Abstract
Streptococcus pyogenes, a major human pathogen, is still considered susceptible to beta-lactams, but for other relevant antibiotics, highly variable resistance rates have been reported. Since no data were available from Iran, we tested 1,335 throat isolates from two different regions of the country for their antibiotic susceptibilities and, for comparison, a collection of 80 strains isolated from 1989 to 1991. Erythromycin resistance was uncommon (0.6%), whereas an overall high rate of tetracycline resistance was found, increasing between 1989–1991 and 1995–1997 from 23 to 42%. The tetracycline-resistant strains belonged to more than 10 different T types, the majority being types 4, 11, and B3264. By conventional M typing of 406 tetracycline-resistant isolates, more than 20 different M types were found. Approximately 50% of the strains were nontypeable by T agglutination as well as serological M typing; however, by genotyping by a combined PCR–capture-enzyme-linked immunosorbent assay, many of these strains were successfully emm typed. We conclude that the high rate of tetracycline resistance among Iranian S. pyogenes isolates is due to multiclonal dissemination of resistance within the streptococcal population rather than epidemic spread of single clones.
Streptococcus pyogenes or the group A streptococcus is a major cause of acute throat and skin infections, which may characteristically be followed by the severe nonsuppurative complications rheumatic fever or acute glomerulonephritis. This pathogen may also give rise to overwhelming invasive disease, such as endometritis, sepsis, necrotizing fasciitis, and a toxic shock-like syndrome.
Penicillins are still the drugs of choice for the treatment of infections caused by S. pyogenes, whereas macrolides are preferentially used for patients allergic or nontolerant to penicillin. The first reports of erythromycin-resistant isolates of S. pyogenes from human clinical sources appeared in 1959 (12). Between 1969 and 1979, a dramatically increased frequency of resistance was reported in Japan (14), and alarming reports have also emerged from other countries during the last decade (8, 23, 27). Resistance of S. pyogenes to tetracycline, no longer used against infections with this species, was initially reported from the United Kingdom in 1954 (13).
From Iran, in particular, surveillance data on the occurrence of antibiotic resistance in S. pyogenes are scarce. The aim of the present study was to screen Iranian clinical strains for their susceptibilities to relevant antibiotics and to examine resistant strains for type and clonal diversity. We therefore tested current isolates from Tehran as well as the northern part of the country, recovered during studies on colonization rates among various populations (9). In addition, a smaller collection of strains isolated from 1989 to 1991 was included to see whether any major changes had occurred during recent years. Somewhat surprisingly, a high rate of tetracycline resistance but an absence of erythromycin resistance was found.
MATERIALS AND METHODS
Bacterial isolates.
In total, 1,335 S. pyogenes strains were isolated from two distinct regions of the country from 1995 to 1997 (9). Of these, 210 were clinical isolates from seven different laboratories, 500 were isolated from schoolchildren in central and south Tehran, and 625 were clinical isolates from three different cities in the north part of Iran (Gilan Province; approximately 3 million inhabitants). All strains were isolated from the throat. In addition, 80 S. pyogenes strains, isolated from the throats of patients with pharyngotonsillitis from 1989 to 1991, were obtained from the Pasteur Institute of Tehran.
Determination of antibiotic susceptibility.
The antibiotic susceptibilities of all strains were investigated by agar dilution with Mueller-Hinton agar (15). Penicillin G, erythromycin, and tetracycline powder were purchased from Sigma (St. Louis, Mo.). The MICs for resistant strains were redetermined by the E-test (Biodisk AB, Solna, Sweden) following the recommendations of the manufacturer. The phenotype of macrolide-lincosamide-streptogramin B (MLS) resistance was tested by disk diffusion (10, 24) with antibiotic disks from Biodisk AB.
T typing of streptococci.
T typing with antisera from the State Serum Institute, Copenhagen, Denmark, was performed by slide agglutination by previously documented methods (11).
Serological M typing of streptococci.
M typing by double diffusion in gels, detection of serum opacity factor (OF), and OF inhibition serotyping were performed by standard procedures at the Public Health Laboratory Service (PHLS)/World Health Organization (WHO) Streptococcal Reference Unit, PHLS, London, United Kingdom (11). A total of 110 tetracycline-resistant strains of different T types from Gilan Province and 296 from Tehran were randomly chosen for M typing.
PCR-capture ELISA for emm typing.
One hundred serologically T- or M-nontypeable strains and 46 M-nontypeable strains of T types 13 or B3264 were tested by a rapid PCR–capture enzyme-linked immunosorbent assay (ELISA) with probes for the identification of 19 important emm gene types of S. pyogenes (21). These included nine novel probes for emm types 9, 13, 22, 77, PT2110, PT2841, PT4245, PT4931, and PT2233. Table 1 shows the published capture probes and primers.
TABLE 1.
Capture probes and primers for emm typing of S. pyogenes
| Serotype or primer designation | Label and sequence |
|---|---|
| Serotype | |
| M, all | Biotin-TTAGTTTWGCAAGTTCTTCAGCTTGTTT |
| M1 | Biotin-TTCTATAACTTCCCTAGGATTACCATC |
| M3 | Biotin-ATGTCTAGGAAACTCTCCATTAACACT |
| M4 | Biotin-GAATCAGCCTGAGGCTTTTTAATCTC |
| M5 | Biotin-CGGGTCATTTATTGTACCCCTAGTC |
| M6 | Biotin-GCTTTGTCCGGGTTTTCTACCGTC |
| M9 | Biotin-GGCGGAAGGGGTTAAGAAGGCGGAA |
| M11 | Biotin-AGCGCTTTGCCCCGCAGCCTTAA |
| M12 | Biotin-GTAGAGTTCTGAACGCTGTTTCAG |
| M13 | Biotin-CCAGAAACAGAACAGCGTTTCTAATGA |
| M22 | Biotin-TTATTAGTTTGCTTTCTTGAGAAAT |
| M28 | Biotin-AAGTCTCAGTACTTTTTGGAGACTCC |
| M58 | Biotin-GCTTTAGCCTTTGCACTATCCGCC |
| M76 | Biotin-TTAGAAACGCTTTTAGAGTTCGCGTC |
| M77 | Biotin-GGTCTGTAATGCGGTTATGTAAGTGAT |
| M78 | Biotin-TTAGTAATACTACGAGAGTTCTGAGAC |
| PT2233 | Biotin-TCGTTTTGATCTAGCAAGTCTTGCA |
| PT4245 | Biotin-ATTCTTTTTCATTATCTTTGACAGA |
| PT4931 | Biotin-TCGATACCTTCTTCTCCAAGTTATTCT |
| Primers | |
| MF | Fluorescein-ATAAGGAGCATAAAAATGGCT |
| MR | AGCTTAGTTTTCTTCTTTGCG |
PFGE.
Selected tetracycline-resistant S. pyogenes strains were typed by pulsed-field gel electrophoresis (PFGE) by standard methods at the PHLS/WHO Streptococcal Reference Unit (25). The strains were grown overnight on horse blood agar plates containing 2% neopeptone at 37°C, and the cells were resuspended in 2 ml of solution I (1.0 M NaCl, 10 mM Tris HCl [pH 7.6]) to a density equivalent to that of a McFarland no. 5 standard. Then, 500 μl of cell suspension was mixed with 500 μl of molten 2% low-melting-point agarose (GIBCO) dissolved in solution I containing mutanolysin (Sigma) at 1.6 μg/ml, and blocks were prepared. The blocks were incubated in 2 ml of solution II (solution I plus 100 mM EDTA) containing mutanolysin at 7.0 μg/ml and lysozyme (Sigma) at 1.0 mg/ml at 37°C for 5 to 6 h. The solution was replaced with lysis buffer (0.5 M EDTA and 1% Sarkosyl [pH 9.5], containing 0.5 mg of proteinase K per ml) and, following three washes in TE (Tris-EDTA) buffer, was incubated for 4 to 5 h in a water bath at 56°C. The blocks were digested overnight at 30°C with the restriction enzyme SmaI (20 U/100 μl; Promega). The digested blocks were electrophoresed at 200 V in 1% molecular biology-grade agarose, using a Bio-Rad CHEF DRII tank, for 24 h at 14°C. The initial and final switch times were 10 and 35 s, respectively. The gel was stained with ethidium bromide, visualized on a UV transilluminator, and photographed.
Dendrogram and relatedness analysis computer program.
The Taxotrone Computer program (Taxotrone Packages) was used for PFGE analyses, according to the instructions of the user's manual, by Patrick A. D. Grimont, Institute Pasteur. The UP (unweighted pair group matching by arithmetic averages) program is usually applied for PFGE unless a high degree of similarity is anticipated; here, the program was applied for determination of whether a number of nontypeable isolates were of the same clone or their patterns agreed with that of a known clone.
RESULTS
Susceptibility to antibiotics.
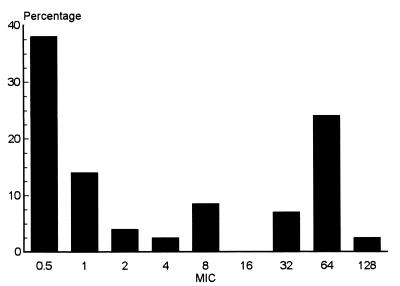
The penicillin G, erythromycin, clindamycin, and tetracycline MICs were determined by agar dilution. All 1,335 strains were susceptible to penicillin G (MIC, ≤ 0.25 μg/ml). Only three strains (0.2%) were resistant to erythromycin (MICs, ≥ 0.25 μg/ml): one was from Gilan Province, and two were from southern Tehran. The latter two strains were resistant to tetracycline and had the inducible MLS phenotype. In contrast, as many as 560 (42.0%) of the strains were resistant to tetracycline (MICs, ≥8.0 μg/ml). The MICs of tetracycline varied between 0.5 and 128 μg/ml; an MIC of 64 μg/ml was observed for most of the resistant strains (Fig. 1).
FIG. 1.
Distribution of tetracycline susceptibility among 1,335 Iranian S. pyogenes isolates recovered from 1995 to 1997. The MICs for these strains varied between 0.5 and 128 μg/ml, as determined by agar dilution.
The distribution of tetracycline resistance among isolates in the districts studied did not differ markedly: of 625 strains isolated from the northern part of Iran, 500 strains recovered from southern Tehran, and 210 strains isolated in central Tehran, the rates of resistance to tetracycline were 43.5, 42.8, and 39.9%, respectively.
In order to find out whether any recent changes in resistance had occurred, a collection of 80 clinical strains isolated in southern Tehran between 1989 and 1991 were tested. Of these, 23.4% were resistant to tetracycline, whereas all were susceptible to penicillin and erythromycin.
T and M typing of tetracycline-resistant isolates.
A majority (n = 406) of the tetracycline-resistant strains were selected for typing. The results of serological T and M typing are shown in Tables 2 and 3. Overall, about 50% of these strains were T nontypeable.
TABLE 2.
Serological T- and M-typing results for tetracycline-resistant Iranian isolates of S. pyogenes
| T type | OF | M type | No. of isolates | Origin |
|---|---|---|---|---|
| 1 | + | 59 | 1 | Tehran |
| 1 | − | 1 | 12 | Tehran |
| 4 | + | 4 | 15 | Gilan |
| 4 | + | 60 | 2 | Tehran |
| 5 | + | NTa | 6 | Gilan |
| 6 | − | 6 | 9 | Tehran |
| 9 | + | 9 | 8 | Tehranb |
| 11 | + | 11 | 19 | Gilan |
| 11 | + | 78 | 2 | Tehran |
| 12 | − | 12 | 31 | Tehran |
| 12 | − | 12 | 6 | Gilan |
| 12 | + | 22 | 5 | Tehran |
| 12 | + | 76 | 2 | Tehran |
| 13 | − | 2110 | 1 | Tehranb |
| 25 | + | 2233 | 2 | Tehran |
| 28 | + | 28R | 6 | Tehran |
| 28 | + | 2841 | 5 | Tehran |
| 28, 12 | − | 12 | 4 | Tehran |
| 28, 11 | − | 12 | 4 | Tehran |
| 13 | + | 13c | 21 | Gilan |
| B3264-3-13 | − | 13c | 5 | Tehran |
| NT | + | 2841 | 1 | Tehran |
| B3264 | + | PT4245 | 25 | Gilan |
| B3264 | − | 2110 | 13 | Tehranb |
| NT | + | 81 | 1 | Tehran |
| NT | + | NT | 37 | Tehran |
| NT | − | NT | 63 | Tehran |
| NT | NDd | ND | 100 | Tehran |
| Total | 406 |
NT, nontypeable.
Tehran strains isolated from 1989 to 1991.
Antiserum against M type 13 was not available (see text).
ND, not done.
TABLE 3.
emm typing by PCR-capture ELISA of 100 T-nontypeable isolates and 46 M-nontypeablea isolates of T type 3264 or 13
| T type | OF | emm type | No. of isolates | Origin |
|---|---|---|---|---|
| NTb | − | 1 | 5 | Tehran |
| NT | − | 6 | 4 | Tehran |
| NT | − | 12 | 15 | Tehran |
| NT | + | 22 | 4 | Tehran |
| NT | + | 76 | 2 | Tehran |
| NT | + | 78 | 6 | Tehran |
| 13 | + | 13 | 21 | Gilan |
| B3264-13-3 | + | 13 | 5 | Tehran |
| B3264 | + | PT4245 | 20 | Gilan |
| NT | − | NT | 25 | Tehran |
| NT | + | NT | 39 | Tehran |
| Total | 146 |
Serologically M nontypeable (see Results section).
NT, nontypeable.
Of 272 strains isolated in Tehran, a predominance of T and M types 1, 6, and 12 was found. Some of these strains displayed more than one T type, as shown in Table 2. The two strains that were resistant to both erythromycin and tetracycline were of T and M type 11.
For 112 isolates collected from Gilan Province, the most common T types were 4, 11, 13, and B3264. M types 4 and PT4245 were common among these strains. T type 13 strains were not recognized by antisera to M type 77 or 81, and OF inhibition tests for several types was negative, although antiserum to M type 13 or OF type 13 was not available, M type 13 appeared to be the most probable (see below).
One hundred T- and M-nontypeable and 46 M-nontypeable isolates were tested by PCR-capture ELISA, by which 82 were successfully emm typed (Table 3). In particular, type PT4245 was established for 20 strains of T type B3264, which agreed with the serological typing of 25 other T type B3264 isolates from the Gilan area. Furthermore, M type 13 was confirmed for the 21 T type 13 strains mentioned above and also for five isolates from Tehran showing T pattern B3264-13-3 (Tables 2 and 3).
Among 22 randomly selected strains from Tehran isolated from 1989 to 1991, only two M types were noted: M9 and PT2110.
PFGE of selected strains.
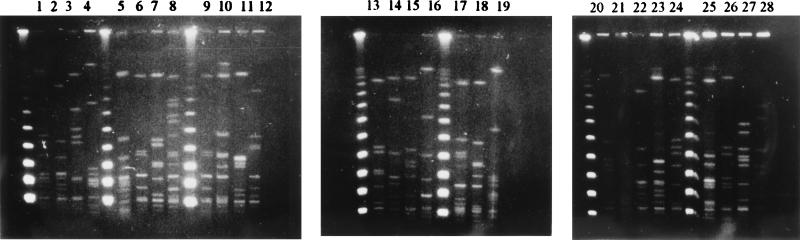
Fifteen nontypeable strains which were isolated in Tehran and which exhibited tetracycline resistance (MIC, 64 μg/ml) were selected for PFGE typing by comparison with strains of known PFGE types; none could be assigned to any of the prevalent types, and all had distinct pulsotypes (Fig. 2 and 3).
FIG. 2.
Clonality test by PFGE restriction patterns of 15 tetracycline-resistant, M-nontypeable S. pyogenes strains and typing by comparison with the patterns of 11 strains of seven prevalent M types (11 pulsotypes). Chromosomal DNA was digested with SmaI. Lanes 1, 4, 5, 6, 7, 13, 16, 17, 19, and 22 to 27, nontypeable strains; lanes 2, 8, 9, and 12, M11 strains (three pulsotypes); lane 3, an M19 strain; lanes 10 and 18, PT2233 strains (two pulsotypes); lane 11, a PT2110 strain; lane 14, an M59 strain; lane 15, an M6 strain; lane 20, a PT2841 strain; lanes 21 and 28, incompletely digested DNA from an M60 and a nontypeable strain, respectively.
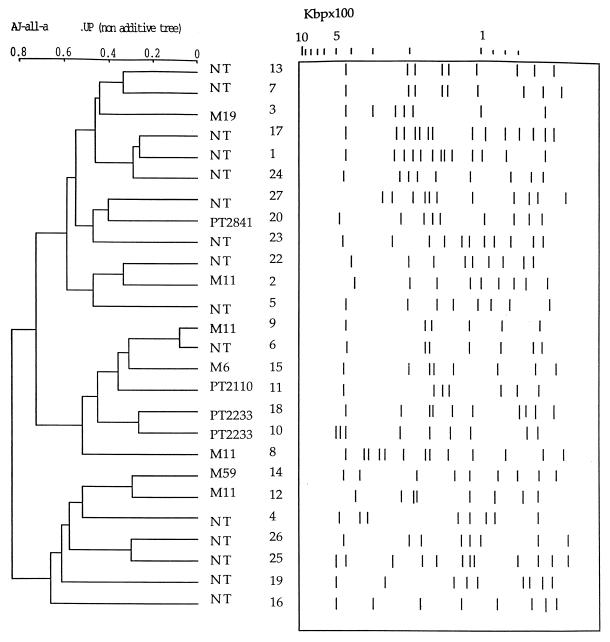
FIG. 3.
Interpretation (Taxotron) of PFGE gel of Fig. 2 and dendrogram derived from the unweighted pair group average linkages (UP) of correlation coefficients between PFGE patterns. Numbers refer to the lane numbers in Fig. 2. NT, nontypeable.
In addition, a number of resistant strains of known T and M types were tested for clonality. Strains showing a maximum of two nonidentical bands were defined as belonging to a common pulsotype. Clonal homogeneity was observed among M types 4 and 13 strains from Gilan as well as M types 6, PT2110, and PT2841 strains from Tehran. However, among M type 12 strains (30 from Tehran and 6 from Gilan), eight different pulsotypes were observed, and of 19 M type 11 strains from Gilan, three pulsotypes were found (Table 4).
TABLE 4.
Summary of PFGE analysis of 134 selected, tetracycline-resistant S. pyogenes isolates
| T type | OF | M type | No. of pulsotypes | No. of isolates | Origin |
|---|---|---|---|---|---|
| 4 | + | 4 | 1 | 15 | Gilan |
| 6 | − | 6 | 1 | 9 | Tehran |
| 11 | + | 11 | 3 | 19 | Gilan |
| 12 | − | 12 | 6 | 30 | Tehran |
| 12 | − | 12 | 2 | 6 | Gilan |
| 13 | + | 13 | 1 | 20 | Gilan |
| B3264 | − | 2110 | 1 | 13 | Tehran |
| 25 | + | 2233 | 2 | 2 | Tehran |
| 28 | + | 2841 | 1 | 5 | Tehran |
| NTa | ± | NT | 15 | 15 | Tehran |
| Total | 134 |
NT, nontypeable.
DISCUSSION
In this large survey of Iranian S. pyogenes isolates we found no penicillin-resistant strains and only a few erythromycin-resistant strains. Penicillin resistance has not been identified in clinical strains thus far, and even the existence of penicillin tolerance in S. pyogenes seems doubtful (17). Since macrolides are fully available and are used as the first choice for patients with penicillin allergy or intolerance (4), the low rate of erythromycin resistance was unexpected, although the absence of erythromycin resistance in smaller samples of isolates from Iran was reported previously (4, 6). High rates of erythromycin resistance in S. pyogenes have recently been reported from Asia and other areas, but a marked decline has been noted in both Japan and Finland, presumably as a consequence of reduced macrolide consumption (5, 22). Due to the virtual absence of erythromycin-resistant strains in the present study, susceptibility testing of MLS drugs other than erythromycin was not performed.
In contrast, our results showed a high rate of tetracycline resistance, 42%, among current Iranian isolates, a level considerably higher than that reported in 1974, 7.8% (6). A continuous rise in the rate of tetracycline resistance during these decades was suggested by the resistance level from 1989 to 1991, 23%, found here. In Iran, tetracycline is not recommended as treatment for streptococcal infections but is widely used as treatment for a variety of human and veterinary infections, for example, brucellosis (18, 20), implying a high total level of consumption. In recent studies, high tetracycline resistance rates among S. pyogenes isolates (41 to 93%) have been reported among smaller samples of isolates from Israeli material (27), Tunisia (14), and Denmark (7), whereas low levels of tetracycline resistance, 7.3 and 1.3%, were reported in a large Spanish survey (16) and from Sweden (26), respectively. In Japan, the rate of tetracycline resistance declined from 60% to less than 20% from 1981 to 1990 (5).
Among the current tetracycline-resistant Iranian S. pyogenes strains there was a predominance of T types B3264, 4, 5, 11, 12, 13, and 14. The results of M typing demonstrated a considerable type diversity among isolates within the Tehran region, whereas in the Gilan region, only a few types, such as M types 4, 11, 13, and PT4245 were found; in the latter case, clonal spread of M types 4 and 11 (Table 4) as well as PT4245 (data not shown) was detected. The multi- and oligoclonal modes of spread of resistant strains in Tehran, a large city with international communications, and the more rural Gilan Province, respectively, were presumably related to the different characters of these regions.
It may be noted that the association of M types PT4245 and PT2110 with T type B3264 has not been previously reported. Interestingly, M type PT2110 and M type 9 were the only types found among the isolates recovered from 1989 to 1991. In a recent Japanese study of 386 S. pyogenes isolates, tetracycline-resistant strains mostly belonged to M types 4, 11, and 13 (3).
For serologically nontypeable strains, emm gene detection by a newly developed PCR-capture ELISA, although so far covering a limited set of types, was successful in many cases. It was notable that for a number of strains that belong to established types, such as M types 1, 6, 12, 13, 22, 76, and 78, typing was possible only by PCR-capture ELISA; this typing problem has been reported previously and has often been ascribed to poor in vitro expression of M protein and other, unknown reasons (1). The PCR-capture ELISA method may thus be useful for molecular typing of S. pyogenes, especially in laboratories without gene sequencing facilities.
PFGE typing of a number of nontypeable strains from Tehran, for all of which the tetracycline MIC was 64 μg/liter, was attempted since it was possible that these strains could belong to a single clone or that their PFGE types could be identified by comparison of their PFGE patterns with those of known strains. However, no common pulsotypes were identified among 15 selected nontypeable strains, and their PFGE patterns were quite distinct from those of strains with defined M types. Furthermore, on testing of more than one strain of certain M types, different PFGE patterns within a single type were also identified for most isolates from Tehran, demonstrating a high degree of diversity of resistant clones.
We conclude from these studies of Iranian S. pyogenes strains that tetracycline resistance has disseminated among several M types. Since tetracycline is not widely used as treatment for streptococcal infections, the spread of tetracycline resistance in S. pyogenes may be particularly relevant from an ecological point of view. Unfortunately, the rates of tetracycline resistance among other pathogens in Iran are largely unknown; it is conceivable, however, that resistance genes have been horizontally transferred to S. pyogenes from other species more directly exposed to tetracycline selective pressure, e.g., viridans group streptococci and other human and animal commensal organisms, as documented in other studies (19). This issue may be further analyzed by determining the genotypes of resistance determinants. Although levels of antibiotic consumption have not been analyzed, our data emphasize the need for a more controlled use of tetracycline in Iran.
ACKNOWLEDGMENTS
The study was supported by the Erik-Philip Sörensen Foundation, Pharmacia & Upjohn, Abbott Scandinavia AB, and Bayer Diagnostica AB.
We thank M. Bastanhag for accepting us to work in the Tehran University of Medical Sciences & Health Services. We are grateful to M. Aslani and A. Shahrokhi for generosity in providing us the opportunity to use the strains that they collected. We are also grateful to the staffs of the laboratories of the Imam Khomeini Hospital, the Pars Hospital, the Children's Hospital, and the Children's Medical Center and of the Azadi Laboratory, the Bahar Laboratory, the Central Laboratory, and the Institute Pasteur for help in collecting streptococcal strains. Our sincere thanks go to M. Deldari and F. Makinejad for isolation of clinical streptococcal strains. We are also grateful to all the staff of schools in Tehran and Gilan who contributed to this work. Finally, we thank R. George, head of the RSIL, Colindale, London, United Kingdom, for support, and G. Hallas, E. Gaworzewska, D. Hathi, C. Dhami, and the other staff members of the PHLS Streptococcal Reference Unit for help and support with the typing work.
REFERENCES
- 1.Beall B, Facklam R, Thompson T. Sequencing emm-specific products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.el Bour M, Fendri C, Ben Hassen A, Kamoun A, Boudabbous A, Ben Redjeb S. Study of antibiotic sensitivity of Streptococcus pyogenes isolated in the hospital milieu. Med Trop. 1993;53:13–17. . (In French.) [PubMed] [Google Scholar]
- 3.Endo M, Kashiwagi Y, Okuno R, Amano Y, Onogawa T. Antibiotic sensitivity and serotypes of group A hemolytic streptococci isolated from clinical specimens and healthy pupils in Japan, 1986–1988. J Jpn Assoc Infect Dis. 1991;65:919–927. doi: 10.11150/kansenshogakuzasshi1970.65.919. [DOI] [PubMed] [Google Scholar]
- 4.Etemadi H, Melakneghad P, Fatolahzadeh B, Jaäfari A, Farahi F, Khadabakshi N. Resistance to erythromycin and tetracycline in streptococci groups A, B and D. Paghohesh Elmi (Sci Res) 1992;5:10–12. . (In Persian.) [Google Scholar]
- 5.Fujita J, Murono K, Yoshikawa M, Murai T. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr Infect Dis J. 1994;13:1075–1078. doi: 10.1097/00006454-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gharagozloo R, Jamshidi M S, Ghadimi H. Microbiological and epidemiological study of streptococcal sore throat at a children's clinic: a one-year study. Pahlavi Med J. 1976;7:334–343. [PubMed] [Google Scholar]
- 7.Hoffman S. The throat carrier rate of group A and other beta-hemolytic streptococci among patients in general practice. Acta Pathol Microbiol Immunol Scand Sect B. 1985;93:347–351. doi: 10.1111/j.1699-0463.1985.tb02899.x. [DOI] [PubMed] [Google Scholar]
- 8.Holmström L, Nyman B, Rosengren M, Wallander S, Ripa T. Outbreak of infections with erythromycin-resistant group A streptococci in child day care centres. Scand J Infect Dis. 1990;22:179–185. doi: 10.3109/00365549009037900. [DOI] [PubMed] [Google Scholar]
- 9.Jasir, A., A. Noorani, A. Mir-Salehian, and C. Schalén. Isolation rates of Streptococcus pyogenes in patients with acute pharyngotonsillitis and among healthy school children. Epidemiol. Infect., in press. [DOI] [PMC free article] [PubMed]
- 10.Jasir A, Schalén C. Survey of macrolide resistance phenotypes in Swedish clinical isolates of Streptococcus pyogenes J. Antimicrob Chemother. 1998;41:135–137. doi: 10.1093/jac/41.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D R. Laboratory diagnosis of group A streptococcal infections. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 12.Lowbury E J L, Hurst L. The sensitivity of staphylococci and other wound bacteria to erythromycin, oleandomycin and spiramycin. J Clin Pathol. 1959;12:163–169. doi: 10.1136/jcp.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowbury E J L, Cason J S. Aureomycin therapy for S. pyogenes in burns. Br Med J. 1954;i:914–915. doi: 10.1136/bmj.2.4893.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama S, Yoshioka H, Fujita K, Takimoto M, Satake Y. Sensitivity of group A streptococci to antibiotics. Am J Dis Child. 1979;133:1143–1145. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Approved standard M2-A5. Performance standards for antimicrobial disk susceptibility test. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 16.Orden B, Martinez R, Lopez de los Mozos A, Franco A. Antibiotic resistance to erythromycin, clindamycin and tetracycline of 573 strains of Streptococcus pyogenes. Enferm Infecc Microbiol Clin. 1996;14:86–89. [PubMed] [Google Scholar]
- 17.Orrling A, Stjernquist-Desatnik A, Schalén C, Kamme C. Treatment failure in streptococcal tonsillitis. An attempt to identify penicillin tolerant Streptococcus pyogenes. Scand J Infect Dis. 1996;28:143–147. doi: 10.3109/00365549609049065. [DOI] [PubMed] [Google Scholar]
- 18.Tehran Research Group of the Institute of Hygiene. The danger of prophylactic usage of antibiotics and chemotherapeutics in animal foods. No. 1924. Tehran, Iran: Tehran University and Institute of Hygiene; 1991. . (In Iranian.) [Google Scholar]
- 19.Roberts M C. Epidemiology of tetracycline-resistance determinants. Trends Microbiol. 1994;2:353–357. doi: 10.1016/0966-842x(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 20.Saadatfar Z. A survey of tetracycline resistance in bacteria due to overuse of the drug in poultry production. Thesis no. 1716. Tehran, Iran: Tehran University; 1988. . (In Persian.) [Google Scholar]
- 21.Saunders N A, Hallas G, Gaworzewska E T, Metherell L, Efstratiou A, Hookey J V, George R. PCR–enzyme-linked immunosorbent assay and sequencing as an alternative to serology for M-antigen typing of Streptococcus pyogenes. J Clin Microbiol. 1997;35:2689–2691. doi: 10.1128/jcm.35.10.2689-2691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seppälä H, Klauka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 23.Seppälä H, Nissinen A, Järvinen H, Huovinen S, Henriksson T, Herva E, Holm S E, Jahkola M, Katila M L, Klankka T, et al. Resistance to erythromycin in group A streptococci. N Engl J Med. 1992;326:292–297. doi: 10.1056/NEJM199201303260503. [DOI] [PubMed] [Google Scholar]
- 24.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 25.Stanley J, Linton D, Desai M, Efstratiou A, George R. Molecular subtyping of prevalent M serotypes of Streptococcus pyogenes causing invasive disease. J Clin Microbiol. 1995;33:2850–2855. doi: 10.1128/jcm.33.11.2850-2855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strömberg A, Schwan A, Cars O. Throat carrier rates of beta-hemolytic streptococci among healthy adults and children. Scand J Infect Dis. 1988;20:411–417. doi: 10.3109/00365548809032477. [DOI] [PubMed] [Google Scholar]
- 27.Weiss I, Gorodnitzky Z, Korenman Z, Yagupsky P. Serotyping and susceptibility to macrolides and other antimicrobial drugs of Streptococcus pyogenes isolated from patients with invasive disease in Southern Israel. Eur J Clin Microbiol Infect Dis. 1997;16:20–23. doi: 10.1007/BF01575115. [DOI] [PubMed] [Google Scholar]