo for depression in patients with coronary artery disease.
| Pharmacological treatment compared to placebo for depression in patients with coronary artery disease | ||||||
| Patient or population: health problem or population Setting: cardiology in‐ and outpatient settings Intervention: Pharmacological Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Pharmacological | |||||
| Depression symptoms ‐ short term assessed with: objective and self‐reported measures of depression; higher scores indicate more severe symptoms | ‐ | SMD 0.83 lower (1.33 lower to 0.32 lower) | ‐ | 750 (8 RCTs) | ⊕⊕⊝⊝ Lowa,b | There is low certainty evidence that pharmacological intervention may result in a large reduction in depression symptoms at the end of treatment |
| Depression remission ‐ short term assessed with: below cut‐point on objective measure of depression (Hamilton Rating Scale for Depression) | 323 per 1000 | 496 per 1000 (412 to 580) | OR 2.06 (1.47 to 2.89) | 646 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | There is moderate certainty evidence that pharmacological intervention probably results in a moderate to large increase in depression remission at the end of treatment. |
| All‐cause mortality ‐ short term assessed with: mortality records | 36 per 1000 | 14 per 1000 (4 to 53) | OR 0.38 (0.10 to 1.47) | 437 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of pharmacological intervention on all‐cause mortality at the end of treatment. In addition to the pooled results, data could not be extracted from 2 studies where no deaths occurred and from 1 trial which remained unclear. |
| Cardiovascular mortality ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for cardiovascular mortality at end of treatment in trials comparing pharmacological intervention versus placebo |
| Myocardial infarction ‐ short term assessed with: standardised criteria for fatal or non‐fatal myocardial infarction | 22 per 1000 | 17 per 1000 (6 to 45) | OR 0.74 (0.26 to 2.09) | 728 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of pharmacological intervention on myocardial infarction at the end of treatment. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_427666962988765745. | ||||||
a Risk of bias rated down one level ‐ trials that contributed to this outcome were rated as unclear or high risk of bias b Inconsistency rated down one level ‐ though confidence intervals generally overlapped, there was considerable unexplained statistical heterogeneity c Imprecision rated down two levels ‐ sparse events and wide confidence intervals encompass an adverse effect to beneficial effect
Summary of findings 4. Summary of findings table ‐ Pharmacological treatment 1 compared to pharmacological treatment 2 for depression in patients with coronary artery disease.
| Pharmacological treatment 1 compared to pharmacological treatment 2 for depression in patients with coronary artery disease | ||||||
| Patient or population: health problem or population Setting: cardiology in‐ and outpatient settings Intervention: Pharmacological intervention 1 Comparison: Pharmacological intervention 2 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Pharmacological intervention 2 | Risk with Pharmacological intervention 1 | |||||
| Depression symptoms ‐ short term (end of treatment) assessed with: objective measure of depression (Hamilton Rating Scale for Depression); higher scores indicate more severe symptoms | Not pooled | Not pooled | Not pooled | 442 (4 RCTs) | ‐ | No meta‐analysis performed due to clinical heterogeneity. The evidence is very uncertain as to whether different pharmacological interventions may result in a reduction in depression symptoms at the end of treatment for: simvastatin compared to atorvastatin (Abbasi 2015); sertraline plus omega‐3 compared to sertraline plus placebo (Carney 2009); paroxetine compared to fluoxetine (Tian 2016); escitalopram compared to Bu Xin Qi (Wang 2020). |
| Depression remission ‐ short term (end of treatment) assessed with: below cut‐points on objective and self‐report measures of depression | Not pooled | Not pooled | Not pooled | 243 (3 RCTs) | ‐ | No meta‐analysis performed due to clinical heterogeneity. The evidence is very uncertain about the effect of pharmacological treatment compared to another pharmacological treatment on depression remission at the end of treatment . |
| All‐cause mortality ‐ short term (end of treatment) assessed with: mortality records | 26 per 1000 | 68 per 1000 (14 to 281) | OR 2.72 (0.51 to 14.49) | 149 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The evidence from 1 trial is very uncertain about the effect of sertraline vs Shugan Jieyu on all‐cause mortality at the end of treatment (Liu 2016). |
| Cardiovascular mortality ‐ short term (end of treatment) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No data for cardiovascular mortality at end of treatment in trials comparing a pharmacological intervention versus another pharmacological intervention |
| Myocardial infarction ‐ short term (end of treatment) assessed with: standardised criteria for fatal and non‐fatal myocardial infarction | Not pooled | Not pooled | Not pooled | 396 (3 RCTs) | ‐ | No meta‐analysis performed due to clinical heterogeneity. The evidence is very uncertain about the effect of pharmacological treatment compared to another pharmacological treatment on the occurrence of myocardial infarction at end of treatment for: sertraline plus omega‐3 compared to sertraline plus placebo (Carney 2009); paroxetine compared to fluoxetine (Tian 2016); escitalopram compared to Bu Xin Qi (Wang 2020). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_428037497253281678. | ||||||
a Risk of bias rated down one level ‐ the trial(s) that contributed to this outcome were rated as unclear or high risk of bias b Imprecision rated down two levels ‐ sparse events and wide confidence intervals encompass an adverse effect to beneficial effect
Comparison 1: Psychological intervention versus control
Thirteen trials studied the effects of a psychological intervention versus control (Barth 2005; Dao 2011; Doering 2007; ENRICHD 2003; Fang 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; U‐CARE 2018; WIDeCAD 2017; Yang 2019; Zarea 2014).
1.1 Primary outcome: depression symptoms
Twelve studies investigated the effects of psychological interventions on short‐term depression symptoms (i.e. the end of treatment) (Barth 2005; Dao 2011; Doering 2007; Fang 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; U‐CARE 2018; WIDeCAD 2017; Yang 2019; Zarea 2014). One study did not report sufficient information to compute effect sizes (Doering 2007). Three studies reported data as change scores (Fang 2003; SPIRR‐CAD 2011; Yang 2019), of which two studies reported end‐of‐treatment scores that could be pooled in analysis of standardised mean difference (SMD) (Fang 2003; SPIRR‐CAD 2011). Meta‐analysis of 10 trials showed that psychological interventions may result in a reduction in depression symptoms at the end of treatment compared to control groups (pooled SMD −0.55, 95% confidence interval (CI) −0.92 to −0.19) (n = 1226) (Analysis 1.1) (Barth 2005; Dao 2011; Fang 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; U‐CARE 2018; WIDeCAD 2017; Zarea 2014). Heterogeneity was considerable (I2 = 88%). One trial reported a significant change in the depression subscale of the HADS for patients' intensive telephone‐based care (Δ −2.20, SD 2.61) compared to usual care (Δ −1.04, SD 2.89) (n = 212) (Yang 2019), which could not be pooled. Sensitivity analyses for Analysis 1.1 (Table 6) indicate minimal change to the pooled SMD and heterogeneity in analyses restricted to non‐major depressive disorder trials and CBT trials. By contrast, analyses restricted to depression‐only trials resulted in an attenuation of the SMD that was no longer significant.
1.1. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 1: Depression symptoms ‐ short term
2. Sensitivity analyses for depression symptoms at end of treatment in psychological versus control trials.
| Comparison | Sensitivity analysis | Study references [n] | SMD | I2 |
| Psychological vs control | None (Analysis 1.1) | Barth 2005; Dao 2011; Fang 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; U‐CARE 2018; WIDeCAD 2017; Zarea 2014 (n = 1226) | −0.55 (95% CI −0.92 to −0.19) | 88 |
| Psychological vs control | Constrained to trials without depression disorders as part of the inclusion criteria | Barth 2005; Dao 2011; Fang 2003; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; U‐CARE 2018; WIDeCAD 2017; Zarea 2014 (n = 1145) | −0.53 (95% CI −0.92 to −0.13) | 89 |
| Psychological vs control | Constrained to depression (e.g. excluding trials with mixed depression and/or anxiety as part of the inclusion criteria) | Barth 2005; Freedland 2009; MoodCare 2011; SPIRR‐CAD 2011; WIDeCAD 2017 (n = 681) | −0.27 (95% CI −0.58 to 0.03) | 65 |
| Psychological vs control | Constrained to cognitive‐behavioural therapy trials | Dao 2011; Freedland 2009; MoodCare 2011; U‐CARE 2018; WIDeCAD 2017 (n = 571) | −0.48 (95% CI −0.77 to −0.19) | 61 |
CI = confidence interval; SMD = standardised mean difference
Eight studies investigated the effects of psychological interventions on medium‐term depression symptoms (i.e. one to six months after treatment) (Dao 2011; Doering 2007; ENRICHD 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; Zarea 2014). One study did not report sufficient information to compute effect sizes (Doering 2007). Meta‐analysis of seven psychotherapy trials showed no benefit compared to control on medium‐term depression symptoms (pooled SMD −0.20, 95% CI −0.42 to 0.01) (n = 2620) (Analysis 1.2) (Dao 2011; ENRICHD 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011; SPIRR‐CAD 2011; Zarea 2014). Heterogeneity was substantial (I2 = 69%).
1.2. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 2: Depression symptoms ‐ medium term
Two trials investigated the effects of psychological interventions on long‐term depression symptoms (i.e. more than six months after treatment) (Freedland 2009; U‐CARE 2018). CBT was not superior to control on long‐term depression symptoms (SMD −0.46, 95% CI −0.96 to 0.04) (n = 282) (Analysis 1.3). There was evidence of considerable heterogeneity (I2 = 72%).
1.3. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 3: Depression symptoms ‐ long term
1.2 Primary outcome: depression remission and response
Three studies reported on depression remission in the short term (i.e. end of treatment) (n = 862) (Freedland 2009; SPIRR‐CAD 2011; Yang 2019). CBT was beneficial compared to usual care (odds ratio (OR) 5.02, 95% CI 1.95 to 12.90) in the study by Freedland 2009. An intensive telephone‐based care programme was beneficial compared to usual care (OR 2.25, 95% CI 1.22 to 4.15) in the study by Yang 2019. Stepwise, fully manualised individual and group psychotherapy was not superior compared to usual care (n = 569) (SPIRR‐CAD 2011). A pooled analysis suggests that psychological intervention results in little to no difference in depression remission at the end of treatment (OR 2.02, 95% CI 0.78 to 5.19) (n = 862) (Analysis 1.4). Statistical heterogeneity remained considerable between studies (I2 = 87%).
1.4. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 4: Depression remission ‐ short term
One trial included only participants with depressive disorders and re‐evaluated participants in the medium term (four months) for depression disorders, but did not report these data (Doering 2007). Only Freedland 2009 (n = 81) reported on medium‐ and long‐term depression remission. No effect was observed in the medium term (i.e. one to six months after end of treatment) (Analysis 1.5). In the same trial, the effect was significant in the long term (i.e. more than six months after end of treatment; OR 5.06, 95% CI 1.96 to 13.08) (Analysis 1.6).
1.5. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 5: Depression remission ‐ medium term
1.6. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 6: Depression remission ‐ long term
No trials reported depression response at any time point.
1.3 Primary outcome: all‐cause and cardiovascular mortality
Two trials reported loss to follow‐up attributable to all‐cause mortality in the short term (McLaughlin 2005; Yang 2019). Few events were recorded (five deaths), and neither trial showed a significant increase or decrease in probability of mortality in the short term. Pooled analysis of the two trials was very uncertain regarding the effect of psychological interventions on all‐cause mortality at end of treatment (OR 0.31, 95% CI 0.05 to 2.02; I2 = 0%) (n = 324) (Analysis 1.7).
1.7. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 7: All‐cause mortality ‐ short term
The SPIRR‐CAD 2011 trial reported all‐cause mortality in the medium term and did not find a significant increase or decrease in probability of mortality (OR 0.66, 95% CI 0.23 to 1.88) (n = 570) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 8: All‐cause mortality ‐ medium term
The ENRICHD 2003 (n = 2481) and Yang 2019 (n = 189) trials reported all‐cause mortality in the long term as an endpoint. No effect between psychotherapy versus usual care was observed in the two trials, and the pooled effect was not significant (OR 0.83, 95% CI 0.48 to 1.42) (n = 2670) (Analysis 1.9). There was evidence of moderate heterogeneity (I2 = 46%).
1.9. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 9: All‐cause mortality ‐ long term
No psychological intervention trial reported the short‐term cardiovascular mortality outcome. The SPIRR‐CAD 2011 trial (n = 570) reported cardiovascular mortality in the medium term and did not find a significant increase or decrease in probability of cardiovascular mortality (Analysis 1.10). Two trials reported on cardiovascular mortality in the long term (ENRICHD 2003; U‐CARE 2018). No effect between CBT versus usual control was observed (OR 0.83, 95% CI 0.62 to 1.10) (n = 2720) (Analysis 1.11). There was no evidence of heterogeneity (I2 = 0%).
1.10. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 10: Cardiovascular mortality ‐ medium term
1.11. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 11: Cardiovascular mortality ‐ long term
1.4 Primary outcome: cardiac events
No psychological intervention trial reported MI as an outcome in the short or medium term. Two trials (n = 2720) reported on MI (ENRICHD 2003; U‐CARE 2018). In U‐CARE 2018, the endpoint was inclusive of acute coronary syndromes (ST and non‐ST elevated MI, and unstable angina). No effect between CBT and control was observed on MI outcome in the long term (OR 1.09, 95% CI 0.73 to 1.65) (n = 2720) (Analysis 1.12). There was no evidence of heterogeneity (I2 = 27%). Only U‐CARE 2018 reported the primary outcomes of heart failure and stroke in the long term (n = 239). U‐CARE 2018 did not report a significant increase or decrease in probability of heart failure (OR 3.82, 95% CI 0.78 to 18.77) (Analysis 1.13) or stroke (OR 2.10, 95% CI 0.19 to 23.52) (Analysis 1.14) in the long term (n = 239), though the number of events were sparse. Two trials reported coronary revascularisation procedure as an outcome in the long term (OR 0.91, 95% CI 0.75 to 1.11) (n = 2780) (Analysis 1.15) without heterogeneity (I2 = 0%) (ENRICHD 2003; U‐CARE 2018). No psychological intervention trial reported angina or arrhythmia as an outcome in the short, medium, or longer term.
1.12. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 12: Myocardial infarction ‐ long term
1.13. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 13: Heart failure ‐ long term
1.14. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 14: Stroke ‐ long term
1.15. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 15: Coronary revascularisation procedure ‐ long term
1.5 Secondary outcome: healthcare and resource utilisation
One trial reported data on the effect of a brief CBT intervention compared to usual care on hospital length of stay after a CABG procedure (mean difference (MD) −1.30, 95% CI −2.53 to −0.07) (n = 97) (Analysis 1.17) (Dao 2011). One trial reported data on the effect of a CBT intervention compared to usual care on hospitalisation for cardiovascular causes (OR 0.92, 95% CI 0.78 to 1.09) (n = 2481) (Analysis 1.16) (ENRICHD 2003).
1.17. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 17: Length of stay ‐ short term
1.16. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 16: Hospitalisations ‐ long term
1.6 Secondary outcome: quality of life
Three studies investigated the effects of psychological interventions on short‐term quality of life (QoL) (Freedland 2009; MoodCare 2011; WIDeCAD 2017). The Physical Component Summary (PCS) and Mental Component Summary (MCS) scores of the Medical Outcomes Study Short‐Form 12/36‐item Health Survey (SF‐12 and SF‐36) were used in MoodCare 2011 and Freedland 2009, respectively. The WIDeCAD 2017 trial utilised the Assessment of Quality of Life scale (AQoL‐8D) at end of treatment, where higher scores indicate lower QoL, therefore these data were not pooled with data from the trials utilising the SF‐12 and SF‐36 (Freedland 2009; MoodCare 2011).
There was no beneficial effect of CBT versus usual care on PCS score (SMD 0.22, 95% CI −0.06 to 0.50) (n = 202) (I2 = 0%). (Analysis 1.18). There was an effect favouring CBT versus usual care on MCS in Freedland 2009. No effect on MCS was reported in MoodCare 2011. The pooled effect of the two trials indicated a moderate effect on MCS favouring CBT versus usual care (SMD 0.51, 95% CI 0.07 to 0.94) (n = 202), with substantial heterogeneity (I2 = 57%) (Analysis 1.19). There was no difference between internet CBT (M = 63.87 ± 16.43) and wait‐list control (M = 63.78 ± 14.42; Cohen's d = 0.00) on total AQoL‐8D scores at end of treatment in the WIDeCAD 2017 trial (n = 34).
1.18. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 18: Quality of life SF‐12/36 physical ‐ short term
1.19. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 19: Quality of life SF‐12/36 mental ‐ short term
Three studies investigated the effects of psychological interventions on medium‐term QoL, using the PCS and MCS scores of the SF‐12/36 (Freedland 2009; MoodCare 2011), and the overall score of the SF‐12 (Dao 2011). The pooled effect of two trials indicated no effect on PCS for psychotherapy (both CBT) versus usual care (SMD 0.18, 95% CI −1.29 to 1.65) (n = 202), without evidence of heterogeneity (I2 = 25%) (Analysis 1.20) (Freedland 2009; MoodCare 2011). The pooled effect of two trials indicated no effect on MCS for psychotherapy (both CBT) versus usual care (SMD 1.21, 95% CI −1.09 to 3.52) (n = 202), with evidence of moderate heterogeneity (I2 = 41%) (Analysis 1.21) (Freedland 2009; MoodCare 2011). In the trial by Dao 2011, no effect for brief CBT versus usual care was found for the SF‐12 overall score (MD −4.00, 95% CI −8.48 to 0.48) (n = 96) (Analysis 1.22). One trial investigated the effects of psychological interventions on long‐term QoL using the PCS and MCS scores of the SF‐36 quantified at nine months (n = 81) (Freedland 2009). No effect was observed on the PCS score in the long term (MD 0.70, 95% CI −3.60 to 5.00) (Analysis 1.23). An effect was reported for the MCS score of the SF‐36 QoL measure (MD 6.70, 95% CI 1.29 to 12.11) (n = 81) (Analysis 1.24). One further study did not report sufficient information to compute effects sizes regarding QoL (ENRICHD 2003).
1.20. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 20: Quality of life SF‐12/36 physical ‐ medium term
1.21. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 21: Quality of life SF‐12/36 mental ‐ medium term
1.22. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 22: Quality of life SF‐12 total ‐ medium term
1.23. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 23: Quality of life SF‐36 physical ‐ long term
1.24. Analysis.

Comparison 1: Psychological intervention versus control, Outcome 24: Quality of life SF‐36 mental ‐ long term
1.7 Secondary outcome: cardiovascular vital signs, biomarkers of platelet activation, ECG wave recording
No trial comparing psychological interventions with control reported cardiovascular vital signs, biomarkers of platelet activation, or ECG wave recording at any follow‐up time point.
1.8 Post hoc outcome: non‐cardiac adverse events
One trial comparing CBT with wait‐list control reported a non‐cardiac adverse event (suicide intent) during the eight‐week intervention and did not attribute this to the intervention (WIDeCAD 2017). One trial reported insufficient information on newly diagnosed severe mental illness (e.g. severe depression, suicide attempt, and psychosis) (SPIRR‐CAD 2011). Otherwise, data were sparse for non‐cardiac adverse effects of psychological interventions for depression in individuals with CAD.
Comparison 2: Psychological intervention versus psychological intervention
In three trials with a total of 219 participants the effects of a specific psychological intervention were compared with the effects of another psychological intervention or clinical management (Brown 1993; Freedland 2009; TREATED‐ACS 2020). Brown 1993 compared 12 weekly sessions of behaviour therapy for patients and their partners by Lewinsohn versus 12 weekly sessions of person‐centred therapy by Rogers. Freedland 2009 compared 12 weekly sessions of CBT versus 12 weekly sessions of supportive stress management. TREATED‐ACS 2020 compared an intervention comprising eight sessions of CBT and four sessions of well‐being therapy versus CM. We could not report pooled estimates for this comparison due to the heterogeneous interventions and different comparators examined in the trials (see Types of interventions). Data are therefore reported as mean differences and described qualitatively in text.
2.1 Primary outcome: depression score
Three studies investigated the effects of psychological intervention compared to another psychological intervention on short‐term depression symptoms (i.e. end of treatment) (Brown 1993; Freedland 2009; TREATED‐ACS 2020). The evidence is very uncertain regarding the effect on end‐of‐treatment depression symptoms for behaviour therapy compared to person‐centred therapy on the BDI (n = 40) (Brown 1993); CBT compared to supportive stress management on the HAM‐D (n = 83) (Freedland 2009); and the combination of CBT and well‐being therapy compared to CM on symptoms measured by the Clinical Interview for Depression (CID) (n = 100) (Analysis 2.1) (TREATED‐ACS 2020).
2.1. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 1: Depression symptoms ‐ short term
Three studies investigated the effects of psychological intervention compared to another psychological intervention or CM on medium‐term depression symptoms (i.e. one to six months after treatment) (Brown 1993; Freedland 2009; TREATED‐ACS 2020). No effect was observed for CBT compared to supportive stress management on symptoms measured by the HAM‐D depression score (n = 83) (Freedland 2009). Behaviour therapy showed a beneficial effect compared to person‐centred therapy on symptoms measured by the BDI (SMD −0.65, 95% CI −1.28 to −0.01) (n = 40) (Brown 1993). No effect was observed for the combination of CBT and well‐being therapy compared to CM on symptoms measured by the CID (n = 100) (Analysis 2.2) (TREATED‐ACS 2020).
2.2. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 2: Depression symptoms ‐ medium term
Three studies investigated the effects of psychological intervention compared to another psychological intervention or CM on long‐term depression symptoms (i.e. more than six months after treatment) (Brown 1993; Freedland 2009; TREATED‐ACS 2020). No effect was observed for CBT compared to supportive stress management on symptoms measured by the HAM‐D (n = 83) (Freedland 2009). Behaviour therapy resulted in a large effect compared to person‐centred therapy on symptoms measured by the BDI (SMD −0.69, 95% CI −1.33 to −0.05) (n = 40) (Brown 1993). No effect was observed for the combination of CBT and well‐being therapy compared to CM on symptoms measured by the CID (n = 100) (Analysis 2.3) (TREATED‐ACS 2020).
2.3. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 3: Depression symptoms ‐ long term
2.2 Primary outcome: depression remission and response
One trial investigated the effects of psychological intervention compared to another psychological intervention on short‐term depression remission (i.e. end of treatment) (Freedland 2009). No effect was observed for CBT compared to supportive stress management on the HAM‐D (n = 83) (Analysis 2.4) (Freedland 2009). No effect was observed for CBT compared to supportive stress management on HAM‐D depression remission in one study (n = 83) in the medium term (i.e. one to six months after end of treatment) (Analysis 2.5) and the long term (i.e. more than six months after end of treatment) (Analysis 2.6) (Freedland 2009). One trial reported depression relapse, but as remission and response rate was unclear data were not extracted (TREATED‐ACS 2020).
2.4. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 4: Depression remission ‐ short term
2.5. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 5: Depression remission ‐ medium term
2.6. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 6: Depression remission ‐ long term
2.3 Primary outcome: all‐cause and cardiovascular mortality
No trials reported all‐cause mortality at any length of follow‐up for this comparison. One trial comparing the combination of CBT and well‐being therapy versus CM reported cardiac death as a cause of attrition or dropout from the study from 18 to 30 months (TREATED‐ACS 2020), though events were sparse (Analysis 2.7) (n = 100).
2.7. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 7: Cardiovascular mortality ‐ long term
2.4 Primary outcome: cardiac events
One trial reported composite cardiac events to 30 months of follow‐up but did not differentiate cardiac events, therefore data could not be analysed (n = 100) (TREATED‐ACS 2020).
2.5 Secondary outcome: healthcare and resource utilisation
No trials reported healthcare and resource utilisation at any length of follow‐up for this comparison.
2.6 Secondary outcome: quality of life
Only Freedland 2009 (n = 83) reported QoL using mean final scores of the SF‐36 subscales PCS and MCS. No effects were observed for CBT compared to supportive stress management in the short term (i.e. end of treatment) for the PCS (Analysis 2.8) (n = 83) and MCS (Analysis 2.9) (n = 83). No effects were observed for CBT compared to supportive stress management in the medium term (i.e. one to six months after end of treatment) for the PCS (Analysis 2.10) (n = 83) and MCS (Analysis 2.11) (n = 83). Likewise, no effects were reported for the PCS (Analysis 2.12) and MCS (Analysis 2.13) in the long term (i.e. more than six months after end of treatment) (n = 83).
2.8. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 8: Quality of life SF‐36 physical ‐ short term
2.9. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 9: Quality of life SF‐36 mental ‐ short term
2.10. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 10: Quality of life SF‐36 physical ‐ medium term
2.11. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 11: Quality of life SF‐36 mental ‐ medium term
2.12. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 12: Quality of life SF‐36 physical ‐ long term
2.13. Analysis.

Comparison 2: Psychological intervention versus psychological intervention/clinical management, Outcome 13: Quality of life SF‐36 mental ‐ long term
2.7 Secondary outcome: cardiovascular vital signs, biomarkers of platelet activation, ECG wave recording
One trial quantified biomarkers of platelet activation (i.e. platelet count, D‐dimer level) at three months but did not report sufficient information to compute effect sizes (TREATED‐ACS 2020).
2.8 Post hoc outcome: non‐cardiac adverse events
Non‐cardiac adverse events were inconsistently and sparsely reported for this comparison. In Freedland 2009, one participant in the supportive stress management group dropped out due to psychiatric complications.
Comparison 3: Pharmacological intervention versus placebo
Thirteen trials studied the effects of a pharmacological intervention versus placebo (ANDROS 2015; CREATE 2007; EsDEPACS 2014; Freeman 1986; Li 2005; Liu 1999; Ma 2019; McFarlane 2001; MIND‐IT 2007; Pizzi 2009; SADHART 2002; Strik 2000; UPBEAT 2012). Minimal information could be extracted from the trial registry of the two trials that were terminated early (ANDROS 2015; Kennedy 2005). Data from CREATE 2007 were restricted to the citalopram and CM versus placebo and CM arms of this trial, thereby excluding data from the arms randomised to psychotherapy (IPT).
3.1 Primary outcome: depression score
Twelve studies investigated the effects of pharmacological interventions on short‐term depression symptoms (i.e. end of treatment) (CREATE 2007; EsDEPACS 2014; Freeman 1986; Li 2005; Liu 1999; Ma 2019; McFarlane 2001; MIND‐IT 2007; Pizzi 2009; SADHART 2002; Strik 2000; UPBEAT 2012). Two trials did not report sufficient information to compute effects sizes (Freeman 1986; MIND‐IT 2007). A pooled analysis of eight trials indicated that pharmacological intervention may result in a large reduction in depression symptoms at the end of treatment versus placebo (SMD −0.83, 95% CI −1.33 to −0.32) (n = 750) (Analysis 3.1) (CREATE 2007; EsDEPACS 2014; Li 2005; Liu 1999; Ma 2019; McFarlane 2001; Pizzi 2009; UPBEAT 2012). There was evidence of considerable heterogeneity between studies (I2 = 90%). Sensitivity analyses for Analysis 3.1 (Table 7) indicated that heterogeneity remained. The pooled SMD was attenuated and no longer significant in three trials undertaken in participants with depressive disorders. There was no attenuation of the pooled SMD in analyses restricted to seven trials undertaken in depression‐only samples (i.e. excluding mixed depression/anxiety). The pooled SMD was modestly attenuated in analyses restricted to six serotonergic antidepressant trials.
3.1. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 1: Depression symptoms ‐ short term
3. Sensitivity analyses for depression symptoms at end of treatment in pharmacological versus placebo trials.
| Comparison | Sensitivity analysis | Study references [n] | SMD | I2 |
| Pharmacological vs placebo | None (Analysis 3.1) | CREATE 2007; EsDEPACS 2014; Li 2005; Liu 1999; Ma 2019; McFarlane 2001; Pizzi 2009; UPBEAT 2012 (n = 750) | SMD −0.83 (95% CI −1.33 to −0.32) | 90 |
| Pharmacological vs placebo | Constrained to trials with major depressive disorders as part of the inclusion criteria |
CREATE 2007; EsDEPACS 2014; Liu 1999 (n = 427) |
SMD −0.48 (95% CI −1.38 to 0.42) | 95 |
| Pharmacological vs placebo | Constrained to depression (e.g. excluding trials with mixed depression and/or anxiety as part of the inclusion criteria) | CREATE 2007; EsDEPACS 2014; Li 2005; Liu 1999; McFarlane 2001; Pizzi 2009; UPBEAT 2012 (n = 695) | SMD −0.76 (95% CI −1.29 to −0.23) | 90 |
| Pharmacological vs placebo | Constrained to serotonergic antidepressant trials | CREATE 2007; EsDEPACS 2014; Liu 1999; McFarlane 2001; Pizzi 2009; UPBEAT 2012 (n = 613) | SMD −0.69 (95% CI −1.27 to −0.11) | 91 |
CI = confidence interval; SMD = standardised mean difference
Two studies reported depression change scores that could not be pooled in the main meta‐analysis of end‐of‐treatment SMDs (SADHART 2002; Strik 2000), and one trial reported both end‐of‐treatment scores and change scores (UPBEAT 2012). A pooled analysis of change scores suggested a small change in depression symptoms (SMD −0.18, 95% CI −0.36 to −0.00) compared to placebo (Analysis 3.2) (n = 482). Heterogeneity was low (I2 = 0%). No trials reported depression symptoms in the medium or long term.
3.2. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 2: Depression symptoms change score ‐ short term
3.2 Primary outcome: depression remission and response
Four studies investigated the effects of pharmacological interventions on short‐term depression remission (i.e. end of treatment) (CREATE 2007; EsDEPACS 2014; MIND‐IT 2007; Strik 2000). One study reported insufficient information on "depressive reductive rate" which was unclear and not extracted (Li 2005). Citalopram showed a beneficial effect compared to placebo in two studies (CREATE 2007; EsDEPACS 2014). Mirtazapine, MIND‐IT 2007 (n = 91), and fluoxetine, Strik 2000 (n = 54), did not show a beneficial effect compared to placebo. Pooled meta‐analysis of four studies indicated that pharmacological intervention probably results in a moderate to large increase in depression remission at the end of treatment versus placebo (OR 2.06, 95% CI 1.47 to 2.89; I2 = 0%) (Analysis 3.3) (n = 646) (CREATE 2007; EsDEPACS 2014; MIND‐IT 2007; Strik 2000).
3.3. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 3: Depression remission ‐ short term
Five trials investigated the effects of pharmacological intervention on depression response, defined as a 50% reduction in depression scores, in the short term (i.e. end of treatment) (CREATE 2007; EsDEPACS 2014; Liu 1999; Pizzi 2009; SADHART 2002). No significant effect was found in one trial (CREATE 2007). An effect favouring pharmacological intervention for depression response versus placebo was found in the other four trials (EsDEPACS 2014; Liu 1999; Pizzi 2009; SADHART 2002). The pooled effect from five trials indicated an effect favouring pharmacological intervention treatment versus placebo (OR 2.73, 95% CI 1.65 to 4.54) (n = 891) with considerable heterogeneity (I2 = 62%) (Analysis 3.4). No trials reported depression remission or depression response in the medium or long term for this comparison.
3.4. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 4: Depression response ‐ short term
3.3 Primary outcome: all‐cause and cardiovascular mortality
Five studies reported all‐cause mortality (EsDEPACS 2014; Liu 1999; McFarlane 2001; MIND‐IT 2007; SADHART 2002). No deaths occurred in two studies in the short term (MIND‐IT 2007 (n = 91); (McFarlane 2001) (n = 27)), and in two trials no effect was observed (Liu 1999; SADHART 2002). Data from one trial after translation remained unclear and could not be extracted (Li 2005). The evidence is very uncertain regarding the effect of pharmacological intervention on all‐cause mortality at end of treatment in two trials (OR 0.38, 95% CI 0.10 to 1.47; I2 = 0%) (n = 437) (Analysis 3.5). Medium‐term all‐cause mortality data was not reported. Two studies reported long‐term all‐cause mortality (EsDEPACS 2014 (n = 300); SADHART 2002 (n = 361)), neither of which showed a survival benefit from pharmacological intervention versus placebo. The pooled effect was not significant (OR 0.89, 95% CI 0.64 to 1.25) (n = 661) (Analysis 3.6) and without heterogeneity (I2 = 0%). One trial reported long‐term cardiovascular mortality and did not find a survival benefit from escitalopram versus placebo (Analysis 3.7) (n = 300) (EsDEPACS 2014).
3.5. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 5: All‐cause mortality ‐ short term
3.6. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 6: All‐cause mortality ‐ long term
3.7. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 7: Cardiovascular mortality ‐ long term
3.4 Primary outcome: cardiac events
Four studies analysed cardiac events (CREATE 2007; EsDEPACS 2014; Liu 1999; SADHART 2002). One trial reported specific cardiac events occurring by the end of treatment in groups randomised to mirtazapine or placebo (MIND‐IT 2007). Serious adverse events were described in the terminated trial (Kennedy 2005). Insufficient information was provided in one trial to adjudicate whether cardiac events were assessed or had occurred (Ma 2019). Three studies reported the occurrence of MI in the short term (CREATE 2007; EsDEPACS 2014; SADHART 2002). The evidence is very uncertain regarding the effects of pharmacological intervention on MI at end of treatment from tree trials (OR 0.74, 95% CI 0.26 to 2.09; I2 = 0%) (n = 728) (Analysis 3.8). Longer‐term MI was not significantly decreased in one trial comparing escitalopram versus placebo (Analysis 3.9) (n = 300) (EsDEPACS 2014).
3.8. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 8: Myocardial infarction ‐ short term
3.9. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 9: Myocardial infarction ‐ long term
There was little to no difference in angina at the end of treatment in trials of sertraline (SADHART 2002) (n = 369), mirtazapine (MIND‐IT 2007) (n = 91), citalopram (CREATE 2007) (n = 142), and escitalopram (EsDEPACS 2014) (n = 217). Meta‐analysis of four studies indicated little to no difference with pharmacological intervention versus placebo in angina pectoris (OR 0.75, 95% CI 0.44 to 1.28; I2 = 0%) (Analysis 3.10) (n = 819) (CREATE 2007; EsDEPACS 2014; MIND‐IT 2007; SADHART 2002). Angina was not reported in the medium to long term for this comparison.
3.10. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 10: Angina ‐ short term
There was little to no difference in heart failure in trials of sertraline (SADHART 2002) (n = 369), mirtazapine (MIND‐IT 2007) (n = 91), and citalopram (CREATE 2007) (n = 142), though the number of events was sparse. Meta‐analysis of three studies indicated little to no difference with pharmacological intervention versus placebo in heart failure in the short term (OR 0.93, 95% CI 0.33 to 2.62; I2 = 0%) (Analysis 3.11) (n = 602) (CREATE 2007; MIND‐IT 2007; SADHART 2002).
3.11. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 11: Heart failure ‐ short term
Arrhythmias were decreased in one trial of fluoxetine compared to placebo at the end of treatment (Liu 1999). Atrial fibrillation was reported as a serious adverse event in the terminated trial (Kennedy 2005). The pooled estimate from two trials showed little to no difference in arrhythmia at end of treatment (OR 0.46, 95% CI 0.01 to 17.06) (Analysis 3.12) (n = 87). The number of events was sparse, and there was considerable heterogeneity between studies (I2 = 71%). Changes to ECG waves, Kennedy 2005; Strik 2000, and heart rate variability, McFarlane 2001, were reported but could not be extracted due to uncertainty in the assessment of arrhythmia endpoints. Ventricular function assessment and endpoints were unclear after translation of one trial, and data could not be extracted (Li 2005).
3.12. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 12: Arrhythmia ‐ short term
There was little to no difference in stroke in one trial comparing sertraline to placebo, SADHART 2002 (n = 369), and one trial comparing escitalopram to placebo, EsDEPACS 2014 (n = 217). One trial of citalopram compared to placebo reported no stroke events in either group (n = 142) (CREATE 2007). The pooled probability estimate of stroke at the end of treatment from the two trials with events indicated little to no difference in stroke (OR 0.99, 95% CI 0.20 to 4.96) (Analysis 3.13) (n = 586). The number of events was sparse, and there was no heterogeneity between studies (I2 = 0%). There was little to no difference in percutaneous coronary intervention procedures in the long term in one trial of escitalopram versus placebo (Analysis 3.14) (n = 300) (EsDEPACS 2014). Evidence of coronary revascularisation interventions for CAD was sparse and not reported in the short and medium term.
3.13. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 13: Stroke ‐ short term
3.14. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 14: Coronary revascularisation procedure ‐ long term
3.5 Secondary outcome: healthcare and resource utilisation
There was little to no difference in healthcare costs at the end of treatment, excluding antidepressant medication with sertraline, in SADHART 2002 (Analysis 3.15) (n = 369). Meta‐analysis of three studies (n = 514) indicated that pharmacological interventions may reduce hospitalisations compared to placebo (OR 0.58, 95% CI 0.39 to 0.85) (Analysis 3.16) without evidence of heterogeneity (I2 = 0%) (MIND‐IT 2007; SADHART 2002; Strik 2000). Any possible effect on hospitalisation was largely attributed to a trial of sertraline (OR 0.59, 95% CI 0.38 to 0.91) (n = 369) (SADHART 2002), whereas no effect was observed in the trials of mirtazapine, MIND‐IT 2007 (n = 91), and fluoxetine, Strik 2000 (n = 54). Emergency room visits at the end of treatment were not reduced in a trial of sertraline (OR 0.58, 95% CI 0.34 to 1.00) (Analysis 3.17) (n = 369) (SADHART 2002).
3.15. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 15: Healthcare costs ‐ short term
3.16. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 16: Hospitalisations ‐ short term
3.17. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 17: Emergency department visits ‐ short term
3.6 Secondary outcome: quality of life
Two trials examined quality of life (EsDEPACS 2014; SADHART 2002). The SADHART 2002 trial (n = 369) investigated quality of life using the Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q) and the SF‐36 comparing sertraline with placebo. Data for the SF‐36 were not reported sufficiently to compute effects sizes. No effect was observed for the Q‐LES‐Q (Analysis 3.18). EsDEPACS 2014 (n = 213) examined short‐ and medium‐term QoL using the WHOQOL‐BREF questionnaire. Escitalopram compared to placebo (n = 213) showed possible short‐term end‐of‐treatment effects on the following WHOQOL‐BREF subscales: physical (MD 6.80, 95% CI 2.77 to 10.83) (Analysis 3.19), psychological (MD 5.60, 95% CI 1.54 to 9.66) (Analysis 3.20), social relationship (MD 4.00, 95% CI 0.03 to 7.97) (Analysis 3.21), and environmental (MD 6.50, 95% CI 2.90 to 10.10) (Analysis 3.22) (EsDEPACS 2014). End‐of‐treatment effects on social and occupational functioning, as well as disability, were reported for a subset of participants in EsDEPACS 2014 (n = 217), but were not extracted here.
3.18. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 18: Quality of life Q‐LES‐Q ‐ short term
3.19. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 19: Quality of life WHOQOL‐BREF Physical ‐ short term
3.20. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 20: Quality of life WHOQOL‐BREF Psychological ‐ short term
3.21. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 21: Quality of life WHOQOL‐BREF Social relationships ‐ short term
3.22. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 22: Quality of life WHOQOL‐BREF Environmental ‐ short term
Escitalopram compared to placebo showed possible medium‐term treatment effects on the WHOQOL‐BREF subscales physical (MD 6.10, 95% CI 1.25 to 10.95) (Analysis 3.23), social relationship (MD 4.80, 95% CI 0.17 to 9.43) (Analysis 3.25), and environmental (MD 5.80, 95% CI 1.54 to 10.06) (Analysis 3.26), but not on the psychological subscale (MD 4.70, 95% CI −0.33 to 9.73) (n = 213) (Analysis 3.24) (EsDEPACS 2014).
3.23. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 23: Quality of life WHOQOL‐BREF Physical ‐ medium term
3.25. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 25: Quality of life WHOQOL‐BREF Social Relationships ‐ medium term
3.26. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 26: Quality of life WHOQOL‐BREF Environmental ‐ medium term
3.24. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 24: Quality of life WHOQOL‐BREF Psychological ‐ medium term
3.7 Secondary outcome: cardiovascular vital signs, biomarkers of platelet activation, ECG wave recording
Three trials reported all BP and heart rate cardiovascular vital signs post‐treatment (CREATE 2007; EsDEPACS 2014; SADHART 2002), and a fourth trial reported heart rate and not BP (McFarlane 2001). Pooled analysis from three trials indicated that pharmacological intervention may result in little to no difference in end‐of‐treatment systolic BP versus placebo (MD −0.24, 95% CI −3.52 to 3.05) (Analysis 3.27) in three trials (n = 675) without substantial heterogeneity (I2 = 32%). Likewise, pharmacological intervention may result in little to no difference in end‐of‐treatment diastolic BP (MD 0.60, 95% CI −1.55 to 2.74) (Analysis 3.28) in three trials (n = 675). There was evidence of moderate heterogeneity between studies (I2 = 43%). Pharmacological intervention may result in little to no difference in end‐of‐treatment heart rate (MD −0.80, 95% CI −2.40 to 0.79) (Analysis 3.29) in four trials (n = 662). There was no evidence of heterogeneity between studies (I2 = 0%).
3.27. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 27: Systolic BP ‐ short term
3.28. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 28: Diastolic BP ‐ short term
3.29. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 29: Heart rate ‐ short term
Seven studies reported platelet biomarkers post‐treatment (CREATE 2007; EsDEPACS 2014; Ma 2019; MIND‐IT 2007; Pizzi 2009; SADHART 2002; UPBEAT 2012), generally from a smaller subset of participants from each trial arm. Two studies reported insufficient data to calculate effect sizes (EsDEPACS 2014; UPBEAT 2012), and additional data for PF4 could be extracted from an online trial data repository (UPBEAT 2012). Two studies reported platelet biomarkers outside of the outcomes of this review (Ma 2019; Pizzi 2009), which may be considered in a future update. Meta‐analysis of three trials showed that pharmacological treatment may reduce βTG at end of treatment (SMD −0.54, 95% CI −0.99 to −0.09) versus placebo (n = 141) (Analysis 3.30). There was evidence of possible heterogeneity (I2 = 36%).
3.30. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 30: Platelet biomarker βTG ‐ short term
Meta‐analysis of three trials showed that pharmacological treatment may result in little to no difference in reduction in PF4 (SMD −0.14, 95% CI −0.48 to 0.19) versus placebo (n = 144) (Analysis 3.31). There was no evidence of heterogeneity between studies (I2 = 0%). Meta‐analysis of two trials showed that pharmacological treatment may result in little to no difference in P‐selectin (SMD −0.31, 95% CI −1.12 to 0.50) versus placebo (n = 121) (Analysis 3.32). There was evidence of considerable between‐study effect sizes (I2 = 79%). Only SADHART 2002 reported PECAM‐1 and TxB2 in a subset of trial participants. SADHART 2002 did not find an effect of pharmacological treatment versus placebo on PECAM‐1 (MD −8.30, 95% CI −18.12 to 1.52) (n = 64) (Analysis 3.33). No effect was observed in SADHART 2002 of pharmacological treatment versus placebo in TxB2 (MD −6.20, 95% CI −15.78 to 3.38) (n = 64) (Analysis 3.34).
3.31. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 31: Platelet biomarker PF4 ‐ short term
3.32. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 32: Platelet biomarker P‐selectin ‐ short term
3.33. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 33: Platelet biomarker PECAM‐1 ‐ short term
3.34. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 34: Platelet biomarker TxB 2 ‐ short term
Six studies performed end‐of‐treatment ECGs and reported wave parameters comparing pharmacological intervention versus placebo at end of treatment (CREATE 2007; EsDEPACS 2014; MIND‐IT 2007; SADHART 2002; Strik 2000; UPBEAT 2012). Three studies reported insufficient data to calculate effect sizes (MIND‐IT 2007; Strik 2000; UPBEAT 2012). A reduction in PR interval was found in the SADHART 2002 trial of sertraline when compared to placebo (MD −6.00, 95% CI −11.84 to −0.16). The pooled effect for PR interval from three studies indicated that pharmacological intervention may result in a small reduction in PR interval (MD −4.35, 95% CI −8.40 to −0.31) (Analysis 3.35) (n = 635) without evidence of heterogeneity (I2 = 0%) (CREATE 2007; EsDEPACS 2014; SADHART 2002). The pooled effect of ECG findings also suggested that pharmacological intervention may result in little to no difference in the QRS interval at the end of treatment (MD 2.37, 95% CI −0.41 to 5.15) (Analysis 3.36) (n = 635) without evidence of heterogeneity (I2 = 0%). Only CREATE 2007 (n = 142) reported the QT interval, finding no evidence of a difference between citalopram and placebo (MD 2.40, 95% CI −9.11 to 13.91). Pooled meta‐analysis from three trials indicated that pharmacological intervention probably results in little to no difference in QTc interval at the end of treatment (MD 2.76, 95% CI −1.96 to 7.47) (Analysis 3.38) (n = 635) without evidence of heterogeneity between studies (I2 = 20%).
3.35. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 35: ECG PR interval ‐ short term
3.36. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 36: ECG QRS interval ‐ short term
3.38. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 38: ECG QTc interval ‐ short term
3.8 Post hoc outcome: non‐cardiac adverse events and pharmacological side effects
One trial reported worsening depression in one participant receiving placebo and CM (CREATE 2007). Otherwise, non‐cardiac adverse events were sparsely reported. Ten studies reported pharmacological side effects (CREATE 2007; EsDEPACS 2014; Kennedy 2005; Li 2005; Ma 2019; MIND‐IT 2007; Pizzi 2009; SADHART 2002; Strik 2000; UPBEAT 2012). Two studies reported insufficient data to calculate effect sizes (Li 2005; Ma 2019). Pharmacological intervention may be associated with an increase in side effects (OR 1.44, 95% CI 1.07 to 1.92) versus placebo (Analysis 3.39) in eight trials (n = 1193) (CREATE 2007; EsDEPACS 2014; Kennedy 2005; MIND‐IT 2007; Pizzi 2009; SADHART 2002; Strik 2000; UPBEAT 2012), without evidence of heterogeneity (I2 = 0%).
3.39. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 39: Non‐cardiac adverse events and side effects ‐ short term
Comparison 4: Pharmacological intervention versus pharmacological intervention
4.1 Primary outcome: depression symptoms
Eight trials compared two active pharmacological interventions against each other (Abbasi 2015; Carney 2009; Divsalar 2018; Liu 2016; Roose 1998; Shahmansouri 2014; Tian 2016; Wang 2020). We did not report pooled estimates for this comparison due to the heterogeneous interventions and comparators examined in the trials. The trials made the following comparisons:
Abbasi 2015 (n = 46) simvastatin versus atorvastatin (each 20 mg/d);
Carney 2009 (n = 122) the add‐on effect of sertraline (50 mg/d) plus omega‐3 (2 g/d) versus sertraline (50 mg/d) and placebo;
Divsalar 2018 (n = 56) sertraline (200 mg/d) plus red yeast rice (2400 mg/d) versus sertraline (200 mg/d) plus placebo;
Liu 2016 (n = 146) Shugan Jieyu plus sertraline placebo versus sertraline plus Shugan Jieyu placebo;
Roose 1998 (n = 81) paroxetine versus nortriptyline;
Shahmansouri 2014 (n = 40) saffron (15 to 30 mg/d) versus fluoxetine (20 to 40 mg/d);
Tian 2016 (n = 46) paroxetine versus fluoxetine (each 20 mg/d); and
Wang 2020 (n = 228) escitalopram (5 to 10 mg/d) versus Bu Xin Qi concoction (400 mL twice a day).
Eight studies examined the differential effects of two pharmacological interventions on short‐term depression symptoms, all using the HAM‐D clinician rating scale (Abbasi 2015; Carney 2009; Divsalar 2018; Liu 2016; Roose 1998; Shahmansouri 2014; Tian 2016; Wang 2020). The evidence is very uncertain as to whether different pharmacological interventions may result in a reduction in depression symptoms at end of treatment (Analysis 4.1) for: simvastatin versus atorvastatin (SMD −0.66, 95% CI −1.25 to −0.06) (n = 46) (Abbasi 2015); paroxetine versus fluoxetine (SMD −1.05, 95% CI −1.67 to −0.43) (n = 46) (Tian 2016); and escitalopram versus Bu Xin Qi (SMD −1.02, 95% CI −1.30 to −0.74) (n = 228) (Wang 2020). The evidence is very uncertain as to whether the add‐on effect of sertraline (50 mg/d) plus omega‐3 (2 g/d) versus sertraline (50 mg/d) and placebo may result in a reduction in depression symptoms at end of treatment (n = 122) (Carney 2009).
4.1. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 1: Depression symptoms ‐ short term
Four trials reported end‐of‐treatment depression change scores (Divsalar 2018; Liu 2016; Roose 1998; Shahmansouri 2014). In the study by Liu 2016, we assumed the data were reported as standard errors and not standard deviation as stated in the article to remain consistent with the P values reported. The evidence is very uncertain regarding the effects of different pharmacological strategies on end‐of‐treatment depression change scores in the four trials (Analysis 4.2) (Divsalar 2018 (n = 50), Liu 2016 (n = 149), Roose 1998 (n = 81), and Shahmansouri 2014 (n = 40)). No trials for this comparison reported medium‐ or longer‐term durability of interventions on depression symptoms.
4.2. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 2: Depression symptoms change score ‐ short term
4.2 Primary outcome: depression remission and response
Three studies examined the differential effects of two pharmacological interventions on depression remission (Carney 2009; Roose 1998; Shahmansouri 2014). In all three trials no differences were observed between groups using the clinician‐rated HAM‐D, Carney 2009; Shahmansouri 2014, or the BDI‐II, Roose 1998 (Analysis 4.3), and the evidence is very uncertain regarding the effects of different pharmacological strategies on end‐of‐treatment depression remission. Four studies examined the differential effects of two pharmacological interventions on depression response (Abbasi 2015; Carney 2009; Roose 1998; Shahmansouri 2014). No differences were observed using the clinician‐rated HAM‐D, Abbasi 2015; Carney 2009; Shahmansouri 2014, or the BDI‐II, Roose 1998 (Analysis 4.4). The study by Liu 2016 (n = 149) reported the number needed for treatment for non‐inferiority, but not depression response nor remission, therefore no data were extracted.
4.3. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 3: Depression remission ‐ short term
4.4. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 4: Depression response ‐ short term
4.3 Primary outcome: all‐cause and cardiovascular mortality
The evidence from one trial is very uncertain regarding the effect of Shugan Jieyu plus sertraline placebo compared to sertraline plus Shugan Jieyu placebo on all‐cause mortality at the end of treatment (n = 149) (Analysis 4.5) (Liu 2016). No trials for this comparison reported medium‐ or longer‐term all‐cause mortality. Likewise, no trials for this comparison reported cardiovascular mortality at any time point.
4.5. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 5: All‐cause mortality ‐ short term
4.4 Primary outcome: cardiac events
Four studies reported the differential effects of two pharmacological interventions on cardiac events at the end of treatment (i.e. short term) (Carney 2009; Roose 1998; Tian 2016; Wang 2020). The number of events were sparse for MI, heart failure, and arrhythmia, whilst no study reported stroke. The evidence is very uncertain regarding the occurrence of MI at end of treatment in trials of: sertraline plus omega‐3 versus sertraline plus placebo (n = 122) (Carney 2009); paroxetine versus fluoxetine (n = 46) (Tian 2016); and escitalopram versus Bu Xin Qi concoction (n = 228) (Wang 2020) (Analysis 4.6). No differences were reported between different pharmacological strategies on end‐of‐treatment angina in the trials by Roose 1998 (n = 81), Tian 2016 (n = 46), or Wang 2020 (n = 228) (Analysis 4.7). No differences were observed between pharmacological interventions in end‐of‐treatment heart failure in the trials reported by Carney 2009 (n = 122) and Wang 2020 (n = 228) (Analysis 4.8). Likewise, no differences were observed between pharmacological interventions in end‐of‐treatment arrhythmia in the trials reported by Carney 2009 (n = 122), Roose 1998 (n = 81), and Wang 2020 (n = 228) (Analysis 4.9). No studies reported the occurrence of MI, angina, heart failure, arrhythmia, or stroke in the medium to longer term. Coronary revascularisation procedure for CAD (angioplasty) at end of treatment did not differ between groups in the trial reported by Carney 2009 (Analysis 4.10) (n = 122).
4.6. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 6: Myocardial infarction ‐ short term
4.7. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 7: Angina ‐ short term
4.8. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 8: Heart failure ‐ short term
4.9. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 9: Arrhythmia ‐ short term
4.10. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 10: Coronary revascularisation procedure ‐ short term
4.5 Secondary outcome: healthcare and resource utilisation
One trial reported emergency room visits, finding no effect for sertraline plus omega‐3 versus sertraline plus placebo (n = 122) (Analysis 4.11) (Carney 2009).
4.11. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 11: Emergency department visits ‐ short term
4.6 Secondary outcome: quality of life
No trials evaluating this comparison reported QoL at any time point.
4.7 Secondary outcome: cardiovascular vital signs, biomarkers of platelet activation, ECG wave recording
Three studies reported the differential effects of pharmacological interventions on cardiovascular vital signs in the short term (Liu 2016; Roose 1998; Tian 2016). Paroxetine may result in a lower systolic BP (MD −10.00, 95% CI −17.10 to −2.90) compared to nortriptyline (n = 63) (Analysis 4.12) (Roose 1998). Systolic BP may not differ for pharmacological strategies employing paroxetine versus fluoxetine (n = 46) (Tian 2016), or Shugan Jieyu versus sertraline (n = 149) (Liu 2016). No differential effects were evident between pharmacological interventions for diastolic BP in the trials by Roose 1998 (n = 63), Tian 2016 (n = 46), and Liu 2016 (n = 149) (Analysis 4.13). Two studies reported heart rate. Paroxetine may result in a lower heart rate (MD −11.00, 95% CI −14.31 to −7.69) compared to nortriptyline (n = 63) (Analysis 4.14) (Roose 1998). No difference in heart rate was observed between Shugan Jieyu plus sertraline placebo and sertraline plus Shugan Jieyu placebo (n = 149) (Liu 2016). No trials reported medium‐ to longer‐term assessment of cardiovascular vital signs for this comparison.
4.12. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 12: Systolic BP ‐ short term
4.13. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 13: Diastolic BP ‐ short term
4.14. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 14: Heart rate ‐ short term
No head‐to‐head comparison of two pharmacological interventions reported on biomarkers of platelet activation.
Two studies reported the differential effects of two pharmacological interventions on ECG waves in the short term (Liu 2016; Roose 1998). Paroxetine may result in a lower PR interval (MD −9.00, 95% CI −16.77 to −1.23) compared to nortriptyline (n = 63) (Analysis 4.15) (Roose 1998). Shugan Jieyu plus sertraline placebo versus sertraline plus Shugan Jieyu placebo was not associated with differences in PR interval (n = 146) (Liu 2016). There may be little to no differences between pharmacological interventions for the QRS (Analysis 4.16) and QTc intervals (Analysis 4.17). No trial reported end‐of‐treatment QT intervals. One trial reported ventricular premature depolarisations (Roose 1998), which was not considered in this review.
4.15. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 15: ECG PR interval ‐ short term
4.16. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 16: ECG QRS interval ‐ short term
4.17. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 17: ECG QTc interval ‐ short term
4.8 Post hoc outcome: non‐cardiac adverse events and pharmacological side effects
In Shahmansouri 2014, one participant allocated to fluoxetine withdrew from treatment due to suicidal ideation. In two trials (Liu 2016 ; Wang 2020), the definition of non‐cardiac adverse events was unclear. Otherwise, non‐cardiac adverse events were sparsely reported. Seven studies reported pharmacological side effects (Abbasi 2015; Carney 2009; Divsalar 2018; Liu 2016; Roose 1998; Shahmansouri 2014; Wang 2020). There may be little to no difference between different pharmacological intervention strategies on side effects at end of treatment in seven trials (Analysis 4.18) (n = 716).
4.18. Analysis.

Comparison 4: Pharmacological intervention versus pharmacological intervention, Outcome 18: Non‐cardiac adverse events and side effects ‐ short term
Comparison 5: Psychological intervention versus pharmacological intervention
We found no trials evaluating psychological intervention versus pharmacological intervention. No analyses were performed.
Subgroup analyses
We planned subgroup analyses to take into account variables such as the population, sex, CAD subtype, time of onset of depression, CAD severity, and risk of bias. Lack of primary data per outcome precluded these analyses. However, as Cochrane Reviews are meant to be updated on a regular basis, these analyses may be feasible in future updates of this review.
Discussion
The current systematic review investigated the effects of psychological and pharmacological interventions on depression outcomes, mortality, cardiac events, healthcare costs and utilisation, health‐related QoL, cardiovascular vital signs, biomarkers of platelet activation, ECG wave parameters, non‐cardiac adverse events, and side effects in CAD patients with comorbid depressive disorder. Our comprehensive search strategy identified 37 RCTs fulfilling the inclusion criteria of the review. Fifteen trials examined psychological interventions, and 21 trials examined pharmacological interventions.
Summary of main results
The results of the current review provide evidence that psychological interventions may result in lower depression symptoms compared to control at end of treatment. However, psychological intervention may result in little to no difference in depression remission in the short term based on three trials. The findings of one trial favoured psychological intervention for depression remission in the long term but not in the medium term. Based on one to two trials per outcome, the evidence is uncertain or sparse for the effects of psychological interventions versus usual care on mortality and cardiac events in the short to longer term. Regarding our secondary outcomes, one trial reported that psychological intervention may result in a reduction in resource utilisation (i.e. length of hospital stay). Though an improved mental QoL favouring psychological intervention versus usual care was found in the short term, no effect on end‐of‐treatment physical QoL was observed.
The evidence base for the comparison of psychological intervention versus other psychological intervention or CM was sparse, and the evidence is very uncertain. Based on four trials, the evidence is very uncertain as to whether there may be differences between the varying approaches (behavioural therapy versus person‐centred therapy; CBT versus supportive stress management; IPT versus CM; CBT and well‐being therapy versus CM) on our primary outcomes. Statistical and methodological heterogeneity precluded the pooling of results to determine effect sizes.
Regarding the comparison pharmacological intervention versus placebo, we found low‐certainty evidence that pharmacological intervention may result in a large reduction in depression symptoms at the end of treatment. There was moderate‐certainty evidence that pharmacological intervention probably results in a moderate to large increase in depression remission at the end of treatment. The evidence is very uncertain regarding the effects of pharmacological intervention on mortality and cardiac events, and no consistent findings were reported. The evidence for our secondary outcomes of hospitalisation rates, emergency room visits, and QoL was sparse but points in the direction of a possible beneficial effect of pharmacological intervention compared to placebo. Evidence on cardiovascular vital signs, platelet biomarkers, and ECG waves was mixed and based on small substudies from the main trials. Pooled meta‐analysis of one to three trials indicated a possible small beneficial effect of pharmacological interventions for βTG and lower PR interval on ECG at the end of treatment. A possible increase in non‐cardiovascular side effects was observed with the pharmacological intervention compared with placebo.
The comparison of pharmacological intervention versus other pharmacological intervention comprised eight trials. The evidence was very uncertain for the effect of different pharmacological agents on depression symptoms at the end of treatment for: simvastatin compared to atorvastatin (Abbasi 2015); sertraline plus omega‐3 compared to sertraline plus placebo (Carney 2009); paroxetine compared to fluoxetine (Tian 2016); and escitalopram compared to Bu Xin Qi (Wang 2020). Statistical and methodological heterogeneity precluded the pooling of results to determine effect sizes.
Overall, there is evidence for a possible beneficial effect of both psychological and pharmacological interventions on depression outcomes at end of treatment. However, the evidence base is still small and did not permit conclusions about the effects of these interventions on most other outcomes, as well as on specific types of psychological approaches and pharmacological agents. Moreover, the settings, samples, interventions, and outcome measures were heterogeneous across the included studies, which hampered the interpretation of meta‐analytical synthesis. Sensitivity analyses for end‐of‐treatment depression symptoms provided mixed results that could not explain heterogeneity between psychological trials (versus control) and pharmacological trials (versus placebo). Moreover, our pre‐planned subgroup analyses were not feasible due to the low number of studies per outcome and methodological and clinical heterogeneity between studies.
Overall completeness and applicability of evidence
This review summarises the evidence regarding depression treatments in a variety of settings. The included trials comprised different CAD samples (MI, CABG, PCI); investigated various types of psychological and pharmacologic interventions; and were located in different countries with different healthcare systems, thus increasing the generalisability of the results. However, the overall completeness is limited, and the applicability of evidence restricted due to four aspects.
Firstly, most of the primary and secondary outcomes were investigated or reported insufficiently. Hence, evidence of treatment effects on these outcomes needs to be interpreted carefully. Moreover, most trials were underpowered to detect effects of depression treatments on mortality and specific cardiac events and were below the optimal information size.
Secondly, we found no studies comparing psychological and pharmacological interventions. Consequently, no conclusions could be drawn on the differential effects of these treatment approaches. A systematic review experimentally comparing the two approaches indicated that overall psychological and pharmacological interventions were equally effective for treating depression, with pharmacotherapy seemingly superior in dysthymia (g = 0.3), as well as compared to non‐directive counselling (g = 0.33), and psychotherapy superior to tricyclic antidepressants (g = 0.21) (Cuijpers 2013). Combining both pharmacotherapy and psychotherapy was superior to pharmacotherapy alone at six months or longer postrandomisation (OR = 2.93), whilst psychotherapy alone resulted in equal depression effects when compared to combined therapy at six months and longer follow‐up (Karyotaki 2016). However, the National Institute for Health and Care Excellence (NICE) guideline on depression in adults with a chronic physical health problem favours the use of psychological interventions as first‐line interventions in patients with minor and mild to moderate depression due to adverse effects of antidepressants and the resulting poor risk‐benefit ratio (NICE 2009). An increase in side effects was observed in trials of antidepressants versus placebo for patients with CAD and depression symptoms.
Thirdly, the samples of the included trials most likely differed regarding subtypes and severity of depression. The included trials comprised participants with a wide range of depressive symptomatology and different aetiology (e.g. dysthymia, minor and major depression, adjustment disorder with depressed mood; Baumeister 2012a). Depressive disorders were present immediately following the cardiac event or up to 12 months after the event. Furthermore, diverse methods and cut‐off points were used to determine trial eligibility and determine depression remission and depression response. The inclusion of mixed samples and those with different depression disorders may have levelled potential effects of depression treatments in participants with specific subtypes of depression (Baumeister 2012a; Baune 2012). For example, when these factors were considered, sensitivity analyses showed changes to the strength and significance of end‐of‐treatment depression symptoms effect sizes. Other research suggests that the onset of depression was previously shown to be a moderator of treatment outcomes in CAD patients (Dickens 2008). Another trial on depression treatment in general highlighted differential responses to psychotherapy versus pharmacotherapy in chronic depressed patients with childhood trauma compared to those without a history of childhood trauma (Nemeroff 2003). Moreover, a patient‐level meta‐analysis concluded that the effects of antidepressant medication are associated with the severity of depressive symptoms, showing minimal effects in mild to moderate depression and substantial benefit in severe depression (Fournier 2010).
Fourthly, the length of psychotherapies examined in the included trials ranged from short‐term four‐session therapies, Barth 2005; Dao 2011, to 12 sessions, Freedland 2009; TREATED‐ACS 2020; single‐ and group‐therapies, Brown 1993; SPIRR‐CAD 2011; and 12‐month telephone support counselling, Yang 2019. The number and intensity of sessions needed to show substantial benefit in psychotherapy should be higher or more specifically tailored to the needs, problems and treatment response of individual patients in order to exploit the full potential of psychological interventions (Carr 2017; Harnett 2010). Hence, the effects found in the included psychological intervention trials may be limited in part due to an insufficient number or intensity of sessions.
Quality of the evidence
The included trials differed with regard to methodological shortcomings (see Risk of bias in included studies) and quality of reporting. Many trials did not adequately describe design aspects such as randomisation procedure, allocation concealment, and blinding. Furthermore, some trials did not report ITT analyses, missing data was common, and selective reporting may have occurred. However, published protocols were not always available, and we were unable to quantify publication bias for our primary outcomes. Low‐quality studies have been associated with exaggerated effects (Cuijpers 2010; Moher 1999). The treatment effects summarised in this review may therefore be overestimated due to poor methodological quality of some of the included trials as well as uncertainty in study quality.
Most pharmacological studies were supported by pharmaceutical industry, and two trials included pharmaceutical company employees in the design, conduct, analysis, and reporting of trial outcomes. It has been shown that studies sponsored by pharmaceutical companies are more likely to have outcomes favouring the sponsor than studies with other sponsors (Lexchin 2003). Furthermore, selective reporting of null findings in industry‐funded RCTs of antidepressant trials has been documented (Turner 2008). Despite our comprehensive search strategy, there may be unpublished trials with non‐significant results or additional trials terminated prematurely.
Another source of bias results from selective reporting of negative findings for prespecified primary outcomes, whilst emphasising positive results from secondary or new outcomes of antidepressant medication trials (Pigott 2010). We were not able to obtain published protocols for all trials included in this review and thus were not able to judge the risk of selective reporting for these studies.
Meta‐analyses regarding depressive outcomes were hampered because depressive symptoms were assessed by a heterogeneous set of clinician‐rated tools and self‐report questionnaires. Furthermore, the included trials reported either final mean scores or mean change scores from baseline to final assessment or did not report sufficient information to compute effect estimates.
The GRADE assessment of primary outcomes in the short term resulted in a range of ratings of certainty of evidence. We assessed the evidence for only one outcome as moderate certainty (depression remission at end of treatment in pharmacological versus placebo trials). Otherwise, the certainty of evidence was very low, low, or could not be determined. The certainty of evidence must be taken into consideration when interpreting the findings of the review (Table 1; Table 2; Table 3; Table 4).
Potential biases in the review process
In the review process we decided to consider the ENRICHD 2003 and Barth 2005 studies as psychological intervention trials, disregarding the fact that participants in these trials were allowed to receive adjunct pharmacologic treatments in addition to the assigned psychological intervention. Hence, it remains unclear to what degree the effects of these studies were impacted by additional pharmacological treatments. Conversely, we excluded the psychological arms (IPT) in the CREATE 2007 trial who received citalopram, placebo, or CM. The results might also be biased by the inclusion of mixed study samples of CAD patients with depression and/or low social support, ENRICHD 2003, and patients with depression and/or anxiety (Brown 1993; Dao 2011; Fang 2003; Freeman 1986; Ma 2019; McLaughlin 2005; U‐CARE 2018; Zarea 2014). As a result of including these trials with mixed study samples and methodological uncertainties, the presented findings might be biased. Moreover, the inclusion of such heterogeneous samples without depression may spuriously increase or decrease the pooled effect sizes. Another source of potential bias may result from the translation process of the included Chinese trials (Fang 2003; Li 2005; Liu 1999). Despite our efforts to translate the Chinese trials accurately, the translations did not result in unambiguous results. Moreover, the limited information provided resulted in unclear risk of bias for these trials (Fang 2003; Li 2005; Liu 1999). Another potential source of bias in the review process concerns the pooling of outcomes by time frame (short, medium, and long term) in accordance with the original review (Baumeister 2011c). This likely results in data below the optimal information size when pooling uncommon cardiovascular events (e.g. stroke). Future updates to this review may consider pooling all time frames together in dichotomous but infrequent primary outcomes of mortality and cardiovascular events. Likewise, combining end‐of‐treatment depression symptom effect sizes with the medium‐term data may result in increased certainty of evidence.
Agreements and disagreements with other studies or reviews
Differences in the trials included in this review compared to previous reviews are attributable to our focus on trials investigating depression treatments in CAD patients with depression. Two psychological intervention trials included in the current review, CREATE 2007; ENRICHD 2003, were also included in the review of Van Straten 2010, which investigated the effects of psychological treatments on depressive symptoms in medical diseases. The authors conducted a meta‐analysis of 23 studies with 10 different medical diseases and concluded that depressive symptoms could be effectively treated with psychological interventions. The results of our review were in a similar direction for CAD patients with depression. However, the evidence is still sparse given the high within‐review variance of findings regarding psychological interventions.
Rayner 2010, a Cochrane Review, systematically reviewed trials investigating the effects of antidepressant medication in treating depression in physically ill people. They concluded that antidepressants were superior to placebo in a meta‐analysis of 51 studies. The evidence of the current review agrees with this finding for the specific group of CAD patients with comorbid depression. Four trials were included in both reviews (CREATE 2007; MIND‐IT 2007; SADHART 2002; Strik 2000).
One systematic review examined collaborative care interventions for patients with CAD and depression (Tully 2015). Based on six included trials, the authors reported a reduction in major cardiac events in the short but not long term, and a small‐to‐moderate effect on depression severity and an increased depression remission rate. A direct comparison of the findings on collaborative care (Tully 2015) with the stand‐alone psychological or pharmacological interventions here is difficult since the methodological heterogeneity between the respective included trials is substantial. There is as yet insufficient evidence to recommend one treatment option over another, suggesting that psychological interventions and pharmacological interventions and more complex collaborative care are recommendable for the treatment of depression in patients with CAD and depression. A network meta‐analysis compared different depression interventions for CAD patients at eight weeks from baseline (Doyle 2021). Both psychological therapy (versus usual care) and antidepressant therapy (versus placebo and usual care) displayed significantly better effects. The strongest effects were evident for combination therapy and exercise; however, only single trials were available. Moreover, the network meta‐analysis included antidepressant versus usual care interventions, which was a prespecified exclusion criterion for the current review, where we only included pharmacological versus placebo trials (Doyle 2021).
Several trials included in our review, Brown 1993; ENRICHD 2003; Freedland 2009; McLaughlin 2005; MoodCare 2011, were included in a Cochrane Review on the effects of psychological interventions in CAD patients in general (i.e. not restricted to depressed CAD patients) (Richards 2017). Overall, psychological interventions had no effect on mortality, but a beneficial effect on depression symptoms (Richards 2017), which is comparable to the current review. Also, Richards 2017 found a 22% reduction in MI for psychological interventions, which cannot be confirmed for the depression‐specific interventions for depressed CAD patients examined in the current review.
The systematic review by Swenson 2006 reported the side effects from antidepressant drug versus placebo trials performed in individuals with chronic illness. No difference was observed between SSRI and placebo for serious and non‐serious cardiovascular events. Swenson 2006 also reported higher dropout due to side effects in participants receiving SSRIs versus placebo. A direct comparison with the current review indicates some consistent results on pharmacological side effects. We found evidence that pharmacological intervention may result in an increase in side effects compared to placebo at end of treatment. However, the evidence is very uncertain as to whether there are differences between different pharmacological agents in end‐of‐treatment side effects.
Authors' conclusions
Implications for practice.
Psychological interventions may result in a reduction in depression symptoms at end of treatment. Pharmacological interventions may result in a reduction in depression symptoms and probably result in a moderate to large increase in depression remission at end of treatment. The National Institute for Health and Care Excellence (NICE) guideline on depression in adults with a chronic physical health problem favours the use of psychological interventions as first‐line interventions in patients with minor and mild‐to‐moderate depression due to the adverse effects of antidepressants and the resulting poor risk‐benefit ratio (NICE 2009). In the primary studies of the current review, antidepressant medications compared to placebo were associated with increased rates of dizziness, diarrhoea, somnolence, sweating, palpitations, libido reduction or sexual difficulties in CREATE 2007; fatigue, appetite changes and weight gain in MIND‐IT 2007; fatigue and increased sexual problems in UPBEAT 2012; and nausea and diarrhoea in SADHART 2002. Nortriptyline had a higher rate of adverse events compared to paroxetine in Roose 1998. These side effects must be weighted against the positive effects on depression outcomes when considering initiating pharmacological treatment in depressed coronary artery disease (CAD) patients. The trials conducted by Carney 2009, Abbasi 2015, Shahmansouri 2014, Divsalar 2018, and Ma 2019 reported no meaningful adverse events for omega‐3 add‐on therapy, simvastatin versus atorvastatin (with a few side effects reported for simvastatin but not for atorvastatin), saffron versus fluoxetine, sertraline augmented with red yeast rice, and Xinkeshu tablets. Conversely, more side effects were observed with escitalopram versus Bu Xin Qi (Wang 2020). There was insufficient evidence to make recommendations on the relative safety of serotonergic drugs with regard to electrocardiogram (ECG) wave parameters. Prolongation of the QTc interval is a possible side effect of selective serotonin reuptake inhibitor (SSRI) drugs (Rochester 2018), and have received a warning from the US Food and Drug Administration (Gerlach 2017). Data from three serotonergic drug versus placebo trials indicated that further investigation is warranted, as two trials did not report sufficient data (MIND‐IT 2007; UPBEAT 2012). The evidence for more specific recommendations is scarce.
There is no evidence to recommend a particular type of psychological intervention (e.g. cognitive‐behavioural therapy (CBT)) on the basis of this review. Specifically, comparable effect sizes were found for psychological interventions and those for CBT‐only interventions on end‐of‐treatment depression symptoms. There was modest attenuation in effect size but still considerable heterogeneity, thereby precluding differential conclusions between CBT and non‐CBT approaches. Similarly, with regard to pharmacological interventions, there is comparable evidence from sensitivity analyses for pharmacological interventions and specifically for serotonergic antidepressants. However, an insufficient number of studies investigating TCAs and the small evidence base regarding cardiac endpoints in the included studies precluded recommendations on the benefits and risks of SSRIs versus other antidepressant drug classes, such as tricyclic antidepressants (TCAs), for the treatment of depression in CAD patients. The TCAs are viewed as highly cardiotoxic in overdose and may therefore worsen outcomes in CAD patients (Lichtman 2008; Taylor 2008).
With regard to initiating treatment for CAD patients with depression, this review focused on psychological and pharmacological interventions as stand‐alone approaches, which neither permit any conclusions on collaborative care (Tully 2015), nor on other treatment options such as exercise (Anderson 2016). For example, UPBEAT 2012 conducted a three‐arm trial comparing sertraline and aerobic exercise with placebo, finding no differences between the active trial arms except for heart rate variability in favour of exercise. It may thus be worth considering evidence‐based alternatives beyond the frequently suggested two psychological and pharmacological approaches, taking patient preferences into account.
Implications for research.
The presence of depression in CAD patients is associated with a high additional burden and a negative medical prognosis (Baumeister 2005; Baumeister 2011a; Baumeister 2015; Dempe 2013; Frasure‐Smith 2003; Haschke 2012; Herrmann‐Lingen 2006; Lichtman 2014). Furthermore, the rather sparse evidence regarding the durability of depression interventions on depression and other outcomes in CAD populations leads to uncertainty in the evidence base. Accordingly, there is a need for further trials focusing on outcomes not yet sufficiently examined. Alternatively, past trials could improve the standard of evidence by reporting long‐term outcomes based on intention‐to‐treat (ITT). Several post hoc analyses of trials included in this review were no longer in ITT or per‐protocol groups, and were ineligible for inclusion in long‐term analyses of depression, mortality, and cardiac events. Rather, post hoc analyses were based on responders to depression treatment and participants with major depression versus those without major depression. This applies at least to medium‐ and long‐term depression, quality of life, mortality, specific cardiac events, and healthcare costs. Moreover, to examine differential effects of depression treatments, more comparative trials of psychological and pharmacological interventions are needed. Finally, there is a need for trials of psychological interventions examining the minimum dose required for a clinical meaningful treatment response.
With regard to the divergent effects of both psychological and pharmacological interventions for depression in CAD patients contingent on depression disorder and mixed samples, a change in the current research agenda away from generic depression patient samples regardless of their specific depression subtype and severity may also be needed (Baumeister 2009a; Baumeister 2009b; Baumeister 2012a; Baune 2012; Bech 2010; Pigott 2010). As summarised earlier (Baumeister 2010b; Lichtenberg 2010), the effectiveness of depression treatments may vary depending on depression subtypes. The evidence of depression treatment in general emphasises that treatment effectiveness should at least be examined for different levels of depression severity (Baumeister 2011b; Fournier 2010; NICE 2009), taking clinical significance of depression into account (Baumeister 2008; Baumeister 2010a; Wakefield 2010). In CAD patients the need for subtyping depression might particularly apply to the differentiation of new‐onset depression, recurrent depression, atypical depression, and treatment‐resistant depression (Dickens 2008; Scherrer 2012).
The current evidence also argues for research efforts beyond the standard treatments that better align with patient needs (Collopy 2021), with a focus on alternatives that improve accessibility, availability, efficacy, and attrition of depression interventions for individuals with CAD and depression. Uncertainty remains regarding the optimal delivery of psychotherapy for depression in CAD via individual or group therapy, or new ways of providing psychological care such as by means of internet‐ and mobile‐based interventions (Bendig 2018; U‐CARE 2018; WIDeCAD 2017). Alternatives to improve accessibility and availability might apply to new biological interventions (Kaster 2016), such as examining the drug‐repurposing potential of standard cardiovascular medications like lipid‐ or blood pressure‐lowering agents (Abbasi 2015; Cipriani 2016), or add‐on therapies using cardiovascular health‐promoting agents such as omega‐3 (Carney 2009).
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2020 | New citation required and conclusions have changed | Updated literature search, 27 new trials included, including new analyses (cognitive‐behavioural therapy (CBT) versus non‐CBT interventions), new outcomes (cardiovascular vital signs and platelet biomarkers as per protocol), new adverse outcomes included (arrhythmia, stroke, electrocardiogram parameters, and drug side effects), conclusions changed. |
| 26 July 2019 | New search has been performed | First updated search for studies and content updated |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 9, 2011
Acknowledgements
We would like to thank the German Federal Ministry of Education and Research for funding the original Cochrane Review (Baumeister 2011c, grant number: 01KG0809), and the Cochrane Heart Group for the comprehensive support. We gratefully acknowledge Erla Magnusdottir, Tatjana Alexander, and Dr Anastasia Ejova for translating Chinese and Russian papers.
Appendices
Appendix 1. Search strategies 2009
CENTRAL, DARE, HTA and EED on The Cochrane Library
#1 MeSH descriptor myocardial ischemia explode all trees #2 MeSH descriptor Myocardial Revascularization explode all trees #3 (ischemi* in All Text near/3 heart in All Text) #4 (ischaemi* in All Text near/3 heart in All Text) #5 (coronary in All Text near/3 disease* in All Text) #6 angina in All Text #7 myocardial next infarct* in All Text #8 heart next infarct* in All Text #9 (coronary in All Text near/3 bypass in All Text) #10 (heart in All Text near/3 disease in All Text) #11 (cardiac in All Text near/3 disease in All Text) #12 chd in All Text #13 cad in All Text #14 (coronary in All Text near/3 angioplasty in All Text) #15 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10) #16 (#11 or #12 or #13 or #14) #17 (#15 or #16) #18 MeSH descriptor depression explode all trees #19 MeSH descriptor Depressive Disorder explode all trees #20 MeSH descriptor Mood Disorders this term only #21 "depression" in Keywords #22 "depressive" in Keywords #23 "Dysthymia" in Keywords #24 dysthymi* in All Text #25 (depressi* in All Text near/3 disorder* in All Text) #26 (depressi* in All Text near/3 symptom* in All Text) #27 mood next disorder* in All Text #28 depression in Record Title #29 antidepress* in All Text #30 anti‐depress* in All Text #31 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27) #32 (#28 or #29 or #30) #33 (#31 or #32) #34 (#17 and #33)
MEDLINE (OVID)
1 exp Myocardial Ischemia/ 2 exp Myocardial Revascularization/ 3 (isch?emi$ adj3 heart).tw. 4 (coronary adj3 disease).tw. 5 angina.tw. 6 myocardial infarct$.tw. 7 heart infarct$.tw. 8 (coronary adj3 bypass$).tw. 9 (heart adj3 disease).tw. 10 (cardiac adj3 disease).tw. 11 chd.tw. 12 CAD.tw. 13 (coronary adj3 angioplasty).tw. 14 or/1‐13 15 Depression/ 16 exp Depressive Disorder/ 17 Mood Disorders/ 18 dysthymi$.tw. 19 (depressi$ adj3 disorder$).tw. 20 (depressi$ adj3 symptom$).tw. 21 mood disorder$.tw. 22 affective disorder$.tw. 23 antidepress$.tw. 24 anti‐depress$.tw. 25 or/15‐24 26 14 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomized.ab. 30 placebo.ab. 31 drug therapy.fs. 32 randomly.ab. 33 trial.ab. 34 groups.ab. 35 or/27‐34 36 (animals not humans).sh. 37 35 not 36 38 26 and 37
EMBASE (OVID)
1 exp ischemic heart disease/ 2 exp coronary artery surgery/ 3 exp percutaneous coronary intervention/ 4 (isch?emi$ adj3 heart).tw. 5 (coronary adj3 disease).tw. 6 angina.tw. 7 myocardial infarct$.tw. 8 heart infarct$.tw. 9 (coronary adj3 bypass$).tw. 10 (heart adj3 disease).tw. 11 (cardiac adj3 disease).tw. 12 chd.tw. 13 CAD.tw. 14 (coronary adj3 angioplasty).tw. 15 or/1‐14 16 exp depression/ 17 affective neurosis/ 18 Mood Disorder/ 19 dysthymi$.tw. 20 (depressi$ adj3 disorder$).tw. 21 (depressi$ adj3 symptom$).tw. 22 mood disorder$.tw. 23 affective disorder$.tw. 24 antidepress$.tw. 25 anti‐depress$.tw. 26 or/16‐25 27 15 and 26 28 controlled clinical trial/ 29 random$.tw. 30 randomized controlled trial/ 31 follow‐up.tw. 32 double blind procedure/ 33 placebo$.tw. 34 placebo/ 35 factorial$.ti,ab. 36 (crossover$ or cross‐over$).ti,ab. 37 (double$ adj blind$).ti,ab. 38 (singl$ adj blind$).ti,ab. 39 assign$.ti,ab. 40 allocat$.ti,ab. 41 volunteer$.ti,ab. 42 Crossover Procedure/ 43 Single Blind Procedure/ 44 or/28‐43 45 (exp animals/ or nonhuman/) not human/ 46 44 not 45 47 27 and 46
PsycINFO
1 exp heart disorders/ 2 heart surgery/ 3 (isch?emi$ adj3 heart).tw. 4 (coronary adj3 disease).tw. 5 angina.tw. 6 myocardial infarct$.tw. 7 heart infarct$.tw. 8 (coronary adj3 bypass$).tw. 9 (heart adj3 disease).tw. 10 (cardiac adj3 disease).tw. 11 chd.tw. 12 CAD.tw. 13 (coronary adj3 angioplasty).tw. 14 or/1‐13 15 exp affective disorders/ 16 "depression (emotion)"/ 17 dysthymi$.tw. 18 (depressi$ adj3 disorder$).tw. 19 (depressi$ adj3 symptom$).tw. 20 mood disorder$.tw. 21 affective disorder$.tw. 22 antidepress$.tw. 23 anti‐depress$.tw. 24 or/15‐23 25 14 and 24 26 random$.tw. 27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or dummy or mask$)).tw. 28 placebo$.tw. 29 crossover.tw. 30 assign$.tw. 31 allocat$.tw. 32 ((clin$ or control$ or compar$ or evaluat$ or prospectiv$) adj25 (trial$ or studi$ or study)).tw. 33 placebo/ 34 treatment effectiveness evaluation/ 35 mental health program evaluation/ 36 experimental design/ 37 clinical trials/ 38 or/26‐37 39 25 and 38
CINAHL (EBSCO)
( (MH "Affective Disorders+") or (TI depression) or dysthymi* or (mood disorder*) or (affective disorder*) or antidepress* or anti‐depress* or (depressi* N3 disorder*) or (depressi* N3 symptom*) ) and ( (MH "Myocardial Ischemia+") or (MH "Myocardial Revascularization+") or Angina or (myocardial infarct*) or (heart infarct*) or coronary or cardiac or chd or CAD or (heart disease) ) and ( (MH "Clinical Trials+") or randomi* or randomly or placebo* or trial )
Appendix 2. Search strategies 2021
CENTRAL, DARE, HTA and EED on The Cochrane Library
#1 MeSH descriptor: [Myocardial Ischemia] explode all trees
#2 MeSH descriptor: [Myocardial Revascularization] explode all trees
#3 (ischemi* near/3 heart)
#4 (ischaemi* near/3 heart)
#5 (coronary near/3 disease*)
#6 angina
#7 myocardial next infarct*
#8 heart next infarct*
#9 (coronary near/3 bypass)
#10 (heart near/3 disease)
#11 (cardiac near/3 disease)
#12 chd
#13 cad
#14 (coronary near/3 angioplasty)
#15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 MeSH descriptor: [Depression] explode all trees
#17 MeSH descriptor: [Depressive Disorder] explode all trees
#18 MeSH descriptor: [Mood Disorders] this term only
#19 depression:kw
#20 depressive:kw
#21 Dysthymia:kw
#22 dysthymi*
#23 (depressi* near/3 disorder*)
#24 (depressi* near/3 symptom*)
#25 mood next disorder*
#26 depression:ti
#27 antidepress*
#28 anti‐depress*
#29 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #15 and #29
MEDLINE OVID
1. exp Myocardial Ischemia/
2. exp Myocardial Revascularization/
3. (isch?emi$ adj3 heart).tw.
4. (coronary adj3 disease).tw.
5. angina.tw.
6. myocardial infarct$.tw.
7. heart infarct$.tw.
8. (coronary adj3 bypass$).tw.
9. (heart adj3 disease).tw.
10. (cardiac adj3 disease).tw.
11. chd.tw.
12. CAD.tw.
13. (coronary adj3 angioplasty).tw.
14. or/1‐13
15. Depression/
16. exp Depressive Disorder/
17. Mood Disorders/
18. dysthymi$.tw.
19. (depressi$ adj3 disorder$).tw.
20. (depressi$ adj3 symptom$).tw.
21. mood disorder$.tw.
22. affective disorder$.tw.
23. antidepress$.tw.
24. anti‐depress$.tw.
25. or/15‐24
26. 14 and 25
27. randomized controlled trial.pt.
28. controlled clinical trial.pt.
29. randomized.ab.
30. placebo.ab.
31. drug therapy.fs.
32. randomly.ab.
33. trial.ab.
34. groups.ab.
35. 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36. exp animals/ not humans.sh.
37. 35 not 36
38. 26 and 37
39.(200907* or 200908* or 200909* or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).ed.
40. 38 and 39
Embase OVID
1. exp ischemic heart disease/
2. exp coronary artery surgery/
3. exp percutaneous coronary intervention/
4. (isch?emi$ adj3 heart).tw.
5. (coronary adj3 disease).tw.
6. angina.tw.
7. myocardial infarct$.tw.
8. heart infarct$.tw.
9. (coronary adj3 bypass$).tw.
10. (heart adj3 disease).tw.
11. (cardiac adj3 disease).tw.
12. chd.tw.
13. CAD.tw.
14. (coronary adj3 angioplasty).tw.
15. or/1‐14
16. exp depression/
17. affective neurosis/
18. Mood Disorder/
19. dysthymi$.tw.
20. (depressi$ adj3 disorder$).tw.
21. (depressi$ adj3 symptom$).tw.
22. mood disorder$.tw.
23. affective disorder$.tw.
24. antidepress$.tw.
25. anti‐depress$.tw.
26. or/16‐25
27. 15 and 26
28. random$.tw.
29. factorial$.tw.
30. crossover$.tw.
31. cross over$.tw.
32. cross‐over$.tw.
33. placebo$.tw.
34. (doubl$ adj blind$).tw.
35. (singl$ adj blind$).tw.
36. assign$.tw.
37. allocat$.tw.
38. volunteer$.tw.
39. crossover procedure/
40. double blind procedure/
41. randomized controlled trial/
42. single blind procedure/
43. 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42
44. (animal/ or nonhuman/) not human/
45. 43 not 44
46. 27 and 45
47. (2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).dd.
48. 46 and 47
PsycINFO
1. exp heart disorders/
2. heart surgery/
3. (isch?emi$ adj3 heart).tw.
4. (coronary adj3 disease).tw.
5. angina.tw.
6. myocardial infarct$.tw.
7. heart infarct$.tw.
8. (coronary adj3 bypass$).tw.
9. (heart adj3 disease).tw.
10. (cardiac adj3 disease).tw.
11. chd.tw.
12. CAD.tw.
13. (coronary adj3 angioplasty).tw.
14. or/1‐13
15. exp affective disorders/
16. "depression (emotion)"/
17. dysthymi$.tw.
18. (depressi$ adj3 disorder$).tw.
19. (depressi$ adj3 symptom$).tw.
20. mood disorder$.tw.
21. affective disorder$.tw.
22. antidepress$.tw.
23. anti‐depress$.tw.
24. or/15‐23
25. 14 and 24
26. random$.tw.
27. factorial$.tw.
28. crossover$.tw.
29. cross‐over$.tw.
30. placebo$.tw.
31. (doubl$ adj blind$).tw.
32. (singl$ adj blind$).tw.
33. assign$.tw.
34. allocat$.tw.
35. volunteer$.tw.
36. control*.tw.
37. "2000".md.
38. or/26‐37
39. 25 and 38
40. (200907* or 200908* or 200909* or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).up.
41. 39 and 40
CINAHL
S43 S41 AND S42
S42 EM 20090715‐20200803
S41 S22 AND S40
S40 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39
S39 TX cross‐over*
S38 TX crossover*
S37 TX volunteer*
S36 (MH "Crossover Design")
S35 TX allocat*
S34 TX control*
S33 TX assign*
S32 TX placebo*
S31 (MH "Placebos")
S30 TX random*
S29 TX (doubl* N1 mask*)
S28 TX (singl* N1 mask*)
S27 TX (doubl* N1 blind*)
S26 TX (singl* N1 blind*)
S25 TX (clinic* N1 trial?)
S24 PT clinical trial
S23 (MH "Clinical Trials+")
S22 S10 AND S21
S21 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20
S20 (heart disease)
S19 CAD
S18 chd
S17 cardiac
S16 coronary
S15 (heart infarct*)
S14 (myocardial infarct*)
S13 Angina
S12 MH "Myocardial Revascularization+"
S11 MH "Myocardial Ischemia+"
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 (depressi* N3 symptom*)
S8 (depressi* N3 disorder*)
S7 anti‐depress*
S6 antidepress*
S5 (affective disorder*)
S4 (mood disorder*)
S3 dysthymi*
S2 TI depression
S1 MH "Affective Disorders+"
The WHO International Clinical Trials Registry Platform (ICTRP) (https://trialsearch.who.int/)
Myocardial Ischemia
Myocardial Revascularization
coronary artery disease
angina
myocardial infarction
heart infarction
coronary artery bypass
heart disease
coronary heart disease
coronary angioplasty
or/1‐10
Depression
Depressive disorder
Mood disorder
dysthymia
depressive
mood disorder
affective disorder
antidepressant
anti‐depressant
or/12‐20
11 and 21
International Standard Randomized Controlled Trial Number Register (ISRCTN,http://isrctn.org)
Myocardial Ischemia.tw
Myocardial Revascularization.tw
coronary artery disease.condition
angina.condition
myocardial infarction.condition
heart infarction.tw
coronary artery bypass.condition
heart disease.condition
coronary heart disease.condition
coronary angioplasty.tw
or/1‐10
Depression.condition
Depressive disorder.condition
Mood disorder.condition
dysthymia.tw
depressive.tw
mood disorder.tw
affective disorder.tw
antidepressant.tw
anti‐depressant.tw
or/12‐20
11 and 21
randomized controlled trial.tw
controlled clinical trial.tw
randomized interventions.tw
U.S. National Library of Medicine ClinicalTrials.gov (clinicaltrials.gov)
Myocardial Ischemia.other term
Myocardial Revascularization.other term
coronary artery disease.condition/disease
angina.condition/disease
myocardial infarction.condition/disease
heart infarction.other term
coronary artery bypass.condition/disease
heart disease.condition/disease
coronary heart disease.condition/disease
coronary angioplasty.other term
or/1‐10
Depression.condition/disease
Depressive disorder.condition/disease
Mood disorder.condition/disease
dysthymia.other term
depressive.other term
mood disorder.other term
affective disorder.other term
antidepressant.other term
anti‐depressant.other term
or/12‐20
interventional studies (clinical trials)
11 and 21 and 22
Data and analyses
Comparison 1. Psychological intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Depression symptoms ‐ short term | 10 | 1226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.92, ‐0.19] |
| 1.2 Depression symptoms ‐ medium term | 7 | 2620 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.42, 0.01] |
| 1.3 Depression symptoms ‐ long term | 2 | 282 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.96, 0.04] |
| 1.4 Depression remission ‐ short term | 3 | 862 | Odds Ratio (IV, Random, 95% CI) | 2.02 [0.78, 5.19] |
| 1.5 Depression remission ‐ medium term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Depression remission ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.7 All‐cause mortality ‐ short term | 2 | 324 | Odds Ratio (IV, Random, 95% CI) | 0.31 [0.05, 2.02] |
| 1.8 All‐cause mortality ‐ medium term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.9 All‐cause mortality ‐ long term | 2 | 2670 | Odds Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.42] |
| 1.10 Cardiovascular mortality ‐ medium term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.11 Cardiovascular mortality ‐ long term | 2 | 2720 | Odds Ratio (IV, Random, 95% CI) | 0.83 [0.62, 1.10] |
| 1.12 Myocardial infarction ‐ long term | 2 | 2720 | Odds Ratio (IV, Random, 95% CI) | 1.09 [0.73, 1.65] |
| 1.13 Heart failure ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.14 Stroke ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.15 Coronary revascularisation procedure ‐ long term | 2 | 2780 | Odds Ratio (IV, Random, 95% CI) | 0.91 [0.75, 1.11] |
| 1.16 Hospitalisations ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.17 Length of stay ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18 Quality of life SF‐12/36 physical ‐ short term | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.06, 0.50] |
| 1.19 Quality of life SF‐12/36 mental ‐ short term | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.07, 0.94] |
| 1.20 Quality of life SF‐12/36 physical ‐ medium term | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐1.29, 1.65] |
| 1.21 Quality of life SF‐12/36 mental ‐ medium term | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 1.21 [‐1.09, 3.52] |
| 1.22 Quality of life SF‐12 total ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.23 Quality of life SF‐36 physical ‐ long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.24 Quality of life SF‐36 mental ‐ long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Psychological intervention versus psychological intervention/clinical management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Depression symptoms ‐ short term | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Depression symptoms ‐ medium term | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Depression symptoms ‐ long term | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Depression remission ‐ short term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Depression remission ‐ medium term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.6 Depression remission ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.7 Cardiovascular mortality ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.8 Quality of life SF‐36 physical ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.9 Quality of life SF‐36 mental ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.10 Quality of life SF‐36 physical ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.11 Quality of life SF‐36 mental ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.12 Quality of life SF‐36 physical ‐ long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.13 Quality of life SF‐36 mental ‐ long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Depression symptoms ‐ short term | 8 | 750 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.33, ‐0.32] |
| 3.2 Depression symptoms change score ‐ short term | 3 | 482 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, ‐0.00] |
| 3.3 Depression remission ‐ short term | 4 | 646 | Odds Ratio (IV, Random, 95% CI) | 2.06 [1.47, 2.89] |
| 3.4 Depression response ‐ short term | 5 | 891 | Odds Ratio (IV, Random, 95% CI) | 2.73 [1.65, 4.54] |
| 3.5 All‐cause mortality ‐ short term | 2 | 437 | Odds Ratio (IV, Random, 95% CI) | 0.38 [0.10, 1.47] |
| 3.6 All‐cause mortality ‐ long term | 2 | 661 | Odds Ratio (IV, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 3.7 Cardiovascular mortality ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.8 Myocardial infarction ‐ short term | 3 | 728 | Odds Ratio (IV, Random, 95% CI) | 0.74 [0.26, 2.09] |
| 3.9 Myocardial infarction ‐ long term | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.10 Angina ‐ short term | 4 | 819 | Odds Ratio (IV, Random, 95% CI) | 0.75 [0.44, 1.28] |
| 3.11 Heart failure ‐ short term | 3 | 602 | Odds Ratio (IV, Random, 95% CI) | 0.93 [0.33, 2.62] |
| 3.12 Arrhythmia ‐ short term | 2 | 87 | Odds Ratio (IV, Random, 95% CI) | 0.46 [0.01, 17.06] |
| 3.13 Stroke ‐ short term | 2 | 586 | Odds Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.96] |
| 3.14 Coronary revascularisation procedure ‐ long term | 1 | 300 | Odds Ratio (IV, Random, 95% CI) | 0.59 [0.32, 1.10] |
| 3.15 Healthcare costs ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.16 Hospitalisations ‐ short term | 3 | 514 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.85] |
| 3.17 Emergency department visits ‐ short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.18 Quality of life Q‐LES‐Q ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.19 Quality of life WHOQOL‐BREF Physical ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.20 Quality of life WHOQOL‐BREF Psychological ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.21 Quality of life WHOQOL‐BREF Social relationships ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.22 Quality of life WHOQOL‐BREF Environmental ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.23 Quality of life WHOQOL‐BREF Physical ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.24 Quality of life WHOQOL‐BREF Psychological ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.25 Quality of life WHOQOL‐BREF Social Relationships ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.26 Quality of life WHOQOL‐BREF Environmental ‐ medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.27 Systolic BP ‐ short term | 3 | 675 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐3.52, 3.05] |
| 3.28 Diastolic BP ‐ short term | 3 | 675 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.55, 2.74] |
| 3.29 Heart rate ‐ short term | 4 | 662 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.40, 0.79] |
| 3.30 Platelet biomarker βTG ‐ short term | 3 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.99, ‐0.09] |
| 3.31 Platelet biomarker PF4 ‐ short term | 3 | 144 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.48, 0.19] |
| 3.32 Platelet biomarker P‐selectin ‐ short term | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.12, 0.50] |
| 3.33 Platelet biomarker PECAM‐1 ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.34 Platelet biomarker TxB 2 ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.35 ECG PR interval ‐ short term | 3 | 635 | Mean Difference (IV, Random, 95% CI) | ‐4.35 [‐8.40, ‐0.31] |
| 3.36 ECG QRS interval ‐ short term | 3 | 635 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐0.41, 5.15] |
| 3.37 ECG QT interval ‐ short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.38 ECG QTc interval ‐ short term | 3 | 635 | Mean Difference (IV, Random, 95% CI) | 2.76 [‐1.96, 7.47] |
| 3.39 Non‐cardiac adverse events and side effects ‐ short term | 8 | 1193 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [1.07, 1.92] |
3.37. Analysis.

Comparison 3: Pharmacological intervention versus placebo, Outcome 37: ECG QT interval ‐ short term
Comparison 4. Pharmacological intervention versus pharmacological intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Depression symptoms ‐ short term | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Depression symptoms change score ‐ short term | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Depression remission ‐ short term | 3 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.4 Depression response ‐ short term | 4 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.5 All‐cause mortality ‐ short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.6 Myocardial infarction ‐ short term | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.7 Angina ‐ short term | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.8 Heart failure ‐ short term | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.9 Arrhythmia ‐ short term | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.10 Coronary revascularisation procedure ‐ short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.11 Emergency department visits ‐ short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.12 Systolic BP ‐ short term | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.13 Diastolic BP ‐ short term | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14 Heart rate ‐ short term | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.15 ECG PR interval ‐ short term | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.16 ECG QRS interval ‐ short term | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.17 ECG QTc interval ‐ short term | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.18 Non‐cardiac adverse events and side effects ‐ short term | 7 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbasi 2015.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 58 Length of follow‐up: no follow‐up Analysis: per‐protocol (6 dropouts in the simvastatin group, 6 dropouts in the atorvastatin group) |
|
| Participants | Location: Iran Number of study centres and setting: patients who had undergone CABG from Psychiatric Clinic of Tehran Heart Center CAD criteria: history of post‐CABG in the last 6 months Depression criteria: patients who met the DSM IV‐TR criteria for diagnosis of MDD, confined to patients with mild‐to‐moderate depression and a HAM‐D score of ≤ 19 Other entry criteria: patients aged 18 to 50 years Exclusion criteria: participants with any diagnosis other than MDD on the DSM‐IV‐TR axis I or II, on any psychotropic medications or presence of any psychotic features, receiving any antidepressant medication in the last month, receiving electroconvulsive therapy during the last 2 months, serum low‐density lipoprotein level of < 80, history of hypothyroidism, hepatic diseases, alcohol or substance (with exception of nicotine) dependence, receiving any statins or any other lipid‐lowering agent during the last 2 months, hypersensitivity to statins, presence of any serious medical condition or neurological problem, high levels of liver aminotransferases, pregnancy and lactations, behavioural intervention therapy Treatment N: 23 (30.4% female, mean age: 56.87 (SD: 6.90)) Control N: 23 (34.8% female, mean age: 57.70 (SD: 7.26)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: 1 tablet of simvastatin once daily (20 mg tablets) Control: 1 tablet of atorvastatin once daily (20 mg tablets) Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression response (50% reduction on HAM‐D), cardiac events, pharmacological side effects (checklist) | |
| Funding | Tehran University of Medical Sciences | |
| Notes | The title says "...placebo‐controlled, randomized trial...", but both groups received a drug treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer generated block randomisation carried out by an independent party |
| Allocation concealment (selection bias) | Low risk | Comment: Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Participants, research investigators, rater and statistician blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: primary outcome (HAMD) rater blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: per‐protocol analysis and authors reported reasons for early drop‐out per group |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial registry measurement points (week 2, 4, 6) differ from reported measurement points (week 3, 6) |
| Other bias | Low risk | Comment: no indication of other bias |
ANDROS 2015.
| Study characteristics | ||
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 2 Length of follow‐up: 6 months Analysis: unclear from trial registry (no analysis was reported, information on dropouts unclear) |
|
| Participants | Location: France Number of study centres and setting: unclear, affiliated with Hôpitaux de Paris CAD criteria: ACS with elevated cardiac enzymes (above the 99th percentile of the upper limit of normal of the laboratory) Depression criteria: depressive symptoms on the BDI short form Other entry criteria: age 18 years and older, without antidepressant therapy for 3 months (valid only for the sertraline and placebo groups), affiliated to a social security scheme (beneficiary or assignee), signed a free and informed consent Exclusion criteria: psychosis, bipolar illness, dementia (Mini‐Mental State Examination score < 23), uncontrolled epilepsy, severe depression (score > 15) with suicidal risk identified by a psychiatrist (urgent treatment for depression needed), patient experienced depression and treated in the last 3 months or currently receiving treatment, treatment with selective and non‐selective monoamine oxidase inhibitors of the group A within 14 days prior to the introduction of sertraline, prothrombin time > 1.5 seconds, platelet rate < 100,000/mm3, hypersensitivity to the active substance or to any of the excipients (anhydrous lactose, pregelatinised corn starch, sodium laurilsulfate, magnesium stearate), treatment with pimozide, genetic galactose intolerance, malabsorption of glucose and galactose, lactase deficiency, women without effective contraception or pregnant or lactating or desiring pregnancy or within 6 months after randomisation, participation in biomedical research on other drugs during the period of participation, unable to follow the treatment Treatment: unclear Control: unclear Comparability of groups: unclear |
|
| Interventions | Intervention 1: sertraline 50 mg/d, which can be increased up to 200 mg/d (maximum dose) Intervention 2: placebo 1 capsule per day, which can be increased up to 4 capsules per day (maximum dose) Control: no depression, no treatment Duration of treatment: 6 months |
|
| Outcomes | Review outcomes: depression symptoms (BDI short form), platelet biomarkers (β‐thromboglobulin) Other outcomes: maximal platelet aggregation (ADP, arachidonic acid, collagen), markers of platelet activation (CD40s), inflammatory markers (interleukin‐6, C‐reactive protein, Fg, myeloperoxydase), tobacco addiction (Fargenström test), bleeding risk (haemoglobin, haematocrit, and follow‐up of haemorrhage) |
|
| Funding | Assistance Publique ‐ Hôpitaux de Paris | |
| Notes | Comment: this study was terminated early after recruitment of 2 participants. No data were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Double blind (Participant, Investigator), not further specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No details reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: No details reported; trial terminated early after recruitment of two participants |
| Other bias | High risk | Comment: The trial was terminated early by the Investigator after recruitment of two participants. The sample size target was 225 |
Barth 2005.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 59 Length of follow‐up: no follow‐up Analysis: per‐protocol (4 participants in the control group dropped out) |
|
| Participants | Location: Germany Number of study centres and setting: 3 cardiac inpatient rehabilitation clinics CAD criteria: patients with MI, CABG, PTCA, unstable angina pectoris; diagnosis based on physician's report; time to randomisation unclear Depression criteria: MDD, dysthymia and depressive adjustment disorder assessed in a 2‐stage procedure: 1) HADS and 2) Structured Clinical Interview for DSM‐IV in all patients with a HADS score of 17 or higher Other entry criteria: none stated Exclusion criteria: poor general health, language and cognitive deficits, bipolar disorder, psychotherapy at residence, psychotic symptoms Treatment: 27 (18.5% female, mean age: 60.8 (SD: 11.1)) Control: 32 (28.1% female, mean age: 55.6 (SD: 10.1)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: brief, individualised, resource‐orientated psychotherapy (4 to 6 sessions of 50 minutes each) comprising patient education, motivation, goal setting, crisis management, modification of dysfunctional cognitions and behaviour, and written recommendations for further outpatient treatment; participants with severe depression were also treated with sertraline Control: usual care Duration of treatment: 3 to 4 weeks during inpatient rehabilitation |
|
| Outcomes | Review outcomes: depression symptoms (Bech Rafaelsen Melancholia Scale, also BDI and HADS) Other outcomes: HADS anxiety score |
|
| Funding | Ministry for Education and Research, Germany, Federal Insurance Authority, Baden‐Württemberg, Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation carried out by methodology center (independent from study staff) |
| Allocation concealment (selection bias) | Low risk | Comment: By sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants and interventionists unmasked. Blinding to psychopharmacological interventions unclear. Possible performance bias with regard to manual adherence of therapists in treatment group, which remains unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: primary outcome (BRMS) interviewers blinded to allocation. All other outcomes patient self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: Table 2 (pg. 6/7): "Only patients with data at both assessments were included in the analysis." |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes as stated in methods section. No protocol or design paper available |
| Other bias | Unclear risk | Comment: In inpatient studies, therapists and clinic staff are not blind to the patients' allocation, which might impact the inpatient treatment of the intervention and the control group |
Brown 1993.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 54 Length of follow‐up: 15 months Analysis: per‐protocol (6 dropouts in intervention arm, 8 dropouts in control arm at 15 months) |
|
| Participants | Location: USA Number of study centres and setting: patients recruited from 5 cardiac rehabilitation departments of medical centres and by newspaper advertisements CAD critieria: MI and/or coronary bypass surgery 4 to 24 months before study; diagnosis based on physician's report; prognosis of no worse than 3.3 based on the New York Heart Association criteria for moderately compromised cardiac status; stable cardiac status with no medical contraindications to increased physical activity according to their physician's report Depression criteria: new‐onset depression and/or anxiety on the SADS; scores of > 13 on the BDI or > 70 on the SCL 90‐R Other entry criteria: spouses, friends, or relative who was willing to participate; age between 43 and 75 years Exclusion criteria: unstable medical condition; chronic, severe depression and/or anxiety preceding the cardiac event; suicidal ideation; changes in county residence; unwillingness or inability to include a partner; pre‐existing psychiatric disorder Treatment N: 20 (45% female, mean age: 63.55 (SD: 7.43)) Control N: 20 (10% female, mean age: 57.65 (SD: 7.82)) Comparability of groups: significant baseline differences regarding age, religion, SCL 90‐R, BDI (with control being more distressed on SCL 90‐R and BDI) |
|
| Interventions | Treatment: behaviour therapy for participants and their partners by Lewinsohn (weekly 1‐hour sessions) in which participants should increase and intensify adaptive behaviours (pleasant activities, relaxation, cognitive restructuring, assertion/anger management, time management) and partners practise positive reinforcement of adaptive behaviours and ignore maladaptive behaviours Control: person‐centred therapy by Rogers (weekly 1‐hour sessions) Duration of treatment: 12 sessions (treatment and control) |
|
| Outcomes | Outcomes: depression symptoms (BDI) Other outcomes: SADS‐C, SCL 90‐R, Minnesota Multiphasic Personality Inventory‐168 (MMPI‐168), Pleasant Events Schedule (PES), Unpleasant Events Schedule (UES), Locke Wallace Marital Adjustment Test, Katz Adjustment Scale |
|
| Funding | Study supported in part by a grant from the American Heart Association. | |
| Notes | Study investigated effects of behaviour therapy of participants and their partners on depression and/or anxiety in comparison to person‐centred therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of patients not stated Comment: therapists supervision provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: Regarding Schedule of Affective Disorders and Schizophrenia (SADS) "None of the therapists conducted the post‐treatment interviews." (p.203) Comment: All other outcomes patient self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Per‐protocol analysis and no drop‐out analysis (14 of 54 patients dropped‐out from baseline to 15‐month follow‐up). Missing data present |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes reported as stated in the methods section; no protocol or design paper available |
| Other bias | Unclear risk | Comment: significant baseline imbalance between groups in baseline depression (BDI) and age Comment: therapists rated and monitored for quality; no efforts regarding therapy quality mentioned; therapist quality analaysis provided |
Carney 2009.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 122 Length of follow‐up: no follow‐up Analysis: ITT with multiple imputation for missing data |
|
| Participants | Location: USA Number of study centres and setting: cardiology practices in St Louis, Missouri and from cardiac diagnostic laboratories affliliated with Washington University School of Medicine CAD criteria: CHD documented by at least 50% stenosis in at least 1 major coronary artery, a history of revascularisation or hospitalisation for an acute coronary syndrome Depression criteria: patients who fullfilled DSM‐IV criteria for current major depressive episode (DISH) and scored 16 or higher on the BDI‐II Other entry criteria: none Exclusion criteria: cognitive impairment, comorbid psychiatric disorders, psychosis, high risk of suicide, current substance abuse, acute coronary syndrome within the previous 2 months, a left ventricular ejection fraction of less than 30%, advanced malignancy or physical inability to participate, use of any antidepressants, anticonvulsants, lithium, or omega‐3 supplements, sensitivity to sertraline or omega‐3, physician or patient refusal Treatment N: 62 (35.5% female, mean age: 58.1 (SD: 9.4)) Control N: 60 (31.7% female, mean age: 58.6 (SD: 8.5)) Comparability of groups: no significant baseline differences between groups except for a higher proportion of aspirin use in the placebo group |
|
| Interventions | Treatment 1: sertraline (50 mg/d) + omega‐3 (2 g/d) Treatment 2: sertraline (50 mg/d) + corn oil capsules (placebo) Duration of treatment: 10 weeks |
|
| Outcomes | Review outcomes: depression score (HAM‐D, also BDI‐II), depression response (50% symptom reduction BDI‐II) and depression remission (BDI‐II ≤ 8), cardiac events, resource utilisation (emergency room visits), pharmacological side effects Other outcomes: anxiety symptoms (Beck Anxiety Inventory), treatment adherence, omega‐3 index |
|
| Funding | Study funded by the National Heart, Lung, and Blood Institute. Other support: GlaxoSmithKline Inc supplied omega‐3 and placebo capsules; Pfizer Inc supplied sertraline |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: permuted‐block‐randomisation (SAS institute, Cary, North Carolina) |
| Allocation concealment (selection bias) | Low risk | Comment: assignments sealed envelopes opened at enrolment by a pharmacist blinded to all assessments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the study pharmacist and the chair of the data and safety monitoring committee were unblinded to group assignment during the trial." (p.1652) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Only the study pharmacist and the chair of the data and safety monitoring committee were unblinded to group assignment during the trial." (p.1652) Quote: "An independent cardiologist, the study investigators, and the study nurses met quarterly to review adverse events. The study pharmacist and the independent cardiologist were informed immediately about serious adverse events and quarterly about routine adverse events" (pg. 1653) Comment: Unclear how HAMD was rated and by whom |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT, reasons for drop‐out given (drop‐out: 4 patients in the sertraline+placebo, 3 patients in the sertraline+Omega‐3) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Secondary outcomes (biomarkers) were not reported ITT |
| Other bias | Unclear risk | Comment: in‐ and exclusion criteria differ from the trial registry |
CREATE 2007.
| Study characteristics | ||
| Methods | RCT design: 2 x 2 factorial trial Total N randomised: 284 Length of follow‐up: no follow‐up Analysis: ITT with last‐observation‐carried‐forward applied for missing data |
|
| Participants | Location: Canada Number of study centres and setting: 9 hospitals with patients being referred from physicians, responded to media advertisements or targeted posters CAD criteria: evidence of CAD based on hospital chart, evidence of a previous hospitalisation for acute MI, or coronary angiographic evidence of 50% or more blockage in at least 1 major coronary artery, or previous revascularisation; patients were not randomised less than 1 week following discharge Depression criteria: current major depressive episode based on the SCID with at least 4 weeks' duration; baseline score of > 19 on the HAM‐D Other entry criteria: adult patients (18 years or older), stable CAD according to physician's clinical judgement Exclusion criteria: coronary bypass surgery planned during the next 4 months, CCS angina class = 4, bipolar disorder, major depression with psychotic features, or evidence of substance abuse or dependency during the previous 12 months, serious suicide risk based on clinical judgement, use of antidepressants, lithium, or anticonvulsants for mood disorder, currently undergoing any form of psychotherapy, absence of response to a previous adequate trial of citalopram or IPT, 2 previous unsuccessful trials of treatment for depression for the index episode, lifetime history of early termination (< 8 weeks) of citalopram because of adverse events or side effects, lifetime history of early termination (< 8 weeks) of 2 other SSRI antidepressants because of adverse events or side effects, significant cognitive problems, depression due to a general medical condition based on clinical judgement, participation in other trials, inability to speak English or French, unable or unwilling to comply with the study regimen Treatment 1 N: 142 (31.0% female, mean age: 59.0 (SD: 9.81)) Treatment 2 N: 142 (23.2% female, mean age: 57.9 (SD: 9.15)) Control 1 N: 142 (18.3% female, mean age: 57.3 (SD: 8.35)) Control 2 N: 142 (26.1% female, mean age: 58.4 (SD: 9.16)) Comparability of groups: significantly more women in IPT compared to CM groups |
|
| Interventions | Treatment 1: IPT + citalopram (20 mg/d to 40 mg/d, tablets) + CM. IPT provided weekly dealing with common problems in CAD patients, including interpersonal conflicts, life transitions, grief, loss, and social isolation (ineligible for this review) Treatment 2: citalopram (20 mg/d to 40 mg/d, tablets) + CM with 20‐ to 25‐minute visits including information about depression and medication use, reassurance, and encouragement of adherence to medication and the study protocol, review of side effects and progress Control 1: IPT + CM + placebo (ineligible for this review) Control 2: placebo administration matched to citalopram condition + CM Duration of treatment: 12 weeks |
|
| Outcomes | Outcomes: depression symptoms (HAM‐D, also BDI‐II), depression remission (HAM‐D ≤ 8), depression response (50% reduction on HAM‐D), cardiovascular vital signs (BP, HR), platelet biomarkers (P‐selectin), ECG waves, pharmacological side effects Other outcomes: Interpersonal Relationships Inventory (IPRI), Functional Performance Inventory (FPI), biomarkers (nitric oxide) |
|
| Funding | Canadian Institute of Health Research; Fondation du Centre Hospitalier de l'Université de Montréal; Fondation de l'Institut de Cardiologie de Montréal Other support: citalopram and matching placebo donated by Lundbeck Canada Inc |
|
| Notes | Factorial design did not allow for 2 randomised comparisons of main effects: 1) IPT vs CM, 2) citalopram vs placebo. Only citalopram vs placebo was included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed in sequentially numbered, site‐specific, sealed opaque envelopes stored at the coordinating center until randomization" (p. 369) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: therapists, patients, site psychiatrists, telephone raters for primary outcome, and other personnel blinded to assignment regarding citalopram treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: therapists, patients, site psychiatrists, telephone raters for primary outcome, and other personnel blinded to assignment regarding citalopram treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat (ITT) analysis with last‐observation‐carried‐forward |
| Selective reporting (reporting bias) | Low risk | Comment: primary and secondary outcomes reported in accordance with the study protocol (ISRCTN15858091) |
| Other bias | Low risk | Comment: no indication of other bias |
Dao 2011.
| Study characteristics | ||
| Methods | RCT design: nested, 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: no follow‐up Analysis: unclear (method of analysis not explicitly stated; analysis not based on total N randomised; 2 dropouts in intervention arm, 2 dropouts in control arm) |
|
| Participants | Location: USA Number of study centres and setting: single centre, Veterans Affairs hospital CAD criteria: patients undergoing first‐time CABG wtihout concomitant valve procedures Depression criteria: BDI‐II of 14 or higher (or anxiety, State–Trait Anxiety Inventory‐Trait score ≥ 40) Other entry criteria: none Exclusion criteria: serious medical illness other than CAD, psychiatric instability (suicidality), schizophrenia, bipolar disorder, active alcoholism or substance abuse, severe cognitive impairment, non‐cardiac illnesses with a poor 1‐year prognosis, previous exposure to CBT within the past year, receiving psychotherapeutic services, unstable antidepressant medication (less than 4 weeks) Treatment N: 48 (22.9% female, mean age: 62.8 (SD: 11.8)) Control N: 49 (20.4% female, mean age: 64.2 (SD: 11.9)) Comparability of groups: no significant baseline differences between groups on demographic variables and measures of depression and anxiety, significantly more diabetes patients in the control group than in the treatment group (Cohen's d = 0.443) |
|
| Interventions | Treatment: brief CBT intervention (Managing Anxiety and Depression using Education and Skills (MADES)). MADES uses a manualised approach to address the needs of patients who have CAD with preoperative depression and/or anxiety. Duration of treatment: 2 sessions before surgery, 1 session after surgery, 1 session 5 days after surgery Control: usual care |
|
| Outcomes | Review outcomes: depression symptoms (BDI‐II), healthcare and resource utilisation (length of stay), quality of life (12‐item Short Form Health survey) Other outcomes: anxiety symptoms (STAI‐Trait) |
|
| Funding | Mental illness research, education and clinical centre | |
| Notes | Mixed study sample (patients with high depression and/or anxiety scores eligible) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Low drop‐out, but reasons for missing data not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol available. Uncertainties regarding data analysis and degrees of freedom (pg.112) |
| Other bias | Unclear risk | Comment: In inpatient studies, therapists and clinic staff are not blind to the patients' allocation, which might impact the inpatient treatment of the intervention and the control group Comment: manualised treatment; all treatment sessions video‐taped and reviewed by lead investigator for adherence and compliance |
Divsalar 2018.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel trial Total N randomised: 101 Length of follow‐up: no follow‐up Analysis: ITT with last‐observation‐carried‐forward (3 dropouts from sertraline/red yeast arm, 3 dropouts from sertraline/placebo arm) |
|
| Participants | Location: Iran Number of study centres and settings: Imam Hospital and Tehran Heart Center CAD criteria: history of coronary angioplasty Depression criteria: diagnosis of major depressive disorder (MDD) based on DSM‐V and HAM‐D score of ≥ 20 Other entry criteria: aged 18 to 60 years old Exclusion criteria: other DSM‐V disorders other than MDD; presence of psychotic features or suicidal ideation; inability to communicate; consumption of psychotropic or antidepressant medications in the last month prior to the study; electroconvulsive therapy in the last 2 months prior to study; lipid‐lowering agents (e.g. statins) in the last 2 weeks prior to the study; hypersensitivity to statins; presence of neurological diseases or serious medical condition or history of hepatic disease or hypothyroidism; elevated serum aminotransferases to serum LDL (80 mg/dL); pregnancy or lactation Treatment: 25 (28% women, mean age: 43.52 (SD: 6.36)) Control: 25 (40% women, mean age: 44.32 (SD: 5.47)) Comparability of groups: no significant baseline differences |
|
| Interventions | Intervention 1: sertraline (200 mg/day) and red yeast rice (2500 mg/day) Intervention 2: sertraline (200 mg/day) and placebo Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), pharmacological side effects (25‐item checklist) | |
| Funding | Tehran University of Medical Sciences (TUMS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computerized random number generation was used |
| Allocation concealment (selection bias) | Low risk | Quote pg. 71 "Computerized random number generation was used by one of the personnel different from raters" Comment: Allocation was achieved using sealed opaque envelopes with sequential numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: All healthcare providers, participants, and caregivers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote pg. 71 "An independent rater was responsible for administration of the HDRS at weeks 0, 3, and 6." Comment: unclear blinding for other outcomes including biomarkers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The reasons for patients who did not complete the intervention were reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available |
| Other bias | High risk | Comment: selection bias evident with 14.9% of participants who were eligible for the study but were excluded prior to randomisation |
Doering 2007.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: not stated Length of follow‐up: 4 months Analysis: per‐protocol (number of participants who dropped out of nested trial unclear; 23 participants did not finish study) |
|
| Participants | Location: USA Number of study centres and setting: 2 urban medical centres CAD criteria: patients undergoing first‐time CABG; time to randomisation not specified Depression criteria: diagnosis of major depression during inpatient treatment or 2 to 4 weeks after hospital admission or minor depression at both interviews diagnosed by the Diagnostic Interview and Structured Hamilton (DISH) Other entry criteria: <= 75 years old, English‐speaking, Mini‐Mental State Examination score of >= 24, available for 6 months follow‐up Exclusion criteria: malignancies or autoimmune disorders Treatment N: 7 (100% female, mean age: 58.6 (SD: 7.6)) Control N: 8 (100% female, mean age: 60.9 (SD: 9.4)) Comparability of groups: treatment group participants had a significantly higher rate of depression history |
|
| Interventions | Treatment: CBT (weekly 1‐hour sessions) by a trained nurse therapist including establishing therapeutic relationship, behavioural activation, active problem‐solving, identification of automatic thoughts, reframing automatic thoughts, learning self‐therapy and relapse prevention Control: usual care comprising usual medical and nursing follow‐up after CABG and an assessment by a psychiatrist who recommended individualised treatment options Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: depression symptoms (BDI), depression disorders (DISH), postoperative illnesses (Modified Health Review) Other outcomes: biomarkers (natural killer cell cytotoxicity, interleukin‐6, C‐reactive protein) |
|
| Funding | Not stated | |
| Notes | Study investigated depressed post‐CABG women; intervention trial was nested in a study inclusive of a non‐depressed comparator group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Outcome assessed by a research assistant blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only those patients who completed all study measures were included in this report." (p. 19) |
| Selective reporting (reporting bias) | Unclear risk | Comment: depression disorders were assessed at follow‐up (DISH) and this data was not reported for intervention and control group who all met criteria for baseline depression disorder. No protocol or design paper available |
| Other bias | Unclear risk | Comment: No efforts regarding nurse therapists protocol adherence reported. Usual care comprised psychiatrists' recommendations for individualised treatment options, but utilised treatments in the control group were not assessed |
ENRICHD 2003.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 2481 Length of follow‐up: evaluations after 6 months and annually thereafter (follow‐up duration 18 to 54 months) Analysis: ITT for mortality and cardiac events (93 participants in the intervention arm did not receive the intervention) |
|
| Participants | Location: USA Number of study centres and setting: Outpatients from 73 hospitals affiliated with 8 clinical centres CAD criteria: acute MI with elevation in 1 or more biomarkers as well as MI‐compatible symptoms or characteristic ECG ST‐T changes or new Q waves; randomisation within 28 days after MI Depression criteria: major depression or dysthymia diagnosis based on the DISH according to modified DSM‐IV criteria Other entry criteria: low perceived social support assessed through the ENRICHD Social Support Instrument (ESSI) Exclusion criteria: patients with acute MI following PCI or CABG, receiving psychotherapy or taking an antidepressant for longer than 14 days but remained depressed, non‐cardiac conditions likely to be fatal within 1 year, too ill to participate, participating in another trial, major psychiatric disorder (including schizophrenia, bipolar disorder, severe dementia, or active substance abuse), at risk for suicide, refusal of participation or physician disallowed participation, could not be enrolled within 28 days, inaccessible for intervention or follow‐up Treatment N: 1238 (43% female, mean age: 61 (SD: 12.6)) Control N: 1243 (44% female, mean age: 61 (SD: 12.5)) Comparability of groups: no significant baseline differences except for the use of ACE inhibitors |
|
| Interventions | Treatment: individual (at least six 1‐hour sessions weekly) and group (weekly 2‐hour sessions) CBT by Beck supplemented by techniques based on social learning theory for participants with low perceived social support; participants with scores > 24 on the HAM‐D or those with less than 50% reduction in BDI score after 5 weeks were referred to study psychiatrist for consideration of pharmacotherapy with sertraline (50 to 200 mg/d) Control: usual care Duration of treatment: individual behavioural intervention up to 6 months with additional 12 weeks for group therapy, adjunctive pharmacotherapy up to 12 months |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), all‐cause mortality, cardiovascular mortality, cardiac events, healthcare and resource utilisation (cardiovascular hospitalisation), quality of life (12‐item Short Form Health survey) Other outcomes: social support and social networks, life satisfaction, change in cardiac risk factor profile, perceived stress, self‐efficacy |
|
| Funding | National Heart, Lung, and Blood Institute Other support: Pfizer Inc provided sertraline for the study |
|
| Notes | Mixed study sample (patients with depression and/or low perceived social support were enrolled) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Automated telephone randomization system using permuted blocks with varying sizes, stratified by clinical center; test for selection bias potentially resulting from unmasking of previous assignments (participants and interventionists were unblinded) with nonsignificant results |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation obtained by an automated telephone randomization system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants and interventionists unmasked Quote: "Psychosocial interventions including those used in ENRICHD cannot be fully blinded" (protocol paper, pg. 4) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Conflicting information reported for blinded outcome assessment. Quote: "Staff who collected, verified, or classified end point data or follow‐up assessments were masked as much as possible" (Berkman, 2003). Quote: "End point data collection, verification and classification, and follow‐up psychosocial assessments are conducted by staff who are blinded to treatment assignment." (protocol paper, 2000) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Depression outcomes analysed per protocol, all other reported outcomes ITT |
| Selective reporting (reporting bias) | High risk | Comment: results of main outcomes reported as described in the design papers of the trial. Secondary outcomes (change in cardiac risk factor profile, perceived stress and self‐efficacy) were not reported per protocol or ITT |
| Other bias | Unclear risk | Comment: Therapy quality and adherence to treatment protocol were monitored by an external organisation (the Beck Institute) Comment: QoL was not assessed at baseline and it thus remains unclear whether or not QoL was balanced in the two groups at baseline |
EsDEPACS 2014.
| Study characteristics | ||
| Methods | RCT design: nested, 3‐arm parallel‐group trial Total N randomised: 300 Length of follow‐up: 6 months Analysis: per‐protocol. ITT stated in protocol paper, and no imputation was performed for end‐of‐treatment results (42 dropouts in intervention group, 45 dropouts in control group including 3 for protocol violation) |
|
| Participants | Location: Korea Number of study centres and setting: Department of Cardiology of Chonnam National University Hospital CAD criteria: patients 2 to 14 weeks after a confirmed ACS episode Depression criteria: diagnosis of major or minor depressive disorder (participants with BDI > 10 and clinically evaluated depression via Mini‐International Neuropsychiatric Interview (MINI) Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 149 (40.9% female, mean age: 60.0 (SD: 11.2)) Control N: 151 (38.4% female, mean age: 60.1 (SD: 10.5)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: flexible doses of daily escitalopram (5, 10, 15, or 20 mg); dose was 10 mg/day (5 mg/day if age ≥ 65) at baseline and could be changed after 4 weeks Control 1: placebo Control 2: usual care (ineligible for this review) Duration of treatment: 24 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D, also Montgomery‐Asperg Depression Rating Scale, BDI), depression remission (HAM‐D ≤ 7), depression response (50% reduction on HAM‐D), all‐cause mortality, cardiac mortality, cardiac events, quality of life (WHOQOL‐BREF), cardiovascular vital signs (BP, HR), platelet biomarkers, ECG waves, pharmacological side effects (unclear how assessed) Other outcomes: clinical global impressions, echocardiography (left ventricular ejection fraction, wall motion), weight, blood biomarkers (troponin I, creatine kinase‐MB, cholesterol, brain derived neurotrophic factor methylation), Big Five Inventory (3 months) |
|
| Funding | Korean Ministry of Health & Welfare; National Research Foundation of Korea; Korean Ministry of Science, ICT and future Planning; National Institute for Health Research Biomedical Research Centre and Dementia Biomedical Research Unit at South London and Maudsley NHS Foundation Trust and King's College London Other support: escitalopram and placebo provided by H. Lundbeck A/S |
|
| Notes | EsDEPACS is a nested placebo‐controlled trial within the Korean Depression in ACS (K‐DEPACS) study. In K‐DEPACS, depressed participants received no treatment; this comparator was ineligible for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computerized random number generator (blocks of four, allocation ratio 1:1), independent party was responsible for generation |
| Allocation concealment (selection bias) | Low risk | Comment: randomization codes provided by a statistician independent of the recruiting clinicians |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome measurement (by nurses) and adverse event monitoring (by psychiatrists) were carried out blind to treatment allocation" (pg. 63) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high drop‐out rate immediately after randomisation including protocol violations |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes from study protocol reported; only personality assessed mid‐treatment is not reported to date |
| Other bias | Low risk | Comment: no indication of other bias |
Fang 2003.
| Study characteristics | ||
| Methods | RCT design: parallel‐group trial Total N randomised: 57 Length of follow‐up: 8 weeks Analysis: unclear |
|
| Participants | Location: China Number of study centres: patients selected from 2 hospitals CAD criteria: MI) confirmed by an electronic radiograph Depression criteria: Sung's self‐rating depressive scale score > 43 Other entry criteria: Sung's self‐rating anxiety scale score > 38 Exclusion criteria: unclear Treatment N: 27 (sex and age distribution unclear) Control N: 30 (sex and age distribution unclear) Comparability of the groups: unclear |
|
| Interventions | Treatment: health education and psychological intervention in addition to standard medication. Health education included basic MI knowledge and related subjects such as healthy diet, exercise, and cholesterol control. Psychological intervention comprised support (5 times a week, 30 to 40 minutes per meeting), various psychological treatments tailored according to the participant's needs (twice a week for 30 to 40 minutes), and mind and body relaxation time using breathing exercises and various relaxation techniques (twice daily, 15 to 20 minutes) Control: usual care Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: depression symptoms (Sung's self‐rating depressive scale) Other outcomes: Sung's self‐rating anxiety scale score, New York Heart Association functional class, left ventricular ejection fraction |
|
| Funding | Unclear | |
| Notes | Comment: translated paper; possible mixed study sample (patients with depression and/or anxiety) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: No sufficient information provided |
| Other bias | Unclear risk | Comment: translated paper |
Freedland 2009.
| Study characteristics | ||
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 123 Length of follow‐up: 6 months Analysis: ITT with multiple imputation |
|
| Participants | Location: USA Number of study centres and setting: patients who had undergone CABG from 3 hospitals CAD criteria: CABG, randomisation within 12 months after surgery Depression criteria: BDI score of 10 or higher and current major or minor depressive episode assessed with the DISH Other entry criteria: patients aged 21 years or older Exclusion criteria: severe psychiatric comorbidities (schizophrenia or bipolar disorder), active alcoholism or substance abuse, severe cognitive impairment, non‐cardiac illnesses with a poor 1‐year prognosis, being too medically ill or living too far away to participate, unable to communicate in English, or receiving ongoing psychotherapeutic services Treatment 1 (CBT) N: 41 (56% female, mean age: 62 (SD: 11)) Treatment 2 (SSM) N: 42 (50% female, mean age: 59 (SD: 10)) Control N: 40 (43% female, mean age: 61 (SD: 9)) Comparability of groups: proportion of African‐American participants in treatment 2 (SSM) was significantly higher than in the other study arms |
|
| Interventions | Treatment 1: individual CBT (weekly 1‐hour sessions) including target problem identification, problem‐solving, behavioural activation, cognitive techniques (challenging distressing automatic thoughts, changing dysfunctional attitudes), self‐therapy and relapse‐prevention skills Treatment 2: SSM (weekly 1‐hour sessions) including patient education regarding stress and coping, practice in progressive muscle relaxation training, controlled breathing and relaxing imagery Control: usual care for depression Duration of treatment: 12 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D, also BDI‐II), depression remission (HAM‐D ≤ 6), quality of life (36‐Item Short Form Health Survey) Other outcomes: anxiety symptoms (Beck Anxiety Inventory), hopelessness (Beck Hopelessness Scale), stress (Perceived Stress Scale), Heart Surgery Questionnaire (HSQ), cognitive function (digit symbol test, Trail Making Test‐part B, paragraph recall, Short Blessed Test) |
|
| Funding | National Institute of Mental Health, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random allocation sequence with block sizes of 3 and 6 |
| Allocation concealment (selection bias) | Low risk | Comment: Concealed in sealed envelopes and revealed to the study coordinator immediately after the participant completed all of the baseline assessments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessors were masked to the participants' group assignments" (p. 389) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Missing data "plausibly missing at random" (p. 389) Comment: Missing outcome data imputed |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes reported in accordance to the study protocol |
| Other bias | Low risk | Comment: 94% of intervention sessions taped for quality assurance; trial fidelity quantified by a Treatment Process Scale after each session. The measure was developed for the study; Comment: manualised CBT treatment with weekly supervision; SSM intervention not manualised |
Freeman 1986.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 107 Length of follow‐up: no follow‐up Analysis: per‐protocol (60 of 107 participants completed the trial) |
|
| Participants | Location: USA Number of study centres and setting: patients from 1 hospital CAD criteria: patients undergoing CABG (assessment method and time to randomisation not specified) Depression criteria: a score of 13 or greater on the Center for Epidemiological Studies ‐ Depression Scale (CES‐D) or a score of 36 or greater on the Spielberger State Anxiety Inventory (SSAI), or both; presence of clinically significant anxiety or depression was confirmed by a semistructured psychiatric interview Other entry criteria: under 65 years of age Exclusion criteria: females of childbearing potential, patients with a history of sensitivity to benzodiazepines, patients with prior or existing evidence of substance abuse, antisocial personality, psychosis, significant uncontrolled systemic disease, cerebral infarction, dementia, or insufficient English Treatment N: 32 (sex and age distribution unclear) Control N: 28 (sex and age distribution unclear) Comparability of groups: treatment group had significantly higher anxiety scores at baseline; no further information regarding group comparability |
|
| Interventions | Treatment: alprazolam (tablets, 2.5 mg/d at bedtime, maximum dose 4.5 mg/d) Control: placebo Duration of treatment: 1 month |
|
| Outcomes | Review outcomes: depression symptoms (CES‐D score, also Zung Self‐Rating Depression Scale) Other outcomes: anxiety symptoms (SSAI, Zung Self‐Rating Anxiety Scale), Global Impression Scale, structured psychiatric semistructured interviews (signs and symptoms of psychosis, cognitive dysfunction, depression, anxiety, and somatisation) |
|
| Funding | Upjohn Company | |
| Notes | Mixed study sample (participants with depression and/or anxiety) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Only 60 of 107 patients completed the trial Comment: 22 early drop‐outs in the alprazolam group (with noncompleters being less distressed than completers preoperatively) Comment: 25 early drop‐outs in the placebo group (with noncompleters being more distressed than completers preoperatively) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available Comment: psychiatric semistructured interviews were performed at all assessment time points (signs and symptoms of psychosis, cognitive dysfunction, depression, anxiety, and somatization) but were not reported |
| Other bias | Unclear risk | Comment: unclear if selection bias present. 60% of 459 patients met entrance criteria and 23% were included. "The remainder were excluded from entering the drug trial by semistructured interview or were rendered ineligible because of surgical complications or withdrawal of consent." (p. 39) Comment: possible baseline imbalance. Treatment group significantly higher anxiety scores at baseline; no further information regarding comparability of groups |
Kennedy 2005.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 19 Length of follow‐up: no follow‐up Analysis: per‐protocol, all randomised participants who took at least 1 dose of investigational medical product (4 of 19 participants completed the trial) |
|
| Participants | Location: Denmark, Estonia, and Norway Number of study centres and setting: multinational and multicentre CAD criteria: had been admitted for chest pains (or other MI symptom) with a diagnosis of evolving MI not less than 3 weeks and not more than 24 weeks prior to screening, as evidenced by either an elevation of biochemical markers of MI (troponin and creatine kinase‐MB fraction) or ECG changes that were unequivocally consistent with an acute, evolving MI, i.e. development of a significant Q‐wave in at least 2 continuous leads Depression criteria: a score of 20 or greater on the SCL‐90‐R at screening and at baseline Other entry criteria: male or female outpatient between 40 and 75 years of age, on the basis of a physical examination, medical history, ECG, and the results of blood biochemistry and haematology tests carried out at the screening visit; the patient is, in the investigator’s opinion, healthy, otherwise than what is part of the MI and its sequelae Exclusion criteria:
Treatment N: 9 (sex and age distribution unclear) Control N: 10 (sex and age distribution unclear) Comparability of groups: unclear as no results shown. Reported "There were no clinically relevant differences in age, sex, weight, or BMI between the treatment groups. At baseline, there were no clinically relevant differences between the placebo and escitalopram groups with respect to medical history or the use of concomitant medication." |
|
| Interventions | Treatment: flexible doses of daily escitalopram (10 or 20 mg); dose was fixed at 10 mg/day at baseline (to 8 weeks) and could be changed to 20 mg/day at 9 weeks according to the participant’s response to treatment, as judged by the investigator Control: placebo Duration of treatment: 24 weeks |
|
| Outcomes | Review outcomes: cardiac events, ECG waves (rhythm, QRS complex, ST‐segment, and T‐wave inversion), pharmacological side effects | |
| Funding | H. Lundbeck A/S, Lundbeck Austria GmbH Other support: study drugs provided by H. Lundbeck A/S Denmark |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This is a double‐blind study; insufficient information provided on blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This is a double‐blind study. Insufficient information provided on blinding and detection |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 83.3% of participants did not complete the study |
| Selective reporting (reporting bias) | High risk | Comment: efficacy data not reported for depressive symptoms; trial was terminated early by the Investigator |
| Other bias | High risk | Comment: trial was terminated early by the Investigator Comment: Protocol reported for Finland, Austria and Denmark. Study recruitment undertaken in Denmark, Estonia, and Norway |
Li 2005.
| Study characteristics | ||
| Methods | RCT design: parallel‐group trial Total N randomised: 87 Length of follow‐up: 6 weeks Analysis: unclear (2 cases dropped out in the intervention group, 3 cases dropped out in the control group) |
|
| Participants | Location: China Number of study centres and setting: hospitalised patients (number of centres unclear) CAD criteria: undergone CABG Depression criteria: self‐rated HAM‐D score > 18 Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 43 (sex and age distribution unclear) Control N: 39 (sex and age distribution unclear) Comparability of groups: unclear |
|
| Interventions | Treatment: St John's wort extract (300 mg, 3 times a day) Control: placebo Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D score), depressive reductive rate (unclear from translation), pharmacological side effects Other outcomes: ventricular function (Tei‐Index) |
|
| Funding | Unclear | |
| Notes | Comment: translated paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided Quote from translation: "placebo‐control and blind evaluation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided. HAMD was described as a self‐rated measure of depression Quote from translation: "placebo‐control and blind evaluation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: No sufficient information provided |
| Other bias | Unclear risk | Comment: translated paper |
Liu 1999.
| Study characteristics | ||
| Methods | RCT design: parallel‐group trial Total N randomised: unclear Length of follow‐up: 4 weeks Analysis: unclear |
|
| Participants | Location: China Number of study centres and setting: patients from 1 hospital CAD diagnosis: MI as confirmed by electrocardiography Depression diagnosis: Center for Epidemiologic Studies ‐ Depression Scale (CES‐D), HAM‐D, diagnosis according to Chinese Classification of Mental Disorders, Second Edition, Revised (CCMD‐2‐R) Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 31 (32% female, mean age unclear) Control N: 37 (27% female, mean age unclear) Comparability of groups: no significant differences |
|
| Interventions | Treatment: fluoxetine Control: placebo Duration of treatment: 4 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression response, all‐cause mortality, cardiac events Other outcomes: heart rate variability |
|
| Funding | Unclear | |
| Notes | Comment: translated paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided Quote: "double‐blind controlled trial" (pg. 210) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: No sufficient information provided |
| Other bias | Unclear risk | Comment: translated paper |
Liu 2016.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 149 Length of follow‐up: no follow‐up Analysis: unclear (27 dropouts in the treatment group, 28 dropouts in the control group) |
|
| Participants | Location: China Number of study centres and setting: single centre, Cardiology of Harbin Medical University Hospital Department CAD criteria: MI in the past month (diagnosis of acute MI according to the Braunwald standard of cardiology); MI patients must comply with symptomatic myocardial ischaemia, and the ECG appears to ischaemic ST‐segment decline or the ST segment elevation or the new pathological Q wave, myocardial enzyme changing observed, such as elevated serum creatine kinase CK‐MB, increased lactate dehydrogenase (LDH1), increased CK (CPK) Depression criteria: depression score HAM‐D ≥ 18 Other entry criteria: none Exclusion criteria: (1) Those suffering from cardiovascular disease: (1.1) acute MI caused by non‐atherosclerotic disease; (1.2) uncontrollable hypertension (systolic BP > 180 mmHg or diastolic BP > 100 mmHg); (1.3) less than 3 months after arterial bypass surgery; (1.4) suffering from arrhythmia; (1.5) suffering from non‐arterial sclerosis (such as anaemia). (2) Those suffering from other somatic diseases: (2.1) an obvious laboratory examination exception; (2.2) distinct hepatic and renal dysfunction; (2.3) with a history of allergies to sertraline, hyperforin, and acanthopanax. (3) Those suffering from mental illness: (3.1) with alcohol or other substance abuse in the last 6 months; (3.2) with psychotic symptoms, psychiatric history or suffering from bipolar disorder, organic mental disorder, dementia, and other diseases; (3.3) frequently receiving benzodiazepines; (3.4) receiving psychological treatment in the last 3 months; (3.5) with suicide attempts; (3.6) receiving monoamine oxidase inhibitor in the last 4 weeks; (3.7) if the patient has a suicide attempt or the depressive symptoms are aggravated in the course of treatment, the psychiatrist would advise the patient to quit the experiment; (3.8) with a history of depression and the HAM‐D24 score higher than 35 before MI. Treatment N: 73 (43.4% female, mean age: 53.4 (SD: 10.3)) Control N: 76 (41.1% female, mean age: 54.1 (SD: 10.8)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: sertraline + placebo of Shugan Jieyu capsule treatment Control: Shugan Jieyu capsule + sertraline placebo Duration of treatment: 24 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), all‐cause mortality, cardiovascular vital signs (BP, HR), ECG waves, pharmacological side effects Other outcomes: clinical global impression, number needed to treat for non‐inferiority |
|
| Funding | None | |
| Notes | Inconsistency in HAM‐D change scores. No significant differences between trial arms were reported, but the data suggest otherwise (likely standard error instead of standard deviation reported). No contact possible in order to clarify results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were numbered chronologically and then labeled by a random number." (p.535) Comment: Group allocation via odd or even number |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided Quote: "We used the double‐blind experiment..." (pg. 537) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: data‐analysis paragraph is missing, insufficient information on data‐analysis (ITT/per protocol) and missing data handling procedure |
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol available; assessment points described (see below) do not correspond with results |
| Other bias | High risk | Comment: data on assessments at 4, 8 and 24 weeks are missing; available results are based on week 12 (middle of the treatment). End of treatment (week 24) not reported |
Ma 2019.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel trial Total N randomised: 312 Length of follow‐up: no follow‐up Analysis: per‐protocol (3 dropouts in treatment group, 2 dropouts in control group) |
|
| Participants | Location: China Number of study centres and settings: patients who were in hospital cardiac centre CAD criteria: CAD confirmed by coronary angiography Depression entry criteria: HADS Anxiety or HADS Depression score ≥ 8 Other entry criteria: Han ethnicity, at least junior middle school level of education Exclusion criteria: chest pain originating from a stomach complaint; sympathetic ganglia compression in the neck; atrial fibrillation; rapid arrhythmia; ejection fraction < 35% by echocardiography; severe liver, kidney, nerve, or coagulation dysfunction; pregnant or lactating women; suspected aortic dissection; previously diagnosed psychiatric patients (including bipolar disorder, manic depression, psychosis, schizophrenia, suicidal tendencies); allergy to Xinkeshu Treatment: 30 (66.67% male, mean age: 61 (SD: 11)) Control: 30 (56.67% male, mean age: 66 (SD: 11)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: Xinkeshu (4 tablets, 3 times a day) Control: placebo Duration of treatment: 12 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HADS Depression, PHQ‐9), platelet biomarkers, pharmacological side effects Other outcomes: HADS Anxiety, cytokine levels |
|
| Funding | National Natural Science Fund and Guangdong Provincial People’s Hospital Fund | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Conflicting report of randomization in the study "random number table" and trial registration "expert bronze camel random envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information provided on allocation concealment Quote: "Randomization was blinded to both the patient and investigator" (pg. 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Insufficient information provided on performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Depression outcome is self‐reported. Otherwise, insufficient information provided on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Out of 60 patients, 2 patients did not complete the intervention (1 patient in each group). 3 patients did not complete the intervention (2 patients in the intervention group, and 1 patient in the control group. The reasons for patients who did not complete the intervention were stated in the result section |
| Selective reporting (reporting bias) | High risk | Comment: drug safety data reported inadequately and cytokine data not reported as either per protocol or ITT |
| Other bias | High risk | Comment: this trial was registered retrospectively after recruitment had commenced ChiCTR‐IPR‐17010940 |
McFarlane 2001.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 38 Length of follow‐up: no follow‐up Analysis: per‐protocol (6 dropouts in the intervention group, 5 dropouts in the control group) |
|
| Participants | Location: Canada Number of study centres and setting: patients admitted to 1 coronary care unit CAD criteria: acute MI; assessment method and time to randomisation not specified Depression criteria: score > 15 on the Inventory to Diagnose Depression (IDD) questionnaire on 2 occasions (just before hospital discharge and 2 weeks later) Other entry criteria: none stated Exclusion criteria: predischarge 24‐hour Holter recordings showing either atrial fibrillation or ventricular etopic beats greater than 100 per hour, congestive heart failure, any life‐threatening comorbid condition, inability to complete the questionnaire, and taking antidepressant medication Treatment N: 12 (33% female, mean age: 56 (SD: 11)) Control N: 15 (47% female, mean age: 56 (SD: 12)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: sertraline (50 mg/d) Control: placebo Duration of treatment: 22 weeks |
|
| Outcomes | Review outcomes: depression symptoms (IDD), all‐cause mortality, cardiovascular vital signs (HR) Other outcomes: HR variability (SD of all 24‐hour N‐N intervals, root mean square of the SD of successive N‐N intervals, low frequency/high frequency ratio, low frequency power in normalised units) |
|
| Funding | Heart and Stroke Foundation of Ontario, Canada | |
| Notes | The intervention trial was nested in a study inclusive of a non‐depressed comparator group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided Quote: "double‐blind, randomized, placebo‐controlled trial" (pg. 618) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11 drop‐outs (3 had side‐effects, 7 non‐compliant, 1 with ectopy) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available Comment: Outcomes reported according to methods section |
| Other bias | Unclear risk | Comment: Inconsistent description of depression change results (p. 619 and p. 620) |
McLaughlin 2005.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: 4 months Analysis: per‐protocol (8 treatment participants dropped out, 12 control participants dropped out) |
|
| Participants | Location: USA Number of study centres and setting: patients with ACS from 2 hospitals CAD diagnosis: ACS assessed by medical chart review in the coronary care unit; time to randomisation not specified Depression diagnosis: score of 7 and more on either subscale of the HADS Other entry criteria: 35 years of age or older, able to speak English, access to a touch‐tone phone Exclusion criteria: mental health care in the prior 3 months, psychoactive drug use during the past year, and diagnosis of substance abuse during the past year Treatment N: 45 (31.1% female, mean age: 59.9 (SD: 10.2)) Control N: 34 (35.3% female, mean age: 60.7 (SD: 9.8)) Comparability of the groups: significantly higher anger scores amongst females in the treatment group, significantly more participants with MI in the treatment group |
|
| Interventions | Treatment: 6 telephone counselling sessions (30 minutes each) with clinicians (psychiatrist, clinical psychologist, internist) comprising review of common fears experienced by those living with chronic medical conditions and identification and management of barriers to adjustment to medical illness; participants with HADS score > 15 were referred for emergent care Control: usual care (received a booklet on coping with cardiac illness typical of those given at hospital discharge and were instructed to contact their primary care physician if they experienced any warning signs of depression; advised to continue follow‐up with their primary care and specialist physicians) Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HADS), all‐cause mortality Other outcomes: anxiety symptoms (HADS), Work and Social Adjustment Scale (WSAS), State‐Trait Anger Expression Inventory (STAXI), clinical global impressions |
|
| Funding | National Institute of Mental Health, USA; Robert Wood Johnson Foundation | |
| Notes | Mixed study sample (patients with depression and/or anxiety) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by coin flip" (p. 1085 in McLaughlin et al., 2005) |
| Allocation concealment (selection bias) | High risk | Quote: "The study coordinator conducted the coin flip and assigned patients to a treatment arm when she contacted study participants by telephone and enrolled consenting participants." (p. 540 in Bambauer et al., 2005) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No sufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Primary outcome was patient self‐report measure (HADS); other blinding not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: The authors describe that multiple imputation methods were used to examine if data were missing at random. But all analyses were reported for the final cohort of 79 patients |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol and design paper available Comment: outcomes consistent in methods and results sections |
| Other bias | Unclear risk | Comment: weekly meetings and supervision of counsellors; monitoring quality not otherwise specified Comment: depression data reported as β value unit change and final HADS depression scores post‐treatment |
MIND‐IT 2007.
| Study characteristics | ||
| Methods | RCT design: nested, 3‐arm parallel‐group trial Total N randomised: 91 Length of follow‐up: no follow‐up Analysis: ITT (10 dropouts in the intervention group, 3 dropouts in the placebo group during the first 8‐week acute treatment phase; 23 dropouts in the intervention group, 15 dropouts in the placebo group during the entire treatment (24 weeks)) |
|
| Participants | Location: Netherlands Number of study centres and setting: patients with a MI from 8 hospitals CAD criteria: MI with typical clinical picture, increase of cardiac enzymes, ECG changes and chest pain for > 20 minutes; time to randomisation 3 to 12 months (to exclude adjustment disorders) Depression criteria: 2‐stage procedure, in which those with 1) score of 10 or more on the BDI were 2) interviewed with the Composite International Diagnostic Interview (CIDI) for major or minor depression diagnosis (psychiatrist confirmed CIDI diagnosis) Other entry criteria: age >= 18 years Exclusion criteria: occurrence of MI whilst hospitalised for another reason except for unstable angina pectoris, lacking capability to participate in study procedures, any disease likely to influence short‐term survival, already receiving psychiatric treatment for depressive disorder, participation in any clinical trial that might intervene with the study, hyperthyroidism, suicidality Treatment N: 47 (12.8% female, mean age: 56.6 (SD: 11.1)) Control N: 44 (18.2% female, mean age: 57.9 (SD: 9.7)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: mirtazapine (30 to 45 mg/d); participants who did not respond and those with relapse were offered open treatment with citalopram Control 1: placebo Control 2: care as usual, pharmacological treatment, non‐pharmacological treatment, or no treatment (not eligible for this review) Duration of treatment: 24 weeks (8 weeks acute plus 16 weeks continuation treatment) |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D, also BDI, depression scale of the SCL 90), depression remission (HAM‐D ≤ 7), all‐cause mortality, cardiac events, hospitalisations, cardiovascular vital signs (BP, HR), platelet biomarkers, ECG waves, pharmacological side effects Other outcomes: clinical global impression, concurrent medication, weight |
|
| Funding | Netherlands Heart Foundation; Organon (Netherlands); Lundbeck (Denmark) | |
| Notes | MIND‐IT trial investigated antidepressant treatment in general versus usual care in patients following MI (N = 331). The intervention arm consisted of double‐blind mirtazapine, open pharmacological treatment, non‐pharmacological treatment, or no treatment. The care‐as‐usual arm comprised pharmacological treatment, non‐pharmacological treatment, or no treatment. We used data for the nested trial investigating mirtazapine versus placebo (n = 91) in this review consistent with the predefined comparisons (i.e. pharmacological intervention vs placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Central randomization service (computer‐generated blocks of four) |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Insufficient information provided Quote: "double‐blind" (Honig 2007, pg. 607) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information provided in primary nested‐trial paper (Honig 2007). Quote: From protocol " A blinded end point committee will judge all possible primary end points" (van den Beek 2002, pg. 223) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results and methods section consistent in Honig (2007) Comment: Analysis of nested‐study trial data not stated in protocol paper (van den Brink, 2002) |
| Other bias | Unclear risk | Comment: in the paper by Honig patients randomised to mirtazapine had higher baseline scores on depression (HAM‐D, p = 0.05) which suggests possible baseline imbalance |
MoodCare 2011.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 121 Length of follow‐up: 6 months, 18 months Analysis: ITT with last‐observation‐carried‐forward (8 dropouts in intervention group, 7 dropouts in control group at 6‐month follow‐up) |
|
| Participants | Location: Australia Number of study centres and setting: multicentre; 6 metropolitan hospitals in the states of Victoria (Austin, St. Vincent's, Geelong, Royal Melbourne Hospitals) and Queensland (Royal Brisbane and Women's and The Prince Charles Hospitals). CAD criteria: clinical diagnosis of ACS (MI (ST segment elevation MI, STEMI or non‐STEMI) or unstable angina confirmed by angiogram) Depression criteria: depression (PHQ‐9) score of 5 to 19 Other entry criteria: age between 21 and 85, fluency in English, availability via the telephone for the duration of the study Exclusion criteria: regular psychological therapy with a mental health professional at the time of admission for ACS, diagnosis of mental health condition which may impact on involvement (including bipolar disorder, psychotic illness of any type, dementia, acute suicidality, severe personality disorder), cognitive impairment impacting ability to participate in the study, diagnosed with a terminal illness, unable to participate in a tele‐based unsupervised mood and lifestyle intervention as confirmed by treating clinician Treatment N: 61 (26.2% female, mean age: 61.0 (SD: 10.2)) Control N: 60 (23.3% female, mean age: 58.9 (SD: 10.7)) Comparability of groups: no significant baseline differences except for a significantly higher proportion of participants in the treatment group being born in Australia and had visited a general practitioner in the past 6 months |
|
| Interventions | Treatment: CBT intervention with structured counselling sessions (2 weeks baseline screening delivered by qualified psychologists, intervention aims to manage depression as well as CHD risk factor behaviours using a tele‐based care management model incorporating CBT counselling; psychologists with at least 2 years of experience deliver the intervention, content: cognitive restructuring, behavioural activation, goal setting, motivational interviewing techniques); participants received 10 sessions lasting 30 to 40 minutes, unless target recovery was achieved prior to programme completion (in this event, the interventionists reviewed the individual case with the senior clinical consultant, and if the participant produced a PHQ score in the normal range for 3 consecutive counselling sessions, after completing at least 4 sessions, the participant was considered to have met target recovery). Additonally, participants received a brief National Heart Foundation of Australia education pamphlet on MI recovery. Control: usual care and a brief National Heart Foundation of Australia education pamphlet on MI recovery Duration of treatment: 6 months |
|
| Outcomes | Review outcomes: depression symptoms (PHQ‐9), quality of life (12‐Item Short Form Health Survey) Other outcomes: cardiac depression (Cardiac Depression Scale), acceptability, feasibility |
|
| Funding | Australian Government Department of Health and Ageing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: automatically generated separate block randomization (integrated into web‐based database), process concealed from investigators |
| Allocation concealment (selection bias) | Low risk | Comment: automatic group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: randomisation schedule was concealed from investigators, participants were asked not to reveal the group to which they were randomised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: project staff who administered telephone questionnaires were blinded to participans´study group. Primary outcome was a self‐report measure (PHQ‐9) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT |
| Selective reporting (reporting bias) | Low risk | Comment: Study protocol available and congruent |
| Other bias | Unclear risk | Comment: manualised intervention and therapist quality assessed by Cognitive Therapy Scale Comment: therapist adherence monitored by audiotape of phonecalls; only 17% reviewed by expert psychiatrist as specified in protocol |
Pizzi 2009.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: 5 months Analysis: per‐protocol (method of analysis not explicitly stated; 3 dropouts in the treatment group, 2 dropouts in the control group) |
|
| Participants | Location: Italy Number of study centres and setting: secondary care referral to visit the Department of International Medicine, Aging and Nephrological Diseases CAD criteria: documented CAD (diagnosis of at least 1 of the following: previous MI, previous or current angina with objective evidence of atherosclerosis, and a previous surgical procedure for coronary revascularisation) Depression criteria: symptoms of depression (BDI ≥ 10) Other entry criteria: none Exclusion criteria: neoplasms, kidney or liver failure, systemic inflammatory disease, uncontrolled hypertension (systolic BP > 180 mmHg or diastolic BP > 100 mmHg), recent AMI or unstable angina, ejection fraction < 50%, current antidepressant treatment, current psychotherapy Treatment N: 47 (53.2% female, mean age: 57.4 (SD: 8.7)) Control N: 48 (47.9% female, mean age: 56.3 (SD: 8.2)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: sertraline (week 1 to 6: 50 mg daily, week 7 to 12: gradually increase to attain a maximum daily dose of 200 mg, depending on each participant's clinical response and tolerance, week 13 to 20: constant dose (= maximum of milligrams reached at the end of week 12) Control: placebo Duration of treatment: 20 weeks |
|
| Outcomes | Review outcomes: depression symptoms (BDI), depression response, platelet biomarkers, pharmacological side effects Other outcomes: inflammatory markers, flow‐dependent endothelium‐mediated dilation |
|
| Funding | No information provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation carried out by a central office, no other information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: No sufficient information provided, steps taken to conceal the allocation unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Double‐blind trial, researchers were blind to patients group allocation during recruitment, data collection and data analyses, physicians who were not involved in the study design performed treatment assignment and implementation of the therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Self‐reported primary outcome (BDI); no other information on blinded outcome assessments for biomarkers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Low drop‐out, authors reported reasons for early drop‐out per group |
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol available but mentioned (p.531) |
| Other bias | Low risk | Comment: No indication of other bias |
Roose 1998.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 81 Length of follow‐up: no follow‐up Analysis: ITT (4 paroxetine participants discontinued, 10 nortriptyline participants discontinued) |
|
| Participants | Location: USA Number of study centres and setting: outpatients from 4 hospitals CAD criteria: MI, CABG, coronary angioplasty, positive stress test, or angiographic evidence of a 75% or greater luminal narrowing of a major coronary artery; time to randomisation unclear Depression criteria: meeting DSM‐IV criteria for major depressive disorder, unipolar subtype, with a score of 16 or greater on the 17‐item HAM‐D Other entry criteria: age >= 18 Exclusion criteria: MI within the past 3 months, a baseline QTc interval of 460 milliseconds or greater, unstable or crescendo angina, receiving drugs with class 1 antiarrhythmic activity or warfarin Treatment 1 N: 41 (12% female, mean age: 57.8 (SD: 11.0)) Treatment 2 N: 40 (22% female, mean age: 57.9 (SD: 12.7)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment 1: paroxetine (+ dummy placebo at night) (age < 65: 20 mg/d for the first 3 weeks; age > 65: 10 mg/d for the first week, 20 mg/d for week 2 and 3; if no response (HAM‐D reduction 50% or HAM‐D <= 8), 30 mg/d at week 4 and 40 mg/d at end of week 5) Treatment 2: nortriptyline (+ dummy placebo in the morning) (25 mg for the first 2 days; 50 mg on day 3; on day 7 plasma level measurement and adjustment of the dose to achieve a nortriptyline plasma level between 203 and 456 nmol/L (80 to 120 ng/mL)) Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression remission (HAM‐D ≤ 8), depression response (50% reduction on HAM‐D), cardiac events, cardiovascular vital signs (BP, HR), ECG waves, pharmacological side effects Other outcomes: heart rate variability (SDNN, pNN50), ventricular premature depolarisations |
|
| Funding | Smith‐Kline Beecham Pharmaceuticals (GlaxoSmithKline) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by permuted blocks of 10" (p. 288) |
| Allocation concealment (selection bias) | Unclear risk | Comment: Reported as a double‐blind study; no other details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Reported as a double‐blind study; with "double dummy" blinding described for patients and physicians and other raters Quote: "To ensure that the treating physician and other raters remained unaware of drug administration, the nortriptyline dose was adjusted by a physician who was not involved in the study" and "the blind was maintained by selecting, on a random basis, patients receiving active paroxetine to have their nortriptyline placebo increased or decreased to mimic the dose adjustment for patients re3cigving active nortriptyline" (Roose 1998, pg. 288) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Reported as a double‐blind study; no other details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results and methods section consistent Comment: No protocol or design paper available |
| Other bias | High risk | Comment: authors of the study involved in design, analysis and reporting were employees of SmithKline Beecham Pharmaceuticals PA |
SADHART 2002.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 369 Length of follow‐up: no follow‐up Analysis: ITT (53 discontinued treatment, 46 discontinued placebo) |
|
| Participants | Location: USA, Europe, Canada, Australia Number of study centres and setting: outpatients from 40 cardiology centres and psychiatry clinics CAD criteria: patients hospitalised for MI or unstable angina in the past 30 days. Criteria for acute MI: at least 1 criterion from each of the following 2 categories: Category A: 1) creatine kinase isoenzyme MB (CK‐MB) level greater than the upper limit of normal, 2) CK or troponin T or troponin 1 level more than 2 times the upper limit of normal, 3) a total lactate dehydrogenase (LDH) level more than 1.5 times the upper limit of normal (with LDH 1 greater than LDH 2). Category B: 1) typical ischaemic symptoms (chest pain or shortness of breath) lasting for more than 10 minutes, 2) ECG evidence of ischaemic ST‐segment depression, ST‐segment elevation, or new pathological Q waves. Criteria for unstable angina: 1) experienced angina of anginal equivalent symptoms at rest, with episodes lasting for at least 10 minutes and leading to hospitalisation, and had ECG documentation of transient ST‐segment elevation or depression of more than 0.5 mm, or had T wave inversion of greater than 1 mm within 12 hours of an episode of chest pain; 2) were hospitalised for symptoms of unstable angina and had known CAD with a documented history of a prior MI, had undergone a prior revascularisation procedure, or had documented coronary artery stenosis greater than 75% in 1 of the major epicardial vessels Depression criteria: major depression according to structured Diagnostic Interview Schedule (DIS) for DSM‐IV, BDI score of 10 or greater Other entry criteria: none Exclusion criteria: uncontrolled hypertension, cardiac surgery anticipated during the next 6 months, MI or unstable angina developed less than 3 months after CABG, resting heart rate of less than 40/min, MI or unstable angina of non‐atherosclerotic aetiology, Killip class III or IV status, persistent clinically significant laboratory abnormalities, renal dysfunction, hepatic dysfunction, other significant non‐cardiac disease, women of childbearing potential not using adequate contraception, current use of class 1 antiarrhythmic medications, use of reserpine, guanethidine, clonidine, methyldopa, anticonvulsants, neuroleptics, antidepressants, benzodiazepines, initiation of psychotherapy in the 3 months prior to study entry, alcohol or substance abuse or dependence in past 6 months, psychotic symptoms, history of psychosis, bipolar disorder, organic brain syndrome, dementia, significant suicide risk Treatment N: 186 (37% female, mean age: 56.8 (SD: 11.1)) Control N: 183 (36% female, mean age: 57.6 (SD: 10.4)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: sertraline 50 mg/d for the first 6 weeks, up to 100 mg/d for weeks 6 to 10, up to 150 mg/d for weeks 10 to 12, up to 200 mg/d for weeks 12 to 24 Control: placebo Duration of treatment: 24 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression response, all‐cause mortality, cardiac events, healthcare costs, hospitalisations, quality of life (Quality of Life Enjoyment and Satisfaction scale (Q‐LES‐Q, Medical Outcomes Study Short‐Form 36), cardiovascular vital signs, platelet biomarkers, ECG waves, pharmacological side effects Other outcomes: left ventricular function, ventricular premature complexes, heart rate variability, clinical global impression |
|
| Funding | Pfizer Inc; Suzanne C. Murphy Foundation; Thomas and Caroline Royster Research Fund; Perry and Martin Granoff Family Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Single‐blind placebo treatment preceded double‐blind randomization to intervention or placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Single‐blind placebo treatment preceded double‐blind randomization to intervention or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Serious adverse events and ECG reporting blinded to treatment allocation; other outcomes unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available |
| Other bias | High risk | Comment: author involved in design, analysis and reporting of data was an employee of Pfizer |
Shahmansouri 2014.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 44 Length of follow‐up: 6 weeks Analysis: per‐protocol, method of analysis not explicitly stated (4 dropouts, 2 in each group) |
|
| Participants | Location: Iran Number of study centres and setting: Psychiatric Clinic of Tehran Heart Center CAD criteria: PCI in the last 6 months Depression criteria: patients who met DSM IV‐TR criteria for diagnosis of MDD (mild‐moderate); HAM‐D = 14 to 22 Other entry criteria: 20 to 65 years of age Exclusion criteria: diagnosis of any other psychiatric disorder on the DSM‐IV axis I or II; patients receiving any other psychotropic medications; patients at high risk for suicide (score ≥ 2 on the suicide item of HAM‐D) were referred to a psychiatrist and were not enrolled in this study. Patients were also excluded if they had received psychotropic agents, alternative medicine, or psychotherapy within 4 weeks or electroconvulsive therapy within 8 weeks prior to entry. Other exclusion criteria were substance abuse or dependence (other than nicotine) within 3 months, serious or life‐threatening illness, thyroid disease, hepatic or renal dysfunction, hypersensitivity to fluoxetine or herbal compounds, pregnancy, lactation, and oral contraception use. Women of child‐bearing age were excluded if they were willing to get pregnant. Treatment N: 22 (50.0% female, mean age 52.05 (SD: 8.92)) Control N: 22 (63.6% female, mean age: 53.10 (SD: 8.47)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment 1: saffron (SaffroMood, IMPIRAN, containing 15 mg of saffron extract); participants would receive 1 capsule every other day for the first week followed by 1 capsule daily for the second week and 2 capsules per day for the rest of the study, for a maximum dose of 30 mg/day Treatment 2: fluoxetine (Abidi, Iran, 20 mg capsule); participants would receive 1 capsule every other day for the first week followed by 1 capsule daily for the second week and 2 capsules per day for the rest of the study, for a maximum dose of 40 mg/day Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression response (50% HAM‐D symptom reduction), depression remission (HAM‐D < 8), pharmacological side effects | |
| Funding | Tehran University of Medical Sciences; IMPIRAN company donated the capsules of SaffroMood | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Random permuted blocks of four, patients were randomly and equally assigned to two groups (fluoxetine or SaffroMood) in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Comment: An independent person who was not involved elsewhere in the research project generated the randomization codes by Excel software. Assignments were kept in sequentially numbered, sealed, opaque envelopes and were opened sequentially only after participant details were written on the envelope. Separate persons were responsible for rating and random allocation of the patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: The patients and their caregivers, the clinician who referred them, the research team investigators who rated the participants and prescribed the medications, and the statistician were all blind to the treatment group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The patients and their caregivers, the clinician who referred them, the research team investigators who rated the participants and prescribed the medications, and the statistician were all blind to the treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Low attrition, no ITT |
| Selective reporting (reporting bias) | Low risk | Comment: Study protocol available and congruent with reported outcomes |
| Other bias | Low risk | Comment: No indication of other bias |
SPIRR‐CAD 2011.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 570 Length of follow‐up: 6 months Analysis: ITT (last‐observation‐carried‐forward) and per‐protocol analysis (24 did not receive intervention; 174 dropouts in intervention group, 90 dropouts in usual care group) |
|
| Participants | Location: Germany Number of study centres and setting: multicentre; 10 tertiary care centres in Germany CAD criteria: documented CAD with recent coronary angiograms Depression criteria: HADS Depression > 7 Other entry criteria: none Exclusion criteria: inability to speak German; severe heart failure (NYHA class IV); scheduled cardiac surgery within the next 3 months; severe depressive episodes according to Structured Clinical Interview for DSM‐IV (SCID) or other severe life‐threatening physical or mental illness Treatment N: 285 (21.4% female, mean age 59.1 (SD: 9.8)) Control N: 285 (20.7% female, mean age 59.3 (SD: 9.3)) Comparability of groups: no baseline‐differences in sociodemographic, clinical, and psychological data |
|
| Interventions | Treatment: stepwise, fully manualised individual and group psychotherapy in addition to usual care by primary physicians or cardiologist, or both. All participants were offered 3 individual supportive‐expressive psychotherapy sessions. Participants' partners were invited for the third session. All participants were reassessed with the HADS after the third session (4 to 6 weeks after inclusion), and those who were still depressed were offered 25 90‐minute sessions of group psychotherapy in closed groups of 6 to 10 participants for approximately 10 months, usually starting 3 to 6 months after randomisation. Control: usual care by primary physicians and/or cardiologist and 1 manualised individual information session, 30 to 45 minutes delivered by trained staff (content of information session: healthy behaviours and psychosocial factors in CAD) Duration of treatment: 18 months |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D, also HADS), depression remission (HADS ≤ 7), all‐cause mortality, cardiovascular mortality Other outcomes: type D personality |
|
| Funding | German Research Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Online randomization service, ALEA, 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "blinding of the intervention to patients and therapists was not possible" Comment: control participants received information session |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Outcome assessments were performed by patients self report (HADS) and face‐to‐face interviews (HAMD, SCID) with trained raters who where masked regarding patients treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: analyses ITT with and without imputation LOC method (per‐protocol also reported, not extracted) Comment: imbalance in loss to follow‐up between groups (174 drop‐outs in intervention group (61.3%), 90 drop‐outs in usual care group (31.7%)) |
| Selective reporting (reporting bias) | Unclear risk | Comment: not all secondary outcomes are reported as yet |
| Other bias | Unclear risk | Comment: changes made from protocol during trial to intervention of implementation i.e. group psychotherapy could commence more than 8 weeks after randomisation Comment: trial fidelity and therapist adherence described in Albus 2011 |
Strik 2000.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 54 Length of follow‐up: no follow‐up Analysis: ITT for primary outcomes (9 withdrawn from control group, 5 withdrawn from treatment group), per‐protocol for cardiologic safety variables |
|
| Participants | Location: Netherlands Number of study centres and setting: patients from 2 hospitals CAD criteria: MI diagnosed by a cardiologist with a clinical picture typical of MI, electrocardiographic changes specific for MI, and a maximum plasma concentration of aspartate aminotransferase (ASAT) of twice the upper normal range (80 units/litre); enrolment 3 to 12 months after MI Depression criteria: patients with a score above the cut‐off on the SCL‐90 Depression Scale (> 22 for men and > 28 for women) were interviewed with the Schedules for Clinical Assessment in Neuropsychiatry; patients meeting DSM‐III‐R criteria for major depressive episode and having a HAM‐D score of > 17 were included Other entry criteria: 18 to 75 years of age Exclusion criteria: any concurrent psychosocial or therapeutic intervention, psychotic symptomatology, a second psychiatric diagnosis, history of mania, current pregnancy or lactation, life‐threatening non‐cardiac physical illness, concurrent use of psychotropic drugs, hypersensitivity to fluoxetine, liver or severe kidney dysfunction, right ventricular filling pressure > 30 mmHg and a low systolic volume or an ATVI < 10 cm Treatment N: 27 (22% female, mean age: 54.1 (SD: 11.3)) Control N: 27 (37% female, mean age: 58.7 (SD: 10.1)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: fluoxetine (acute treatment period of 9 weeks and continuation period of 16 weeks; 20 to 60 mg/d) Control: placebo Duration of treatment: maximum of 25 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), depression remission (HAM‐D < 7), cardiac events, resource utilisation (hospitalisation), cardiovascular vital signs (BP, HR), ECG waves, pharmacological side effects Other outcomes: SCL‐90 Hostility Scale score, concurrent use of medications, cognitive performance, echocardiography (LVEF, ATVI, E/A ratio) |
|
| Funding | Eli Lilly, Dutch Prevention Fund, Maastricht University Hospital Research Fund | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" (pg. 785) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind" (pg. 785) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 14 patients meeting inclusion criteria did not complete the trial, but did not differ from participants in age, gender, or maximum ASAT Comment: Intention‐to‐treat for primary outcomes |
| Selective reporting (reporting bias) | High risk | Comment: Many outcomes not or only partially reported Comment: No protocol or design paper available |
| Other bias | Low risk | Comment: No indication of other bias |
Tian 2016.
| Study characteristics | ||
| Methods | RCT design: 3‐arm parallel‐group trial nested within observational cohort Total N randomised: 46 (not including inactive comparator) Length of follow‐up: no follow‐up Analysis: per‐protocol (attrition per group allocation not specified; 16/308 recruited patients dropped out) |
|
| Participants | Location: China Number of study centres and setting: patients from Coronary Care Unit in Qian Fo Shan Hospital of Shandong University Medical School CAD criteria: acute MI with: ischaemic chest pain for > 30 minutes but < 24 hours, persistent ST‐segment elevation >= 0.1 mV, ST‐segment depression or T‐wave inversion in 2 adjacent electrocardiography leads, and significantly elevated blood levels of biomarkers for myocardial injury (creatine kinase‐MB and troponin I) Depression criteria: patients with a score above the cut‐off on the HAM‐D‐17 and a self‐test score using the Self‐Rating Depression Scale (cut‐off not specified) Other entry criteria: none specified Exclusion criteria: more than 85 years old, infection, allergic disorder, endocrine disease, malignancy, autoimmune disease, rheumatic heart disease, severe liver disease, renal failure, history of drug abuse, or having been prescribed an anti‐inflammatory or immunosuppressant drug except aspirin in the past 3 weeks prior to MI. Other comorbid heart diseases ineligible (atrial fibrillation, myocarditis, endocarditis, valvular heart disease, or requirement of an implanted pacemaker) Treatment 1: paroxetine N: 27 (43% female, mean age: 63.4 (SD: 10.7)) Treatment 2: fluoxetine N: 27 (48% female, mean age: 61.7 (SD: 10.4)) Comparability of groups: no significant baseline differences evident |
|
| Interventions | Treatment 1: paroxetine: 10 mg/d initially and increased to 20 mg/d within 1 week Treatment 1: fluoxetine: 10 mg/d initially and increased to 20 mg/d within 1 week Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), cardiac events, cardiovascular vital signs (BP) | |
| Funding | Tackle Key Problems in Science and Technology Program of Shandong Province | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No sufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated as randomized, double‐blind study with no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: stated as randomized, double‐blind study with no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Psychiatrists rated depression (HAMD) at baseline after myocardial infarction, no information provided on follow‐up assessments or blinding to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No flow chart or reasons for attrition provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol paper or trial registry |
| Other bias | High risk | Comment: no trial registry provided for this trial |
TREATED‐ACS 2020.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: 30 months Analysis: ITT and imputation not specified (8 dropouts from intervention group, 10 dropouts from CM group) |
|
| Participants | Location: Italy Number of study centres and setting: Maggiore Hospital in Bologna and San Giovanni Battista Hospital in Torino CAD criteria: first episode of acute MI or unstable angina. MI defined by cardiac symptoms (presence of acute chest, epigastric, neck, jaw, or arm pain or discomfort or pressure without apparent non‐cardiac source) and signs (acute congestive heart failure or cardiogenic shock in the absence of non‐CHD causes) associated with ECG findings (characteristic evolutionary ST‐T changes or new Q waves) and/or cardiac biomarkers (blood measures of myocardial necrosis, specifically CK, CK‐MB, CK‐MBm, or troponin, cTn). Unstable angina defined by cardiac symptoms (chest pain lasting less than 20 minutes) with likely ECG findings (ST‐segment depression and abnormal T‐wave) in the absence of myocardial necrosis biomarkers Depression criteria: a current diagnosis of at least 1 of the following: major or minor depression, dysthymia according to DSM‐IV criteria, and demoralisation according to Diagnostic Criteria for Psychosomatic Research criteria Other entry criteria: Mini‐Mental State Examination score higher than 24, written informed consent provided by the patient to participate Exclusion criteria: history of bipolar disorder (DSM‐IV criteria), major depression with psychotic features, history of substance abuse or dependency during the previous 12 months, serious suicide risk, current use of antidepressants, current treatment with any form of psychotherapy |
|
| Interventions | Intervention 1: CBT in combination with WBT and lifestyle modification. CBT involves: identifying and correcting inaccurate thoughts associated with depressed feelings (cognitive restructuring); helping participants to engage in enjoyable activities more often (behavioural activation); enhancing problem‐solving skills; providing instruction and guidance in specific strategies for solving problems. WBT involves techniques to overcome impairments in environmental mastery, purpose in life, personal growth, autonomy, self‐acceptance, and positive relations with others. Lifestyle modification not further specified. Intervention 2: clinical management, consisting of reviewing the participant's clinical status and providing the participant with support and advice if necessary |
|
| Outcomes | Primary: depression symptoms measured by Paykel's 20‐item change version of the Clinical Interview for Depression (CID). Depressive symptoms subscale of Kellner's Symptom Questionnaire Secondary outcomes: frequency of negative cardiac outcomes, such as rehospitalisations due to cardiac complications, acute MI, unstable angina, angioplasty, cardiac surgery, and cardiac mortality occurring after the first episode of ACS |
|
| Funding | Compagnia di San Paolo di Torino, Italy | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT00998400 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Treatment allocation was accomplished through random computerized assignment that allocated 50% of the patients to each treatment group, not further specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: Assignment concealed until the time of group assignment, not further specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Outcomes included self‐report and structured interviews, as well as biomarkers. Assignment concealed until the time of group assignment; blinding not further specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Patients were assessed by 2 clinical psychologists, who were blind to treatment assignment, at pretreatment and posttreatment, and 3, 6, 12, and 30 months after the end of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 of 48 patients in each group dropped‐out from baseline to end of treatment, and 22 patients total dropped‐out before 30‐month follow‐up Comment: All analyses were performed by using intention‐to‐treat analysis, where missing values were managed by means of a multiple‐imputations procedure |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section and trial registry NCT00998400 were reported Comment: Biomarker analyses included in the paper were not stated in the trial registry NCT00998400 Comment: No design paper available |
| Other bias | Unclear risk | Comment: exact p values not reported for baseline comparisons between intervention groups. Possible imbalance at baseline, psychotherapy group taking less cardiac medications and reporting more personal growth; psychotherapy group reporting more depression + demoralisation Comment: therapists trained in intervention; no efforts regarding therapy quality mentioned |
U‐CARE 2018.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel design Total N randomised: 3928 Length of follow‐up: 14 weeks Analysis: ITT with multiple imputation by chained equations for depression, ITT without missing data for cardiovascular mortality and cardiac events (28 dropouts intervention group, 10 dropouts control group at 12 months) |
|
| Participants | Location: Sweden Number of study centres and settings: 25 Swedish hospitals CAD criteria: recent MI < 3 months Depression entry criteria: > 7 on 1 or both of the 2 HADS subscales Other entry criteria: < 75 years old Exclusion criteria: scheduled for coronary artery bypass surgery; unable to use computer, internet, email, or mobile phone; unable to read Swedish; expected to live < 1 year; anticipated to show poor compliance (e.g. substance abuse or not showing up to the cardiac nurse visit); self‐reported severe depression or suicidal ideation (MARDS‐S total score > 34 or MARDS‐S item 9 > 3); participating in another behavioural intervention trial Treatment: 117 participants (44% women, mean age: 58.4 (SD: 9)) Control: 122 participants (36% women, mean age: 60.8 (SD: 7.8)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: therapist‐guided internet CBT treatment Control: usual treatment Duration of treatment: 14 weeks |
|
| Outcomes | Review outcomes: depression symptoms (Montgomery–Åsberg Depression Rating Scale, also HADS), cardiovascular mortality, cardiac events Other outcomes: anxiety symptoms (HADS Anxiety), behavioural activation (Behavioral Activation for Depression Scale), cardiac anxiety (Cardiac Anxiety Questionnaire), adherence to treatment |
|
| Funding | Swedish Research Council | |
| Notes | Mixed study sample (patients with symptoms of depression or anxiety were recruited) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence generation with stratification by clinical centre Quote "1:1 allocation, using a computer‐generated code" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Randomization occurred automatically in the internet‐based portal |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: technical and telephone support staff blind to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details on outcome assessment blinding; primary outcomes self‐reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Intention‐to‐treat as the main analysis. Reasons for drop‐out not provided in Norlund or Humphries |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported (depression and anxiety) Comment: secondary endpoints in protocol not yet reported ‐ quality of life, stress behaviors, fatigue, sleep pattern, posttraumatic stress, posttraumatic growth, health economy aspects, cost‐effectiveness of the intervention, major adverse cardiac events |
| Other bias | High risk | Comment: There was a change in the inclusion criteria during the study due to low recruitment numbers. The HADS threshold was lowered from ≧10 to >7 on any subscale of the HADS. The recruitment target in the protocol was 500 (eventual recruitment n = 239) Comment: manualised treatment (online) and therapist support was provided for the online modules in patients allocated to treatment; no information on therapists adherence or quality Comment: email and telephone prompts provided; unclear if to intervention and control groups |
UPBEAT 2012.
| Study characteristics | ||
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 101 Length of follow‐up: no follow‐up Analysis: ITT with multiple imputation (4 dropouts from intervention group, 1 dropout from control group) |
|
| Participants | Location: USA Number of study centres and setting: physician referrals, community‐based screenings, mass media advertisements CAD criteria: documented CHD (e.g. prior MI, revascularisation procedure, or significant (> 70% stenosis) coronary atherosclerosis) Depression criteria: BDI ≥ 7 Other entry criteria: age 35 years or older Exclusion criteria: presence of another primary mental disorder diagnosis; medical comorbidities that would preclude participation in the trial (e.g. significant musculoskeletal disease, cancer); current psychotherapy; use of antidepressants or other psychotropic medications; history of inability to tolerate or benefit from sertraline; use of dietary supplements or herbal therapies with psychoactive indications; current active alcohol or drug abuse or dependence; active suicidal intent; participation in regular excercise > 1 day/week Treatment N: 40 (63% male, mean age: 63.4 (SD: 10.2)) Control N: 37 (65% male, mean age: 64.7 (SD: 11.0)) Comparability of groups: no baseline differences |
|
| Interventions | Treatment: sertraline once daily, dosage dependent on clinical response, but participants usually started at 50 mg and progressed up to 200 mg, contingent on therapeutic response and the presence of side effects Control 1: placebo Control 2: aerobic exercise (3 classes per week, 16 weeks); ineligible for inclusion in this review Duration of treatment: 4 months |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), platelet biomarkers, ECG waves, pharmacological side effects | |
| Funding | National Heart, Lung, and Blood Institute Other support: sertraline and matching placebo pills were supplied by Pfizer Inc NY |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants assigned to exercise, sertraline, or placepo in a pre‐determined 2:2:1 ratio. Randomization was performed centrally by computer with conditional randomization (stratified by age [35 to 59 vs ≥60], CHD status (prior MI vs. no MI) and depression severity [HAM‐D score > 18 vs. ≤ 18)]; patients were provided with sealed envelopes containing their group assignments" Comment: no |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: treating psychiatrist blinded to pill condition; only research pharmacist was aware of which patients were assigned to sertraline or to placebo Comment: group randomized to exercise were unblinded and not used in this review |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: primary HAMD assessments were performed blinded to treatment allocation Quote: "Outcome assessors were unaware of patients’ treatment assignments" (pg. 1056) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Reasons for drop‐out provided; 1 patient randomized to sertraline and 4 patients randomized to placebo did not complete the study Quote: "unless otherwise indicated, treatment effects were analyzed following the intent‐to‐treat principle..." (pg. 1056) |
| Selective reporting (reporting bias) | Unclear risk | Comment: predetermined endpoints are reported; no protocol paper available Comment: measures of variance (e.g. SD or SE) not reported for biomarkers in paper, only p values for between group comparisons ‐ e.g. active treatment vs. placebo, thereby incorporating the exercise group which were not relevant to this review. Biomarker data obtained from trial repository |
| Other bias | Low risk | Comment: No indication of other bias |
Wang 2020.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 280 Length of follow‐up: 4 weeks after the 8‐week intervention Analysis: per‐protocol (27 dropouts from intervention group, 25 dropouts from control group) |
|
| Participants | Location: China Number of study centres and setting: outpatient clinic and hospitalised patients from the Cangzhou Center Hospital, the Tangshan Hospital of Traditional Chinese Medicine, and the Beijing Huilongguan Hospital CAD criteria: angina pectoris diagnosed according to the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina, and standardisation of diagnosis and treatment of unstable angina pectoris published by the Chinese Medical Association in 2000 Depression criteria: depression was diagnosed according to the Chinese Classification and Diagnostic Criteria of Mental Disorders, Third Edition, and depression symptoms were assessed with the HAM‐D, total score 20 or higher CAD and depression standardised in Guidelines for Diagnosis and Treatment of Common Disease in Traditional Chinese Internal Medicine, First Edition: “Xiong Bi and Xin Tong syndrome”, “palpitation”, “insomnia”, “hysteria”, “depressive psychosis”, “consumptive disease”, and “sweating syndrome” Other entry criteria: age 40 years or older; TCM criteria for "Qi deficiency and blood stasis"; not yet received antidepression drugs, or received antidepression treatment but discontinued for at least 1 month; agreement to participate in the study; without any other acute diseases and severe complications Exclusion criteria: MI or acute or severe heart failure; advanced malignancy; a physical impairment that would prevent participation; cognitive impairment, comorbid major psychiatric disorders, psychosis, a high risk of suicide or current substance abuse; severe arrhythmia such as atrial fibrillation, atrial flutter, high atrioventricular block, sick sinus syndrome, frequent ventricular premature or ventricular tachycardia; history of epilepsy; current use of an antidepressant or anticonvulsant; patient with a HAM‐D score of > 35; physician or patient refusal |
|
| Interventions | Intervention 1: flexible doses of daily escitalopram (Lexapro): 5 mg/d for mild depression HAM‐D 20 to 23, and 10 mg/d for moderate depression HAM‐D > 24 Intervention 2: Bu Xin Qi herbal decoction 400 mL orally (2 times per day); Dang shen Codonopsis pilosula (root) 15 mg/d, Fu lin Poria cocos 15 mg/d, Bai zhu Bighead atractylodes rhizome (rhizome) 15 mg/d, Huang qi Astragalus membranaceus (Fisch) Bge. (root) 12 mg/d, Dang gui Angelica sinensis (root) 20 mg/d, Gui zhi Cassia twig (dried twigs) 10 mg/d, Gan cao Radix liquiritiae (root) 18 mg/d Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: depression symptoms (HAM‐D), cardiac events, pharmacological side effects | |
| Funding | Supported by the Scientific Research Plan Project of Administration of Traditional Chinese Medicine of Hebei Province of China (Project No. 2016117) and Scientific Research Plan Project of Cangzhou City of Hebei Province of China (Project No. 151302047) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomization was carried out by using random‐number tables with a block size of 4. The assignment was carried out at the trial coordination center. |
| Allocation concealment (selection bias) | High risk | Comment: Described as an open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Described as an open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding not described for depression, major adverse cardiac events or biomarkers; described as open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: per‐protocol analysis that excluded patients for non‐compliance, adverse events Comment: reason for drop‐out described ‐ 27 drop‐outs in escitalopram and 25 in Bu Xin Qi decoction |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial protocol paper available |
| Other bias | Unclear risk | Comment: unclear how study drugs were obtained Comment: p values for between group baseline differences in comorbidities were not reported |
WIDeCAD 2017.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 34 Length of follow‐up: no follow‐up Analysis: ITT with multiple imputation (5 dropouts from intervention arm, 3 dropouts from control arm) | |
| Participants | Location: Germany, Switzerland, and Austria Number of study centres and setting: not specified. Recruitment involved medical specialists in clinics, heart institutions and foundations, self‐help and groups for people living with CAD. CAD criteria: self‐reported diagnosis of CAD Depression criteria: PHQ‐9 score ≥ 5 Other entry criteria: age 18 years or older, access to internet, sufficient German language skills Exclusion criteria: acute suicidality; concurrent or lifetime diagnosis of schizophrenia, psychosis or bipolar disorder Treatment: not specified Control: not specified All participants: 34 (35% female, mean age: 56.4 years (SD: 10.2) Comparability of groups: no comparisons performed |
|
| Interventions | Intervention 1: participants received treatment as usual and immediate access to a guided (e‐coach; trained psychologists) internet‐ and mobile‐based cognitive‐behavioural self‐help programme. The programme consisted of 7 lessons, 6 out of 9 optional lessons and 1 booster session after 4 weeks; participants worked on 1 to 2 lessons per week and received feedback after each lesson by e‐coaches (trained psychologists); the main components of the programme included psychoeducation, behavioural activation, problem‐solving, and cognitive restructuring Wait‐list control group: participants received standard care and were given access to the same programme as the intervention group after a waiting time (2 months); participants in the wait‐list control group worked on the programme without guidance |
|
| Outcomes | Review outcomes: depression symptoms (PHQ‐9), quality of life (Assessment of Quality of Life), non‐cardiac adverse events (negative effects of psychotherapy) Secondary outcomes: anxiety symptoms (GAD‐7), satisfaction (Client Satisfaction Questionnaire‐8), adherence (discontinuation rate), fear of disease progression (Fear of Progression Questionnaire) |
|
| Funding | No external financing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was created by an automated web‐based program, sealed envelope (https://www.sealedenvelope.com)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "There was no way that researchers involved in the study could foresee
allocation of individual participants." Quote: "Not otherwise to the study associated staff enrolled and assigned participants (Laura Simmelbauer, LS and Karolin Bauer, KB)." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "There was no way that researchers involved in the study could foresee allocation of individual participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details on outcome assessment blinding; primary outcomes self‐reported. Quote: All surveys were conducted online via the “Unipark” platform (www. unipark.de)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15 of 18 (83.3%) patients randomised to the intervention did not continue the intervention at 8 weeks |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes as stated in methods section. No protocol or design paper available |
| Other bias | High risk | Comment: 53% of eligible participants were excluded after not providing consent Comment: the trial was terminated after recruitment of 34 participants and deemed unfeasible; target sample 122 |
Yang 2019.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel group Total N randomised: 224 Length of follow‐up: 12 months Analysis: ITT with last‐observation‐carried‐forward |
|
| Participants | Location: China Number of study centres and settings: 1 hospital CAD criteria: at least 1 coronary artery having a stenosis greater than the cutoff point of 50% by coronary angiography Depression entry criteria: HADS Depression > 8 and SDS score > 50 Other entry criteria: > 18 years of age; life expectancy > 1 year; available to be followed up regularly Exclusion criteria: treated with antidepressants within 3 months before enrolment; history of other mental disorders (e.g. dementia, schizophrenia, schizotypal affective disorder, delusional disorder, bipolar affective disorder, alienation, schizoid personality disorders, etc.); imminent risk of suicide and risk of suicide attempts; uncontrolled hypertension, cardiac arrhythmia, or unstable angina pectoris; history of severe pulmonary and renal comorbidities, heart failure, tumours, or other life‐threatening diseases; pregnancy or lactation Treatment: 112 (27.7% female, mean age: 61.25 (SD: 8.6)) Control: 112 (28.6% female, mean age: 60.85 (SD: 10.8)) Comparability of groups: no significant baseline differences |
|
| Interventions | Treatment: patients’ intensive telephone‐based care program Control: usual care Duration of treatment: 12 months |
|
| Outcomes | Review outcomes: depression symptoms (HADS Depression), depression remission (HADS Depression), all‐cause mortality | |
| Funding | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Block‐randomization generated in SAS Comment: Randomization was performed by an independent analyzer who was not involved in other parts of the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation was done by a randomized module by a medical and statistical service company (Shanghai Qeejen Bio‐tech Co) Comment: Randomization was performed by an independent analyzer who was not involved in other parts of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants unblinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Nurses who were in charge of the primary depression outcome (HADS‐D) were blinded to allocation Comment: Unclear whether outcome assessor for death and cardiac events were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Reasons for drop‐out described for both groups during treatment phase and longer‐term follow‐up Comment: Analyses were ITT with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results of all main outcomes reported as described in the methods. No protocol available. Comment: there is a discrepancy between the all‐cause mortality reported in the CONSORT flow chart and the survival analyses reported in the results section. No numbers at risk were reported with the Kaplan‐Meier survival plot |
| Other bias | Unclear risk | Comment: no monitoring of intervention quality was reported for study‐trained counsellors |
Zarea 2014.
| Study characteristics | ||
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 74 Length of follow‐up: 2 and 4 months' post‐treatment Analysis: per‐protocol (method of analysis not stated, also no information on participant flow chart) |
|
| Participants | Location: Iran Number of study centres and setting: Al‐Zahra Heart Hospital CAD criteria: all patients who were candidates for coronary artery bypass referred to the research environment on a non‐emergency basis (being in a bypass list) Depression criteria: moderate to severe depression and anxiety scores (HADS) Other entry criteria: no history of mental illness, interested in participating in the study (i.e. the tendency of the patient and their family to participate in the intervention), lack of previous bypass surgery, aged between 35 and 70 years, ability to communicate verbally and ability to speak Persian Exclusion criteria: lack of co‐operation of patients and families during the intervention, failure to perform coronary artery bypass surgery for various reasons, mortality during the study, failure to attend therapeutic communication sessions (absence of 2 or more sessions) Treatment N: 37 (29.7% female, 100% aged 51 to 70 years) Control N: 37 (51.4% female, 100% aged 51 to 70 years) Comparability of groups: not stated. Different gender distribution |
|
| Interventions | Treatment: therapeutic communication sessions were held for the intervention group based on Peplau's model at 4 stages, including orientation, identification, exploitation, and resolution. In total, 7 sessions were held individually with the consent of the participant and their family at the hospital and the participant's home. It should be noted that during therapeutic communication, duration of each session varied given the location and participant's needs. Control: not stated Duration of treatment: not stated. Quote: "...duration of each session was variable..." (p 161), total treatment duration unclear |
|
| Outcomes | Review outcomes: depression symptoms (HADS Depression) Other outcomes: anxiety symptoms (HADS Anxiety) |
|
| Funding | None | |
| Notes | Mixed study sample (patients with depression and/or anxiety were enrolled) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: The subjects were randomly divided into test and control groups using a coin toss |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants and therapists unblinded Comment: Unclear the number and type of staffs involved in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The procedures for obtaining outcome data are unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No information on participant flow or attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trial registry congruent with reported outcomes for depression and anxiety (HADS) Comment: Depression and anxiety (HADS) data reported as unadjusted means and analysis of covariance across all timepoints |
| Other bias | Unclear risk | Comment: No indication of intervention fidelity and monitoring |
ACE: angiotensin‐converting enzyme ACS: acute coronary syndrome BDI: Beck Depression Inventory BMI: body mass index BP: blood pressure CABG: coronary artery bypass graft CAD: coronary artery disease CBT: cognitive‐behavioural therapy CCS: Canadian Cardiovascular Society Angina Class CHD: coronary heart disease CK‐MB: creatine kinase myocardial band CM: clinical management DISH: Depression Interview and Structured Hamilton ECG: electrocardiogram HADS: Hospital Depression and Anxiety Scale HAM‐D: Hamilton Rating Scale for Depression HR: heart rate IPT: interpersonal psychotherapy ITT: intention‐to‐treat LDL: low‐density lipoprotein LVEF: left ventricular ejection fraction MI: myocardial infarction NYHA: New York Heart Association PCI: percutaneous coronary intervention PHQ‐9: Patient Health Questionnaire PTCA: percutaneous transluminal coronary angioplasty RCT: randomised controlled trial SADS: Schedule of Affective Disorders and Schizophrenia SCID: Structured Clinical Interview for Depression SCL 90‐R: Symptom Checklist 90‐Revised SD: standard deviation SSM: supportive stress management SSRI: selective serotonin reuptake inhibitor STAI: State‐Trait Anxiety Inventory TCM: Traditional Chinese Medicine WBT: well‐being therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedimanesh 2017 | Study included participants without comorbid depression. |
| ACHD‐CARE 2015 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| ACTonHEART 2014 | Study included participants without comorbid depression. |
| Beating Heart Problems 2014 | Study included participants without comorbid depression. |
| Black 1998 | Study included participants without comorbid depression. The study investigated psychologically distressed patients (depression was not explicitly assessed). |
| Boese 2013 | Intervention not specifically a psychological or pharmacological treatment for depression. Intervention not delivered by a trained professional ("Peer counselor with CAD trained in attentive listening and sharing experiences"). |
| BraveHeart 2013 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| Bucknall 1988 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| BY.PASS Study 2013 | Study included participants without comorbid depression. |
| CADENCE 2016 | Study investigated collaborative interventions for depression treatment versus usual care, which was not a predefined comparison of this review. |
| Carney 2012 | Study investigated collaborative interventions for depression treatment versus usual care, which was not a predefined comparison of this review. |
| Carney 2019 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| CHAMPS 2016 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| Chang 2020 | Study included participants without comorbid depression. |
| Child 2010 | The psychological intervention was not allocated as part of an RCT. |
| CHIP 2011 | Study included participants without comorbid depression. |
| Chung 2010 | Intervention not specifically a psychological or pharmacological treatment for depression (home‐based deep breathing). |
| CODIACS 2013 | Study investigated patient‐preference, stepped‐care depression treatment versus usual care, which was not a predefined comparison of this review. |
| COINCIDE 2012 | Study investigated collaborative interventions for depression treatment versus usual care, which was not a predefined comparison of this review. |
| COPES 2010 | Study investigated patient‐preference, stepped‐care depression treatment (including no treatment, problem‐solving therapy and/or pharmacotherapy) versus usual care, which was not a predefined comparison of this review. |
| Doering 2013 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| ENHANCED 2016 | Study included participants without comorbid depression. |
| Fu 2006 | Control group unclear (treatment with "Shierkang tablets") |
| Giltay 2011 | Study included participants without comorbid depression. |
| González‐Jaimes 2003 | Study included participants without comorbid depression. The study investigated patients with acute myocardial infarction and adjustment disorder with depressed mood (DSM‐IV: 309.0), but not depression. |
| Haberka 2013 | Study included participants without comorbid depression. |
| Haybar 2018 | Study included participants without comorbid depression. |
| Huffman 2011 | Study investigated collaborative care versus usual care, which was not a predefined comparison of this review. |
| I‐CARE 2018 | Study included participants without comorbid depression. |
| InterHerz 2012 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| Jang 2018 | Intervention not specifically a psychological or pharmacological treatment for depression (mindfulness‐based art therapy). |
| Kachkovskii 2006 | Control group unclear |
| Keeping‐Burke 2013 | Study included participants without comorbid depression. |
| Li 2014 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
| Li 2020 | Study included participants without comorbid depression. |
| Liang 2019 | Study included participants without comorbid depression. |
| Lin 2014 | Study investigated collaborative care versus usual care, which was not a predefined comparison of this review. |
| Lv 2016 | Study compared a combination of psychotherapy and pharmacological interventions (CBT, escitalopram, and alprazolam) versus a combination of pharmacological interventions (escitalopram and alprazolam), which was not a predefined comparator of this review. |
| Ma 2010 | Control group unclear |
| Malik 2002 | The pharmacological intervention was not allocated as part of an RCT. |
| Mazereeuw 2016 | Study included participants without comorbid depression. |
| MindfulHeart 2014 | Study included participants without comorbid depression. |
| Mohapatra 2005 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
| MOSAIC 2013 | Study investigated collaborative care versus enhanced usual care, which was not a predefined comparison of this review. |
| MOTIV‐CABG 2013 | Study included participants without comorbid depression. |
| Nikrahan 2019 | Study included participants without comorbid depression. |
| Norris 2009 | Intervention not specifically a psychological or pharmacological treatment for depression. The study investigated the effectiveness of providing follow‐up information regarding mental health services to depressed patients after cardiac catheterisation. |
| Oldridge 1991 | Intervention not specifically a psychological or pharmacological treatment for depression (cardiac rehabilitation). |
| Oranta 2010 | Study included participants without comorbid depression. |
| O’Doherty 2015 | Not a randomised trial; allocation to intervention or wait‐list control group was not by randomisation |
| Park 2013 | Study included participants without comorbid depression. |
| PATHWAY Group MCT | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| PATHWAY Home MCT | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| Pogosova 2004 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
| Pogosova 2009 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
| Rakowska 2015 | Study included participants without comorbid depression. |
| Rollman 2009 | Study investigated telephone‐delivered collaborative care versus usual care, which was not a predefined comparison of this review. |
| Schneider 2020 | Study included participants without comorbid depression. Quote: "The inclusion of people below clinical thresholds of depression and/or general anxiety may have weakened the results and limited generalizability among clinical settings" |
| Schrader 2005 | Study investigated the effectiveness of different forms of communication between hospital psychiatric services and general practitioners of depressed cardiac patients; sample of heart disease patients not restricted to CAD. |
| Sogolitappeh 2009 | The psychological intervention was not allocated as part of an RCT. |
| Soucy 2019 | Study investigated behavioural activation for depression versus physical activity, which was not a predefined comparison of this review. |
| STEP‐IN‐AMI‐2013 | Study included participants without comorbid depression. |
| Stern 1983 | Study compared counselling versus exercise therapy, which was not a predefined comparison of this review. |
| Strokova 2012 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
| SU.FOL.OM3 2012 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| SUPRIM 2011 | Study included participants without comorbid depression. |
| TAKE Heart 2010 | Intervention not specifically a psychological or pharmacological treatment for depression. |
| TEAMcare 2010 | Study investigated a collaborative intervention for depression versus usual care, which was not a predefined comparison of this review. |
| Tsai 2012 | Study included participants without comorbid depression. |
| UPBEAT‐UK 2014 | Intervention consisted of collaborative care (case management, information provision and referral to other health professionals via the Improving Access to Psychological Therapy programme, and behaviour change techniques), which was not a predefined comparison of this review. |
| Vasiuk 2010 | The pharmacological intervention was not allocated as part of an RCT. |
| Veith 1982 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| WELL.ME 2012 | Study investigated a sample of heart disease patients that was not restricted to CAD. |
| Zeng 2001 | Study compared pharmacological treatment versus usual care, which was not a predefined comparison of this review. |
CAD: coronary artery disease CBT: cognitive‐behavioural therapy DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Ahangarezaiezadeh 2017.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 60 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: heart clinic in Takab city CAD criteria: diagnosis of coronary heart disease by a physician cardiologist Depression criteria: unclear. Depression Anxiety and Stress Scales‐21 used to compare groups pre and post intervention. Other entry criteria: 20 to 65 years of age, provides written informed consent Exclusion criteria: concurrent participation in educational programmes or other treatment; antianxiety or antidepression medication and tranquilisers; drug abuse; other mental disorder; chronic disease (progressive neurologic diseases such as Parkinson's, Alzheimer's, multiple sclerosis, cancer, diabetes); undergoing open‐heart surgery |
| Interventions | Intervention: positive‐thinking training delivered as group therapy in 8 sessions (90 min each) over 8 weeks Control: not specified |
| Outcomes | Depression, anxiety and stress, not further specified |
| Notes | http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2016022026347n2 |
Cai 2012.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 126 Length of follow‐up: 4 weeks of intervention with no follow‐up Analysis: unclear (method of analysis not explicitly stated; dropouts unclear) |
| Participants | Location: China Number of study centres and setting: unclear CAD criteria: unstable angina, not further specified Depression criteria: unclear. Clinical Global Impression used to quantify change. Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 66 (no demographics reported) Control N: 60 (no demographics reported) Comparability of groups: no comparisons reported |
| Interventions | Treatment: flupentixol melitracen (trade name Deanxit), 1 or 2 pieces for 4 weeks Control: unclear Duration of treatment: 4 weeks |
| Outcomes | Cinical global improvement in depression and anxiety, incidence of angina pectoris and malignant cardiovascular events |
| Notes | Conference abstract only. Department of Cardiology, Affiliated Hospital of Gannan Medical College, JiangXi, GanZhou, China |
CoroDep 2019.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 150 Length of follow‐up: length of intervention unclear, follow‐up 6 months after PCI Analysis: unclear (method of analysis not explicitly stated; dropouts unclear) |
| Participants | Location: Croatia Number of study centres and setting: unclear, affiliated with Klinički Bolnički Centar Zagreb CAD criteria: percutaneous coronary intervention due to angina pectoris or myocardial infarction Depression criteria: unclear Other entry criteria: 18 to 70 years old, without antidepressant drugs or major tranquilisers more than 1 year Exclusion criteria: symptoms of myocardial infarction lasting more than 12 hours, left ventricular ejection fraction less than 40%, earlier presence of cardiomyopathy, acute infection Treatment N: not specified (no demographics reported) Control N: not specified (no demographics reported) Comparability of groups: no comparisons reported |
| Interventions | Treatment 1: sertraline, from 50 to 200 mg/day Treatment 2: escitalopram, from 10 to 20 mg/day Duration of treatment: not specified |
| Outcomes | Montgomery Asberg Depression Scale, Hamilton Rating Scale for Depression, Beck Depression Inventory, EuroQol, other outcomes not relevant to this review (GRACE score, Duke Activity Index, Seattle Angina Questionnaire, brain‐derived neurotrophic factor) |
| Notes | Described as an unmasked trial and that the two interventions will be reported as a single group |
Gu 2017.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 146 Length of follow‐up: no follow‐up Analysis: unclear (method of analysis not explicitly stated; dropouts unclear) |
| Participants | Location: China Number of study centres and setting: unclear CAD criteria: stable ischaemic heart disease and exercise‐induced myocardial ischaemia Depression criteria: unclear. General distress and BDI‐II measured Other entry criteria: unclear Exclusion criteria: unclear Treatment N: sample size unclear (no demographics reported) Control N: sample size unclear (no demographics reported) Comparability of groups: no comparisons reported |
| Interventions | Treatment: mindfulness‐based stress reduction programme for 2.5 hours 2 times per week Control: usual care Duration of treatment: 12 weeks |
| Outcomes | Depression measured by the BDI‐II, ECG quantified wall motion abnormalities and HRV |
| Notes | Clinical College, Hainan Medical University Affiliated Hospital, Hainan, China |
BDI: Beck Depression Inventory
CAD: coronary artery disease
ECG: electrocardiogram
PCI: percutaneous coronary intervention
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ahmadi 2018.
| Study name | Effectiveness of Rumination‐Focused Cognitive‐Behavioral Therapy on improvement depression and anxiety in patients with coronary heart disease |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 32 Length of follow‐up: 8 weeks Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: not stated, affiliated with Taleghani Educational Hospital, Tehran, Iran CAD criteria: coronary heart disease Depression criteria: major depressive disorder, single episode, moderate, criteria not further specified Other entry criteria: 20 to 80 years of age, ability to read and write and do homework treatment Exclusion criteria: drug addiction, severe mental disorder, psychiatric medication, attending other psychotherapy |
| Interventions | Intervention group: CBT based on rumination. This treatment was introduced by Edward Watkins and includes 10 sessions of 45 minutes. Duration of treatment: 8 weeks Control group: no treatment other than conventional treatments |
| Outcomes | Depression: at the beginning of the intervention, 8 weeks after the intervention, quantified by the BDI‐II |
| Starting date | 22 June 2018 |
| Contact information | Dr Abbas MasjediArani Taleghani Educational Hospital, Tabnak St Velenjak Region, Chamran High Way, Tehran, Iran 1985711151 Tehran Iran (Islamic Republic of) T: +98 912 575 2870 E: doctormasjedi@yahoo.com Affiliation: Shahid Beheshti University of Medical Sciences Seyed Mojtaba Ahmadi Taleghani Educational Hospital, Tabnak St Velenjak Region, Chamran High Way, Tehran, Iran 1985711151 Tehran Iran (Islamic Republic of) T: +98 930 640 7071 E: mojtabakmahmadi@yahoo.com Affiliation: Shahid Beheshti University of Medical Sciences |
| Notes |
Ardakani 2020.
| Study name | The study of of escitalopram ʼs effectiveness on treatment of mild to moderate depressive disorder and improvement of quality of life in patients who are undergone coronary artery bypass graft surgery (CABG): a randomized double blind placebo controlled trial |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 41 Length of follow‐up: end of treatment, 8 weeks Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: 1, Tehran Heart Center Hospital, Tehran, Iran CAD criteria: undergone coronary artery bypass graft surgery Depression criteria: BDI scores between 10 and 20, confirmed through clinical interview by a psychiatrist (ICD F32.8) Other entry criteria: 18 to 75 years of age Exclusion criteria: previous history of intolerance to SSRI, psychosis or dementia or cognitive impairment, severe liver disease, high risk of postoperative cardiac complications such as bleeding, participating in other trials, history of bipolar disorder, patients treated with escitalopram or another antidepressant during the previous month, recent alcohol and substance abuse, pregnancy and lactation |
| Interventions | Intervention: escitalopram 10 mg/d Control: placebo, not further specified Duration of treatment: 8 weeks |
| Outcomes | Primary: change in depressive symptoms as a result of intervention effects on BDI‐II, quality of life quantified with the 36‐Item Short Form Health Survey Secondary: checklist of drug side effects |
| Starting date | 22 November 2020 |
| Contact information | Dr Mohammad Reza khodaie Ardakani Razi Psychiatric Hospital, Shahre Rey, Tehran, Iran T: +98 21 3340 1604 E: kh.ardakani@uswr.ac.ir |
| Notes |
COMBAT‐DS 2021.
| Study name | Online cognitive behavioral therapy for depressive symptoms in rural patients with cardiac disease (COMBAT‐DS) |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 500 (also states 150 per group; stratified by sex) Length of follow‐up: 12 months from baseline Analysis: not specified |
| Participants | Location: USA Number of study centres and setting: not specified, affiliated with University of Kentucky, University of California, Patient‐Centered Outcomes Research Institute CAD criteria: physician‐documented acute coronary syndrome event Depression criteria: at least moderate depressive symptoms (PHQ‐9 ≥ 10) Other entry criteria: rural dwelling Exclusion criteria: cognitive impairment, major psychiatric comorbidities that might require additional treatment, presence of non‐CHD conditions likely to be fatal within next year |
| Interventions | Intervention 1: video‐conferenced CBT (vcCBT) consisting of 8 face‐to‐face video‐conferencing sessions via tablet computers lasting approximately 45 minutes each Intervention 2: iCBT is self‐directed cognitive‐behavioural therapy using an interactive internet programme, MoodGYM, which does not include direct interactions with a therapist Duration of treatment: 3 months |
| Outcomes | Primary: change in depressive symptoms as a result of intervention effects on PHQ‐9 Seconday: all‐cause hospitalisation rates |
| Starting date | 3 August 2021 |
| Contact information | Debra K Moser PhD, RN Professor and Linda C Gill Endowed Chair of Nursing University of Kentucky, Lexington, Kentucky, United States T: 859‐323‐6687 E: dmoser@uky.edu Misook Chung University of Kentucky, Lexington, Kentucky, United States T: 8593236687 E: misook.chung@uky.edu Rana Rahman, PhD, RN University of Kentucky, Lexington, Kentucky, United States T: 8593236656 E: rana.lindsay‐rahman@uky.edu |
| Notes |
eMindYourHeart 2021.
| Study name | eMindYourHeart |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 188 Length of follow‐up: 3 and 9 months after end of treatment or usual care Analysis: "modified" ITT |
| Participants | Location: Denmark Number of study centres and setting: cardiac rehabilitation settings at hospitals and municipalities delivering cardiac rehabilitation across 5 regions in Denmark CAD criteria: diagnosis of ischaemic heart disease (ICD codes I20 to I25) Depression criteria: score ≥ 8 on depression or anxiety or both scales of the HADS Other entry criteria: ≥ 18 years of age, access to a computer or smartphone, ability to use a computer or smartphone, and proficient in the Danish language Exclusion criteria: severe psychiatric disorders (i.e. borderline personality disorder, schizophrenia, bipolar disorder), severe cognitive difficulties (e.g. severe brain damage, mental retardation, or dementia) that would prevent patients from participating, endorsement of suicidal ideation with daily suicidal thoughts (PHQ‐9 item 9 > 2), participating in other intervention studies (unless they are clinical studies (e.g. medication trials)), or seeing a psychologist or mental health professional for the treatment of depression and anxiety |
| Interventions | Intervention: between 10 and 12 sessions of CBT and includes aspects of acceptance and commitment therapy and compassion‐focused therapy (i.e. an introductory module, 9 core treatment modules, 2 optional modules related to sleep and lifestyle changes) with telephone support from a psychologist Control group: no treatment other than usual care (cardiac rehabilitation) Duration of treatment: 3 months |
| Outcomes | Primary: symptoms of depression, measured with HADS at the end of the intervention (i.e. 3 months) Secondary: symptoms of anxiety measured with HADS at 3 months; symptoms of depression and anxiety at 6 and 12 months’ follow‐up; quality of life (HeartQoL) at 3, 6, and 12 months' follow‐up; trial dropout (number of participants who dropped out in either arm at 3 months); and cost‐effectiveness/cost‐utility Other measures reported in protocol: Cardiac Anxiety Questionnaire (CAQ‐18), PHQ‐9, UCLA Loneliness Scale, Multidimensional Scale of Perceived Social Support (MSPSS), Brief Illness Perceptions Questionnaire (B‐IPQ), quality of life (HeartQoL), Perceived Stress Scale, Health Complaints Scale, physical activity, patient engagement in cardiac rehabilitation, Negative Effects Questionnaire |
| Starting date | 1 June 2020 |
| Contact information | Susanne S Pedersen, PhD T: +4565507992 E: sspedersen@health.sdu.dk Affiliation: Department of Psychology, University of Southern Denmark, Campusvej 55, DK‑5230 Odense M, Denmark |
| Notes |
Firouzjaei 2017.
| Study name | Cognitive‐behavioral therapy for cardiovascular disease |
| Methods | RCT design: 2‐arm parallel‐group trial. Study described as randomised controlled design comparing a problem‐solving therapy with usual care group (control group); also stated as "not randomized". Unclear group allocation Total N randomised: 24 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: single centre, Shahid Modarres Hospital, Tehran CAD criteria: ischaemic heart disease, ICD‐10 codes I20 to I25 Depression criteria: meeting the diagnostic criteria for depression due to other medical condition according to DSM‐5 and a minimum score of 10 on the BDI‐II Other entry criteria: sufficient knowledge of the Persian language, age under 70 years, minimum diploma education, minimum score of 26 on the Dysfunctional Attitudes Scale‐26 Exclusion criteria: already receiving psychotherapy for mental health problems, taking psychiatric drugs during the last 6 months, the presence of severe depressive symptoms (indicated by a score above 40 on the items of the BDI‐II) |
| Interventions | Intervention: problem‐solving therapy based on Robert Leahy protocol. No further details provided. Usual care: 2 educational sessions on cardiovascular disease and a depression brochure. Educational sessions under the supervision of cardiologist included: 1) medical education about heart disease and CABG surgery; 2) general education about health care after heart bypass surgery; 3) having a good diet programme; 4) having a daily walking programme Duration of treatment: 50 days |
| Outcomes | Depression measured by the BDI‐II Dysfunctional attitudes measured by the Dysfunctional Attitudes Scale‐26 Social support measured by the Multidimensional Scale of Perceived Social Support All outcomes assessed pre‐ and postintervention. |
| Starting date | 13 August 2017 |
| Contact information | Nima Hajitabar Firouzjaei Shahid Beheshti University of Medical Science Teharan ‐ Evin ‐ Student bouleward‐ Arabi street‐ Medical College, Shahid Beheshti University of Medical Science Tehran T: +98 21 6670 5780 E: nimahajitabar@yahoo.com |
| Notes |
Geng 2018.
| Study name | Effect of guanxin danshen dropping pill on clinical anxiety, depression, heart rate variability and cardiovascular prognosis in patients with coronary heart disease after PCI combined with anxiety or depression |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: China Number of study centres and setting: Guangdong General Hospital CAD criteria: CHD, any stable angina pectoris, unstable angina pectoris, non‐ST‐elevation myocardial infarction, acute ST‐segment elevation myocardial infarction, PCI patients (completed or required) Depression criteria: patients who scored between 5 to 14 points on the PHQ‐9, or score of between 5 and 14 points on the Generalized Anxiety Disorder‐7 Other entry criteria: voluntarily participate in the trial, signed informed consent and ability to understand and voluntarily sign informed consent, aged between 18 and 75 years, can co‐operate with the treatment Exclusion criteria: cachexia state; or combined lung, liver, kidney, haematopoietic system, immune system, and other serious primary disease and dysfunction, angina pectoris not relieved or NYHA cardiac function grade IV, recurrent anxiety, depression patients, electrolyte imbalance acid‐base imbalance, history of epilepsy, or patients with organic mental disorders, other mental illness such as: bipolar affective disorder, schizophrenia, and other patients, alcohol and drug abuse and addiction within 1 year, use of other antidepressant and anxiolytic drugs within 4 weeks before assessment, pregnant or lactating women, or planned pregnancy, had previous coronary revascularisation, participated in other drug clinical trials within 3 months, researchers believe that patient is not suitable for clinical trials, patients with cognitive impairment, patients with suicidal tendencies |
| Interventions | Guanxin danshen drop pills. No further information available. Duration of treatment: not specified |
| Outcomes | Symptoms of anxiety and depression Heart rate variability during cardiopulmonary exercise Major adverse cardiovascular events (MACE) |
| Starting date | 1 August 2017 |
| Contact information | Qingshan Geng 106 Second Zhongshan Road, Guangzhou, Guangdong, China T: +86 13922205818 E: mahuannovel@163.com |
| Notes | http://www.chictr.org.cn/showproj.aspx?proj=20507 |
Hamzehpour 2020.
| Study name | Treatment of depressive symptoms in patients after coronary artery bypass graft surgery |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 100 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: 1 Ayatollah Rouhani Hospital, Iran CAD criteria: undergoing coronary artery bypass graft surgery Depression criteria: unclear. Depression measured with BDI. ICD code stated in health conditions studied (F32.0) Major depressive disorder, single episode, mild Other entry criteria: willingness to participate in the study Exclusion criteria: cognitive impairment, history of substance use, bipolar disorder |
| Interventions | Intervention 1: bupropion 75 mg/d increased to 150 mg/d Intervention 2: unclear from text. Likely citalopram or escitalopram 5 mg/d increased to 20 mg/d Duration of treatment: 8 weeks |
| Outcomes | Primary: BDI measured at baseline and end of therapy; no other outcomes specified |
| Starting date | 25 July 2020 |
| Contact information | Romina Hamzehpour Ayatollah Rouhani Hospital, Daneshgah Square, Ganjafrooz Avenue T: +98 11 3233 8301 E: r.hamzehpour@mubabol.ac.ir |
| Notes |
Irfan 2020.
| Study name | The effect of culturally adapted CBT‐based guided self help in depressed patients with myocardial infarction |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 140 Length of follow‐up: 8 weeks Analysis: ITT with last‐observation‐carried‐forward |
| Participants | Location: Pakistan Number of study centres and setting: Punjab Institute of Cardiology, Lahore CAD criteria: myocardial infarction Depression criteria: score of 8 or more on HADS and fulfilling criteria of Major Depressive Disorder using DSM‐5 Other entry criteria: 18 to 65 years of age Exclusion criteria: patients using alcohol or drugs, significant cognitive impairment (intellectual disability or dementia), active psychosis, patients who have received CBT during the previous 12 months |
| Interventions | Intervention: culturally adapted cognitive‐behavioural therapy (CaCBT)‐based guided self‐help (using the book Khushi Aur Khatoon) Usual care: not specified Duration of treatment: 8 weeks |
| Outcomes | Change in HADS Depression Subscale score from baseline to end of therapy Change in World Health Organization Disability Assessment Schedule (WHODAS 2.0) score from baseline to end of therapy |
| Starting date | 22 October 2019 |
| Contact information | Muhammad Irfan, Professor of Psychiatry and Director Research, Peshawar Medical College Farooq Naeem, PhD, University of Toronto |
| Notes |
Jazayeri 2017.
| Study name | Effect of hesperidin supplementation on depressive symptoms, serum levels of BDNF and cortisol in patients after myocardial infarction |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 70 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: not specified, affiliated with Iran University of Medical Sciences, Tehran CAD criteria: myocardial infarction in past 6 weeks Depression criteria: BDI score of 10 or higher Other entry criteria: willingness to co‐operate and signed informed consent, no use of supplements during the last 3 months (including omega‐3, Q10, antioxidants, and vitamins), absence of any allergies (gastrointestinal and skin) to any type of antioxidant supplements and vitamins, age 30 to 70 years, do not use more than 1 blood orange or more than 2 other citrus fruits a day, body mass index between 25 to 40 Exclusion criteria: major psychiatric disorder history, treatment of depression within past 12 months, consumes more than 500 mL per day of flavonoid‐rich beverages include tea, coffee, or citrus juice, major cognitive disorders or impaired cognitive function, lack of follow‐up after discharge, tobacco smoking, uncontrolled metabolic disease, pregnancy and lactation, use of heparin or warfarin, uncontrolled chronic diseases (hepatic failure, renal failure, diabetes, etc.), digestive disorders, compliance below 80% |
| Interventions | Intervention 1: hesperidin capsules 200 mg/d Control: placebo containing starch Duration of treatment: 3 months |
| Outcomes | Primary: depression measured by the BDI Secondary: serum levels of BDNF and cortisol |
| Starting date | 5 May 2017 |
| Contact information | Dr Shima Jazayeri Vice chancellor for research, Iran University of Medical Sciences, Besides Milad Tower, Hemmat, Tehran, Iran (Islamic Republic of) T: +98 21 8670 4805 E: Jazayeri.sh@iums.ac.ir; sh_jaz@yahoo.com |
| Notes |
Luberto 2021.
| Study name | MBCT via group videoconferencing for acute coronary syndrome patients with depressive symptoms: a pilot RCT |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 50 Length of follow‐up: 3 months Analysis: not specified |
| Participants | Location: USA Number of study centres and setting: Department of Psychiatry, Massachusetts General Hospital CAD criteria: acute coronary syndrome specified by medical records or patient confirmation, or both Depression criteria: current elevated depression symptoms (PHQ‐9 greater than or equal to 5) Other entry criteria: 35 to 85 years of age, access to high‐speed internet Exclusion criteria: active suicidal ideation or past‐year psychiatric hospitalisation (per patient report or medical record review, or both); non‐English‐speaking; cognitive impairments preventing informed consent per medical record review and/or cognitive screen less than or equal to 4; patient deemed to be unable to complete the study protocol or has a condition that would likely interfere with the study |
| Interventions | Intervention 1: 8 virtually delivered MBCT sessions (approximately 1.5 hours each) to regulate distress and choose healthy behaviours, as well as learn about cardiac health Intervention 2: attention‐matched control comprising 8 weekly virtual group sessions that focus on cardiac health and depression education Duration of treatment: 8 weeks |
| Outcomes | Depression measured by the HADS and PHQ‐9 Quality of life measured by PROMIS‐Physical Function and 1 item of 12‐Item Short Form Health Survey Inflammatory biomarkers, interleukin 6, tumour necrosis factor‐α, and C‐reactive protein measured by whole dried blood spot sample collection All outcomes measured 1 week before and after the intervention and at 3 months postintervention. |
| Starting date | April 2021 |
| Contact information | Christina Luberto, Assistant Professor, Department of Psychiatry, Harvard Medical School; Massachusetts General Hospital T: 617‐643‐9453 E: cluberto@mgh.harvard.edu Elyse Park, PhD, MPH T: 617‐724‐6836 E: epark@mgh.harvard.edu |
| Notes |
Ma 2014.
| Study name | The therapeutic effect of statins on patients with depression after acute coronary syndrome |
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 180 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: China Number of study centres and setting: Tongji Hospital affiliated to Tongji University, Shanghai CAD criteria: diagnostic criteria of ACS based on guidelines, including ST elevation myocardial infarction, non‐ST elevation myocardial infarction, and unstable angina Depression criteria: based on PHQ, not further specified Other entry criteria: between 30 and 90 years of age Exclusion criteria: previous history of mental illness, suicidal tendencies, severe hepatic and renal dysfunction, anxiety state (HADS Anxiety score more than 7), left ventricular ejection fraction less than 0.45, haemodynamic instability, LDL‐c more than 3.37 mmol/L, allergic to statins or sertraline |
| Interventions | Intervention 1: rosavastatin calcium 20 mg/d Intervention 2: sertraline 25 to 50 mg/d Intervention 3: rosavastatin calcium 5 mg/d Duration of treatment: not specified |
| Outcomes | Not specified |
| Starting date | 14 December 2014 |
| Contact information | Wenlin Ma
NO.389 Xincun Rd, Putuo district, Shanghai, China T: +86 18621892703 E: mawenlin@medmail.com.cn |
| Notes |
Mohammadian 2018.
| Study name | The efficacy of cognitive‐behavioral therapy based on rumination on depression, anxiety and hostility in cardiac patients |
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 48 Length of follow‐up: 2 months Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: not stated, affiliated with Shahid Beheshti University of Medical Sciences CAD criteria: open‐heart surgery for coronary heart disease Depression criteria: a mild or moderate score on the depression questionnaire (PHQ‐9 total score greater than 5) or anxiety questionnaire (Beck Anxiety Inventory total score greater than 8) or aggression questionnaire (Buss‐Perry Aggression Questionnaire total score greater than 40) Other entry criteria: 40 to 70 years of age, ability to participate in the rehabilitation programme and doing assignments with a diagnosis of cardiologist and rehabilitation specialist, reading and writing skills, no other physical illness that limits attendance at meetings, no change in psychiatric medications from 1 month before intervention, no history of receiving psychotherapy before intervention Exclusion criteria: cognitive disorders such as Alzheimer's and dementia, severe mental disorder (personality disorders, psychotic disorders, and dissociative disorders), thyroid problems |
| Interventions | Intervention 1 (intervention group): 12 sessions of CBT based on rumination devised to treat depression for 1 session per week Intervention 2 (rehabilitation group): 40 sessions of rehabilitation, with 3 sessions per week. Intervention 3 (control group): this group will be selected from the waiting list and will not receive any intervention Duration of treatment: 12 weeks in Intervention 1; 13 weeks in Intervention 2 |
| Outcomes | Depression pre‐ and postintervention quantified by the BDI. Unclear if PHQ‐9 is captured at end of treatment |
| Starting date | 15 July 2018 |
| Contact information | Rasoul Mohammadian 10th Alley, Resalat street 5981944451 Shahindezh Iran (Islamic Republic of) T: +98 44 4632 1250 E: rasoul.2828@gmail.com Shahid Beheshti University of Medical Sciences |
| Notes |
Moudi 2016.
| Study name | The efficacy of citalopram in depression and quality of life in the patients with cardiac disease |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 40 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: 2 cardiac care units affiliated to Babol University of Medical Sciences, Babol CAD criteria: admission in cardiac care units because of acute coronary syndrome, including acute myocardial infarction or unstable angina, ICD‐10 code I121 Depression criteria: not specified Other entry criteria: age more than 18 years, informed consent Exclusion criteria: past history of intolerance to SSRIs, severe and life‐threatening medical conditions that inhibit participation of the patient in the duration of the study, severe congestive heart failure with diagnosis of the cardiologist, dependence on alcohol or other drugs, psychosis or dementia or intellectual disability, pregnancy, lactation, or planned pregnancy during study, history of elevated mood |
| Interventions | Intervention: citalopram 20 mg/d Control: weekly psychiatric visit without any antidepressant drug Duration of treatment: 8 weeks |
| Outcomes | Depression measured by the HADS Quality of life measured by the 36‐Item Short Form Health Survey Quality of Life Questionnaire |
| Starting date | |
| Contact information | Sussan Moudi Department of Psychiatry, Shahid Yahyanejad Hospital Babol, Iran T: +98 11323268823 E: sussan.mouodi@gmail.com |
| Notes |
Qiaoning 2019.
| Study name | Effect of guanxindanshen dropping pills on quality of life and cardiovascular prognosis of patients with depression or anxiety after PCI for coronary heart disease |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 200 Length of follow‐up: not specified Analysis: not specified |
| Participants | Location: China Number of study centres and setting: 6 hospital recruitment sites: Xiyuan Hospital, CACMS (Beijing); Shanghai Tongji Hospital (Shanghai); Fuwai Hospital, CAMS & PUMC (Beijing); Guangdong Provincial People's Hospital (Guangzhou); Beijing First Hospital of Integrated Chinese and Western Medicine (Beijing); The Affiliated Hospital of Changchun University of TCM (Changchun) CAD criteria: diagnostic criteria of CHD (not specified) and complete PCI procedure Depression criteria: PHQ‐9 score 5 to 14 or Generalized Anxiety Disorder‐7 Scale score 5 to 14, or both Other entry criteria: voluntary participation and informed consent, age 18 to 75 years, able to co‐operate with examination and treatment Exclusion criteria:
|
| Interventions | Intervention: guanxin danshen dropping pills, therapeutic dose or intake not specified Control: placebo, unclear |
| Outcomes | Primary: depression measured by the PHQ‐9, major adverse cardiac events, angina measured by the Seattle Angina Questionnaire Secondary: quality of life measured with the 12‐Item Short Form Health Survey, blood pressure, vital signs, microcirculation and inflammatory‐related indicators, adverse events |
| Starting date | 1 March 2017 |
| Contact information | Yang Qiaoning Xiyuan Hospital, CACMS 1 Xiyuan Caochang, Haidian District, Beijing, China T: +86 15101072110 E: 15101072110@126.com |
| Notes |
Sourizahi 2017.
| Study name | Comparison of therapeutic effect of sertraline and supportive psychotherapy in comparison with placebo in coronary heart disease patients with mild to moderate depression |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 90 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: Iran Number of study centres and setting: not specified. Investigators are affiliated with Zahedan University of Medical Sciences. CAD criteria: coronary heart disease, not further specified Depression criteria: patients mild or moderate depression who fulfill ICD criteria for any: F32 Depressive episode, F32.0 Mild depressive episode, or F32.1 Moderate depressive episode Other entry criteria: no serious medical illness, no use of psychiatric drugs Exclusion criteria: heart medications affecting the mood |
| Interventions | Intervention: sertraline 50 mg/d with weekly dose 150 to 200 mg per week, plus supportive supportive psychotherapy consisting of: discussion about depression in response to the diagnosis of heart disease, controlled diaphragmatic breathing and gradual muscle relaxation training, exposure and cognitive therapy, behavioural activation, pleasant activity scheduling Control: sertraline placebo (vitamin C) |
| Outcomes | Depression level measured by the HAM‐D |
| Starting date | 22 December 2017 |
| Contact information | Mohammadislam Sourizahi Zahedan University of Medical Sciences Zahedan, Dr Hesabi square 9816743463 Zahedan Iran (Islamic Republic of) T: +98 54 3329 5715 E: dr.sourizahi58@zaums.ac.ir |
| Notes |
Wang 2015.
| Study name | Intervention study of the patients with mild depressive mood after percutaneous coronary intervention in the treatment of the patients with collateral vessels |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: sample size and allocation ratio not specified Length of follow‐up: not specified Analysis: not specified |
| Participants | Location: China Number of study centres and setting: not specified, affiliated with Dongzhimen Hospital CAD criteria: previous PCI surgery for coronary heart disease Depression criteria: accordance with the diagnostic criteria of TCM syndrome differentiation of depression, accordance with the diagnostic criteria of depressive episode of Western medicine; depression symptoms defined by score of 8 to 35 on the HAM‐D Other entry criteria: stable vital signs, the consciousness is clear, has certain expression ability, aged 18 to 80 years old, patient is willing to try and co‐operate, provides informed consent Exclusion criteria: suicide risk, language difficulties, disturbance of consciousness, dementia, aphasia, deafness, agnosia influence emotional expression and not according to the prescribed medication, patients with severe or unstable heart, liver, kidney, endocrine, thyroid dysfunction, blood, and other diseases in the department of internal medicine; history of epilepsy, cerebral trauma; alcohol and drug dependence in the past year; depressive episode secondary to other mental illness or physical disease; serious psychiatric symptoms, such as hallucinations, paranoia, and other symptoms; pregnant women or likely to become pregnant during the trial; history of allergy to paroxetine hydrochloride; participation in other clinical trials of drugs; cannot take medication according to doctor's advice |
| Interventions | Intervention: paroxetine hydrochloride, not further specified Control: placebo, not further specified |
| Outcomes | Primary: 5‐HT content, HAM‐D scores Secondary: Seattle Angina Questionnaire |
| Starting date | 1 January 1990 |
| Contact information | Wang Zhen 11 North 3rd Ring Road, Chaoyang District, Beijing, China Dongzhimen Hospital T: +86 13001240944 E: 1282174720@qq.com |
| Notes |
Yang 2020.
| Study name | The efficacy and safety of Ginkgo biloba dropping pills in the treatment of coronary heart disease with stable angina pectoris and depression |
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 72 Length of follow‐up: no follow‐up Analysis: not specified |
| Participants | Location: China Number of study centres and setting: not specified, affiliated with Foshan Chancheng Central Hospital (Guangdong), The First Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong), Luohu District People's Hospital (Guangdong), and The Second Affiliated Hospital of Guizhou University of Chinese Medicine (Guizhou) CAD criteria: coronary heart disease and at least 1 of (history of myocardial infarction; coronary artery revascularisation; coronary radiography or coronary angiography with at least 1 coronary artery stenosis and lumen stenosis ≥ 50%; or cardiac magnetic resonance imaging or radionuclide myocardial perfusion imaging or cardiac color Doppler diagnosis coronary heart disease with myocardial ischaemia); stable angina pectoris treated for at least 4 weeks Depression criteria: diagnostic criteria for depressive episode in the ICD‐10 (World Health Organization) Other entry criteria: 18 to 75 years of age, informed consent, not used any food that has an impact on intestinal flora such as foods containing probiotics (e.g. yogurt) or drugs (e.g. antibiotics) in the past 7 days Exclusion criteria: acute myocardial events, unstable angina pectoris, severe heart failure; serious arrhythmia; severe or poorly controlled hypertension (systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg); sitting blood pressure and systolic blood pressure ≤ 85 mmHg or symptomatic hypotension, severe primary diseases such as liver, kidney, and haematopoietic system, or serious diseases affecting patient's survival (such as tumour, etc.); serious suicidal tendency (HAM‐D item 3 ≥ 3); bipolar disorder depressive episode in patients with epilepsy history, or depression secondary to other mental or physical diseases; alcohol and drug dependence within 1 year; abnormal liver and kidney function (ALT and/or AST > 3 times of the upper normal limit, and/or CRE > 2 times of the upper normal limit); patients who are currently taking antianxiety drugs; pregnant women, lactating women, women of child‐bearing age who do not take effective contraceptive measures, or who plan to conceive during the trial, and whose pregnancy test results are positive before the test; those who have participated in clinical trials of other new drugs within 30 days before screening; other reasons unsuitable to participate (researcher determined); allergic to the ingredients contained in Ginkgo biloba dropping pills |
| Interventions | Intervention: sertraline 50 mg/d with weekly dose 150 to 200 mg per week Ginkgo biloba dropping 63 mg x 5 pills, x 3 per day (total 945 mg/d) Control: matching placebo, not further specified |
| Outcomes | Primary:
Secondary:
|
| Starting date | 20 September 2020 |
| Contact information | Zhongqi Yang T: 0086‐020‐36591222 E: Yang_zhongqi@163.com |
| Notes |
BDI: Beck Depression Inventory BDNF: brain‐derived neurotrophic factor CABG: coronary artery bypass graft CAD: coronary artery disease CBT: cognitive‐behavioural therapy CHD: coronary heart disease DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition HADS: Hospital Anxiety and Depression Scale HAM‐D: Hamilton Depression Rating Scale ICD: International Classification of Diseases ITT: intention to treat NYHA: New York Heart Association PCI: percutaneous coronary intervention PHQ‐9: Patient Health Questionnaire PROMIS: Patient‐Reported Outcomes Measurement Information System RCT: randomised controlled trial SSRI: selective serotonin reuptake inhibitor TCM: Traditional Chinese Medicine
Differences between protocol and review
We omitted subgroup analyses due to the small numbers of trials investigating the various outcomes. For the same reason we did not create funnel plots or test them for asymmetry.
We included new adverse outcomes (arrhythmia, stroke) in the current review that were not reported in the previous version (Baumeister 2011c).
We reported secondary outcomes, including the effects of depression treatment on cardiac parameters (e.g. vital signs, platelet biomarkers), that were not reported in the previous version of the review (Baumeister 2011c), but that were prespecified in the protocol.
We added new secondary outcomes (adverse effects) for electrocardiogram (ECG) parameters, adverse mental health outcomes, and pharmacological side effects in this update.
Contributions of authors
Phillip Tully: trials search and selection, data extraction, entering data into review, data analysis, GRADE classification, drafting the updated review
Ser Yee Ang: trials search and selection, data extraction, entering data into review, data analysis, drafting the updated review
Emily Jo Lynn Lee: trials search and selection, data extraction, entering data into review, data analysis, drafting the updated review
Eileen Bendig: trials search and selection, data extraction, entering data into review, data analysis, drafting the updated review
Natalie Bauereiss: trials search and selection, data extraction, entering data into review, data analysis, drafting the updated review
Jürgen Bengel: drafting of protocol, trials search and selection, drafting the original Cochrane Review
Harald Baumeister: drafting of protocol, developing of search strategy, trials search and selection, data extraction, entering data into review, data analysis, drafting the review, updating the review
Sources of support
Internal sources
No sources of support provided
External sources
-
German Federal Ministry of Education and Research, Germany
Funding the review project
-
National Health and Medical Research Council of Australia, Australia
Fellowship support to Dr Tully
-
Freemasons Foundation Centre for Men's Health, Australia
Ser Yee Ang and Emily Jo Lynn Lee were supported by research scholarships provided by the Freemasons Foundation Centre for Men's Health to undertake this Cochrane Review update.
Declarations of interest
Phillip Tully: Dr Tully reports receiving salary from the National Health and Medical Research Council of Australia. Dr Tully reports receiving salary and his institution received grant payment from the Alzheimer's Drug Discovery Foundation. Dr Tully has received royalties from Springer and Lambert Academic Publishing. Dr Tully reports receiving payment for development of educational presentations from the Mental Health Professionals Network. Dr Tully reports receiving payment for editorial services from Elsevier.
Ser Yee Ang: none to declare.
Emily JL Lee: none to declare.
Eileen Bendig: EB is an author of an included study, but was not involved in the data extraction or ratings of bias or quality for that study.
Natalie Bauereiss: NB is an author of an included study, but was not involved in the data extraction or ratings of bias or quality for that study.
Jürgen Bengel: JB is an author of an included study, but was not involved in the data extraction or ratings of bias or quality for that study.
Harald Baumeister: HB is an author of an included study, but was not involved in the data extraction or ratings of bias or quality for that study.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abbasi 2015 {published data only}
- Abbasi SH, Mohammadinejad P, Shahmansouri N, Salehiomran A, Beglar AA, Zeinoddini A, et al. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: a double-blind, placebo-controlled, randomized trial. Journal of Affective Disorders 2015;183:149-55. [DOI] [PubMed] [Google Scholar]
ANDROS 2015 {published data only (unpublished sought but not used)}
- NCT02463110. Acute myocardial necrosis and depression: antiplatelet effect of reuptake inhibition of serotonin. clinicaltrials.gov/show/nct02463110 (first posted 4 June 2015).
Barth 2005 {published and unpublished data}
- Barth J, Paul J, Härter M, Bengel J. Inpatient psychotherapeutic treatment for cardiac patients with depression in Germany: short-term results. GMS Psycho-Social-Medicine 2005;2:1-8. [PMC free article] [PubMed] [Google Scholar]
Brown 1993 {published data only (unpublished sought but not used)}
- Brown MA, Munford AM, Munford PR. Behavior therapy of psychological distress in patients after myocardial infarction or coronary bypass. Journal of Cardiopulmonary Rehabilitation and Prevention 1993;13:201-10. [Google Scholar]
Carney 2009 {published data only}
- Bot M, Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, et al. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. Journal of Psychosomatic Research 2011;71:13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease. JAMA 2009;302:1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Steinmeyer BC, Freedland KE, Rubin EH, Rich MW, Harris WS. Baseline blood levels of omega-3 and depression remission: a secondary analysis of data from a placebo-controlled trial of omega-3 supplements. Journal of Clinical Psychiatry 2016;77:e138-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
CREATE 2007 {published data only}
- Frasure-Smith N, Koszycki D, Swenson JR, Baker B, Zyl L. Design and rationale for a randomized, controlled trial of interpersonal psychotherapy and citalopram for depression in coronary artery disease (CREATE): erratum. Psychosomatic Medicine 2007;69(2):216. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Koszycki D, Swenson JR, Baker B, Zyl LT, Laliberté MA, et al. Design and rationale for a randomized, controlled trial of interpersonal psychotherapy and citalopram for depression in coronary artery disease (CREATE). Psychosomatic Medicine 2006;68:87-93. [DOI] [PubMed] [Google Scholar]
- Lespérance F, Frasure-Smith N, Koszycki D, Laliberté MA, Zyl LT, Baker B, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian cardiac randomized evaluation of antidepressant and psychotherapy efficacy (CREATE) trial. JAMA 2007;29:367-79. [DOI] [PubMed] [Google Scholar]
- Zyl LT, Lesperance F, Frasure-Smith N, Malinin AI, Atar D, Laliberte MA, et al. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian cardiac randomized evaluation of antidepressant and psychotherapy efficacy trial (CREATE) biomarker sub-study. Journal of Thrombosis and Thrombolysis 2009;27(1):48-56. [DOI] [PubMed] [Google Scholar]
Dao 2011 {published data only}
- Dao TK, Youssef NA, Armsworth M, Wear E, Papathopoulos KN, Gopaldas R. Randomized controlled trial of brief cognitive behavioral intervention for depression and anxiety symptoms preoperatively inpatients undergoing coronary artery bypass graft surgery. Journal of Thoracic and Cardiovascular Surgery 2011;142:109-15. [DOI] [PubMed] [Google Scholar]
Divsalar 2018 {published data only}
- Divsalar P, Noorbala AA, Zadeh EM, Jafarinia M, Shakiba M, Shahmansouri N, et al. Red yeast rice as an adjunct to sertraline for treatment of depression in patients with percutaneous coronary intervention: placebo-controlled trial. Advances in Integrative Medicine 2018;5(2):69-74. [DOI: 10.1016/j.aimed.2018.01.001] [DOI] [Google Scholar]
Doering 2007 {published data only (unpublished sought but not used)}
- Doering LV, Cross R, Vredevoe D, Martinez-Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Alternative Therapies in Health and Medicine 2007;13:18-21. [PubMed] [Google Scholar]
ENRICHD 2003 {published data only (unpublished sought but not used)}
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA 2003;289:3106-16. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease (ENRICHD): baseline characteristics. American Journal of Cardiology 2001;88(3):316-22. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease (ENRICHD) study intervention: rationale and design. Psychosomatic Medicine 2001;63(5):747-55. [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods. American Heart Journal 2000;139:1-9. [DOI] [PubMed] [Google Scholar]
- Mendes de Leon CF, Czajkowski SM, Freedland KE, Bang H, Powell LH, Wu C, et al. The effect of a psychosocial intervention and quality of life after acute myocardial infarction: the enhancing recovery in coronary heart disease (ENRICHD) clinical trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2006;26:9-13. [DOI] [PubMed] [Google Scholar]
- Saab PG, Bang H, Williams RB, Powell LH, Schneiderman N, Thoresen C, et al. The impact of cognitive behavioral group training on event-free survival in patients with myocardial infarction: the ENRICHD experience. Journal of Psychosomatic Research 2009;67:45-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
EsDEPACS 2014 {published data only}
- Kim JM, Bae KY, Kang HJ, Kim SW, Shin IS, Hong YJ, et al. Design and methodology for the Korean observational and escitalopram treatment studies of depression in acute coronary syndrome: K-DEPACS and EsDEPACS. Psychiatry Investigations 2014;11:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Bae KY, Stewart R, Jung BO, Kang HJ, Kim SW, et al. Escitalopram treatment for depressive disorder following acute coronary syndrome: a 24-week double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2015;76(1):62-8. [DOI: 10.4088/JCP.14m09281] [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Bae KY, Kang HJ, Kim SW, Shin IS, et al. Correlates and escitalopram treatment effects on sleep disturbance in patients with acute coronary syndrome: K-DEPACS and EsDEPACS. Sleep 2015;38(7):1105-11. [DOI: 10.5665/sleep.4822] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Bae KY, Kang HJ, Kim SW, Shin IS, et al. Effects of depression co-morbidity and treatment on quality of life in patients with acute coronary syndrome: the Korean depression in ACS (K-DEPACS) and the escitalopram for depression in ACS (EsDEPACS) study. Psychological Medicine 2015;45:1641-52. [DOI: 10.1017/S003329171400275X] [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kang HJ, Bae KY, Kim SW, Shin IS, et al. BDNF methylation and depressive disorder in acute coronary syndrome: the K-DEPACS and EsDEPACS studies. Psychoneuroendocrinology 2015;62:159-65. [DOI: 10.1016/j.psyneuen.2015.08.013] [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kang HJ, Kim SY, Kim JW, Lee HJ, et al. Long-term cardiac outcomes of depression screening, diagnosis and treatment in patients with acute coronary syndrome: the DEPACS study. Psychological Medicine 2021;51(6):964-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, et al. Effect of escitalopram vs placebo treatment for depression on long-term cardiac outcomes in patients with acute coronary syndrome: a randomized clinical trial. JAMA 2018;320(4):350-8. [DOI: 10.1001/jama.2018.9422] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kang HJ, Bae KY, Kim SW, Shin IS, Yoon JS, et al. Social support deficit and depression treatment outcomes in patients with acute coronary syndrome: findings from the EsDEPACS study. International Journal of Psychiatry in Medicine 2019;54(1):39-52. [DOI: 10.1177/0091217418791439] [DOI] [PubMed] [Google Scholar]
Fang 2003 {published data only}
- Fang R, Jiang Y, Song J, Cheng G, Xue G. Control study of general psychological intervention for patients with myocardial infarction. Chinese Journal of Clinical Rehabilitation 2003;7:1382-3. [Google Scholar]
Freedland 2009 {published data only}
- Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Dávila-Roman VG, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Archives of General Psychiatry 2009;66:387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE. Treatment of depression after coronary bypass surgery. www.controlled-trials.com/mrct/trial/438737 (accessed 14 December 2009).
Freeman 1986 {published data only (unpublished sought but not used)}
- Freeman AM, Fleece L, Folks DG, Sokol RS, Hall KR, Pacifico AD, et al. Alprazolam treatment of postcoronary bypass anxiety and depression. Journal of Clinical Psychopharmacology 1986;6:39-41. [PubMed] [Google Scholar]
Kennedy 2005 {unpublished data only}
- EUCTR2004-000990-78-AT. A double-blind, multicentre, randomised, parallel-group, placebo-controlled study assessing the efficacy and safety of escitalopram in post-myocardial infarction patients suffering from depressive symptoms. www.who.int/trialsearch/trial2.aspx?Trialid=euctr2004-000990-78-at (date of registration 23 September 2004).
Li 2005 {published data only}
- Li YM, Li GY, Wang XL, Yu LF. Rehabilitation effect of St John's wort extract on depression and myocardial function after coronary artery bypass grafting: a randomized grouping, placebo-control and blind evaluation. Zhongguo Linchuang Kangfu 2005;9:38-9. [Google Scholar]
Liu 1999 {published data only}
- Liu JD, Zheng H. Efficacy of fluoxetine in the treatment of depression in patients with acute myocardial infarction. Journal of Clinical Psychological Medicine 1999;9:210-1. [Google Scholar]
Liu 2016 {published data only}
- Liu W, Qin J. Clinical efficacy and safety of the ShuganJieyu capsule in patients with acute myocardial infarction and depression. International Journal of Psychiatry in Medicine 2016;51:534-43. [DOI] [PubMed] [Google Scholar]
Ma 2019 {published data only}
- Ma H, Wang Y, Xue Y, Huang D, Kong Y, Zhao X, et al. The effect of Xinkeshu tablets on depression and anxiety symptoms in patients with coronary artery disease: results from a double-blind, randomized, placebo-controlled study. Biomedicine and Pharmacotherapy 2019;112:108639. [DOI: 10.1016/j.biopha.2019.108639] [DOI] [PubMed] [Google Scholar]
McFarlane 2001 {published data only (unpublished sought but not used)}
- McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. American Heart Journal 2001;142(4):617-23. [DOI] [PubMed] [Google Scholar]
McLaughlin 2005 {published data only (unpublished sought but not used)}
- Bambauer KZ, Aupont O, Stone PH, Locke SE, Mullan MG, Colagiovanni J, et al. The effect of a telephone counseling intervention on self-rated health of cardiac patients. Psychosomatic Medicine 2005;67:539-45. [DOI] [PubMed] [Google Scholar]
- McLaughlin TJ, Aupont O, Bambauer KZ, Stone P, Mullan MG, Colagiovanni J. Improving psychologic adjustment to chronic illness in cardiac patients: the role of depression and anxiety. Journal of General Internal Medicine 2005;20:1084-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
MIND‐IT 2007 {published data only (unpublished sought but not used)}
- Honig A, Kuyper AM, Schene AH, Melle JP, Jonge P, Tulner DM, et al. Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosomatic Medicine 2007;69:606-13. [DOI] [PubMed] [Google Scholar]
- Schins A, Hamuly KK, Scharp S, Lousberg R, Van Melle J, Crijns H, et al. Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients. Life Sciences 2004;76(6):637-50. [DOI] [PubMed] [Google Scholar]
- Van den Brink RH, Melle JP, Honig A, Schene AH, Crijns HJ, Lambert FP, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). American Heart Journal 2002;144:219-25. [PubMed] [Google Scholar]
- Van Melle JP, Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, et al. Effects of antidepressant treatment following myocardial infarction. British Journal of Psychiatry 2007;190:460-6. [DOI] [PubMed] [Google Scholar]
- Zuidersma M, Conradi HJ, Melle JP, Ormel J, Jonge P. Depression treatment after myocardial infarction and long-term risk of subsequent cardiovascular events and mortality: a randomized controlled trial. Journal of Psychosomatic Research 2013;74:25-30. [DOI] [PubMed] [Google Scholar]
MoodCare 2011 {published data only}
- O'Neil A, Hawkes AL, Chan B, Sanderson K, Forbes A, Hollingsworth B, et al. A randomised, feasibility trial of a tele-health intervention for acute coronary syndrome patients with depression (’MoodCare’): study protocol. BMC Cardiovascular Disorders 2011;11:1471-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil A, Taylor B, Hare DL, Sanderson K, Cyril S, Venugopal K, et al. Long-term efficacy of a tele-health intervention for acute coronary syndrome patients with depression: 12-month results of the MoodCare randomized controlled trial. Preventive Cardiology 2015;22:1111-20. [DOI] [PubMed] [Google Scholar]
- O'Neil A, Taylor B, Sanderson K, Cyril S, Chan B, Hawkes AL, et al. Efficacy and feasibility of a tele-health intervention for acute coronary syndrome patients with depression: results of the “MoodCare” randomized controlled trial. Annals of Behavioral Medicine 2014;48:163-74. [DOI] [PubMed] [Google Scholar]
Pizzi 2009 {published data only}
- Pizzi C, Mancini S, Angeloni L, Fontana F, Costa GM. CORRIGENDUM: Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clinical Pharmacology & Therapeutics 2009;86:683. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Mancini S, Angeloni L, Fontana F, Costa GM. Sertraline ameliorates endothelial dysfunction and inflammation in coronary heart disease patients with depression. European Heart Journal 2009;30(Suppl 1):520. [Google Scholar]
- Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clinical Pharmacology and Therapeutics 2009;86:527-32. [DOI] [PubMed] [Google Scholar]
Roose 1998 {published data only (unpublished sought but not used)}
- Nelson JC, Kennedy JS, Pollock BG, Laghrissi-Thode F, Narayan M, Nobler MS, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. American Journal of Psychiatry 1999;156:1024-8. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology 2000;20(2):137-40. [DOI] [PubMed] [Google Scholar]
- Roose S, Pollock B, Kennedy J, Nelson J, Gergel I, McCafferty J. Paroxetine in the treatment of depressed patients with ischaemic heart disease. In: Sixth World Congress of Biological Psychiatry, Nice, France. June 22 27. 1997.
- Roose S, Pollock B, Kennedy J, Nelson J, McCafferty J, Gergel I. Paroxetine versus nortriptyline in ischemic disease. In: 150th Annual Meeting of the American Psychiatric Association San Diego, California, USA 17 22 May. 1997.
- Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Pollock BG, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998;279:287-91. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pesce V, Jayaraman A, Roose S. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long-term heart rate variability measures. Biological Psychiatry 2002;52(5):418-29. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Roose S, Mallavarapu M, Radhakrishna RK, Pesce V. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology 2002;46(3):125-35. [DOI] [PubMed] [Google Scholar]
SADHART 2002 {published data only (unpublished sought but not used)}
- Glassman AH, Bigger T Jr, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression. Archives of General Psychiatry 2009;66:1022-9. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina: ERRATUM. JAMA 2002;288(14):1720. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9. [DOI] [PubMed] [Google Scholar]
- Lattanzio F, Cherubini A, Furneri G, Di Bari M, Marchionni N. Sertraline treatment for depression associated with acute coronary syndromes: a cost analysis from the viewpoint of the Italian healthcare system. Aging: Clinical and Experimental Research 2008;20(1):76-80. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Glassman AH, Harrison DJ. Pharmacoeconomic analysis of sertraline treatment of depression in patients with unstable angina or a recent myocardial infarction. Journal of Clinical Psychiatry 2005;66:346-52. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003;108(8):939-44. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Suckow RF, Cooper TB, O'Connor CM, Malinin AI, Krishnan KR, et al. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. American Journal of Psychiatry 2005;162(6):1165-70. [DOI] [PubMed] [Google Scholar]
- Swenson JR, O'Connor CM, Barton D, Zyl LT, Swedberg K, Forman LM, et al. Influence of depression and effect of treatment with sertraline on quality of life after hospitalization for acute coronary syndrome. American Journal of Cardiology 2003;92:1271-6. [DOI] [PubMed] [Google Scholar]
Shahmansouri 2014 {published data only}
- Shahmansouri S, Farokhnia M, Abbasi SH, Kassaian SE, Tafti AA, Gougol A, et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patient. Journal of Affective Disorders 2013;155:216-22. [DOI] [PubMed] [Google Scholar]
SPIRR‐CAD 2011 {published data only}
- Albus C, Beutel ME, Deter H-C, Fritzsche K, Hellmich M, Jordan H, et al. A stepwise psychotherapy intervention for reducing risk in coronary artery disease (SPIRR-CAD) - rationale and design of a multicenter, randomized trial in depressed patients with CAD. Journal of Psychosomatic Research 2011;71:215-22. [DOI] [PubMed] [Google Scholar]
- Fangauf SV, Herbeck Belnap B, Meyer T, Albus C, Binder L, Deter H-C, et al. Associations of NT-proBNP and parameters of mental health in depressed coronary artery disease patients. Psychoneuroendocrinology 2018;96:188-94. [DOI] [PubMed] [Google Scholar]
- Fritzsche K, Albus C, Jordan J, Beutel ME, Wiltink J, Herrmann-Lingen C. Individual and couple intervention for depressive coronary heart patients. Therapy manual, quality assurance and first experiences within the framework of a randomized controlled trial [Einzel- und Paarintervention für depressive Koronarpatienten. Therapiemanual, Qualitätssicherung und erste Erfahrungen im Rahmen einer randomisierten kontrollierten Interventionsstudie]. Psychotherapeut 2011;56:325-36. [Google Scholar]
- Herrmann-Lingen C, Beutel ME, Bosbach A, Deter H-C, Fritzsche K, Hellmich M, et al. A stepwise psychotherapy intervention for reducing risk in coronary artery disease (SPIRR-CAD): results of an observer-blinded, multicenter, randomized trial in depressed patients with coronary artery disease. Psychosomatic Medicine 2016;78:704-15. [DOI] [PubMed] [Google Scholar]
- ISRCTN76240576. A stepwise psychotherapy intervention for reducing risk in coronary artery disease - a randomised controlled trial (SPIRR-CAD). doi.org/10.1186/ISRCTN76240576 (date assigned 27 March 2008).
Strik 2000 {published data only (unpublished sought but not used)}
- Strik JJ, Honig A, Klinkenberg E, Dijkstra J, Jolles J. Cognitive performance following fluoxetine treatment in depressed patients post myocardial infarction. Acta Neuropsychiatrica 2006;18(1):1-6. [DOI] [PubMed] [Google Scholar]
- Strik JJ, Honig A, Lousberg R, Lousberg AH, Cheriex EC, Tuynman-Qua HG, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosomatic Medicine 2000;62:783-9. [DOI] [PubMed] [Google Scholar]
- Strik JJ, Praag HM, Honig A. Depression after first myocardial infarction. A prospective study on incidence, prognosis, risk factors and treatment. Tijdschrift Voor Gerontologie en Geriatrie 2003;34(3):104-12. [PubMed] [Google Scholar]
Tian 2016 {published data only}
- Tian X, Wang Q, Guo R, Xu L, Chen QM, Hou Y. Effects of paroxetine-mediated inhibition of GRK2 expression on depression and cardiovascular function in patients with myocardial infarction. Neuropsychiatric Disease and Treatment 2016;12:2333-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
TREATED‐ACS 2020 {published data only (unpublished sought but not used)}
- Rafanelli C, Gostoli S, Buzzichelli S, Bergerone S, Gaita F, Roncuzzi R, et al. Results of the TREATED-ACS: a randomized controlled trial on the effectiveness of Cognitive Behavioral Therapy, combined with Well-Being Therapy, in improving depression and reducing cardiac risk among patients with acute coronary syndrome. Psychotherapy and Psychosomatics 2019;88(Suppl 1):106-7. [Google Scholar]
- Rafanelli C, Gostoli S, Buzzichelli S, Guidi J, Sirri L, Gallo P, et al. Sequential combination of cognitive-behavioral treatment and well-being therapy in depressed patients with acute coronary syndromes: a randomized controlled trial (TREATED-ACS Study). Psychotherapy and Psychosomatics 2020;89(6):345-56. [DOI] [PubMed] [Google Scholar]
U‐CARE 2018 {published data only}
- Humphries SM, Wallert J, Norlund F, Held C, Olsson E. 12-month follow-up of the U-CARE heart trial: using internet-based cognitive behavioural therapy to treat patients experiencing depression and anxiety after myocardial infarction. In: 25th World Congress of the International College of Psychosomatic Medicine, ICPM 2019 Italy , editors(s). Psychotherapy and Psychosomatics. Vol. 88. 2019.
- Humphries SM, Wallert J, Norlund F, Wallin E, Burell G, Essen L, et al. Internet-based cognitive behavioral therapy for patients reporting symptoms of anxiety and depression after myocardial infarction: U-CARE Heart randomized controlled trial twelve-month follow-up. Journal of Medical Internet Research 2021;23:e25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlund F, Olsson EM, Burell G, Wallin E, Held C. Treatment of depression and anxiety with internet-based cognitive behavior therapy in patients with a recent myocardial infarction (U-CARE Heart): study protocol for a randomized controlled trial. Trials 2015;16:154. [DOI: 10.1186/s13063-015-0689-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlund F, Wallin E, Olsson EM, Wallert J, Burell G, Essen L, et al. Internet-based cognitive behavioral therapy for symptoms of depression and anxiety among patients with a recent myocardial infarction: the U-CARE Heart randomized controlled trial. European Journal of Preventive Cardiology 2018;25(2 Suppl 1):S63-4. [DOI: 10.1177/2047487318786178] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlund F, Wallin E, Olsson EM, Wallert J, Burell G, Essen L, et al. Internet-based cognitive behavioral therapy for symptoms of depression and anxiety among patients with a recent myocardial infarction: the U-CARE heart randomized controlled trial. Journal of Medical Internet Research 2018;20(3):e88. [DOI: 10.2196/jmir.9710] [DOI] [PMC free article] [PubMed] [Google Scholar]
UPBEAT 2012 {published data only}
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. Journal of the American College of Cardiology 2012;60:1053-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Sherwood A, Rogers SD, Babyak MA, Doraiswamy PM, Watkins L, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study - rationale, design, and methodological issues. Clinical Trials 2007;4:548-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang Y, Liu YJ, Li F-E, Guo Z, Wang J, Comorbid Depression and Coronary Heart Disease Research Group of Cangzhou Central Hospital. A Chinese herbal formula shows beneficial effects on comorbid depression and coronary heart disease based on the philosophy of psycho-cardiology. Journal of Herbal Medicine 2020;19:100303. [CHICTR-INC: 17012872] [Google Scholar]
WIDeCAD 2017 {published data only (unpublished sought but not used)}
- Bendig E, Bauereiss N, Buntrock C, Habibovic M, Ebert DD, Baumeister H. Lessons learned from an attempted randomized-controlled feasibility trial on “WIDeCAD” - an internet-based depression treatment for people living with coronary artery disease (CAD). Internet Interventions 2021;24:100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang L, Wang X, Cui X. Patients' intensive telephone-based care program reduces depression in coronary artery disease patients and may contribute to favorable overall survival by decreasing depression. Journal of Cardiovascular Nursing 2019;34:236-43. [DOI] [PubMed] [Google Scholar]
Zarea 2014 {published data only}
- Zarea K, Maghsoudi S, Dashtebozorgi B, Hghighizadeh MH, Javadi M. The impact of Peplau's therapeutic communication model on anxiety and depression in patients candidate for coronary artery bypass. Clinical Practice and Epidemiology in Mental Health 2014;10:159-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abedimanesh 2017 {published data only}
- Abedimanesh N, Ostadrahimi A, Bathaie SZ, Abedimanesh S, Motlagh B, Jafarabadi MA, et al. Effects of saffron aqueous extract and its main constituent, crocin, on health-related quality of life, depression, and sexual desire in coronary artery disease patients: a double-blind, placebo-controlled, randomized clinical trial. Iranian Red Crescent Medical Journal 2017;19:e13676. [Google Scholar]
ACHD‐CARE 2015 {published data only}
- Kovacs AH, Bandyopadhyay M, Grace SL, Kentner AC, Nolan RP, Silversides CK, et al. Adult congenital heart disease-coping and resilience (ACHD-CARE): rationale and methodology of a pilot randomized controlled trial. Contemporary Clinical Trials 2015;45:385-93. [DOI] [PubMed] [Google Scholar]
- Kovacs AH, Grace SL, Kentner AC, Nolan RP, Silversides CK, Irvine MJ. Feasibility and outcomes in a pilot randomized controlled trial of a psychosocial intervention for adults with congenital heart disease. Canadian Journal of Cardiology 2018;34:766-73. [DOI] [PubMed] [Google Scholar]
ACTonHEART 2014 {published data only}
- Spatola CA, Manzoni GM, Castelnuovo G, Malfatto G, Facchini M, Goodwin CL, et al. The ACTonHEART study: rationale and design of a randomized controlled clinical trial comparing a brief intervention based on Acceptance and Commitment Therapy to usual secondary prevention care of coronary heart disease. Health and Quality of Life Outcomes 2014;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beating Heart Problems 2014 {published data only}
- Turner A, Murphy BM, Higgins RO, Elliott PC, Le Grande MR, Goble AJ, et al. An integrated secondary prevention group programme reduces depression in cardiac patients. European Journal of Preventive Cardiology 2014;21:153-62. [DOI] [PubMed] [Google Scholar]
Black 1998 {published data only}
- Black JL, Allison TG, Williams DE, Rummans TA, Gau GT. Effect of intervention for psychological distress on rehospitalization rates in cardiac rehabilitation patients. Psychosomatics 1998;39:134-43. [DOI] [PubMed] [Google Scholar]
Boese 2013 {published data only}
- Boese A, Bock S, Kielblock B, Siegmund-Schultze E, Kröner-Herwig B, Herrmann-Lingen C. Randomised controlled trial of a telephone-based peer support intervention to reduce depressive symptoms and improve social support in women with CHD. Journal of Psychosomatic Research 2013;74:539-62. [Google Scholar]
BraveHeart 2013 {published data only}
- Hambridge JA, Turner A, Baker AL. BraveHeart begins: pilot results of group cognitive behaviour therapy for depression and anxiety in cardiac patients. Australian and New Zealand Journal of Psychiatry 2009;43:1171–7. [DOI] [PubMed] [Google Scholar]
- Turner A, Hambridge J, Baker A, Bowman J, McElduff P. Randomised controlled trial of group cognitive behaviour therapy versus brief intervention for depression in cardiac patients. Australian and New Zealand Journal of Psychiatry 2013;47:235-43. [DOI] [PubMed] [Google Scholar]
Bucknall 1988 {published data only}
- Bucknall C, Brooks D, Curry PVL, Bridges PK, Bouras N, Ankier SI. Mianserin and trazodone for cardiac patients with depression. European Journal of Clinical Pharmacology 1988;33:565-9. [DOI] [PubMed] [Google Scholar]
BY.PASS Study 2013 {published data only}
- Rosendahl J, Tigges-Limmer K, Gummert J, Dziewas R, Albes JM, Strauss B. Bypass surgery with psychological and spiritual support (the BY.PASS Study): results of a pragmatic trial based on patients' preference. Psychotherapy and Psychosomatics 2013;82:35-44. [DOI] [PubMed] [Google Scholar]
CADENCE 2016 {published data only}
- Richards SH, Campbell JL, Dickens C, Anderson R, Gandhi M, Gibson A, et al. A feasibility study and pilot RCT to establish methods for assessing the acceptability, and clinical effectiveness and cost effectiveness of enhanced psychological care in cardiac rehabilitation services for patients with new onset depression compared with treatment as usual: the CADENCE Study. Health Technology Assessments 2017;22:1-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SH, Campbell JL, Dickens C, Anderson R, Gandhi M, Gibson A, et al. Enhanced psychological care in cardiac rehabilitation services for patients with new-onset depression: the CADENCE feasibility study and pilot RCT. Health Technology Assessment 2018;22(30):1-220. [DOI: 10.3310/hta22300] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SH, Dickens C, Anderson R, Richards DA, Taylor RS, Ukoumunne OC, et al. Assessing the effectiveness of Enhanced Psychological Care for patients with depressive symptoms attending cardiac rehabilitation compared with treatment as usual (CADENCE): a pilot cluster randomised controlled trial. Trials 2018;19(1):211. [DOI: 10.1186/s13063-018-2576-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SH, Dickens C, Anderson R, Richards DA, Taylor RS, Ukoumunne OC, et al. Assessing the effectiveness of enhanced psychological care for patients with depressive symptoms attending cardiac rehabilitation compared with treatment as usual (CADENCE) : study protocol for a pilot cluster randomised controlled trial. Trials 2016;17(1):59. [DOI: 10.1186/s13063-016-1184-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder R, Richards SH, Campbell JL, Richards DA, Dickens C, Gandhi M, et al. Development and refinement of a complex intervention within cardiac rehabilitation services: experiences from the CADENCE feasibility study. Pilot and Feasibility Studies 2017;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2012 {published data only}
- Carney RM, Freedland KE, Steinmeyer BC, Rubin EH, Ewald G. Collaborative care for depression symptoms in an outpatient cardiology setting: a randomized clinical trial. International Journal of Cardiology 2016;219:164–71. [NCT: 01552889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2019 {published data only}
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. A randomized placebo-controlled trial of omega-3 and sertraline in depressed patients with or at risk for coronary heart disease. Journal of Clinical Psychiatry 2019;80:19m12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
CHAMPS 2016 {published data only}
- Tully PJ, Turnbull DA, Horowitz JD, Beltrame JF, Selkow T, Baune BT, et al. Cardiovascular health in anxiety or mood problems study (CHAMPS): study protocol for a randomized controlled trial. Trials 2016;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2020 {published data only}
- Chang Z, Guo A, Zhou A, Sun TW, Ma LL, Gardiner FW, et al. Nurse-led psychological intervention reduces anxiety symptoms and improves quality of life following percutaneous coronary intervention for stable coronary artery disease. Australian Journal of Rural Health 2020;28:124-31. [DOI] [PubMed] [Google Scholar]
Child 2010 {published data only}
- Child A, Sanders J, Sigel P, Hunter MS. Meeting the psychological needs of cardiac patients: an integrated stepped-care approach within a cardiac rehabilitation setting. British Journal of Cardiology 2010;17:175-9. [Google Scholar]
CHIP 2011 {published data only}
- Thieszen CL, Merrill RM, Aldana SG, Diehl HA, Mahoney ML, Greenlaw RL, et al. The coronary health improvement project (CHIP) for lowering weight and improving psychosocial health. Psychological Reports 2011;109:338-52. [DOI] [PubMed] [Google Scholar]
Chung 2010 {published data only}
- Chung LJ, Tsai PS, Liu BY, Chou KR, Lin WH, Shyu YK, et al. Home-based deep breathing for depression in patients with coronary heart disease: a randomised controlled trial. International Journal of Nursing Studies 2010;47:1346-53. [DOI] [PubMed] [Google Scholar]
CODIACS 2013 {published data only}
- Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, et al. Centralized, stepped, patient preference based, depression treatment for patients after acute coronary syndrome. CODIACS vanguard randomized controlled trial. European Heart Journal 2013;173:997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
COINCIDE 2012 {published data only}
- Coventry PA, Lovell K, Dickens C, Bower P, Chew-Graham C, Cherrington A, et al. Collaborative Interventions for Circulation and Depression (COINCIDE): study protocol for a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease. Trials 2012;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry PA, Lovell K, Dickens C, Bower P, Chew-Graham C, Cherrington A, et al. Update on the collaborative interventions for circulation and depression (COINCIDE) trial: changes to planned methodology of a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease. Trials 2013;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
COPES 2010 {published data only}
- Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemporary Clinical Trials 2008;29:231-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms. Archives of Internal Medicine 2010;170:600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Chaplin WF, Rieckmann N, Burg MM, Davidson KW. The effect of enhanced depression care on anxiety symptoms in acute coronary syndrome patients: findings from the COPES trial. Psychotherapy & Psychosomatics 2012;81:245-7. [DOI: 10.1159/000332439] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronish IM, Rieckmann N, Burg MM, Edmondson D, Schwartz JE, Davidson KW. The effect of enhanced depression care on adherence to risk-reducing behaviors after acute coronary syndromes: findings from the COPES trial. American Heart Journal 2012;164:524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Shaffer JA, Rieckmann N, Schwartz JE, Kronish IM, Ladapo JA, et al. Long-term outcomes of enhanced depression treatment in patients with acute coronary ayndromes. American Journal of Medicine 2014;127:1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doering 2013 {published data only}
- Doering LV, Chen B, Bodán RC, Magsarili MC, Nyamathi A, Irwin MR. Early cognitive behavioral therapy for depression after cardiac surgery. Journal of Cardiovascular Nursing 2013;28:370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ENHANCED 2016 {published data only}
- Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation 2016;133:1341-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Sherwood A, Mabe S, Watkins L, Hinderliter A, Blumenthal JA. Physical activity and psychosocial function following cardiac rehabilitation: one-year follow-up of the ENHANCED study. General Hospital Psychiatry 2017;49:32-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2006 {published data only}
- Fu YX, Meng HQ, Luo QH. Interventional effect of fluoxetine on patients with acute myocardial infarction accompanied by anxiety and depression. Chinese Journal of Clinical Rehabilitation 2006;10:16-7. [Google Scholar]
Giltay 2011 {published data only}
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94:1442-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
González‐Jaimes 2003 {published data only}
- Gonzalez-Jaimes EI, Turnbull-Plaza B. Selection of psychotherapeutic treatment for adjustment disorder with depressive mood due to acute myocardial infarction. Archives of Medical Research 2003;34:298-304. [DOI] [PubMed] [Google Scholar]
Haberka 2013 {published data only}
- Haberka M, Mizia-Stec K, Mizia M, Gieszczyk K, Chmiel A, Sitnik-Warchulska K, et al. Effects of n-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacological Reports 2013;65:59-68. [DOI] [PubMed] [Google Scholar]
Haybar 2018 {published data only}
- Haybar H, Javid AZ, Haghighizadeh MH, Valizadeh E, Mohaghegh SM, Mohammadzadeh A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clinical Nutrition ESPEN 2018;26:47-52. [DOI] [PubMed] [Google Scholar]
Huffman 2011 {published data only}
- Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26-33. [DOI] [PubMed] [Google Scholar]
I‐CARE 2018 {published data only}
- Li S, Blumenthal JA, Shi C, Millican D, Li X, Du X, et al. I-CARE randomized clinical trial integrating depression and acute coronary syndrome care in low-resource hospitals in China: design and rationale. American Heart Journal 2018;202:109-15. [NCT: 02195193] [DOI] [PubMed] [Google Scholar]
InterHerz 2012 {published data only (unpublished sought but not used)}
- Messerli-Bürgy N, Barth J, Berger T. The InterHerz project - a web-based psychological treatment for cardiac patients with depression: study protocol of a randomized controlled trial. Trials 2012;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2018 {published data only}
- Jang SH, Lee JH, Lee HJ, Lee SY. Effects of mindfulness-based art therapy on psychological symptoms in patients with coronary artery disease. Journal of Korean Medical Science 2018;33(12):e88. [DOI: 10.3346/jkms.2018.33.e88] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kachkovskii 2006 {published data only}
- Kachkovskii MA, Kriukov NN. Treatment of depression in patients with myocardial infarction with tianeptine. Kardiologiia 2006;46:21-6. [PubMed] [Google Scholar]
Keeping‐Burke 2013 {published data only}
- Keeping-Burke L, Purden M, Frasure-Smith N, Cossette S, McCarthy F, Amsel R. Bridging the transition from hospital to home: effects of the VITAL telehealth program on recovery for CABG surgery patients and their caregivers. Research in Nursing & Health 2013;36:540-53. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li CJ, Zhang G, Zhang Y, Cong H, Zhang BL. Clinical efficacy of Xinkeshu Pian on coronary heart disease and mood disorder complications after PCI. International Journal of Clinical and Experimental Medicine 2014;7:1887-90. [PMC free article] [PubMed] [Google Scholar]
Li 2020 {published data only}
- Li X, Yang S, Wang Y, Yang B, Zhang J. Effects of a transtheoretical model-based intervention and motivational interviewing on the management of depression in hospitalized patients with coronary heart disease: a randomized controlled trial. BMC Public Health 2020;20:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2019 {published data only}
- Liang H, Liu L, Hu H. The effects of mindfulness-based stress reduction on the mental states, sleep quality, and medication compliance of patients with acute myocardial infarction after percutaneous coronary intervention. International Journal of Clinical and Experimental Medicine 2019;12:13514-23. [Google Scholar]
Lin 2014 {published data only}
- Lin EH, Korff M, Peterson D, Ludman EJ, Ciechanowski P, Katon W. Population targeting and durability of multimorbidity collaborative care management. American Journal of Managed Care 2014;20:887-95. [PMC free article] [PubMed] [Google Scholar]
Lv 2016 {published data only}
- Lv J, Zhang X, Ou S, Gu S, Su Z, Tong S, et al. Influence of cognitive behavioral therapy on mood and quality of life after stent implantation in young and middle-aged patients with coronary heart disease. International Heart Journal 2016;57:167-72. [DOI] [PubMed] [Google Scholar]
Ma 2010 {published data only}
- Ma W, Hu D, Liu G, Jiang J, Deng B, Liu R, et al. Effect of patient-specific intervention of depression on quality of life after acute coronary syndrome. Circulation 2010;122:251. [Google Scholar]
Malik 2002 {published data only}
- Malik NA. Comparison of treatment to influence depression as a risk factor for ischemic heart disease with new generation antidepressants. In: XII World Congress of Psychiatry, Yokohama, Japan. Aug 24 9, 2002.
Mazereeuw 2016 {published data only}
- Mazereeuw G, Herrmann N, Oh PI, Ma DW, Wang CT, Kiss A, et al. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: analyses from a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology 2016;36(5):436-44. [CLINICALTRIALS.GOV ID: NCT00981383] [DOI] [PMC free article] [PubMed] [Google Scholar]
MindfulHeart 2014 {published data only}
- Nyklíček I, Dijksman S, Lenders P, Fonteijn W, Koolen J. A brief mindfulness based intervention for increase in emotional well-being and quality of life in percutaneous coronary intervention (PCI) patients: the MindfulHeart randomized controlled trial. Journal of Behavioral Medicine 2014;37:135-44. [DOI: 10.1007/s10865-012-9475-4] [DOI] [PubMed] [Google Scholar]
Mohapatra 2005 {published data only}
- Mohapatra PK, Kar N, Kar GC, Behera M. Effectiveness of sertraline in treatment of depression in a consecutive sample of patients with acute myocardial infarction: six month prospective study on outcome. Clinical Practice and Epidemiology in Mental Health 2005;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
MOSAIC 2013 {published data only}
- Huffman JC, Beach SR, Suarez L, Mastromauro CA, DuBois CM, Celano CM, et al. Design and baseline data from the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized controlled trial. Contemporary Clinical Trials 2013;36:488-501. [DOI] [PubMed] [Google Scholar]
MOTIV‐CABG 2013 {published data only}
- Chocron S, Vandel P, Durst C, Laluc F, Kaili D, Chocron M, et al. Antidepressant therapy in patients undergoing coronary artery bypass grafting: the MOTIV-CABG trial. Annals of Thoracic Surgery 2013;95:1609-18. [DOI] [PubMed] [Google Scholar]
Nikrahan 2019 {published data only}
- Nikrahan GR, Eshaghi L, Massey CN, Hemmat A, Amonoo HL, Healy B, et al. Randomized controlled trial of a well-being intervention in cardiac patients. General Hospital Psychiatry 2019;61:116-24. [DOI] [PubMed] [Google Scholar]
Norris 2009 {published data only}
- Norris CE, Patterson L, Galbraith D, Hegadoren KM. All you have to do is call; a pilot study to improve the outcomes of patients with coronary artery disease. Applied Nursing Research 2009;22:133-7. [DOI] [PubMed] [Google Scholar]
Oldridge 1991 {published data only}
- Oldridge N, Guyatt G, Jones N, Crowe J, Singer J, Feeny D, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. American Journal of Cardiology 1991;67:1084-9. [DOI] [PubMed] [Google Scholar]
- Oldridge N, Streiner D, Hoffmann R, Guyatt G. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Medicine and Science in Sports and Exercise 1995;27:900-5. [PubMed] [Google Scholar]
Oranta 2010 {published data only}
- Oranta O, Luutonen S, Salokangas RK, Vahlberg T, Leino-Kilpi H. Depression-focused interpersonal counseling and the use of healthcare services after myocardial infarction. Perspectives in Psychiatric Care 2012;48:47-55. [DOI] [PubMed] [Google Scholar]
- Oranta O, Luutonen S, Salokangas RK, Vahlberg T, Leino-Kilpi H. The effects of interpersonal counselling on health-related quality of life after myocardial infarction. Journal of Clinical Nursing 2011;20:3373-82. [DOI] [PubMed] [Google Scholar]
- Oranta O, Luutonen S, Salokangas RK, Vahlberg T, Leino-Kilpi H. The outcomes of interpersonal counselling on depressive symptoms and distress after myocardial infarction. Nordic Journal of Psychiatry 2010;64:78-86. [DOI] [PubMed] [Google Scholar]
- Oranta O, Luutonen S, Salokangas RKR, Vahlberg T, Leino-Kilpi H. Nurse-led interpersonal counseling for depressive symptoms in patients with myocardial infarction. Cardiology (Switzerland) 2013;126:104. [Google Scholar]
O’Doherty 2015 {published data only}
- O’Doherty V, Carr A, McGrann A, O’Neill JO, Dinan S, Graham I, et al. A controlled evaluation of mindfulness-based cognitive therapy for patients with coronary heart disease and depression. Mindfulness 2014;6:405–16. [Google Scholar]
Park 2013 {published data only}
- Park JH, Tahk SJ, Bae SH, Son YJ. Effects of a psychoeducational intervention for secondary prevention in Korean patients with coronary artery disease: a pilot study. International Journal of Nursing Practice 2013;19:295-305. [DOI] [PubMed] [Google Scholar]
PATHWAY Group MCT {published data only}
- Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial. Trials 2018;19(1):215. [DOI: 10.1186/s13063-018-2593-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
PATHWAY Home MCT {published data only}
- Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Metacognitive therapy home-based self-help for cardiac rehabilitation patients experiencing anxiety and depressive symptoms: study protocol for a feasibility randomised controlled trial (PATHWAY Home-MCT). Trials 2018;19(1):444. [CLINICALTRIALS.GOV:: NCT03129282] [DOI: 10.1186/s13063-018-2826-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pogosova 2004 {published data only}
- Pogosova GV, Zhidko NI, Krasnitsky VB, Tikhomirova EA, Odintsova AS, Akhmedjanov NM, et al. Clinical efficacy of tianeptine in patients with ischemic heart disease and comorbid depression. Kardiologiia 2004;44:20-4. [PubMed] [Google Scholar]
Pogosova 2009 {published data only}
- Pogosova GV, Koltunov IE, Karpova AV, Eliseeva NA, Sapunova ID. Clinical efficacy of escitalopram in patients with ischemic heart disease and comorbid depression. Kardiologiia 2009;49:4-8. [PubMed] [Google Scholar]
Rakowska 2015 {published data only}
- Rakowska JM. Brief strategic therapy in first myocardial infarction patients with increased levels of stress: a randomized clinical trial. Anxiety, Stress, & Coping 2015;28(6):687-705. [DOI] [PubMed] [Google Scholar]
Rollman 2009 {published data only}
- Donohue JM, Belnap BH, Men A, He F, Roberts MS, Schulberg HC, et al. Twelve-month cost-effectiveness of telephone-delivered collaborative care for treating depression following CABG surgery: a randomized controlled trial. General Hospital Psychiatry 2014;36(5):453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA 2009;302:2095-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF 3rd. The Bypassing the Blues treatment protocol: stepped collaborative care for treating post-CABG depression. Psychosomatic Medicine 2009;71:217-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2020 {published data only}18912772
- Schneider LH, Hadjistavropoulos HD, Dear BF, Titov N. Efficacy of internet-delivered cognitive behavioural therapy following an acute coronary event: a randomized controlled trial. Internet Interventions 2020;21:100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrader 2005 {published data only}
- Cheok F, Schrader G, Banham D, Marker J, Hordacre AL. Identification, course, and treatment of depression after admission for a cardiac condition: rationale and patient characteristics for the Identifying Depression As a Comorbid Condition (IDACC) project. American Heart Journal 2003;146:978-84. [DOI] [PubMed] [Google Scholar]
- Schrader G, Cheok F, Hordacre AL, Marker J, Wade V. Effect of psychiatry liaison with general practitioners on depression severity in recently hospitalised cardiac patients: a randomised controlled trial. Medical Journal of Australia 2005;182:272-6. [DOI] [PubMed] [Google Scholar]
- Wade V, Cheok F, Schrader G, Hordacre AL, Marker J. Depression after cardiac hospitalisation - the Identifying Depression as a Comorbid Condition (IDACC) study. Australian Family Physician 2005;34:985-9. [PubMed] [Google Scholar]
Sogolitappeh 2009 {published and unpublished data}
- Sogolitappeh FN, Aliloo MM, Kheyroddin JB, Tabrizi MT. Effectiveness of group life skills training on decreasing anxiety and depression among heart patients, after bypass surgery. Iranian Journal of Psychiatry and Clinical Psychology 2009;15:50-6. [Google Scholar]
Soucy 2019 {published data only}
- Soucy I, Provencher MD, Fortier M, McFadden T. Secondary outcomes of the guided self-help behavioral activation and physical activity for depression trial. Journal of Mental Health 2019;28(4):410–8. [DOI] [PubMed] [Google Scholar]
STEP‐IN‐AMI‐2013 {published data only}
- Roncella A, Giornetti A, Cianfrocca C, Pasceri V, Pelliccia F, Denollet J, et al. Rationale and trial design of a randomized, controlled study on short-term psychotherapy after acute myocardial infarction: the STEP-IN-AMI trial (short term psychotherapy in acute myocardial infarction). Journal of Cardiovascular Medicine 2009;10:947-452. [DOI] [PubMed] [Google Scholar]
- Roncella A, Pristipino C, Cianfrocca C, Scorza S, Pasceri V, Pelliccia F, et al. One-year results of the randomized, controlled, short-term psychotherapy in acute myocardial infarction (STEP-IN-AMI) trial. International Journal of Cardiology 2013;170:132-9. [DOI] [PubMed] [Google Scholar]
Stern 1983 {published data only}
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Archives of Internal Medicine 1983;143:1719-25. [PubMed] [Google Scholar]
Strokova 2012 {published data only}
- Strokova EV, Naumova EA, Shvarts JG. Compliance to long-term treatment of cardiologic patients with mild to moderate depression: ineffectiveness of antidepressive therapy with pirlindol in randomized study. Rational Pharmacotherapy 2012;8:45-50. [Google Scholar]
SU.FOL.OM3 2012 {published data only}
- Andreeva VA, Galan P, Torrès M, Julia C, Hercberg S, Kesse-Guyot E. Supplementation with B vitamins or N-3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomized trial. American Journal of Clinical Nutrition 2012;96:208-14. [DOI] [PubMed] [Google Scholar]
SUPRIM 2011 {published data only}
- Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svärdsudd K. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: secondary prevention in Uppsala primary health care project (SUPRIM). Archives of Internal Medicine 2011;171:134-40. [DOI] [PubMed] [Google Scholar]
TAKE Heart 2010 {published data only}
- Eckert K, Schrader G, Wilkinson D, Askew D, Dick M, Wade T, et al. Detection and management of depression in patients with chronic heart disease: the Take Heart in Primary Care Cluster Randomised Controlled Trial. Heart, Lung and Circulation 2010;19:240-1. [Google Scholar]
TEAMcare 2010 {published data only}
- Katon W, Lin EH, Von Korff M, Ciechanowski P, Ludman E, Young B, et al. Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: the design of the TEAMcare study. Contemporary Clinical Trials 2010;31:312-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2012 {published data only}
- Tsai ST, Chou FH. The effectiveness of multimedia nursing education on reducing illness-related anxiety and uncertainty in myocardial infarction patients after percutaneous coronary intervention. Hu Li Za Zhi 2012;59:43-53. [DOI] [PubMed] [Google Scholar]
UPBEAT‐UK 2014 {published data only}
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT Nurse-Delivered Personalized Care Intervention for People with Coronary Heart Disease Who Report Current Chest Pain and Depression: A Randomised Controlled Pilot Study. PLoS ONE 2014;9:e98704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee A, Haddad M, Barley E, Ashworth M, Brown J, Chambers J, et al. A pilot randomised controlled trial of personalised care for depressed patients with symptomatic coronary heart disease in South London general practices: the UPBEAT-UK RCT protocol and recruitment. BMC Psychiatry 2012;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vasiuk 2010 {published data only}
- Vasiuk IuA, Lebedev AV, Dovzhenko TV, Semiglazova MV. Affective disorders in acute myocardial infarction and possibilities of their correction with tianeptin. Terapevticheskii Arkhiv 2010;82:28-33. [PubMed] [Google Scholar]
Veith 1982 {published data only}
- Veith RC, Raskind MA, Caldwell JH, Barnes RF, Gumbrecht G, Ritchie JL. Cardiovascular effects of tricyclic antidepressants in depressed patients with chronic heart disease. New England Journal of Medicine 1982;306:954-9. [DOI] [PubMed] [Google Scholar]
WELL.ME 2012 {published data only}
- Compare A, Kouloulias V, Apostolos V, Peña WM, Molinari E, Grossi E, et al. Retraction Note: WELL.ME - Wellbeing therapy based on real-time personalized mobile architecture, vs. cognitive therapy, to reduce psychological distress and promote healthy lifestyle in cardiovascular disease patients: study protocol for a randomized controlled trial. Trials 2018;19:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compare A, Kouloulias V, Apostolos V, Peña WM, Molinari E, Grossi E, et al. WELL.ME - Wellbeing therapy based on real-time personalized mobile architecture, vs. cognitive therapy, to reduce psychological distress and promote healthy lifestyle in cardiovascular disease patients: study protocol for a randomized controlled trial. Trials 2012;13:157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Zeng 2001 {published data only}
- Zeng W, Ma H, Liang Q, Dong Y, Ye H, Zhang Y. The influence of antidepressive therapy on short-term prognosis in elderly patients with unstable angina and depression. Zhonghua Nei Ke Za Zhi 2001;40:809-10. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ahangarezaiezadeh 2017 {published data only (unpublished sought but not used)}
- Ahangarezaiezadeh S, Oladrostam N, Nemotplahi A. The effect of positive thinking on stress, anxiety and depression in coronary heart disease. Nursing and Midwifery Journal 2017;15:339-48. [Google Scholar]
- IRCT2016022026347N2. The effect of positive thinking on stress, anxiety and depression in coronary patients. www.who.int/trialsearch/trial2.aspx?Trialid=irct2016022026347n2 (first received 13 July 2016).
Cai 2012 {published data only (unpublished sought but not used)}
- Cai JM, Xie DY. Clinical analysis on deanxit treating unstable angina patients with anxiety-depression. Heart 2012;98 Suppl 2:GW23-e1783. [Google Scholar]
CoroDep 2019 {published data only}
- NCT03841474. The changes in functional recovery and brain neurotrophic factor six months after percutaneous coronary intervention in cardiovascular patients with depression. clinicaltrials.gov/ct2/show/NCT03841474 (first received 15 February 2019). [NCT: 03841474]
Gu 2017 {published data only (unpublished sought but not used)}
- Gu Y, Zhu Y. Effects of mindfulness-based stress reduction on markers of cardiovascular risk in patients with ischemic heart disease a randomized controlled trial. Journal of the American College of Cardiology 2017;70(16):C166. [DOI: DOI: 10.1016/j.jacc.2017.07.592] [Google Scholar]
References to ongoing studies
Ahmadi 2018 {published data only (unpublished sought but not used)}
- IRCT20180312039056N2. Effectiveness of rumination-focused cognitive-behavioral therapy on improvement depression and anxiety in patients with coronary heart disease. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180312039056N2 (first received 14 October 2018).
Ardakani 2020 {published data only}IRCT20140126016374N2
- IRCT20140126016374N2. The study of of escitalopram's effectiveness on treatment of mild to moderate depressive disorder and improvement of quality of life in patients who are undergone coronary artery bypass graft surgery (CABG) – a randomized double blind placebo controlled trial. en.irct.ir/trial/49571?revision=159827 (first received 11 November 2020). [IRCT: 20140126016374N2]
COMBAT‐DS 2021 {published data only}
- NCT04986969. Online Cognitive Behavioral Therapy for Depressive Symptoms in Rural Patients With Cardiac Disease (COMBAT-DS). clinicaltrials.gov/ct2/show/NCT04986969 (first received 3 August 2021).
eMindYourHeart 2021 {published data only}
- Pedersen SS, Andersen CM, Ahm R, Skovbakke SJ, Kok R, Helmark C, et al. Efficacy and cost-effectiveness of a therapist-assisted web-based intervention for depression and anxiety in patients with ischemic heart disease attending cardiac rehabilitation (eMindYourHeart trial): a randomised controlled trial protocol. BMC Cardiovascular Disorders 2021;21:20. [HTTPS://CLINICALTRIALS.GOV/: NCT04172974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Firouzjaei 2017 {published data only (unpublished sought but not used)}
- IRCT2017080835568N1. The effectiveness of cognitive behavioral therapy on symptoms of depression, dysfunctional attitudes and level of social support in patients with cardiovascular disease. en.irct.ir/trial/26838 (first received 13 August 2017). [WHO CLINICAL TRIAL ID: IRCT2017080835568N1]
Geng 2018 {published data only (unpublished sought but not used)}
- ChiCTR1800014291. Effect of guanxin danshen dropping pill on clinical anxiety, depression, heart rate variability and cardiovascular prognosis in patients with coronary heart disease after PCI combined with anxiety or depression. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1800014291 (first received 4 January 2018). [WHO CLINICAL TRIAL ID: chictr1800014291]
Hamzehpour 2020 {published data only}IRCT20190525043700N2
- Hamzehpour R. Comparison of bupropion and ecitalopram efficacy on depressive symptoms after coronary artery bypass graft surgery. en.irct.ir/trial/49626 (registration date 25 July 2020).
Irfan 2020 {published data only}
- NCT04347525. The effect of culturally adapted cognitive behaviour therapy (CaCBT)-based guided self help in depressed patients with myocardial infarction. A randomised controlled trial. clinicaltrials.gov/show/NCT04347525 (first received 15 April 2020).
Jazayeri 2017 {published data only}IRCT201702262394N36
- Jazayeri S. Effect of hesperidin supplementation on depressive symptoms, serum levels of BDNF and cortisol in patients after myocardial infarction. www.irct.ir/trial/2090 (registration date 26 April 2017). [IRCT: 201702262394N36]
Luberto 2021 {published data only}
- MBCT via group videoconferencing for acute coronary syndrome patients with depressive symptoms: a pilot RCT. Ongoing study. April 2021. Contact author for more information.
Ma 2014 {published data only (unpublished sought but not used)}
- Ma WL. The therapeutic effect of statins on patients with depression after acute coronary syndrome. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPR-14005672 (date of registration 15 December 2014). [WHO CLINICAL TRIAL ID: ChiCTR-IPR-14005672]
Mohammadian 2018 {published data only}
- Mohammadian R. The efficacy of cognitive-behavioral therapy based on rumination on depression, anxiety and hostility in cardiac patients. https://www.irct.ir/trial/36370 (registration date 27 March 2019)).
Moudi 2016 {published data only (unpublished sought but not used)}
- Moudi S. The efficacy of citalopram in depression and quality of life in the patients with cardiac disease. en.irct.ir/trial/19730 (registration date 30 September 2016). [WHO CLINICAL TRIAL ID: IRCT2016091722991N6]
Qiaoning 2019 {published data only}ChiCTR1900023457
- Effect of guanxindanshen dropping pills on quality of life and cardiovascular prognosis of patients with depression or anxiety after PCI for coronary heart disease. Ongoing study. 1 March 2017. Contact author for more information.
Sourizahi 2017 {published data only (unpublished sought but not used)}
- Sourizahi M. Comparison of therapeutic effect of sertraline and supportive psychotherapy in comparison with placebo in coronary heart disease patients with mild to moderate depression. en.irct.ir/trial/27596 (registration date 26 December 2017). [WHO CLINICAL TRIAL ID: IRCT20171105037241N2]
Wang 2015 {published data only (unpublished sought but not used)}
- Wang Z. Intervention study of the patients with mild depressive mood after percutaneous coronary intervention in the treatment of the patients with collateral vessels based on the hypothesis of. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-15007378 (date of registration 11 November 2015). [WHO CLINICAL TRIAL ID: ChiCTR-INR-15007378]
Yang 2020 {published data only (unpublished sought but not used)}
- The efficacy and safety of Ginkgo biloba dropping pills in the treatment of coronary heart disease with stable angina pectoris and depression. Ongoing study. 20 September 2020. Contact author for more information.
Additional references
Abberger 2017
- Abberger B, Haschke A, Tully PJ, Forkmann T, Berger J, Wirtz M, et al. Development and validation of parallel short forms PaSA-cardio for the assessment of general anxiety in cardiovascular rehabilitation patients using Rasch analysis. Clinical Rehabilitation 2017;31(1):104-14. [DOI] [PubMed] [Google Scholar]
Alpert 2000
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined - a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Journal of the American College of Cardiology 2000;36:959-69. [DOI] [PubMed] [Google Scholar]
Anderson 2016
- Anderson L, Oldridge N, Thompson DR, Zwisler A-D, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD001800. [DOI: 10.1002/14651858.CD001800.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antman 2004
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004;44:e82-292. [PubMed] [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 3rd edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Appleton 2015
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega‐3 fatty acids for depression in adults. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD004692. [DOI: 10.1002/14651858.CD004692.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Psychological Society 2018
- The Australian Psychological Society. Evidence-Based Psychological Interventions in the Treatment of Mental Disorders: a Literature Review. 4th edition. The Australian Psychological Society Ltd, 2018. [Google Scholar]
Barth 2004
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosomatic Medicine 2004;66:802-13. [DOI] [PubMed] [Google Scholar]
Barth 2013
- Barth J, Munder T, Gerger H, Nüesch E, Trelle S, Znoj H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLoS Medicine 2013;10:e1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumeister 2005
- Baumeister H, Balke K, Härter M. Psychiatric and somatic comorbidities are negatively associated with quality of life in physically ill patients. Journal of Clinical Epidemiology 2005;58:1090-100. [DOI] [PubMed] [Google Scholar]
Baumeister 2008
- Baumeister H, Morar V. The impact of clinical significance criteria on subthreshold depression prevalence rates. Acta Psychiatrica Scandinavica 2008;118:443-50. [DOI] [PubMed] [Google Scholar]
Baumeister 2009a
- Baumeister H, Maercker A, Casey P. Adjustment disorders with depressed mood: a critique of its DSM-IV and ICD-10 conceptualization and recommendations for the future. Psychopathology 2009;42:139-47. [DOI] [PubMed] [Google Scholar]
Baumeister 2009b
- Baumeister H, Kufner K. It is time to adjust the adjustment disorder category. Current Opinion in Psychiatry 2009;22:409-12. [DOI] [PubMed] [Google Scholar]
Baumeister 2010a
- Baumeister H. A clinical significance criterion is essential for diagnosing subthreshold depression. American Journal of Psychiatry 2010;167:866. [DOI] [PubMed] [Google Scholar]
Baumeister 2010b
- Baumeister H, Parker G. A second thought on subtyping major depression. Psychotherapy and Psychosomatics 2010;79:388-9. [DOI] [PubMed] [Google Scholar]
Baumeister 2011a
Baumeister 2011b
- Baumeister H. Inappropriate prescriptions of antidepressant drugs in patients with subthreshold to mild depression: time for the evidence to become practice. Journal of Affective Disorders 2012;139(3):240-3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Baumeister 2012a
- Baumeister H, Parker G. Meta-review of depressive subtyping models. Journal of Affective Disorders 2012;139:126-40. [DOI] [PubMed] [Google Scholar]
Baumeister 2012b
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD008381. [DOI: 10.1002/14651858.CD008381.pub2] [DOI] [PubMed] [Google Scholar]
Baumeister 2015
- Baumeister H, Haschke A, Munzinger M, Hutter N, Tully PJ. Inpatient and outpatient costs in patients with coronary artery disease and mental disorders: a systematic review. BioPsychoSocial Medicine 2015;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baune 2012
- Baune B, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, et al. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models.. Translational Psychiatry 2012;2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bech 2010
- Bech P. Is the antidepressive effect of second-generation antidepressants a myth? Psychological Medicine 2010;40:181-6. [DOI] [PubMed] [Google Scholar]
Bendig 2018
- Bendig E, Bauereiss N, Ebert DD, Snoek F, Andersson G, Baumeister H. Internet-based interventions in chronic somatic disease. Deutsches Arzteblatt International 2018;115:659-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosco 2021
- Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Medical Research Methodology 2021;21(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2017
- Carney RM, Freedland KE. Depression and coronary heart disease. Nature Reviews Cardiology 2017;14:145-55. [DOI] [PubMed] [Google Scholar]
Carr 2017
- Carr MM, Saules KK, Koch EI, Waltz TJ. Testing the dose-response curve in a training clinic setting: use of client pretreatment factors to minimize bias in estimates. Training and Education in Professional Psychology 2017;11:26-32. [Google Scholar]
Chen 2019
- Chen SF, Chien YH, Chen PC, Wang IJ. Association of age with risk of major depression among patients with chronic kidney disease over midlife: a nationwide cohort study in Taiwan. International Psychogeriatrics 2019;31(8):1171-9. [DOI] [PubMed] [Google Scholar]
Cipriani 2016
- Cipriani A, Saunders K, Attenburrow MJ, Stefaniak J, Panchal P, Stockton S, et al. A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Molecular Psychiatry 2016;21:1324-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic, 1988. [Google Scholar]
Collopy 2021
- Collopy CM, Cosh SM, Tully PJ. Screening and referral is not enough: a qualitative exploration of barriers to access and uptake of mental health services in patients with cardiovascular diseases. BMC Health Services Research 2021;21(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2008a
- Cuijpers P, Straten A, Andersson G, Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology 2008;76:909-22. [DOI] [PubMed] [Google Scholar]
Cuijpers 2008b
- Cuijpers P, Straten A, Andersson G, Oppen P. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. Journal of Clinical Psychiatry 2008;69:1675-85. [DOI] [PubMed] [Google Scholar]
Cuijpers 2010
- Cuijpers P, Straten A, Bohlmeijer E, Hollon SD, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychological Medicine 2010;40:211-23. [DOI] [PubMed] [Google Scholar]
Cuijpers 2013
- Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry 2013;12:137-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2016
- Cuijpers P. Are all psychotherapies equally effective in the treatment of adult depression? The lack of statistical power of comparative outcome studies. Evidence-Based Mental Health 2016;19:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2006
- Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute working group report. Annals of Behavioral Medicine 2006;32:121-6. [DOI] [PubMed] [Google Scholar]
Davison 2003
- Davison GC, Neale JM. Abnormal Psychology. New York, NY: John Wiley & Sons, 2003. [Google Scholar]
de Boer 1995
- Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. International Clinical Psychopharmacology 1995;10:19-23. [DOI] [PubMed] [Google Scholar]
Dempe 2013
- Dempe C, Jünger J, Hoppe S, Katzenberger ML, Möltner A, Ladwig KH, et al. Association of anxious and depressive symptoms with medication nonadherence in patients with stable coronary artery disease. Journal of Psychosomatic Research 2013;74:122–7. [DOI] [PubMed] [Google Scholar]
Dickens 2008
- Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. New onset depression following myocardial infarction predicts cardiac mortality. Psychosomatic Medicine 2008;70:450-5. [DOI] [PubMed] [Google Scholar]
Doyle 2021
- Doyle F, Freedland KE, Carney RM, Jonge P, Dickens C, Pedersen SS, et al. Hybrid systematic review and network meta-analysis of randomized controlled trials of interventions for depressive symptoms in patients with coronary artery disease. Psychosomatic Medicine 2021;83:423-31. [DOI] [PubMed] [Google Scholar]
Firth 2019
- Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675-712. [DOI] [PubMed] [Google Scholar]
Fournier 2010
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity. A patient-level meta-analysis. JAMA 2010;303:47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2006
- Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. European Heart Journal 2006;27:1341-81. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 2003
- Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry 2003;60:627-36. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 2003a
- Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry 2003;60(6):627-36. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 2008
- Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of cardiac events in patients with stable coronary artery disease. Archives Of General Psychiatry 2008;65(1):62-71. [DOI] [PubMed] [Google Scholar]
Gerlach 2017
- Gerlach LB, Kales HC, Maust DT, Chiang C, Stano C, Choe HM, et al. Unintended consequences of adjusting citalopram prescriptions following the 2011 FDA warning. American Journal of Geriatric Psychiatry 2017;25(4):407-14. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed prior to 7 December 2021). Available at gradepro.org.
Harnett 2010
- Harnett P, O'Donovan A, Lambert MJ. The dose response relationship in psychotherapy: implications for social policy. Clinical Psychologist 2010;14:39-44. [Google Scholar]
Härter 2007
- Härter M, Baumeister H, Reuter K, Jacobi F, Höfler M, Bengel J, et al. Increased 12-month prevalence rates of mental disorders in patients with chronic somatic diseases. Psychotherapy and Psychosomatics 2007;76:354-60. [DOI] [PubMed] [Google Scholar]
Härter 2007a
- Härter M, Baumeister H. Etiology of mental disorders in chronic somatic diseases [Ätiologie psychischer Störungen bei chronischen körperlichen Erkrankungen]. In: Härter M, Baumeister H, Bengel J, editors(s). Psychische Störungen bei körperlichen Erkrankungen. Heidelberg: Springer, 2007:1-13. [Google Scholar]
Haschke 2012
- Haschke A, Hutter N, Baumeister H. Indirect costs in patients with coronary artery disease and mental disorders: a systematic review and meta-analysis. International Journal of Occupational Medicine and Environmental Health 2012;25:319-29. [DOI] [PubMed] [Google Scholar]
Herrmann‐Lingen 2006
- Herrmann-Lingen C, Buss U. Anxiety and depression in patients with coronary heart disease. In: Jordan J, Bardé B, Zeiher AM, editors(s). Contributions Toward Evidence-Based Psychocardiology: a Systematic Review of the Literature. Washington, DC: American Psychological Association, 2006:125-57. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hillis 2011
- Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124(23):e652-735. [DOI] [PubMed] [Google Scholar]
Jani 2013
- Jani BD, Purves D, Barry S, Cavanagh J, McLean G, Mair FS. Challenges and implications of routine depression screening for depression in chronic disease and multimorbidity. PLoS ONE 2013;8:e74610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joynt 2003
- Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biological Psychiatry 2003;54:248-61. [DOI] [PubMed] [Google Scholar]
Karyotaki 2016
- Karyotaki E, Smit Y, Holdt Henningsen K, Huibers MJ, Robays J, Beurs D, et al. Combining pharmacotherapy and psychotherapy or monotherapy for major depression? A meta-analysis on the long-term effects. Journal of Affective Disorders 2016;194:144-52. [DOI] [PubMed] [Google Scholar]
Kaster 2016
- Kaster MP, Moretti M, Cunha MP, Rodrigues AL. Novel approaches for the management of depressive disorders. European Journal of Pharmacology 2016;771:236–40. [DOI] [PubMed] [Google Scholar]
Kendler 2009
- Kendler KS, Gardner CO, Fiske A, Gatz M. Major depression and coronary artery disease in the Swedish twin registry: phenotypic, genetic, and environmental sources of comorbidity. Archives of General Psychiatry 2009;66(8):857-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2010
- Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychological Medicine 2010;40(2):225-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J In: Higgins JPT & Green S (editors). Chapter 6: Searching for studies. In: Higgins JP & Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from training.cochrane.org/handbook.
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ (Clinical Research Ed.) 2003;326(7400):1167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichtenberg 2010
- Lichtenberg P, Belmaker RH. Subtyping major depressive disorder. Psychotherapy and Psychosomatics 2010;79:131-5. [DOI] [PubMed] [Google Scholar]
Lichtman 2008
- Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768-75. [DOI] [PubMed] [Google Scholar]
Lichtman 2014
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350-69. [DOI] [PubMed] [Google Scholar]
Matte 2016
- Matte DL, Pizzichini MM, Hoepers AT, Diaz AP, Karloh M, Dias M, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respiratory Medicine 2016;117:154-61. [DOI] [PubMed] [Google Scholar]
Mezuk 2015
- Mezuk B, Heh V, Prom-Wormley E, Kendler KS, Pedersen NL. Association between major depression and type 2 diabetes in midlife: findings from the Screening Across the Lifespan Twin Study. Psychosomatic Medicine 2015;77(5):559-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Jadad AR. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technology Assessment 1999;29:823-32. [PubMed] [Google Scholar]
Musselman 1998
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of General Psychiatry 1998;55:580-92. [DOI] [PubMed] [Google Scholar]
Nemeroff 2003
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. PNAS 2003;100:14293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Depression in adults with a chronic physical health problem: recognition and management Clinical guideline [CG91]. www.nice.org.uk/guidance/CG91 (accessed 26 July 2011).
Nicholson 2006
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 146 538 participants. European Heart Journal 2006;27(23):2763-74. [DOI] [PubMed] [Google Scholar]
Nieuwsma 2017
- Nieuwsma JA, Williams JW Jr, Namdari N, Washam JB, Raitz G, Blumenthal JA, et al. Diagnostic accuracy of screening tests and treatment for post-acute coronary syndrome depression: a systematic review. Annals of Internal Medicine 2017;167(10):725-35. [DOI] [PubMed] [Google Scholar]
Ormel 2007
- Ormel J, Von Korff M, Burger H, Scott K, Demyttenaere K, Huang YQ, et al. Mental disorders among persons with heart disease - results from World Mental Health surveys. General Hospital Psychiatry 2007;29:325-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrak 2015
- Petrak F, Baumeister H, Skinner TC, Brown A, Holt RI. Depression and diabetes: treatment and health-care delivery. Lancet Diabetes & Endocrinology 2015;3(6):472-85. [DOI] [PubMed] [Google Scholar]
Pigott 2010
- Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: current status of research. Psychotherapy and Psychosomatics 2010;79:267-79. [DOI] [PubMed] [Google Scholar]
Rayner 2010
- Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD007503. [DOI: 10.1002/14651858.CD007503.pub2] [DOI] [PubMed] [Google Scholar]
Richards 2017
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD002902. [DOI: 10.1002/14651858.CD002902.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rochester 2018
- Rochester MP, Kane AM, Linnebur SA, Fixen DR. Evaluating the risk of QTc prolongation associated with antidepressant use in older adults: a review of the evidence. Therapeutic Advances in Drug Safety 2018;9(6):297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollman 2009
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA 2009;302:2095-103. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2017
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology 2017;70(1):1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadock 2009
- Sadock BJ, Sadock VA, Ruiz P, editor(s). Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th edition. Philadelphia; London: Lippincott Williams & Wilkins, 2009. [Google Scholar]
Scherrer 2003
- Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosomatic Medicine 2003;65(4):548-57. [DOI] [PubMed] [Google Scholar]
Scherrer 2012
- Scherrer JF, Chrusciel T, Garfield LD, Freedland KE, Carney RM, Hauptman PJ, et al. Treatment-resistant and insufficiently treated depression and all-cause mortality following myocardial infarction. British Journal of Psychiatry 2012;200(2):137-42. [DOI] [PubMed] [Google Scholar]
Schulman‐Marcus 2016
- Schulman-Marcus J, Shah T, Swaminathan RV, Feldman DN, Wong SC, Singh HS, et al. Comparison of recent trends in patients with and without major depression and acute ST-elevation myocardial infarction. American Journal of Cardiology 2016;118(6):779-84. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Scott 2013
- Scott KM, Jonge P, Alonso J, Viana MC, Liu Z, O'Neill S, et al. Associations between DSM-IV mental disorders and subsequent heart disease onset: beyond depression. International Journal of Cardiology 2013;168(6):5293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skala 2006
- Skala JA, Freedland KE, Carney RM. Coronary heart disease and depression: a review of recent mechanistic research. Canadian Journal of Psychiatry 2006;51:738-45. [DOI] [PubMed] [Google Scholar]
Stenman 2014
- Stenman M, Holzmann MJ, Sartipy U. Relation of major depression to survival after coronary artery bypass grafting. American Journal of Cardiology 2014;114(5):698-703. [DOI] [PubMed] [Google Scholar]
Swenson 2006
- Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Canadian Journal of Psychiatry 2006;51(14):923-9. [DOI] [PubMed] [Google Scholar]
Taragano 2005
- Taragano FE, Bagnatti P, Allegri RF. A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of "vascular depression". International Psychogeriatrics 2005;17:487-98. [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor D. Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety. Acta Psychiatrica Scandinavica 2008;118:434-42. [DOI] [PubMed] [Google Scholar]
Thombs 2008
- Thombs BD, Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008;300(18):2161-71. [DOI] [PubMed] [Google Scholar]
Tully 2012
- Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. Journal of Geriatric Cardiology 2012;9(2):197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tully 2015
- Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2015;5:e009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358:252-60. [DOI] [PubMed] [Google Scholar]
Van Straten 2010
- Van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. Journal of Psychosomatic Research 2010;69:23-32. [DOI] [PubMed] [Google Scholar]
Wakefield 2010
- Wakefield JC, Schmitz MF, Baer JC. Does the DSM-IV clinical significance criterion for major depression reduce false positives? Evidence from the National Comorbidity Survey Replication. American Journal of Psychiatry 2010;167:298-304. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical description and diagnostic guidelines. 10th edition. Geneva: World Health Organization, 1992. [Google Scholar]
Whooley 2008
- Whooley MA, Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008;300:2379-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkley 2020
- Winkley K, Upsher R, Stahl D, Pollard D, Brennan A, Heller S, et al. Systematic review and meta-analysis of randomized controlled trials of psychological interventions to improve glycaemic control in children and adults with type 1 diabetes. Diabetic Medicine 2020;37(5):735-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Baumeister 2011c
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD008012. [DOI: 10.1002/14651858.CD008012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


