Abstract
Coronavirus is a family of viruses that can cause diseases such as the common cold, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS). The universal outbreak of coronavirus disease 2019 (COVID-19) caused by SARS coronaviruses 2 (SARS-CoV-2) has become a global pandemic. The β-Coronaviruses, which caused SARS-CoV-2 (COVID-19), have spread in more than 213 countries, infected over 81 million people, and caused more than 1.79 million deaths. COVID-19 symptoms vary from mild fever, flu to severe pneumonia in severely ill patients. Difficult breathing, acute respiratory distress syndrome (ARDS), acute kidney disease, liver damage, and multi-organ failure ultimately lead to death. Researchers are working on different pre-clinical and clinical trials to prevent this deadly pandemic by developing new vaccines. Along with vaccines, therapeutic intervention is an integral part of healthcare response to address the ongoing threat posed by COVID-19. Despite the global efforts to understand and fight against COVID-19, many challenges need to be addressed. This article summarizes the current pandemic, different strains of SARS-CoV-2, etiology, complexities, surviving medications of COVID-19, and so far, vaccination for the treatment of COVID-19.
Keywords: Coronavirus complexities, COVID-19, Coronavirus variants, Global pandemic, SARS-CoV-2, Therapeutic interventions, Vaccination, β-Coronavirus
Graphical Abstract
1. Introduction
The current outbreak of infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is termed coronavirus disease 2019 (COVID-19) [1]. The World Health Organization (WHO) characterized the illness as a pandemic, spreading rapidly and considerably in many people [2], [3]. To date, SARS-CoV-2 has infected the highest number of people, 81 + million (81,475,053), and has been declared a global emergency (pandemic) by the WHO [4]. Unlike epidemics, pandemics are worldwide and can spread over several countries. Previously, the H1N1 influenza outbreak was declared as a pandemic in 2009. The emergence of the novel SARS-CoV-2 was initially reported on December 31, 2019, in the Huanan Seafood Market, where livestock animals are traded in Wuhan State of Hubei Province in China; it has become the focus of global attention [5]. At present, the virus officially known as COVID-19 has spread to 213 countries of the world. The highest numbers of COVID-19 infection cases are reported from the USA, India, Brazil, Russia, Peru, Columbia, Spain, France, Iran, and the United Kingdom.
The mode of transmission of COVID-19 outbreak initially was through the animals, after a few initially infected individuals reported from Seafood Market; subsequently, all other transmissions are said to be human to human [6]. Furthermore, the outbreak emerged so rapidly due to transmission from humans to humans that it flew from China to other countries within a few days. There are copious ways through which virus transfers from human to human, i.e., via coughing or sneezing droplets, surfaces of public transport, restaurants, and other public places like toilets, elevators, and bus stops, etc. [7], [8].
Some therapeutic moieties used previously for viral infections are being tried for COVID-19 patients [9], [10]. New drug candidates are also in the development phase but are subject to certain limitations [11], [12]. More than 80 companies and academic research groups are currently working on 3370 registered trials for drug testing and vaccine development for COVID-19, and the Drug Regulatory Authority of Pakistan (DRAP) also approved five clinical studies on COVID-19 patients. Additionally, more than 75 therapeutic agents based on RNAi, recombinant proteins, and peptides are under 600 planned clinical trials [13], [14], most of them are monoclonal antibodies, and a few are polyclonal antibodies [15], [16]. UK has become the first to approve Pfizer/BioNTech COVID-19 vaccine. Some trials with umbilical cord immunoglobulin G (IgG) and stem cell therapy are at the stage of early development, and researchers are testing them against COVID-19 symptoms; however, the debate continues over the best approach.
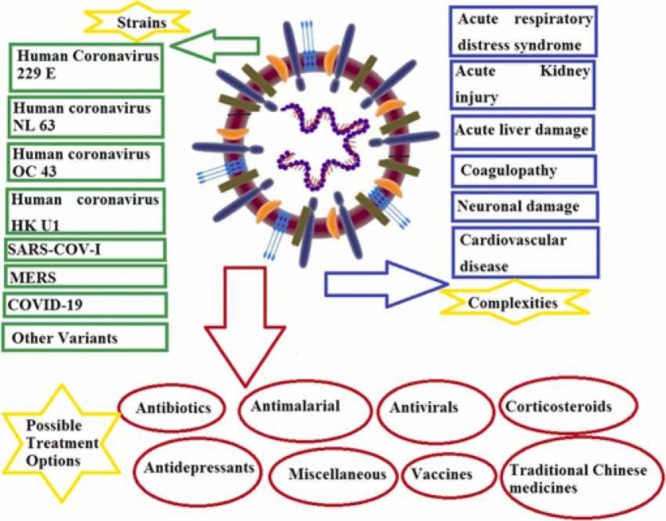
In this review, we summarize the historical background and emergence of different SARS CoV variants in 2020, recurrence in 2021, and highlight the evidence for viral recombination between the other coronaviruses (CoVs) present in animal populations, which may have resulted in the evolution and emergence of novel CoVs that are transmissible and lethal to humans. Our primary focus was an outbreak of SARS-CoV-2, etiology, and complications of COVID-19. Finally, we summarize the possible treatment and prevention options, challenges, and future perspectives of COVID-19 in detail.
2. Coronavirus history/ background
Human coronaviruses were first identified in the mid-1960s. Until now, seven coronaviruses’ types have been documented. Three recent examples of such coronaviruses are SARS-CoV-1, MERS-CoV, and 2019-nCoV, which are involved in the pathogenesis of severe respiratory infections. COVID-19 is manifested by high-grade fever, dry cough, and pneumonia of unknown causes in people with an inadequate immune system [17]. A recent study identified that animal-to-human transmission could be halted more quickly than the transfer from humans to humans [18]. Still, experts warn that the COVID-19 should not be downplayed or linked to the seasonal flu. Relatively, respiratory disease due to COVID-19 is analogous to severe pneumonia, and in severely ill cases, patients experience difficulty breathing and need to be hospitalized and put on ventilators in some critical cases. Seasonal flu has a mortality rate of 0.1%, while COVID-19 mortality rate so far is > 2%, making it 20 times more lethal than the seasonal flu [19]. People infected with COVID-19 had experienced multiple symptoms, including fever, cough, sore throat, and chest pain, and sputum production. Additionally, muscle ache, respiratory symptoms, headache, and renal injury are also observed by various research groups working on COVID-19. COVID-19 pandemic is a complex problem, and significant complications of COVID-19 include acute respiratory distress [20], arrhythmia, shock, acute kidney injury (AKI), or acute renal failure (ARF), acute cardiac injury, liver dysfunction, and secondary infection. A new, bizarre symptom includes pink eye (conjunctivitis), blue-tinged lips, anosmia, confusion, delirium, inability to wake or stay awake, skin rashes, and "Covid-toes [21].
Historically, the contagious maladies have been considered the highest menace to the community health and resulted in added years of life lost from untimely death than any other malady [22], [23]. In 2005, WHO established an Emergency Committee due to the rapid developments in the appearance of novel contagious diseases with a pandemic perspective. Within one decade, four severe infectious public health disasters of transnational trepidation have been publicized. The possible reasons behind the amplified frequency of these pandemics might be the snowballing international trade and expedition, interruption of ecosystems from confrontations or economic advances that brings humans into interaction with beforehand unrecognized infectious microbes, and humans are confronted with eternal contagious encounter and an amassed occurrence of pandemic menaces to global health [24]. Generally, infectious public health hazards are caused by microbes, which are either zoonotic or vector-borne. Since the beginning of the 21st century, various infectious outbreaks have occurred; out of these, the most prominent is Ebola [25], Influenza [26], Zika virus [27], SARS [28], Middle East respiratory syndrome (MERS) [29] and most recently, COVID-19. The same family of CoVs already responsible for MERS and SARS causes the COVID-19. Among the several CoVs that are pathogenic to humans, most are associated with mild clinical symptoms [30], with two notable exceptions: SARS coronavirus (SARS-CoV-2), a novel β-coronavirus that emerged in Guangdong, Southern China, in November 2002 [31] resulted in more than 8000 human infections, 774 deaths in 37 countries during 2002–03 [32] and MERS coronavirus first detected in Saudi Arabia in 2012 [33] and was responsible for 2494 laboratory-confirmed cases of infection and 858 fatalities since September 2012, including 38 deaths in South Korea following a single introduction [34], [35].
In late December 2019, several patients with viral pneumonia were epidemiologically associated with the Huanan seafood market of Wuhan, in the Hubei Province of China, where several non-aquatic animals such as birds and rabbits were on sale before the outbreak. A novel, human-infecting coronavirus [36] provisionally named 2019 novel coronavirus (2019-nCoV) was identified using next-generation sequencing (NGS).
3. Diverse strains of infectious human coronaviruses
Human coronaviruses are shared all over the world. Coronaviruses are enveloped, single-stranded, non-segmented, positive-sense RNA viruses with the most significant viral genome (26–32 kb) among RNA viruses [37]. Large surface projections were observed on coronaviruses, spike proteins providing the typical crown-like structure (crown=corona) on electron microscopic evaluation. Another unique attribute of coronaviruses is that they have a multiplicity of receptors containing protein receptors as well as sugar receptors [38]. Coronaviruses are host-specific and can infect humans, mammals, and birds. Coronaviruses have four distinct genera named alpha-, beta-, gamma-, and delta-coronavirus. Coronaviruses are further subdivided genotypically into three groups. Viruses from groups I and II require mammals hosts, while group III viruses are birds specific [39]. Human coronaviruses were first identified in the mid-1960 s. Until now, seven coronaviruses’ types have been documented. Occasionally, due to evolution, coronaviruses can transmit from animals to humans and reproduce a new human coronavirus. Three recent examples of such transmission are SARS-CoV-1, MERS-CoV, and 2019-nCoV. A comparison of all corona variants is explained in Table 1 .
Table 1.
Comparison of Different Types, Clinical manifestation, Incubation period, and incidence of Corona variants.
| Types of corona variant | Subgroup | Clinical manifestation | Incubation Period | Year | Incidence | References |
|---|---|---|---|---|---|---|
| HCoV-229E | Alpha-coronavirus | General malaise, headache, nasal discharge, sneezing, sore throat, fever, and cough. | 2–5 days | 1966 | N/A | [47], [189], [190] |
| HCoVOC43 | Beta-coronavirus | General malaise, headache, nasal discharge, sneezing, sore throat, fever, and cough. | 2–5 days | 1967 | N/A | [191], [192] |
| HCoV-NL63 | Alpha-coronavirus | Mild respiratory disease is similar to the common cold, cough, rhinorrhea, tachypnea, fever, hypoxia, obstructive laryngitis (croup). | 2–4 days | 2004 | N/A | [193], [194], [195] |
| HCoV-HKU1 | Beta-coronavirus | Upper respiratory tract fever, running nose, and cough lower respiratory tract, fever, productive cough, and dyspnea. | 2–4 days | 2005 | N/A | [196], [197] |
| Severe acute respiratory syndrome coronavirus (SARS-CoV). | Beta-coronavirus | Fever > 37.8 °C (100,0°F), chills, headache, malaise, myalgia, lethargy, sore throat, pneumonia (direct viral or secondary bacterial), non-productive cough, dyspnea, respiratory distress, Diarrhea (30–40% of patients). | 2–14 days | 2003 | 9% died, much higher for those over 60 years old, with mortality rates approaching 50% for this subset of patients | [198] |
| Middle East respiratory syndrome coronavirus | Beta-coronavirus | A severe respiratory illness characterized by fever, cough dyspnea, chills, sore throat, myalgia, arthralgia, pneumonia, diarrhea and vomiting (one-third of patients), acute renal impairment. | 2–13 days | 2012 | The case fatality rate is 35–40% | [134], [199], [200], [201], [202] |
| Novel coronavirus COVID-19 | Beta- coronavirus | Asymptomatic, mild infection: fever, dry cough, malaise, dehydration.Severe infection: high fever, shortness of breath, chest pain, hemoptysis | 2–14 days | 2019 | ~ 468,644 cases (Dec 2019 – March 2020) | [203], [204], [205] |
3.1. Human coronavirus 229E (alpha-coronavirus)
Researchers at the University of Chicago isolated ether sensitive RNA virus in 1966 and named it Human Coronavirus 229E (HCoV-229E). The HCoV-229E strain was isolated after characterizing 05 novel agents from the respiratory tract of human beings experiencing the common cold [40]. HCoV-229E is a species of coronavirus and a member of the genus α-coronavirus and subgenus Duvinacovirus. HCoV-229E is antigenically distinct from all identified Human myxoviruses and distributed globally [41]. HCoV-229E is a single-stranded RNA, enveloped positive-sense virus transmitted through droplet-respiration [42].
3.2. Human coronavirus NL63 (alpha-coronavirus)
Novel HCoV-NL63 belongs to species of Human coronavirus, genus alpha-coronavirus and subgenus setracovirus. HCoV-NL63 was primarily identified in the Netherlands in late 2004 from a child suffering from bronchiolitis, fever, coryza, and conjunctivitis [43]. HCoV-NL63 is an enveloped, positive-sense, single-stranded RNA virus and enters the host through the angiotensin-converting enzyme 2 (ACE2) receptor [42]. Diseases linked to this virus include mild to moderate upper respiratory tract infections, severe lower respiratory tract infections, croup, and bronchiolitis. Patients with respiratory illness, immune-compromised, children, and people of old age are the primary victim of this virus [44].
3.3. Human coronavirus OC43 (beta-coronavirus)
Almost 50 years ago, HCoV-OC43 and HCoV-229E were recognized, which mainly cause the common cold in humans. HCoV-OC43 was isolated in 1967 from volunteers at the Common Cold Unit in Salisbury, United Kingdom. The nasopharyngeal swab of a patient suffering from common cold was collected, and strain OC43 was isolated from that specimen [45]. Although there is no serological cross-reactivity between 229E and OC43 but HCoV-OC43 patients had almost the same clinical symptoms as 229E [46]. A research group performed reverse transcriptase-polymerase chain reaction (RT-PCR)-hybridization in the M gene of coronaviruses and described that this hybridization is 40-times more sensitive for HCoV-229E while for HCoV-OC43, it is 100 times more sensitive as compared to the viral isolation technique [47]. HCoV-OC43 is an enveloped virus, pleiomorphic with a genome size of around 30.5 RNA molecules. It is a coronavirus species and a member of the genus beta-coronavirus and subgenus embecovirus [48].
3.4. Human coronavirus HKU1 (beta-coronavirus)
Human Coronavirus HKU1 was discovered in 2005 from patients with pneumonia symptoms in Hong Kong. HCoV-HKU1 was detected from the nasopharyngeal aspirates of patients by RT-PCR of the pol gene of coronaviruses [49]. In another article, Woo et al. reported the discovery of HCoV-HKU1 in January 2005 from nasopharyngeal aspirates of a 71-year-old man who had just come back from Shenzhen, China. This man suffered from pneumonia, and quantitative RT-PCR results showed that the amount of HCoV-HKU1 RNA was 8.5–9.6 × 106 copies per mL in his sample [50]. HCoV-HKU1 belongs to species of coronavirus which originated from infected mice and differentiated from other members of the genus beta-coronavirus and subgenus embecovirus due to the presence of the hemagglutinin esterase (HE) gene [51]. HCoV-HKU1 coronavirus employed an N-acetyl-9-O-acetylneuraminic acid receptor to enter the host. These are enveloped, positive-sense, single-stranded RNA viruses. Symptoms related to HCoV-HKU1 are the common cold, asthma, bronchitis, pneumonia exacerbation, and chronic obstructive pulmonary disease (COPD) [52].
3.5. SARS coronavirus (SARS-CoV-1)
SARS coronavirus is also known as SARS-related coronavirus, and severe acute respiratory syndrome coronavirus (SARS-CoV)− 1 transmit the infection to bats, humans, and palm civets [53], [54]. SARS-CoV-1 belongs to the genus beta-coronavirus that infects the epithelial cells within the lungs. ACE2 is the primary human receptor for the attachment of this virus [55]. SARS is caused by the strain of SARS-CoV-1, first identified in February 2003 in China (Guangdong Province) [56]. In April 2003 National Microbiology Laboratory (NML) in Canada and the Centre for Disease Control and Prevention (CDC) [57] in the United States identified the genome of SARS-CoV-1 as an enveloped, positive-sense, single-stranded RNA virus [58], [59].
3.6. MERS coronavirus
MERS-related coronavirus belongs to genus beta-coronavirus and subgenus merbecovirus. It is the first beta-coronavirus belonging to lineage C that infects humans. MERS-CoV genomes were phylogenetically classified into two clades, clade A and B. Initial cases of MERS were of clade A clusters, while new cases that were genetically distinct belong to clade B clusters [60]. MERS-CoV is initially known as the 2012 novel coronavirus (2012-nCoV) or simply novel coronavirus (nCoV), belong to species of coronavirus which infects humans, bats, and camels [54], [61]. MERS is a respiratory infection caused by MERS CoV first identified in the 2012 outbreak in Saudi Arabia. MERS-CoV infection globally spread to over 25 countries with high fatalities leading to high-level public health threats [62]. It was found that bats were the reservoirs for MERS-CoV, which make it zoonotic, but the human-to-human transmission was also observed. MERS-CoV transmission from camels to humans was also observed in early 2010 [63]. WHO listed MERS coronavirus as a future epidemic for urgent research and development [64].
3.7. COVID-19 (SARS-CoV-2)
SARS-CoV-2 has been renamed by the International Committee on Taxonomy of Viruses (ICTV) [65], [66] to the formerly named 2019-nCoV [53], [67]. However, a group of virologists in China suggested its name as human coronavirus 2019 (HCoV-19) instead of SARS-CoV-2 to differentiate the virus from SARS-CoV-1 [68]. This disease has been entitled the 6th Public Health Emergency of International Concern (PHEIC) by the WHO [69], [70].
According to taxonomic classification, SARS-CoV-2 is categorized as a strain of the species SARS-CoV-1 [71]. The SARS-CoV-2 is a new β-coronavirus belonging to the family of enveloped, positive-sense, single-stranded RNA viruses, approximately 1250 nm in diameter, similar to SARS-CoV-1 and MERS-CoV. SARS-CoV-2 genome is 86.9% identical to bat SARS-like CoV genome and is 26–32 kilobases distinctive from human SARS-CoV-1 and MERS-CoV, respectively [72], [73], [74], [75]. SARS-CoV-2 has club-shaped surface projections giving a typical crown-like appearance to the virus. These structural proteins are of 05 types: spike (S), hemagglutinin-esterase (HE), membrane (M), envelope (E), and nucleocapsid (N). SARS-CoV-2 enters into the human cell with the help of S protein, supported by HE. The S, M, and E proteins generate the viral envelope. N proteins are responsible for forming RNA and viral assembly complex after their replication [76].
The transmission of SARS-CoV-2 is assumed analogous to influenza and other respiratory pathogens in humans; during coughing and sneezing, droplets are transferred to the individuals close to the infected symptomatic individuals. SARS-CoV-2 is highly contagious, and the virus remains communicable outsides the host. Usually, coronaviruses are vulnerable to strong acid, basic pH, and heat but seem more stable at 4 °C [77], [78]. The life cycle of SARS-CoV-2 fluctuates from a few hours to a few days based on temperature, humidity, and type of residing surface. SARS-CoV-2 possibly remained viable on plastic and steel for up to 03 days, cardboard surface for 01 days, on copper for 04 h [79]. SARS-CoV-2 has also been found in stool samples from infected people [80].
3.8. Other variants
Throughout the COVID-19 pandemic, several genetic variations of SARS-CoV-2 have emerged and circulated worldwide after the first wave of COVID-19. Variants of the SARS-CoV-2 linked to increased transmission, immune evasion, or severe disease have prompted a global rise in genomic surveillance. These variants are.
I) Alpha (α-variant), B.1.1.7 (UK).
II) Concerning the change in α variant (E484K).
III) Beta (β-variant), B.1.351 (South-Africa).
IV) Gamma (γ-variants), P.1 and P.2 (Brazil).
V) Eta (η-variant) (B.1.525).
VI) Epsilon (ε-variant), B.1.429/497 (California).
VII) Delta Kappa (Δ/k variant), B.1.617 (India).
VIII) 501. V2 variant [81].
VIX) N501Y [82].
However, the US Department of Health and Human Services (HHS) formed a SARS-CoV-2 Interagency Group (SIG) to increase collaboration between the CDC, National Institute of Health (NIH), Food and Drug Administration (FDA), Biomedical Advanced Research and Development Authority (BARDA), and the Department of Defense (DoD). This interagency group focused on rapidly characterizing new variations and their potential influence on crucial SARS-CoV-2 countermeasures, such as vaccines, treatments, and diagnostics [83].
3.8.1. Variant of concern (VOC)
The variant of concern (VOC) is a variant with indications of higher transmissibility, more severe disease (e.g., more hospitalizations or deaths), considerable reduction in neutralization by antibodies developed after previous infection or vaccination, reduced efficiency of therapies or vaccinations, or diagnostic detection failures [84].
-
1.
Alpha (α-variant)
-
2.
B.1.1.7
Another variant, the B.1.1.7 lineage, often termed as 20I/501Y.V1 or VOC 202012/01, was identified by the WHO working evolution group collaborating with UK medical authorities. This strain has been found in the Netherlands, Denmark, and Australia, and it is expected to play a role in the upcoming pandemic [85].
-
1.
B.1.1.7 and Q sublineages
-
2.
β variant, B.1.351 and sublineages (B.1.351.2, B.1.351.3)
It was discovered in December 2020 that the B.1.351 lineage, also known as 20 H/501Y.V2 or VOC 202012/02, originated in South Africa.
-
3.
B.1.617.2 and all AY sublineages
-
4.
Gamma γ P.1 and P.1 sublineages
At least 45 nations have detected the P.1 lineage, which is sometimes referred to as 20 J/501Y.V3 or B.1.1.28. Four Brazilians traveling to Japan in December 2020 were found to have this variant, which is a prevalent circulating variant throughout Brazil (>72%) [86].
-
•
B.1.1.529 (Omicron)
On November 26, 2021, the Technical Advisory Group on SARS-CoV-2 Virus Evolution (TAG-VE) gathered to analyze the SARS-CoV-2 variant B.1.1.529. On November 24, 2021, the B.1.1.529 variant was initially reported to WHO by South Africa. There are numerous mutations in this variation, some of which are problematic. The TAG-VE recommended that this variant must be declared as a VOC. The WHO has identified B.1.1.529 as a VOC, called Omicron, based on the information given indicating a harmful shift in COVID-19 epidemiology [87], [88].
3.8.2. Variant of interest (VOI)
Variant having specific genetic markers associated with alterations in receptor binding, lower neutralization by antibodies developed against recent infection or vaccination, the impaired success of treatments, potential diagnostic effects, or projected increase in transmissibility or disease severity.
-
1.
Eta η variant (B.1.525) (B.1.526)
-
2.
Kappa Δ/k variant, B.1.617.1 and B.1.617.3
They are classed as such since there is data that they transmit fastly, induce more severe infections, or circumvent previously acquired immunity better than circulating disease variants. Earlier this month, the UK government classified the B.1.617.2 subtype as a variation of concern in the United Kingdom. There has been an increase from 202 to 520 B.1.617.2 infections in just one week [89].
3.8.3. Variant of high consequence (VOI)
It is not uncommon for a variety of significant consequences to continue spreading despite prophylactic measures. Preventive interventions or medical countermeasures (MCMs) are much less effective for a variant of high consequence when compared to previously circulated variants.
-
•
Currently, there are no SARS-CoV-2 variants that rise to the level of high consequence.
4. Etiology and severity of coronavirus
While discussing the origin of SARS-CoV-2, it is assumed that bats are the cause of this infection, as the sequences of SARS-related CoVs have been identified in Chinese horseshoe bats. Two bats were found to have the SARS-CoVs with a genomic sequence similar to SARS-CoV-2 than any other virus identified to date. They also act through the same ACE2 receptors as the human coronavirus, confirming that SARS-CoV-2 originated from bats [42]. The expression of ACE2 is significantly increased in patients with diabetes mellitus and hypertension being treated with ACE inhibitors, which produces an up-regulation of ACE2 receptors. Contrary to initial reports, the American College of Cardiology (ACC) has reported no data to support the claim that ACE inhibitors increase the risk of COVID-19 infection [90]. Human lung epithelial cells are severely infected when the virus attacks the respiratory system. The virus can invade macrophages and dendritic cells but only leads to mild infection [91]. Despite this mild nature of the infection, various pro-inflammatory cytokines and chemokines are activated that may contribute to disease and can be identified in the blood of SARS-CoV-2 infected individuals [92]. The precise mechanism of lung injury and the cause of severe disease in humans is still unknown [93]. The symptoms may appear from 2 to 14 days after the exposure. Based on current epidemiological findings, the incubation period is 1–14 days and, in some cases, 3–7 days. The virus can spread through direct contact during the incubation period [94]. Most people with robust immune systems recover without treatment, while older people and patients with pre-existing diseases like cardiovascular disease (CVD), diabetes, chronic respiratory disease, and cancer are more prone to develop serious illnesses [95].
A study reported by Rasmussen and co-workers described 23–32% of patients with severe pneumonia were admitted to the intensive care unit (ICU) while 17–29% of patients developed acute respiratory distress [96]. COVID-19 has been classified as mild, moderate, severe, and critical based on clinical symptoms [97]. Mild disease patients suffer from upper respiratory tract viral infection with mild fever, dry cough, sore throat, headache, muscle pain, and nasal congestion. 81% of cases of COVID-19 are mild with no sign of severe disease like dyspnea [98]. Moderate disease patients present with cough, shortness of breath, tachypnea with no severe symptoms. Severe disease symptoms include pneumonia, acute respiratory distress syndrome, sepsis, and septic shock [99]. However, patients also experience a decrease in lymphocytes circulating in the blood [100]. About 5% of severe disease patients develop respiratory failure, septic shock, and multiple organ failure. Data from the Chinese Centers for Disease Control and Prevention (China CDC) [57] suggest that the mortality rate for severely diseased patients is 49% [98]. COVID-19 also has affected pregnant women, and clinical manifestations were the same as in the non-pregnant adults. A clinical case report described 18 cases of COVID-19 infection in pregnant women in the third trimester with symptoms of fetal distress and preterm delivery. Another study of 12 pregnant women reports a 25% mortality rate. Clinical complications include acute respiratory distress in four patients, three with disseminated intravascular coagulopathy, three with renal failure, and two with secondary bacterial pneumonia and sepsis [96].
Previous studies reported that SARS-CoV-1 infection caused male infertility. SARS-CoV-2 also binds the same ACE2 receptors as SARS-CoV-1, and the expression of ACE2 is upregulated in testicular cells [101]. The findings suggested that orchitis is the complication of SARS-CoV-1, and spermatogenesis could be affected after the infection. SARS-CoV-2 also infects children, and clinical data confirmed pediatric patients have mild symptoms. Current data reports that the mean age of onset in children is from 1.5 months to 17 years, mainly with no fever and pneumonia symptoms. Most pediatric patients recover from the disease within 1–2 weeks [102].
5. COVID-19 complexities
COVID-19 pandemic is a complex problem, and it should not be considered a single unit but as a heterogeneous group of infections. Several disease-related and patient-related factors are involved in the development of COVID-19. Complications of COVID-19 include acute respiratory distress (ARD) [20], arrhythmia, shock, AKI, acute cardiac injury, liver dysfunction, and secondary infection. The poor clinical outcome was related to disease severity [103].
5.1. Acute respiratory distress syndrome
The pathophysiology of unusual high-risk acute respiratory distress syndrome (ARDS) in SARS-CoV-1 or MERS-CoV infection has not been completely understood. Previous studies have indicated that high levels of pro-inflammatory cytokines in serum (e.g., IL6, IL12, IFNγ, IP10, and MCP1) were associated with pulmonary inflammation and extensive lung damage in SARS patients [104]. The progression to ARDS shows worsening of respiratory symptoms and ultimately leads to respiratory failure. ARDS occurs as a complication within one week of known clinical signs. The values of the partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) are used to differentiate ARDS severity based on different degrees of hypoxia. PaO2/FiO2 less than 100 mmHg indicates severe ARDS. PaO2/FiO2 values between 100 mmHg and 200 mmHg are indicative of moderate ARDS. PaO2/FiO2 values between 200 mmHg and 300 mmHg support the diagnosis of mild ARDS. Levels of aspartate transaminase (AST) and alanine transaminase (ALT) at the time of admission correlate with clinical worsening of symptoms to ARDS. Therefore, higher levels at admission result in rapid respiratory deterioration to ARDS [98]. A research group from China studied the pathological features of a patient who died from severe infection with SARS-CoV-2 by postmortem biopsies. The patient was a 50-year-old man admitted with fever, chills, dry cough, and difficulty breathing. After oxygen therapy, the patient was not stable and died due to sudden cardiac arrest. Biopsy samples were taken from the lungs of the patient. Left lung tissue biopsy showed pulmonary edema and hyaline membrane formation indicative of ARDS. Inflammatory infiltrates of interstitial mononuclear cells were observed in both lungs [105].
5.2. Acute kidney injury
Although ARDS is the main complication of COVID-19, the involvement of other organs needs to be considered. After respiratory infection, the infiltrated virus may go into the bloodstream, accumulate in the kidney and cause damage to renal resident cells. RNAaemia, characterized by a positive result for RT-PCR in the plasma sample, was found in 15% COVID-19 patients. It was reported that 6.7% of patients with SARS in 2003 developed acute renal impairment, and death due to SARS in patients with AKI was 91.7%. Thus, the kidney impairment and outcome in patients infected by SARS-CoV-2, which resembles SARS in 2003, were urgently warranted [106]. A study of Wuhan General Hospital, China, reported that 27.06% of patients with COVID-19 had abnormal glomerular filtration rate (GFR) and patients who are aged or have comorbidities more commonly developed acute renal failure. They performed the autopsy of six COVID-19 subjects and observed renal function. Different degrees of acute tubular necrosis, luminal brush border damage, and vacuole degeneration were found in different areas of all six renal tissue samples. Severe infiltration of lymphocytes in the tubulointerstitium was seen in two cases, and moderate infiltration was observed in three patients, and the remaining patient showed an absence of lymphocyte infiltration impairment. It is indicated that the SARS-CoV-2 virus can directly infect human renal tubules and, as a result, lead to acute renal tubular injury. Moreover, improved estimated GFR (eGFR) would increase the survival of COVID-19 patients with acute renal failure. Therefore, it is strongly recommended that applying potential interventions, including continuous renal replacement therapies to protect kidney function in COVID-19 patients, particularly for acute respiratory failure (ARF) cases, maybe a key approach to reducing mortality [107].
5.3. Acute liver damage
With the outbreak of COVID-19, patients with severe infection seem to have higher rates of liver damage. The viral infection of liver cells might directly cause liver dysfunction in patients with coronavirus infections. It is also possible that immune-mediated inflammation, such as cytokine elevation and pneumonia-associated hypoxia, might contribute to liver injury or even develop into liver failure in patients with COVID-19. Approximately 2–10% of patients with COVID-19 showed the symptoms of diarrhea, and SARS-CoV-2 RNA has been detected in stool and blood samples. This evidence implicates the possibility of viral presence in the liver. Data from the Fifth Medical Center of PLS General Hospital, Beijing, China, indicate that 2–11% of patients with COVID-19 had liver comorbidities, and 14–53% cases reported abnormal levels of alanine aminotransferase and AST during disease progression [108]. In a study published in The Lancet by Huang and colleagues, the elevation of AST was observed in eight (62%) of 13 patients in the ICU compared with seven (25%) of 28 patients who did not require care in the ICU [109]. Another large cohort, including 1099 patients from 552 hospitals in 31 provinces or provincial municipalities, had more severe patients with disease and abnormal liver aminotransferase levels than less severe patients [110]. Moreover, patients with liver cirrhosis or liver cancer are at risk of SARS-CoV-2 infection because of their systemic immuno-compromised status. The severity, mortality, and incidence of complications in these patients, including secondary infection, hepatic encephalopathy, upper gastrointestinal bleeding, and liver failure, need to be examined in large-cohort clinical studies.
5.4. Coagulopathy
Patients with severe SARS-CoV-2 infection can develop a coagulopathy state with fulminant activation of the coagulation cascade, resulting in widespread microvascular thrombosis and depletion of coagulation factors. This is characterized by thrombocytopenia, prolongation of the partial thromboplastin time, the elevation of D-dimer, and decreased fibrinogen levels [111]. A study reports a 69-year-old man with a history of hypertension, diabetes, and stroke presented with fever, cough, dyspnea, diarrhea, and the headache were diagnosed COVID-19 on RT-PCR. There were clinically significant coagulopathy and antiphospholipid antibodies in the patient's blood. Patient examination showed ischemia in the lower limbs as well as in the digits of the left hand. Patient laboratory reports indicated leukocytosis, thrombocytopenia, and elevated prothrombin time and partial thromboplastin time with high levels of fibrinogen and d-dimer. Serological test analysis revealed the presence of anticardiolipin IgA antibodies as well as anti–β2-glycoprotein I IgA and IgG antibodies. The presence of these antibodies may lead to thrombotic events in COVID-19 patients [112].
5.5. Neuronal damage
Clinicians have become more concerned that the pandemic will result in many individuals suffering from long-term illnesses and disabilities [113]. The neurological dysfunction is getting worse as the pandemic has progressed. Stroke, brain hemorrhage, and memory loss have all been added to the list recently. Host defense mechanisms can be activated by cerebrovascular inflammation, which can contribute to neurological disorders [114]. As the pandemic ramped up, many experts, including Alysson Muotri, a La Jolla-based neuroscientist at the University of California, San Diego, Michael, and his colleagues, began accumulating case reports of neurological problems connected to COVID-19 [115].
A study published in Brain (a Journal of Neurology) suggests that SARS-CoV-2 infection is related to neurological and neuropsychiatric diseases. The patients are separated into five categories based on their clinical, neurological, and laboratory aspects, and the results are summarized. COVID-19 has a broad spectrum of neurological consequences, including CNS disorders such as encephalitis, acute disseminated encephalomyelitis (ADEM) with hemorrhage and necrotic change, transverse myelitis, ischemic stroke, and Guillain-Barré syndrome (GBS). Acute hemorrhagic leukoencephalopathy (AHLE) is a kind of ADEM that requires a decompressive craniectomy [116]. In another study, He and his colleagues analyzed clinical details for 125 persons in the United Kingdom who reported COVID-19-related neurological or mental problems in a study published in Psychiatry (The Lancet). Sixty-two percent had suffered damage to the brain's blood supply, such as strokes and hemorrhages, and 31% had altered mental states, such as disorientation or prolonged unconsciousness, which was occasionally accompanied by encephalitis, or brain tissue swelling. Psychosis developed in ten patients who had changed mental states [117]. In a recent paper, Wan et al. explain typical signs of neurological dysfunctions linked with SARS-CoV-2, including encephalopathy, aphasia, meningitis, prosopoplegia, encephalitis, sensory loss, GBS, dysarthria, skeletal, muscular symptoms, and acute confusion [118].
To date, several research have looked into the relationship between SARS-CoV-2 and neurological illnesses like schizophrenia [119], dysphoria [120], delirium [121], epilepsy [122], depression [123], [124], bipolar disorder [125], Alzheimer's disease (AD) [126], [127], Parkinson's disease (PD) [128], and obsessive-compulsive disorder (OCD) [129].
5.6. Cardiovascular damage
COVID-19 can induce cardiovascular events such as myocardial damage, arrhythmias, myocardial infarction, and pulmonary embolism. Many individuals with coronavirus disease 2019 (COVID-19) have pre-existing CVD or suffer from acute myocardial injury even during illness [120]. Though severe acute pulmonary edema is the hallmark of severe COVID-19 disease, cardiac events can appear in a variety of ways, each posing its own set of treatment problems. Myocardial infarction and right ventricular failure are the most prevalent signs, but heart failure, cardiovascular shock, encephalopathy, arrhythmia, and vascular thrombosis have also been reported [121]. Though severe acute pulmonary edema is the hallmark of severe COVID-19 disease, cardiac events can appear in a variety of ways, each posing its own set of treatment problems. Myocardial infarction and right ventricular failure are the most prevalent signs, but heart failure, cardiovascular shock, encephalopathy, arrhythmia, and vascular thrombosis have also been reported [122].
6. COVID-19 preventive measures and management
The current clinical management of the COVID-19 consists of preventive measures and control of infection and supportive care, including oxygen therapy and mechanical ventilatory support when indicated.
6.1. Therapeutic interventions
All therapeutic options have been based on the previous experiences of treating SARS, MERS, or other influenza viruses previously reported. Possible treatments options for COVID-19 are summarized here. Furthermore, symptomatic treatments remained the only key to success in treating COVID-19 patients. The drugs mentioned below would be helpful, and the efficacy needs to be further confirmed.
6.1.1. Antibiotics as adjuvant therapy
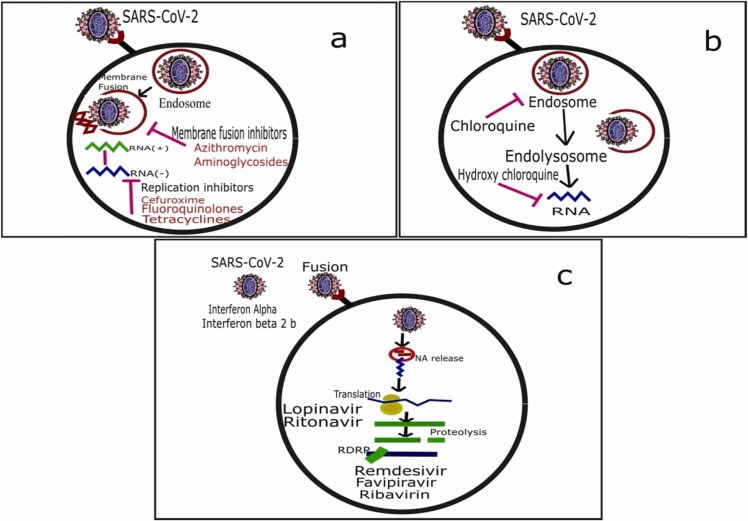
Antibiotics are used as adjuvant therapy depending on the clinical manifestations of patients. Prompt use of antibiotics to prevent infection and to strengthen the immune system might reduce complications and mortality. A single-center study, conducted at Jinyintan Hospital in Wuhan, China, recruited 99 COVID-19 patients. All patients were tested for nine respiratory pathogens, including Acinetobacter baumannii, Klebsiella pneumonia, and Aspergillus flavus. Severely ill patients had co-infection of bacteria. Most patients were given antibiotic treatment, 25% were given a single antibiotic, and 45% were treated with combination therapy. The antibiotics were used based on the results of bacterial culture and drug sensitivity. The antibiotics used were cephalosporin, quinolones, carbapenems, linezolid, and tigecycline against methicillin-resistant Staphylococcus aureus (MRSA). The duration of antibiotic treatment was 3–17 days. By the end of the study, 31% of patients were discharged, and 11 (11%) patients died; all other patients were still in hospital [130]. In a recent trial with patients on COVID-19 treatment, 100% of patients treated with hydroxychloroquine in combination with the macrolide antibiotic azithromycin were cured compared with 57.1% patients treated with hydroxychloroquine alone and 12.5% in the control group. Depending on their clinical presentation, azithromycin (500 mg on day1 followed by 250 mg per day, the next four days) was added as adjuvant therapy to prevent bacterial super-infection. Study results indicated the synergistic effect of the combination of hydroxychloroquine and azithromycin. Azithromycin has also shown activity in vitro against Zika and Ebola viruses and prevents bacterial super-infections when administered to patients suffering from viral infection [131]. Antibiotics' probable mechanism of action against SARS-CoV-2 infection is depicted in Fig. 1 (a).
Fig. 1.
Potential mechanisms of action of antibiotics (a) antimalarial (b) and antivirals (c) for the treatment of COVID-19.
6.1.2. Antimalarial
Chloroquine has been used globally for more than 70 years as an antimalarial drug, and it is part of the WHO model list of essential medicines. It is less costly and has an approved clinical safety profile. Chloroquine's potential mechanism of action against SARS-CoV-2 infection has been depicted in Fig. 1 (b). Researchers have repositioned chloroquine and hydroxychloroquine as a therapeutic regimen to hunt for new pharmacologic drugs that would be effective against the SARS-CoV-2 virus [132]. A group of Chinese researchers investigated the effect of chloroquine in vitro by using Vero E6 cells infected by SARS-CoV-2. The results indicated that chloroquine was highly influential in decreasing viral replication, with an effective concentration (EC) of 6.90 μM that is easily attainable with standard dosing due to its better penetration in lung tissues. The authors demonstrated that chloroquine blocks virus infection by increasing endosomal pH and interfering with the glycosylation of the cellular receptor of SARS-CoV-1. The authors also speculated that the known immunomodulant action of the drug might improve the antiviral effect in vivo [133].
Additionally, chloroquine repurposing was investigated in two different hospitals in China. Preliminary data suggested that approximately 100 COVID-19 infected patients treated with chloroquine showed a more rapid decrease in fever and improved lung computed tomography images [134]. Patients recovered in a short period compared with control groups, with control groups no noticeable adverse severe effects. The Chinese medical advisory board has suggested chloroquine inclusion in the COVID-19 treatment guidelines on seeing these results. As a result, chloroquine is probably the first molecule used in China and abroad as a first-line agent for treating severe COVID-19 infections [135].
Another Chinese research group conducting multicenter trials on the efficacy of chloroquine in COVID-19 associated pneumonia reported that chloroquine phosphate had proven efficacy and acceptable safety in more than 100 patients enrolled in the trials [136]. According to the risk-benefit ratio, the high security and the low expenditure of chloroquine in the context of the current COVID-19 outbreak make it a suitable candidate in the highly stressed health care system [137]. Recently, Wang and colleagues concluded from their clinical isolate of SARS-CoV-2 that chloroquine is highly effective in controlling COVID-19 infection in vitro, and its safety should be tested in patients infected with the coronavirus. There are 16 clinical trials registered in the Chinese clinical trial registry for chloroquine and hydroxychloroquine use in treating COVID-19 infected patients [138].
WHO confirmed in a published report "clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected" that there is currently no evidence from randomized control trials of specific drug treatment of COVID-19 and that unregistered therapies should be administered only after ethically-approved clinical trials under strict monitoring? There is sufficient pre-clinical rationale and evidence regarding the effectiveness of chloroquine in treating COVID-19, but clinical data is required for its use in infected patients.
Hydroxychloroquine (an analog of chloroquine) has been demonstrated to have an anti-SARS-CoV-1 activity in vitro [139]. Hydroxychloroquine's clinical safety profile is better than that of chloroquine (during long-term use) and allows a higher daily dose with fewer concerns about drug-drug interactions. Study results showed that hydroxychloroquine is efficient in clearing a viral nasopharyngeal load of SARS-CoV-2 in COVID-19 patients in only three to six days, in most patients. A significant difference was observed between hydroxychloroquine-treated patients and controls starting even on day 3 post-infection. These results are of great importance because a recent study in China has shown that the mean duration of viral shedding in patients suffering from COVID-19 was 20 days (even 37 days for the most extended duration) [131]. As of March 30, 2020, the US FDA has given emergency approval of antimalarial drugs chloroquine and hydroxychloroquine to treat COVID-19 [140].
6.1.3. Antivirals
According to the National Health Commission (NHC) of the People's Republic of China guidelines, IFN-α, lopinavir/ritonavir are recommended as antiviral therapy in COVID-19 treatment [141]. Remdesivir may be the best option for treating COVID-19 as it has proven efficacy in treating MERS-CoV compared with lopinavir/ritonavir in animal studies [142]. On May 7, 2020, remdesivir was approved for use in Japan [143]. The research was conducted on the first Korean COVID-19 patient from China on January 20, 2020. He presented the symptoms of chills and muscle pain. He developed a fever and dry cough on days 5 and 7, respectively. Lopinavir/ritonavir was started on10th day of illness.
Interestingly, viral load started to reduce from the next day of lopinavir/ritonavir administration, and no detectable coronavirus titers have been observed [144]. Another study reported that the ribonucleoside analog, β-D-N4-hydroxycytidine (NHC, EIDD-1931), had shown broad-spectrum antiviral activity against SARS-CoV-2, MERS-CoV, SARS-CoV-1, and other related Bat-CoVs, as well as showed increased efficacy against the coronavirus resistant to any other nucleoside analog inhibitor. In a study, mice infected with SARS-CoV-1 or MERS-CoV showed improved respiratory conditions with the reduced viral load when treated with prophylactic and therapeutic doses of EIDD-2801. The superior efficacy of NHC/EIDD-2801 against multiple coronaviruses and better oral bioavailability emphasizes its potential application as an effective antiviral agent against SARS-CoV-2 and any other zoonotic coronaviruses [145].
Favipiravir is a member of RNA polymerase inhibitor antiviral drugs. It blocks the replication of RNA viruses by converting them to the active phosphoribosylated form in cells Fig. 1 (c). This active form is recognized as a substrate by viral RNA polymerase, thus inhibiting RNA polymerase activity [146]. An open-label controlled clinical trial on favipiravir for the treatment of COVID-19 initiated by the Clinical Medical Research Center of the National Infectious Diseases (NCID) and Third People's Hospital of Shenzhen on February 14, 2020, had achieved promising results. The initial results from 80 patients (including the experimental and control groups) indicated that favipiravir had more potent antiviral action than lopinavir/ritonavir. No significant adverse reactions were seen in the favipiravir treatment group, and it had significantly fewer adverse effects than the lopinavir/ritonavir group [147]. Neuraminidase inhibitors (NAIs) such as oral oseltamivir, inhaled zanamivir, and intravenous peramivir are also suggested as antiviral drugs in influenza. Oral oseltamivir has been widely used for COVID-19 cases in China hospitals. It has been found that neuraminidase inhibitors are effective as empirical treatment in MERS-CoV infection. However, evidence regarding their use in the treatment of COVID-19 is lacking [142]. Molnupiravir is Covid's first oral antiviral medication to see positive outcomes in clinical trials. Molnupiravir is the first antiviral medicine for Covid that can be taken as a tablet instead of injection, according to Merck, Sharp, and Dohme (MSD) and Ridgeback Biotherapeutics. The UK medicine regulator has given its approval to the first oral treatment designed to treat symptomatic Covid. It will first be administered to both vaccinated and unvaccinated patients in countrywide research, with additional data on its usefulness gathered before any decision to order more. To be most effective, the medicine must be taken within five days of the onset of symptoms [148].
Pfizer stated on November 5, 2021, that its COVID-19 treatment pill (brand name Paxlovid) was shown to be very effective in preventing severe disease in at-risk people who took the therapy immediately after exhibiting symptoms in a critical clinical trial, making it the second antiviral pill to show efficacy against COVID. When taken within three days of the onset of symptoms, Pfizer's medication reduced the chance of hospitalization or death by 89%. Thirty tablets are administered over five days as part of the treatment. This comprises ten ritonavir pills, an ancient H.I.V. medicine that makes Pfizer's drug last longer in the body. (Merck's therapy is a five-day course of 40 pills)[149]. So far, possible antiviral and other FDA-approved/ under clinical trials treatments for COVID-19 are mentioned in Table 2.
Table 2.
FDA-approved/ under clinical trials treatments for COVID-19.
| Sr. No | Drug Name | Type | Sponsors and Collaborators | Previous work | Phase of Development | References |
|---|---|---|---|---|---|---|
| 1. | TAK-888 | Plasma-derived drug | Takeda Pharmaceutical Company Ltd. | H-IGs are used to treat severe acute viral respiratory infections. | Preclinical | [206] |
| 2. | Avigan® (Favipiravir) | Influenza Antiviral Drug | FUJIFILM Toyama Chemical Co. Ltd., China | – | Approved | [207] |
| 3. | Febuxostat (FBX)) | Non-purine xanthine oxidase (XO) | Lotfollah Davoodi, Mazandaran University of Medical Sciences, Iran | – | Open-label clinical trial | [208], [209] |
| 4. | Voxelotor (brand name Oxbryta) | Sickle cell drug | Global Blood Therapeutics | – | Early Phase 1 | [210] |
| 5. | No name yet | Monoclonal antibodies | Vir Biotechnology Inc. VIR, and Biogen Inc. BIIB | 05 product candidates are targeting Hepatitis B, influenza A, HIV, and tuberculosis. | Preclinical | [211] |
| 6. | Remdesivir | Treatment | Gilead Sciences Inc. GILD | Hepatitis-C in Sovaldi, HIV drugs including Truvada for pre-exposure prophylaxis (PrEP). | Phase 3 clinical trials | [212] |
| 7. | Prezcobix (Darunavir/cobicistat) | Antiviral | Johnson & Johnson JNJ, | Antiviral used for the treatment of HIV; already on the market | Phase 3 clinical trials | [213] |
| 8. | No name yet | Monoclonal antibodies | Regeneron Pharmaceuticals Inc. REGN. | This technology was previously used to create a cocktail of antibodies against Ebola. | Preclinical | [214] |
| 9. | Kevzara (sarilumab) & Dupixent (dupilumab) | Drug Treatment | Regeneron Pharmaceuticals and Sanofi | Previously developed a treatment for rheumatoid arthritis | Phase 2/3 clinical trial | [215] |
| 10. | Remdesivir | Antiviral drug remdesivir | Gilead Sciences, Inc. | – | Approved | [216] |
| 11. | Actemra/RoActemra | Drug Treatment | Company: Roche Holding AG ROG | Rheumatoid arthritis drug | Phase 3 | [217] |
| 12. | Methylprednisolone | Corticosteroid Drugs | Peking Union Medical College Hospital, China | – | Phase 2/Phase 3 | [218] |
| 13. | Intravenous immunoglobulin | Antibody | Peking Union Medical College Hospital & Tongji Hospital, Huazhong University of Science and Technology, China | – | Phase 2/Phase 3 | [219] |
| 14. | MSCs | Mesenchymal stem cells Therapy | Beijing 302 Hospital, Innovative Precision Medicine Group (IPM), Wuhan Jinyintan Hospital, Tianjin Haihe Hospital, Vcanbio Cell & Gene Engineering Corp. Ltd., Wuhan Union Hospital, China | – | Phase 3 | [220] |
| 15. | MSCs-derived exosomes | Mesenchymal Stem Cells Therapy | Shanghai Public Health Clinical Center, Wuhan Jinyintan Hospital, Cellular Biomedicine Group Ltd., China | – | Phase 1 | [221] |
| 16. | NestCell® | Mesenchymal stem cells Therapy | Azidus Brasil, Cellavita Pesquisa Científica Ltda, Hospital Vera Cruz | – | Phase 1 | [222] |
| 17. | No name yet | Dental pulp mesenchymal stem cells | CAR-T (Shanghai) Biotechnology Co., Ltd. China | – | Early Phase 1 | [223] |
| 18. | Washed microbiota transplantation | Cell therapy | The Second Hospital of Nanjing Medical University, China | – | N/A | [224] |
| 19. | Mesenchymal stem cells, Ruxolitinib | Cell therapy, anti-inflammatory | Tongji Hospital, China | – | Recruiting;From2020–01–31 To2020–12–31 | [225] |
| 20. | Umbilical cord blood mononuclear cells | Cell therapy | Xiangyang First People's Hospital, China | – | [226] | |
| 21. | Human umbilical cord mesenchymal stem cell | Stem cells Therapy | Shanghai East Hospital, China | – | Phase 1 | [227] |
| 22. | UC-MSCs | Umbilical cord blood mesenchymal stem cell therapy | Wuhan Hamilton Biotechnology Co., Ltd, China and Wuhan Union Hospital, China | – | Not Applicable | [228] |
| 23. | TZLS-501 | Anti-interleukin-6 receptor (anti-IL6R) monoclonal antibody (mAb) | Tiziana Life Sciences | – | N/A | [229] |
| 24. | Kaletra | HIV drugs lopinavir and ritonavir | ABBVIE | – | a randomized, controlled, open-label trial | [230], [231] |
| 25. | Leronlimab | Group of HIV drugs called CCR5 antagonists. | CytoDyn | – | Phase 2 for HIV and fast-tracked for COVID-19 | [232] |
| 26. | APN01 | A recombinant version of the human ACE2 2 (rhACE2) | APEIRON Biologics | – | Phase 2 | [233] |
| 27. | Jakavi (Ruxolitinib) | Treat cytokine storm in patients with severe Covid-19 | Novartis and Incyte | – | Phase 3 clinical trials | [234] |
| 28. | Hydroxychloroquine | Antimalarial | Duke Clinical Research Institute (DCRI) | – | randomized clinical trial | [235] |
| 29. | Newgen beta-gluten probiotic composite powder | Probiotics | – | N/A | [236] | |
| 30. | Fingolimod | Sphingosine 1 phosphate (S1P) analog, | First Affiliated Hospital of Fujian Medical University,& Wan-Jin Chen, Fuzhou, China | – | Phase 2 | [237], [238] |
| 31. | Eculizumab(Soliris) | A distal complement inhibitor. | Hudson Medical & Thomas Pitts, M.D., Hudson Medical | – | Treatment IND/Protocol | [239] |
| 32. | Ivermectin | Anti-parasitic drug | Monash University | – | Pre-clinical testing | [240] |
| 33. | (Danoprevir) | Hepatitis C virus protease inhibitor | The Ninth Hospital of Nanchang, China | Phase 4 | [241] | |
| 34. | Galidesivir | Nucleoside RNA polymerase inhibitor | BioCryst Pharmaceuticals | Developmental stage | [242] | |
| 35. | Thalidomide | Immunomodulatory agents. | First Affiliated Hospital of Wenzhou Medical University, China | Phase 2 | [243] | |
| 36. | Triazavirin | guanine nucleotide analog antiviral | Health Commission of Heilongjiang Province, China | Phase 3 | [244] | |
| 37. | Baricitinib | Janus kinase (JAK) inhibitors | Prato hospital | Phase 3 | [245] | |
| 38. | Aviptadil (RLF-100) | Vasoactive Intestinal Polypeptide VIP | NeuroRx, Relief Therapeutics | Phase 3Orphan drug designation | [246] | |
| 39. | Actemra (Tocilizumab) | Monoclonal antibody | Roche,Medical institutions worldwide | Approved | [247] | |
| 40. | Sotrovimab | Monoclonal Antibodies | GSK &Vir Biotechnology | Phase 3 | [248] | |
| 41. | MK-4482, EIDD-2801 (Molnupuravir) | Antiviral | Dohme Corp &Merck Sharp | Phase 3 | [249] |
6.1.4. Corticosteroids
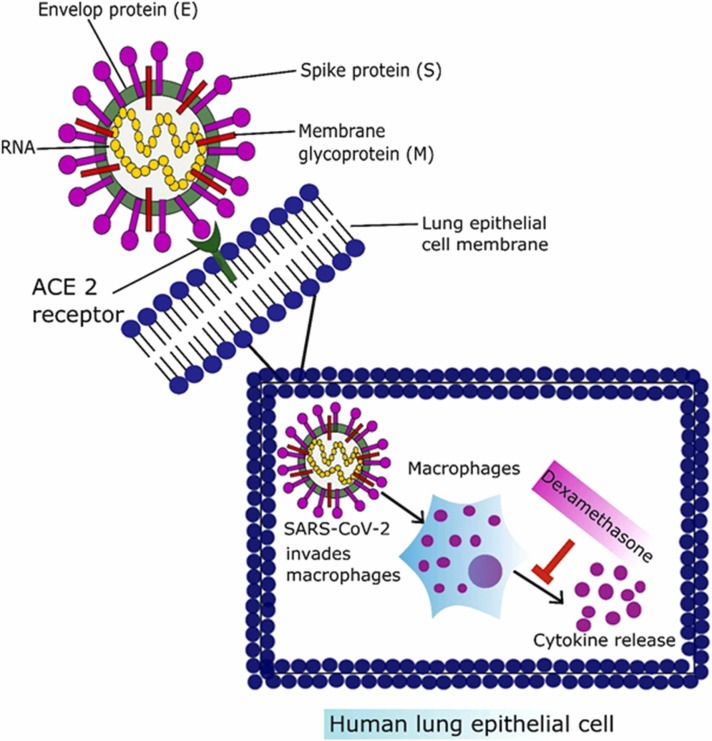
Corticosteroids are used extensively to treat various respiratory system disorders due to their anti-inflammatory, and immune suppression properties Fig. 2. Despite their established use in respiratory disorders, there is debate over their role in managing respiratory symptoms associated with COVID-19 [150]. Various studies have been conducted on the use of corticosteroids in COVID-19 management and demonstrated a reduction in mortality rate compared to patients who did not receive corticosteroids [151]. Along with their therapeutic potential, corticosteroids use has adverse effects. A study reported immunity suppression and increased viral load, and delayed clearance of coronavirus from the body [152]. The latest development in the COVID-19 treatment came from the Randomized Evaluation of COVID-19 therapy Trial (RECOVERY Trial) on June 16, 2020. Researchers from Oxford University started working in March 2020 as a randomized clinical trial to evaluate potential therapeutic options for COVID-19. Above 11,500 COVID-19 positive patients have been recruited from 175 National Health Service (NHS) hospitals in the UK. The use of dexamethasone was started on June 8, 2020, for ten days at a dosing rate of 6 mg per day to calculate therapeutic potential compared to 4321 patients without taking dexamethasone. Dexamethasone reduced the mortality rate by one-third in patients on a ventilator and by one-fifth in patients on oxygen therapy [153]. The Oxford RECOVERY Trial tested various therapeutic options, including low dose corticosteroid (dexamethasone), antivirals, hydroxychloroquine, and azithromycin, in a randomized manner. Out of all tested therapeutic moieties, the only dexamethasone succeeded in reducing the COVID-19 associated death rate [154]. Dexamethasone is an anti-inflammatory and immunosuppressive corticosteroid drug that the FDA has licensed. Dexamethasone has been declared a "significant development" for the COVID-19 in the present epidemic. The use of steroidal dexamethasone has been highlighted as a recent breakthrough in lowering the mortality rate in severe COVID-19 cases [153]. Early findings showed that this drug could decrease the mortality risk from 40% to 28% in ventilated patients and from 25% to 20% for patients on oxygen therapy. Dexamethasone use did not produce any significant side effects, and use was ineffective in mild COVID-19 cases [155]. The current administration of corticosteroids should be reserved for patients with severe conditions related to cytokine storm, including ARDS, renal failure, acute cardiac injury, and elevated serum levels of D-dimers [156].
Fig. 2.
Entry of SARS-CoV-2 inside the lung epithelial cell and the mechanism of action of corticosteroids.
6.1.5. Antidepressants
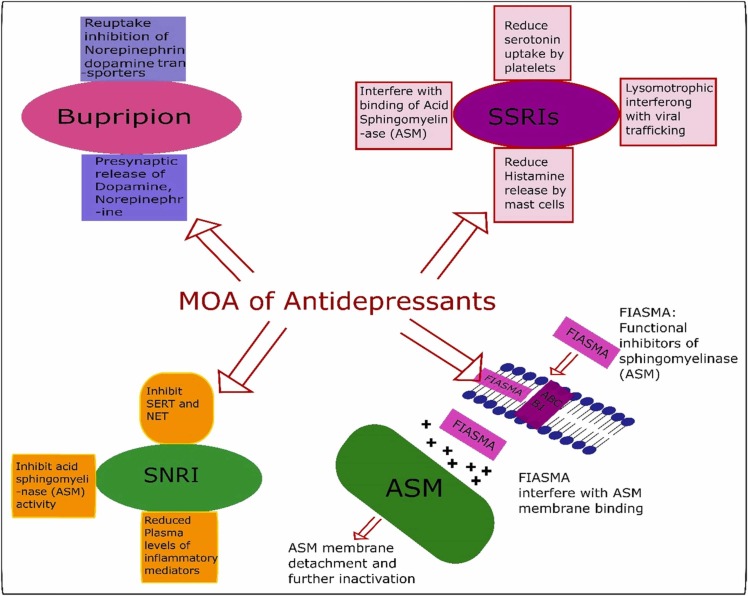
According to the researchers of the latest analysis on using an antidepressant for COVID-19, the medicine significantly lowers hospitalizations and mortality. Selective serotonin reuptake inhibitors (SSRIs) include fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram [157], and serotonin-norepinephrine reuptake inhibitors (SNRIs) and Dopamine Reuptake Inhibitors, e.g., Bupropion has been used for SARS CoV-2 [158]. The drug, called fluvoxamine, is a medication that is used to treat depression and obsessive-compulsive disorder. It is an SSRI and agonist of the serotonin-1 receptor (S1R)[159]. Fluvoxamine has a number of potential pathways for treating COVID-19 disease, including anti-inflammatory and antiviral properties Fig. 3. However, it is also known to reduce immunological responses and tissue damage, and researchers attribute its success in the new experiment to these features. COVID-19-related mortality was reduced by about 90% among trial participants who took medicine as advised and did so in the early stages of the condition, while the requirement for intense COVID-19-related medical treatment dropped by roughly 65%. A huge win for medicine repurposing!" Vikas Sukhatme, a drug repurposing researcher at Emory University School of Medicine in Atlanta, Georgia, said in an e-mail to Nature. Those at high risk of collapse and are unable to receive monoclonal antibodies should be treated with fluvoxamine [160].
Fig. 3.
Various antidepressants mechanisms of action against SARS-COV-2.
According to the latest clinical-trial results, a low-cost, high-effective, and widely available medicine used to treat mental illness reduces the chance of death from COVID-19 and the demand for patients with the condition to need intense medical care. For this investigation, the research team assessed 9803 potential volunteers. Fluvoxamine was given to 741 participants and a placebo to 756. A randomized, placebo-controlled, adaptive platform trial in Minas Gerais, Brazil, was conducted to see if fluvoxamine could prevent COVID-19 progression and hospitalization in outpatients with laboratory-confirmed SARS-CoV-2. This is only the second trial to indicate that a repurposed medicine had a significant therapeutic benefit in the early treatment population. Fluvoxamine may minimize the risk of clinical deterioration in COVID-19 outpatients, according to a small placebo-controlled, randomized experiment, indicating the need for larger randomized, placebo-controlled research. In high-risk outpatients with early diagnosed COVID-19, treatment with fluvoxamine (100 mg twice daily for 10 days) reduced the requirement for hospitalization, defined as retention in a COVID-19 emergency setting or transfer to a tertiary hospital [161].
Zimniak et al. discovered that compared to other SSRIs such as escitalopram and paroxetine, fluoxetine has antiviral effects toward SARS-CoV-2. Fluoxetine also stops cytokines from being released. Consequently, the 5-HT reuptake receptor has no relevance to the antiviral effect. The studies state that fluoxetine therapy decreased viral protein expression, signaling that the drug works proximal to gene expression. Fluoxetine successfully suppressed SARS-CoV-2 replication at a dose of 0.8 g/mL, with an EC50 of 0.38 g/mL [162].
Antidepressants also worked as strong inhibitors of an enzyme called acid sphingomyelinase. This enzyme works by breaking down a few of the fatty molecules on cell surfaces, making them highly susceptible; the COVID-19 virus uses this enzyme to help it invade cells. Hence, the antidepressants effectively prevented the virus from entering [163].
6.1.6. Miscellaneous drugs
Besides these, other medical treatments, including benzodiazepines [164], a potent pan-AKT-kinase drug (Capivasertib), have a role in SARS-CoV-2 S protein pseudo-type virus and VSV-dG in Vero cells [165]. Lithium has been demonstrated to: a) impede the reproduction of numerous types of viruses, including ones that are related to the SARS-CoV-2 virus; b) boost the immune response by lowering lymphopenia, and c) fight inflammation via avoiding the cytokine storm [166]. Besides, antiepileptic's include carbamazepine, valproic acid, and gabapentin [158]. Attempts have also been made to evaluate clozapine therapy and its link to an increased risk of COVID-19 infection and severe outcomes [167].
6.2. Vaccination strategies
Current therapy of COVID-19 with the existing practice of antivirals and antimalarial drugs mainly focused on symptomatic treatment and respiratory maintenance. Given the lack of effective antiviral and antimalarial therapy against COVID-19, researchers have stressed the need for novel development and manufacturing platforms that can be readily adapted to this new pathogen. So, we would need a system that links genotype to phenotype to keep up with the sequence data generation. In pharmaceutical research, everything takes time, and it takes longer if you have to start from zero. Several platforms are under development for COVID-19. Vaccine development is a lengthy, expensive process. Attrition is high, and it typically takes multiple candidates and many years to produce a licensed vaccine [168]. As reported by the WHO, vaccines are one of the most effective ways to prevent diseases by boosting the body's immune system to defend the body against infectious pathogens like bacteria and viruses.
The projections on the surface of COVID-19 enable the virus to enter human cells. In developing a vaccine that targets SARS-CoV-2, scientists are looking at these projections intensely. In creating a vaccine for SARS-CoV-2, scientists need to find a viable antigen to stimulate the body's immune system against the infection. Many companies worldwide are working on a SARS-CoV-2 vaccine, developing different ways to boost the immune system summarized in Table 3. Adjuvants are also employed for elderly patients to amplify their immune system, and it is expected that by studying adjuvants to boost a vaccine, the elderly can be vaccinated with a mix of ingredients that would supercharge their immunity [169].
Table 3.
FDA-approved/ under clinical trials vaccines for COVID-19.
| Sr. No | Vaccine Name | Type | Sponsors and Collaborators | Previous work | Phase of Development | References |
|---|---|---|---|---|---|---|
| 1. | BNT162 | Vaccine | Pfizer Inc. &BioNTech SE | – | Approved | [250] |
| 2. | mRNA-1273 | Vaccine | Moderna | – | Approved | [251] |
| 3. | Sputnik V | Muscle Injection | Gamaleya Research Institute | Approved | [252] | |
| 2. | No name yet | Oral recombinant Vaccine | Vaxart Inc. VXRT, + 2.35% | – | Phase III clinical trials | [253], [254] |
| 3. | No name yet | Recombinant vaccines. | Sanofi SNY,& BARDA | – | Preclinical | [255] |
| 4. | No name yet | Matrix-M™ adjuvant with COVID-19 vaccine to enhance immune responses. | Novavax Inc. NVAX, | Ebola vaccine, NanoFlu™, ResVax™, | Phase I clinical trials | [256] |
| 5. | AS03 Adjuvant System | A pandemic adjuvant platform for vaccines | GlaxoSmithKline (GSK), and CEPI | brought to market vaccines for human papillomavirus (HPV) and the seasonal flu. | Preclinical studies | [257] |
| 6. | TBD vaccine is under development, so there is no specific name at this time,” | Vaccine | Johnson & Johnson JNJ, and BARDA | – | Phase 1 clinical trial | [258] |
| 7. | vaccine for the novel coronavirus | Vaccine | Heat Biologics Inc. HTBX, | – | Preclinical | [259] |
| 8. | INO-4800 | Type: DNA-based vaccine | Inovio Pharmaceuticals Inc. INO | – | Preclinical | [260] |
| 9. | mRNA-1273 | RNA-based vaccine | Biotechnology company Moderna, Inc. | – | Phase III clinical trial | [261], [262] |
| 10. | gp96 vaccine | Vaccination | Heat Biologics & University of Miami | – | Early development | [263] |
| 11. | BX-25 | Vaccination | BIOXYTRAN | – | Pre-clinical trial | [264] |
| 12. | S-Trimer vaccine | Vaccination | Clover & Dynavax | – | Pre-clinical trials | [265] |
| 13. | TNX-1800 | Vaccination | Tonix Pharmaceuticals | – | Initial evaluation | [266] |
| 14. | No name yet | Infectious Bronchitis Virus (IBV) vaccine | MIGAL and IIBR | – | Pre-clinical trials | [267] |
| 15. | Pitt-Co-Vacc | Vaccination uses lab-made pieces of viral protein to build immunity in the same way as a flu. | Pittsburgh School of Medicine | – | Animal studies | [204] |
| 16. | Covaxin | Derived from a strain of the novel coronavirus | Bharat Biotech, India Institute of Medical Sciences in Patna, and Post-Graduate Institute of Medical Sciences in Rohtak | – | Phase I/II clinical trials | [268] |
| 17. | AZD1222 | Vaccine | The University of Oxford, Vaccitech, Astra Zeneca (UK-based global biopharmaceutical company) | – | Approved | [269], [270]. |
For the development of vaccines, various technologies are being used, both well-known and brand new for human vaccines, such as viral vector vaccine, protein-based vaccine, virus vaccine, and nucleic acid vaccine.
6.2.1. Viral vector vaccines
Inserting a pathogen protein gene into another virus can infect a person without producing disease. The safe virus serves as a platform or "vector" to deliver the protein that stimulates an immune response. The safe virus is then administered as a vaccination. Some organisms replicate (reproduce) in the body, whereas others do not.
6.2.2. Protein-based vaccines
Like a vaccination, a pure protein is taken from the virus (either living or inactivated). Coronaviruses use this protein as their "spike" in just the same way as virus-like particles. Hepatitis B, shingles, and other viral illnesses can be prevented with protein vaccinations that have been in use for decades. As a means of eliciting an immune response, these vaccines deliver proteins, together with immunotherapy that stimulate the immune system of the body, straight to the cells of a person. [170].
6.2.3. Virus vaccines
In this technique, a virus is chosen, changed (weakened), or inactivated totally to prevent disease transmission.
6.2.4. Nucleic acid vaccines
This is a new technique – no other vaccinations for human use have employed this – and it's less stable than DNA. It is possible to use nucleic acid rather than just the virus, protein antigen, or virus that expresses protein. DNA plasmid: penetrates the nucleus, is translated to mRNA for protein expression, and injected or mRNA [171].
6.3. Traditional Chinese medicine (TCM)
Traditional Chinese remedies (TCM) have a key role in preventing and controlling COVID-19, with a successful cure rate. TCM includes decoctions, Chinese patent medicine, acupuncture, and other characteristic therapies that can reduce inflammation and alleviate immune response by regulating immune-related pathways and cytokine action-related pathways [172], [173]. Ideally, SARS-CoV-2 infection can be stopped by the blockade of ACE2, and fortunately, TCM-derived compounds could interact with ACE2 receptors, i.e., used for the cellular entrance of SARS-CoV-2. Additionally, TCM can inhibit cytokine storm; based on historical records of TCM against SARS and H1N1 influenza, and there are pretty compelling pieces of evidence supporting the notion that TCM has a beneficial effect in the treatment or prevention of COVID-19 [130], [174]. The anti-SARS activity of TCM has been revealed by performing a screening analysis of hundreds of Chinese medicinal herbs. Some examples are extracts of Lycoris radiate (family Amaryllidaceae), Artemisia annual (family Asteraceae), Pyrrosia lingua (is an epiphytic fern in the family Polypodiaceae), and Lindera aggregata (genus Lindera) [175]. Moreover, it has been reported that naturally occurring triterpene glycosides, i.e., saikosaponins (A, B2, C, and D) isolated from medicinal plants such as bupleurum spp. (family Apiaceae), heteromorphic spp. (family Apiaceae) and Scrophularia scorodonia (family Scrophulariaceae) exert significant antiviral activity against HCoV‐22E9 [176]. Myricetin, scutellarein, and phenolic compounds of Isatis indigotica and Torreya nucifera possess natural enzyme inhibitors (such as the nsP13 helicase and 3CL protease), which are very effective against SARS [177]. Additionally, baicalin, a Chinese herbal compound, also had anti-SARS activity (123). Water extract of most TCM such as Houttuynia cordata (also known as fish mint, fish leaf, rainbow plant, chameleon plant, heartleaf, fish wort, Chinese lizard tail, or bishop’s weed) exhibits several antiviral mechanisms against SARS by inhibiting the viral 3CL protease and also by blocking the viral RNA‐dependent RNA polymerase activity [178]. In a study, Luo and colleagues have reported 10 most commonly used Chinese herbs that played a substantial role in the fight against COVID-19 to consist of; Astragalus membranaceus, Atractylodis Rhizoma, Agastache rugosa, Cyrtomium fortune, Fructus forsythia, Glycyrrhizae uralensis, Lonicerae Japonicae Flos, Rhizoma Atractylodis Macrocephalae, Radix platycodonis, and Saposhnikoviae divaricate [179]. Another research reported that the most frequently used Chinese herb, licorice root, which contains glycyrrhizin, potently inhibits the replication of SARS viruses [180]. Though there are scads of publications on TCM treatments for SARS yet HNC’s guideline did not recommend TCM products owing to the shortage of safety data and trials [181]. Toxicological studies are also insufficient for Chinese herbal medicines, yet some reported that some herbs used in TCM contain nephrotoxins and mutagens [182], [183].
7. Drugs with failed clinical trials
To date, 05 ongoing clinical trials of COVID-19 drugs have not demonstrated positive results, as reported by Global Data [184]. Thus far, hydroxychloroquine or chloroquine is one of the most prominent examples that recently failed to benefit hospitalized patients, and even developed side effects in a retrospective study (Phase II clinical trial). Patients treated with hydroxychloroquine also had a higher mortality rate in clinical trials. In the US clinical trials, Patients treated with hydroxychloroquine also had a higher mortality rate [185]. The other clinical trial drug is darunavir (HIV treatment) and cobicistat (phase III clinical trial) for COVID-19 pneumonia. These trials have the expected end date of August 31, 2020."Pharma company Johnson & Johnson claimed that darunavir presented no evidence of activity against the COVID-19 testing thus far [186]. Additionally, WHO also discontinue the trials of lopinavir/ritonavir for the treatment of COVID-19. These drug trial results show little or no reduction in the mortality of hospitalized COVID-19 patients when compared to the standard of care [187]. Remdesivir (antiviral drug) also reported failure in its first randomized clinical trial and was halted early due to poor statistical acceptance [188]. However, this does not spell the end of the road for the drug discovery towards COVID-19 treatment, and many ongoing trials will offer a clear representation of therapy soon.
8. Conclusion and future perspectives
This article has summarized different variants of coronaviruses with particular emphasis on SARS-CoV-2. Our primary focus was on etiology, complexities, preventive measures, therapeutic interventions, and future challenges in the fight against SARS-CoV-2 associated COVID-19. SARS-CoV-2 is linked with severe complications, including ARDS, liver damage, AKI, acute cardiac injury, arrhythmia, shock, and neuronal disorders. Clinicians either used antivirals, antibiotics, and antimalarial drugs as new treatments (monotherapy) or adjuncts to existing treatments (combined therapy) to improve therapeutic effectiveness against COVID-19. However, some treatment options have not been successful when being clinically translated. Despite the considerable progress in the prevention and treatment options, we face challenges in the fight against COVID-19. Execution of the clinical trials on a large patient population is needed to generalize the treatment outcomes.
Conflicts of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments/funding statements
This work was financed by the Fundamental Research Funds for the Central Universities (No.31920180028) and the Qinghai Fundamental Scientific and Technological Research Plan (#. 2018-ZJ-730, 2019-SF-134), China. We are highly thankful to Dr. Sobia Noreen (Ph.D., Scholar) for critically reviewing the text of this paper. Furthermore, all the authors of the manuscript also thank and acknowledge their respective Universities and Institutes.
References
- 1.Beck M., Tobin D. The 2019/2020 Novel Corona Virus Outbreak: An International Health Management Perspective. Open Public Health J. 2020;13(1) [Google Scholar]
- 2.Mouffak S., et al. Recent Advances in Management of COVID-19: a review. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2021.112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar D., Trivedi N. Disease-drug and drug-drug interaction in COVID-19: risk and assessment. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2021.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letchumanan V., et al. The rising fear of Wuhan Virus ‘2019-nCoV’. Prog. Microbes Mol. Biol. 2020;3(1) [Google Scholar]
- 5.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T., et al. Transmission dynamics of 2019 novel coronavirus (2019-nCoV) 2020. [Google Scholar]
- 7.Ahmad T., et al. COVID-19: Zoonotic aspects. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berretta A.A., et al. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury A., et al. A secondary approach with conventional medicines and supplements to recuperate current COVID-19 status. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2021.111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An X., et al. The direct evidence and mechanism of traditional Chinese medicine treatment of COVID-19. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2021.111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S., Ashtey B. Investigational treatments for COVID-19. Evaluation. 2020;14(47):19. [Google Scholar]
- 12.Dos Santos W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trafton A. MIT News: Cambridge,; England: 2020. An experimental peptide could block Covid-19, in Massachusetts Institute of Technology, MIT News. [Google Scholar]
- 14.Institute, M. COVID-19 Treatment and Vaccine Tracker. 2020 March 26, 2020,; Available from: https://milkeninstitute.org/sites/default/files/2020–03/Covid19-Tracker-3–36-20-FINAL.pdf.
- 15.Hodgson, J., The pandemic pipeline. nature biotechnology, 2020. [DOI] [PubMed]
- 16.ELIZABETHS. EATON, Clinical trials for coronavirus have begun. First up: approved antivirals, malaria treatments and steroids . 2020, Biocentury.
- 17.Shi, Y., et al., COVID-19 infection: the perspectives on immune responses. 2020, Nature Publishing Group. [DOI] [PMC free article] [PubMed]
- 18.Rodriguez-Morales A.J., et al. History is repeating itself: probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infez Med. 2020;28(1):3–5. [PubMed] [Google Scholar]
- 19.Faust J.S., del Rio C. Assessment of Deaths From COVID-19 and From Seasonal Influenza. JAMA Intern. Med. 2020;180(8):1045–1046. doi: 10.1001/jamainternmed.2020.2306. [DOI] [PubMed] [Google Scholar]
- 20.J. Stebbing, et al., COVID-19: combining antiviral and anti-inflammatory treatments. The Lancet Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed]
- 21.B.S. LaMotte, Covid-19: What we now know about the disease caused by the novel coronavirus. 2020 July 17, 2020; Available from: 〈https://edition.cnn.com/2020/07/17/health/covid-19-coronavirus-need-to-know-now-wellness-trnd/index.html〉.
- 22.Organization W.H. World Health Organization; 2008. The global burden of disease: 2004 update. [Google Scholar]
- 23.Organization W.H. World Health Organization; 2009. World Health Statistics 2009 (Chinese) [Google Scholar]
- 24.Fauci A.S., Morens D.M. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012;366(5):454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 25.Gomes M.F., et al. Assessing the international spreading risk associated with the 2014 West African Ebola outbreak. PLoS Curr. 2014:6. doi: 10.1371/currents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., et al. Influenza Research Database: an integrated bioinformatics resource for influenza virus research. Nucleic Acids Res. 2017;45(D1):D466–D474. doi: 10.1093/nar/gkw857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauer F., et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré syndrome: systematic review. PLoS Med. 2017;14:1. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCloskey B., Heymann D.L. SARS to novel coronavirus–old lessons and new lessons. Epidemiol. Infect. 2020:148. doi: 10.1017/S0950268820000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudas G., et al. MERS-CoV spillover at the camel-human interface. Elife. 2018;7 doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su S., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10(12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan-Yeung M., Xu R. Vol. 8. epidemiology,; SARS: 2003. pp. S9–S14. (Respirology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaki A.M., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 34.Lee J., Chowell G., Jung E. A dynamic compartmental model for the Middle East respiratory syndrome outbreak in the Republic of Korea: a retrospective analysis on control interventions and superspreading events. J. Theor. Biol. 2016;408:118–126. doi: 10.1016/j.jtbi.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.Y., et al. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect. Dis. 2017;17(1):498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.N. Zhu et al., A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 2020. [DOI] [PMC free article] [PubMed]
- 37.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang J., et al. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020;16(3) doi: 10.1371/journal.ppat.1008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, X., et al., Human Coronaviruses: General Features. 2019.
- 40.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 41.King A.M., et al. Virus Taxon. Ninth Rep. Int. Comm. Taxon. Virus. 2012:486–487. [Google Scholar]
- 42.Fehr A.R., Perlman S. Springer,; 2015. Coronaviruses: an overview of their replication and pathogenesis, in Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Der Hoek L., et al. Identification of a new human coronavirus. Nat. Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Der Hoek L., Pyrc K., Berkhout B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol. Rev. 2006;30(5):760–773. doi: 10.1111/j.1574-6976.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vijgen L., et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79(3):1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez S.R., Robinson C.C., Holmes K.V. Detection of four human coronaviruses in respiratory infections in children: A one‐year study in Colorado. J. Med. Virol. 2009;81(9):1597–1604. doi: 10.1002/jmv.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vabret A., et al. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J. Virol. Methods. 2001;97(1–2):59–66. doi: 10.1016/S0166-0934(01)00343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vabret A., et al. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin. Infect. Dis. 2003;36(8):985–989. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vabret A., et al. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin. Infect. Dis. 2006;42(5):634–639. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo P.C., et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo P.C., et al. Coronavirus genomics and bioinformatics analysis. viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005;24(11):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 53.A.E. Gorbalenya , Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv, 2020.
- 54.Wong A.C., et al. Global epidemiology of bat coronaviruses. Viruses. 2019;11(2):174. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drosten C., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 57.(CDC), c.f.d.c. Symptoms of Coronavirus. 2020; Available from: 〈https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html〉.
- 58.C.f .D. Control and Prevention, Remembering SARS: A Deadly Puzzle and the Efforts to Solve It. Atlanta, GA: CDC. Online document at: www. cdc. gov/about/history/sars/feature. htm Accessed March, 2016. 25.
- 59.Snijder E.J., et al. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331(5):991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu D.K., et al. MERS coronaviruses in dromedary camels, Egypt. Emerg. Infect. Dis. 2014;20(6):1049. doi: 10.3201/eid2006.140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Memish Z.A., Zumla A.I., Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N. Engl. J. Med. 2013;369(9):884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 62.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs. 2019;9(1):35. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kindler E., et al. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio. 2013;4(1):e00611–e00612. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zumla A., et al. ‘One Health’approach for turning the tide against the Middle East respiratory syndrome coronavirus and other zoonotic pathogens with epidemic potential. Elsevier,; 2016. Taking forward a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.F. Wu, , S. Zhao , and B. Yu , A new coronavirus associated with human respiratory disease in China.[published on February 03, 2020]. Nature. [DOI] [PMC free article] [PubMed]
- 66.Wu Y., et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020 doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavakoli A., Vahdat K., Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19) Emerg. Infect. Dis. 21st Century ISMJ. 2020;22(6):432–450. [Google Scholar]
- 68.Jiang S., et al. A distinct name is needed for the new coronavirus. Lancet. 2020 doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan J.F.-W., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Team E.E., editor. Vol. 25. sixth public health emergency of international concern. Eurosurveillance; 2020. (Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouadma L., et al. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020:1–4. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu N., et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kandeil A., et al. Middle East respiratory syndrome coronavirus infection in non-camelid domestic mammals. Emerg. Microbes Infect. 2019;8(1):103–108. doi: 10.1080/22221751.2018.1560235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.J. Harcourt et al., Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Novel Coronavirus Disease 2019, United States.
- 76.Ujike M., et al. The contribution of the cytoplasmic retrieval signal of severe acute respiratory syndrome coronavirus to intracellular accumulation of S proteins and incorporation of S protein into virus-like particles. J. Gen. Virol. 2016;97(8):1853. doi: 10.1099/jgv.0.000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabenau H., et al. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194(1–2):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamarre A., Talbot P.J. Effect of pH and temperature on the infectivity of human coronavirus 229E. Can. J. Microbiol. 1989;35(10):972–974. doi: 10.1139/m89-160. [DOI] [PubMed] [Google Scholar]
- 79.van Doremalen N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.M.L.D. Holshue et al., FirstCaseof2019NovelCoronavirusintheUnitedStates. NEnglJ Med2020. 10.1056/NEJMoa2001191, 2020.
- 81.Nguyen K.V. Problems associated with antiviral drugs and vaccines development for COVID-19: approach to intervention using expression vectors via GPI anchor. Nucleosides, Nucleotides Nucleic Acids. 2021:1–32. doi: 10.1080/15257770.2021.1914851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wise, J., Covid-19: New coronavirus variant is identified in UK. 2020, British Medical Journal Publishing Group. [DOI] [PubMed]
- 83.National Center for Immunization and Respiratory Diseases (NCIRD), D.o.V.D. SARS-CoV-2 Variant Classifications and Definitions. 2021 Sept. 7, 2021 sep.11, 2021]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- 84.Khateeb J., Li Y., Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care. 2021;25(1):1–8. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arif T.B. The 501. V2 and B. 1.1. 7 variants of coronavirus disease 2019 (COVID-19): A new time-bomb in the making? Infect. Control Hosp. Epidemiol. 2021:1–2. doi: 10.1017/ice.2020.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang H., et al. Nature Publishing Group,; 2021. SARS-CoV-2 N501Y variants of concern and their potential transmission by mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Organization W.H. WHO,; 2021. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. [Google Scholar]
- 88.Callaway E. Heavily mutated Omicron variant puts scientists on alert, in Nature. Nature. 2021 doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 89.Vaidyanathan G. Coronavirus variants are spreading in India—what scientists know so far. Nature. 2021;593(7859):321–322. doi: 10.1038/d41586-021-01274-7. [DOI] [PubMed] [Google Scholar]
- 90.Wan Y., et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94:7. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spiegel M., et al. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J. Gen. Virol. 2006;87(7):1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- 92.Law H.K., et al. Chemokine up-regulation in sars-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lau Y.L., Peiris J.M. Pathogenesis of severe acute respiratory syndrome. Curr. Opin. Immunol. 2005;17(4):404–410. doi: 10.1016/j.coi.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo Y.-R., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.organization, W.h. Coronavirus. 2020 [cited 2020 22 March]; Available from: 〈https://www.who.int/health-topics/coronavirus#tab=tab_1〉.
- 96.Rasmussen S.A., et al. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y., et al. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J. Med. Virol. 2020 doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hassan S.A., et al. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cures. 2010;12:3. doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cascella M., et al. StatPearls Publishing.,; 2020. Features, Evaluation and Treatment Coronavirus (COVID-19), in StatPearls [Internet]. [PubMed] [Google Scholar]
- 100.Yang M., et al. Hematological findings in SARS patients and possible mechanisms. Int. J. Mol. Med. 2004;14(2):311–315. [PubMed] [Google Scholar]
- 101.Fan C., et al. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. medRxiv. 2020 [Google Scholar]
- 102.Shen K., et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet. Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong C., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Y., et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv. 2020:20023242. 2020.02.18. [Google Scholar]
- 107.Diao B., et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020 doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guan, W.-j., et al., Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv, 2020.
- 111.Lee, A.Y., et al. COVID-19 and Coagulopathy. 2020 [cited 2020 11 April]; Available from: https://www.hematology.org/covid-19/covid-19-and-coagulopathy.
- 112.Zhang, Y., et al., Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. 2020. [DOI] [PMC free article] [PubMed]
- 113.van Vuren E.J., et al. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed. Pharmacother. 2021 doi: 10.1016/j.biopha.2020.111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naveed M., Zhou Q.-G., Han F. Cerebrovascular inflammation: A critical trigger for neurovascular injury? Neurochem. Int. 2019;126:165–177. doi: 10.1016/j.neuint.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 115.Marshall M. How COVID-19 can damage the brain. Nature. 2021:342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- 116.Paterson R.W., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varatharaj A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wan D., et al. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct. Target. Ther. 2021;6(1):406. doi: 10.1038/s41392-021-00818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barlati S., Nibbio G., Vita A. Schizophrenia during the COVID-19 pandemic. Curr. Opin. Psychiatry. 2021;34(3):203–210. doi: 10.1097/YCO.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perl L., et al. Effects of the COVID19 Pandemic on Transgender and Gender Non-Conforming Adolescents’ Mental Health. Psychiatry Res. 2021 doi: 10.1016/j.psychres.2021.114042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mendes A., et al. Delirium in Older Patients With COVID-19: Prevalence, Risk Factors, and Clinical Relevance. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2021 doi: 10.1093/gerona/glab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuroda N. Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav. 2021 doi: 10.1016/j.yebeh.2021.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al Omari O., et al. Prevalence and predictors of depression, anxiety, and stress among youth at the time of COVID-19: an online cross-sectional multicountry study. Depress Res. Treat. 2020 doi: 10.1155/2020/8887727. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Islam M.A., et al. Depression and anxiety among university students during the COVID-19 pandemic in Bangladesh: A web-based cross-sectional survey. PloS One. 2020;15(8) doi: 10.1371/journal.pone.0238162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khatib M.Y., et al. Managing a patient with bipolar disorder associated with COVID‐19: A case report from Qatar. Clin. Case Rep. 2021;9(4):2285–2288. doi: 10.1002/ccr3.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li, J., et al., Resilience of Alzheimer’s Disease to COVID-19. Journal of Alzheimer's Disease, 2020 (Preprint): p. 1–7. [DOI] [PMC free article] [PubMed]
- 127.Naughton S.X., Raval U., Pasinetti G.M. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J. Alzheimer’S. Dis. 2020;76(1):21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fearon, C. and A. Fasano, Parkinson’s disease and the COVID-19 pandemic. Journal of Parkinson's Disease, 2021(Preprint): p. 1–14. [DOI] [PMC free article] [PubMed]
- 129.Acenowr C.P., Coles M.E. OCD during COVID-19: Understanding clinical and non-clinical anxiety in the community. Psychiatry Res. 2021;300 doi: 10.1016/j.psychres.2021.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen H., Du Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints. 2020 [Google Scholar]
- 131.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Saghir S.A., et al. Chloroquine and hydroxychloroquine for the prevention and treatment of COVID-19: A fiction, hope or hype? An updated review. Ther. Clin. risk Manag. 2021;17:371. doi: 10.2147/TCRM.S301817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corman V.M., et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Devaux C.A., et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 137.Colson P., Rolain J.-M., Raoult D. Chloroquine for the 2019 novel coronavirus. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biot C., et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49(9):2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 140.Winsor, M. and E. Shapiro. Coronavirus live updates: FDA gives anti-malaria drugs emergency approval to treat COVID-19. 2020 [cited 2020 30 March]; Available from: https://abcnews.go.com/Health/coronavirus-live-updates-fda-anti-malaria-drugs-emergency/story?id=69867398.
- 141.Wang, F.-S. Safety and Efficiency of Mesenchymal Stem Cell in Treating Pneumonia Patients Infected With 2019 Novel Coronavirus. 2020 February 26, 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04252118?show_xprt=Y.
- 142.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Bioscience trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 143.Administration., U.F.a.D., Remdesivir EUA Letter of Authorization. 2020.
- 144.Lim J., et al. Case of the index patient who caused tertiary transmission of Coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35(6) doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sheahan T.P., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic. epidemic bat Corona bioRxiv. 2020 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discoveries & Therapeutics. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 147.Cai, Q., et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering, 2020. [DOI] [PMC free article] [PubMed]
- 148.Reed, B.J., Molnupiravir: First pill to treat Covid gets approval in UK, in BBC News 2021, BBC News Services.
- 149.Robbins R. The New York Times,; 2021. Pfizer Says Its Antiviral Pill Is Highly Effective in Treating Covid, in The New York Times. [Google Scholar]
- 150.McCreary E.K., Pogue J.M. Oxford University Press,; US: 2020. Coronavirus disease 2019 treatment: a review of early and emerging options. in Open Forum Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chu C., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noreen S., Maqbool I., Madni A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2021 doi: 10.1016/j.ejphar.2021.173854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cain D.W., Cidlowski J.A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat. Rev. Immunol. 2020:1–2. doi: 10.1038/s41577-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yong, S.J. Biology of Dexamethasone: The First Lifesaving Drug for Covid-19. 2020 [cited 2020 2July]; Available from: https://medium.com/@shinjieyong/biology-of-dexamethasone-the-first-lifesaving-drug-for-covid-19–357ed9daaf7a.
- 156.Soy M., et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheuma. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pashaei Y. Drug repurposing of selective serotonin reuptake inhibitors: Could these drugs help fight COVID-19 and save lives? J. Clin. Neurosci. 2021;88:163–172. doi: 10.1016/j.jocn.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bilbul M., et al. Psychopharmacology of COVID-19. Psychosomatics. 2020;61(5):411–427. doi: 10.1016/j.psym.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sukhatme V.P., et al. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front. Pharmacol. 2021;12:763. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sidik, S.M., Common antidepressant slashes risk of COVID death, study says 2019, Nature. [DOI] [PubMed]
- 161.Reis, G., et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. The Lancet Global Health, 2021. [DOI] [PMC free article] [PubMed]
- 162.Zimniak M., et al. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021;11(1):1–5. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Le Corre P., Loas G. , Repurposing functional inhibitors of acid sphingomyelinase (fiasmas): an opportunity against SARS‐CoV‐2 infection? J. Clin. Pharm. Ther. 2021 doi: 10.1111/jcpt.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hoertel N., et al. Association between benzodiazepine receptor agonist use and increased mortality among patients hospitalized for COVID-19: results from an observational study. medRxiv. 2021 [Google Scholar]
- 165.Sun F., et al. Capivasertib restricts SARS-CoV-2 cellular entry: a potential clinical application for COVID-19. Int. J. Biol. Sci. 2021;17(9):2348. doi: 10.7150/ijbs.57810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rajkumar R.P. Lithium as a candidate treatment for COVID‐19: promises and pitfalls. Drug Dev. Res. 2020;81(7):782–785. doi: 10.1002/ddr.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dragoi A.M., et al. Clozapine: an updated overview of pharmacogenetic biomarkers, risks, and safety—particularities in the context of COVID-19. Brain Sci. 2020;10(11):840. doi: 10.3390/brainsci10110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gouglas D., et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Health. 2018;6(12):e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dolgin E. How protein-based COVID vaccines could change the pandemic. Nature. 2021:359–360. doi: 10.1038/d41586-021-03025-0. [DOI] [PubMed] [Google Scholar]
- 171.Silveira M.M., Moreira G.M.S.G., Mendonça M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ren J., Zhang A.-H., Wang X.-J. Traditional Chinese Medicine for COVID-19 Treatment. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsai K.-C., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang Y., et al. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li S.-y, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng P.W., et al. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ryu Y.B., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lau K.-M., et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luo H., et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020:1–8. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cinatl J., et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Han, Y., et al., Application of integrative medicine protocols on treatment of coronavirus disease 2019. Chi Tradit Herbal Drugs. 1, 2020. 5.
- 182.Ng A.W., et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017;9(412):eaan6446. doi: 10.1126/scitranslmed.aan6446. [DOI] [PubMed] [Google Scholar]
- 183.Zeng Z.P., Jiang J.G. Analysis of the adverse reactions induced by natural product‐derived drugs. Br. J. Pharmacol. 2010;159(7):1374–1391. doi: 10.1111/j.1476-5381.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wikipedia. RECOVERY Trial. 2020 17 June 2020,; Available from: 〈https://en.m.wikipedia.org/wiki/RECOVERY_Trial〉.
- 185.Margaret Harris, D.B., WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19, in WHO News release. 2020, WHO.
- 186.Bell, J. Majority of Covid-19 clinical trials showing ‘encouraging’ signs, says analyst. 2020 15 MAY 2020; Available from: 〈https://www.clinicaltrialsarena.com/analysis/covid-19-clinical-trials-results-2/〉.
- 187.Matthew Herper, E.R., Data show panic and disorganization dominate the study of Covid-19 drugs , in STAT News. 2020.
- 188.News, B. Hopes dashed as coronavirus drug remdesivir 'fails first trial'. 2020 23 April 2020; Available from: https://www.〈bbc.com/news/world-52406261〉.
- 189.Pene F., et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003;37(7):929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hendley J.O., Fishburne H.B., Gwaltney J.M., Jr. Coronavirus infections in working adults: eight-year study with 229 E and OC 43. Am. Rev. Respir. Dis. 1972;105(5):805–811. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 191.Cabeça T.K., Granato C., Bellei N. Epidemiological and clinical features of human coronavirus infections among different subsets of patients. Influenza Other Respir. Virus. 2013;7(6):1040–1047. doi: 10.1111/irv.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dijkman R., et al. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J. Virol. 2013;87(11):6081–6090. doi: 10.1128/JVI.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chiu S.S., et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 2005;40(12):1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bastien N., et al. Human coronavirus NL63 infection in Canada. The. J. Infect. Dis. 2005;191(4):503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van der Hoek, L., et al., Croup is associated with the novel coronavirus NL63. PLoS medicine, 2005. 2(8). [DOI] [PMC free article] [PubMed]
- 196.Woo P.C., et al. More and more coronaviruses: human coronavirus HKU1. Viruses. 2009;1(1):57–71. doi: 10.3390/v1010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kanwar A., Selvaraju S., Esper F. Open forum infectious diseases. Oxford University Press,; 2017. Human coronavirus-HKU1 infection among adults in Cleveland, Ohio. in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sørensen M.D., et al. Severe Acute Respiratory Syndrome (SARS) Development of Diagnostics and Antivirals. Ann. N. Y. Acad. Sci. 2006;1067(1):500–505. doi: 10.1196/annals.1354.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Organization W.H. WHO,; Geneva: 2017. WHO MERS-CoV global summary and assessment of risk. [Google Scholar]
- 200.Group W.M.-C.R. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013:5. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Arabi Y.M., et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014;160(6):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 202.Group W.M.-C.R. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;12:5. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lai C.-C., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sohrabi C., et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carlos W.G., et al. Novel wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. care Med. 2020;201(4):P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 206.Yun-mi, K., Takeda begins developing COVID-19 plasma therapy TAK-888, in Korea Biomedical Review(KBR) 2020.
- 207.(EPR), H.B. Influenza antiviral Avigan® (favipiravir) to enter Phase III trials in COVID-19 patients. 2020 3 April 2020; Available from: 〈https://www.europeanpharmaceuticalreview.com/news/116308/the-influenza-antiviral-avigan-favipiravir-to-enter-phase-iii-trials-in-covid-19-patients/〉.
- 208.Davoodi L., et al. Febuxostat therapy in outpatients with suspected COVID‐19: A clinical trial. Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Medha. Gout Drug Febuxostat Useful For Treatment Of COVID-19. 2020 16 July 2020; Available from: 〈https://medicaldialogues.in/medicine/news/gout-drug-febuxostat-useful-for-treatment-of-covid-19–67701?infinitescroll=1〉.
- 210.Saha, D.S. Can Voxelotor Help SCD Patients Tide Over Blood Shortage In COVID 19 Season? 2020 7 Aug 2020; Available from: 〈https://medicaldialogues.in/medicine/news/voxelotor-may-help-scd-patients-tide-over-blood-shortage-in-covid-19-season-68349〉.
- 211.Devereux, L. Vir Biotechnology Announces Intent to Collaborate with Biogen on Manufacturing of Antibodies to Potentially Treat COVID-19. 2020; Available from: 〈https://www.biospace.com/article/releases/vir-biotechnology-announces-intent-to-collaborate-with-biogen-on-manufacturing-of-antibodies-to-potentially-treat-covid-19/〉.
- 212.Releases G.P. Gilead Sciences Initiates Two Phase 3 Studies of Investigational Antiviral Remdesivir for the Treatment of COVID-19. Gilead Sci., Inc. 2020 [Google Scholar]
- 213.Newswire C.P. Bloomberg Software company: NEW BRUNSWICK,; N.J, USA: 2020. Johnson & Johnson Launches Multi-Pronged Response to Coronavirus Global Public Health Threat. [Google Scholar]
- 214.HERPER, M., Regeneron says potential Covid-19 drugs could start human tests by early summer, in Biotech. 2020.
- 215.TARRYTOWN, N.Y.a.C. REGENERON AND SANOFI BEGIN GLOBAL KEVZARA® (SARILUMAB) CLINICAL TRIAL PROGRAM IN PATIENTS WITH SEVERE COVID-19. 2020 March 16, 2020 Available from: 〈https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-begin-global-kevzarar-sarilumab-clinical〉.
- 216.RELEASE, F.N., FDA Approves First Treatment for COVID-19, F.O.o.M. Affairs, Editor. 2021, FDA US Food and Drug Administration.
- 217.Ltd, F.H.-L.R. Roche initiates Phase III clinical trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 pneumonia. 2020; Available from: 〈https://www.roche.com/media/releases/med-cor-2020–03-19.htm〉.
- 218.Hospital, P.U.M.C. Glucocorticoid Therapy for Critically Ill Patients With Severe Acute Respiratory Infections Caused by Noval Coronovirus 2019-nCoV: a Prospective, Randomized Controlled Trial. 2020 February 13, 2020; Available from: https://www.clinicaltrials.gov/ct2/show/NCT04244591.
- 219.Taisheng Li, W.C. A Randomized, Open-label, Controlled, Single-center Study to Evaluate the Efficacy of Intravenous Immunoglobulin Therapy in Patients With Severe 2019- nCoV Pneumonia. 2020 February 7, 2020; Available from: 〈https://clinicaltrials.gov/ct2/show/NCT04261426〉.
- 220.Wang C., et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Qu J.-m. A Pilot Clinical Study on Aerosol Inhalation of the Exosomes Derived From Allogenic Adipose Mesenchymal Stem Cells in the Treatment of Severe Patients With Novel. Corona Pneumonia. 2020 〈https://clinicaltrials.gov/ct2/show/NCT04276987?show_xprt=Y〉 2020 February 25. (Available from) [Google Scholar]
- 222.Florentino de Araujo Cardoso Filho, L.F. Exploratory Clinical Study to Assess the Efficacy of NestCell® Mesenchymal Stem Cell to Treat Patients With Severe COVID-19 Pneumonia. 2020 March 23, 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04315987?show_xprt=Y.
- 223.Zhou, X. Clinical Study of Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells. 2020 March 10, 2020; Available from: 〈https://clinicaltrials.gov/ct2/show/NCT04302519?show_xprt=Y〉.
- 224.University, T.S.H.o.N.M. Washed Microbiota Transplantation for Patients With 2019-nCoV Infection: a Randomized, Double-blind, Placebo-controlled Study. 2020; Available from: https://www.ichgcp.net/clinical-trials-registry/NCT04251767.
- 225.Harrison, C., Coronavirus puts drug repurposing on the fast track. Nature Biotechnology, 2020. [DOI] [PubMed]
- 226.Bin, P. Safety and efficacy of umbilical cord blood mononuclear cells conditioned medium in the treatment of severe and critically novel coronavirus pneumonia (COVID-19): a randomized controlled trial 2020 2020–02-12 Available from: http://www.chictr.org.cn/showprojen.aspx?proj=49062.
- 227.Li, Z.L.a.Q. A Singlecenter, Randomized, Open Lable, Intervention Controlled Clinical Study on the Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cells for the Treatment of Severe Viral Pneumonia. 2020 February 25, 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04282928?show_xprt=Y.
- 228.Yang Jin. Clinical Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Novel Coronavirus Severe Pneumonia. 2020 [cited 2020 30–03-2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04273646.
- 229.writers, H.I.s. Tiziana Life Sciences Accelerate Development of Potential Covid-19 Drug. 2020 14 Mar 2020; Available from: https://www.hospimedica.com/coronavirus-crisis/articles/294781127/tiziana-life-sciences-accelerate-development-of-potential-covid-19-drug.html.
- 230.Liu, A. AbbVie's HIV drug Kaletra stumbles in COVID-19 trial, but one analyst begs to differ. 2020 Mar 19, 2020 Available from: https://www.fiercepharma.com/pharma-asia/does-abbvie-s-hiv-drug-kaletra-also-works-covid-19-maybe-not-nejm-study-finds.
- 231.Cao B., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Inc C. Treatment with CytoDyn's Leronlimab Indicates Significant Trend Toward Immunological Restoration in Severely Ill COVID-19 Patients. Cytodyn. 2020 [Google Scholar]
- 233.Biologics, A. APEIRON Biologics Initiates Phase II Clinical Trial of APN01 for Treatment of COVID-19. 2020 April 02, 2020; Available from: https://www.globenewswire.com/news-release/2020/04/02/2010465/0/en/APEIRON-Biologics-Initiates-Phase-II-Clinical-Trial-of-APN01-for-Treatment-of-COVID-19.html.
- 234.Pharmaceuticals, C.T.-A. Novartis and Incyte to trial Jakavi for Covid-19. 2020 3 APRIL 2020; Available from: https://www.clinicaltrialsarena.com/news/novartis-incyte-jakavi-covid-19/.
- 235.Pharmaceuticals, C.T.-A. DCRI to trial hydroxychloroquine to prevent Covid-19. 2020 2 APRIL 2020; Available from: https://www.clinicaltrialsarena.com/news/dcri-hydroxychloroquine-covid-19/.
- 236.Registry, C.c.T. Evaluation of the effect of taking Newgen beta-gluten probiotic composite powder to nutrition intervention of patients with novel coronavirus pneumonia (COVID-19). 2020 [cited 2020 28–07-2020]; Available from: http://www.chictr.org.cn/showprojen.aspx?proj=50462.
- 237.Foerch C., et al. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smart patients, I. Efficacy of Fingolimod in the Treatment of New Coronavirus Pneumonia (COVID-19). 2020 [cited 2020; Available from: https://www.smartpatients.com/trials/NCT04280588.
- 239.medicine, N.U.N.A.o. Soliris to Stop Immune Mediated Death In Covid 19 Infected Patients. A Trial of Distal Complement Inhibition. 2020 March 30, 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04288713.
- 240.Caly L., et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen H., et al. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine. 2020;99:48. doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Khalil A., Kamar A., Nemer G. Thalidomide-revisited: are COVID-19 patients going to be the latest victims of yet another theoretical drug-repurposing? Front. Immunol. 2020;11:1248. doi: 10.3389/fimmu.2020.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kováč I.M.J.Č.G., Hudecová M.P.L. Triazavirin might be the new hope to fight Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Ceska a Slov. Farm.: Cas. Ceske Farm. Spol. a Slov. Farm. Spol. 2021;70(1):18–25. [PubMed] [Google Scholar]
- 245.Marconi V.C., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. The Lancet. Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Beshay S., et al. Rapid Clinical Recovery from Critical COVID-19 Pneumonia with Vasoactive Intestinal Peptide Treatment. The. J. Heart Lung Transplant. 2021;40(4):S501. [Google Scholar]
- 247.Samaee H., et al. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int. Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cosdon, N. Sotrovimab Reduces Risk of Disease Progression in High-Risk Adults with Symptomatic COVID-19. 2021 November 1, 2021; Available from: https://www.contagionlive.com/view/sotrovimab-reduces-risk-of-disease-progression-in-high-risk-adults-with-symptomatic-covid-19.
- 249.Holman W., et al. Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2. Trials. 2021;22(1):1–7. doi: 10.1186/s13063-021-05538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mahase E. British Medical Journal Publishing Group.,; 2020. Covid-19: UK approves Pfizer and BioNTech vaccine with rollout due to start next week. [DOI] [PubMed] [Google Scholar]
- 251.Mahase E. Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ: Br. Med. J. (Online) 2020:371. [Google Scholar]
- 252.Burki T.K. The Russian vaccine for COVID-19. Lancet Respir. Med. 2020;8(11):e85–e86. doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Biehn B. Vaxart Announces Initiation of Coronavirus Vaccine Program, in Vaxart. Inc. (VXRT) 2020 [Google Scholar]
- 254.Biehn B. GLOBE NEWSWIRE-Vaxart, Inc,; 2020. Norovirus Gastroenteritis Costs an Estimated $10.6 Billion Each Year in the United States. [Google Scholar]
- 255.SANDI WONG. COVID-19 update: Sanofi enters vaccine race with BARDA collaboration; plus J&J-BARDA expansion, clinical and diagnostic advances, and more . 2020; Available from: https://www.biocentury.com/article/304464/covid-19-update-sanofi-enters-vaccine-race-with-barda-collaboration-plus-j-amp-j-barda-expansion-clinical-and-diagnostic-advances-and-more.
- 256.Novavax. Novavax Advances Development of Novel COVID-19 Vaccine. 2020; Available from: https://ir.novavax.com/news-releases/news-release-details/novavax-advances-development-novel-covid-19-vaccine.
- 257.Taylor, N.P. GSK makes adjuvant available to coronavirus vaccine project. 2020; Available from: https://www.fiercebiotech.com/biotech/gsk-makes-adjuvant-available-to-coronavirus-vaccine-project.
- 258.Taylor, N.P. J&J allies with BARDA to accelerate coronavirus vaccine program. 2020; Available from: https://www.fiercebiotech.com/biotech/j-j-allies-barda-to-accelerate-coronavirus-vaccine-program.
- 259.Waldman D. Heat Biologics and University of Miami Developing Proprietary COVID-19 Diagnostic Test Under Collaborative Research Agreement. Heat. Biol., Inc. 2020 [Google Scholar]
- 260.Robert Carlson, M. INO-4800 DNA Coronavirus Vaccine. 2020 03/28/2020 Available from: https://www.precisionvaccinations.com/vaccines/ino-4800-dna-coronavirus-vaccine.
- 261.Routh J. National Institute of Allergy and Infectious Diseases,; Maryland, US State: 2020. NIH clinical trial of investigational vaccine for COVID-19 begins, in NIH News in Health. [Google Scholar]
- 262.DISEASES, N.N.I.O.A.A.I. COVID-19 Vaccine Phase 1 Trial Results: Safe, Generates High Levels of Neutralizing Antibodies. 2020 AUGUST 6, 2020; Available from: https://scitechdaily.com/covid-19-vaccine-phase-1-trial-results-safe-generates-high-levels-of-neutralizing-antibodies/.
- 263.Westlund, R. Immunology researchers at the University of Miami Miller School of Medicine are collaborating with North Carolina-based Heat Biologics, Inc. to develop a vaccine for the novel coronavirus COVID-19. 2020 March 18, 2020; Available from: https://physician-news.umiamihealth.org/miller-school-and-heat-biologics-to-collaborate-on-covid-19-vaccine/.
- 264.Terry M. As Coronavirus Threat Continues. Biopharma Acad. Gear Test. Dev. Vaccin. Ther., Biospace. 2020 [Google Scholar]
- 265.Corporation D.T. Dynavax and Clover Biopharmaceuticals Announce Research Collaboration to Evaluate Coronavirus (COVID-19) Vaccine Candidate with CpG 1018 Adjuvant. Dynavax. 2020 [Google Scholar]
- 266.Corp T.P.H. Tonix Pharmaceuticals Holding Corp,; NEW YORK: 2020. Tonix Pharmaceuticals Reports Fourth Quarter and Full Year 2019 Financial Results and Operational Highlights. [Google Scholar]
- 267.MAAYAN JAFFE-HOFFMAN, A.R. , Israeli scientists: Coronavirus vaccine to be tested on humans by June 1, in The Jerusalem Post. 2020, The Jerusalem Post.
- 268.Ray, A. India's first COVID-19 vaccine: Covaxin human trial starts well. 10 updates. 2020 17 Jul 2020; Available from: https://www.livemint.com/news/india/india-s-first-covid-19-vaccine-covaxin-human-trial-starts-well-key-updates-11594977657239.html.
- 269.Writer, P.S. AZD1222 delivers strong immune response in phase I/II Covid-19 vaccine trial. 2020 20 July 2020; Available from: 〈https://www.pharmaceutical-business-review.com/news/azd1222-astrazeneca-oxford-trial/〉.
- 270.Agency, A. UK coronavirus antibody test found to be a success. 2020 July 18, 2020; Available from: 〈https://tribune.com.pk/story/2255523/uk-coronavirus-antibody-test-found-to-be-a-success〉.