Abstract
Objective
Studies suggesting that vulnerability increased short-term mortality in older patients with COVID-19 enrolled hospitalized patients and lacked COVID-negative comparators. Aim of this study was to examine the relationship between frailty and 1-year mortality in older patients with and without COVID-19, hospitalized and nonhospitalized.
Design
Cohort study.
Setting and Participants
Patients over 75 years old accessing the emergency departments (ED) were identified from the ED archives in Florence, Italy.
Methods
Vulnerability status was estimated with the Dynamic Silver Code (DSC). COVID-19 hospital discharges (HC+) were compared with non-COVID-19 discharges (HC-). Linkage with a national COVID-19 registry identified nonhospitalized ED visitors with (NHC+) or without COVID-19 (NHC-).
Results
In 1 year, 48.4% and 33.9% of 1745 HC+ and 15,846 HC- participants died (P < .001). Mortality increased from 27.5% to 64.0% in HC+ and from 19.9% to 51.1% in HC- across DSC classes I to IV, with HC+ vs HC- hazard ratios between 1.6 and 2.2. Out of 1039 NHC+ and 18,722 NHC- participants, 18% and 8.7% died (P < .001). Mortality increased from 14.2% to 46.7% in NHC+ and from 2.9% to 26% in NHC- across DSC; NHC+ vs NHC- hazard ratios decreased from 5.3 in class I to 2.0 in class IV.
Conclusions and Implications
In hospitalized older patients, mortality increases with vulnerability similarly in the presence and in the absence of COVID-19. In nonhospitalized patients, vulnerability-associated excess mortality is milder in individuals with than in those without COVID-19. The disease reduces survival even when background risk is low. Thus, apparently uncomplicated patients deserve closer clinical monitoring than commonly applied.
Keywords: Frailty, vulnerability, COVID-19, Dynamic Silver Code, long-term mortality, prognostic assessment
Since its beginning, the SARS-CoV-2 pandemic has severely hit older patients, in whom COVID-19 mortality reaches stunning proportions.1, 2, 3 An advanced age has been identified as a major negative prognostic determinant in the course of COVID-19, independent of disease-specific predictors.4, 5, 6 Specifically, an exceeding COVID-19 mortality has been reported in subsets of older patients at an increased background risk of death, generically defined as frail.7, 8, 9, 10, 11, 12, 13, 14 Consequently, recommendations have been issued to consider frailty in the decision-making process on whether or not to increase the level of care in older patients with COVID-19.15 However, in the given context the use of the term frailty may be questioned because assessment of an increased risk status was based on tools, such as the Clinical Frailty Scale (CFS), that rely on comorbidities and dependency more than on the construct of frailty as a predisability condition, accepted in most current literature.16, 17, 18, 19 Therefore, the term vulnerability will be used hereinafter, even when frailty had been used in the original reports.
Other studies showed that CFS-assessed vulnerability did not contribute to predicting death in older persons hospitalized with COVID-19.20 , 21 Yet, the evidence provided so far is unsatisfactory. Most of the studies considered only hospitalized patients and were limited to hospital mortality,7, 8, 9, 10, 11, 12, 13, 14 providing no information on the role of vulnerability in individuals not requiring hospitalization nor on long-term survival. Moreover, they usually lacked non-COVID-19 comparators and, finally, assessed vulnerability a posteriori on the basis of some operator-dependent tool, such as the CFS. Assessing the excess risk associated with COVID-19 in vulnerable older patients is, therefore, a substantially unsolved issue.
In the community hospitals of the Central District for Healthcare Services of Tuscany (Azienda USL Toscana Centro, ATC) and in the Azienda Ospedaliero-Universitaria Careggi (AOUC), an academic hospital caring for adult patients in Florence, real-time, automated prognostic stratification of persons aged 75+ years accessing the emergency department (ED) is provided by the Dynamic Silver Code (DSC). Using only administrative data, the tool is able to predict short- and long-term survival22 , 23: more recently, it has been shown to also reflect pre-existing functional status, specifically inability to walk.24 Thus, although not a direct measure of frailty, the DSC expresses an increased vulnerability to adverse outcomes strictly associated with poor physical functioning.
This study was conducted to evaluate the role of pre-existing vulnerability, as represented by the DSC, on long-term mortality in a large cohort of older persons seeking care in the ED during the pandemic, separately in hospitalized and not hospitalized persons, comparing patients diagnosed with COVID-19 to those with other diagnoses.
Methods
Study Design and Data Source
A concurrent cohort study design was applied, using data obtained from the administrative archives of the ATC and the AOUC and the database of the Italian National Health Institute (Istituto Superiore di Sanità, ISS) of individuals diagnosed with COVID-19.
The ATC serves a population of approximately 1.6 million residents in central Tuscany, where the AOUC and 13 community hospitals are located. From the ATC and AOUC archives collecting all ED accesses and hospital discharges, we selected patients age 75+ years whose ED database record reported the DSC, accessing an ED in the area between March 1 and November 15, 2020. In addition, we also consulted the local demographics registry to obtain mortality data.
The ISS database25 is the national registry of all the confirmed cases of COVID-19, based on reverse transcriptase–polymerase chain reaction testing. It reports when, but not where (ie, hospital, community clinic, or patient's home), the diagnosis was made.
Assembly of Study Cohorts
Two different approaches were applied to select eligible patients, depending on whether ED access was followed or not by hospital admission.
Hospitalized patients were identified by linking the ED database with the hospital discharge database, using a unique identifier that does not allow personal identification. Linkage was limited to cases accessing the ED not earlier than 2 months prior hospitalization; in case of multiple ED access, the one closest to admission was kept. Elective hospitalizations were excluded. In this database, diagnoses are coded following the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The diagnosis of COVID-19, deriving from a positive reverse transcriptase–polymerase chain reaction testing, was adjudicated when an ICD-9-CM code 078.89 was reported as primary or secondary discharge diagnosis, or as a subsequent diagnosis when the primary diagnosis was consistent with an acute respiratory disease or a viral infection (ICD-9-CM codes 484.8, 466, 490, 519.8, 518.82, 518.81, 518.84, 480, 480.8, 480.9, 487.0, or 480.3). These hospitalized COVID-19 (HC+) patients were compared with all other nonelective, non-COVID-19 admissions (HC-).
Among patients registered in the ED database but not admitted to the hospital, those with COVID-19 (nonhospitalized COVID-19 cases, NHC+) were identified by linkage, using again the anonymous identifier, with the ISS database. When a participant had more than 1 ED access, the closest to the date of COVID-19 diagnosis was considered. ED records not linking with the ISS database were considered for comparison, as referring to nonhospitalized patients without COVID-19 (NHC-) patients.
Assessment of Vulnerability
Vulnerability was assessed with the DSC, a tool that, using only administrative data (age, sex, previous hospitalizations, and drug prescriptions), predicts short- and long-term mortality in 75+ years old residents of the ATC area accessing the ED.23 The DSC, originally validated in 2 different cohorts of more than 180,000 and 4400 individuals,23 is provided by a software incorporated into the application routinely used by ED clinicians in all the hospitals in the ATC and in the AOUC. When an eligible patient is triaged, the software queries the repository of health care data, links the archives contained in the repository, and extracts the information required. The lag time between occurrence of events contributing to the DSC and their registration in the repository is approximately 2 weeks. From the scores assigned to each item (Supplementary Table 1), the DSC is calculated and shown onto the computer screen, together with the corresponding risk class (class I: score 0–10; class II: score 11–25; class III: score 26–34; class IV: score 35+).24 In previous studies, 1-year mortality was approximately 2, 3, and more than 5 times greater in individuals in DSC class II, III, and IV than in those in class I, independent of the discharge diagnosis of the index hospitalization.22 Besides mortality, also the level of physical impairment has been shown to increase progressively across the four DSC classes.24 Thus, the tool is a valid measure of background risk or vulnerability and, similar to its parent instrument,26 allows performing risk adjustment when comparing therapeutic interventions.
Mortality Ascertainment
Vital status was ascertained from the ATC demographics registry as of March 31, 2021.
Analytic Procedures
Statistical analysis was performed with STATA v 16.1 (StataCorp, 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). Interval variables were expressed as mean ± standard error (SEM) or median and interquartile range (IQR), depending on the distribution, and categorical variables as percentages.
Main analyses were performed separately in hospitalized and nonhospitalized participants. The student t-test was used to compare normally distributed variables between 2 groups, the Mann-Whitney test for non-normally distributed variables, and the χ 2 test to compare relative frequencies, considering trends as appropriate. Survival analysis was performed with Cox proportional hazards models with hazard ratio (HR) and 95% CI, to compare mortality across DSC classes, separately in individuals with and without COVID-19, and between persons with and without the disease within DSC classes. The assumption of proportionality of hazards over time was verified with the Schoenfeld residuals and comparing the survival functions for each covariate pattern; the fitting of the models was evaluated using the Cox-Snell residuals. Interaction between diagnosis of COVID-19 and DSC class was tested with Wald test. Because the DSC incorporates demographics and some data on comorbidities, these variables were not entered in multivariable analyses, to prevent over-correction.
Protection against type I error was set at alpha level of 0.05.
Results
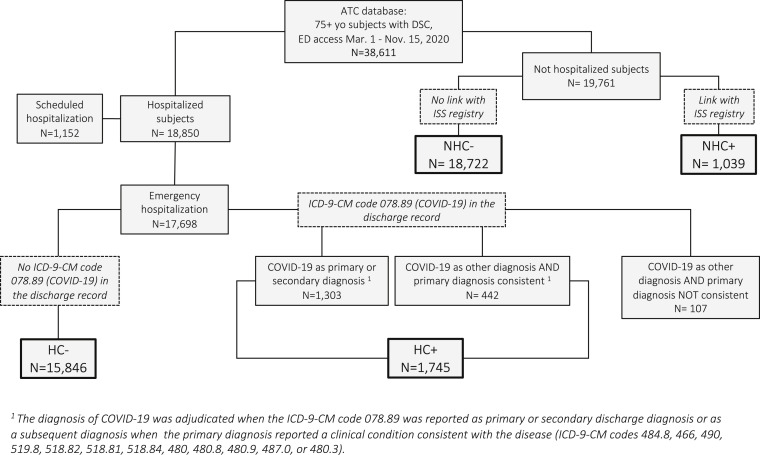
Figure 1 reports the study cohort assembly, after exclusion of repeated ED accesses. A total of 38,611 patients age 75+ years had at least 1 ED access between March 1 and November 15, 2020 registered in the ATC and AOUC archives, from which the DSC could be extracted. Of them, 17,698 had emergency hospitalization, 1152 elective hospitalization, and 19,761 were not hospitalized. Among patients with emergency hospitalization, those with an ICD-9-CM code 078.89 as their primary or secondary diagnosis, together with those in whom this code was a subsequent diagnosis and the primary diagnosis reported an ICD-9-CM code consistent with acute respiratory disease or viral infection, represented the group of hospitalized participants with adjudicated diagnosis of COVID-19 (HC+, n = 1745). The HC+ group was compared with patients whose emergency hospitalization was not due to COVID-19 (HC-, n = 15,846).
Fig. 1.
Flow-chart of the study cohort assembly. HC+ and HC- are hospitalized participants with/without adjudicated diagnosis of COVID-19, based on discharge records. NHC+ and NHC- are nonhospitalized participants with/without diagnosis of COVID-19, based on linkage with the ISS registry.
Of the 19,761 patients whose ED access was not followed by hospital admission, 1039 could be linked to records in the ISS registry of COVID-19 cases and represented the group of nonhospitalized COVID-19 (NHC+) participants, whereas 18,722 could not be linked and were considered as nonhospitalized non-COVID-19 (NHC-) comparators.
Overall Assessment of Mortality Risk
Over the entire follow-up, 8134 (21.8%) participants died. Increasing DSC class, the diagnosis of COVID-19, and hospital admission predicted independently the risk of death (Supplementary Table 2).
Hospitalized Participants
The characteristics of HC+ and HC- participants are shown in Table 1 . HC+ participants were younger than HC-, with a similar proportion of men. The distribution across DSC classes and the duration of hospital stay differed significantly between the 2 groups. The 10 most common discharge diagnoses in HC- are reported in Supplementary Table 3.
Table 1.
Comparison of the Characteristics of Participants Who Were or Were Not Diagnosed With COVID-19 Separately in Those Who Were or Were Not Hospitalized
| HC+ (n = 1745) | HC- (n = 15,846) | P Value | NHC+ (n = 1039) | NHC- (n = 18,722) | P Value | |
|---|---|---|---|---|---|---|
| Age (y) | 84 ± 5.6 | 85 ± 5.7 | <.001 | 84 ± 6.1 | 83 ± 5.5 | <.001 |
| Male sex | 852 (48.8) | 6839 (43.1) | <.001 | 417 (40.1) | 7751 (41.4) | .432 |
| DSC class (score) | ||||||
| I (<10) | 541 (31.0) | 4599 (29.0) | <.001 | 352 (33.9) | 8233 (44.0) | <.001 |
| II (11‒25) | 616 (35.3) | 6270 (39.6) | 396 (38.1) | 6468 (34.6) | ||
| III (26‒34) | 360 (20.6) | 3525 (22.2) | 199 (19.2) | 2834 (15.1) | ||
| IV (≥35) | 228 (13.1) | 1452 (9.2) | 92 (8.6) | 1187 (6.3) | ||
| Length of hospital stay (d) | 11 [6, 19] | 7 [5, 11] | <.001 | / | / | / |
| Mortality | 845 (48.4) | 5372 (33.9) | <.001 | 291 (28.0) | 1629 (8.7) | <.001 |
Data are mean ± SEM, median [IQR], or n (%).
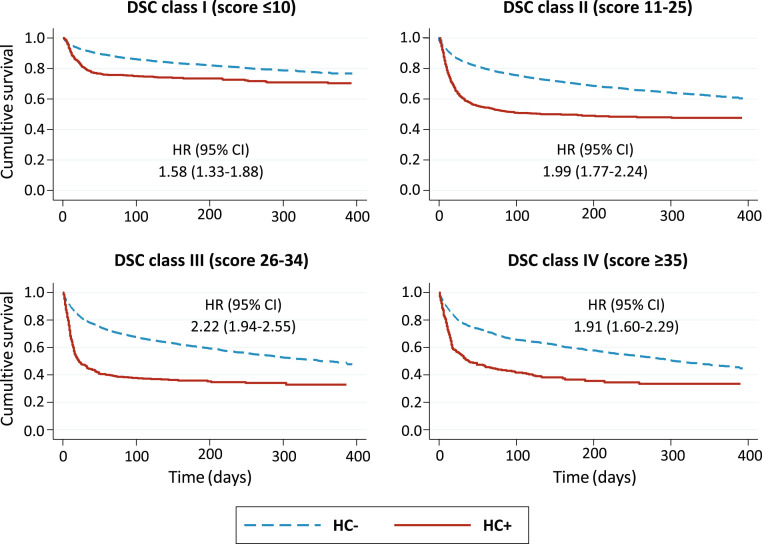
Over a median follow-up duration of 206 (86–293) days, 48.4% of the HC+ and 33.9% of the HC- participants died (Table 1). Interaction between DSC class and the diagnosis of COVID-19 was slightly significant (P = .034). In HC+ participants, mortality increased from 27.5% in DSC class I to 51.1% in class II and 65.3% in class III, then it declined mildly (64.0%) in class IV, with 2-fold to 3-fold greater hazards of death in class II–IV vs class I. In HC- patients, the absolute risk of death was always lower than in HC+ within each DSC class and increased progressively across DSC classes, from 19.9% in class I through 51.1% in class IV. HRs had a similar stepwise increase, from 1.9 to 2.9 (Table 2 ). Thus, in analyses stratified by DSC class, the excess mortality associated with COVID-19, although always significant, was comparable within each DSC stratum, with HRs ranging between 1.6 and 2.2 (Figure 2 ).
Table 2.
Mortality and Risk of Death by DSC Class Separately in Participants Who Were or Were Not Hospitalized and Were or Were Not Diagnosed With COVID-19
| Hospitalized |
Non-hospitalized |
|||||
|---|---|---|---|---|---|---|
| Participants | Deaths (%) | HR (95% CI) | Participants | Deaths (%) | HR (95% CI) | |
| All COVID-positives | 1745 | 845 (48.4) | / | 1039 | 291 (28.0) | / |
| DSC class I (score ≤10) | 541 | 149 (27.5) | 1 | 352 | 50 (14.2) | 1 |
| DSC class II (score 11-25) | 616 | 315 (51.1) | 2.24 (1.84-2.72) | 396 | 114 (28.8) | 2.25 (1.62-3.14) |
| DSC class III (score 26-34) | 360 | 235 (65.3) | 3.37 (2.75-4.14) | 199 | 84 (42.2) | 3.50 (2.46-4.96) |
| DSC class IV (score ≥35) | 228 | 146 (64.0) | 3.08 (2.45-3.87) | 92 | 43 (46.7) | 3.72 (2.47-5.59) |
| All COVID-negatives | 15,846 | 5372 (33.9) | / | 18,722 | 1626 (8.7) | / |
| DSC class I (score ≤10) | 4599 | 913 (19.9) | 1 | 8233 | 235 (2.9) | 1 |
| DSC class II (score 11-25) | 6270 | 2131 (34.0) | 1.88 (1.74-2.03) | 6468 | 634 (9.8) | 3.55 (3.05-4.12) |
| DSC class III (score 26-34) | 3525 | 1587 (45.0) | 2.65 (2.45-2.88) | 2834 | 448 (15.8) | 5.86 (5.00-6.86) |
| DSC class IV (score ≥35) | 1452 | 741 (51.0) | 2.85 (2.59-3.15) | 1187 | 309 (26.0) | 9.70 (8.18-11.49) |
Fig. 2.
Survival curves of hospitalized COVID-19 vs non-COVID-19 (HC+, HC-) participants, separately in each DSC class.
Nonhospitalized Participants
The characteristics of NHC+ and NHC- participants are presented in Table 1. NHC+ participants were older than NHC-, with a similar proportion of men. The distribution across DSC classes was also different between the 2 groups.
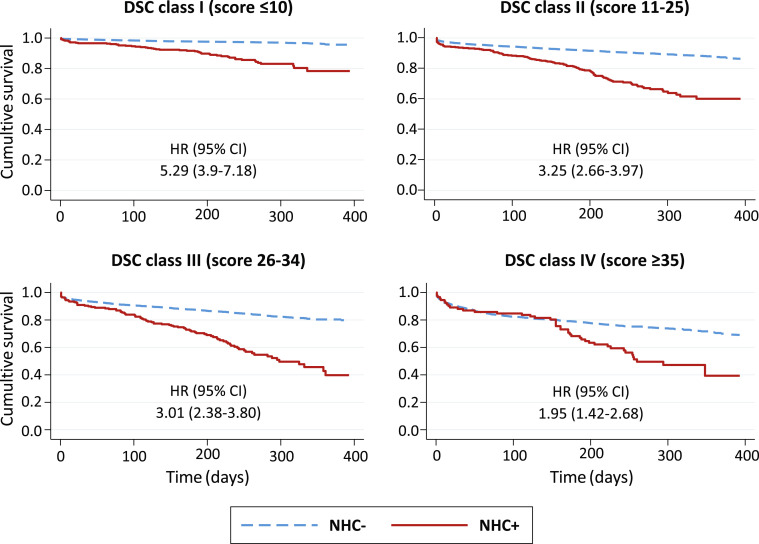
Throughout a median (IQR) observation time of 247 (190–302) days, 28% of the NHC+ participants and 8.7% of the NHC- participants died (P < .001). Interaction between DSC class and the diagnosis of COVID-19 in nonhospitalized participants was highly significant (P < .001). Mortality increased stepwise across DSC classes in both groups, yet more sharply in NHC-, from 14.2% in class I to 46.7% in class IV among NHC+, and from 2.9% in class I to 26% in class IV among NHC- (Table 2). Compared with class I, the hazard of death across classes II–IV was 2.3, 3.5, and 3.7 greater in NHC+, and 3.6, 5.9, and 9.7 greater in NHC- (Table 2). The excess mortality associated with COVID-19 decreased progressively with advancing DCS class, from an HR of 5.3 in class I to an HR of 2.0 in class IV (Figure 3 ).
Fig. 3.
Survival curves of non-hospitalized COVID-19 vs non-COVID-19 (NHC+, NHC-) participants, separately in each DSC class.
Time Course of Mortality
Mortality gradient between participants with and without COVID-19 had different time courses in hospitalized and nonhospitalized individuals. In HC+ of all DSC classes, the risk of death increased dramatically in the first month after enrollment, plateauing in the following months (Figure 2). Conversely, in nonhospitalized participants the survival curves separated progressively in classes I–III and diverged substantially only after the third month in class IV (Figure 3).
Discussion
In this large cohort of older patients accessing the EDs of Tuscany, we examined whether vulnerability, expressed by the DSC, modulated the risk of death associated with COVID-19 several months after ED access, separately in individuals who were or were not hospitalized. At the same time, we evaluated the excess risk of death associated with COVID-19, balancing background risk with the DSC. In hospitalized participants, mortality increased 2- to 3-fold with advancing DSC class, similarly in the presence and in the absence of COVID-19. The mild decline in the risk of death observed in DSC class IV HC+ participants compared with class III, can be ascribed to the lower precision of the estimates in the smaller group of HC+ patients. Conversely, in nonhospitalized participants, the diagnosis of COVID-19 increased the risk of death within each DSC class, but to a greater extent in the first than in the last classes (ie, more in individuals with lower background risk).
Several studies7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and systematic reviews27 , 28 analyzed the relationship between frailty and COVID-19 mortality. However, the tool usually applied for this purpose was the CFS, which incorporates dependency as a measure of “frailty”, where in fact dependency is to be considered as an outcome of the frailty status.16 Therefore, we questioned this use of the term frailty, instead of vulnerability. With few exceptions,20 , 21 the available evidence suggests that CFS-defined vulnerable individuals have an increased COVID-19 short-term mortality. In particular, a systematic review of 34 articles, with more than 18,000 hospitalized patients, reported that, compared with individuals with CFS of 1-3, mortality was 2-fold and 3-fold greater in those with CFS of 4–5 and 6–9, respectively.28 Nevertheless, in a retrospective cohort study of 1071 patients age 65+ years, increasing vulnerability was associated with greater 30-day mortality in COVID-negative, but not in COVID-positive participants: because the diagnosis of COVID-19 enhanced the risk of death, the authors concluded that the disease strongly influences per se survival, beyond well-established prognostic indicators.20 Consistently with the majority of previous studies enrolling only COVID-19 participants, we found that vulnerability, as estimated from the DSC, increases long-term mortality in older patients hospitalized with COVID-19, but not more than in patients hospitalized with other diagnoses.
The relationship between vulnerability and COVID-19 differed substantially in nonhospitalized patients, as the mortality gradient across DSC classes, although always detectable independent of the diagnosis, was less pronounced in participants with COVID-19 than in others. Thus, COVID-19 had a relatively more severe impact on survival in participants with lower background risk, as shown by decreasing HRs of NHC+ vs NHC- across DSC classes.
We also observed a different time course of COVID-19 mortality in patients who were or were not hospitalized. The brisk decline in survival in HC+ reflects the well-known severity of the disease in its acute phase. Yet, the slowly progressive separation between NHC+ and NHC- survival curves was unexpected and suggested that COVID-19 may eventually lead to a fatal outcome, even when no need for hospitalization was initially devised. It should be emphasized that COVID-negative individuals accessing the ED in the pandemic period probably had more severe conditions, thus, minimizing the difference with COVID-positive individuals, than the average population of ED visitors in nonpandemic times. Overall, our findings alert toward long-term consequences of the disease in otherwise well older patients, whose initial clinical presentation may appear noncritical.
Many patients with COVID-19 recover slowly and remain symptomatic long after the acute phase,29 but long-term sequelae are sometimes unrelated to the initial severity of the disease.30 It has been hypothesized that a “long-COVID” syndrome might affect a fairly large number of patients.30 In a recent series of 958 COVID-19-convalescent, never hospitalized young individuals, first examined in a post-COVID outpatient clinic 6 weeks after the diagnosis, 442 persons were followed-up at 4 months and 353 at 7 months: shortness of breath and fatigue were present in as many as 9%–10% at 4 months and 14%–15% at 7 months.31 Putting this evidence and our findings together, we would speculate that some insidious, possibly undetected, post-COVID syndrome might develop in old age, ultimately increasing the risk of death in the long term.
Most previous studies assessed the relationship between vulnerability and COVID-19 only in hospitalized patients without COVID-negative comparators, and limited to survival until discharge or, at most, at 30 days after admission. Thus, compared with the existing literature, this study has several strengths. We could assess the impact of vulnerability on long-term survival in both hospitalized and nonhospitalized older persons with COVID-19, compared with participants without COVID-19. We assembled a large, population-based sample of individuals older than those in most previous studies. Finally, because the CFS and other vulnerability screening tools are usually applied a posteriori and require some degree of skills, they might present issues of reliability and validity. Furthermore, they largely depend on the quality of the data collected, which may be suboptimal when taking history from an older patient. Conversely, the DSC is objective, completely operator-independent, and can also be obtained in noncollaborating patients.
The study has limitations. We had no other information, besides that conveyed by the DSC, on associated chronic comorbidities. In nonhospitalized participants, we could not ascertain the reason for ED access and its precise timing in relation to COVID-19 diagnosis, as well as the mode and cause of death. Because the DSC is available only following ED access, we could not extend our evaluation to patients with COVID-19 who received care in the community without accessing the ED: in particular, it is possible that extremely vulnerable older persons, such as those living in nursing homes, received neither a diagnosis of COVID-19 nor an ED admission during the months of the pandemic. This might limit the external validity of our findings. Finally, our findings depict the natural history of the disease as it appeared before the widespread application of vaccination programs, which fortunately has dramatically reduced COVID-19 mortality in older individuals.
Conclusions and Implications
In hospitalized patients age 75+ years, the increase in long-term mortality with progressive vulnerability, as documented by the DSC, is similar in presence and in absence of COVID-19. Conversely, in patients who are not hospitalized after ED access, the increase in long-term risk of death associated with worsening DSC class is greater in the absence than in the presence of COVID-19. In other terms, the disease appears to compromise long-term survival of older patients proportionally more when initial clinical status presents as noncritical and hospitalization is not devised. As a consequence, these apparently uncomplicated patients deserve closer clinical monitoring than commonly thought. Further studies are required to understand the pathophysiological mechanisms underlying this epidemiologic evidence.
Footnotes
The authors declare no conflicts of interest.
The study was partly supported by the Tuscany Region, grant B19C21000380002.
Appendix
Supplementary Table 1.
Variables Included in the DSC With Corresponding Scores, Obtained From Cox Regression Model Predicting 1-Year Death in 90,039 Individuals Age 75+ Years21
| Variables | Score |
|---|---|
| Age (y) | |
| 75‒79 | 0 |
| 80‒84 | 8 |
| 85+ | 23 |
| Sex | |
| Female | 0 |
| Male | 5 |
| Number of drugs in previous 3 mo | |
| 0‒3 | 0 |
| 4‒5 | 1 |
| 6‒8 | 2 |
| 9+ | 6 |
| Main diagnostic group in previous (6 months) hospital admission | |
| No admission | 0 |
| Cardiovascular disease/others | 19 |
| Cancer | 42 |
| Respiratory disease | 28 |
| Days from previous (6 mo) hospital admission | |
| No admission | 0 |
| 30‒180 | 8 |
| 0‒30 | 0 |
Supplementary Table 2.
Mortality and Risk of Death by DSC Class, COVID-19 Diagnosis, and Hospitalization
| Participants N = 37,352 |
Deaths (%) n = 8134 (21.8) |
HR (95% CI) | |
|---|---|---|---|
| DSC score | |||
| DSC class I (score ≤10) | 13.725 | 1347 (9.8) | 1 |
| DSC class II (score 11‒25) | 13.750 | 3194 (23.2) | 2.25 (2.11‒2.4) |
| DSC class III (score 26‒34) | 6.918 | 2354 (34) | 3.32 (3.1‒3.55) |
| DSC class IV (score ≥35) | 2.959 | 1239 (41.9) | 3.83 (3.55‒4.14) |
| COVID-19 diagnosis | |||
| No | 34.568 | 6998 (20.2) | 1 |
| Yes | 2.784 | 1136 (40.8) | 2.17 (2.04‒2.31) |
| Hospitalization | |||
| No | 19.761 | 1917 (9.7) | 1 |
| Yes | 17.591 | 6217 (35.3) | 3.69 (3.5‒3.88) |
Supplementary Table 3.
The 10 Most Common Discharge Diagnoses in the 15,846 COVID-19-Free Hospitalized Participants
| ICD-9 Codes | Description | n | % |
|---|---|---|---|
| 518.81 | Acute respiratory failure | 1163 | 7.34 |
| 428.0 | Congestive heart failure | 428 | 2.70 |
| 434.01 | Cerebral thrombosis with cerebral infarction | 392 | 2.47 |
| 428.1 | Left heart failure | 365 | 2.30 |
| 518.84 | Acute and chronic respiratory failure | 362 | 2.28 |
| 820.20 | Closed fracture of trochanteric section of neck of femur | 357 | 2.25 |
| 410.71 | Subendocardial infarction, initial episode of care | 281 | 1.77 |
| 995.91 | Sepsis | 270 | 1.70 |
| 486 | Pneumonia, organism unspecified | 262 | 1.65 |
| 820.02 | Closed fracture of midcervical section of neck of femur | 235 | 1.48 |
References
- 1.Our World in Data Excess mortality during the coronavirus pandemic (COVID-19) https://ourworldindata.org/excess-mortality-covid
- 2.Eurostat Excess mortality, monthly data. https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_mexrt&lang=en
- 3.Islam N., Shkolnikov V.M., Acosta R.J., et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021;373:n1137. doi: 10.1136/bmj.n1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parohan M., Yaghoubi S., Seraji A., et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;23:1416–1424. doi: 10.1080/13685538.2020.1774748. [DOI] [PubMed] [Google Scholar]
- 6.Palmieri L., Vanacore N., Donfrancesco C., et al. Italian National Institute of Health COVID-19 Mortality Group Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrés-Esteban E.M., Quintana-Diaz M., Ramírez-Cervantes K.L., et al. Outcomes of hospitalized patients with COVID-19 according to level of frailty. PeerJ. 2021;9:e11260. doi: 10.7717/peerj.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitt J., Carter B., Vilches-Moraga A., et al. COPE Study Collaborators The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marengoni A., Zucchelli A., Vetrano D.L., et al. Beyond chronological age: frailty and multimorbidity predict in-hospital mortality in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2021;76:e38–e45. doi: 10.1093/gerona/glaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomaard L.C., van der Linden C.M.J., van der Bol J.M., et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 2021;50:631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smet R., Mellaerts B., Vandewinckele H., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932.e1. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilis M., Chagrot N., Koeberle S., et al. Older adults with SARS-CoV-2 infection: utility of the clinical frailty scale to predict mortality. J Med Virol. 2021;93:2453–2460. doi: 10.1002/jmv.26766. [DOI] [PubMed] [Google Scholar]
- 13.Koduri G., Gokaraju S., Darda M., et al. Clinical frailty score as an independent predictor of outcome in COVID-19 hospitalised patients. Eur Geriatr Med. 2021;12:1065–1073. doi: 10.1007/s41999-021-00508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sablerolles R.S.G., Lafeber M., van Kempen J.A.L., et al. COMET research team. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021;2:e163–e170. doi: 10.1016/S2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NICE guideline COVID-19 rapid guideline: critical care in adults (NG 159) https://www.nice.org.uk/guidance/ng159 [PubMed]
- 16.Hoogendijk E.O., Afilalo J., Ensrud K.E., Kowal P., Onder G., Fried L.P. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 17.Pilotto A., Azzini M., Cella A., et al. Italian Geriatric Society Hospital and Community (SIGOT) Study Group The multidimensional prognostic index (MPI) for the prognostic stratification of older inpatients with COVID-19: a multicenter prospective observational cohort study. Arch Gerontol Geriatr. 2021;95:104415. doi: 10.1016/j.archger.2021.104415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dent E., Martin F.C., Bergman H., et al. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 20.Owen R.K., Conroy S.P., Taub N., et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2021;50:307–316. doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miles A., Webb T.E., Mcloughlin B.C., et al. Outcomes from COVID-19 across the range of frailty: excess mortality in fitter older people. Eur Geriatr Med. 2020;11:851–855. doi: 10.1007/s41999-020-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balzi D., Carreras G., Tonarelli F., et al. Real-time utilisation of administrative data in the ED to identify older patients at risk: development and validation of the Dynamic Silver Code. BMJ Open. 2019;9:e033374. doi: 10.1136/bmjopen-2019-033374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Bari M., Carreras G., Giordano A., et al. Long-term survival after hospital admission in older Italians: comparison between geriatrics and internal medicine across different discharge diagnoses and risk status. J Gerontol A Biol Sci Med Sci. 2021;76:1333–1339. doi: 10.1093/gerona/glaa147. [DOI] [PubMed] [Google Scholar]
- 24.Di Bari M., Giordano A., Tonarelli F., et al. Estimating prognosis and frailty in persons aged 75+ in the emergency department: further validation of Dynamic Silver Code. J Am Med Dir Assoc. 2022;23:87–91. doi: 10.1016/j.jamda.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Istituto superiore di Sanità - Sorveglianza Covid-19. https://covid-19.iss.it/
- 26.Di Bari M., Balzi D., Fracchia S., et al. Decreased usage and increased effectiveness of percutaneous coronary intervention in complex older patients with acute coronary syndromes. Acute Myocardial Infarction in Florence 2 (AMI Florence-2) Working Group. Heart. 2014;100:1537–1542. doi: 10.1136/heartjnl-2013-305445. [DOI] [PubMed] [Google Scholar]
- 27.Dumitrascu F., Branje K.E., Hladkowicz E.S., et al. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. 2021;69:2419–2429. doi: 10.1111/jgs.17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastora S., Kounidas G., Perrott S., et al. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: a systematic review and meta-analysis. E Clin Med. 2021;36:100896. doi: 10.1016/j.eclinm.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oronsky B., Larson C., Hammond T.C., et al. A Review of Persistent Post-COVID Syndrome (PPCS) Clin Rev Allergy Immunol. 2021:1–9. doi: 10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend L., Dowds J., O'Brien K., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18:997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Augustin M., Schommers P., Stecher M., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]