Abstract
Objective:
To compare the corrosion behavior of commercially available surface modified nickel titanium (NiTi) arch wires with respect to a conventional NiTi and to evaluate its association with surface characteristics.
Materials and Methods:
Five types of surface modified arch wires and a conventional NiTi arch wire, all from different manufacturers, were evaluated for their corrosion resistance from breakdown potential in an anodic polarization scan in Ringer's solution. Surface characteristics were determined from scanning electron microscopy, atomic force microscopy, and energy dispersive analysis. One-way analysis of variance and post hoc Duncan's multiple range tests were used to evaluate statistical significance.
Results:
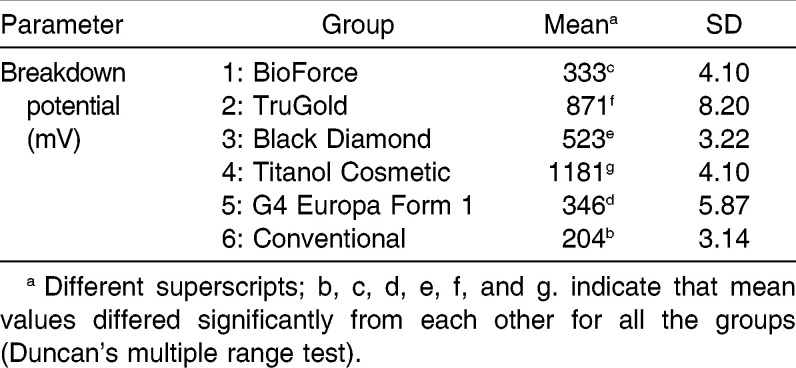
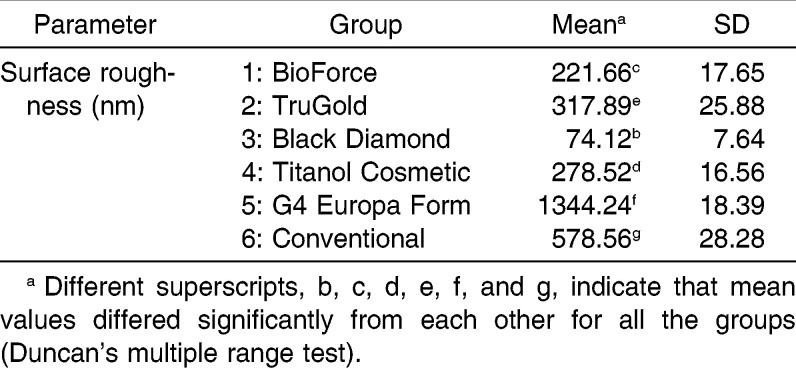
Surface modified NiTi wires showed significant improvement in corrosion resistance and reduction in surface roughness values. Breakdown potentials increased in the order of group 6 (conventional; 204 mV) < group 1 (nitride; 333 mV) < group 5 (epoxy resin; 346mV) < group 3 (oxide; 523 mV) < group 2 (gold; 872 mV) < group 4 (Teflon; 1181 mV), but root mean square (RMS) roughness values, which indicated surface roughness, followed a different pattern: group 3 (oxide; 74.12 nm) < group 1 (nitride; 221.651 nm) < group 4 (Teflon; 278.523 nm) < group 2 (gold; 317.894 nm) < group 5 (epoxy resin; 344.236 nm) < group 6 (conventional; 578.555 nm).
Conclusions:
Surface modification of NiTi wires proved to be effective in improving its corrosion resistance and decreasing surface roughness. However, neither factor could maintain a direct, one-to-one relationship. It meant that the type and nature of coating material can effectively influence the anticorrosive features of NiTi wires, compared with its surface roughness values.
Keywords: Corrosion, Surface modified NiTi, Root mean square roughness value, Anodic polarization, Breakdown potential
INTRODUCTION
Orthodontic appliances undergo corrosion in the oral cavity, which is a continuous degradation process brought out by wide variations in temperature, pH, microbial, and salivary factors. Corrosion of nickel titanium (NiTi) arch wires raises major concern because of their high nickel content (47% to 50% nickel) and associated biocompatibility issues.1 Because nickel atoms are not strongly held in NiTi alloy as in other intermetallic compounds,2 its release into the oral cavity during clinical use is reported to cause several types of adverse reactions ranging from mild hypersensitive responses, such as contact dermatitis, to extremes of cytotoxic, mutagenic, and carcinogenic changes. NiTi arch wires are constantly exposed to the oral cavity and carry greater risk for toxic effects,3 unlike nickel alloy implants inserted elsewhere in the body, where a protective fibrous capsule forms around them. Corrosion also affects the physical properties of NiTi wires,4 including their crucial shape memory and super-elasticity features,5 which are vital for clinical performance.6 Moreover, corrosion products can increase wire-bracket frictional coefficients and decelerate tooth movement.7,8 In acidic pH, hydrogen ions penetrate into the NiTi alloy and form brittle titanium hydrides, which cause arch wire fracture.9 Similarly, fluoride ions present in various anticaries preparations are also known to increase their susceptibility to corrosion.10
NiTi arch wires are made from an ingot through multiple stages of heat treatment and by different types of wire drawing methods.2 This causes formation of several oxides of titanium and nickel3 (TiO2, TiO, Ti2O5, and NiO), which provide the inherent corrosion resistance.11 Even though this mechanism renders good anticorrosive properties for NiTi alloys,12 such oxides are not infallible and can initiate corrosion on disruption.13 The high surface roughness of NiTi is cited as one reason for its corrosive characteristics.14–16 NiTi arch wires have a rough surface compared with other arch wire materials, such as stainless steel, chromium cobalt, and titanium molybdenum alloys; this rough surface is expressed as a parameter known as the root mean square roughness (RMS) value, which is usually determined by profilometric and optical methods.17,18 However, certain other studies have related corrosion characteristics of NiTi wires to the residual stresses incorporated into it during manufacturing and not to its surface roughness.7,19,20 This means the effects of surface features on corrosive features of NiTi wires are not conclusively known. In this context, surface modification of NiTi arch wires is considered a favorable option for improving its surface roughness and thereby its corrosion resistance and biocompatibility.21 Additional benefits expected are better esthetics and reduced frictional coefficients.22 Nitride ions, metals, oxides and esthetic materials have therefore been used by different manufacturers to modify the surface of NiTi arch wires. A comprehensive study comparing the efficacy of such coatings on the corrosion behavior of NiTi wires has not been reported so far.
One of the common methods used for corrosion testing is potentiostatic anodic polarization scan, which determines the transition of a sample from a passive to an active state. This involves assessment of the corrosion resistance of arch wires based on their breakdown potential, the point at which the material loses its protective surface layer and corrosion is initiated.20 The aim of this study, therefore, was to determine the corrosion behavior of commercially available surface modified NiTi wires along with a conventional type in an in vitro setup and to find out whether there were any correlations with surface topographic features.
MATERIALS AND METHODS
Five study groups consisted of five types of surface modified nickel titanium wires and the control group consisted of conventional NiTi in 0.016″ dimensions from different manufacturers (n = 6 per group). Group 1, BioForce Ionguard NiTi (GAC International, Bohemia, NY); group 2, TruGold NiTi (Ortho Technology, Inc, Tampa, Fla); group 3, Black Diamond NiTi (Class One Orthodontics, Lubbock, Tex); group 4, Titanol Cosmetic NiTi (Forestadent, Pforzheim, Germany); group 5, G4 Nickel Titanium Europa Form 1 (G&H Wire Company, Franklin, Ind); and group 6 (control), conventional NiTi (Ortho Organizers, San Marcos, Calif).
Preliminary surface analysis of the arch wires was done with scanning electron microscopy (SEM) at 500× magnification and elemental mapping with energy dispersive analysis (EDS) (SNE-3000M model, SEC, Woncheon-Dong Suwon, South Korea, Korea) at three different areas. Standardless analyses (qualitative/semiquantitative with ZAF correction) were performed with a compositional accuracy of ±1% percent. Surface roughness was evaluated with Solver Pro EC Atomic Force Microscope (AFM; NT-MDT Company, Zelenograd, Moscow). All measurements were carried out in contact mode using a standard conical silicon tip attached to a cantilever with a force constant of 5 nNm−1 with a frequency range from 50 to 150 Hz. The radius of curvature of the tip was 10 nm, and the cone angle was <22°. The scan area was 50 × 50 µm of each sample at three different locations. Averages of these from six wire samples per group were taken to express the surface roughness as the RMS value in nanometers using the equipment's proprietary software.
The electrochemical testing was done as per the method commonly used for potentiostatic anodic polarization experiments.22 A 30-mm-long arch wire sample was clamped to a crocodile clip brazed to a stainless steel rod. The steel rod and crocodile clips were covered with a nonconducting tape to avoid contact with the solution. The electrolyte was Ringer's solution23 (NaCl, 9.00 g/L; KCl, 0.43 g/L; CaCl2, 0.24 g/L; and NaHCO3, 0.20 g/L; pH 7.4). A 1000 mL round bottom flask was modified; into this a test electrode (sample), platinized auxiliary electrodes, a Luggin capillary with salt-bridge connection to the reference electrode, and a thermometer were inserted. The saturated calomel electrode was the reference electrode for measuring electrode potential. The Luggin capillary was kept at a distance of 2 mm from the working electrode to avoid ohmic drop. The temperature of the polarization cell was maintained at 37°C by a thermostated water bath.
The samples were immersed in the electrolyte long enough to achieve a steady state between electrolyte and specimen, which was around 30 minutes. The changes in potential during that time were monitored with respect to the saturated calomel electrode using the Solartron Electrochemical Interface Model 1287, (Solartron Metrology Ltd; Leicestershire, UK) and the stable potential achieved was taken as the open circuit potential (OCP). After determining OCP, specimens were anodically polarized from a starting potential, 0.200 mV below OCP, at a scan rate of 10 mV/min. Applied electrode potential vs current density plots were obtained using the proprietary Corrware software (Solartron Metrology Ltd; Leicestershire, UK), and the potential at which there was a sharp increase in anodic current density in the curve was taken as the breakdown potential.
RMS values and breakdown potentials of the samples (n = 6 per group) were subjected to one-way analysis of variance (P < .05) along with post hoc comparison with Duncan's multiple range test to elucidate multiple comparisons among different groups.
RESULTS
The open circuit potential and its corresponding breakdown potential for each group are illustrated in the representative graphs in Figures 1 and 2. Significant differences (P < .001) in breakdown potentials were observed among the study groups (Table 1). Surface modified NiTi wires had higher values of breakdown potential than the conventional type, which reflected their superior corrosion resistance.
Figure 1.
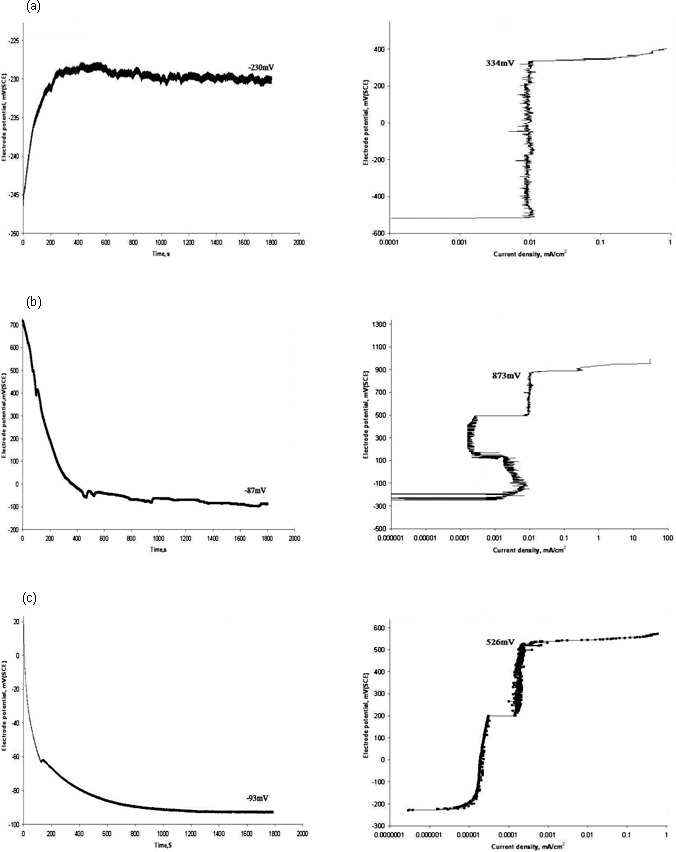
Graphs of electrochemical analysis with open circuit potential (left) and corresponding breakdown potential (right) for arch wires. (a) Group 1, (b) Group 2, and (c) Group 3.
Figure 2.
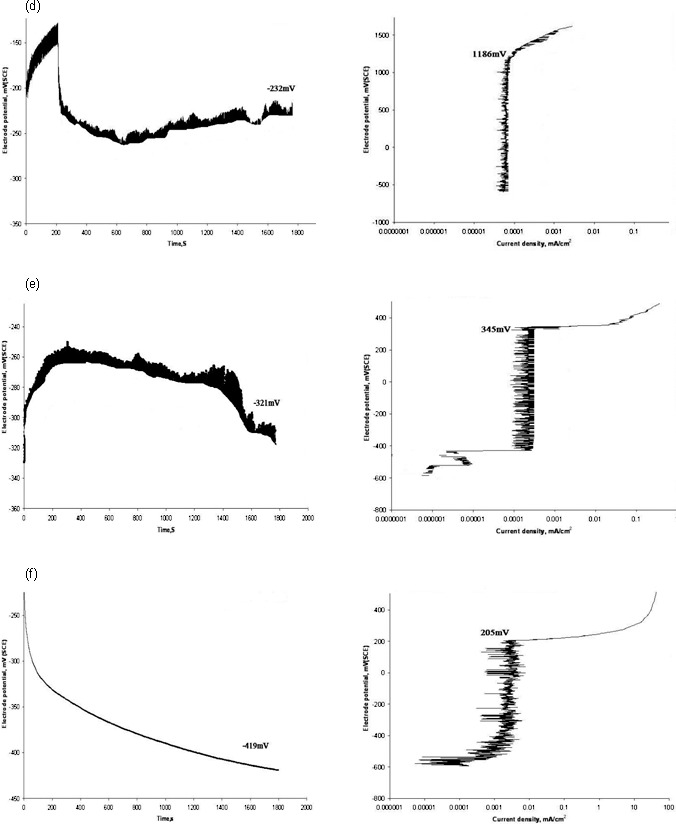
Graphs of electrochemical analysis with open circuit potential (left) and corresponding breakdown potential (right) for arch wires. (d) Group 4, (e) Group 5, and (f) Group 6.
Table 1.
One-Way Analysis of Variance of Mean Breakdown Potential Values for Different Groups (n = 6 per Group; F value = 32682.285; and P < .001)
Energy dispersive analysis of arch wires is shown in Figure 3. Conventional NiTi showed principal constituent elements: nickel and titanium, along with such trace elements as aluminum, iron, and chromium. Nitride ions predominated on the surface of group 1. Presence of gold was conspicuous for group 2. Barium, carbon, and oxygen were seen on group 3. Fluoride ions were noticed in the Teflon layer in group 4. Epoxy resin modified wires (group 5) demonstrated carbon and oxygen on the surface. SEM images are presented in Figure 4. Conventional NiTi showed a rough topography with striations and longitudinal grooving. Group 1 (nitride) had a coated texture with disruptions. Group 2, which had gold coating displayed a matted finish in the longitudinal axis of the wire. The Black Diamond NiTi (Group 3) showed a smooth exterior with relatively few imperfections. The Teflon layer of group 4 showed surface elevations and depressions. Group 5 had resin cover with irregularities on the wire surface.
Figure 3.
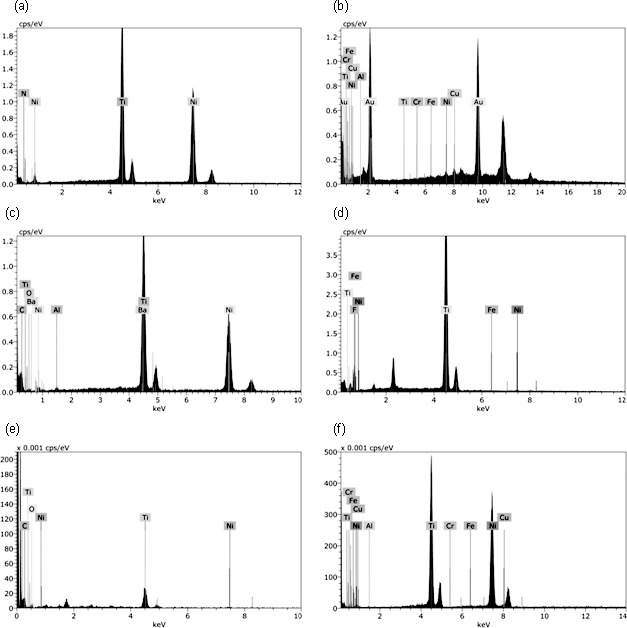
Energy dispersive analysis of wires from (a) Group 1, (b) Group 2, (c) Group 3, (d) Group 4, (e) Group 5, and (f) Group 6.
Figure 4.
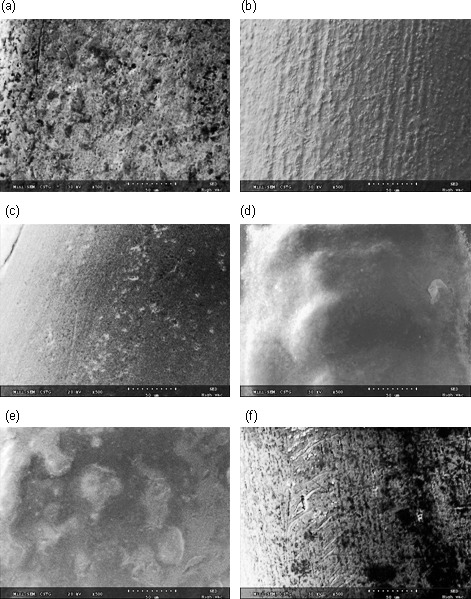
Scanning electron micrographs of wires at 500× magnification for (a) Group 1, (b) Group 2, (c) Group 3, (d) Group 4, (e) Group 5, and (f) Group 6.
Figure 5 shows the three-dimensional AFM views of arch wires with RMS values that showed significant (P<.001) differences among groups (Table 2). Interruptions in the nitride layer of group 1 and the conglomeration of gold particles in group 2 were visible in AFM. Group 3, which had an oxide coating and a plain surface, had the lowest RMS value (74.12 nm). The uneven nature of the Teflon coating (group 4) and the surface discrepancies of the resin layers (group 5) were apparent in the AFM. Conventional NiTi (group 6) showed high surface roughness compared with other groups (Table 2).
Figure 5.
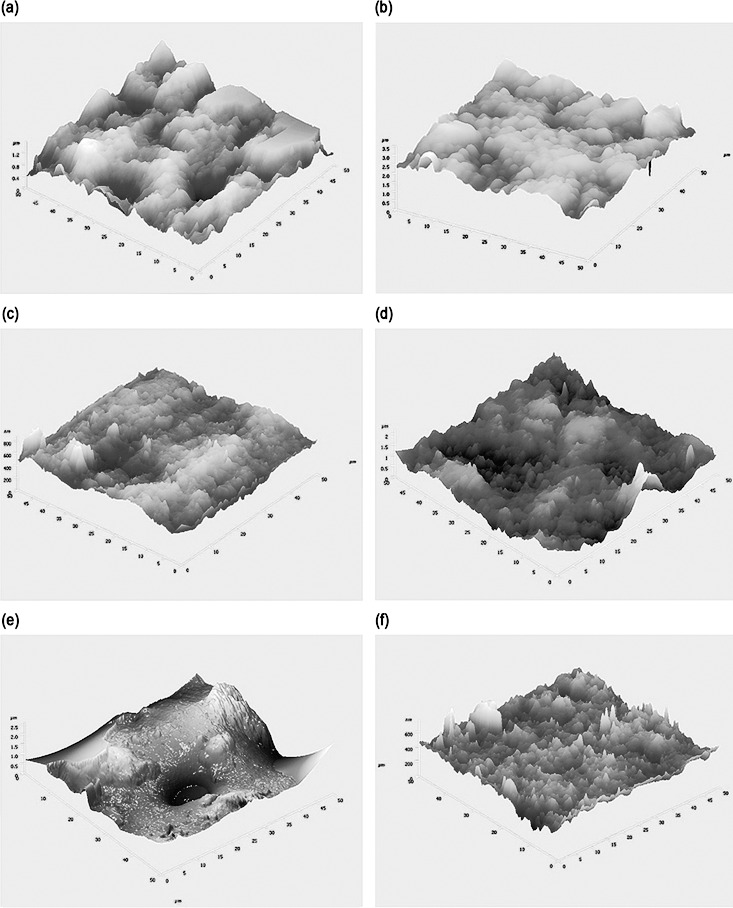
Three-dimensional atomic force microscope views of wires: (a) Group 1, RMS value = 221.66 nm; (b) Group 2, RMS value = 317.89 nm; (c) Group 3, RMS value = 74.12 nm; (d) Group 4, RMS value = 278.52 nm; (e) Group 5, RMS value = 344.24 nm; and (f) Group 6, RMS value = 578.56 nm.
Table 2.
One-Way Analysis of Variance of Mean Surface Roughness Values for Different Groups (n = 6 per group; F value = 98.053; and P < .001)
DISCUSSION
In vitro anodic polarization studies simulate the natural corrosion mechanism that usually happens over a protracted duration of time. In this method, the open circuit potential of the samples with respect to an electrolyte is initially determined. Artificial saliva,19 saline,14 or Ringer's solution23 are the common electrolytes used for corrosion studies. Samples of different groups had OCP values (−87 to −321 mV) significantly different from that of the control (−419 mV). OCP served as a guiding point for initiating the anodic polarization scan to determine the breakdown potential, which represented the minimum potential at which pitting or crevice corrosion can begin in a material.20 The lowest breakdown potential (204 mV) for conventional NiTi indicated its higher propensity for corrosion among other groups. SEM images brought out the surface roughness on conventional NiTi that is usually generated during wire fabrication. From a corrosion point of view, surface pits are related to the galvanic type of corrosion.3
Corrosion resistance of epoxy and nitride wires, along with conventional wires, was previously studied by Kim and Johnson.22 They reported a higher breakdown potential (1800 mV) for epoxy resin wires but could not find any advantages for nitride ion deposited wires compared with conventional (both had 300 mV). Conversely, in our evaluation, both resin and nitride ion modified wires showed higher breakdown potentials (346 mV and 333 mV, respectively) than conventional (204 mV). This can be attributable to the differences in products from different manufacturers used in both studies. Possibilities of such disparities among different NiTi wire products and with different electrolyte media have been cited by Lee and coworkers7 and Huang.19 The improved corrosion resistance shown by nitride ion modified wires in our investigation was akin to findings of Tan et al.21 Besides, we noticed superior anticorrosive features for black oxide coated wires (group 3; 526 mV) compared with the control.
Currently, there is an increasing trend of using esthetic materials like Teflon, gold, and epoxy resins as wire surface modifying materials and therefore their corrosion perspectives are worth evaluating. In the present study, Teflon (1181 mV) and gold (872 mV) coated wires demonstrated maximum corrosion resistance indicating their suitability as an esthetic coating material for NiTi wires. Though the Teflon layer showed an uneven surface in the SEM and AFM images, it still had the highest breakdown potential values. It proved that the type of coating material, rather than surface roughness values, should play a decisive role in expressing anticorrosive features. On the other hand, resin coated wires, though they performed better than the control, could not match the breakdown potential (345 mV) of the Teflon (1186 mV) and gold (872 mV) groups. Notwithstanding the differences in breakdown potentials among different groups, our results proved that coatings on NiTi wires can bring out significant changes in their corrosion behavior, which can be of benefit to clinical practice.
Surface roughness of orthodontic arch wires has been studied from the perspectives of friction, corrosion, and esthetics.17 Conventional NiTi showed the highest surface roughness values (578.555 nm) with AFM, which was well within the range of values (100–1300 nm) reported earlier.18,19 Even though group 2 with gold modification showed reasonably good resistance against corrosion (872 mV), it did not feature a smooth topography in the SEM and AFM views. This disproportionate relation was true for group 1 also, where the nitride ion deposited wires registered a low RMS value (221 nm) but did not demonstrate a better breakdown potential. This may be attributable to the fewer but discrete uncoated areas that were visible on the surface in SEM and AFM views. A similar disparity in surface roughness value and breakdown potential was also observed for the Black Diamond modified NiTi, which had an oxide layer. This group had the lowest surface roughness (74.12 nm), which presented barium, carbon, and oxygen in the EDS analysis, but it did not exhibit a higher breakdown potential. Similarly, Teflon and resin modified wires (groups 4 and 5) could not establish a direct relationship between breakdown potential and surface roughness.
Comparing different brands of commercially available NiTi wires, Huang19 analyzed the relationship between surface roughness and corrosion resistance and found surface roughness with AFM and corrosion resistance from polarization resistance,7,19 measured in artificial saliva at normal and acidic pH. Polarization resistance was deduced from the linear polarization slope of potential vs current density plot. Huang noticed differences in corrosion resistance among different brands of conventional NiTi wires and attributed it to stresses incorporated into it during wire drawing and manufacturing and not to the surface roughness values. In our experiment, we had representative samples from all the commercially available surface modified NiTi wires, where corrosion resistance was determined from the breakdown potential. We, too, could not find a direct relationship between RMS values and breakdown potential.
To summarize, corrosion resistance had a material specific pattern in our experiment: group 6 (conventional) < group 1 (nitride) < group 5 (epoxy resin) < group 3 (oxide) < group 2 (gold) < group 4 (Teflon). On the other hand, surface roughness followed a different order: group 3 (oxide) < group 1 (nitride) < group 4 (Teflon) < group 2 (gold) < group 5 (epoxy resin) < group 6 (conventional). Obviously, the progressions did not have a one-to-one relation. This suggested that although surface modification can improve surface roughness and corrosion resistance of NiTi wires as two different entities, both the parameters cannot be correlated to elicit a direct relationship. Increase in breakdown potential achieved seems to be more dependent on the material used for coating. This means, changes in surface roughness values cannot be taken as a determining factor in predicting corrosion behavior of surface modified NiTi wires. Also, in vitro findings can only give an estimate of the actual efficacy of coatings, which can differ in clinical use.
CONCLUSIONS
Surface modified NiTi arch wires showed significant improvement in corrosion resistance compared with conventional NiTi.
Similarly, surface roughness values also underwent considerable modification with coating.
Nonetheless, corrosion resistance assessed by breakdown potential and surface roughness calculated from root mean square values of surface modified wires did not show a direct relation.
It is suggested that surface roughness is not a critical parameter for evaluating corrosion resistance for surface modified NiTi wires. The type and nature of coating materials like nitride ions, metals, oxides, Teflon, and resins had a more influential role in predicting the corrosive potential of NiTi wires than their surface roughness values.
ACKNOWLEDGMENTS
The authors are grateful to Dr Kamachi Mudali, senior scientist at Corrosion Science Division, Indira Gandhi Centre for Atomic Research, Department of Atomic Energy, Governmentt of India, Kalpakkam, Tamil Nadu, PhD, for giving necessary assistance in the experiment and to Kurian Mathew Abraham, PhD, for the statistics.
REFERENCES
- 1.Locci P, Lilli C, Marinucci L, et al. In vitro cytotoxic effects of orthodontic appliances. J Biomed Mater Res. 2000;53:560–567. doi: 10.1002/1097-4636(200009)53:5<560::aid-jbm16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Brantley WA. Orthodontic wires. In: Brantley WA, Eliadas T, editors. Orthodontic Materials Scientific and Clinical Aspects. Stuttgart, Germany: Thieme; 2001. pp. 77–103. [Google Scholar]
- 3.Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222–237. doi: 10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.von Fraunhofer JA. Corrosion of orthodontic devices. Semin Orthod. 1997;3:198–205. doi: 10.1016/s1073-8746(97)80070-9. [DOI] [PubMed] [Google Scholar]
- 5.Kusy RP. A review of contemporary archwires: their properties and characteristics. Angle Orthod. 1997;67:197–207. doi: 10.1043/0003-3219(1997)067<0197:AROCAT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Biermann MC, Berzins DW, Bradley TG. Thermal analysis of as-received and clinically retrieved copper-nickel-titanium orthodontic archwires. Angle Orthod. 2007;77:499–503. doi: 10.2319/0003-3219(2007)077[0499:TAOAAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Lee TH, Huang TK, Lin SY, Chen LK, Chou MY, Huang HH. Corrosion resistance of different nickel-titanium archwires in acidic fluoride-containing artificial saliva. Angle Orthod. 2010;80:547–553. doi: 10.2319/042909-235.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ. Corrosion of orthodontic appliances–should we care. Am J Orthod Dentofacial Orthop. 2008;133:584–592. doi: 10.1016/j.ajodo.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama K, Hamada K, Moriyama K, Asaoka K. Degradation and fracture of Ni-Ti superelastic wire in an oral cavity. Biomaterials. 2001;22:2257–2262. doi: 10.1016/s0142-9612(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 10.Walker MP, White RJ, Kula KS. Effect of fluoride prophylactic agents on the mechanical properties of nickel-titanium-based orthodontic wires. Am J Orthod Dentofacial Orthop. 2005;127:662–669. doi: 10.1016/j.ajodo.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Iijima M, Endo K, Ohno H, Yonekura Y, Mizoguchi I. Corrosion behavior and surface structure of orthodontic Ni-Ti alloy wires. Dent Mater J. 2001;20:103–113. doi: 10.4012/dmj.20.103. [DOI] [PubMed] [Google Scholar]
- 12.Rondelli G, Vicentini B. Evaluation by electrochemical tests of the passive film stability of equiatomic Ni-Ti alloy also in presence of stress-induced martensite. J Biomed Mater Res. 2000;51:47–54. doi: 10.1002/(sici)1097-4636(200007)51:1<47::aid-jbm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Huang HH, Chiu YH, Lee TH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–3592. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 14.Hunt NP, Cunningham SJ, Golden CG, Sheriff M. An investigation into the effects of polishing on surface hardness and corrosion of orthodontic archwires. Angle Orthod. 1999;69:433–440. doi: 10.1043/0003-3219(1999)069<0433:AIITEO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Oshida Y, Sachdeva RC, Miyazaki S. Microanalytical characterization and surface modification of TiNi orthodontic archwires. Biomed Mater Eng. 1992;2:51–69. [PubMed] [Google Scholar]
- 16.Widu F, Drescher D, Junker R, Bourauel C. Corrosion and biocompatibility of orthodontic wires. J Mater Sci Mater Med. 1999;10:275–281. doi: 10.1023/a:1008953412622. [DOI] [PubMed] [Google Scholar]
- 17.Kusy RP, Whitley JQ, Mayhew MJ, Buckthal JE. Surface roughness of orthodontic archwires via laser spectroscopy. Angle Orthod. 1988;58:33–45. doi: 10.1043/0003-3219(1988)058<0033:SROOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Bourauel C, Fries T, Drescher D, Plietsch R. Surface roughness of orthodontic wires via atomic force microscopy, laser specular reflectance, and profilometry. Eur J Orthod. 1998;20:79–92. doi: 10.1093/ejo/20.1.79. [DOI] [PubMed] [Google Scholar]
- 19.Huang HH. Variation in corrosion resistance of nickel-titanium wires from different manufacturers. Angle Orthod. 2005;75:661–665. doi: 10.1043/0003-3219(2005)75[661:VICRON]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Kao CT, Huang TH. Variations in surface characteristics and corrosion behaviour of metal brackets and wires in different electrolyte solutions. Eur J Orthod. 2010;32:555–560. doi: 10.1093/ejo/cjp146. [DOI] [PubMed] [Google Scholar]
- 21.Tan L, Dodd RA, Crone WC. Corrosion and wear-corrosion behavior of NiTi modified by plasma source ion implantation. Biomaterials. 2003;24:3931–3939. doi: 10.1016/s0142-9612(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Montero-Ocampo C, Lopez H, Salinas Rodriguez A. Effect of compressive straining on corrosion resistance of a shape memory Ni-Ti alloy in Ringer's solution. J Biomed Mater Res. 1996;32:583–591. doi: 10.1002/(SICI)1097-4636(199612)32:4<583::AID-JBM11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]