CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Dear editors,
Mounting evidence has demonstrated that the genetic factors play an important role in PD (Parkinson's disease). Previous studies have identified several genetic variants that are associated with Parkinson's disease in eastern China. 1 , 2 Recently, Uladzislau Rudakou and colleagues identified a rare 3′ UTR variant (rs945006601), a rare 5′ UTR variant (rs1034608171), the rare nonsynonymous variants that drive the SYT11, FGF20, GCH1 associated with PD in three cohorts, respectively. 3 In addition, they found that common variants in MAPT, TMEM175, BST1, SNCA, and GPNMB are linked with PD. Since race is an important factor for the genetic association test, we investigated these rare variants in SYT11, FGF20, and GCH1 genes in our eastern Chinese Han population.
In our study, 516 patients with sporadic PD (mean age: 61.35 ± 10.56; male/female: 289/227) and 498 ethnicity‐, age‐, and sex‐matched healthy controls (mean age: 60.14 ± 11.44; male/female: 287/211) were included by two movement disorder specialists according to Movement Disorder Society clinical diagnostic criteria in the second affiliated hospital of Zhejiang University of Medicine (Hangzhou, China). 4 All participants have provided written informed consent. The ethics of this study was approved by the ethics committee of the second affiliated hospital of Zhejiang University of Medicine.
All blood samples were collected, and then genomic DNA was extracted from peripheral blood leucocytes using QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer's protocol. We sequenced these rare variants using the MassArray and KASP assays, and the details of the primers were provided in Tables S1 and S2. The results were analyzed using a MassARRAY Analyzer (Agena Bioscience) and an ABI 7900 Analyzer (Applied Biosystems), respectively. We used Pearson's chi‐square test to compare allelic frequencies of these 10 rare variants between PD and healthy controls. All statistical analyses were conducted using SPSS20.0 (IBM). We predicted the pathogenicity of the variant (position: 55312562; c.C552T, p.R186C) by CADD, Polyphen2, SIFT, MutationTaster in VarCards website (http://varcards.biols.ac.cn/). The threshold values were set for the harmfulness of the variants: CADD>15; Polyphen2 > 0.86; SIFT<0.05.
In our cohort, we did not find any significantly different rare variants between PD patients and healthy controls reported by Uladzislau Rudakou. One nonsynonymous variant (position: 55312562; c.C552T, p.R186C) in the GCH1 gene was found in eight PD patients and four healthy controls (Table 1). The p.R186C variant is located in the GTP cyclohydrolase 1 domain and is highly conserved across different species (Figure 1), suggesting that the p.R186C variant might change the function of GCH1 and contribute to the pathogenesis of PD. Moreover, all pathogenicity predictions in silico (CADD, Polyphen2, SIFT, MutationTaster) indicated that the p.R186C variant was harmful to PD (Figure 1). However, there is no statistically significant difference between PD patients and healthy controls in our cohort.
TABLE 1.
Variants of SYT11, FGF20, and GCH1 genes identified in our cohort
| Gene | Position | dbSNP ID | Ref/Alt | PD | Control | p | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| SYT11 | 155852207 | rs945006601 | G/C | 1030/0 | 998/0 | ||
| FGF20 | 16859615 | rs1034608171 | G/C | 1032/0 | 998/0 | ||
| GCH1 | 55312498 | NA | A/T | 1032/0 | 1000/0 | ||
| 55312559 | NA | G/C | 1032/0 | 1000/0 | |||
| 55312562 | NA | G/A | 1012/8 | 986/4 | 0.269 | 1.949 (0.585–6.492) | |
| 55332087 | NA | C/T | 1032/0 | 1000/0 | |||
| 55310826 | rs104894434 | A/G | 1032/0 | 998/0 | |||
| 55312502 | rs200891969 | C/T | 1012/0 | 986/0 | |||
| 55310817 | rs41298442 | T/C | 1020/0 | 992/0 | |||
| 55326454 | rs756256944 | C/T | 1032/0 | 996/0 |
Abbreviations: Alt, alternate allele; OR, odds ratio; PD, Parkinson's disease; Ref, reference allele.
FIGURE 1.
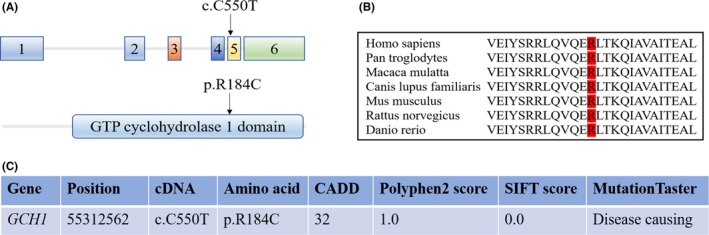
Schematic of human GCH1 and pathogenetic analysis of c.C552T, p.R186C variant. (A) The c.C552T, p.R186C variant locates in GCH1 gene and GCH1 protein. (B) The position and the surrounding sequence of GCH1 p.R186C are highly conserved across different species. (C) Pathogenicity prediction of c.C552T, p.R186C variant in silico. Threshold values for deleteriousness: CADD greater than 15; Polyphen2 greater than 0.86; SIFT less than 0.05 [Colour figure can be viewed at wileyonlinelibrary.com]
The SYT11 protein, synaptotagmin‐11, is a synaptotagmin isoform, which regulates membrane trafficking in synaptic transmission. 5 Genetic studies have reported that the mutations in the SYT11 gene were linked with PD and schizophrenia. 6 , 7 Previous studies have suggested that the variants in the nearby GBA gene drive the SYT11 GWAS association with PD. 8 However, after exclusion of all GBA variant carriers, Uladzislau Rudakou and colleagues found that the rs945006601 in the SYT11 gene remained statistically significant between PD patients and healthy controls. Therefore, the variant rs945006601 in the SYT11 gene linked with PD was independent of GBA variants. Conversely, in our eastern Chinese Han population, we did not find rs945006601 which likely indicated the important effect of ethnic context in SYT11 variants.
The protein encoded by FGF20, fibroblast growth factor 20, is a neurotrophic factor preferentially expressed in the substantia nigra pars compacta. FGF20 can significantly improve the survival of cultured dopaminergic neurons via activating the MAPK pathway. FGF20 protects against the loss of dopaminergic neurons in the rat model of PD. 9 Mounting evidence has indicated that variants in the FGF20 gene were risk factors for PD in different ethnic populations. 10 , 11 Uladzislau Rudakou et al. found a novel rare variant (rs1034608171) located in the promoter region of the FGF20 gene which drives the significant association with PD. However, this variant did not exist in our PD patients and healthy controls. The relatively smaller sample size or different ethnic context might have contributed to our failure in detecting this variate.
The GCH1 gene, which encodes GTP cyclohydrolase 1, belongs to the GTP cyclohydrolase family of enzymes known to be involved in the biosynthesis of tetrahydrobiopterin. Tetrahydrobiopterin is a cofactor for tyrosine hydroxylase that is a rate‐limiting enzyme for dopamine biosynthesis. Therefore, variants in the GCH1 gene could cause dopa‐responsive dystonia. 12 Moreover, many genetic association studies have also identified GCH1 variants that increased the risk for PD. 13 , 14 In our cohort, we only found one nonsynonymous variant (c.C552T, p.R186C) that exists in eight Parkinson's disease patients and four healthy controls. Moreover, analysis of conservation across different species and prediction of pathogenesis all suggested that the p.R186C variant may contribute to the pathogenesis of PD.
Taken together, our results indicated that the 10 variants in SYT11, FGF20, GCH1 genes may not be causative variants for PD in eastern China. However, p.R186C variant likely increases the risk of PD. In consideration of the different results due to the diverse ethnic contexts, additional genetic association studies with larger samples and different ethnic cohorts are needed to identify the role of the variants in SYT11, FGF20, GCH1 gene in PD.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
We express our gratitude to all the patients and their families who participated in this study.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Shen YT, Wang JW, Wang M, et al. BST1 rs4698412 allelic variant increases the risk of gait or balance deficits in patients with Parkinson's disease. CNS Neurosci Ther. 2019;25(4):422‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao T, Wu J, Zheng R, et al. Assessment of three essential tremor genetic loci in sporadic Parkinson's disease in Eastern China. CNS Neurosci Ther. 2020;26(4):448‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rudakou U, Yu E, Krohn L, et al. Targeted sequencing of Parkinson's disease loci genes s SYT11, FGF20 and other associations. Brain. 2020;144(2):462‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591‐1601. [DOI] [PubMed] [Google Scholar]
- 5. Xie Z, Long J, Liu J, Chai Z, Kang X, Wang C. Molecular mechanisms for the coupling of endocytosis to exocytosis in neurons. Front Mol Neurosci. 2017;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue S, Imamura A, Okazaki Y, et al. Synaptotagmin XI as a candidate gene for susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(3):332‐340. [DOI] [PubMed] [Google Scholar]
- 7. Sesar A, Cacheiro P, Lopez‐Lopez M, et al. Synaptotagmin XI in Parkinson's disease: new evidence from an association study in Spain and Mexico. J Neurol Sci. 2016;362:321‐325. [DOI] [PubMed] [Google Scholar]
- 8. Blauwendraat C, Bras JM, Nalls MA, et al. Coding variation in GBA explains the majority of the SYT11‐GBA Parkinson's disease GWAS locus. Mov Disord. 2018;33(11):1821‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itoh N, Ohta H. Roles of FGF20 in dopaminergic neurons and Parkinson's disease. Front Mol Neurosci. 2013;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun XY, Wang L, Cheng L, et al. Genetic analysis of FGF20 in Chinese patients with Parkinson's disease. Neurol Sci. 2017;38(5):887‐891. [DOI] [PubMed] [Google Scholar]
- 11. Sadhukhan D, Das G, Biswas A, et al. Evaluation of FGF 20 variants for susceptibility to Parkinson's disease in Eastern Indians. Neurosci Lett. 2018;675:68‐73. [DOI] [PubMed] [Google Scholar]
- 12. Cao L, Zheng L, Tang WG, et al. Four novel mutations in the GCH1 gene of Chinese patients with dopa‐responsive dystonia. Mov Disord. 2010;25(6):755‐760. [DOI] [PubMed] [Google Scholar]
- 13. Mencacci NE, Isaias IU, Reich MM, et al. Parkinson's disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137(Pt 9):2480‐2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan HX, Zhao YW, Mei JP, et al. GCH1 variants contribute to the risk and earlier age‐at‐onset of Parkinson's disease: a two‐cohort case‐control study. Transl Neurodegener. 2020;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
Data available on request from the authors.


