Abstract
Background
Coronavirus disease 2019 (COVID-19), an acute, sometimes severe respiratory illness caused by a novel coronavirus has led to a vast pandemic with an astonishing spread rate. Its treatment is unknown, its mortality is significant, and its socioeconomic complications are uncontrollable. Although there is still little known about the pathogenesis of the disease, severe cases of COVID-19 are usually associated with cytokine release syndrome and high serum levels of inflammatory cytokines, which are believed to be a major cause of mortality in these patients. Different pathways cause inflammation and the release of cytokines. One of these pathways is the Bruton tyrosine kinase (BTK) pathway, which is essential for the production of several anti-inflammatory cytokines. Theoretically, the inhibition of BTK signaling can reduce cytokine levels and subsequent anti-inflammatory effects.
Objective
This review aims to investigate the role of the BTK pathway in the pathogenesis of COVID-19 and the possible effects of its inhibition in the treatment of this disease.
Methods
This narrative review provides information regarding the use of BTK inhibitors in patients with COVID-19 and discusses whether clinicians should consider these medications while managing their patients based on the literature. Data were gathered using the PubMed, Scopus, and Web of Science databases.
Results
Some data support the use of BTK inhibitors for treating COVID-19.
Conclusions
It is recommended that patients continue their medications in this class if they develop COVID-19 and were receiving these agents before the disease developed. The use of BTK inhibitors might enable patients to experience less severe immune responses to the COVID-19. Well-designed studies are needed to evaluate the effectiveness of BTKis in the management of COVID-19. (Curr Ther Res Clin Exp. 2022; 82:XXX–XXX) © 2022 Elsevier HS Journals
Key words: Bruton tyrosine kinase inhibitor, COVID-19, SARS-CoV-2
Introduction
Coronaviruses are a large family of respiratory viruses that can cause a wide range of illnesses, from the common cold to the Middle East respiratory syndrome and severe acute respiratory symptoms (SARS). In late 2019, a new kind of coronavirus was named SARS-CoV-2 by the World Health Organization, and the resulting disease was called coronavirus disease 2019 (COVID-19). The condition has quickly become a pandemic and has involved almost every country in the world.1
The pathophysiology of COVID-19 has not yet been clearly defined. The recent evidence proposes that the excessive host immune response may play a role in the pathogenesis of the disease.2 Severe cases of COVID-19 are usually associated with cytokines release syndrome and significant serum levels of inflammatory cytokines that are believed to be the leading cause of mortality for these patients.3 The Bruton tyrosine kinase (BTK) pathway is crucial for the production of several anti-inflammatory cytokines,4 and inhibiting BTK signaling reduces cytokine levels and consequently has an anti-inflammatory effect. Impairments to BTK signal regulation in lung macrophages may be a significant pathophysiological component of SARS-CoV-2-induced lung injuries. Therefore, inhibiting the BTK pathway may reduce the excessive and harmful immune response in the severe form of COVID-19 and the respiratory complications resulting from it.
Tyrosine kinases and their role in the immune system
Tyrosine kinase is an enzyme whose role is to transfer a phosphate group from the adenosine triphosphate molecule to a protein. This enzyme acts as an off–on key in many cellular activities.5 Tyrosine kinases are a subset of protein kinases whose role is to bind phosphate groups to other amino acids such as serine and threonine. The phosphate group is attached to the amino acid tyrosine in proteins, and the phosphorylation of proteins by kinases is the main stage in cell signal transduction.5
The function of tyrosine kinases
Tyrosine kinases catalyze the phosphorylation of tyrosine residues in proteins. The phosphorylation of tyrosine residues, in turn, alters the function of proteins existing in them.6 The phosphorylation of tyrosine residues controls a wide range of properties existing in proteins, such as enzyme activity, intracellular localization, and interactions between molecules. Moreover, tyrosine kinases are used in many signal transduction cascades in which extracellular signals are transmitted through the cell membrane to the cytoplasm and often to the nucleus, where gene expression may be modified. Finally, mutations can activate some tyrosine kinases, leading to an unstoppable functional state that may play a role in the onset or progression of cancer.7
Tyrosine kinases are involved in various processes, pathways, and functions, and they are responsible for several critical events in the body. Receptor tyrosine kinases are involved in intermembrane signaling, whereas intracellular tyrosine kinases play a role in signal transmission to the nucleus. Tyrosine kinase activity in the nucleus includes cell cycle control and the properties of transcription factors. Thus, tyrosine kinase activity is involved in mitogenesis (the induction of mitosis in a cell). The proteins available in the cytosol and the nucleus are phosphorylated in the tyrosine residues during this process.7
BTK
BTK has a vital role in producing mature B cells because it is essential for the transmission of signals from the pre-B cell receptor, which is formed after the successful rearrangement of the heavy chain immunoglobulin.8 It, also, has a role in the activation of mast cells via immunoglobulin E receptor with high affinity.9 BTK contains a pleckstrin-homology domain that binds trisphosphate (PIP3- 3,4,5) to phosphatidylinositol. Binding BTK induces PIP3 to phosphorylate phospholipase C, which in turn hydrolyzes PIP2 (an inositol phosphatidyl) to the 2-second messengers, inositol triphosphate, and diacylglycerol, which subsequently regulates the activities of the downstream proteins in B cell.9
Tyrosine kinase inhibitors and COVID-19
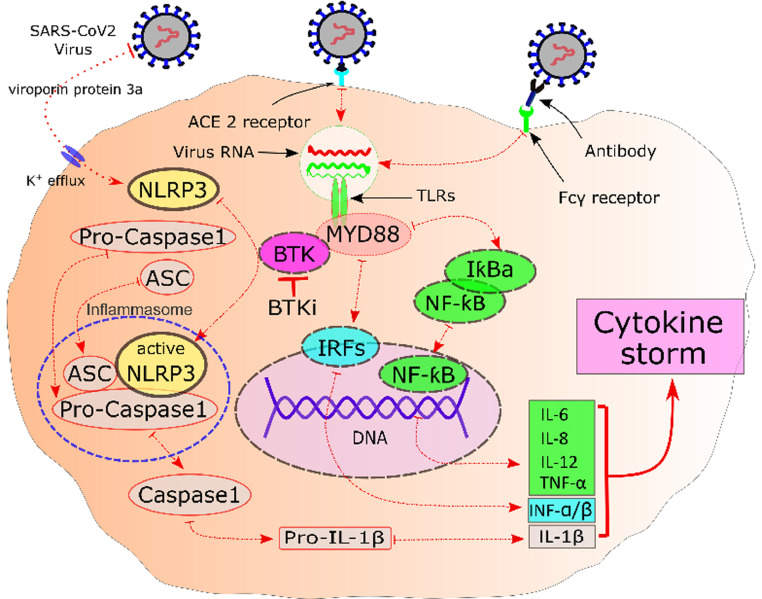
Nicolson et al10 showed that the use of tyrosine kinase inhibitors can simultaneously reduce clotting and inflammatory responses effectively. It has also been observed in influenza mouse models that BTK inhibitors (BTKis) can save mice from acute fatal lung injuries. Sick mice with respiratory failure that had not received the medication were in complete contrast to mice receiving ibrutinib according to tomography and histology findings compatible with lung injury. The control mice lost weight and died, whereas all ibrutinib-treated mice regained their weight and survived. The main point was that decreased infiltration of inflammatory cells; proinflammatory cytokines; and chemoattractants such as interleukin 1b, interleukin 6 keratinocyte-derived chemokine/chemokine C-X-C motif ligand 1 (KC/CXCL1), tumor necrosis factor alpha, and monocyte chemoattractant protein-1 (MCP-1), were observed in the lung tissues of mice treated with ibrutinib.11 The serum samples collected from patients with chronic lymphocytic leukemia (CLL), Waldenström's macroglobulinemia (WM), and chronic graft versus host disease (GVHD) after ibrutinib monotherapy indicated significant reductions in proinflammatory cytokines and chemoattractants, which are highly present in the lungs of SARS-CoV-2 and SARS-CoV-1 patients.12 Therefore, the dysregulation of the BTK signal in lung macrophages may be a leading pathophysiological component of SARS-CoV-2-related lung damage.13 Thus, inhibiting the BTK pathway could reduce the excessive and inappropriate immune response associated with severe COVID-19 and prevent related respiratory complications. In a review article written by Otsuka and Senio,14 the authors suggest treating patients with COVID-19 with administration of BTKis. The Figure 1 shows the proposed role of the BTK pathway in COVID-19.
Figure 1.
Macrophage cell and proposed Bruton tyrosine kinase (BTK)-dependent signaling pathways of hyperinflammation in severe COVID-19. ACE = angiotensin-converting enzyme; ASC = apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD); BTKi = BTK inhibitor; Fcγ = Fc-gamma; IКBa = inhibitor of kappa light chain gene enhancer in B cells alpha; INF = interferon; IRF7 = interferon regulatory factor 7; IL = interleukin; MYD88 = myeloid differentiation primary response 88; NF-КB = nuclear factor-κB; NLRP3 = NLR family pyrin domain containing 3; TNF = tumor necrosis factor; TLR = toll-like receptors.
BTK inhibitors, including ibrutinib, acalabrutinib, and zanubrutinib, are commonly used to treat CLL, WM, and GVHD. It has been shown that they have potent anti-inflammatory effects, leading to decreased levels of inflammatory cytokines, which are usually present in severe COVID-19.15
Thousands of patients with CLL, B-cell lymphoma, and chronic GVHD, who have developed less severe complications of COVID-19 are now being treated with tyrosine kinase inhibitors.16 In a study performed by Reda et al17 on 2902 patients from 6 treatment centers for CLL patients, it was shown that the symptoms of COVID-19 had developed in < 1% of patients taking ibrutinib. Scarfo et al18 also concluded that BTKis had a protective effect against the development of severe forms of COVID-19 in patients with CLL.
Ibrutinib
Ibrutinib is a first-generation BTKi that inhibits the function of B cell antigen receptors and cytokines via the selective and irreversible inhibition of BTK. As a result, it can inhibit malignancies due to the abnormal proliferation of B cells. Ibrutinib showed beneficial effects against lung injury in COVID-19 hypoxic patients in a retrospective case series conducted by Treon et al.12 The authors described the treatment results of 6 patients with WM who had received ibrutinib and had developed COVID-19. Five of these 6 patients had mild symptoms, did not need to be admitted to the hospital, and recovered quickly. One of these 6 patients required admission and mechanical ventilation. However, he eventually recovered completely. By accepting the limitations of this small study, the authors hypothesized that ibrutinib might protect against lung damage in patients infected with SARS-CoV-2, and suggested that BTKis be continued in patients with WM infected with COVID-19.12
In a case report conducted by Lin et al,19 ibrutinib showed beneficial effects against acute lung injury in a patient with CLL and COVID-19. However, in that study, the patient was receiving tocilizumab as well. Currently, there are 3 ongoing clinical trials (NCT04375397, NCT04665115, and NCT04439006) evaluating the effectiveness of ibrutinib in patients with COVID-19.20, 21, 22 Ibrutinib is available as an oral once-daily formulation and is approved to treat CLL, WM, marginal zone lymphoma, and chronic GVHD.23
Acalabrutinib
Acalabrutinib (a second-generation BTKi) was approved in October 2017 to treat recurrent mantle cell lymphoma; also, 95% improvement has been reported regarding its use for recurrent cases of CLL. Acalabrutinib and its active metabolite, ACP-5862, form a covalent bond with cysteine at the active site of BTK and inhibit BTK enzymatic activity.24
Roschewski et al25 conducted an observational study using acalabrutinib on 19 hospitalized patients who needed supplemental oxygen due to severe COVID-19. Initially, 18 of these patients required additional oxygen, but within 3 days, improvements in their oxygenation were noted. The biomarkers of inflammation (eg, interleukin 6 and C-reactive protein) returned to their normal levels in most of the treated patients within 10 to 14 days. At the end of treatment, 8 patients (72.7%) out of the 11 who needed supplemental oxygen were breathing comfortably in room air. Four patients (50%) out of 8 were extubated under a ventilator. Finally, 25% of intubated patients breathed comfortably in room air at the end of their treatment without any significant side effects.25 Currently, a clinical trial (NCT04497948) is ongoing to evaluate the efficacy of acalabrutinib with the best care in COVID-19 patients.26 Acalabrutinib is available as a twice-daily oral formulation.
Zanubrutinib
Zanubrutinib is a second-generation BTKi recommended to treat mantle cell lymphoma and can be taken orally after using another BTKi family drug. It is believed that zanubrutinib has fewer toxicities than the first-generation class.27 There is currently 1 clinical trial evaluating the effectiveness of zanubrutinib in COVID-19 hospitalized patients (NCT04382586).28 Zanubrutinib is available as an oral formulation that can be administered once or twice daily.
We believe that ibrutinib may be associated with better patient adherence considering its once-daily dosing. However, its use may be associated with more severe adverse effects. Zanubrutinib is also available as a once-daily formulation. Moreover, fewer adverse effects may be developed regarding its use because it is a second-generation BTKi. It is recommended to wait for the results of ongoing clinical trials before making a firm choice among these agents for treating COVID-19.
Side effects of BTKis
The use of first-generation BTKis (eg, ibrutinib) is associated with bleeding disorders, infections, atrial fibrillation, and diarrhea. Contrarily, second-generation BTKis are associated with less toxic side effects than ibrutinib.23 The second-generation BTKis (eg, acalabrutinib and zanubrutinib) were designed to increase efficacy and reduce adverse events.
It is noteworthy that the use of BTKis requires careful consideration of their effects on the host's immune system. A high rate of serious infectious complications has been identified following their use in some prospective clinical trials in patients with cancer. In 1 study, infectious complications occurred in 56% of patients who received ibrutinib alone and 52% of those who received a combination treatment including ibrutinib. Approximately 1 in 5 patients developed pneumonia, which was the leading cause of death due to infection. Many cases of pneumonia occurred due to opportunistic factors.29 However, these adverse effects are primarily reported in patients in cancer. Considering the short-term course of treatment of these agents in COVID-19, the significance of these adverse effects is not especially prominent. The Table 1 summarizes the BTKi characteristics and the ongoing clinical trials evaluating their effects in COVID-19 patients.
Table 1.
Summary of the mentioned Bruton tyrosine kinase inhibitor characteristics and the ongoing clinical trials evaluating their effects in patients with COVID-19.
| Medication | FDA approval | Available form | Approved or recommended indications | Ongoing clinical trials in COVID-19 | Side effects |
|---|---|---|---|---|---|
| Ibrutinib | November 13, 2013 | 70 and 140 mg capsules | MCL, CLL, SLL WM, MZL, GVHD |
3 Studies Ibrutinib for the treatment of COVID-19 in patients requiring hospitalization Study of oral ibrutinib capsules to assess respiratory failure in adult participants with severe acute respiratory syndrome Coronavirus-2 and Pulmonary Injury Ibrutinib for the treatment of patients with b-cell malignancies who are infected with coronavirus disease 2019 |
Low platelet count, diarrhea, neutropenia, anemia, fatigue, musculoskeletal pain, muscle spasms, joint pain, swelling of the extremities, fever, upper respiratory tract infection |
| Acalabrutinib | October 31, 2017 | 100 mg capsules | MCL CLL SLL |
1 Study Acalabrutinib study with best supportive care in participants hospitalized with COVID-19 |
Headache, nausea, vomiting, abdominal pain, diarrhea, tiredness, or muscle aches |
| Zanubrutinib | November 14, 2019 | 80 mg capsules | MCL | 1 Study COVID-19 infection and pulmonary distress treatment with zanubrutinib in hospitalized participants |
Headache, nausea, vomiting, abdominal pain, diarrhea, tiredness, or muscle aches |
| Vecabrutinib (SNS-062) | Preclinical and clinical investigation | CLL | No | ||
| Evobrutinib | Preclinical and clinical investigation | Multiple sclerosis | No | ||
| Fenebrutinib | Preclinical and clinical investigation | Rheumatoid arthritis systemic lupus erythematosus, and chronic spontaneous urticaria | No | ||
| Spebrutinib | Preclinical and clinical investigation | Rheumatoid arthritis (Phase II) and B-cell lymphoma (Phase I) | No | ||
| Tirabrutinib ONO-4059 | Preclinical and clinical investigation | NHL | No | ||
| HM71224 | Preclinical and clinical investigation | Autoimmune diseases | No | ||
| ABBV-105 | Preclinical and clinical investigation | Lupus erythematosus | No | ||
| LOXO-305 | Preclinical and clinical investigation | CLL, SLL, NHL | No | ||
| Orelabrutinib (ICP-022) | Preclinical and clinical investigation | Diffuse large B cell lymphoma; Lymphoma; marginal zone B-cell lymphoma; multiple sclerosis; WM | No |
CLL = chronic lymphocytic leukemia; FDA = Food and Drug Administration; GVHD = chronic graft versus host disease; MCL = mantle cell lymphoma; ML = Marginal zone lymphoma; NHL = non-Hodgkin's lymphoma; SLL = small lymphocytic lymphoma; WM = Waldenström's macroglobulinemia.
Management of patients with cancer during the pandemic
As the number of patients with COVID-19 being treated with BTKis increases, the prospective continuation or discontinuation BTKis should be considered in these patients. During the outbreak of COVID-19, the risk of increased secondary infections and impaired humoral immunity due to decreased B-cell activity regarding using these agents might favor discontinuing BTKis. However, the potential benefits of reducing the severe inflammatory reaction due to SARS-CoV-2 by weakening macrophage tendency to M1 status seem to outweigh the potential risk of humoral immunodeficiency. Despite the increased risk of secondary infections, it is recommended that BTKis are continued in patients with COVID-19 and cancer.30 Another point to consider is that BTKis are not evaluated as monotherapy of COVID-19 management regardless of the patients’ comorbidities. Hence, they should not be used in this regard.
In a study conducted by the CLL Association, there was a conflict among CLL experts regarding the administration of BTKis: 40% of the experts agreed with the continuation of BTKis, whereas the other 60% believed that they should be discontinued in patients with COVID-19 or continued only in specific clinical scenarios. The potential protective anti-inflammatory effects of the BTKis against the theoretical risk of suppressing the humoral immune system have to be considered to ensure the right decision is made.31
In a study conducted on 8 patients with CLL and COVID-19 at the same time, it was shown that despite these patients’ immune disorders, the administration of BTKis led most of them to be discharged from the hospital and reduced the need for their admission to an intensive care unit.16 Also, in a study conducted by Chong et al15 on patients with concomitant cancer and SARS-CoV-2 infection, it was suggested that despite the increased infection rate in these patients, continuing treatment with tyrosine kinase inhibitors should be considered.
In another meeting held to decide the best treatment for patients with WM during the COVID-19 pandemic, it was recommended that treatments with tyrosine kinase inhibitors should be continued even if the patient is utilizing other forms of therapy and had COVID-19.31
Conclusions
BTK plays an essential role in the activation of B cells and without its activation, these cells would not be able to play an appropriate role in immune reactions. BTK also plays a role in macrophage function. In COVID-19, the overactivation of immune cells causes the overproduction of cytokines, resulting in cytokine storms. This phenomenon increases the severity of the disease and can lead to acute respiratory distress syndrome. The use of BTKis can help patients experience less severe responses to the SARS-CoV-2 virus and overcome the disease by inhibiting this process. Well-designed studies, especially multicentered double-blind clinical trials, are needed to evaluate the effectiveness of BTKis in the management of COVID-19. Currently, we have to wait for the results of ongoing clinical trials before making any firm treatment decisions.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
M. Rezaei was responsible for conceptualization, methodology, and writing (review and editing). S. Barati was responsible for conceptualization, methodology, and writing the original draft. A. Babamahmoodi was responsible for methodology and writing (review and editing). F. Dastan was responsible for conceptualization, methodology, and writing (review and editing). M. Marjani was responsible for methodology and writing (review and editing).
Literature search: All authors; Figure creation: A Babamahmoudi, M Rezaei; Study design: All authors; Data Collection: All authors; Data interpretation: M Marjani, F Dastan; Writing: F Dastan, S Barati; Review and editing: All authors
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity. 2020;109 doi: 10.1016/j.jaut.2020.102433. [published Online First: 2020/03/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoudi S, Rezaei M, Mansouri N, et al. Immunologic features in coronavirus disease 2019: functional exhaustion of T cells and cytokine storm. Journal of clinical immunology. 2020;40(7):974–976. doi: 10.1007/s10875-020-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wu Z, Li J-W, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. International journal of antimicrobial agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra PK, Palma M, Buechel B, et al. Sterile particle-induced inflammation is mediated by macrophages releasing IL-33 through a Bruton's tyrosine kinase-dependent pathway. Nature materials. 2019;18(3):289–297. doi: 10.1038/s41563-018-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science (New York, NY) 1988;241(4861):42–52. doi: 10.1126/science.3291115. [published Online First: 1988/07/01] [DOI] [PubMed] [Google Scholar]
- 6.Radha V, Nambirajan S, Swarup G. Association of Lyn tyrosine kinase with the nuclear matrix and cell-cycle-dependent changes in matrix-associated tyrosine kinase activity. European journal of biochemistry. 1996;236(2):352–359. doi: 10.1111/j.1432-1033.1996.00352.x. [published Online First: 1996/03/01] [DOI] [PubMed] [Google Scholar]
- 7.Schaller MD, Borgman CA, Cobb BS, et al. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proceedings of the National Academy of Sciences of the United States of America1992;89(11):5192-6. doi: 10.1073/pnas.89.11.5192 [published Online First: 1992/06/01] [DOI] [PMC free article] [PubMed]
- 8.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [published Online First: 1999/12/10] [DOI] [PubMed] [Google Scholar]
- 9.Geier CB, Sauerwein KMT, Leiss-Piller A, et al. Hypomorphic Mutations in the BCR Signalosome Lead to Selective Immunoglobulin M Deficiency and Impaired B-cell Homeostasis. Front Immunol. 2018;9:2984. doi: 10.3389/fimmu.2018.02984. -84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolson PL, Welsh JD, Chauhan A, et al. A rationale for blocking thromboinflammation in COVID-19 with Btk inhibitors. Platelets. 2020;31(5):685–690. doi: 10.1080/09537104.2020.1775189. [DOI] [PubMed] [Google Scholar]
- 11.Florence JM, Krupa A, Booshehri LM, et al. Inhibiting Bruton's tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. American journal of physiology Lung cellular and molecular physiology. 2018;315(1):L52–l58. doi: 10.1152/ajplung.00047.2018. [published Online First: 2018/03/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912–1915. doi: 10.1182/blood.2020006288. [published Online First: 2020/04/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshikawa T, Hill T, Li K, et al. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. Journal of virology. 2009;83(7):3039–3048. doi: 10.1128/jvi.01792-08. [published Online First: 2008/11/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuka R, Seino K-i. Macrophage activation syndrome and COVID-19. Inflammation and Regeneration. 2020;40(1):19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong EA, Roeker LE, Shadman M, et al. BTK Inhibitors in Cancer Patients with COVID-19: "The Winner Will be the One Who Controls That Chaos" (Napoleon Bonaparte) Clinical cancer research: an official journal of the American Association for Cancer Research. 2020;26(14):3514–3516. doi: 10.1158/1078-0432.ccr-20-1427. [published Online First: 2020/04/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibaud S, Tremblay D, Bhalla S, et al. Protective Role of BTK Inhibitors in Patients with Chronic Lymphocytic Leukemia and COVID-19. British Journal of Haematology. 2021 doi: 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reda G, Noto A, Cassin R, et al. Reply to “CLL and COVID-19 at the Hospital Clinic of Barcelona: an interim report” Analysis of six hematological centers in Lombardy. Leukemia. 2020;34(9):2531–2532. doi: 10.1038/s41375-020-0966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin AY, Cuttica MJ, Ison MG, et al. Ibrutinib for chronic lymphocytic leukemia in the setting of respiratory failure from severe COVID-19 infection: Case report and literature review. Ejhaem. 2020 doi: 10.1002/jha2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrutinib for the Treatment of COVID-19 in Patients Requiring Hospitalization 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04439006.
- 21.Ibrutinib for the Treatment of Patients With B-Cell Malignancies Who Are Infected With Coronavirus Disease 2019 (COVID-19) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04665115?cond=ibrutinib+covid&draw=2&rank=2.
- 22.Study of Oral Ibrutinib Capsules to Assess Respiratory Failure in Adult Participants With Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and Pulmonary Injury (iNSPIRE) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04375397?cond=ibrutinib+covid&draw=2&rank=1.
- 23.Owen C, Berinstein NL, Christofides A, et al. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233. doi: 10.3747/co.26.4345. -e40[published Online First: 2019/04/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montalban X, Arnold DL, Weber MS, et al. Placebo-Controlled Trial of an Oral BTK Inhibitor in Multiple Sclerosis. The New England journal of medicine. 2019;380(25):2406–2417. doi: 10.1056/NEJMoa1901981. [published Online First: 2019/05/11] [DOI] [PubMed] [Google Scholar]
- 25.Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Science Immunology. 2020;5(48) doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acalabrutinib Study With Best Supportive Care in Participants Hospitalized With COVID-19 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04497948.
- 27.Tam C, Grigg AP, Opat S, et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: Initial report of a phase 1 first-in-human trial. Blood. 2015;126 [Google Scholar]
- 28.Covid-19 Infection and Pulmonary Distress Treatment With Zanubrutinib in Hospitalized Participants 2020 [Available from: https://clinicaltrials.gov/ct2/results?cond=Covid19&term=Zanubrutinib+&cntry=&state=&city=&dist=.
- 29.Tillman BF, Pauff JM, Satyanarayana G, et al. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. European Journal of Haematology. 2018;100(4):325–334. doi: 10.1111/ejh.13020. [DOI] [PubMed] [Google Scholar]
- 30.Chong EA, Roeker LE, Shadman M, et al. BTK inhibitors in cancer patients with COVID19:" The winner will be the one who controls that chaos"(Napoleon Bonaparte) Clinical Cancer Research. 2020 doi: 10.1158/1078-0432.CCR-20-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koffman B, Mato A, Byrd JC, et al. Management of CLL patients early in the COVID-19 pandemic: An international survey of CLL experts. American journal of hematology. 2020 doi: 10.1002/ajh.25851. [DOI] [PMC free article] [PubMed] [Google Scholar]