Abstract
Objective:
To evaluate how therapy with a fixed functional appliance affects airway dimensions, dentoalveolar changes, and tongue and hyoid positions.
Materials and Methods:
A retrospective study was carried out on 46 pre- and posttreatment lateral cephalometric radiographs of 23 post-peak Class II patients (12 girls, 11 boys) treated with a Forsus Fatigue Resistant Device (FRD) appliance. The radiographies were taken at the start and at the end of Forsus FRD appliance therapy when a Class I or overcorrected Class I canine and molar relationship was achieved. The process took an average of 5 months 13 days ± 1 month 4 days. Skeletal and dental parameters were measured using Dolphin software, and the sagittal airway area was measured by AutoCAD software.
Results:
Analyses of the pre- and posttreatment means revealed that there was no statistically significant skeletal correction of the sagittal malocclusion; increase of lower incisor inclination, decrease of upper incisor inclination, decrease of interincisal angle, and rotation of occlusal plane all contributed to the reduction of overjet. The tongue area and intermaxillary space area increased in response to these dentoalveolar changes; however, there was no statistically significant change in the hyoid position or the oropharyngeal area between the two time points.
Conclusions:
The dentoalveolar changes produced by Forsus FRD appliance did not cause any significant posterior airway changes in young adult patients.
Keywords: Airway, Functional therapy, Forsus FRD appliance, Class II malocclusion
INTRODUCTION
Changes induced in the airway after orthodontic treatment of growing1–3 and nongrowing subjects4,5 have been investigated. The importance of the airway dimension is that it is related to breathing disorders. The current view is that adenotonsillar hypertrophy, which constricts the airway, is the major cause of sleep-disordered breathing in otherwise healthy children.6,7 Sleep-disordered breathing may affect pulmonary ventilation, oxygenation, sleep quality, sweating, and nocturnal enuresis.8,9 In children, behavioral problems and impaired cognitive performance may also be encountered.10 Sleep disorders are also reported in patients with chronic orofacial pain and headache, and sleep bruxism is one of the common sleep disorders.11
The aim of fixed functional therapy in young adults is to achieve a neutral bite by dentoalveolar compensation for the skeletal discrepancy; mainly, the upper dentition is moved to the distal and the lower dentition is moved to the mesial with a clockwise rotation of the occlusal plane and without creating pronounced vertical skeletal changes, only inhibiting the sagittal growth potential of maxilla. The minimal skeletal mandibular effects depend on the device used and the age and growth capacity of the patient.12–17 The lower incisors procline and intrude, while the lower molars move significantly in a mesial and vertical direction. One device that generates these effects is the Forsus Fatigue Resistant Device (FRD; 3M Unitek, Monrovia, Calif), which is a semirigid three-piece telescoping spring for Class II correction.16,17
As there are studies reporting on changes of the posterior airway after dental movements only,18–22 the amount of dental movement induced by these appliances in post-peak patients is large enough to justify the investigation of related changes of the airway. Most patients undergoing fixed functional therapy have retrognathic mandibles, and because this is noted as one of the risk factors for sleep apnea, it is logical to investigate how these appliances potentially affect the airway.23 Therefore, the aim of this study is to evaluate how therapy with a fixed functional appliance affects airway dimensions, dentoalveolar changes, and tongue and hyoid positions using cephalometric radiographs of a group of patients in the post-peak growth stage. The null hypothesis is that there is no change in the airway parameters with Forsus FRD treatment.
MATERIALS AND METHODS
The material for this retrospective study consisted of 46 pre- and posttreatment lateral cephalometric radiographs of 23 Class II patients (12 girls and 11 boys) ranging in age from 12 to 20 years (mean ± SD = 17.3 ± 4.2 years). The radiographs were taken with Planmeca Promax 3D (Planmeca OY, Helsinki, Finland) and obtained from the archives of Yeditepe University Dental School, Department of Orthodontics. Ethics approval for the study was obtained from the Yeditepe University Ethics Committee.
All patients in the clinic's archive who presented with ANB more than 4°, overjet more than 5 mm, Angle Class II molar and canine relationships, no missing teeth, no history of nasal or pharyngeal obstruction or related surgical treatments, no significant residual growth potential evident on the cervical vertebral maturation index (CVMI stage 5 and CVMI stage 6, which correspond to post-peak growth period), and clinically normal vertical skeletal pattern (SN/MP angle in the 28°–35° range), and who had received Forsus treatment, were included. The patients had received Class II correction with a Forsus FRD. Because patients selected were at the post-peak developmental stage, there was no control group designed to account for growth changes. Lateral cephalometric radiographies were taken in a natural head posture at the start and end of FRD appliance treatment when a Class I or overcorrected Class I canine and molar relationship was achieved; this occurred in a mean time of 5 months 13 days ± 1 month 4 days.
Data Collection
Skeletal and dental measurements were digitized using Dolphin Imagining 11.0 Software (Dolphin Imaging and Management Solutions, Chatsworth, Calif), and the sagittal airway area was measured by AutoCAD 2012 software (AutoCAD, Autodesk, Inc, San Rafael, Calif). The customized cephalometric analysis, including measurements from the Steiner, Ricketts, and Tweed analyses was used. Cephalometric and anatomic landmarks and reference lines are shown at Figure 1.
Figure 1.
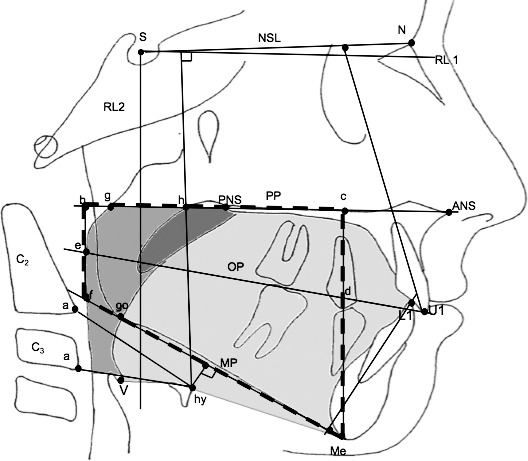
The cephalometric points, reference lines, and areas used in the study. Soft palate is black, tongue is light grey, and oropharyngeal area is dark grey.
Definitions of Cephalometric and Anatomic Landmarks
The following landmarks were used: Hy, most anterior point on the hyoid bone; V (Vallecula), most profound point in the curvature of the depression just behind the root of the tongue between the folds in the throat; a, most anteroinferior point on corpus of C2 and C3; g, point on the nasal surface of the soft palate at the level of the maxillary plane (opposite point to h); h, point on the posterior pharyngeal wall at the same horizontal level as point g; e, point of intersection of the occlusal plane with the posterior pharyngeal wall; U1, incisal edge of the upper middle incisor; L1, incisal edge of the lower middle incisor.
Following are definitions of the reference lines used in the study: NSL, line passing through the Sella and Nasion points; RL 1, reference line 7° to NSL; RL 2, reference line perpendicular to NSL and intersecting the Sella point; MP, mandibular plane, that is, the line tangent to the lower border of the body of the mandible through Menton; PP, palatal plane, that is, the line connecting the anterior nasal spine and posterior nasal spine; OP, anatomic occlusal plane.
Definitions of Linear Measurements (mm)
Following are the definitions of linear measurements used: b-f, posterior intermaxillary space height (perpendicular to PP), that is, the length of a perpendicular line from the maxillary plane to the mandibular plane, which passes through the point where the occlusal plane intersects the posterior pharyngeal wall; c-Me, anterior intermaxillary space height (perpendicular to PP), that is, the length from the maxillary plane to Menton; intermaxillary length measured along the occlusal plane from the incisors to where it intersects the pharyngeal wall posteriorly; hy-NL, the perpendicular distance from NSL to hyoid; hy-MP, the perpendicular distance from MP to hyoid; hy-aC2, the linear distance between hy and aC2; hy-aC3, the linear distance between hy and aC3.
Definitions of Area Measurements
Following are the are measurements used24: black soft palate area bounded superiorly by PP; oropharyngeal area, the dark grey oropharyngeal area, bounded superiorly by a backward extension of the maxillary plane and inferiorly by a line parallel to palatal plane drawn through the tip of epiglottis; tongue area: light grey area enclosed posteriorly by the oropharynx and uvula, superiorly by the hard palate, and anteriorly by the lingual aspects of the anterior teeth and lingual mandibular symphyseal contour. The inferior border is the line extending from the vallecula to the most anterior point on the hyoid body and the line from the most anterior point on the hyoid bone to the menton, and IMA is the intermaxillary space area delineated by a trapezium drawn through the palatal and mandibular planes and points e and d.
Definitions of Angular Measurements
Following are the angular measurements used: NSL/U1, inclination of the maxillary incisors to NSL; RL1/U1, inclination of the maxillary incisors to RL1; IA, interincisal angle between inclinations of the maxillary and mandibular incisors; NSL/OP, the angle between the NSL and occlusal plane; IMPA, inclination of the mandibular incisors to the mandibular plane; and RL2/L1, inclination of the mandibular incisors to RL2.
Data Analyses
Statistical calculations were carried out with NCSS software for Windows (Kaysville, Utah) and MedCalc version 10.2 (Ostend, Belgium). Besides descriptive statistics (mean, standard deviation), in order to test the statistical significance of the difference between the means of the first and second measurements of the variables, a paired sample t-test was used. The power of the study was calculated on the basis of the sample size of the two groups and an effect size equal to 1. The power exceeded 0.95 at an alpha level of 0.05. To detect method error, a randomly selected 20 radiographs were digitized and measured a second time 15 days later by the same operator, and intraclass correlation coefficients were calculated.
RESULTS
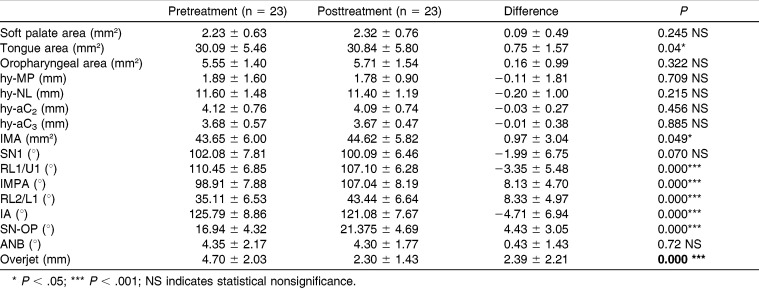
The operator was consistent in measuring the radiographs; intraclass correlation coefficient, calculated for the repeated measurements of randomly selected 20 radiographs, was more than 0.99 for skeletal and dentoalveolar and more than 0.98 for the airway area measurements. Although there was no statistical correction of skeletal Class II as seen in the change of ANB, the overjet showed significant reduction at the end of treatment. The analyses of pre- and posttreatment means revealed statistically significant changes as follows: tongue area, intermaxillary space area, IMPA, RL2, and SN-OP variables increased, and RL1/U1 and interincisal angle decreased, after treatment with an FRD appliance (Table 1). Statistically significant dentoalveolar changes point to an increase of lower incisor inclination, decrease of upper incisor inclination, decrease of interincisal angle, and rotation of the occlusal plane, all contributing to the reduction of overjet. The tongue area and intermaxillary space area increased in response to these dentoalveolar changes; however, there was no statistically significant change in the hyoid position or the oropharyngeal area between the two time points.
Table 1.
Pre - and Posttreatment Means, Means of Difference, Related Standard Deviations, and P Values
DISCUSSION
Recent research suggests that airway changes induced by orthodontic movements affect the room for the tongue, thereby affecting the position of the hyoid bone and causing a subsequent change in the dimensions of the posterior airway. In the present study, the interincisal angle increased mainly because of the forward movement of the lower incisors, which might have given the tongue a greater area. Our results, while pointing to a statistically significant increase of tongue space (0.75 ± 1.57 mm) and intermaxillary space (0.97 ± 3.04 mm), did not reveal any clinically significant increase in the area of the tongue, any consequent increase in the posterior oropharyngeal airway, or a change in the position of the hyoid bone. The reason for the lack of airway change may be that the anterior dental changes were not large enough to affect the tongue and subsequently the airway, or the increase of the tongue space caused by the forward movement of the lower dentition may not be as effective on the airway as is a decrease in the same space.
The study with the most similar purpose and method to our study is the cephalometric study by Kinzinger et al.15 They investigated two fixed functional appliances (FMA and Herbst appliance) for their effect on the morphology of the airway. The appliances had a similar effect on airway depth. It was found that increases in anterior facial height were related to increases in upper posterior airway width. On the other hand, increases in posterior facial height and the mandible's forward displacement correlated inversely to the decreases in depth in the central and lower posterior airway.
Because there were no expected growth changes and associated skeletal corrections—only dental changes—for our sample of patients in only 5 months of treatment,25 studies regarding airway changes after dental movements are appropriate for us to compare our results with. There are inconsistencies between studies reporting on the changes of airway dimensions due to dental movements during orthodontic therapy. Although some studies give very detailed information on the type of anchorage and how it was provided,18–20 others do not mention these factors.21,22 However, all of the studies reported changes in incisor inclination at the end of treatment, albeit using different measurement variables.
Similar to our results, some studies do not report any change in the posterior airway dimensions after orthodontic treatment with extractions.21,22 One of these is a recent study on cone beam computed tomography, which reported no change in the airway between extraction and non-extraction groups.22 The amount of tooth movement was comparatively less in their study than in similar studies. The extraction group had 6° and 3 mm of retrusion, and the non-extraction group had 3° and 1.5 mm of protrusion of the maxillary incisors. The mandibular incisors were retruded 4° and 2 mm in the extraction group and were 5° and 1 mm protruded in the non-extraction group. In another cephalometric study, where upper and lower premolars were extracted for the treatment of bimaxillary proclination, the upper incisor inclination had decreased by 10°, and the lower incisor inclination had decreased by 9.7°.21 Even though there was a tongue length decrease of 1.75 mm, no effect was found on the airway, similar to the result of the present study.
On the other hand, some studies have reported a change in the airway as a result of incisor movements.18–20 In a cephalometric study by Germec-Cakan et al.,18 the authors concluded that airway dimensions increased 1.5 mm in patients where 6 mm of mesial molar movement occurred, decreased 3 mm when the incisors were retracted more than 10°, and did not change when these teeth remained stable during treatment. They explained their result with adaptational changes of the tongue without any change in the hyoid bone position. The amount of mesial movement that created a change in the airway in the non-extraction group of Germec-Cakan et al.18 is similar to the amount of dental movement found in our study; however, we have not seen any airway changes. Even though their sample consisted of Class I subjects, the initial conditions of the study groups were not compared; therefore, it is not clear whether the differences found were due to the specific condition of each group or by treatment.
In another study, the tip of the upper incisor was retracted by a mean of 6.84 mm and the lower incisor was retracted by 4.95 mm, with a corresponding significant decrease in airway parameters.19 The hyoid bone moved in a posterior and inferior direction. These authors stated that any factors that could influence the posture and position of tongue and soft palate might displace them backward. The authors reported that the retraction of anterior teeth decreased the arch length, causing dorsal movement of the anterior boundary of oral cavity.
In the tomography study by Chen et al.,20 the mean retraction amount of the maxillary incisors was 7.64 mm. They found a significant correlation between the amount of incisor retraction and the amount of hyoid retraction, which they thought might be the reason for the narrowing of the hypopharynx.
Even though there is an inherent limitation of this study, as the nasopharyngeal airway is a three-dimensional structure and was evaluated two dimensionally on lateral cephalometric films, such films have been used extensively to study airway dimensions.26,27 A high correlation between posterior airway size on cephalometric radiographs and pharyngeal volume measured on tomographic scans has been reported.28 Furthermore, Miles et al.29 reported a high reliability of identification of the commonly used cephalometric landmarks and measurements.
CONCLUSION
In this study the dentoalveolar changes produced by the Forsus FRD did not cause any significant posterior airway changes in young adult patients.
REFERENCES
- 1.Sayınsu K, Isik F, Arun T. Sagittal airway dimensions following maxillary protraction: a pilot study. Eur J Orthod. 2006;28:184–189. doi: 10.1093/ejo/cji095. [DOI] [PubMed] [Google Scholar]
- 2.Cakirer B, Kucukkeles N, Nevzatoglu S, Koldas T. Sagittal airway changes: rapid palatal expansion versus Le Fort I osteotomy during maxillary protraction. Eur J Orthod. 2012;34:381–389. doi: 10.1093/ejo/cjr023. [DOI] [PubMed] [Google Scholar]
- 3.Ozbek MM, Memikoglu TU, Gogen H, Lowe AA, Baspinar E. Oropharyngeal airway dimensions and functional-orthopedic treatment in skeletal Class II cases. Angle Orthod. 1998;68:327–336. doi: 10.1043/0003-3219(1998)068<0327:OADAFO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Gokce SM, Gorgulu S, Gokce HS, et al. Changes in posterior airway space, pulmonary function and sleep quality, following bimaxillary orthognathic surgery. Int J Oral Maxillofac Surg. 2012;41:820–829. doi: 10.1016/j.ijom.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Enacar A, Aksoy AU, Sencift Y, Haydar B, Aras K. Changes in hypopharyngeal airway space and in tongue and hyoid bone positions following the surgical correction of mandibular prognathism. Int J Adult Orthod Orthognath Surg. 1994;9:285–290. [PubMed] [Google Scholar]
- 6.Marcus CL. Pathophysiology of childhood obstructive sleep apnea: current concepts. Respir Physiol. 2000;119:143–154. doi: 10.1016/s0034-5687(99)00109-7. [DOI] [PubMed] [Google Scholar]
- 7.Katyal V, Pamula Y, Martin AJ, et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2013;143:20–30. doi: 10.1016/j.ajodo.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Carroll JL. Obstructive sleep-disordered breathing in children: new controversies, new directions. Clin Chest Med. 2003;24:261–282. doi: 10.1016/s0272-5231(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 9.Tran KD, Nguyen CD, Weedon J, Goldstein NA. Child behavior and quality of life in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg. 2005;131:52–57. doi: 10.1001/archotol.131.1.52. [DOI] [PubMed] [Google Scholar]
- 10.Blunden S, Lushington K, Lorenzen B, Martin J, Kennedy D. Neuro-psychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J Pediatr. 2005;146:780–786. doi: 10.1016/j.jpeds.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Yamaguchi T, Okura K, Abe S, Lavigne GJ. Sleep less and bite more: sleep disorders associated with occlusal loads during sleep. J Prosthodont Res. 2013;57(2):69–81. doi: 10.1016/j.jpor.2013.03.001. doi:10.1016/j.jpor.2013.03.001. Epub April 17, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Nalbantgil D, Arun T, Sayinsu K, Fulya I. Skeletal, dental and soft-tissue changes induced by the Jasper Jumper appliance in late adolescence. Angle Orthod. 2005;75:426–436. doi: 10.1043/0003-3219(2005)75[426:SDASCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Ruf S, Pancherz H. Dentoskeletal effects and facial profile changes in young adults treated with the Herbst appliance. Angle Orthod. 1999;69:239–246. doi: 10.1043/0003-3219(1999)069<0239:DEAFPC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Kinzinger G, Diedrich P. Skeletal effects in Class II treatment with the Functional Mandibular Advancer (FMA) J Orofac Orthop. 2005;66:469–490. doi: 10.1007/s00056-005-0524-2. [DOI] [PubMed] [Google Scholar]
- 15.Kinzinger G, Czapka K, Ludwig B, Glasl B, Gross U, Lisson J. Effects of fixed appliances in correcting Angle Class II on the depth of the posterior airway space: FMA vs. Herbst appliance—a retrospective cephalometric study. J Orofac Orthop. 2011;72:301–320. doi: 10.1007/s00056-011-0035-2. [DOI] [PubMed] [Google Scholar]
- 16.Bilgiç F, Hamamci O, Başaran G. Comparison of the effects of fixed and removable functional appliances on the skeletal and dentoalveolar structures. Aust Orthod J. 2011;27:110–116. [PubMed] [Google Scholar]
- 17.Franchi L, Alvetro L, Giuntini V, Masucci C, Defraia E, Baccetti T. Effectiveness of comprehensive fixed appliance treatment used with the Forsus Fatigue Resistant Device in Class II patients. Angle Orthod. 2011;81:678–683. doi: 10.2319/102710-629.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germec-Cakan D, Taner T, Akan S. Uvulo-glossopharyngeal dimensions in non-extraction, extraction with minimum anchorage, and extraction with maximum anchorage. Eur J Orthod. 2011;33:515–520. doi: 10.1093/ejo/cjq109. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Iia P, Anderson NK, Wang L, Lin J. Changes of pharyngeal airway size and hyoid bone position following orthodontic treatment of Class I bimaxillary protrusion. Angle Orthod. 2012;82:115–121. doi: 10.2319/011011-13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Hong L, Wang CL, et al. Effect of large incisor retraction on upper airway morphology in adult bimaxillary protrusion patients. Angle Orthod. 2012;82:964–970. doi: 10.2319/110211-675.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Maaitah E, El Said N, Abu Alhaija ES. First premolar extraction effects on upper airway dimension in bimaxillary proclination patients. Angle Orthod. 2012;82:853–859. doi: 10.2319/101711-646.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valiathan M, El H, Hans MG, Palomo MJ. Effects of extraction versus non-extraction treatment on oropharyngeal airway volume. Angle Orthod. 2010;80:1068–1074. doi: 10.2319/010810-19.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jena AK, Singh SP, Utreja AK. Sagittal mandibular development effects on the dimensions of the awake pharyngeal airway passage. Angle Orthod. 2010;80:1061–1067. doi: 10.2319/030210-125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vig PS, Cohen AM. The size of the tongue and the intermaxillary space. Angle Orthod. 1974;44:25–28. doi: 10.1043/0003-3219(1974)044<0025:TSOTTA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Phan KL, Bendeus M, Hägg U, Hansen K, Rabie AB. Comparison of the headgear activator and Herbst appliance effects and post-treatment changes. Eur J Orthod. 2006;28:594–604. doi: 10.1093/ejo/cjl052. [DOI] [PubMed] [Google Scholar]
- 26.van Vlijmen OJ, Kuijpers MA, Bergé SJ, et al. Evidence supporting the use of cone-beam computed tomography in orthodontics. J Am Dent Assoc. 2012;143:241–252. doi: 10.14219/jada.archive.2012.0148. [DOI] [PubMed] [Google Scholar]
- 27.Linder-Aronson S, Henriksson CO. Radiocephalometric analysis of anteroposterior nasopharyngeal dimensions in 6- to 12-year-old mouthbreathers compared with nose breathers. Pract Otorhinolaryngol. 1973;35:19–29. doi: 10.1159/000275083. [DOI] [PubMed] [Google Scholar]
- 28.Zamora N, Llamas JM, Cibrián R, Gandia JL, Paredes V. Cephalometric measurements from 3D reconstructed images compared with conventional 2D images. Angle Orthod. 2011;8:856–864. doi: 10.2319/121210-717.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles PG, O'Reilly M, Close J. The reliability of upper airway landmark identification. Aust Orthod J. 1995;14:3–6. [PubMed] [Google Scholar]