Abstract
Objectives:
To evaluate, by using cone beam computed tomography, the skeletal, dental, oropharyngeal (OP) airway volume, and nasal passage (NP) volume changes that occur after rapid maxillary expansion (RME).
Materials and Methods:
Two groups were selected, each with 35 patients (15 males, 20 females), an RME group (mean age, 14.02 ± 1.46 years) and a control group (mean age, 14.10 ± 1.44 years). The RME group consisted of patients with maxillary constriction who were treated with Hyrax palatal expanders, and the control group comprised age- and sex-matched patients who underwent comprehensive orthodontic treatment without the use of a rapid maxillary expander.
Results:
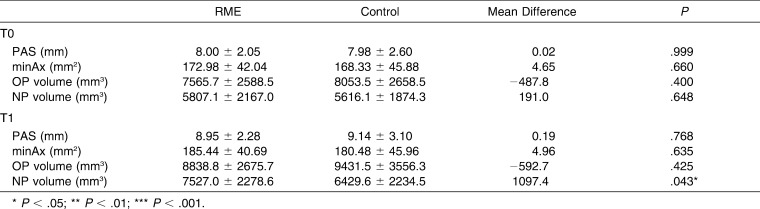
All of the transverse skeletal (medial orbital width, lateral nasal width, maxillary width, and mandibular width) and interdental (intermolar, interpremolar, and intercanine) parameters were significantly enlarged in the RME group. A statistically significant increase in airway variables was seen in both groups between pretreatment (T0) and final records (T1). The mean increase of NP airway volume for the RME group (1719.9 ± 1510.7 mm3) was twofold compared with the control group (813.6 ± 1006.7 mm3), and no intergroup significant difference was found for the OP volume.
Conclusions:
Rapid maxillary expansion creates a significant increase in nasal passage airway volume but no significant change in the oropharyngeal airway volume.
Keywords: CBCT, Airway, Expansion, RME, Three-dimensional, Cone beam CT
INTRODUCTION
Rapid maxillary expansion (RME) is a well-documented orthodontic treatment modality for correcting transverse maxillary deficiency. It was first introduced by Angell in the 19th century, and it was suggested as a treatment for respiratory disturbances and cited for its effects over the maxilla.1 Over the years, the method described by Angell was attempted with varying success, and it was finally reintroduced by Haas,2,3 who suggested that the application of RME created considerable changes in the nasomaxillary complex. Wertz4 also stated that patients presenting with nasal stenosis located in the anteroinferior portion of the nasal chambers would benefit from maxillary suture opening. Another detrimental effect of maxillary constriction is its role in the pathophysiology of obstructive sleep apnea, where the retro position of the tongue may cause a narrow oropharyngeal airway.5,6 Furthermore, RME is known to affect the position of the mandible, which may also change the size and volume of the oropharyngeal (OP) airway.7
The effects of RME over nasal size and volume have been researched with various procedures such as lateral and anteroposterior radiographs,8–12 acoustic rhinometric methods13–16 and multislice computed tomography.17–20 Recent advances in cone beam computed tomography (CBCT) and related software have made it possible to visualize and measure the upper airway as a solid structure. Lower costs, lower radiation dose, shorter scanning time, and overall accuracy have made CBCT technology a preferred method to assess the airway.21–24 Studies have also used this technology to evaluate the effects of RME over nasal airway.25–27 However, most of the RME studies have not taken into account nasal and oropharyngeal airway volumes.28,29 The objectives of this study were to evaluate the skeletal, dental, OP airway volume, and nasal passage (NP) volume changes that occur after RME treatment.
MATERIALS AND METHODS
The experimental protocol used in this study was approved by the Institutional Review Board of Case Western Reserve University in Cleveland, Ohio. All participants were obtained from the existing patient database of the Department of Orthodontics and were treated in the same facility. A total of 264 patient records consisting of initial and final records, including CBCT scans, photographs, and medical history forms, were available.
All CBCT images were acquired using a custom Hitachi CB Mercuray scanner (Hitachi Medical Systems America Inc, Twinsburg, Ohio). The custom modification allowed the low settings of 2 mA, 120 kVp, using a 12-inch field of view (F Mode).21,23 Each patient's image data consisted of 512 slices, with a slice thickness of 0.377 mm, a resolution of 1024×1024 pixels, and 12 bits per pixel (4096 gray scale). The images were taken in natural head position, with teeth in maximum intercuspation, and at the end of the exhalation period when the patient was not swallowing. Each scan was 9.6 seconds long, with a single rotation around the patient's head.
Inclusion criteria for the experimental group were complete records, nonextraction treatment plan, and use of a Hyrax maxillary expander as part of the treatment provided. Exclusion criteria were history of craniofacial deformities, pharyngeal pathology and/or nasal obstruction, snoring, obstructive sleep apnea, adenoidectomy, and tonsillectomy. Any CBCT scans in which the airways were not clear, were not fully contained in the volume, or contained artifacts were also excluded. From the selected records, a group of 35 patients (15 females and 20 males) who underwent RME and a control group of 35 patients (15 females and 20 males) who underwent comprehensive orthodontic treatment were selected. All participants had bilateral Class I malocclusion. The RME group consisted of patients with maxillary constriction treated with Hyrax maxillary expanders, and the control group was a sample matched for age, sex, and treatment duration who underwent regular orthodontic treatment without expanders (Table 1). Expansion protocol consisted of twice per day screw activation until a slight amount of overcorrection was achieved. Screws were then stabilized, and the expander was passively left in place for 4–6 months. All subjects were treated with fixed edgewise appliances and had good occlusion at the end of treatment.
Table 1.
Comparison of Ages and Treatment Times of RME and Control-Group Subjects by Gender
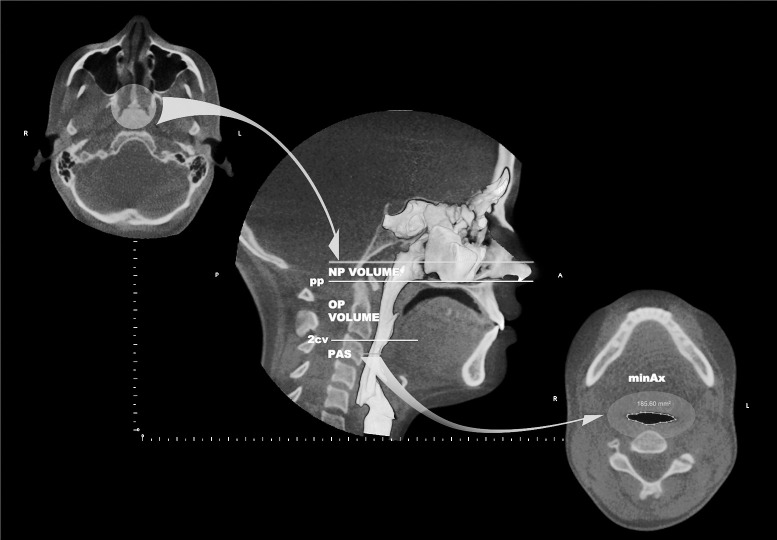
Lateral cephalograms for all patients were obtained from CBCT data using InVivo Dental 5.0 (Anatomage Inc, San Jose, Calif) for pretreatment (T0) and posttreatment (T1) intervals. Dolphin Imaging 11.0 (Dolphin Imaging & Management Solutions, Chatsworth, Calif) was used for lateral cephalometric analysis (Figure 1). Custom transverse skeletal (Figure 2), dental (Figure 3), and three-dimensional volumetric (Figure 4) measurements were performed using InVivo Dental 5.0. Mesiobuccal cusp tips of first molars, buccal cusp tips of first premolars, and cusp tip of canines were used for interdental measurements (Figure 3). The superior limit of the OP airway is the palatal plane (ANS-PNS), extending to the posterior wall of the pharynx, and the inferior limit is a line parallel to the palatal plane, touching the most anteroinferior point of the second cervical vertebrae. The superior limit of the NP airway is the last slice before the nasal septum fuses with the posterior wall of the pharynx, viewed on the axial slice first and then projected to the sagittal view. The inferior limit is the palatal plane (Figure 4).22 Two constriction measurements also calculated were the posterior airway space (PAS), defined as the most constricted space behind the base of the tongue and limited by soft tissues, and the minAx, defined as the representation of PAS on the axial slice (Figure 4). All measurements were completed by an experienced orthodontist.
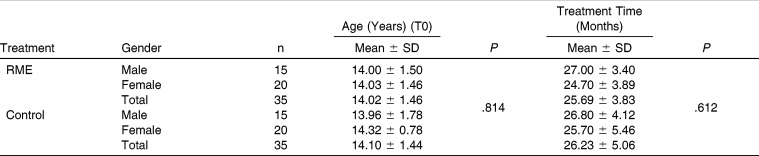
Figure 1.
Cephalometric tracing illustrating measurements performed. 1, SNA in degrees; 2, SNB in degrees; 3, ANB in degrees;, 4, maxillary depth (FH-NaPerp) in degrees; 5, maxillary skeletal (A-NaPerp) in millimeters; 6, skeletal profile convexity (NaApOG) in degrees; 7, midface length (Co-A) in millimeters; 8, effective mandibular length (Co-Gn) in millimeters; 9, lower facial height (ANS-Me) in millimeters, 10, GoGnSN in degrees; 11, palatal plane-facial height in degrees; 12, palatal plane-mandibular plane in degrees.
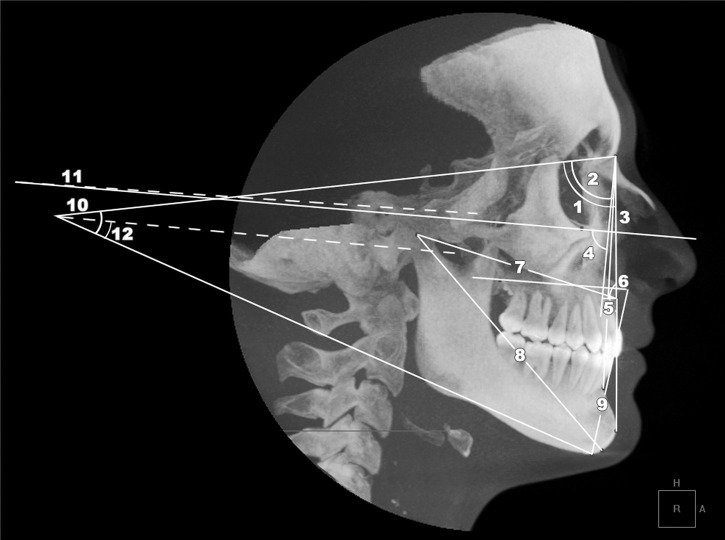
Figure 2.
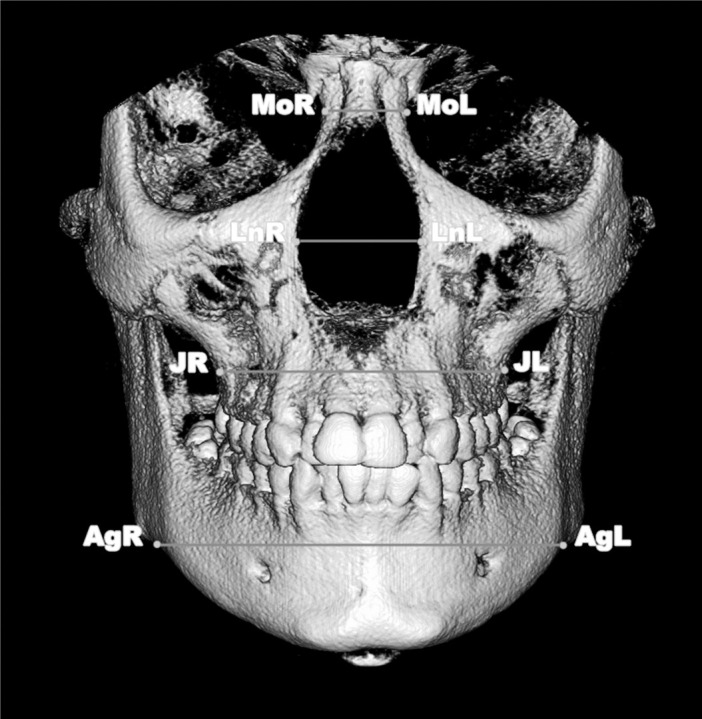
Three-dimensional measurements of transverse skeletal variables. MoL-Mor (medial orbital width in millimeters), LnL-LnR (lateral nasal width), JL-JR (most superior aspects of the concavity of the maxillary bone as it joins the zygomatic process (maxillary width) in millimeters), AgL-AgR (mandibular width in millimeters).
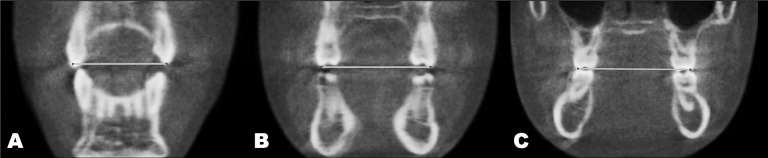
Figure 3.
Interdental measurements performed using the coronal view. (A) Intercanine width, or the distance between the cusp tips of canines (B) Interpremolar width, or the distance between the buccal cusp tips of first premolars. (C) Intermolar width, or the distance between the mesiobuccal cusp tips of the right and left first molars.
Figure 4.
Superior and inferior limits of OP and NP volume, PAS, and minAx (area of the most constricted region at the base of the tongue). The circle on the left illustrates the last axial slice before the nasal septum fuses with the posterior wall of the pharynx. In the center image, the top line represents the mentioned axial slice on the sagittal view (superior border of NP). In the lower right image, the area in evidence represents the PAS on the axial view; pp (line passing from palatal plane), 2cv (line passing from the most anteroinferior aspect of the second cervical vertebrae and parallel to pp).
To estimate operator reliability, 15 randomly selected records were reevaluated after a week of preliminary data collection. The error for linear and angular measurements was measured using the Dahlberg formula30 as follows:
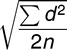 |
Independent sample t-tests were used to validate the consistency of both groups at T0 in terms of age and to compare the outcome variables at T1. Paired sample t-tests were used to compare changes from T0 to T1. The SPSS Statistics 17.0 (SPSS Inc, Chicago, Ill) software was used for all statistical analyses.
RESULTS
Reliability results showed an error of 0.47 mm, 2.85 mm2, and 0.50° for linear, areal, and angular variables, respectively, and an intraclass correlation of 0.93 for the OP and 0.89 for the NP volumes. The Kolmogorov–Smirnov test was applied and showed that all data were normally distributed.
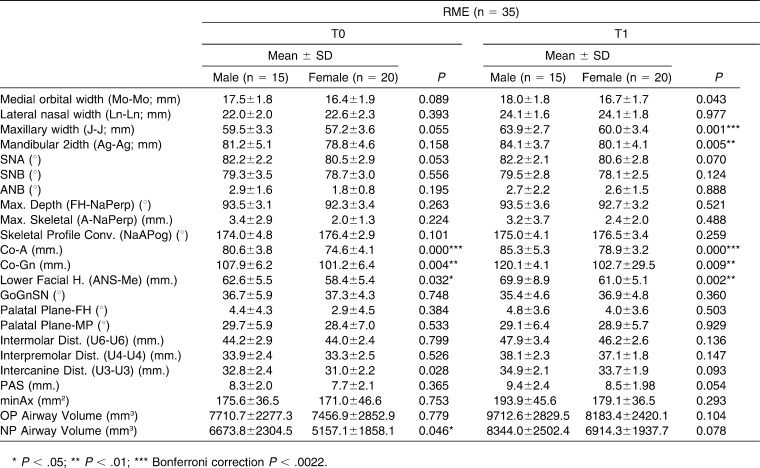
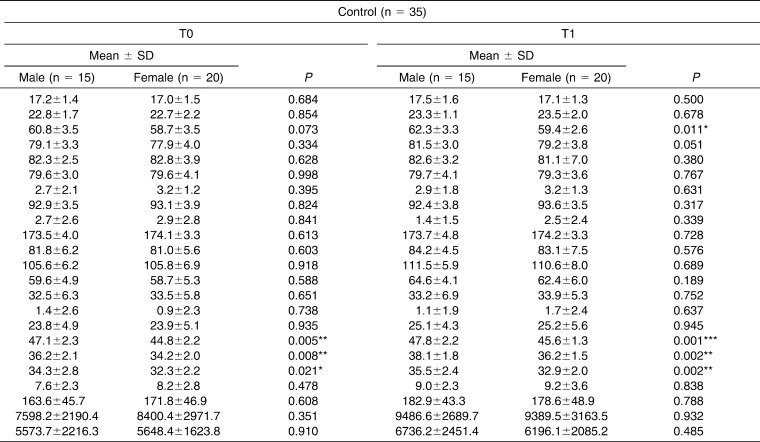
Table 1 shows the sample demographics and confirms that both groups were matched on age, sex, and treatment duration. Table 2 shows all variables separate for males and females, showing the males having larger values, probably because of size differences.
Table 2.
Demographic Data and Comparison of Variables Between Males and Females at T0 and T1 Intervals for RME and Control Groups (Independent Samples t-Test)
Table 2.
Extended.
All of the transverse skeletal (medial orbital width, lateral nasal width, maxillary width and mandibular width) and interdental (intermolar, interpremolar, and intercanine) parameters were enlarged significantly in the RME group, whereas significant enlargement was also observed for all interdental variables and for lateral nasal width, maxillary width, and mandibular width for the control group. A statistically significant increase for airway variables was also evident in both groups between T0 and T1, which can probably be attributed to growth.
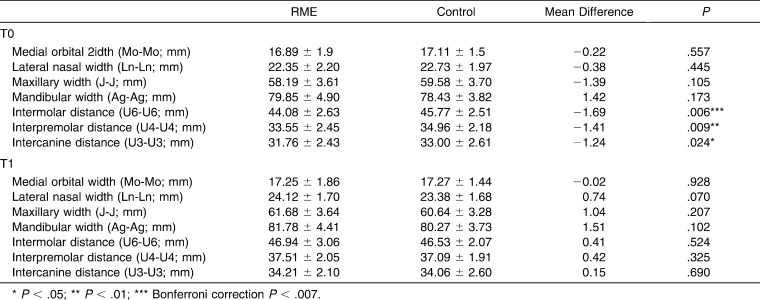
Table 3 shows no skeletal transverse differences between the groups at either T0 or T1, but maxillary values in the control group were larger at T0 and smaller at T1. The dental transverse measurements show that the RME group was significantly smaller at T0 and transversely normalized after expansion; there were no significant differences at T1.
Table 3.
Comparisons Between RME and Control Group for Transverse Skeletal and Dental Measurements
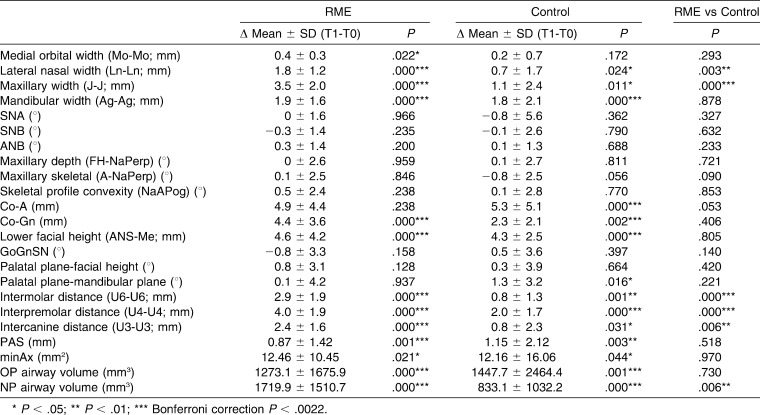
Table 4 shows that the increases in lateral nasal width and maxillary width were significantly higher in the RME group. This was also true for the interdental variables. Although both groups displayed a significant increase for OP and NP airway, only NP airway showed a significant difference between the groups. The mean increase of NP airway volume in the RME group (1719.9 ± 1510.7 mm3) was twofold compared with the control group (813.6 ± 1006.7 mm3) (Table 4).
Table 4.
Mean Differences Between T0 and T1 Intervals
No significant difference was observed between the groups for PAS, minAx, and OP and NP airway volume at T0 and for PAS, minAx, and OP airway volume at T1. At T1 the mean NP airway volume was significantly larger in the RME group (Table 5).
Table 5.
Mean OP and NP Volumes at T0 and T1 Intervals
DISCUSSION
Skeletal and dental effects of RME have been extensively researched in the literature. The current study analyzed three-dimensional skeletal and dental changes after RME treatment in teenage subjects. The present study used images taken before and after comprehensive orthodontic treatment in order to analyze the effects of a Hyrax maxillary expander. Given that all experimental subjects had orthodontic treatment in addition to RME, treatment may also have played a significant role in the results seen. To factor out changes introduced by comprehensive orthodontic treatment, a matched control sample was used.
Weissheimer et al.31 compared the immediate effects of RME in the transverse plane using CBCT and reported an increase in all maxillary transverse dimensions, which coincides with the findings of the present study. A systematic review by Lagravere et al.32 concluded that RME did not produce clinically significant anteroposterior or vertical changes in the long term compared with results for control subjects. Although in the present study there were significant changes in the skeletal parameters for both RME and control subjects, the intergroup comparison did not reveal any significant differences, which is in agreement with the reported systematic review.
The immediate and long-term effects of RME over the upper airway have been shown in previous studies.9,18,27,28,32,33 The literature shows that patients presenting with maxillary constriction tend to have a higher nasal airway resistance.34 The present study does not show a difference in nasal air passage volume at T0, but this may be due to the fact that patients with clinically normal respiratory functions for selected for both groups. The maxilla forms most of the lateral walls of the nasal cavity; therefore, an increase in volume in the nasal cavity would be an expected RME effect. The series of events that cause this phenomenon is mainly the triangular11,35 or parallel36 opening of the median palatal suture, which increases the width of the nasal floor and results in an increased volume of the nasal cavity. The present study shows a twofold increase in the NP volume after RME. This finding suggests that RME may be able to improve the breathing pattern by reducing nasal resistance, but further studies are needed to confirm such anatomical and functional correlations.
Farronato et al.7 reported that the mandibular position changes in various directions when RME is applied to patients with different malocclusions. These differences in mandibular position may affect the OP airway size, shape, and volume.37 The present study did not find significant changes in mandibular position between the RME and control groups.
Zhao et al.28 assessed the changes of the OP airway on 24 patients with maxillary constriction treated with RME and compared them to 24 age- and sex-matched patients and found no significant increase. They concluded that RME would not enlarge OP airway volume. Malkoç et al.29 evaluated the effects of mandibular symphyseal distraction osteogenesis followed by RME on pharyngeal size and concluded that RME did not significantly affect the pharyngeal dimensions. The present study confirms such findings and also found no effect on pharyngeal airway when using RME.
CONCLUSION
Rapid maxillary expansion creates a significant increase in nasal passage volume, but no significant change is observed in the oropharyngeal airway region.
REFERENCES
- 1.Angell EC. Treatment of irregularities of the permanent or adult teeth. Dental Cosmos. 1860;1:540–544. [Google Scholar]
- 2.Haas AJ. Rapid expansion of the maxillary dental arch and nasal cavity by opening the mid palatal suture. Angle Orthod. 1961;31:73–89. [Google Scholar]
- 3.Haas AJ. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965;35:200–217. doi: 10.1043/0003-3219(1965)035<0200:TTOMDB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Wertz RA. Changes in nasal airflow incident to rapid maxillary expansion. Angle Orthod. 1968;38:1–11. doi: 10.1043/0003-3219(1968)038<0001:CINAIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Johal A, Conaghan C. Maxillary morphology in obstructive sleep apnea: a cephalometric and model study. Angle Orthod. 2004;74:648–656. doi: 10.1043/0003-3219(2004)074<0648:MMIOSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Nowara W, Lowe A, Wiegand L, Cartwright R, Perez-Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995;18:501–510. doi: 10.1093/sleep/18.6.501. [DOI] [PubMed] [Google Scholar]
- 7.Farronato G, Giannini L, Galbiati G, Maspero C. Sagittal and vertical effects of rapid maxillary expansion in Class I, II, and III occlusions. Angle Orthod. 2011;81:298–303. doi: 10.2319/050410-241.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CH, Font B. Skeletal and dental changes in the sagittal, vertical, and transverse dimensions after rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2004;126:569–575. doi: 10.1016/j.ajodo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Kurt G, Altug-Atac AT, Atac MS, Karasu HA. Changes in nasopharyngeal airway following orthopedic and surgically assisted rapid maxillary expansion. J Craniofac Surg. 2010;21:312–317. doi: 10.1097/SCS.0b013e3181cf5f73. [DOI] [PubMed] [Google Scholar]
- 10.Buccheri A, Dilella G, Stella R. Rapid palatal expansion and pharyngeal space. Cephalometric evaluation. Prog Orthod. 2004;5:160–171. [PubMed] [Google Scholar]
- 11.Basciftci FA, Mutlu N, Karaman AI, Malkoc S, Kucukkolbasi H. Does the timing and method of rapid maxillary expansion have an effect on the changes in nasal dimensions. Angle Orthod. 2002;72:118–123. doi: 10.1043/0003-3219(2002)072<0118:DTTAMO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Altug-Atac AT, Atac MS, Kurt G, Karasud HA. Changes in nasal structures following orthopaedic and surgically assisted rapid maxillary expansion. Int J Oral Maxillofac Surg. 2010;39:129–135. doi: 10.1016/j.ijom.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Halicioglu K, Kilic N, Yavuz I, Aktan B. Effects of rapid maxillary expansion with a memory palatal split screw on the morphology of the maxillary dental arch and nasal airway resistance. Eur J Orthod. 2010;32:716–720. doi: 10.1093/ejo/cjp164. [DOI] [PubMed] [Google Scholar]
- 14.Bicakci AA, Agar U, Sokucu O, Babacan H, Doruk C. Nasal airway changes due to rapid maxillary expansion timing. Angle Orthod. 2005;75:1–6. doi: 10.1043/0003-3219(2005)075<0001:NACDTR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Babacan H, Sokucu O, Doruk C, Ay S. Rapid maxillary expansion and surgically assisted rapid maxillary expansion effects on nasal volume. Angle Orthod. 2006;76:66–71. doi: 10.1043/0003-3219(2006)076[0066:RMEASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Gordon JM, Rosenblatt M, Witmans M, et al. Rapid palatal expansion effects on nasal airway dimensions as measured by acoustic rhinometry. A systematic review. Angle Orthod. 2009;79:1000–1007. doi: 10.2319/082108-441.1. [DOI] [PubMed] [Google Scholar]
- 17.Deeb W, Hansen L, Hotan T, Hietschold V, Harzer W, Tausche E. Changes in nasal volume after surgically assisted bone-borne rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2010;137:782–789. doi: 10.1016/j.ajodo.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Haralambidis A, Ari-Demirkaya A, Acar A, Kucukkeles N, Ates M, Ozkaya S. Morphologic changes of the nasal cavity induced by rapid maxillary expansion: a study on 3-dimensional computed tomography models. Am J Orthod Dentofacial Orthop. 2009;136:815–821. doi: 10.1016/j.ajodo.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Habersack K, Karoglan A, Sommer B, Benner KU. High-resolution multislice computerized tomography with multiplanar and 3-dimensional reformation imaging in rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2007;131:776–781. doi: 10.1016/j.ajodo.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Tausche E, Deeb W, Hansen L, Hietschold V, Harzer W, Schneider M. CT analysis of nasal volume changes after surgically-assisted rapid maxillary expansion. J Orofac Orthop. 2009;70:306–317. doi: 10.1007/s00056-009-9910-5. [DOI] [PubMed] [Google Scholar]
- 21.Palomo JM, Rao PS, Hans MG. Influence of CBCT exposure conditions on radiation dose. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:773–782. doi: 10.1016/j.tripleo.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 22.El H, Palomo JM. Measuring the airway in 3 dimensions: a reliability and accuracy study. Am J Orthod Dentofacial Orthop. 2010;137(4 suppl):S50e51–59. doi: 10.1016/j.ajodo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Kwong JC, Palomo JM, Landers MA, Figueroa A, Hans MG. Image quality produced by different cone-beam computed tomography settings. Am J Orthod Dentofacial Orthop. 2008;133:317–327. doi: 10.1016/j.ajodo.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 24.Osorio F, Perilla M, Doyle DJ, Palomo JM. Cone beam computed tomography: an innovative tool for airway assessment. Anesth Analg. 2008;106:1803–1807. doi: 10.1213/ane.0b013e318172fd03. [DOI] [PubMed] [Google Scholar]
- 25.Gohl E, Nguyen M, Enciso R. Three-dimensional computed tomography comparison of the maxillary palatal vault between patients with rapid palatal expansion and orthodontically treated controls. Am J Orthod Dentofacial Orthop. 2010;138:477–485. doi: 10.1016/j.ajodo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Christie KF, Boucher N, Chung CH. Effects of bonded rapid palatal expansion on the transverse dimensions of the maxilla: a cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2010;137(4 suppl):S79–S85. doi: 10.1016/j.ajodo.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Görgülü S, Gokce SM, Olmez H, Sagdic D, Ors F. Nasal cavity volume changes after rapid maxillary expansion in adolescents evaluated with 3-dimensional simulation and modeling programs. Am J Orthod Dentofacial Orthop. 2011;140:633–640. doi: 10.1016/j.ajodo.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Nguyen M, Gohl E, Mah JK, Sameshima G, Enciso R. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010;137:S71–S78. doi: 10.1016/j.ajodo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Malkoç S, Üşümez S, Işeri H. Long-term effects of symphyseal distraction and rapid maxillary expansion on pharyngeal airway dimensions, tongue, and hyoid position. Am J Orthod Dentofacial Orthop. 2007;132:769–775. doi: 10.1016/j.ajodo.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 30.Dahlberg G. Statistical Method for Medical and Biological Students. New York, NY: Interscience Publications; 1940. [Google Scholar]
- 31.Weissheimer A, de Menezes LM, Mezomo M, Dias DM, de Lima EM, Rizzatto SM. Immediate effects of rapid maxillary expansion with Haas-type and hyrax-type expanders: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2011;140:366–376. doi: 10.1016/j.ajodo.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Lagravere MO, Heo G, Major PW, Flores-Mir C. Meta-analysis of immediate changes with rapid maxillary expansion treatment. J Am Dent Assoc. 2006;137:44–53. doi: 10.14219/jada.archive.2006.0020. [DOI] [PubMed] [Google Scholar]
- 33.Baratieri C, Alves M, Jr, de Souza MM, de Souza Araujo MT, Maia LC. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing. Am J Orthod Dentofacial Orthop. 2011;140:146–156. doi: 10.1016/j.ajodo.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Cistulli PA, Sullivan CE. Influence of maxillary morphology on nasal airway resistance in Marfan's syndrome. Acta Otolaryngol. 2000;120:410–413. doi: 10.1080/000164800750000658. [DOI] [PubMed] [Google Scholar]
- 35.Timms DJ. A study of basal movement with rapid maxillary expansion. Am J Orthod. 1980;77:500–507. doi: 10.1016/0002-9416(80)90129-3. [DOI] [PubMed] [Google Scholar]
- 36.Ballanti F, Lione R, Baccetti T, Franchi L, Cozza P. Treatment and posttreatment skeletal effects of rapid maxillary expansion investigated with low-dose computed tomography in growing subjects. Am J Orthod Dentofacial Orthop. 2010;138:311–317. doi: 10.1016/j.ajodo.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Hong JS, Hwang YI, Park YH. Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofacial Orthop. 2010;137:306. e301–e311; discussion 306–307. doi: 10.1016/j.ajodo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 38.El H, Palomo JM. Airway volume for different dentofacial skeletal patterns. Am J Orthod Dentofacial Orthop. 2011;139:e511–e21. doi: 10.1016/j.ajodo.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 39.El H, Palomo JM. An airway study of different maxillary and mandibular sagittal positions. Eur J Orthod. 2013;35:262–270. doi: 10.1093/ejo/cjr114. [DOI] [PubMed] [Google Scholar]