Abstract
Objective:
To investigate whether adding ethanolic extracts of propolis (EEP) might influence the antibacterial and mechanical (shear-peel band strength [SPBS]) properties of conventional glass ionomer cement (GIC) used in orthodontic band cementation.
Materials and Methods:
The cement was divided into four groups: one using the original composition and three with 10%, 25%, and 50% EEP added to the liquid and then manipulated. An antimicrobial assay, broth-dilution method was used to determine the antibacterial capacity of the GIC containing EEP. Eighty teeth were used for the mechanical assay, and an Instron testing machine was used to evaluate the SPBS. Kolmogorov-Smirnov and Kruskal-Wallis tests were used for statistical analyses.
Results:
GIC with the addition of 25% and 50% EEP activated inhibition of Streptococcus mutans (ATCC 25175) growth, but this effect did not occur in the group to which 10% EEP was added or in the control GIC group. There was no significant difference between the groups in terms of SPBS (P > .05).
Conclusions:
The addition of EEP may increase antibacterial properties without negatively modifying the mechanical properties of conventional GIC.
Keywords: Antibacterial properties, Shear-peel band strength, GIC
INTRODUCTION
During the orthodontic treatment period, practitioners expect to find plaque accumulation around the orthodontic fixed appliances, primarily around brackets and at the cervical margins of the bands, because of the patients' difficulty in maintaining hygiene and the large number of sites available for microbial colonization.1 A problem experienced by many orthodontists and patients is the development of enamel demineralization and even caries and hyperplastic gingivitis in these areas after the use of brackets and bands bonded and cemented to teeth. Orthodontic cements have been used for band cementation, and although they enhance the adhesion between the band and teeth, unfavorable properties are found in many of these cements, such as high solubility in oral fluids and low bond strengths, which may contribute to demineralization beneath the bands.2–4
Glass ionomer cements (GICs) are the most commonly used material for band cementation. This material exhibits a continuous release and uptake of fluoride, which creates certain antibacterial activities.5–7 Conventional GIC has an antibacterial effect against a small spectrum of microorganisms and a low bactericide potential. Therefore, GIC may not prevent plaque proliferation and development of caries and periodontal disease in some patients.6–8 Caries is a disease usually associated with Streptococcus mutans. Therefore, it is important to evaluate the interaction of orthodontic cements with these bacteria along with their effect on adhesion, bacterial viability, and biofilm formation.9,10
Reports in the literature show that propolis is a natural material with bactericidal activity, and several in vitro and some in vivo studies have shown that it has potential for use in the treatment of bacterial diseases.11–13 Furthermore, concerning bactericidal activity, scholars conducting epidemiologic studies have detected that propolis has many pharmacologic properties, such as antifungal, antiviral, antioxidant, antitumor, and anti-inflammatory effects among others.12 These researchers have associated two mechanisms with the anti-caries properties of propolis: antimicrobial activity against cariogenic bacteria and inhibition of glucosyltransferase activity.13 Although several studies have demonstrated propolis's variable activity against different bacteria and its antibacterial activity on oral microorganisms, very few researchers have studied its effect on the mechanical properties of oral appliances.11,12 The purpose of this study was to evaluate the antibacterial and mechanical properties of GIC with ethanolic extracts of propolis (EEP).
MATERIALS AND METHODS
Preparation of Propolis Extract
Pure propolis was produced for use in this study by honeybees (Apis mellifera L) in Bingöl, Turkey. Propolis samples were frozen at −20°C. These samples were ground (ZM 200, Retsch, Haan, Germany) and bottled in 25 g portion. Then each 25 g sample of propolis was dissolved in 250 mL of ethanol 80% (vol/vol) using a magnetic mixer for 24 hours at room temperature. Next, rough particles were removed from the propolis extract using a filter, and the suspension was separated by centrifugation at 8800 rpm for 30 minutes to produce EEP. Samples were kept at room temperature in the dark until they were used.
Preparation of Propolis Containing GIC
EEP was added to the GIC liquid in the following proportions: 10%, 25%, 50%. Samples were then prepared containing the conventional GIC liquid and the three new solutions. Preparation of propolis containing GIC, assessment of shear-peel band strength (SPBS), and analysis of antibacterial tests were done blindly.
Antimicrobial Assay
The microorganisms and growth condtions
The antimicrobial activity of GIC with EEP was tested on S mutans (ATCC 25175) that was obtained by Refik Saydam National Public Health Agency in Ankara, Turkey. S mutans is a gram-positive bacterium that causes tooth decay. The test organism was maintained on brain heart infusion agar (Merck, Darmstadt, Germany) at +4°C until it was used. The microorganism cultures were activated by transferring them into the brain heart infusion broth, and then they were incubated at 37°C for 2 hours; hence, the overnight culture of S mutans was obtained.
Minimal Inhibitory Concentrations (MICs)
For the antimicrobial assay, we used the broth dilution method developed by Andrews14 to determine the antibacterial capacity of GIC with EEP. The MIC value of GIC, EEP, and GIC with EEP (50%, 25%, 10%) for S mutans was described as the lowest concentration where the microorganism was inhibited.
After the GIC samples with different EEP concentrations were prepared by adding 1 unit powder (GIC) and 1 drop of liquid containing each of the concentrations, they were mixed and dried and placed into groups with three different concentrations of GIC with EEP. Using the stock solutions of GIC and GIC with EEP (50%, 25%, 10%), we prepared standard antibiotics using sterile Mueller Hinton broth (MHB), which contains 10% dimethyl sulfoxide.
Twofold serial dilutions of the GIC and GIC with EEP in stock solutions were arranged in test tubes containing 1000 µg/mL to 3.9 µg/mL of sterile MHB. We ended up with a series of test tubes in which the density of antibacterial substances was reduced in each step. In other words, the first tube's density was 1000 µg/mL, and the density of the last tube was 3.9 µg/mL. These tubes containing fresh MHB and 100 µL of overnight culture of each of the microorganisms were standardized to approximately 108 colony-forming units/mL (using McFarland no. 0.5). Then we studied the contents of these tubes to find out which had the lowest concentration of antibacterial activity and the lowest MIC value.
Mechanical Assay
Eighty healthy human permanent premolars extracted for orthodontic reasons were collected from the Surgery Clinic of the Faculty of Dentistry of Inonu University. The following criteria were used for tooth selection: no cracks, restorations, surface defects, or caries. After extraction, the teeth were stored in a 0.5% chloramine T solution for 1 week and then transferred to distilled water kept at room temperature until the experiments took place (a maximum of 4 months).
The premolars were randomly divided into four groups. Each group received equal numbers of maxillary and mandibular premolars. These teeth were then placed in a plastic model and filled with cold-cure acrylic resin approximating the height of normal bone. The resin was allowed to set, leaving only a few millimeters of the crown protruding between the band and the resin. The axial plane of each tooth was oriented parallel to the plunger of the testing machine. This configuration allowed all forces to be directed parallel to the long axis of the tooth during debanding. The teeth were cleaned with water/pumice slurry in brushes at a slow speed for 15 seconds, then rinsed and dried with an air stream for 10 seconds each.
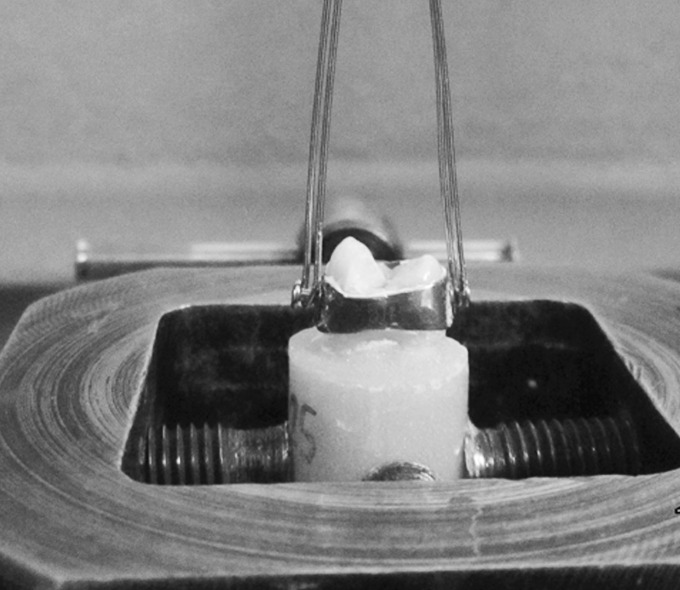
Stainless steel orthodontic premolar bands (Victory Series, 3M Unitek, Monrovia, Calif) with buccal and lingual attachments were fitted and seated around the teeth, including adaptation of the margins with a band seater. All bands were selected and placed by one researcher to eliminate any operator bias in band positioning and fitting. Each band was cemented in place with its specific cement (Figure 1). Excess cement was removed from the occlusal and cervical margins of the band so that it would not influence the results.
Figure 1.

A specimen was prepared for the SPBS test.
All specimens were then stored in distilled water at 37°C for 24 hours before band retention was measured because maximum band retention is obtained 24 hours after cementation. An Instron testing machine (TSTM 02500, Elista, Turkey) was used for the SPBS test at a crosshead speed of 1 mm/min. The force was applied using a 0.9-mm diameter stainless steel wire (Dentaurum, Pforzheim, Germany) from attachments on the buccal and lingual surface of the bands (Figure 2). The maximum load necessary to deband was recorded in newtons. For surface area measurements, bands were turned into a flat surface by cutting. Then the bands were scanned using a two-dimensional browser (Epson Stylus SX235W, Suwa, NGN, Japan). The data obtained from scanning were transferred into the software program 3D Doctor version 4.0 (Able Software Corp, Lexington, Ky) (Figure 3). The surface area of the bands was calculated in mm2 and then converted into megapascals as a ratio of newtons to surface area of the bands.
Figure 2.
The force was applied using a 0.9-mm diameter stainless steel wire from attachments on the buccal and lingual surface of bands.
Figure 3.
The surface area of the bands was calculated using the 3D Doctor software program.
Statistical Analysis
The data were analyzed using SPSS for Windows, version 14.0 (SPSS Inc, Chicago, Ill). Values are expressed as median (min-max). Suitability of normal distribution of data in each of the groups was performed with the Kolmogorov-Smirnov test. The Kruskal-Wallis test was used to compare the four groups. The level of significance was set at P < .05.
RESULTS
Table 1 shows the MIC of groups against the S mutans strains. According to the results obtained using the broth dilution method, EEP enhances the antibacterial effect of GIC. The MIC values of 25% and 50% EEP added to GIC and pure EEP were 125 µg/mL, 31.2 µg/mL, and 15.7 µg/mL, respectively. In particular, the MIC value of 50% EEP added to GIC significantly decreased the bacterial levels, while MIC values of the pure and 10% EEP were larger than 1000 µg/mL. From these results, it could be seen that pure propolis has a strong antibacterial activity and a positive impact on the antibacterial properties of GIC.
Table 1.
MICs of Groups Against the S mutans Strains
Mechanical test results showed that the values did not have a normal distribution. Although groups 1 and 2 (1.6 and 1.7 megapascals, respectively) displayed higher SPBS numbers than the others, no statistically significant difference was observed (P >.05) among the groups (P = .07). Adding propolis enhanced the mechanical properties of GIC, but this effect was not statistically significant (Table 2).
Table 2.
Comparison of the SPBS
DISCUSSION
Recently, propolis has been the subject of increasing scientific interest because of its diverse range of biological properties. Propolis extracts have been recognized for their wide range of pharmacologic activities, including prevention of oral diseases.15,16 Researchers have shown that propolis has antibacterial, antiviral, and antifungal properties.17–19 Moreover, studies have shown that propolis offers substantial protection against cariogenic bacteria and oral pathogens.20 Many products on the world market contain propolis, such as ethanol extracts, toothpastes, and mouth rinses.
The cariogenic microbacteria include S mutans, Lactobacillus, and some Actinomyces species. There is a significant increase in the levels of cariogenic bacteria in the saliva and plaque of patients undergoing fixed appliance treatment.21,22 However, during the initial phase of caries growth, S mutans is the most frequently associated microorganism.11 S mutans was used in this study to examine antibacterial activity because it is considered to be the primary organism responsible for enamel demineralization.
The agar diffusion test, direct-contact test, and broth-dilution methods were used to perform the antibacterial evaluation. Growth of the S mutans was investigated using the broth-dilution method in the present study, following the method of a previous study by Andrews,14 because the broth-dilution method with a liquid culture medium is like the oral environment and GIC is a solid material that is difficult to make soluble in a solid culture medium such as the agar diffusion test.
Chlorhexidine, antibiotics, and titanium dioxide nanoparticles were added to GIC in previous studies. Adding chlorhexidine to GIC increased the antibacterial properties and had no deleterious effect on the mechanical properties (diameter tensile strength, shear bond strength) of GIC.6,23,24 Adding antibiotics to GIC enhanced antibacterial activity, but Yesilyurt et al.25 showed that the antibiotic negatively affected the mechanical properties (compressive and bond strength) of GIC. Castilho et al.26 found that antibiotics have negative effects on compressive strength, but the finding was not statistically significant. Elsaka et al.27 observed that titanium dioxide nanoparticles improved the antibacterial properties of GIC and increased the bond strength insignificantly. This study showed that adding EEP to GIC increased antibacterial activity of GIC against S mutans.
Kouidhi et al.28 found that EEP has positive effects, especially against S mutans, S mitis, S oralis, S pyogenes, S sanguis, S salivarius, S constellatus, and Gemella morbillorum. In this study, we found that adding EEP to GIC increases the antibacterial activity of GIC.
All of the researchers mentioned earlier found increased antibacterial activity and no negative impact on mechanical characteristics. Propolis, a natural material, has similar properties, and currently, most researchers think that standardized preparations of propolis are safe and less toxic than many other synthetic medicines.29,30
We found that in this study the MIC value of Turkish propolis was 15.7 µg/mL. MIC values of Brazilian and Tunisian propolis against S mutans were 50–400 µg/mL and 8–32 µg/mL, respectively.28,31 This change in the MIC values is related to differences in the regional ecology. Its chemical composition changes its antibacterial activity, but all samples exhibited significant antibacterial and antifungal activity.16 Kujumgiev et al.12 tested the antibacterial activity of 11 different propolis samples from different geographical regions and found that all samples were active against gram-positive bacterial test strains.
In our study, we evaluated antibacterial and mechanical properties of propolis. The results of the mechanical test revealed that adding propolis increased SPBS, but this change was not statistically significant. Troca et al.32 used a diametral tensile strength test to show that adding propolis has a negative effect on GIC. We think these controversies may come from the directions of forces of the test methods. Propolis may enhance the chemical adhesion of GIC. There are very few studies about how propolis affects the mechanical characteristics of cements. For a better understanding of mechanical properties, further studies should be performed. According to our experience, we found adding propolis to the liquid of GIC makes the liquid less viscous and prolongs the working time. However, the longer working time was at the low level, so this would not create any problems during the clinical application. We think these results should encourage the use of propolis in clinical practice.
CONCLUSIONS
Adding EEP increases the antibacterial effects of orthodontic cements.
Adding EEP insignificantly enhances the mechanical properties.
REFERENCES
- 1.Mizrahi E. Enamel demineralization following orthodontic treatment. Am J Orthod. 1982;82:62–67. doi: 10.1016/0002-9416(82)90548-6. [DOI] [PubMed] [Google Scholar]
- 2.Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96:423–427. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 3.Mizrahi E. Surface distribution of enamel opacities following orthodontic treatment. Am J Orthod. 1983;84:323–331. doi: 10.1016/s0002-9416(83)90348-2. [DOI] [PubMed] [Google Scholar]
- 4.Artun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod. 1986;8:229–234. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
- 5.Millett DT, Doubleday B, Alatsaris M, et al. Chlorhexidine-modified glass ionomer for band cementation? An in vitro study. J Orthod. 2005;32:36–42. doi: 10.1179/14653120522502078. [DOI] [PubMed] [Google Scholar]
- 6.Farret MM, de Lima EM, Mota EG, Oshima HM, Barth V, de Oliveira SD. Can we add chlorhexidine into glass ionomer cements for band cementation. Angle Orthod. 2011;81:496–502. doi: 10.2319/090310-518.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millett DT, Duff S, Morrison L, Cummings A, Gilmour WH. In vitro comparison of orthodontic band cements. Am J Orthod Dentofacial Orthop. 2003;123:15–20. doi: 10.1067/mod.2003.48. [DOI] [PubMed] [Google Scholar]
- 8.Hoszek A, Ericson D. In vitro fluoride release and the antibacterial effect of glass ionomers containing chlorhexidine gluconate. Oper Dent. 2008;33:696–701. doi: 10.2341/08-20. [DOI] [PubMed] [Google Scholar]
- 9.Matalon S, Slutzky H, Weiss EI. Antibacterial properties of 4 orthodontic cements. Am J Orthod Dentofacial Orthop. 2005;127:56–63. doi: 10.1016/j.ajodo.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.DeSchepper EJ, White RR, von der Lehr W. Antibacterial effects of glass ionomers. Am J Dent. 1989;2:51–56. [PubMed] [Google Scholar]
- 11.Duailibe SA, Goncalves AG, Ahid FJ. Effect of a propolis extract on Streptococcus mutans counts in vivo. J Appl Oral Sci. 2007;15:420–423. doi: 10.1590/S1678-77572007000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 13.Hayacibara MF, Koo H, Rosalen PL, et al. In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. J Ethnopharmacol. 2005;101:110–115. doi: 10.1016/j.jep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 15.Duarte S, Rosalen PL, Hayacibara MF, et al. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Arch Oral Biol. 2006;51:15–22. doi: 10.1016/j.archoralbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Koo H, Rosalen PL, Cury JA, Park YK, Ikegaki M, Sattler A. Effect of Apis mellifera propolis from two Brazilian regions on caries development in desalivated rats. Caries Res. 1999;33:393–400. doi: 10.1159/000016539. [DOI] [PubMed] [Google Scholar]
- 17.Topcuoglu N, Ozan F, Ozyurt M, Kulekci G. In vitro antibacterial effects of glass-ionomer cement containing ethanolic extract of propolis on Streptococcus mutans. Eur J Dent. 2012;6:428–433. [PMC free article] [PubMed] [Google Scholar]
- 18.Amoros M, Simoes CM, Girre L, Sauvager F, Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J Nat Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 19.Silici S, Koc NA, Ayangil D, Cankaya S. Antifungal activities of propolis collected by different races of honeybees against yeasts isolated from patients with superficial mycoses. J Pharmacol Sci. 2005;99:39–44. doi: 10.1254/jphs.fpe05002x. [DOI] [PubMed] [Google Scholar]
- 20.Feres M, Figueiredo LC, Barreto IM, Coelho MH, Araujo MW, Cortelli SC. In vitro antimicrobial activity of plant extracts and propolis in saliva samples of healthy and periodontally-involved subjects. J Int Acad Periodontol. 2005;7:90–96. [PubMed] [Google Scholar]
- 21.Poeta P, Igrejas G, Goncalves A, et al. Influence of oral hygiene in patients with fixed appliances in the oral carriage of antimicrobial-resistant Escherichia coli and Enterococcus isolates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:557–564. doi: 10.1016/j.tripleo.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003;7:181–188. doi: 10.1007/s00784-003-0236-1. [DOI] [PubMed] [Google Scholar]
- 23.de Castilho AR, Duque C, Negrini TD, et al. In vitro and in vivo investigation of the biological and mechanical behaviour of resin-modified glass-ionomer cement containing chlorhexidine. J Dent. 30 2012 doi: 10.1016/j.jdent.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Sanders BJ, Gregory RL, Moore K, Avery DR. Antibacterial and physical properties of resin modified glass-ionomers combined with chlorhexidine. J Oral Rehabil. 2002;29:553–558. doi: 10.1046/j.1365-2842.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 25.Yesilyurt C, Er K, Tasdemir T, Buruk K, Celik D. Antibacterial activity and physical properties of glass-ionomer cements containing antibiotics. Oper Dent. 2009;34:18–23. doi: 10.2341/08-30. [DOI] [PubMed] [Google Scholar]
- 26.de Castilho AR, Duque C, Negrini Tde C, et al. Mechanical and biological characterization of resin-modified glass-ionomer cement containing doxycycline hyclate. Arch Oral Biol. 2012;57:131–138. doi: 10.1016/j.archoralbio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: influence on physical and antibacterial properties. J Dent. 2011;39:589–598. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Kouidhi B, Zmantar T, Bakhrouf A. Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe. 2010;16:566–571. doi: 10.1016/j.anaerobe.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Moreno MIN, Zampini IC, Ordonez RM, Jaime GS, Vattuone MA, Isla MI. Evaluation of the cytotoxicity, genotoxicity, mutagenicity, and antimutagenicity of propolis from Tucuman, Argentina. J Agric Food Chem. 16 2005;53:8957–8962. doi: 10.1021/jf0513359. [DOI] [PubMed] [Google Scholar]
- 30.Sonmez S, Kirilmaz L, Yucesoy M, Yucel B, Yilmaz B. The effect of bee propolis on oral pathogens and human gingival fibroblasts. J Ethnopharmacol. 2005;102:371–376. doi: 10.1016/j.jep.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Koo H, Rosalen PL, Cury JA, et al. Effect of a new variety of Apis mellifera propolis on mutans streptococci. Curr Microbiol. 2000;41:192–196. doi: 10.1007/s0028400101170. [DOI] [PubMed] [Google Scholar]
- 32.Troca VBPB, Fernandes KBP, Terrile AE, Marcucci MC, de Andrade FB, Wang LD. Effect of green propolis addition to physical-mechanical properties of glass ionomer cements. J Appl Oral Sci. 2011;19:100–105. doi: 10.1590/S1678-77572011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]