Abstract
Progress in the field of soft devices—i.e., haptics, robotics, and human-machine interfaces (HRHMIs)—has its basis in the science of polymeric materials and chemical synthesis. However, in examining the relevant literature, we find that most developments have been enabled by off-the-shelf materials used either alone or as components of physical blends and composites. In this Progress Report, we take the position that a greater awareness of the capabilities of synthetic chemistry will accelerate the capabilities of HRHMIs. Conversely, an awareness of the applications sought by engineers working in this area may spark the development of new molecular designs and synthetic methodologies by chemists. We highlight several applications of active, stimuli-responsive polymers, which have demonstrated or shown potential use in HRHMIs. These materials share the fact that they are products of state-of-the-art synthetic techniques. The Progress Report is thus organized by the chemistry by which the materials were synthesized, including controlled radical polymerization, metal-mediated cross-coupling polymerization, ring-opening polymerization, various strategies for crosslinking, and hybrid approaches. These methods can afford polymers with multiple properties (i.e. conductivity, stimuli-responsiveness, self-healing and degradable abilities, biocompatibility, adhesiveness, and mechanical robustness) that are of great interest to scientists and engineers concerned with soft devices for human interaction.
1. Introduction
Human interaction with the physical world is mediated in nearly every natural and artificial context by organic structures. Natural environments, including those that appear abiotic, such as desert landscapes and the surface of the ocean, teem with living organisms, along with their organic metabolites and byproducts of degradation. Most indoor surfaces are composed of or coated with polymers and other organic media. Even uncoated surfaces like glass and metal are usually covered with adventitiously adsorbed molecules from the atmosphere. Engineered organics—particularly in the form of polymers—are ubiquitous as structural materials and surface coatings. Moreover, they are critical components of electronic devices (e.g., in LCDs[1,2] and OLEDs[3,4] and as the smudge-resistant coatings of smartphone glass) and as materials used in the key steps of their fabrication (e.g., photoresists used in semiconductor manufacturing).[5,6] It is not hyperbole to say that modern life is enabled by innovation in molecular design.[7] It thus stands to reason that any future technology intended to interface with human users would be built on a foundation laid by molecular—and especially polymer—engineering.
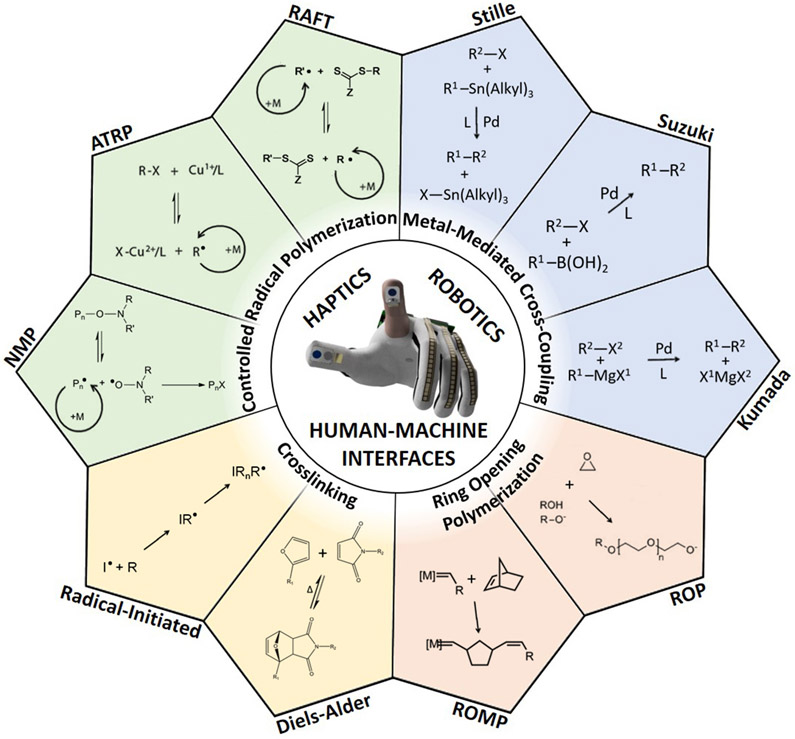
At the core of all innovation that has its roots in molecular engineering is the science of chemical synthesis (Figure 1).[8,9] That is, the covalent assembly of complex polymeric and molecular architectures from simple starting materials. The state of the art in chemical synthesis comes as the result of over a century of work by scientists investigating new reactivity, reagents, and spectroscopic techniques to determine the structures of what has been synthesized. To an outsider, the daily practice of a synthetic chemist looks very different from that of, for example, a mechanical engineer. Reflux condensers, stir plates, Schlenk lines, silica gel columns, test tube racks, and chromatography plates litter the fume hood of a chemist, who spends most of the day in head-to-shin protective equipment. The jargon is also unique. “Chemical shift,” “nucleophile,” “enantiomeric excess”—along with countless named reactions—are heard with far more frequency than “force,” “voltage,” or “potential energy.” Moreover, common scientific ideas have different connotations. For example, the value of the “dielectric constant” of a medium has different practical relevance to chemists (usually used in reference to solubility) and engineers (usually used in reference to capacitance, or to the facility of charge-carrier transport in semiconductors).[10,11]
Figure 1.
Polymer chemistry for haptics, soft robotics, and human-machine interfaces.
There are some areas of technology in which a closer association between fields can generate advancements that would be impossible without cross pollination. However, differences in methods and language may serve as barriers to communication and collaboration. These barriers are especially prominent on university campuses, where chemistry and engineering are usually located in different academic divisions.[12] In this Progress Report, we argue that the space of advanced functional materials that could be synthesized using the tools of organic and organometallic chemistry is underdeveloped in many of the most exciting, up-and-coming areas of experimental technology. These technologies include haptics, robotics (especially soft robotics), and mechanical forms of human-machine interfaces (in this paper, we refer to all of these technologies collectively as “HRHMIs”). Common to these applications is the need for materials exhibiting actuation, stimuli-response, electrical conductivity, biocompatibility, various dielectric and mechanical properties, and repeatable behavior under cyclic loading or stimuli.
As haptics and robotics have their intellectual roots in mechanical and electrical engineering, the components needed to construct an actuator are usually designed from off-the-shelf materials with well-known properties. Silicone elastomers, epoxy resins, thermoplastic polyurethane, and acrylic polymers are ubiquitous.[13-16] Such materials, however, are characterized by a limited range of function. To achieve new sensations in haptics, improved performance in soft robotics, and new modes of human-machine interaction requires materials with a much broader range of responsiveness than is currently available. While some capabilities can be produced by physical blending of common materials (e.g., achieving conductivity in an elastomer by mixing it with metallic particles), to affect the type of transformative effects needed to advance these technologies requires the design of new materials at the molecular level. Moreover, the types of chemical structures that can produce the range of responses we seek often cannot be obtained using simple reactions performed routinely in engineering laboratories—e.g., polyesterification, silanization, and crosslinking reactions performed using kits (as in silicone rubber and two-part epoxy). To achieve real structural diversity requires the full range of state-of-the-art techniques now available to chemists. These methods include controlled radical polymerization, metal-mediated cross-coupling polymerization, ring-opening polymerization, and approaches which combine such methodologies.
The value of an approach which marries synthetic chemistry with one or more engineering disciplines is apparent when one considers the enormous advances made by the fields of OLEDs and semiconductor manufacturing. In the field of OLEDs—now a multi-billion dollar industry—molecular engineering has produced emitters with increasingly greater efficiencies and stabilities, and emissive spectra that are more accurately tuned for optimal visual perception.[3] In semiconductor manufacturing—which ultimately supports the entirety of entertainment, consumer electronics, and information technology—it is the chemistry of photoresists, along with light sources, and imaging optics that have enabled the industry to keep pace with Moore’s Law.[17]
In this Progress Report, we have identified several types of polymeric materials for use or potential use in haptics, robotics, and mechanical human-machine interfaces (by specifying mechanical properties, e.g., actuation, variable friction, and extreme elasticity, we are excluding materials used primarily for neural interfacing and other audiovisual forms of human-machine interfacing which are well established).[14,18] Our focus on polymers is not meant to exclude interesting properties of advanced materials based on small molecules—e.g., liquid crystals or molecular semiconductors of the type found in OLEDs—but rather to retain focus. Polymers have the advantage over small molecules of mechanical robustness. That is, in the context of a human-machine interface, a polymer is less likely to “rub off” on a user’s hand. Moreover, the polymers we have chosen to cover have a range of electrical, mechanical, and chemical functionality. What unites the materials is not their structures nor their potential applications, but rather the sophistication of the molecular design and the chemistry that enabled it. We focus in particular on polymerization methodologies which may not be well known outside of the community of synthetic polymer chemistry. Such a focus precludes detailed analysis of the figures-of-merit needed for any particular application: e.g., response time, conductivity, stopping force, and other parameters. Nevertheless, our hope is that this Progress Report serves as a bridge to researchers in both synthetic chemistry and engineering, who may not have knowledge of each other’s capabilities nor challenges.
2. Synthetic Techniques
Techniques of synthetic polymer chemistry occupy a range of sophistication, from those requiring specialized equipment, glassware, physical infrastructure, and personal protective equipment, to those which can be performed at home, i.e., two-part epoxy resins and other construction adhesives. As a general rule, obtaining function of the type needed for stimuli-response requires control over the molecular structure, which requires control over the reaction conditions. Thus, some degree of sophistication and often substantial experience is required to design the synthetic route and execute the steps required in the laboratory. Furthermore, only a select few types of polymerization methodologies can afford the control over molecular structure needed to produce the desired structure and thus response to stimulus.
There are many ways to classify methods of polymerization, but the most common classification bins them according to their fundamental kinetic mechanism. The broadest two categorizations which together encompass all polymerization methodologies are those which proceed by step-growth and chain-growth mechanisms. In a step-growth polymerization, each monomer in the reaction mixture has two or more reactive groups, which can react with any other monomer and any other polymer chain in the reaction mixture.[19] Step-growth polymerizations are typically equilibrium reactions, so the final polymeric product is a result of balancing the forward versus the reverse reactions (i.e., removal of a byproduct of condensation, e.g., H2O, increases the molecular weight of the product). An important characteristic of the product of a step-growth polymerization is a relatively large distribution in the degree of polymerization (i.e., number of monomer residues in each chain) of each polymer chain at the conclusion of the reaction. That is, the theoretical dispersity of a linear polymer upon consumption of all reactive groups is 2, as predicted by Carothers[20] (a dispersity of 1 means that the polymer chains all have exactly the same length). Additionally, the reaction must go to near-completion in order to achieve large degrees of polymerization, as the initial stages of reaction consist of monomers and short-chain oligomers reacting with each other. The control in distribution of molecular weight afforded by step-growth is thus inferior to that provided by other methodologies, but it is the only way that produce certain types of materials, including most semiconducting polymers that have a low bandgap and high charge-carrier mobility.[21] Common polymers formed through step-growth polymerization include polyesters, nylons, polyurethanes, polysiloxanes and polycarbonates.
In contrast to step-growth mechanisms, in which any monomer or polymer chain can react with any other monomer or chain in the mixture, the reactivity in chain-growth mechanism is restricted. Unlike step-growth chemistry, chain growth polymerization requires an initiation step; after initiation, a monomer may only react with the growing end of a polymer chain, termed the propagating center. Chain-growth mechanisms can be further subdivided into uncontrolled and controlled mechanisms. The quintessential uncontrolled chain-growth mechanism is free-radical polymerization, the process by which the vast majority of polymers are manufactured, in terms of tonnage, worldwide. Common examples of commodity polymers produced by free-radical polymerization include polyethylene,[22] polystyrene,[23] polyvinyl chloride,[24] and polymethyl methacrylate.[25] In addition to mechanisms mediated by a radical process, uncontrolled chain-growth polymerization can also be initiated and propagated using cationic species, coordination of metals, and relief of ring strain. In uncontrolled chain-growth polymerizations, after an initiation event, the polymer chain grows extremely rapidly, followed by an irreversible termination event. Since propagation is so rapid, fully formed chains appear in the mixture relatively early during the course of the reaction, even though there remains a large concentration of unreacted monomers. Thus increasing the reaction time increases the yield, even though the average degree of polymerization remains constant. Because termination of a polymer chain can take place regardless of its length, the distribution of molecular weight is difficult to control. Given that polymers produced by such mechanisms have limited structural sophistication and thus are of limited use in the fabrication of actuators (other than as structural components), we will not cover these materials in this Progress Report unless this simple chemistry is complemented by a further form of chemical functionalization.
In controlled chain-growth mechanisms, which include living and quasi-living processes, the initiation of each polymer chain occurs at roughly the same timepoint, and each chain grows roughly at the same rate. This process results in a narrower dispersity of chain lengths compared to uncontrolled chain-growth polymerization. A relatively uniform molecular weight distribution is an important property when considering structure-activity relationships that are dependent on chain length. Polymerizations mediated by an anionic process proceed this way.[26] However, some of the most versatile materials are made using techniques collectively referred to as controlled radical polymerization (CRP), whose substrates include vinyl halides, acrylates, acrylamides, and styrenic monomers.[27,28] In a controlled radical process, the key step is the reversible deactivation of polymerization at the propagating center. The reaction is paused by converting the propagating center into a dormant state, which is typically favored energetically by a factor of ≥106. When the dormant state becomes transiently active, a small number of monomers may add to the growing polymer chain before deactivation reoccurs. Since, the propagating center is in the dormant state most of the time, this mechanism leads to the approximately uniform growth of all chains in the mixture. The three most common types of CRP methodologies are nitroxide-mediated polymerization (NMP),[29-31] atom transfer radical polymerization (ATRP),[32,33] and reversible addition-fragmentation chain-transfer polymerization (RAFT).[34,35] These processes are differentiated foremost by the way in which the dormant state is achieved. They also have strengths and weaknesses in terms of the compatibility of substrates and solvents, the ease with which fully formed polymer chains can be grafted to solid surfaces (“graft to”), or can use a solid as a support for polymerization (“graft from”), as well as other trade-offs.
Another polymerization strategy that follows controlled chain-growth behavior under the right conditions is ring-opening metathesis polymerization (ROMP).[36] Advantages of this process include ambient reaction conditions, rapid polymerization times and the retention of double bonds along the backbone, which can be further derivatized. ROMP-based polymerizations necessarily require monomers that have both ring strain and a double bond, the most common of which are norbornene- and cyclooctene-derived. However, a wide range of monomers are rapidly becoming available to provide additional functionality to ROMP-based polymers.
With the level of molecular control afforded by highly controllable polymerization methodologies, it is possible to generate materials with a range of novel functionality. Below, we highlight several examples of polymers exhibiting stimuli-responsive behavior which may find use in haptics, soft robotics, or mechanical forms of human-machine interfaces. We have organized this section by method of polymerization (they key steps of which are illustrated in Figure 1). Due to considerations of space, we were not able to include every interesting demonstration. Additionally, we were not able to provide complete details as to the macroscopic properties of the solid materials, i.e., force generated, actuation speed, or stress-strain behavior. For this information, we refer the reader to the original research articles. However, we believe we have given the reader the flavor of what can be achieved with the appropriate application-driven design of material, whose synthesis is enabled by a state-of-the-art synthetic technique.
2.1. Controlled Radical Polymerization
In CRP, control is achieved by reversible deactivation of the reactive center. It is an extremely broad class of reactions, which taken together encompass a wide scope of monomers, solvents, and reaction conditions, while preserving relative simplicity and low cost. One potential drawback for the inexperienced chemist is the need to maintain an oxygen-free environment during the course of the polymerization in order to prevent premature termination of the propagating radical species. However, techniques have been developed that increase the tolerance of CRP to oxygen. These developments potentially make these techniques more accessible to researchers outside of polymer chemistry.[37] The living characteristic of CRP allows for flexibility in designing novel architectures, such as branched polymers, star polymers, and di- and multi-block copolymers. This flexibility is enabled by the living character of CRP reactions, which is amenable to the addition of chemically distinct monomers. In the examples from the literature featured in this section, the power of CRP—as opposed to free-radical polymerization—is derived from this ability to control the molecular structure. For example, many types of electronic or stimuli-responsive polymers require chemically distinct blocks and covalent incorporation of specific chemical moieties through modification of the end groups. Such materials cannot generally be made by free-radical techniques, and thus various modes of CRP are needed. Many examples of CRP exist, but only the most popular ones are featured here: NMP, ATRP, and RAFT.
2.1.1. Nitroxide-Mediated Polymerization
NMP is perhaps conceptually the simplest form of CRP.[38] Its defining characteristic is the use of a nitroxide—a type of stable radical species that will not react with monomer—which forms reversible bonds with the radical residing at the reactive end of a growing polymer chain. The nitroxide usually takes the form of an alkoxyamine, with the exemplar being ((2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO). Many alkoxyamine initiators are commercially available, and this availability, along with the ease of performing the deactivation in situ, make NMP one of the most convenient CRPs.[39] Like RAFT, NMP does not require the use of transition metals, which could complicate interfacing of polymers with biological systems. Moreover, compared to RAFT, NMP has a much lower potential for odor generated during the synthesis and discoloration of the final product, both of which are associated with the thiocarbonylthio-containing RAFT agent. However, despite its simplicity, NMP has the most limited substrate scope.
While examples of NMP-synthesized polymers for HRHMIs are not numerous, some authors have found success with this approach. For example, Jangu et al. have reported electromechanical actuators based on ionically conductive imidazolium-containing triblock copolymers.[40] This class of self-assembled, nanoscale block copolymers function as solid-state polyelectrolytes in which the first and third blocks contribute to mechanical reinforcement, and the middle block contributes to the ionic conductivity. When ionic liquid was added into the triblock membrane, actuation speed matched those made from samples of Nafion, the material used as a benchmark for such measurements.[40]
2.1.2. Atom Transfer Radical Polymerization
Like NMP, ATRP also produces a dormant state by way of reversible bonding with a radical species.[41,42] In ATRP, activation begins when a terminal carbon-centered radical capable of monomer incorporation and chain propagation is generated by homolytic abstraction of a terminal halogen, usually chlorine or bromine, by a transition metal complex (e.g., Cu, Fe, Ru, Ni, Os),[43,44] with the metal in the lower of two oxidation states. The dormant species is reformed when the metal halide is transferred back to the propagating chain at the terminal radical. The design of the transition metal complex—especially its ligand environment—allows for control of the equilibrium constant between active and dormant states, which determines the overall growth rate.[45,46] Control is achieved through this “reversible deactivation” that prevents individual chains from propagating faster than the others. ATRP has a significant advantage in that polymer chains can be initiated from molecules or at solid surfaces functionalized with alkyl halides (usually tertiary bromides). Limitations of ATRP are associated with the use of transition metals, which are air-sensitive and toxic. Moreover, ATRP has a limited scope in protic media (e.g., water), although recent work has advanced the methodology of aqueous ATRP considerably.[47]
In one example of ATRP used to synthesize polymers of potential use in HRHMIs, Liu and co-workers synthesized poly[2-(methacryloyloxy)ethyltrimethylammonium chloride] brushes from cotton threads, followed by electroless deposition of metal ions to produce highly durable conductive yarns.[48] The applications of this relatively large scale approach to mechanically reliable, wearable fibers has a wide variety of applications extendable to HRHMIs. A smaller scale surface-initiated ATRP process was recently reported by Malakooti and co-workers to produce components of stretchable thermoelectric devices.[49] The authors reported solution-processable, surface-initiated ATRP from the oxidized surface of liquid metal eutectic gallium droplets. This “graft-from” process enhanced the stability and distribution of droplets, allowing for up to 50 wt% loading into elastomeric, wearable devices. Specifically, the authors demonstrated self-powered thermoelectrics for biosensing and heart-rate monitoring in extreme-cold conditions (to establish large enough gradients in temperature for acceptable generation of power). Adhesiveness is another important property for human-machine interfaces. While our group and others have reported extensively on the intrinsic mechanical properties of conjugated polymers,[50-54] the literature on adhesive behavior of these materials is substantially less developed. Baek et al. addressed this by grafting poly(n-butyl acrylate) side chains to poly(phenylene vinylene)s (PPVs) using activators regenerated by electron transfer (ARGET) ATRP.[55] Resulting copolymers were solution processable, exhibited solid-state optoelectronic properties, and served as a dry adhesive without any additives.
2.1.3. Reversible Addition-Fragmentation Chain-Transfer Polymerization
The RAFT process is widely used in the construction of diverse polymeric structures for HRHMIs. RAFT was developed by CSIRO in 1998,[34] and is known for its experimental simplicity,[56] compatibility with wide range of monomers,[57] controllability of the polymeric architectures,[56] an independence from transition metals,[57] compatibility with many solvents (water included),[58,59] and facile modification of the end group.[60] Unlike NMP and ATRP, which use a reversible capping agent to provide the dormant state, RAFT uses reversible chain transfer. The chain transfer agent (the RAFT agent) takes the form of a thiocarbonylthio molecule flanked by two versatile substituents, R and Z. R is reversibly cleaved during the polymerization process and plays a role in re-initiating the reaction. The principal role of the Z group is to modify the kinetics of the reaction. It can also be used as a functional handle, e.g., tether a growing chain to a surface, or to modify the molecular environment of the polymer chain end. Retention of the thiocarbonylthio groups in the propagating polymer is responsible for the living character of RAFT polymerization. Moreover, it renders the process suitable for synthesizing block copolymers, end functional polymers, and tethering growing chains to a surface. The reaction is initiated using common radical initiators, including 2,2′-azobis(2-methylpropionitrile) (AIBN) and 4,4′-azobis(4-cyanovaleric acid) ACVA. The absence of transition metals in the RAFT process is particularly attractive for biocompatibility, which is favorable in cases where the HRHMI makes contact with biological tissue. Furthermore, many of the common RAFT chain transfer agents are commercially available and can be readily derivatized.[61,62] Moreover, when compared to other CRPs, it excels over a much wider temperature range as well as being less affected by impurities.[63]
In an example from our laboratory on the use of RAFT to produce an electronic block copolymer for an application in haptics, Kayser et al. modified the poly(styrene sulfonate) (PSS) portion of the familiar conductive polymer poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS)[64] in a process of internal plasticization. Despite being well known for its relatively high conductivity among organic materials, the mechanical properties of commercial formulations of PEDOT:PSS are poor (and inconsistent).[53] Furthermore, additives—some of which are toxic—are commonly added to reach the highest values for conductivity.[65-67] The block copolymer demonstrated in this work, poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)-b-poly(poly(ethylene glycol)methyl ether acrylate) (PEDOT:PSS-b-PPEGMEA), was synthesized by our group to address these drawbacks.[68] Its key characteristic is that it achieves high stretchability and toughness, along with useful levels of conductivity, with no additives needed. As shown in Figure 2a, PSS was first polymerized by RAFT in the presence of an ACVA initiator. Bearing the thiocarbonylthio groups, the synthesized PSS could be further utilized as a macro RAFT agent for introducing the second building block of the copolymer. Hence, RAFT then continued in the presence of more initiator and PEGMEA prepolymer to yield the block copolymer scaffold. The final step was oxidative polymerization of PEDOT within the scaffold.
Figure 2.
Virtual texture generated using an elastomeric conductive block copolymer in a wireless multimodal haptic glove. a) RAFT polymerization of PSS-b-PPEGMEA diblock copolymer followed by oxidative polymerization of PEDOT in the block copolymer scaffold. b) The glove features three actuators, one of which is an electrotactile device composed of the conductive block copolymer for interfacing with a robotic hand or VR environment. c) Eight test panels in VR with permutations of the three sensation types (rough/smooth, soft/hard, warm/cool). Participants use the glove to control the virtual hand interacting with the panels. Adapted with permission.[69] Copyright 2020, Wiley-VCH, GmbH & Co, KGaA.
Subsequent to this work, Keef and co-workers used this material for electrotactile electrodes in the fingertips of a “haptic glove” (Figure 2b).[69] The electrotactile effect refers to the generation of tactile signals in the sensory neurons of the skin by using an electrical stimulus.[70] In such an application, organic materials are favorable to traditional inorganic materials because of the advantages of facile, low-temperature processing, oxide-free interfaces, low impedance relative to metals, and high deformability.[71-74] Our group successfully demonstrated the transmission of electrotactile signals to the fingertips of the wearer in order to recreate sensations of smoothness and roughness, which were achieved based on the intermittency of the electrical signal. In order to show that human participants could distinguish these sensations in the presence of other haptic signals, we combined the electrotactile effects produced by the conductive polymer with vibrotactile motors and thermoelectric devices to simulate surface hardness and temperature (Figure 2b). When equipped with flex sensors and commercial motion capture hardware, the glove could be used to control a hand in VR. Using the virtual environment, it was possible to impart sensations such as warmth, coolness, hardness, and softness. To assess the capability of the participants to discern sensations produced by the haptic glove, volunteers were asked to touch “mystery” panels with having 23 = 8 permutations of the binary gradations of rough vs. smooth, warm vs. cool, and hard vs. soft (Figure 2c). Experimenters found that participants were 85% accurate in identifying the correct input textures (smoothness/roughness). Additionally, the haptic glove was also capable of transmitting tactile signals from a robotic hand to the participant, which has promising applications—albeit in the far future—for applications such as telehealth or medical training.
While conductivity is a key property for electrotactile electrodes in haptic devices, the property sought for most materials used in soft robotics is mechanical actuation. In these purposes, RAFT can provide a variety of polymer architectures that provide the means to incorporation of stimuli-responsive moieties.[75-77] Hydrogels in particular are valuable for a range of potential HRHMIs. Hydrogels are 3D solids composed of mostly water in a matrix of chemically or physically crosslinked hydrophilic polymer chains. They have already shown significant value as biomaterials in a host of applications in tissue engineering, and are promising for HRHMIs predominantly due to their biocompatibility, mechanical softness, and the potential for their properties to be tailored by synthesis.[78] However, in the context of HRHMIs, the mechanical stability of hydrogels under cyclic loading is notably poor. In an effort to increase the stability against fatigue, Jiang and co-workers employed RAFT to design a polymer matrix with the ability to form crosslinks reversibly.[79] This dynamism was achieved using a backbone of poly(methacrylic acid-co-oligo(ethylene glycol) methacrylate) (poly(MAA-co-OEGMA) synthesized by RAFT, modified with photochromic anthracene moieties (Figure 3a). The presence of strong hydrogen bonding moieties (Figure 3b), as well as the π-π hydrophobic interactions between anthracene molecules, afforded the ability to be healed at modestly elevated temperature. Tensile testing demonstrated the striking efficacy of the healing ability of the hydrogel: after 8 min of annealing at 35 °C, the hydrogel returned to its original strength. Moreover, the anthracene unit was bonded to the main chain using an imine linkage, which undergoes cis-trans isomerization upon radiation. This isomerization changes the density of the polymer and thus permits mechanical actuation. Figure 3c demonstrates the macroscopic effect of isomerization and subsequent π-π interactions of anthracene when exposed to visible light. This transformation of light to mechanical energy is due to the increase in density of the side exposed to light relative to the unexposed side. Furthermore, the hydrogel has another advantage over traditionally cross linked photoresponsive hydrogels; it can be dissolved and recycled after any amount of selective irradiation (Figure 3c).
Figure 3.
Strong, healable, and recyclable visible-light-responsive hydrogel actuators. a) Reaction scheme of the anthracene-based small molecule and the post-modification of poly(MAA-co-OEGMA). b) Chemical structure of the hydrogel and non-covalent interactions of the designed chemical groups. c) Photographs showing the recycling and reprogrammable process of the hydrogel, featuring a helical structure formed by irradiation with patterned light. Adapted with permission.[79] Copyright 2020, Wiley-VCH, GmbH & Co, KGaA.
In an application of RAFT-enabled synthesis to thermally responsive actuators, Jingquan and co-workers modified the RAFT agent with pyrene prior to polymerization.[80] The authors successfully demonstrated the synthesis of an end-modified polymeric anchor for non-covalent π-π stacking interactions with chemical vapor deposition (CVD)-graphene (Figure 4a). The researchers chose the thermally responsive poly(N-isopropylacrylamide) (PNIPAAm), whose key characteristic is the presence of a lower critical solution temperature (LCST), at 32 °C.[81,82] The effect of the LCST is to give the polymer decreased solubility at elevated temperature due to a change in conformation from an extended, hydrate state to a shrunken, dehydrated state. When integrated with CVD-synthesized graphene, the PNIPAAm generated a composite film that could generate internal stress upon changing the temperature (Figure 4b, 4c). Importantly, the researchers found that the deformation was reversible and controllable by manipulation of the environmental temperature. When the temperature was lowered to 28 °C, the shapes of the composite films returned to their initial states. Finally, an electrical application for actuators was demonstrated. When the temperature was higher than the LCST, the thermo-responsive CVD graphene-PNIPAAm composite film deformed to close the electrical circuit and turned on the LED light.[80]
Figure 4.
Thermoresponsive actuators based on graphene modified with pyrene-terminated PNIPAAm. a) Reaction scheme of the pyrene-modified RAFT agent and the subsequent polymerization of pyrene-terminated polymer, PNIPAAm. b) A schematic illustration demonstrating molecular restructuring and the induced actuation upon temperature change. c) Thermal deformation experiments of the CVD graphene-PINPAAm film on adhesive tape. Adapted with permission.[80] Copyright 2017, Wiley-VCH, GmbH & Co, KGaA.
2.2. Metal-Mediated Cross-Coupling
Metal-mediated cross-coupling reactions, including those named after Stille, Suzuki, and Kumada, are versatile solution-phase reactions.[83] In these processes, two organic groups, R1 and R2, are bonded together to form a new carbon-carbon bond using a catalyst based on a transition metal, usually palladium, platinum, or nickel, which is surrounded by ligands coordinated to the metal center. Common to all such schemes is the use of an R1-M precursor, where M is a main-group center (e.g., tin, boron, magnesium), and an R2-X precursor, where X is a halide (e.g., bromide). Typically, R2 is unsaturated to prevent unwanted elimination of the beta hydride. Upon successful coupling, the M and X groups are ejected as MX (typically a waste product). The name of the reaction is associated with the identity of the M group (e.g., for M = SnR3, the reaction is a “Stille coupling”). Bifunctional reactants, e.g., X-R-M, or M-R1-M combined with X-R2-X, are thus able to undergo polycondensation reactions to form polymers. The requirement that at least one of the monomers be unsaturated lends the technique well to the synthesis of fully π-conjugated polymers, which have an uninterrupted arrangement of alternating double- and single-bonds along the backbone. This structural motif gives rise to a bandgap, along with semiconducting properties.[84]
For most examples of metal-mediated cross-coupling reactions, the polymerization proceeds by a step-growth process. The ability to tune the bandgap and to achieve the highest possible charge-carrier mobilities requires the alternating arrangement of electron-rich and electron-poor monomer residues. This arrangement produces an electronic structure in which a “push-pull” mechanism has the net effect of bringing the frontier molecular orbitals closer to one another (usually < 2 eV). These materials are variously designated as “low-bandgap” or “donor-acceptor” polymers, which, as a class, are believed to represent the future of semiconducting polymers in applications such as solar cells, thin-film transistors, and biosensors.[85] Thus innovation in metal-mediated cross-coupling directly benefits organic bioelectronics, which is a highly inviting application for future polymer-based HRHMIs. (One notable exception to the rule that metal-mediated polycondensation reactions proceed by step-growth kinetics is the Grignard-metathesis (GRIM) polymerization, commonly used in the synthesis of regioregular poly(3-alkylthiophene) (P3AT). In this process, the transition metal catalyst remains associated with the polymer chain, growing as monomers are inserted one at a time. Thus, the polymerization follows a quasi-living, chain-growth behavior).[86] Although uncommon, metal-mediated cross-coupling can also exhibit a chain growth nature, which could be used to better control the molecular weight and distribution of conjugated polymers, as well as introduce facility in designing block copolymers.[87] Very recently, a chain-growth polymerization of a benzotriazole based n-type conjugated polymer was achieved using a Suzuki cross-coupling with commercially available Pd-dialkylbiarylphosphine catalyst.[88] End group tolerance was also reported, suggesting this method could be used with other monomers.
An example of how a metal-mediated polycondensation reaction can be used in concert with ring-opening polymerization (ROP) for potential bioelectronic applications is shown in Figure 5. In particular, the key goal was to produce a polymer with high charge-carrier mobility, stretchability, and toughness, and for this application, degradability under simulated physiological conditions. Our work in development of a new polymerization strategy was motivated in contrast to an approach based on compositing and blending, which often due to substantial phase separation does not achieve the desired properties.[89-92] Recently, Sugiyama and co-workers from our group confronted the issues facing composite systems with a single component block copolymer (BCP) with a semiconducting diketopyrrolopyrrole (DPP)-based unit and a stretchable, degradable polycaprolactone unit.[93] Along with the absence of plasticizing additives which may be toxic, this block copolymer has the potential for applications in HRHMIs. Tunable stretchability is key to reach the mechanical compliance of human tissue, which would enable enhanced contact as well as survival during stretching cycles.[94-97] The ability to degrade in the body is also significant in order to eliminate the need for a second surgery to retrieve an implanted device.
Figure 5.
Stretchable and degradable semiconducting block copolymers. a) Stille polymerization of BCPs containing DPP-based polymers and an aliphatic polyester. b) The effect of PCL content in PDPP-b-PCL on mechanical properties. c) Schematic of the OFET structure and the relationship between charge-carrier mobility and PCL content with standard deviations of three distinct measurements. Adapted with permission.[93] Copyright 2018 American Chemical Society.
The three synthetic steps are shown in Figure 5a: first, Stille coupling polymerized DPP-Br with a thiophene bis-stannane and end-capping agent. Next, ε-caprolactone (ε-CL) in the presence of 1,6-hexanediol (HDO) underwent (ROP) to generate polycaprolactone (PCL). The final step generated the single block copolymer of the DPP-based polymer and the insulating PCL using a diisocyanate linking agent that covalently bound both segments with a urethane linkage. To predict the durability of this material in actual devices, stress–strain curves were obtained from polymer films suspended on water. PCL content was varied from 0% to 75%, with a clear increase in ultimate extensibility and mechanical toughness (Figure 5b). Films of 50% and 75% PCL could even withstand elongations above 50% without fracture. The extent of cracking at 100% elongation in these single-component films was also significantly less when compared against same-weight ratio films of simple blends (PCL mixed with PDPP). However, increasing the PCL content was expected to reduce the charge-carrier mobility. To measure this effect, the hole mobilities of top-contact, bottom-gate field-effect transistors (FETs) with the polymer films as the active layers were obtained (Figure 5c). Block copolymers containing isolated thiophenes rings (PCB-T) exhibited a trend of decreasing charge-carrier mobility with increasing mechanical toughness, yet those containing fused thienothiophene rings (PCB-TT) retained their mobilities and toughness with weight fractions up to 90% PCL. The BCPs were also degradable, with 50% DPP/PCL films disintegrating after 3 d in 0.5 NaOH.
2.3. Ring-Opening Polymerization
Ring-opening polymerization is the process by which cyclic monomers open to form linear chains. The stereotypical reaction is ring-opening of the simplest epoxide, ethylene oxide, to form poly(ethylene oxide) (PEO). This and all other ROP processes follow a chain-growth mechanism, though they can be either uncontrolled (e.g., cationic polymerization of PEO) or living (e.g., anionic polymerization of PEO, organocatalytic ROP of cyclic esters), the later provide structures with the degree of control needed for HRHMIs. A special type of ROP is that based on metal-mediated olefin cross-metathesis. This methodology, ROMP, has found tremendous value in a range of applications in engineering plastics and biomaterials,[98,99] and has the level of control and tolerance to functional groups needed to facilitate the synthesis of active polymers for HRHMIs.
2.3.1. Conventional Ring-Opening Polymerization
Although ring-opening polymerization has been used for over a century,[100] researchers are still taking advantage of the simple nature of ROP to develop novel materials and applications. For example, Zotzmann and co-workers fabricated a temperature-controlled, reversible shape-memory polymer (SMP) based on ROP of two polymer segments.[101] The authors engineered a triple-shape effect into their material while maintaining reversibility of the transformation between two temporary states and the permanent state. This effect was achieved through multiphase polymer networks with two distinct crystallizable segments, poly(ω-pentadecalactone) (PPD) and poly(ε-caprolactone) (PCL). To maintain distinct properties in the same material, it was necessary to interconnect the two networks at their termini rather than have a blend of both polymers. Their approach was to perform ROP of each cyclic monomer separately, yielding star-shaped precursors with -OH end groups ready for the final crosslinking step. Reversibility of their triple-state design enabled the fabrication of soft actuators fit for more complex shape-shifting than common two-state SMPs.
2.3.2. Ring-Opening Metathesis Polymerization
Like ROP, ROMP is driven by the release of cyclic ring strain. However, the initiation step in ROMP is the coordination of the metal alkylidene catalyst to the cyclic olefin.[36] Following [2+2] cycloaddition and cycloreversion, a new metal alkylidene is generated that can subsequently coordinate and incorporate additional monomer units, which constitutes the growing polymer chain. Various transition metal catalysts can be used to catalyze ROMP (e.g., W, Mo, Re, and Ti),[102] however catalysts based on ruthenium are most widely used due to their stability in air and tolerance toward a wide scope of functional groups.[102] Monomers are limited to cyclic olefins with high ring strain such as cyclobutene, cyclooctene, and norbornene.[36] In fact, fully conjugated polyacetylenes were achieved through ROMP by the Grubbs group, using cyclooctatetraenes as the monomers.[103] On the other hand, control of tacticity and the cis/trans isomer of the main-chain olefins are still challenges.[104]
Otherwise, ROMP is considered very useful in producing a range of block copolymers and resulting self-assembled architectures, as well as the synthesis of amino acid and peptide acid based polymers for biodegradable and biocompatible applications.[104] Another advantage of ROMP is its control of the end group, which enables the synthesis of versatile block copolymers.[105] Furthermore, some groups have even achieved a quasi-living ROMP available to continue polymerizing through other techniques such as ATRP and RAFT.[105] These advantages are prerequisites to achieve self-assembly of certain ROMP polymers yielding interesting architectures. In a rare example of ROMP for HRHMI’s, Kim et al. exhibited hierarchical superstructures of azobenzene-based polynorbornenes to fabricate remote-controllable actuators.[106] A film of this material coated with Ag served as a photoresponsive electrode sensitive to either visible or UV light. When interfaced with an LED and illuminated, different colors could be produced.
In the following featured work, Dean and co-workers performed “frontal” ROMP (FROMP) of 1,5-cyclooctadiene (COD) and dicyclopentadiene (DCPD) to yield copolymers with tunable thermomechanical properties.[107] Frontal polymerization (FP) refers to a technique in which monomer is converted at a localized, heated region, which continues to propagate along a direction driven by the exothermic nature of polymerization.[108] It requires no solvent and can be run at ambient conditions, making it an energy-efficient alternative to the traditional production of bulk rubbers. These traditional methods typically require high temperatures and extra processing steps due to the dependence of solvents.[23,109] In this work, FP in concert with ROMP allowed for rapid synthesis of spatially varied compositions in a single sample of material. When the authors increased the concentration of DCPD monomer, which has two reactive sites available for crosslinking after ROMP (Figure 6a), a very wide range of properties emerged. The glass-transition temperature (Tg) increased from −90°C to 114°C, the tensile modulus increased by 3 orders of magnitude, and the strength increased 40-fold. Dean et al. took advantage of this range of properties to fabricate a shape memory polymer. Figure 6b highlights the gradient in concentration of the monomer in the reaction vessel, which translated to a gradient of Tg and other mechanical properties after FROMP. Consecutive mechanical actuation across the three levels was achieved by raising the temperature from the programmed state to the permanent state (fixed at the time of FROMP crosslinking) (Figure 6c). This recent work extends the possibilities of not only using ROMP for soft-robotics applications, but, notably, for the convenient bulk production of mechanically robust, functional materials.
Figure 6.
Frontal ring-opening metathesis polymerization of copolymers with tunable thermomechanical properties and shape memory actuation. a) Copolymerization of 1,5-cyclooctadiene and dicyclopentadiene yields a copolymer with side groups available for further crosslinking and mechanical programming. b) Rapid synthesis of a polymer with a Tg gradient based on monomer concentration. c) Sequential thermal actuation and recycling of a shape memory polymer directed by a Tg gradient. Adapted with permission.[107] Copyright 2020, American Chemical Society.
2.4. Crosslinking
While not a strategy for polymerization per se, crosslinking is a key strategy used to form polymers with mechanical resilience, and thus a critical area for chemical innovation in materials used for HRHMIs. Crosslinking is used in the synthesis of hydrogels, epoxies, silicone rubber, and many other examples of solid structures that are not solids in their uncrosslinked forms (e.g., glassy and semicrystalline polymers are solids even when uncrosslinked). In this section, we focus on strategies for crosslinking that have the characteristic of reversibility for dynamic reconfiguration of materials.
2.4.1. Crosslinking Using Diels-Alder Adducts
A key advantage of soft robotics in comparison to traditional robotics is the ability of soft systems to navigate diverse terrains and to be resilient upon exposure to mechanical insults. Moreover, their mechanical softness makes them uniquely suited for safe contact with humans. The adaptability and resilience of soft robotic systems arise from their elastomeric components. These soft components, however, are susceptible to damage from highly concentrated forces, e.g., puncture. To address this challenge, Terryn et al. has developed a series of pneumatic actuators composed of a single-component healable polymer (Figure 7a).[110] The novelty of the approach arises from reversible Diels-Alder (DA) crosslinking available in selected functional groups (conjugated diene and substituted alkene) in the precursors, furan and maleimide respectively (Figure 7b). The resulting elastomer is originally cross linked in the second synthetic step at 90 °C, but when the Diels-Alder adducts are broken when damaged upon puncture, they can be reformed by heating at 80 °C in 20-40 min, and the solid is healed upon cooling (Figure 7c). With the same design, the authors demonstrated that a range of mechanical properties can be achieved by varying the spacer length of prepolymer, so long as the end groups are furan and maleimide. The material was effectively incorporated into artificial muscle-like actuators as well as grippers which could grasp weights of different shapes. Lastly, the healable elastomer could be recycled upon dissolution in chloroform and re-casting.
Figure 7.
Healable soft pneumatic robots by reversible Diels-Alder (DA) linkages. a) The synthesis of the thermoreversible covalent networks. The first step is an irreversible epoxy-amine reaction between Jeffamine (poly(propylene glycol) bis(2-aminopropyl ether)) and furfuryl glycidyl ether, followed by the reversible Diels-Alder crosslinking between maleimide and furan groups. b) The equilibrium DA reaction: At low temperatures, the network is formed due to high conversion to the DA bonds. At high temperatures, these DA bonds fall apart, and the mobility of the polymer chains increases. Thermoreversible elastomeric network is formed by cross-links of the reversible DA bond. c) Macroscopic cuts (8.6 mm) in the self-healing membrane of the pleated pneumatic artificial muscle can be healed entirely using a thermal treatment procedure. After this treatment, the muscle is again airtight and recovers its functionality. Adapted with permission.[110] Copyright 2017, American Association for the Advancement of Science.
2.4.2. Radical Initiated Crosslinking
Free-radical-initiated crosslinking is ubiquitous in the chemistry of hydrogels and is familiar to biochemists in the preparation of poly(acrylamide) gels for electrophoresis. In the example discussed here, it is used in the preparation of a liquid crystal elastomer (LCE). Like molecular (non-polymeric) liquid crystalline media, an LCE contains a rigid mesogen responsible for liquid crystalline phase transitions between the glass transition temperature and the melting temperature. If the mesogens are aligned by stretching or shear, the bulk structure undergoes large reversible mechanical deformation upon application of a thermal stimulus. Other stimuli to achieve deformation include electric fields and light (using a chromophore to convert light energy into localized heating).[111,112] LCEs are thus potentially useful in applications as artificial muscles[113] or in haptic applications such as reconfigurable braille displays.[114,115]
In the example highlighted here, Lee and co-workers fabricated thermoresponsive, 3D shape-morphing LCEs in the macroscale as well as microscale.[116] The aza-Michael addition-based step growth polymerization used here was developed by the White group in 2015.[117,118] First, a commercially available LC monomer underwent chain extension with n-butylamine, which resulted in a diacrylate-functionalized poly(β-amino ester)-type LC oligomer. Exposure to UV crosslinked the diacrylate linkages (Figure 8a). The liquid crystalline phases adopted by the structure can interconvert in response to thermal signals; the LCE explored here achieved the nematic phase upon heating to 65 °C. 3D shaped, millimeter sized objects of LCE were formed and their reversible shape changes were demonstrated (Figure 8b). Furthermore, the transmittance of the LCE changed drastically from 2% at 25 °C to 73% at 120 °C, a characteristic which is potentially relevant for thermochromic applications (Figure 8c). Radical-initiated crosslinking of the LC prepolymer also permitted the fabrication of high-aspect-ratio microstructures. For example, micropillars fabricated by UV imprinting also exhibited actuation in response to temperature, and changes in height were confirmed by confocal microscopy (Figure 8d,e).
Figure 8.
Mechanically programmed 2D and 3D liquid crystal elastomers at macro- and microscale using two-step photocrosslinking. a) LCE synthesis by crosslinking of amine chain extender and monomer. b) A bulk, reversible shape change of 3D-shaped LCE during heating and cooling. Scale bar indicates 5 mm. c) Change in transmittance as a function of temperature: the inset shows the photographs of the patterned LCE at 25 and 120 °C. d) 3D topography images and height profiles of LCE micropillars at 25 and 120 °C recorded by a laser scanning confocal microscope. The height of the pillars decreases from 46 to 8 μm. Adapted with permission.[116] Copyright 2020, Royal Society of Chemistry.
2.5. Hybrid Synthetic Strategies and Supramolecular Structures
Hydrogel-based composites are a powerful class of materials, having applications in a wide variety of industries.[119] Conductive hydrogels in particular are of great interest for bioelectronic applications.[120] One of the challenges of this material for wearable electronics and HMIs is overcoming their poor adhesive properties. Conductive hydrogels are often designed by incorporating conductive fillers such as graphene, carbon nanotubes, and small molecules.[121-123] However, incorporation of these materials produces materials that often suffer from poor durability, flexibility, and other mechanical properties necessary for wearable and implantable devices. Recently, Di and co-workers offered a route to improved adhesion through a hydrogel based on polydopamine-grafted polysuccinimide networked with nanoclay and PNIPAAm.[124] To synthesize the base polymer network of their multi-component hydrogel, they polymerized L-aspartic acid to yield polysuccinimide, from which polydopamine and ethanolamine moieties were grafted to yield a catechol-modified polypeptide (PDAEA) (Figure 9a). The hydrogel was prepared by polymerizing NIPAM in the presence of clay, sodium pyrophosphate, and PDAEA in water. Many hydrogen bonding moieties contributed to the self-adhesiveness as well as self-healing ability (Figure 9b). In addition to exhibiting many robust mechanical properties, such as recovery from tension and compression and adhesion to a variety of different surfaces, they also highlighted reversible, tunable adhesion within 9 °C. For haptic devices, this control of adhesion could be useful in recreating a sensation of tack, which could be generated independently from friction arising from roughness.[114] Additionally, the hydrogel could self-heal without any external stimuli after only 5 minutes and continue to withstand stretching (Figure 9c). Finally, the hydrogel maintained a lit LED despite tension up to 200%.
Figure 9.
Tough, conductive, reversibly adhesive hydrogels based on PNIPAM and a dopamine modified polypeptide. a) Ring-opening polymerization of dopamine-conjugated PDAEA. b) Schematic demonstrating the fabrication of a PNIPAM-clay-PDAEA hydrogel. Hydrogen bonding interactions between the clay, PDAEA chains, and PNIPAM chains are shown. c) The self-healing behavior of the PNIPAM-clay(7.5%)-PDAEA(1%) hydrogel. Adapted with permission.[124] Copyright 2020, Royal Society of Chemistry.
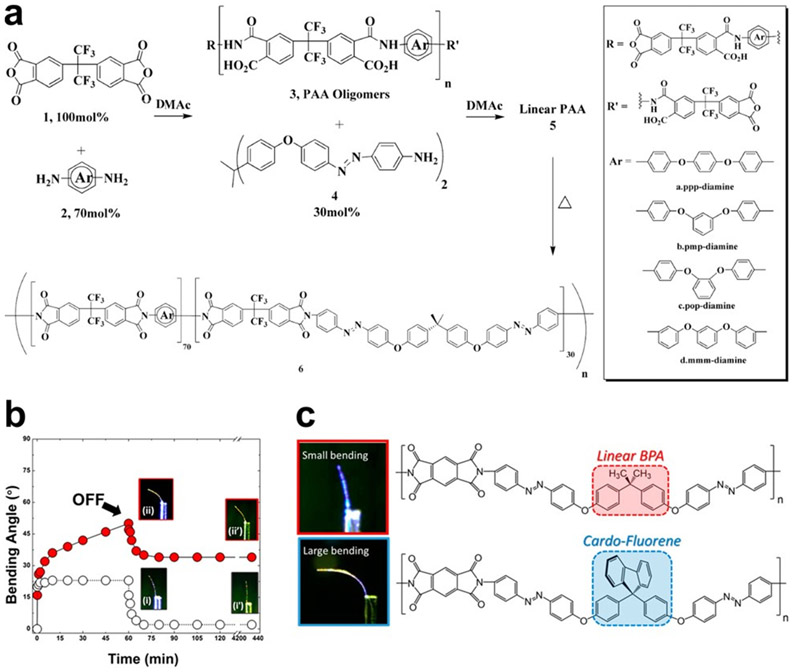
The next two demonstrations focus on the tunability of polymers containing azobenzene groups, which undergo mechanical actuation in response to light.[125]. Photoisomerization of azobenzene in glassy polymeric materials have been examined for potential utility in optics[126,127] and mechanics[125,128] actuators, and sensors.[129] White and co-workers showed several examples for the molecular design principles to produce a large force and shape change in a single material using the azobenzene photochemistry. As reviewed by Torkelson[130] and Teboul,[131] the sensitivity of azobenzene photochemistry to the local environment of the polymeric host has been shown to depend on the crystallinity, free volume, and molecular architecture. In one of the reports by White et al.,[16] they molecularly engineered linear and cross-linked azobenzene-functionalized polyimides to enhance both photomechanical work and motion (Figure 10a). They showed that segmental rotational motion of benzene rings strongly depends on whether the phenylene groups in the aromatic polyimides are interconnected in the meta, ortho, or para position. The polyimides prepared with meta connectivity had a higher rotational mobility, resulting from the rotational freedom of the phenylene rings, consequently influenced on segmental mobility.[132] This work showed how specific chemical modifications affect the segmental mobility of materials and can combine the properties of simultaneously large displacement and force generation (Figure 10b).
Figure 10.
Azobenzene-functionalized polyimides synthesized by ring-opening polymerization to enhance photomechanical work and motion a) Synthesis of linear polyimides. b) Photogenerated bending angle for meta (○) and para (red circle) isomers. The films were subjected to 445 nm light at 120 mW/cm2 intensity for 1 h and subsequently relaxed in dark. Inserts are labeled (i) and (ii) to denote the diimide structure composed of (i) meta (○) and (ii) para (red circle) isomers. Images marked with (′) were taken after 3 days of relaxation in darkness. c) The effects of bulky substituents on the degree of photomechanical response. Adapted with permission.[16,133] Copyright 2014 and 2017 American Chemical Society.
In another report, the authors demonstrated the sensitivity of azobenzene photochemistry to the free volume of their polymeric actuators.[133] Free volume is a factor known to be critically important in allowing the photoisomerization of azobenzene in macromolecular systems.[134] To increase the free volume in their photomechanically active material they designed a cardo or “loop” pendant bulky substituent which will interfere with the polymer chains proximity. They developed a series of photomechanically active azobenzene-functionalized polyimides bearing a new bis(azobenzene-diamine) monomer containing a 9,9-diphenylfluorene cardo structure (azoCBODA), labeled as cardo-fluorene (highlighted in blue, Figure 10c). Similar to their previous report, polymerization occurred through the reaction between diamine (azoCBODA) and dianhydride functionalized monomers, to create a poly(amic acid). Finally heating and curing the polymers generated the imidized-polymer film. Next, applying a 60 mW/cm2 445 nm light on the sample resulted in isomerization or reorientation of the azobenzene chromophore in the microlevel, and in a cantilever bending angle from 0° to ~90° in 30 min (Figure 10c). Importantly, a reversible actuation is highly desired, and all their samples exhibit fast relaxation in 30 min and almost full recovery in 2 h. This research demonstrated that as the fractional free volume is higher, the macromolecular environment is prone to more efficient photoisomerization or reorientation, enabling the molecular-level photochemistry to be transduced through the network to result in large scale macroscopic photomechanical responses visualized as cantilever bending or quantified using stress measurements.
3. Outlook
The critical role of interdisciplinarity research in the resolution of complex problems is well known, and reflected in the rise of large-scale, multi-departmental research centers. However, despite the obvious benefits of working together, barriers in terminology and mutual unfamiliarity of methods have the effect of reducing the chances for successful collaboration. The field of synthetic organic chemistry, along with mechanical and electrical engineering, have joined forces to produce entire new fields in the past (e.g., much of materials science, microelectronics fabrication, microelectromechanical systems, and display technologies), and will do so again in the future. We have identified the young fields of haptics, soft-robotics, and human-machine interfaces as being prime examples in which the challenges faced by engineers, and the capabilities of synthetic chemists, are in good alignment, but currently underexplored. We have identified several examples from the literature that have showcased ways in which polymeric materials designed on the molecular scale and synthesized using state-of-the-art methodologies exhibit properties that would be difficult or impossible to achieve using off-the-shelf materials or their composites.
In general, figures of merit needed for a particular application should be at the forefront of molecular design. For the scope of HRHMIs, which covers a broad range of devices and applications, it is challenging to define engineering performance benchmarks without studying each example individually. Nevertheless, there are some desired properties shared between these fields of study. High conformability, for example, is essential to ensure proper contact between a device and untraditional substrates. This property is difficult to quantify and is not only dependent on the modulus and adhesion of the device but on the substrate geometry and texture as well.[135] Other figures of merit to isolate include adhesive force, cycling stability, and extent of biocompatibility. On the other hand, there are well-defined property requirements for interfacing with human skin for wearable devices.[14] These include fingertip spatial resolution (1 mm),[136] temporal resolution (20-40 ms),[137] vibration frequency limit (700 hz),[138] and 1-point discrimination threshold pressure (10kPa). An ideal actuatable soft material must also accommodate extremely short response times (< 1 ms)[139] to render tactile sensations most accurately. These requirements are most important for determining a material’s mechanical recovery time as well as degree and force of deformation upon actuation. These two are considerably challenging and begin to highlight some of the steepest limitations of polymeric materials for HRHMIs. However, in this emerging interdisciplinary arena, identification of many limitations is expected. One of the main attractions of soft robots is their ability to excel in various unpredictable or harsh terrains, however, environmental conditions such as moisture, heat, oxidizing atmosphere, and radiation are common enemies of electronic polymers.[140] Production of conjugated polymers and other highly modified polymers is often on the sub-gram scale in laboratories, which is another serious limitation in implementation. Chemists and engineers will also need to address serious concerns such as leeching of chemical components and decreased versatility of actuatable devices due to high energy inputs required. Resolving confounding variables in actuation and sensation, such as light/temperature and simulated textures, is another issue for haptics. This is precluded by the already limited library of realistic, simulated textures.
Despite these many challenges, our tone is hopeful. While one might be dubious of a polymer’s ability to achieve multiple properties simultaneously, the superior tunability of polymers down to the molecular level is what has enabled polymers to be one of the most versatile engineering materials. In fact, in the 21st century alone, specially designed polymers have yielded tremendous breakthroughs in a diverse range of engineering fields.[141] For example, conjugated polymers have evolved from being barely-conductive “brick dust” to possessing high conductivity,[142] stretchability, and toughness.[71] This example alone has enabled a myriad of applications such as skin-conforming electronics[143] and biodegradable electronics.[144,145] In particular, PEDOT:PSS has received ubiquitous praise for its high conductivity, oxide-free interfaces, and low impedance in electrodes.[146] Even so, its capabilities continue to expand as the polymer community takes advantage of its ripeness for many other chemical modifications.[147-149] Shape memory polymers, another successful example, have evolved to switch reversibly between two or more independent shapes with stimuli beyond simple heating.[150] Hydrogels, once the equivalent of laboratory “Jell-O,” are being capitalized on for their self-healing properties, high degrees of actuation, and surprising capability for mechanical stability.[151] Developments in the community extend into the processing steps as well, such as with additive manufacturing routes that add an additional level of complexity and function at the structural level.[152] In conclusion, as long as polymer scientists continue to take greater command of the molecular domain, state-of-the-art materials will continue to enable previously unheard-of applications.
Though we acknowledge some drawbacks in the nascent stages of polymers in haptics, soft robotics, and human-machine interfaces, understanding the rapid, recent, and multi-branched evolution of multifunctional polymers gives us hope for the future. We believe, however, only through a closer interaction between chemists and engineers, that the next generation in the evolution of advanced, multifunctional polymers can be achieved. It is our hope that this Progress Report creates new intellectual ties and reinforces existing ones.
Acknowledgements
This work was supported by the National Institutes of Health Director's New Innovator Award number 1DP2EB022358 and the supplemental award 4DP2EB022358 from the National Institute of Bioimaging and Bioengineering through the Presidential Early Career Award for Scientists and Engineers (PECASE) to D.J.L. Psychophysical work discussed in connection with Figure 1 was supported by the National Science Foundation under grant number CBET-1929748. D.C.C. and J.K.P. are supported by the National Science Foundation through the UC San Diego Materials Research Science and Engineering Center (MRSEC, grant DMR-2011924). Referenced work that was performed in our laboratory was conducted in part at the San Diego Nanotechnology Infrastructure (SDNI), a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542148). R.B. was supported in part by the Zuckerman-CHE STEM Leadership Program.
References
- [1].Schadt M, Jpn. J. Appl. Phys 2009, 48, 03B001. [Google Scholar]
- [2].Chigrinov V, Liquid Crystal Devices: Physics and Applications; Artech-House: Boston, USA, 2004. [Google Scholar]
- [3].Sirringhaus H, Adv. Mater 2014, 26, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kulkarni AP, Tonzola CJ, Babel A, Jenekhe SA, Chem. Mater 2004, 16, 4556. [Google Scholar]
- [5].Huang J, Dahlgren DA, Hemminger JC, Langmuir 1994, 10, 626. [Google Scholar]
- [6].Bratton D, Yang D, Dai J, Ober CK, Polym. Adv. Technol 2006, 17, 94. [Google Scholar]
- [7].Kagan CR, Fernandez LE, Gogotsi Y, Hammond PT, Hersam MC, Nel AE, Penner RM, Willson CG, Weiss PS, ACS Nano 2016, 10, 9093. [DOI] [PubMed] [Google Scholar]
- [8].Nicolaou KC, Sorenson EJ, Classics in Total Synthesis: Targets, Strategies, Methods; Wiley-VCH: Weinheim, Germany, 1996. [Google Scholar]
- [9].Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA, Chem. Soc. Rev 2012, 41, 5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao XY, Liu HJ, Wang MZ, In Recent Advances in Dielectric Materials; Huang A, Ed.; Nova Science Publishers, Inc., 2009; pp. 323–368. [Google Scholar]
- [11].Wilson JN, Chem. Rev 1939, 25, 377. [Google Scholar]
- [12].Rivnay J, Owens RM, Malliaras GG, Chem. Mater 2014, 26, 679. [Google Scholar]
- [13].Rogers JA, Someya T, Huang Y, Science (80-. ). 2010, 327, 1603. [DOI] [PubMed] [Google Scholar]
- [14].Hammock ML, Chortos A, Tee BC-K, Tok JB-H, Bao Z, Adv. Mater 2013, 25, 5997. [DOI] [PubMed] [Google Scholar]
- [15].Muth JT, Vogt DM, Truby RL, Mengüç Y, Kolesky DB, Wood RJ, Lewis JA, Adv. Mater 2014, 26, 6307. [DOI] [PubMed] [Google Scholar]
- [16].Wie JJ, Wang DH, Lee KM, Tan LS, White TJ, Chem. Mater 2014, 26, 5223. [Google Scholar]
- [17].Shaw JM, Gelorme JD, LaBianca NC, Conley WE, Holmes SJ, IBM J. Res. Dev 1997, 41, 81. [Google Scholar]
- [18].Organic Semiconductors in Sensor Applications; Bernards DA; Malliaras GG; Owens RM, Eds.; Springer Berlin Heidelberg, 2008. [Google Scholar]
- [19].Dove AP, Meier MAR, Macromol. Chem. Phys 2014, 215, 2135. [Google Scholar]
- [20].Hiemenz P, Lodge T, Polymer chemistry; 2nd ed.; CRC Press: Boca Ranton, 2011. [Google Scholar]
- [21].Bolognesi A, Pasini MC, In Semiconducting Polymers: Chemistry, Physics and Engineering; 2006; pp. 1–68. [Google Scholar]
- [22].Aggarwal SL, Sweeting OJ, Chem. Rev 1957, 57, 665. [Google Scholar]
- [23].Maul J, Frushour BG, Kontoff JR, Eichenauer H, Ott K-H, Schade C, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- [24].Millan J, Smets G, Die Makromol. Chemie 1969, 121, 275. [Google Scholar]
- [25].Biondi M, Borzacchiello A, Netti PA, Chem. Eng. J 2010, 162, 776. [Google Scholar]
- [26].Quirk RP, Rubber Chem. Technol 2020, 93, 1. [Google Scholar]
- [27].Parkatzidis K, Wang HS, Truong NP, Anastasaki A, Chem 2020, 6, 1575. [Google Scholar]
- [28].Grajales S, Moad G, Rizzardo E, Controlled Radical Polymerization Guide 2012. [Google Scholar]
- [29].Hawker CJ, Bosman AW, Harth E, Chem. Rev 2001, 101, 3661. [DOI] [PubMed] [Google Scholar]
- [30].Hawker CJ, J. Am. Chem. Soc 1994, 116, 11185. [Google Scholar]
- [31].Benoit D, Chaplinski V, Braslau R, Hawker CJ, J. Am. Chem. Soc 1999, 121, 3904. [Google Scholar]
- [32].Braunecker WA, Matyjaszewski K, Prog. Polym. Sci 2007, 32, 93. [Google Scholar]
- [33].Haddleton DM, Crossman MC, Dana BH, Duncalf DJ, Heming AM, Kukulj D, Shooter AJ, Macromolecules 1999, 32, 2110. [Google Scholar]
- [34].Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH, Macromolecules 1998, 31, 5559. [Google Scholar]
- [35].Lowe AB, McCormick CL, Prog. Polym. Sci 2007, 32, 283. [Google Scholar]
- [36].Bielawski CW, Grubbs RH, Prog. Polym. Sci 2007, 32, 1. [Google Scholar]
- [37].Yeow J, Chapman R, Gormley AJ, Boyer C, Chem. Soc. Rev 2018, 47, 4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maric M, Curr. Org. Chem 2018, 22, 1264. [Google Scholar]
- [39].Lee N, Wooley K, Sigma-Aldrich Co. LLC. Materials Science. 2012,. [Google Scholar]
- [40].Jangu C, Wang J-HH, Wang D, Sharick S, Heflin JR, Winey KI, Colby RH, Long TE, Macromol. Chem. Phys 2014, 215, 1319. [Google Scholar]
- [41].Matyjaszewski K, Tsarevsky NV, J. Am. Chem. Soc 2014, 136, 6513. [DOI] [PubMed] [Google Scholar]
- [42].Matyjaszewski K, Macromol. Symp 1998, 134, 105. [Google Scholar]
- [43].Di Lena F, Matyjaszewski K, Prog. Polym. Sci 2010, 35, 959. [Google Scholar]
- [44].Braunecker WA, Brown WC, Morelli BC, Tang W, Poli R, Matyjaszewski K, Macromolecules 2007, 40, 8576. [Google Scholar]
- [45].Tang W, Kwak Y, Braunecker W, Tsarevsky NV, Coote ML, Matyjaszewski K, J. Am. Chem. Soc 2008, 130, 10702. [DOI] [PubMed] [Google Scholar]
- [46].Tsarevsky NV, Braunecker WA, Brooks SJ, Matyjaszewski K, Macromolecules 2006, 39, 6817. [Google Scholar]
- [47].Fantin M, Isse AA, Gennaro A, Matyjaszewski K, Macromolecules 2015, 48, 6862. [Google Scholar]
- [48].Liu X, Chang H, Li Y, Huck WTS, Zheng Z, ACS Appl. Mater. Interfaces 2010, 2, 529. [DOI] [PubMed] [Google Scholar]
- [49].Malakooti MH, Kazem N, Yan J, Pan C, Markvicka EJ, Matyjaszewski K, Majidi C, Adv. Funct. Mater 2019, 29, 1906098. [DOI] [PubMed] [Google Scholar]
- [50].Printz AD, Savagatrup S, Burke DJ, Purdy TN, Lipomi DJ, RSC Adv. 2014, 4, 13635. [Google Scholar]
- [51].Savagatrup S, Printz AD, Rodriquez D, Lipomi DJ, Macromolecules 2014, 47, 1981. [Google Scholar]
- [52].Savagatrup S, Printz AD, O’Connor TF, Zaretski AV, Lipomi DJ, Chem. Mater 2014, 26, 3028. [Google Scholar]
- [53].Savagatrup S, Chan E, Renteria-Garcia SM, Printz AD, Zaretski AV, O’Connor TF, Rodriquez D, Valle E, Lipomi DJ, Adv. Funct. Mater 2015, 25, 427. [Google Scholar]
- [54].Savagatrup S, Makaram AS, Burke DJ, Lipomi DJ, Adv. Funct. Mater 2014, 24, 1169. [Google Scholar]
- [55].Baek P, Kerr-Phillips T, Damavandi M, Chaudhary OJ, Malmstrom J, Chan EWC, Shaw P, Burn P, Barker D, Travas-Sejdic J, Eur. Polym. J 2016, 84, 355. [Google Scholar]
- [56].Quinn JF, Chaplin RP, Davis TP, J. Polym. Sci. Part A Polym. Chem 2002, 40, 2956. [Google Scholar]
- [57].Moad G, Rizzardo E, Thang S, Sigma-Aldrich Co. LLC. Materials Science 2012,. [Google Scholar]
- [58].McCormick CL, Lowe AB, Acc. Chem. Res 2004, 37, 312. [DOI] [PubMed] [Google Scholar]
- [59].Hill MR, Carmean RN, Sumerlin BS, Macromolecules 2015, 48, 5459. [Google Scholar]
- [60].Willcock H, O’Reilly RK, Polym. Chem 2010, 1, 149. [Google Scholar]
- [61].Chong YK, Krstina J, Le TPT, Moad G, Postma A, Rizzardo E, Thang SH, Macromolecules 2003, 36, 2256. [Google Scholar]
- [62].Chiefari J, Mayadunne RTA, Moad CL, Moad G, Rizzardo E, Postma A, Skidmore MA, Thang SH, Macromolecules 2003, 36, 2273. [Google Scholar]
- [63].Moad G, Rizzardo E, Thang SH, Polymer (Guildf). 2008, 49, 1079. [Google Scholar]
- [64].Elschner A, Kirchmeyer S, Lövenich W, Merker U, Reuter K, PEDOT: Principles and applications of an intrinsically conductive polyme; CRC Press, 2010. [Google Scholar]
- [65].Peters JM, Gonzalez FJ, Chem. Res. Toxicol 2011, 24, 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Krafft MP, Riess JG, Curr. Opin. Colloid Interface Sci 2015, 20, 192. [Google Scholar]
- [67].Santos AG, Ribeiro BD, Alviano DS, Coelho MAZ, RSC Adv. 2014, 4, 37157. [Google Scholar]
- [68].Kayser LV, Russell MD, Rodriquez D, Abuhamdieh SN, Dhong C, Khan S, Stein AN, Ramírez J, Lipomi DJ, Chem. Mater 2018, 30, 4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Keef CV, Kayser LV, Tronboll S, Carpenter CW, Root NB, Finn M, O’Connor TF, Abuhamdieh SN, Davies DM, Runser R, Meng YS, Ramachandran VS, Lipomi DJ, Adv. Intell. Syst 2020, 2, 2000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Root SE, Carpenter CW, Kayser LV, Rodriquez D, Davies DM, Wang S, Tan STM, Meng YS, Lipomi DJ, ACS Omega 2018, 3, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Root SE, Savagatrup S, Printz AD, Rodriquez D, Lipomi DJ, Chem. Rev 2017, 117, 6467. [DOI] [PubMed] [Google Scholar]
- [72].Xia Y, Whitesides GM, Annu. Rev. Mater. Sci 1998, 28, 153. [Google Scholar]
- [73].Whitesides GM, Isr. J. Chem 2016, 56, 66. [Google Scholar]
- [74].Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM, Chem. Rev 2005, 105, 1103. [DOI] [PubMed] [Google Scholar]
- [75].Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S, Chem. Soc. Rev 2013, 42, 7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Moad G, Rizzardo E, Thang SH, In Australian Journal of Chemistry; 2012; Vol. 65, pp. 985–1076. [Google Scholar]
- [77].Perrier SS, Macromolecules 2017, 50, 38. [Google Scholar]
- [78].Le X, Lu W, Zhang J, Chen T, Adv. Sci 2019, 6, 1801584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jiang Z, Tan ML, Taheri M, Yan Q, Tsuzuki T, Gardiner MG, Diggle B, Connal LA, Angew. Chemie Int. Ed 2020, 59, 7049. [DOI] [PubMed] [Google Scholar]
- [80].Jiang D, Zhu H, Yang W, Cui L, Liu J, Sensors Actuators, B Chem. 2017, 239, 193. [Google Scholar]
- [81].Balamurugan SS, Bantchev GB, Yang YM, McCarley RL, Angew. Chemie-International Ed 2005, 44, 4872. [DOI] [PubMed] [Google Scholar]
- [82].Liu JQ, Yang WR, Tao L, Li D, Boyer C, Davis TP, J. Polym. Sci. Part a-Polymer Chem 2010, 48, 425. [Google Scholar]
- [83].Leone AK, Mueller EA, McNeil AJ, J. Am. Chem. Soc 2018, 140, 15126. [DOI] [PubMed] [Google Scholar]
- [84].Coropceanu V, Cornil J, da Silva Filho DA, Olivier Y, Silbey R, Brédas JL, Chem. Rev 2007, 107, 926. [DOI] [PubMed] [Google Scholar]
- [85].Dou L, Liu Y, Hong Z, Li G, Yang Y, Chem. Rev 2015, 115, 12633. [DOI] [PubMed] [Google Scholar]
- [86].Malika JEL, Sauvé G, McCullough RD, Adv. Mater 2004, 16, 1017. [Google Scholar]
- [87].Yokozawa T, Ohta Y, Chem. Rev 2016, 116, 1950. [DOI] [PubMed] [Google Scholar]
- [88].Bautista MV, Varni AJ, Ayuso-Carrillo J, Tsai C-H, Noonan KJT, ACS Macro Lett. 2020, 9, 1357. [DOI] [PubMed] [Google Scholar]
- [89].Lipomi DJ, Chong H, Vosgueritchian M, Mei J, Bao Z, Sol. Energy Mater. Sol. Cells 2012, 107, 355. [Google Scholar]
- [90].Roth B, Savagatrup S, De Los Santos NV, Hagemann O, Carlé JE, Helgesen M, Livi F, Bundgaard E, Søndergaard RR, Krebs FC, Lipomi DJ, Chem. Mater 2016, 28, 2363. [Google Scholar]
- [91].Wang G-JN, Shaw L, Xu J, Kurosawa T, Schroeder BC, Oh JY, Benight SJ, Bao Z, Adv. Funct. Mater 2016, 26, 7254. [Google Scholar]
- [92].Oh JY, Rondeau-Gagné S, Chiu YC, Chortos A, Lissel F, Wang GJN, Schroeder BC, Kurosawa T, Lopez J, Katsumata T, Xu J, Zhu C, Gu X, Bae WG, Kim Y, Jin L, Chung JW, Tok JBH, Bao Z, Nature 2016, 539, 411. [DOI] [PubMed] [Google Scholar]
- [93].Sugiyama F, Kleinschmidt AT, Kayser LV, Alkhadra MA, Wan JMH, Chiang ASC, Rodriquez D, Root SE, Savagatrup S, Lipomi DJ, Macromolecules 2018, 51, 5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chortos A, Bao Z, Skin-inspired electronic devices. Mater. Today 2014, 17, 321–331. [Google Scholar]
- [95].Wagner S, Bauer S, MRS Bull. 2012, 37, 207. [Google Scholar]
- [96].Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Il Kim T, Chowdhury R, Ying M, Xu L, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang Y, Coleman T, Rogers JA, Science (80-. ). 2011, 333, 838. [DOI] [PubMed] [Google Scholar]
- [97].Shyu TC, Damasceno PF, Dodd PM, Lamoureux A, Xu L, Shlian M, Shtein M, Glotzer SC, Kotov NA, Nat. Mater 2015, 14, 785. [DOI] [PubMed] [Google Scholar]
- [98].Church DC, Pokorski JK, Angew. Chemie 2020, 132, 11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lee PW, Isarov SA, Wallat JD, Molugu SK, Shukla S, Sun JEP, Zhang J, Zheng Y, Lucius Dougherty M, Konkolewicz D, Stewart PL, Steinmetz NF, Hore MJA, Pokorski JK, J. Am. Chem. Soc 2017, 139, 3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Leuchs H, Berichte der Dtsch. Chem. Gesellschaft 1906, 39, 857. [Google Scholar]
- [101].Zotzmann J, Behl M, Hofmann D, Lendlein A, Adv. Mater 2010, 22, 3424. [DOI] [PubMed] [Google Scholar]
- [102].Kahveci MU, Yagci Y, Avgeropoulos A, Tsitsilianis C, In Reference Module in Materials Science and Materials Engineering; Elsevier, 2016. [Google Scholar]
- [103].Gorman CB, Ginsburg EJ, Grubbs RH, J. Am. Chem. Soc 1993, 115, 1397. [Google Scholar]
- [104].Sutthasupa S, Shiotsuki M, Sanda F, Polym. J 2010, 42, 905. [Google Scholar]
- [105].Leitgeb A, Wappel J, Slugovc C, Polymer (Guildf). 2010, 51, 2927. [Google Scholar]
- [106].Kim D-Y, Shin S, Yoon W-J, Choi Y-J, Hwang J-K, Kim J-S, Lee C-R, Choi T-L, Jeong K-U, Adv. Funct. Mater 2017, 27, 1606294. [Google Scholar]
- [107].Dean LM, Wu Q, Alshangiti O, Moore JS, Sottos NR, ACS Macro Lett. 2020, 15, 819. [DOI] [PubMed] [Google Scholar]
- [108].Robertson ID, Yourdkhani M, Centellas PJ, Aw JE, Ivanoff DG, Goli E, Lloyd EM, Dean LM, Sottos NR, Geubelle PH, Moore JS, White SR, Nature 2018, 557, 223. [DOI] [PubMed] [Google Scholar]
- [109].Groover MP, Fundamentals of Modern Manufacturing: Materials, Proceses, and Systems, 6th Edition; 7th ed.; John Wiley & Sons, 2015. [Google Scholar]
- [110].Terryn S, Brancart J, Lefeber D, Van Assche G, Vanderborght B, Sci. Robot 2017, 2, 16. [DOI] [PubMed] [Google Scholar]
- [111].He Q, Wang Z, Wang Y, Minori A, Tolley MT, Cai S, Sci. Adv 2019, 5, eaax5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jiang H, Li C, Huang X, Nanoscale 2013, 5, 5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ohm C, Brehmer M, Zentel R, Liquid crystalline elastomers as actuators and sensors. Adv. Mater 2010, 22, 3366–3387. [DOI] [PubMed] [Google Scholar]
- [114].Carpenter CW, Dhong C, Root NB, Rodriquez D, Abdo EE, Skelil K, Alkhadra MA, Ramírez J, Ramachandran VS, Lipomi DJ, Mater. Horizons 2018, 5, 70. [Google Scholar]
- [115].Bar-Cohen Y, In Electroactive Polymer Actuators and Devices (EAPAD) 2010; SPIE, 2010; Vol. 7642, p. 764206. [Google Scholar]
- [116].Lee J, Guo Y, Choi YJ, Jung S, Seol D, Choi S, Kim JH, Kim Y, Jeong KU, Ahn SK, Soft Matter 2020, 16, 2695. [DOI] [PubMed] [Google Scholar]
- [117].Ware TH, White TJ, Polym. Chem 2015, 6, 4835. [Google Scholar]
- [118].Ware TH, McConney ME, Wie JJ, Tondiglia VP, White TJ, Science (80-. ). 2015, 347, 982. [DOI] [PubMed] [Google Scholar]
- [119].Ahmed EM, J. Adv. Res 2015, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Guiseppi-Elie A, Biomaterials 2010, 31, 2701. [DOI] [PubMed] [Google Scholar]
- [121].Zhang X, Pint CL, Lee MH, Schubert BE, Jamshidi A, Takei K, Ko H, Gillies A, Bardhan R, Urban JJ, Wu M, Fearing R, Javey A, Nano Lett. 2011, 11, 3239. [DOI] [PubMed] [Google Scholar]
- [122].Tee BCK, Wang C, Allen R, Bao Z, Nat. Nanotechnol 2012, 7, 825. [DOI] [PubMed] [Google Scholar]
- [123].Wang Y, Huang F, Chen X, Wang XW, Bin Zhang W, Peng J, Li J, Zhai M, Chem. Mater 2018, 30, 4289. [Google Scholar]
- [124].Di X, Hang C, Xu Y, Ma Q, Li F, Sun P, Wu G, Mater. Chem. Front 2020, 4, 189. [Google Scholar]
- [125].Yu Y, Nakano M, Ikeda T, Nature 2003, 425, 145. [DOI] [PubMed] [Google Scholar]
- [126].Yesodha SK, Pillai CKS, Tsutsumi N, Prog. Polym. Sci 2004, 29, 45. [Google Scholar]
- [127].Wang P, Ming H, Zhang JY, Liang ZC, Lu YH, Zhang QJ, Xie JP, Tian YP, Opt. Commun 2002, 203, 159. [Google Scholar]
- [128].van Oosten CL, Bastiaansen CW, Broer DJ, Nat Mater 2009, 8, 677. [DOI] [PubMed] [Google Scholar]
- [129].Datskos PG, Sepaniak MJ, Tipple CA, Lavrik N, Sensors and Actuators B-Chemical 2001, 76, 393. [Google Scholar]
- [130].Victor JG, Torkelson JM, Macromolecules 1987, 20, 2241. [Google Scholar]
- [131].Teboul V, Accary JB, Chrysos M, Phys. Rev. E 2013, 87. [Google Scholar]
- [132].Mi Y, Stern SA, Trohalaki S, J. Memb. Sci. 1993, 77, 41. [Google Scholar]
- [133].Baczkowski ML, Wang DH, Lee DH, Lee KM, Smith ML, White TJ, Tan LS, ACS Macro Lett. 2017, 6, 1432. [DOI] [PubMed] [Google Scholar]
- [134].Lee BKM, Koerner H, Wang DH, Tan LS, White TJ, Vaia RA, Macromolecules 2012, 45, 7527. [Google Scholar]
- [135].Oribe S, Yoshida S, Kusama S, ichiro Osawa S, Nakagawa A, Iwasaki M, Tominaga T, Nishizawa M, Sci. Rep 2019, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Van Boven RW, Johnson KO, Neurology 1994, 44, 1074. [DOI] [PubMed] [Google Scholar]
- [137].Craig JC, Baihua X, Percept. Psychophys 1990, 47, 22. [DOI] [PubMed] [Google Scholar]
- [138].Dahiya RS, Metta G, Valle M, Sandini G, IEEE Trans. Robot 2010, 26, 1. [Google Scholar]
- [139].Saal HP, Delhaye BP, Rayhaun BC, Bensmaia SJ, Proc. Natl. Acad. Sci. U. S. A 2017, 114, E5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mcculloch I, Heeney M, Chabinyc ML, Delongchamp D, Kline RJ, Cölle M, Duffy W, Fischer D, Gundlach D, Hamadani B, Hamilton R, Richter L, Salleo A, Shkunov M, Sparrowe D, Tierney S, Zhang W, Adv. Mater 2009, 21, 1091. [Google Scholar]
- [141].Wang K, Amin K, An Z, Cai Z, Chen H, Chen H, Dong Y, Feng X, Fu W, Gu J, Han Y, Hu D, Hu R, Huang D, Huang F, Huang F, Huang Y, Jin J, Jin X, Li Q, Li T, Li Z, Li Z, Liu J, Liu J, Liu S, Peng H, Qin A, Qing X, Shen Y, Shi J, Sun X, Tong B, Wang B, Wang H, Wang L, Wang S, Wei Z, Xie T, Xu C, Xu H, Xu ZK, Yang B, Yu Y, Zeng X, Zhan X, Zhang G, Zhang J, Zhang MQ, Zhang XZ, Zhang X, Zhang Y, Zhang Y, Zhao C, Zhao W, Zhou Y, Zhou Z, Zhu J, Zhu X, Tang BZ, Advanced functional polymer materials. Mater. Chem. Front 2020, 4, 1803–1915. [Google Scholar]
- [142].Swager TM, 2017.
- [143].Wang M, Baek P, Akbarinejad A, Barker D, Travas-Sejdic J, Conjugated polymers and composites for stretchable organic electronics. J. Mater. Chem. C 2019, 7, 5534–5552. [Google Scholar]
- [144].Feig VR, Tran H, Bao Z, ACS Cent. Sci 2018, 4, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tran H, Feig VR, Liu K, Wu HC, Chen R, Xu J, Deisseroth K, Bao Z, ACS Cent. Sci 2019, 5, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sessolo M, Khodagholy D, Rivnay J, Maddalena F, Gleyzes M, Steidl E, Buisson B, Malliaras GG, Adv. Mater 2013, 25, 2135. [DOI] [PubMed] [Google Scholar]
- [147].Yeon C, Kim G, Lim JW, Yun SJ, RSC Adv. 2017, 7, 5888. [Google Scholar]
- [148].Yang X, Kirsch J, Olsen EV, Fergus JW, Simonian AL, Anti-fouling PEDOT:PSS modification on glassy carbon electrodes for continuous monitoring of tricresyl phosphate. Sensors Actuators, B Chem. 2013, 177, 659–667. [Google Scholar]
- [149].Tseghai GB, Mengistie DA, Malengier B, Fante KA, Van Langenhove L, Sensors 2020, 20, 1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhang H, Wang D, Wu N, Li C, Zhu C, Zhao N, Xu J, ACS Appl. Mater. Interfaces 2020, 12, 9833. [DOI] [PubMed] [Google Scholar]
- [151].Ko H, Javey A, Acc. Chem. Res 2017, 50, 691. [DOI] [PubMed] [Google Scholar]
- [152].Nadgorny M, Ameli A, Functional Polymers and Nanocomposites for 3D Printing of Smart Structures and Devices. ACS Appl. Mater. Interfaces 2018, 10, 17489–17507. [DOI] [PubMed] [Google Scholar]