Abstract
Levocetirizine, a third-generation antihistamine, and montelukast, a leukotriene receptor antagonist, exhibit remarkable synergistic anti-inflammatory activity across a spectrum of signaling proteins, cell adhesion molecules, and leukocytes. By targeting cellular protein activity, they are uniquely positioned to treat the symptoms of COVID-19. Clinical data to date with an associated six-month follow-up, suggests the combination therapy may prevent the progression of the disease from mild to moderate to severe, as well as prevent/treat many of the aspects of ‘Long COVID,’ thereby cost effectively reducing both morbidity and mortality. To investigate patient outcomes, 53 consecutive COVID-19 test (+) cases (ages 3–90) from a well-established, single-center practice in Boston, Massachusetts, between March – November 2020, were treated with levocetirizine and montelukast in addition to then existing protocols [2]. The data set was retrospectively reviewed. Thirty-four cases were considered mild (64%), 17 moderate (32%), and 2 (4%) severe. Several patients presented with significant comorbidities (obesity: n = 22, 41%; diabetes: n = 10, 19%; hypertension: n = 24, 45%). Among the cohort there were no exclusions, no intubations, and no deaths. The pilot study in Massachusetts encompassed the first COVID-19 wave which peaked on April 23, 2020 as well as the ascending portion of the second wave in the fall. During this period the average weekly COVID-19 case mortality rate (confirmed deaths/confirmed cases) varied considerably between 1 and 7.5% [37]. FDA has approved a multicenter, randomized, placebo-controlled, Phase 2 clinical trial design, replete with electronic diaries and laboratory metrics to explore scientific questions not addressed herein.
Keywords: COVID-19, Long COVID, Levocetirizine, Montelukast, Therapeutic, Anti-inflammatory
1. Introduction
The coronavirus 2019 (COVID-19) pandemic has been partially contained under a backdrop of substantial resources allocated by international parties to resolve the problem. Presently, definitive treatment for COVID-19 infection remains both limited and costly, particularly for patients with mild to moderate disease. The heterogenous clinical features of COVID-19 range from an asymptomatic presentation to acute respiratory distress syndrome (ARDS) and multi-organ system failure; untreated the disease can progress to pneumonia, ARDS, sepsis, shock, and death. The insidious progression is accompanied in some patients by an excessive inflammatory response underscored by an increase in proinflammatory cytokine levels [3], [4] termed ‘cytokine storm.’ The advent of the SARS-CoV-2 (COVID-19) pandemic presents a challenge in identifying a therapeutic that will derail viral replication/target cellular protein activity and effectively mitigate symptoms without causing concurrent host toxicity (see Table 1 ).
Table 1.
Summary of key characteristics of levocetirizine and montelukast.
| Levocetirizine | Montelukast |
|---|---|
|
|
1.1. Synergistic anti-inflammatory combination therapy for COVID-19
Coronaviruses are a large group of enveloped, positive sense (immediately translated by the host cell), single-stranded RNA viruses belonging to the order Nidovirales. SARS-CoV-2 has been designated within the order as the seventh discrete coronavirus species capable of causing human disease. The virus is characterized by a long incubation period between 5 and 14 days. Initial symptoms are varied, ranging from none to typical viral presentations including fever, cough, shortness of breath, fatigue, myalgia, headache, anosmia, and diarrhea [3], [5]. Contemporary and evolving COVID-19 research has identified the treatment of inflammation caused by the virus as a cornerstone of therapy [6]. The anti-inflammatory synergy between levocetirizine, a third-generation antihistamine, and montelukast, a leukotriene receptor antagonist, was discovered by B. Chandler May, MD, JD, MS, FCLM during the 2009 H1N1 pandemic (US Patent No. 9044479). The combination is ideally positioned to treat COVID-19 symptoms, addressing multiple targets within the inflammatory pathway including: histamine, leukotriene D4 (LTD4), NF-kB, ICAM-1, VCAM-1, IL-4, IL-6, IL-8, RANTES, GM-CSF, TLR-3, AP-1, and eosinophil and neutrophil quantity and migration [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18].
1.2. Synergistic NF-kB inhibition
The downregulation of NF-kB is considered a key mechanism of action (MOA) for relief of COVID-19 symptoms and mitigation of inflammation as NF-kB plays a critical role in mediating responses to a remarkable diversity of external stimuli; providing at least in part, regulation of cytokine release triggered by infection. Equally if not more important, is recognition of the NF-kB family of transcription factors as pivotal across the spectrum of not only inflammation, but also immunity, cell proliferation, differentiation, cell survival, and cell death. NF-kB is expressed in almost all cell types and tissues. Specific binding sites are present in the promoters and/or enhancers of a large number of genes including: cytokines/chemokines and their modulators, immunoreceptors, proteins involved in antigen presentation, cell adhesion molecules, acute phase proteins, stress response genes, cell surface receptors, regulators of apoptosis, growth factors, ligands and their modulators, early response genes, transcription factors and regulators, viruses, and enzymes [19].
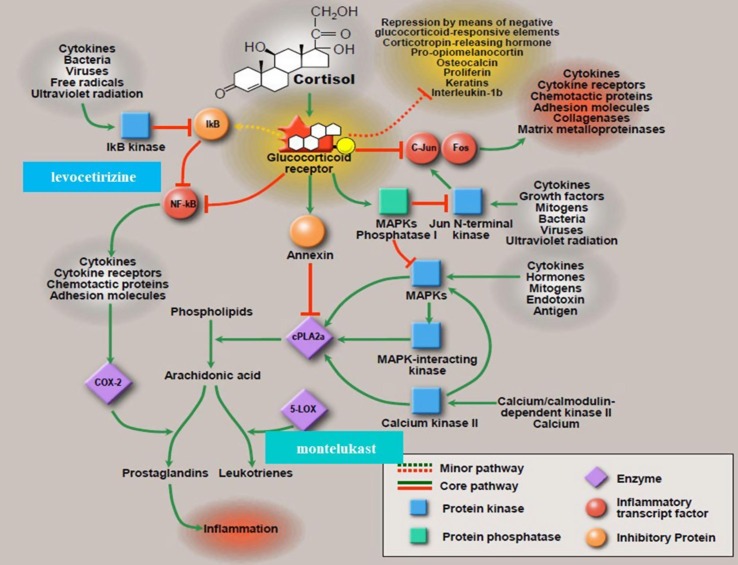
Data from DeDiego et al. illustrated the importance of the downregulation of NF-kB in coronavirus infected mice with SARS-CoV-1 (2002) severe acute respiratory distress syndrome [20]. The authors found that pulmonary pathology was significantly less in infected mice treated with each of NF-kB inhibitors CAPE (caffeic acid phenethyl ester) and parthenolide. A higher reduction of pathology was observed in the mice treated simultaneously with both inhibitors; reduction in pulmonary pathology correlated with a higher survival rate (no treatment: 16.7% survival; CAPE: 44.4%; parthenolide: 33.3%; combined treatment: 55.6% survival) and reduced proinflammatory cytokines in the lung. Viral titers in the lung homogenates were similar in both untreated and treated animals, suggesting the reduction in proinflammatory cytokines after treatment with NF-kB inhibitors was not a consequence of reduced virus replication. One advantage of antivirals that target cellular protein activity in contrast to viral proteins lies in an effect not likely to be negated by mutations in the virus genome. This research illustrated the activation of the NF-kB signaling pathway as a major contribution to inflammation following SARS-CoV-1 (2002) infection with the acknowledgement that NF-kB inhibitors have the potential as promising therapeutics in infections caused by SARS-CoV and other pathogenic coronaviruses [20]. Fig. 1 depicts, in part, the mechanism of action associated with the combination levocetirizine and montelukast.
Fig. 1.
Proposed Mechanism of Action of the Combination of Levocetirizine and Montelukast - NF-kB as a family of transcription factors plays a critical role in mediating responses to a remarkable diversity of external stimuli, inflammation, cell survival, and cell death (www.bu.edu/nf-kb). NF-kB is found throughout the animal kingdom - a master regulator of the inflammatory response; Fig. 1: Steroid Pathway - Adapted by permission [1].
1.3. Levocetirizine mechanism of action
Levocetirizine, a third-generation antihistamine, classically downregulates the H1 receptor on the surface of mast cells and basophils to block the IgE-mediated release of histamine. Histamine has been well characterized by its effects on the body, including in part, its function as a neurotransmitter, dilation of blood vessels which in turn increases permeability and lowers blood pressure, contraction of smooth muscle in the lung, uterus, and stomach, and as a source of sneezing, itching, and congestion. Levocetirizine is considered by pharmacologists an ‘insurmountable’ H1 receptor antagonist [23]. It has been objectively established as the most potent of the five modern generation antihistamines (levocetirizine, cetirizine, fexofenadine, loratadine, and desloratadine) through histamine wheal and flare data [10], [24], [25], [26], [27].
Levocetirizine, given its low volume of distribution and high receptor occupancy, is also among a select group of H1 receptor antagonists which can inhibit NF-kB and activator protein-1 (AP-1) activity through H1 receptor-dependent and independent mechanisms [9], [21], [22]. Induction of such activity follows in a dose-dependent manner to decrease, inter alia, tumor necrosis factor-α induced production of the chemokine RANTES (Regulated upon activation, normal T cell expressed and presumably secreted). RANTES expression, mediated exclusively through NF-kB, attracts eosinophils, monocytes, mast cells and lymphocytes, activates basophils, and induces histamine release from these cells.
1.4. Montelukast mechanism of action
Montelukast functions at the CysLT1 receptor to inhibit the physiologic action of leukotriene D4 (LTD4). Leukotrienes are protein mediators of inflammation similar to histamine; however, 100-1000x more potent on a molar basis than histamine in the lung. LTD4 is the most potent cysteinyl leukotriene in contracting smooth muscle, thereby producing bronchoconstriction. Contemporary cell and animal science support the use of montelukast in patients with acute respiratory distress syndrome [28], [29].
At the molecular level, distinct from CysLTR1 antagonism, montelukast has also been reported to inhibit the activation of NF-kB in a variety of cell types including monocytes/macrophages, T cells, epithelial cells, and endothelial cells, thereby interfering with the generation of multiple proinflammatory proteins [17]. Separately, Robinson, et al. found that montelukast independently inhibited resting and GM-CSF-stimulated eosinophil adhesion to VCAM-1 under flow conditions [14].
1.5. Montelukast potential dual effect - enzyme inhibition and COVID-19 virus entry
An expanding body of molecular science favorably supports montelukast as a potential therapeutic in the treatment of COVID-19. Multiple in silico and in vitro studies have depicted the dual potential of montelukast to inhibit the SAR-CoV-2 main proteinase 3CLpro as well as viral entry into the host cell (Spike/ACE2). The anti-inflammatory drugs: montelukast, ebastine, a second-generation antihistamine, and steroid, Solu-Medrol (methylprednisolone) exhibit remarkable affinities to 3CLpro. 3CLpro plays an essential role in processing polyproteins, the resultant products of which are subsequently utilized in the production of new virions. Additionally, there is a known clinical crossover between ebastine and levocetirizine, the latter considered more potent [27], [30], [31], [32], [33], [34].
1.6. Levocetirizine and montelukast safety/quality of life
Montelukast has been safely and extensively used throughout the world since 1998. In certain patient populations, particularly children, are reports of an increase incidence of neuropsychiatric events (NAE). As such, FDA issued a black box warning in the Spring of 2020 pertaining to use in allergic rhinitis. However, observational studies, including the FDA’s own Sentinel study which examined asthma patients 6 years and older [30], found no increased risk of mental health side effects with montelukast compared to inhaled corticosteroids (ICS). Moreover, in those with a psychiatric history, montelukast patients exhibited a decreased risk of outpatient depression compared to ICS patients; additional data found no statistical association (inpatient depressive disorder and self-harm) between montelukast and serious NAEs, across age, sex, and time strata [35]. The absence of adverse outcomes was consistent with results from clinical trials and well-conducted observational studies [36], [37], [38]. In their conclusion, from the totality of the observational evidence, including well-conducted observational studies, montelukast was not suggestive of a risk [35]. Prudence; however, dictates that patients considered for therapy undergo a mental health screening.
Levocetirizine has also been used extensively across the globe beginning with a successful launch in Europe at the turn of the century. It remains the only antihistamine in the world to demonstrate improved quality of life across all treatment domains (Short Form Health Survey−36 (SF-36); p < 0.001) in a series of 421 patients with allergy/asthma treated for six months [39]. The SF-36 addresses multiple domains: physical functioning, role limitation to due physical health, bodily pain, social functioning, general mental health, role limitation due to emotional problems, vitality/fatigue, and general health perception.
The two molecules are titratable, i.e., levocetirizine from 5 mg to 20 mg/day and montelukast from 10 mg to 40 mg/day and are underscored by millions of days of patient use. In the United States, both are considered Pregnancy Category B (dosed once daily – levocetirizine 5 mg; montelukast 10 mg). In the context of treating a potentially life-threatening infectious disease, the combination appears remarkably suited as a therapeutic in the COVID-19 treatment paradigm.
2. Materials and methods
2.1. Data collection and analysis
Machelle Wilchesky, PhD, McGill University, Lead Investigator for a COVID-19 Symptom Montelukast Trial, provided the research framework for the pilot data and its release here. All patients were screened for psychological conditions using the Patient Health Questionnaire-4 (PHQ-4) [40]. Patients testing (+) for COVD-19 within the clinical practice or hospital and subsequently referred to Holly Gallivan, MD, MPH, FACS, FAAOA by another provider, were sequentially seen and treated with the combination of levocetirizine and montelukast. All patients were accepted for treatment regardless of presenting symptoms; no patients were excluded due to underlying comorbidities. Follow-up consisted of a minimum six-month period.
3. Results
A descriptive analysis of 53 COVID-19 (+) patients from a well-established single-center otolaryngology and allergy practice is presented in Table 2 . The pilot study in Massachusetts encompassed the first COVID-19 wave which peaked on April 23, 2020 as well as the ascending portion of the second wave in the fall. During this period the average weekly COVID-19 case mortality rate (confirmed deaths/confirmed cases) varied considerably between 1 and 7.5% [37]. Among the patient population were 32 females and 21 males. The mean age among males was 55 and females, 51. Fifteen patients (28%) were between the ages of 66 and 90; 11 patients (21%) were under 30. Thirty-four cases were considered mild (64%), 17 moderate (32%), and 2 (4%) severe. Moderate was defined as shortness of breath (difficulty breathing) with or without any of the other symptom of mild COVID-19. Clinical signs suggestive of moderate illness with COVID-19 were defined as a respiratory rate ≥ 20 breaths per minute, saturation of oxygen (SpO2) > 93% on room air at sea level, and heart rate ≥ 90 beats per minute. In the 18 hospitalized patients (34%), therapy was initiated upon diagnosis. The 2 severe cases received remdesivir as well as levocetirizine and montelukast, the latter of which were initiated on hospital day 9. With the exception of one patient with nasal polyps, steroids were not part of the treatment paradigm. In addition, no patient received monoclonal antibodies. Within the combined outpatient and inpatient cohort, 22 were considered obese (BMI > 30, 41%), 10 had diabetes (19%) and 24 had hypertension (45%). During the course of the illness 66% had a fever (n = 35; >100.4 °F, 38 °C), 50% had a headache (n = 25/50) and 29% had loss of the sense of smell/taste (n = 15/52). Fifty-one of 53 patients were considered a clinical cure on therapy with restoration of their overall status to a pre-infection baseline within 2 weeks. Two patients, ages 73 and 80, continued to complain of fatigue for a period of time post discontinuation of therapy. The 73-year-old male diagnosed in March 2020, improved in 10 days although continued to exhibit a dry cough for months. The 80-year male, post subdural hematoma with a neurological deficit, was diagnosed in the hospital on day 3; however, did well and also recovered from the virus on combination therapy. Importantly, most patients treated with co-administration of levocetirizine and montelukast had symptom resolution within 7 days. Subjects with symptom resolution after 7 days typically had comorbidities that required a longer treatment period. Notably, there were no comorbidity exclusions, no intubations, no deaths, and no reported treatment-related safety findings. In addition, no one in the study exhibited ‘Long COVID’ symptoms greater than three months.
Table 2.
Clinical overview, symptoms, and comorbidities in 53 COVID-19 (+) patients.
|
Clinical Overview |
Symptoms |
Comorbidities |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Outcome (cured, still symptomatic, still very ill, deceased) | Initial severity of symptoms (mild moderate severe) | Cough | Thoracic Tightness | Fever | Loss of smell taste | Headache | Obesity | Diabetes | Hypertension | ||||||
| (Y/N) | (Y/N) | (Y/N) | (Y/N) | (Y/N) | (Y/N) | (Y/N) | (Y/N) | ||||||||||
| M | 54 | CURE | MOD | Y | Y | Y | N | N | Y | N | Y | ||||||
| M | 69 | CURE | MILD | Y | Y | Y | N | Y | N | N | N | ||||||
| M | 58 | CURE | MOD | Y | N | Y | N | N | N | N | Y | ||||||
| M | 63 | CURE | MOD | Y | Y | Y | N | Y | N | Y | |||||||
| M | 62 | CURE | MOD | Y | N | Y | N | Y | N | Y | |||||||
| F | 47 | CURE | MILD | N | N | Y | Y | N | N | N | N | ||||||
| F | 24 | CURE | MILD | Y | N | N | N | Y | N | N | N | ||||||
| F | 40 | CURE | MILD | N | N | N | N | Y | N | N | N | ||||||
| F | 56 | CURE | MILD | Y | Y | Y | N | Y | N | N | N | ||||||
| F | 73 | FATIGUE | MILD | Y | N | Y | Y | Y | N | N | N | ||||||
| M | 31 | CURE | MILD | N | N | N | Y | N | N | N | N | ||||||
| M | 44 | CURE | MOD | Y | N | N | N | N | N | N | N | ||||||
| M | 40 | PARTIAL SMELL | MOD | Y | Y | Y | Y | Y | Y | N | N | ||||||
| M | 61 | CURE | MILD | Y | N | Y | Y | N | Y | N | Y | ||||||
| F | 52 | CURE | MILD | N | N | N | Y | N | N | N | N | ||||||
| M | 87 | CURE | MOD | Y | N | Y | N | N | Y | ||||||||
| F | 51 | CURE | MILD | N | Y | Y | N | N | N | N | N | ||||||
| F | 60 | CURE | MILD | Y | N | Y | N | N | N | N | N | ||||||
| F | 64 | CURE | MILD | Y | Y | Y | N | Y | Y | N | N | ||||||
| M | 70 | CURE | MILD | Y | Y | Y | N | N | Y | Y | Y | ||||||
| F | 18 | CURE | MILD | Y | N | N | N | N | N | N | N | ||||||
| M | 80 | CURE | MOD | Y | N | Y | N | Y | Y | N | Y | ||||||
| M | 83 | CURE | MILD | N | N | N | N | N | Y | Y | Y | ||||||
| M | 47 | CURE | MILD | N | N | N | Y | N | N | N | N | ||||||
| F | 41 | CURE | MILD | N | N | N | Y | N | N | N | N | ||||||
| M | 71 | CURE | MOD | Y | Y | Y | N | Y | Y | N | Y | ||||||
| F | 80 | FATIGUE | MOD | Y | N | y | N | Y | Y | Y | Y | ||||||
| F | 17 | CURE | MILD | N | N | N | N | N | N | N | N | ||||||
| F | 50 | CURE | MILD | Y | Y | N | Y | N | N | N | N | ||||||
| M | 32 | CURE | MOD | Y | Y | Y | N | Y | Y | Y | Y | ||||||
| F | 55 | CURE | SEVERE | Y | Y | Y | N | N | Y | N | Y | ||||||
| F | 66 | CURE | MILD | Y | Y | Y | N | N | N | N | N | ||||||
| F | 73 | CURE | MILD | Y | N | Y | N | Y | Y | Y | Y | ||||||
| F | 70 | CURE | MILD | Y | N | Y | N | N | Y | Y | Y | ||||||
| M | 23 | CURE | MOD | Y | Y | Y | N | Y | N | N | N | ||||||
| F | 75 | CURE | MOD | Y | Y | Y | Y | Y | Y | N | Y | ||||||
| F | 75 | CURE | MOD | Y | Y | Y | N | Y | Y | N | Y | ||||||
| M | 89 | CURE | MOD | Y | N | Y | N | Y | N | Y | Y | ||||||
| M | 21 | CURE | MILD | Y | N | Y | N | Y | Y | N | N | ||||||
| F | 69 | CURE | SEVERE | Y | Y | Y | N | Y | Y | Y | Y | ||||||
| F | 67 | CURE | MILD | N | N | N | Y | N | N | Y | Y | ||||||
| M | 55 | CURE | MILD | Y | N | N | N | Y | Y | Y | Y | ||||||
| M | 58 | POLYPS | MOD | Y | Y | Y | Y | Y | N | N | Y | ||||||
| F | 22 | CURE | MILD | N | N | N | Y | Y | Y | N | N | ||||||
| F | 21 | CURE | MILD | Y | N | Y | Y | Y | N | N | N | ||||||
| F | 55 | CURE | MILD | Y | Y | N | Y | N | N | N | N | ||||||
| F | 26 | CURE | MILD | Y | Y | N | N | N | N | N | N | ||||||
| F | 56 | CURE | MILD | N | N | N | N | N | N | N | N | ||||||
| F | 90 | CURE | MOD | Y | Y | Y | N | Y | Y | N | Y | ||||||
| F | 83 | CURE | MILD | Y | N | Y | N | N | N | N | Y | ||||||
| F | 29 | CURE | MILD | Y | N | Y | N | N | N | N | N | ||||||
| F | 23 | CURE | MILD | Y | N | Y | N | Y | N | N | N | ||||||
| F | 3 | CURE | MILD | N | N | N | N | Y | N | N | N | ||||||
| N = 53 | Data Summary | ||||||||||||||||
| MOD | 17 | Y 40 | Y 21 | Y 35 | Y | 15 | Y | 25 | Y | 22 | Y | 10 | Y | 24 | |||
| M | 21 | MILD | 34 | N 13 | N 32 | N 18 | N | 37 | N | 25 | N | 31 | N | 43 | N | 29 | |
| F | 32 | SEVERE | 2 | ||||||||||||||
4. Discussion
To investigate patient outcomes, 53 consecutive COVID-19 test (+) cases (ages 3–90) from a well-established, single-center practice in Boston, Massachusetts, between March – November 2020, were treated with levocetirizine and montelukast in addition to then existing protocols [2]. In review, thirty-four patients (64%) were considered mild, 17 (32%) moderate, and 2 (4%) severe. The 2 severe hospital cases also received remdesivir. One patient with nasal polyps received steroids and no one received monoclonal antibodies. No patient progressed to intubation or death. Many allergy and asthma patients had co-existing morbidities including obesity, diabetes and hypertension, which increased their risk for major complications associated with COVID-19, yet notably recovered well from the virus. Early treatment, particularly in younger patients, enhanced the clinical response, with resolution of headache and fever within the first 48 hours following initiation of therapy. Analyzed collectively, the data support improved patient outcomes for those treated with the combination of levocetirizine and montelukast over patients who were either left untreated or treated with the then existing protocols. Most patients treated with co-administration of levocetirizine and montelukast experienced symptom resolution within 7 days versus 10–14 days or longer reported by untreated symptomatic patients [2]. These data suggest the combination therapy, underscored by their uniquely synergistic mechanisms of action, contributes to symptom relief for patients testing positive for COVID-19. The data also suggest the two drugs can be safely co-administered in COVID-19 patients over a wide age range (3–90), even those with significant comorbidities.
Early in the pilot study levocetirizine was used interchangeably with cetirizine; however, the paradigm was subsequently refined to include only levocetirizine with montelukast. Cetirizine exists as a racemic mixture of levocetirizine [(R)-enantiomer] and dextrocetirizine [(S)-enantiomer]. The S-enantiomer is tenfold less active than levocetirizine and competes with the H1 receptor to defeat the otherwise clinically remarkable and titratable properties associated with the R-enantiomer. Levocetirizine has twice the affinity of cetirizine for the H1 receptor [10], [26], [50].
4.1. Dosing
The current study utilized commercially available products and the respective adult doses for the treatment of allergy and asthma, i.e., levocetirizine 5 mg and montelukast 10 mg orally, once a day. In general, therapy was continued for 14 days. The three-year-old pediatric patient was treated with levocetirizine 1.25 mg and montelukast 4 mg daily, also for 14 days. Patients with significant comorbidity were treated for thirty days or longer, depending upon their underlying diagnoses (e.g., asthma, allergy, nasal polyps, etc.). Clinical experience with the treatment of COVID-19 outside the pilot study as well as treatment of multiple other inflammatory disease states (e.g., sepsis, traumatic brain injury, traumatic lung injury, vasculitis) over the past 10 years, suggests a potentially higher, yet safe dosing regimen may foreshorten the nature and extent of the COVID-19 presentation, particularly if therapy is initiated early (within 5 days of the onset of symptoms/diagnosis). Such patients are less likely to progress to pneumonia or require hospitalization, parameters which have been defined in the Phase 2 trial design.
4.2. Decreased potential for a drug-drug interaction
Levocetirizine and montelukast are characterized in part by different metabolic pathways which significantly decreases the potential for a drug-drug interaction. The extent of metabolism of levocetirizine in humans is less than 14% with 77% excreted unchanged through the kidney. The minimal biotransformation of levocetirizine in the liver is low and likely of no clinical relevance [51]. As such, differences resulting from genetic polymorphisms or the concomitant intake of hepatic drug metabolizing enzyme inhibitors are expected to be negligible [41]. Separately, montelukast is predominantly metabolized through the relatively minor CYP450 2C8 pathway and excreted in the bile [46]. Metabolic interaction of levocetirizine with montelukast or other extensively transformed drugs is unlikely.
4.3. Limitations and strengths of the pilot study
Limitations of the pilot study include the absence of a placebo arm, respectfully considered within the ethical constraints of the underlying disease. Regarding statistics, data was collected from March – November 2020, a period in time when there was insufficient testing, potentially inflating the treatment effect. Without controls, the extent of this effect is difficult to quantify. Further study is warranted.
Strengths include the mitigation of symptoms, particularly given the intrinsic mechanism of action of montelukast, inter alia, its ability to improve breathing. Moreover, treatment was offered to all patients regardless of age, comorbidities, and time from presentation of symptoms to time to the initiation of therapy. FDA accepted the initial data as positive proof of concept, suggested, and subsequently approved a multicenter, randomized, placebo-controlled, Phase 2 clinical trial design, replete with electronic diaries and laboratory metrics to explore scientific questions not addressed herein.
4.4. Conclusion
Presently, one cornerstone in the COVID-19 treatment paradigm lies in the effective attenuation of inflammation elicited by the virus. Levocetirizine and montelukast, unlike many single target therapeutics, safely attenuate not only histamine and leukotriene D4, respectively, but also synergistically mitigate inflammation across a spectrum of signaling proteins, cell adhesion molecules, and leucocytes: NF-kB, ICAM-1, VCAM-1, IL-4, IL-6, IL-8, RANTES, GM-CSF, TLR-3, AP-1, and eosinophil and neutrophil quantity and migration. Moreover, both molecules in the United States are considered Pregnancy Category B and underscored by millions of days of patient use (montelukast, 1998 FDA approval; levocetirizine, 2007 FDA approval).
As new COVID variants evolve in a global environment, one of many attributes of the repurposed combination lies in the ability to target cellular protein activity in contrast to viral proteins, an effect not likely to be negated by mutations in the virus genome. Levocetirizine and montelukast appear to offer a significant addition to the treatment of COVID-19, effectively mitigating symptoms without creating concurrent host toxicity. Cumulative data to date suggests the uniquely synergistic combination may reduce the progression and duration as well as prevent/treat many of the aspects of ‘Long COVID,’ thereby cost-effectively reducing both the morbidity and mortality associated with the disease.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: B. Chandler May, MD, JD, MS, FCLM is a practicing physician and CEO of Inflammatory Response Research, Inc. (IRR, Inc.) a drug development company focused on the combination of levocetirizine and montelukast for the treatment of inflammatory disorders. The current retrospective study utilized commercially available levocetirizine and montelukast from sources unrelated to IRR, Inc; independently prescribed by Kathleen Holly Gallivan, MD, MPH, FACS, FAAOA. Dr. Gallivan has no financial interests to report.
Acknowledgements
We would like to thank Machelle Wilchesky, PhD, McGill University, lead investigator of the COVID-19 Symptom Montelukast Trial, for providing the research framework for the pilot data and allowing for its release here. We would also like to thank Arianne Johnson, PhD, Cottage Health Research, Santa Barbara, CA, and Heather Stevens, Cottage Health Library, Santa Barbara, CA. for their advice and expertise in the critique, analysis, and preparation of the manuscript.
References
- 1.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 2.Hospital MG. SARS-COV-2 Infection Statuses and Resolution COVID-19, CoV-Presumed, CoV-Risk, and CoV-Exposed. June 2020.
- 3.Chen J., Qi T., Liu L.i., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., Song Z., Zeng Y., Shen Y., Shi Y., Zhu T., Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Morales A.-J.-C.-O.-J., Gutierrez-Ocampo E. Clincial, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34:1–13. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahmi A.N.A.S.G., Salem H.A. Levocetirizine pretreatment mitigates lipopolysaccharide-induced lung inflammation in rats. Biomed Res. Int. 2018;2018:1–9. doi: 10.1155/2018/7019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogli S., Stefanelli F., Neri T., Bardelli C., Amoruso A., Brunelleschi S., Celi A., Breschi M.C. Montelukast prevents microparticle-induced inflammatory and functional alterations in human bronchial smooth muscle cells. Pharmacol. Res. 2013;76:149–156. doi: 10.1016/j.phrs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Giustizieri M.L., Albanesi C., Fluhr J., Gisondi P., Norgauer J., Girolomoni G. H1 histamine receptor mediates inflammatory responses in human keratinocytes. J. Allergy Clin. Immunol. 2004;114(5):1176–1182. doi: 10.1016/j.jaci.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 10.Hair P.I., Scott L.J. Levocetirizine: a review of its use in the management of allergic rhinitis and skin allergies. Drugs. 2006;66(7):973–996. doi: 10.2165/00003495-200666070-00017. [DOI] [PubMed] [Google Scholar]
- 11.Jang Y.J., Wang J.H., Kim J.S., Kwon H.J., Yeo N.-K., Lee B.-J. Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells. Antiviral Res. 2009;81(3):226–233. doi: 10.1016/j.antiviral.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Mullol J., Callejas F.B., Méndez-Arancibia E., et al. Montelukast reduces eosinophilic inflammation by inhibiting both epithelial cell cytokine secretion (GM-CSF, IL-6, IL-8) and eosinophil survival. J. Biol. Regul. Homeost. Agents. 2010;24(4):403–411. [PubMed] [Google Scholar]
- 13.Peters-Golden M., Henderson W.R. Leukotrienes. N. Engl. J. Med. 2007;357(18):1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 14.Robinson A.J., Kashanin D., O'Dowd F., Williams V., Walsh G.M. Montelukast inhibition of resting and GM-CSF-stimulated eosinophil adhesion to VCAM-1 under flow conditions appears independent of cysLT(1)R antagonism. J. Leukoc. Biol. 2008;83(6):1522–1529. doi: 10.1189/jlb.1007717. [DOI] [PubMed] [Google Scholar]
- 15.Stelmach I., Jerzynska J., Kuna P. A randomized, double-blind trial of the effect of treatment with montelukast on bronchial hyperresponsiveness and serum eosinophilic cationic protein (ECP), soluble interleukin 2 receptor (sIL-2R), IL-4, and soluble intercellular adhesion molecule 1 (sICAM-1) in children with asthma. J. Allergy Clin. Immunol. 2002;109(2):257–263. doi: 10.1067/mai.2002.121456. [DOI] [PubMed] [Google Scholar]
- 16.Tahan F., Jazrawi E., Moodley T., Rovati G.E., Adcock I.M. Montelukast inhibits tumour necrosis factor-alpha-mediated interleukin-8 expression through inhibition of nuclear factor-kappaB p65-associated histone acetyltransferase activity. Clin. Exp. Allergy. 2008;38(5):805–811. doi: 10.1111/j.1365-2222.2008.02963.x. [DOI] [PubMed] [Google Scholar]
- 17.Theron A.J., Steel H.C., Tintinger G.R., Gravett C.M., Anderson R., Feldman C. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function. J Immunol Res. 2014;2014:1–16. doi: 10.1155/2014/608930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tintinger G.R., Feldman C., Theron A.J., Anderson R. Montelukast: more than a cysteinyl leukotriene receptor antagonist? Sci. World J. 2010;10:2403–2413. doi: 10.1100/tsw.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.www.bu.edu/NF-kB. NF-kB. 2021.
- 20.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88(2):913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roumestan C., Henriquet C., Gougat C., Michel A., Bichon F., Portet K., Jaffuel D., Mathieu M. Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms. Clin. Exp. Allergy. 2008;38(6):947–956. doi: 10.1111/j.1365-2222.2008.02990.x. [DOI] [PubMed] [Google Scholar]
- 22.S.M.Q. Ying, A.B. Kay, The effect of levocetirizine on histamine and cytokine-induced upregulation of eotaxin by endothelial cells, in: Proceedings of the XXI Congress of the European Academy of Allergy and Clincial Immunology; 2001; Naples, France; 2001.
- 23.Chen C. Physicochemical, pharmacological and pharmacokinetic properties of the zwitterionic antihistamines cetirizine and levocetirizine. Curr. Med. Chem. 2008;15(21):2173–2191. doi: 10.2174/092986708785747625. [DOI] [PubMed] [Google Scholar]
- 24.Church M.K., Church D.S. Pharmacology of antihistamines. Indian J. Dermatol. 2013;58(3):219–224. doi: 10.4103/0019-5154.110832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estelle F., Simons R., Mcmillan J., Simons K. A double-blind, single-dose, crossover comparison of cetirizine, terfenadine, loratadine, astemizole, and chlorpheniramine versus placebo: suppressive effects on histamine-induced wheals and flares during 24 hours in normal subjects. J. Allergy Clin. Immunol. 1990;86(4):540–547. [PubMed] [Google Scholar]
- 26.Gillman S., Gillard M., Benedetti M.S. The concept of receptor occupancy to predict clinical efficacy: a comparison of second generation H1 antihistamines. Allergy Asthma Proc. 2009;30(4):366–376. doi: 10.2500/aap.2009.30.3226. [DOI] [PubMed] [Google Scholar]
- 27.Grant J.A., Riethuisen J.-M., Moulaert B., DeVos C. A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann. Allergy Asthma Immunol. 2002;88(2):190–197. doi: 10.1016/S1081-1206(10)61995-3. [DOI] [PubMed] [Google Scholar]
- 28.Davino-Chiovatto J.E., Oliveira-Junior M.C., MacKenzie BreAnne, Santos-Dias A., Almeida-Oliveira A.R., Aquino-Junior J.C.J., Brito A.A., Rigonato-Oliveira N.C., Damaceno-Rodrigues N.R., Oliveira A.P.L., Silva A.P., Consolim-Colombo F.M., Aimbire F., Castro-Faria-Neto H.C., Vieira R.P. Montelukast, leukotriene inhibitor, reduces LPS-induced acute lung inflammation and human neutrophil activation. Arch. Bronconeumol. 2019;55(11):573–580. doi: 10.1016/j.arbres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Suetrong B. Successful treatment of refractory pediatric severe ARDS with high-dose montelukast. Crit. Care Med. 2019;47(1):1244. [Google Scholar]
- 30.Abu-Saleh A.-A., Awad I.E., Yadav A., Poirier R.A. Discovery of potent inhibitors for SARS-CoV-2's main protease by ligand-based/structure-based virtual screening, MD simulations, and binding energy calculations. PCCP. 2020;22(40):23099–23106. doi: 10.1039/d0cp04326e. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S., Singh B., Kumari P., Kumar P.V., Agnihotri G., Khan S., Kant Beuria T., Syed G.H., Dixit A. Identification of multipotent drugs for COVID-19 therapeutics with the evaluation of their SARS-CoV2 inhibitory activity. Comput. Struct. Biotechnol. J. 2021;19:1998–2017. doi: 10.1016/j.csbj.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Štekláč M., Zajaček D., Bučinský L. 3CL(pro) and PL(pro) affinity, a docking study to fight COVID19 based on 900 compounds from PubChem and literature. Are there new drugs to be found? J. Mol. Struct. 2021;1245:130968. doi: 10.1016/j.molstruc.2021.130968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copertino D.C., Duarte R.R.R., Powell T.R., Mulder Rougvie M., Nixon D.F. Montelukast drug activity and potential against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Med. Virol. 2021;93(1):187–189. doi: 10.1002/jmv.26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.S. Durdagi, T. Avsar, M.D. Orhan, et al., The neutralization effect of montelukast on SARS-CoV-2 is shown by multiscale in silico simulations and combined in vitro studies, Molecular Therapy 2021 Oct 19;S1525-0016(21)00521-9. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 35.Sansing-Foster V. Neuropsychiatric adverse events and montelukast: observational safety analyses. FDA. 2019:1–37. [Google Scholar]
- 36.Ali M.M., O'Brien C.E., Cleves M.A., Martin B.C. Exploring the possible association between montelukast and neuropsychiatric events among children with asthma: a matched nested case-control study. Pharmacoepidemiol. Drug Saf. 2015;24(4):435–445. doi: 10.1002/pds.3758. [DOI] [PubMed] [Google Scholar]
- 37.Philip G., Hustad C.M., Malice M.-P., Noonan G., Ezekowitz A., Reiss T.F., Knorr B. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J. Allergy Clin. Immunol. 2009;124(4):699–706.e8. doi: 10.1016/j.jaci.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Schumock G.T., Stayner L.T., Valuck R.J., Joo M.J., Gibbons R.D., Lee T.A. Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: a nested case-control study. J. Allergy Clin. Immunol. 2012;130(2):368–375. doi: 10.1016/j.jaci.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 39.Bachert C., Bousquet J., Canonica G.W., Durham S.R., Klimek L., Mullol J., Van Cauwenberge P.B., Van Hammée G. Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J. Allergy Clin. Immunol. 2004;114(4):838–844. doi: 10.1016/j.jaci.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K., Spitzer R.L., Williams J.B., Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 41.Sanofi. XYZAL Prescribing Information, vol. 19, 2016.
- 42.Sharma V.K., Gupta V., Pathak M., Ramam M. An open-label prospective clinical study to assess the efficacy of increasing levocetirizine dose up to four times in chronic spontaneous urticaria not controlled with standard dose. J. Dermatolog. Treat. 2017;28(6):539–543. doi: 10.1080/09546634.2016.1246705. [DOI] [PubMed] [Google Scholar]
- 43.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain R.K. Cetirizine and astemizole in allergic rhinitis a comparative study. Indian J. Otolaryngol. Head Neck Surg. 1999;51(3):94–98. doi: 10.1007/BF02996544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Z., Vorperian V.R., Gong Q., Zhang S., January C.T. Block of HERG potassium channels by the antihistamine astemizole and its metabolites desmethylastemizole and norastemizole. J. Cardiovasc. Electrophysiol. 1999;10(6):836–843. doi: 10.1111/j.1540-8167.1999.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 46.Merck. Singulair Prescribing Information, 2020.
- 47.Storms W., Michele T.M., Knorr B., et al. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clin. Exp. Allergy. 2001;31(1):77–87. doi: 10.1046/j.1365-2222.2001.00969.x. [DOI] [PubMed] [Google Scholar]
- 48.Sarzi-Puttini P., Giorgi V., Sirotti S., et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 49.Chen Y., Li Y., Wang X., Zou P. Montelukast, an Anti-asthmatic Drug, Inhibits Zika Virus Infection by Disrupting Viral Integrity. Front. Microbiol. 2019;10:3079. doi: 10.3389/fmicb.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gandon J.M., Allain H. Lack of effect of single and repeated doses of levocetirizine, a new antihistamine drug, on cognitive and psychomotor functions in healthy volunteers. Br. J. Clin. Pharmacol. 2002;54(1):51–58. doi: 10.1046/j.1365-2125.2002.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tillement Jean-Paul, Testa Bernard, Brée Françoise. Compared pharmacological characteristics in humans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonists. Biochem. Pharmacol. 2003;66(7):1123–1126. doi: 10.1016/s0006-2952(03)00558-6. [DOI] [PubMed] [Google Scholar]