Abstract
Introduction
The coronavirus disease-2019 (COVID-19) has emerged as a novel infection which has spread rapidly across the globe and currently presents a grave threat to the health of the cancer patient.
Objective
The aim of this meta-analysis was to evaluate the proportion of hematological cancer patients with the SARS-CoV-2 infection during the COVID-19 pandemic.
Method
A comprehensive literature review was performed on PubMed, Web of Science, Scopus, EKB SciELO, SID, CNKI and Wanfang databases to retrieve all relevant publications up to January 31, 2021. Observational studies, consecutive case-series and case-control studies were included. The proportion for hematological cancer patients with COVID-19 was estimated using the odds ratios (ORs) and 95% confidence interval (95% CIs).
Results
Fourteen studies with a total of 3,770 infected cancer patients and 685 hematological cancer cases with COVID-19 were selected. Combined data revealed that the overall proportion of hematological cancer patients with COVID-19 was 16.5% (95% CI 0.130 - 0.208, p ≤ 0.001). The stratified analysis by ethnicity showed that the proportion was 18.8% and 12.4% in Caucasian and Asian hematological cancer patients with COVID-19, respectively. Moreover, subgroup analysis by country of origin showed that its proportion was the highest in the United Kingdom (22.5%), followed by France (17.1%) and China (8.2%).
Conclusion
This meta-analysis result indicated that the proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic was 16.5%. Further larger sample sizes and multicenter studies among different ethnic groups are necessary to get a better assessment of the proportion.
Keywords: SARS-CoV-2, COVID-19, Hematological Cancer, Infection, Pandemic
Introduction
The coronavirus disease 2019 (COVID-19) outbreak started in Wuhan, China in late 2019 and has rapidly spread globally and become a major health threat to human life in many ways. 1, 2, 3 The identified virus is a new strain of coronavirus-enveloped non‐segmented positive sense RNA virus and has been named Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS‐CoV‐2) by the World Health Organization (WHO). 4, 5, 6 As of December 2019, 103,950,353 cases of COVID-19 have been reported, including 2,248,179 deaths worldwide. 3 The United States has 4% of the world's population, but represents approximately 20% of worldwide COVID-19 cases and mortality. 7 Most patients with the SARS-CoV-2 infection are asymptomatic or exhibit mild-to-moderate symptoms, but approximately 15% progress to severe pneumonia and 5% require intensive care unit (ICU) management. 8,9
COVID-19 brings a huge burden to healthcare facilities, especially in patients with an underlying disease. 10,11 It is reported that vulnerable patients, such as those with old age, hypertension, cardiovascular disease, diabetes mellitus and malignancy and the immunocompromised, are presumed to be at increased risk of severe COVID-19 outcomes due to the underlying disease and treatment regimen. 4,12,13 Recent publications have indicated an increased risk of severe infection, poorer outcomes and worse prognosis in infected cancer patients. 14, 15, 16, 17, 18 More importantly, cancer patients with the SARS-CoV-2 infection had a higher risk of severe events, such as requiring invasive ventilation and death, than infected patients without cancer. 19 The United Kingdom Coronavirus Cancer Monitoring Project (UKCCMP) showed that cancer patients with the SARS-CoV-2 infection with advanced age and with other comorbidities had a significantly higher mortality rate. 20 It seems that newly diagnosed cancer patients might not get treatment on time during the COVID-19 pandemic. 21 Some studies revealed that the type of hematologic malignancy was associated with higher COVID-19 mortality than solid cancer. 19 A study based on combined data showed that the risk of death in pediatric hematological cancer patients with the SARS-CoV-2 infection was significantly lower than that of adult patients 22, which confirmed previous reports that increasing age is highly correlated with the risk of death due to the infection. 23,24
Several epidemic and clinical studies have been consulted on patients with hematologic malignancies in different population mortalities in order to understand the epidemiology of COVID-19. 14,19,25 There are few data on the risk of developing COVID-19 in hematological cancer patients. 25 However, those studies results were not inconclusive and data on the proportion of hematological cancer patients with the SARS-CoV-2 infection are lacking. A meta-analysis combines and appraises the available data from different sources to answer a specific research question. 26 Although meta-analyses usually include randomized clinical trials (RCTs) and case controls to get a more precise assessment on the effect of a treatment or a disease risk factor, they have also been extensively utilized during the COVID-19 pandemic to synthesize the disease outcomes in different groups of individuals. 10,27, 28, 29 Thus, this meta-analysis was carried out to evaluate the proportion of hematological cancer patients with the SARS-CoV-2 infection during the COVID-19 pandemic.
Method
Identification of relevant studies
Ethical approval or patient consent was not needed because this is a meta-analysis in which all data were extracted from published literature. We have performed a comprehensive computer bibliographic search on PubMed/MEDLINE, Google Scholar, EMBASE, Cochrane Library database, SciELO, Springer Link, Chinese Biomedical Database (CBD), China National Knowledge Infrastructure (CNKI) platforms, VIP, Chinese literature (Wan Fang) and China Science and Technology Journal database and Egyptian Knowledge Bank (EKB) Journals to identify all relevant studies concerning the proportion of the SARS-CoV-2 infection in patients with lung cancer published up to January31, 2021. We used the combination of the following search terms and keywords: (‘’Coronavirus Disease 2019’’ OR ‘’COVID-19’’ OR ‘’Severe Acute Respiratory Syndrome Coronavirus 2’’ OR ‘’SARS-CoV-2’’) AND (‘’chronic lymphocytic leukemia’’ OR ‘’CLL’’ OR ‘’multiple myeloma’’ OR ‘’MM’’ OR ‘’non-Hodgkin lymphoma’’ OR ‘’NHL’’ OR ‘’acute lymphoblastic leukemia’’ OR ‘’ALL’’ OR ‘’acute myeloid leukemia’’ OR ‘’AML’’ OR ‘’chronic myeloid leukemia’’ OR ‘’CML’’ OR ‘’Hodgkin lymphoma’’ OR ‘’HL’’) AND (‘’Pediatrics’’ OR ‘’Children’’ OR ‘’Adults’’ OR ‘’Female’’ OR ‘’Male’’). We restricted our search to pediatric and adult patients. The search was limited to English, Farsi and Chinese languages. Moreover, a manual search in all references of retrieved articles and reviews was carried out for more relevant articles.
Inclusion and exclusion criteria
The following inclusion criteria were used to select literatures for the meta-analysis: 1) consecutive case-series, case-control and cohort studies; 2) studies on COVID-19 prevalence in cancer patients with the SARS-CoV-2 infection, and; 3) sufficient data were presented to calculate the odds ratio (OR) with a 95% confidence interval (CI). The following exclusion criteria were used: 1) studies on only hematological cancers; 2) usable data not reported; 3) case only studies and non-consecutive case series; 4) in vitro studies; 5) studies with no available data and no contact with the authors; 6) posters, abstracts, letters, conference papers, non-standard data presentation, reviews and meta-analyses, and; 7) duplicate publications.
Data extraction
Two authors independently performed the publication search in the database. Subsequently, they reviewed the titles and abstracts of the selected studies in the primary search and extracted the necessary data into a form. When the authors were not in agreement, a third author was involved to reach an agreement. For each study, the following data ere extracted: first author's name, year of publication, country or region, ethnicity (the ethnicity was classified as Caucasian, Asian, African or mixed population), total numbers of infected cancer patients with the SARS-CoV-2 infection, age (mean and range), female/male ratio, number of hematological cancer patients with the SARS-CoV-2 infection and cancer treatment in total cases (surgical, radiotherapy, chemotherapy, targeted therapy and immunotherapy). If a duplicate publication or the same population (obvious overlap) was found in the primary literature search, the article with the larger sample size was selected for further analysis. The corresponding author for additional information, if the essential data was found to be incomplete, was contacted by email or telephone for any missing data.
Quality assessment
Before the inclusion of selected studies in the meta-analysis, the methodological quality of the selected studies in the meta-analysis was performed using the Newcastle-Ottawa scale (NOS). The NOS ranges from zero to nine stars selection of patients (4 points), comparability of the groups (2 points) and ascertainment of exposure (3 points). Each selected study was interpreted to be of low quality (for scores ≤ 4), moderate quality (for scores 5 - 6) or high quality (for scores ≥ 7). Two authors assessed the quality of included studies independently and all disagreements were resolved by discussion or by consulting a third party.
Data synthesis
The proportion of hematological cancer patients with the SARS-CoV-2 infection was assessed using odds ratios (ORs) with 95% confidence intervals (CIs) based on the COVID-19 infection between total cancer patients and hematological cancer patients. The Z-test was employed to assess the significance of pooled ORs, in which p < 0.05 was defined as the significance threshold. We utilized the I2 statistics (range of 0 to 100%; I2 = 0 - 25%, no heterogeneity; I2 = 25 - 50%, moderate heterogeneity; I2 = 50 –75%, large heterogeneity; I2 = 75 - 100%, extreme heterogeneity) and the χ2 based Q-statistic (p ≤ 0.10) to assess between-study heterogeneities. If between-study heterogeneity existed statistically, a random effect model (DerSimonian and Laird method) was adopted. Otherwise, a fixed-effect model (Mantel-Haenszel method) was used to combine the pooled data in the absence of heterogeneity. Stratified analysis was performed based on by ethnicity and country of origin to evaluate ethnic-specific results. A visual inspection of the funnel plot was used to assess whether our combined results could have been influenced by publication biases. Moreover, Egger's test was performed to assess the publication bias statistically, in which p < 0.05 was considered statistically significant. If the publication bias tests indicated bias existed, the Duval and Tweedie ‘‘trim and fill’’ method was used to adjust the bias. In the current meta-analysis, the Comprehensive Meta-Analysis (CMA) software version 2.0 (Biostat, USA) was used to combine the results of single studies and calculations of all the statistical analyses.
Results
Characteristics of eligible studies
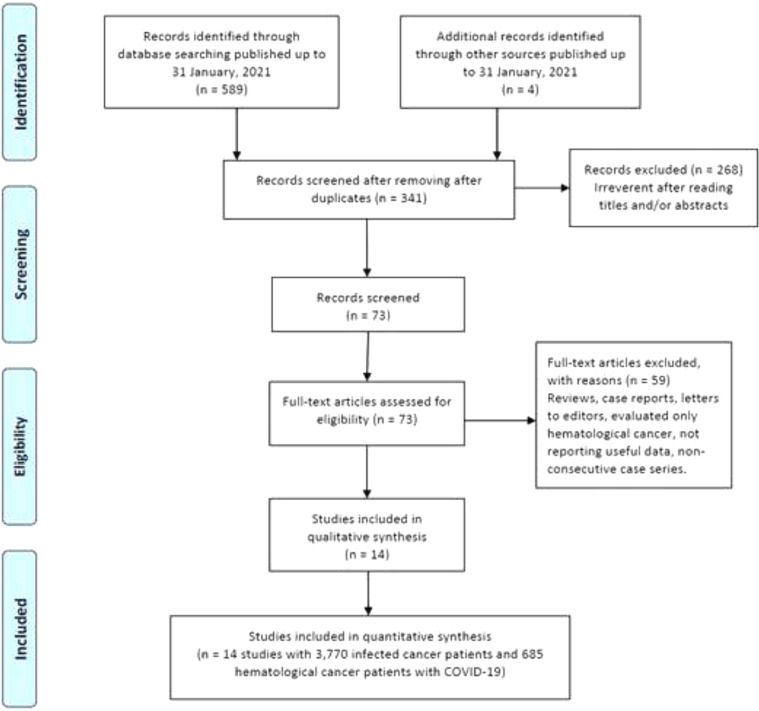
As shown in Figure 1, our initial search yielded 593 studies retrieved through the publication search. After removing duplicated studies, 341 studies were remained for further assessment. Subsequently, 268 studies were excluded after assessment of the titles and abstracts. In sequence, 59 studies were excluded because of not reporting useful data for the analysis, reviews, case reports, non-consecutive or case series, or were on only hematological cancers and we failed to contact their corresponding authors. Finally, a total of 14 studies 12,14,36, 37, 38, 39,15,20,30, 31, 32, 33, 34, 35 with a total 3,770 infected cancer patients and 685 hematological cancer cases with COVID-19 were ultimately found to be eligible for inclusion. The main characteristics of the selected studies are presented in Table 1. All of the included articles were written in English and published between 2020 and 2021. The sample size of all infected cancer patients ranged from 18 to 928 and 1 to 169 for hematological cancer patients. The study patients were from the China, France, United States, United Kingdom, Canada, Iran and Pakistan. Of them, eight studies were performed among Caucasians (with 2,848 all cancer patients and 544 hematological cancer patients) and six studies among Asians (with 922 all cancer patients and 141 hematological cancer patients). As shown in Table 1, quality scores ranged from 6 to 9, indicating that all included studies had high-quality scores.
Figure 1.
Flowchart of literature search and selection process in the meta-analysis.
Table 1.
Main characteristics of the included studies in the meta-analysis.
| First Author/Year | Country (Ethnicity) | Sample Size⁎⁎ | Age (range) | F/M | HC | Cancer treatment in total cases |
NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Radiotherapy | Chemotherapy | Targeted therapy | Immunotherapy | |||||||
| Yang et al., 2020 | China (Asian) | 205 | 63(56-70) | 109/96 | 22 | 4(2.0) | 9(4.4) | 31(15.1) | 12(5.9) | 4(2.0) | 8 |
| Tian et al., 2020 | China (Asian) | 232 | 64(58-69) | 113/119 | 12 | 197(84.9) | 214(92.2) | 32(13.8) | 8 | ||
| Liang et al., 2020 | China (Asian) | 18 | 62(56-68) | 6/12 | 1 | 1(5.6) | 0(0.0) | 2(11.1) | 2(11.1) | 1(5.6) | 8 |
| Dai et al., 2020 | China (Asian) | 105 | 64(55-69) | 48/57 | 9 | 8(7.6) | 13 (12.4) | 17(16.2) | 4(3.8) | 6(5.7) | 8 |
| Ali et al., 2020 | Pakistan (Asian) | 201 | 45(18–78) | 115/86 | 33 | 22(10.9) | 13(6.5) | 146(72.6) | 2(1) | 0(0.0) | 8 |
| Aznab et al., 2020 | Iran (Asian) | 161 | NA | NA | 64 | NA | NA | NA | NA | NA | 6 |
| Kuderer et al., 2020 | Caucasian* | 928 | 66(57-76) | 412/516 | 167 | 32(3.4) | 12(1.3) | 160(17.2) | 75(8.1) | 38(4.1) | 9 |
| Lee et al., 2020 | UK (Caucasian) | 800 | 69(59-76) | 349/449 | 169 | 29(3.6) | 76(9.5) | 281(35.1) | 72(9.0) | 44(5.5) | 9 |
| Russeli et al., 2020 | UK (Caucasian) | 156 | 65 | 66/90 | 28 | NA | NA | 45(55.6) | 5(6.2) | 7(8.6) | 7 |
| Bhogal et al., 2021 | UK (Caucasian) | 179 | 72(61-81) | 74/105 | 52 | 39(21.8) | 34(19.0) | 117(65.4) | 8 | ||
| Barlesi et al., 2020 | France (Caucasian) | 137 | 61(21-90) | 79/58 | 24 | 0(0.0) | 0(0.0) | 48(35.0) | 18(13.1) | 12(8.8) | 8 |
| Albiges et al., 2020 | France (Caucasian) | 178 | 60(52-71) | 102/76 | 30 | NA | NA | NA | NA | NA | 6 |
| Mehta et al., 2020 | USA (Caucasian) | 218 | 69(10-92) | 91/127 | 54 | 0(0.0) | 49(22.5) | 42(19.3) | 0(0.0) | 5(2.3) | 8 |
| Elkrief et al., 2020 | Canada (Caucasian) | 252 | 73(4-95) | 125/127 | 20 | NA | NA | NA | NA | NA | 6 |
NA: Not Available; F/M: Female/Male; HC: Hematological Cancer; NOS: Newcastle-Ottawa Scale.
Europe, USA, Asia;
All cancer cases with SARS-CoV-2 Infection.
Quantitative data synthesis
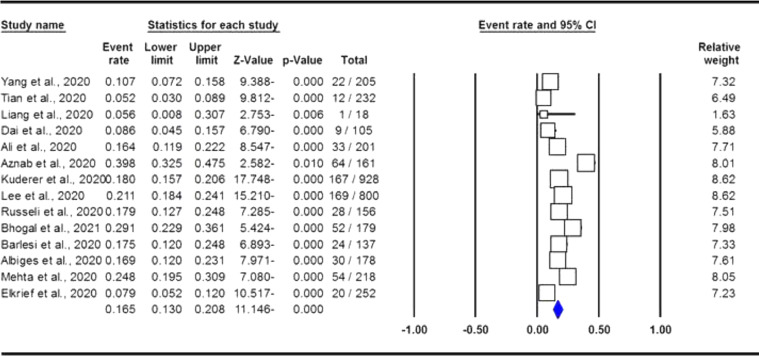
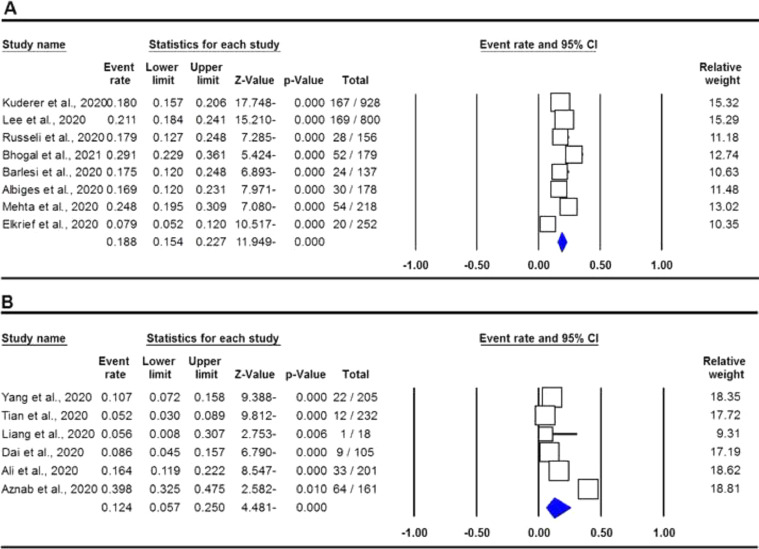
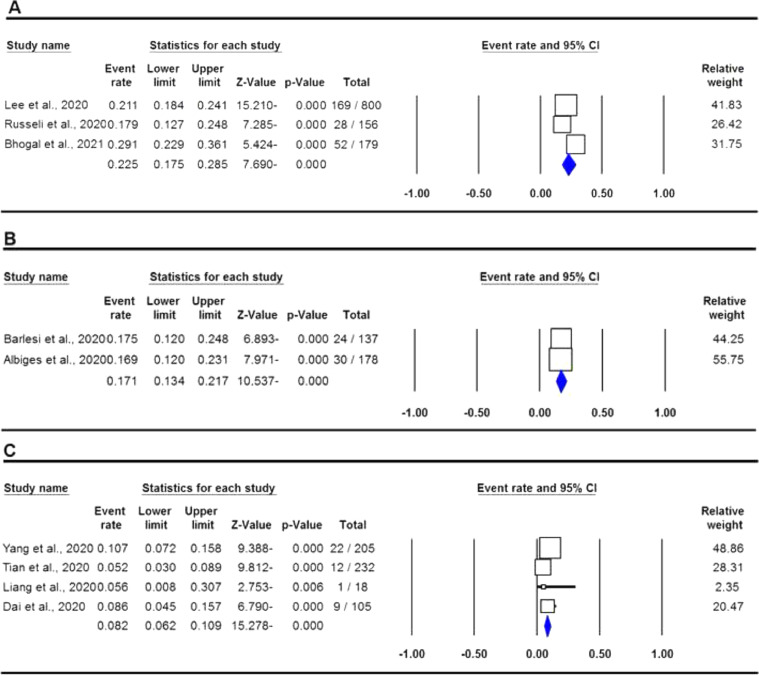
The pooled data on the proportion of hematological cancer patients with the SARS-CoV-2 infection are shown in Table 2. The I2 and the Q-statistic tests showed that there was a significant heterogeneity. Thus, a random effect model (DerSimonian and Laird method) was used to assess the proportion of hematological cancer patients with the SARS-CoV-2 infection. When all eligible studies were included, the pooled data revealed that the proportion of infected hematological cancer patients during the COVID-19 pandemic was 16.5% (95% CI 0.130 - 0.208, p ≤ 0.001, Figure 2) globally. Moreover, a stratified analysis by ethnicity and country of origin was performed. The subgroup analysis by ethnicity showed that the proportion of hematological cancer patients with the SARS-CoV-2 infection was 18.8% (95% CI 0.154 - 0.227, p ≤ 0.001, Figure 3A) in Caucasian and 12.4% in Asian (95% CI 0.057 - 0.250, p ≤ 0.001, Figure 3B), respectively. Moreover, the subgroup analysis by country of origin revealed that the proportion of infected hematological cancer patients was highest in the United Kingdom (22.5%, 95% CI 0.175 - 0.285, Figure 4A), followed by France (17.1%, 95% CI 0.134 - 0.217, Figure 4B) and China (8.2%, 95% CI 0.062 - 0.109, Figure 4C), respectively.
Table 2.
Summary estimates for proportion of hematological cancer patients with SARS-CoV-2 Infection.
| Subgroup | Type of Model | Heterogeneity |
Odds Ratio |
Publication Bias |
|||||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | PH | OR | 95% CI | Ztest | POR | PBeggs | PEggers | ||
| Overall | Random | 89.33 | ≤ 0.001 | 0.165 | 0.130-0.208 | -11.146 | ≤ 0.001 | 0.028 | 0.141 |
| Ethnicity | |||||||||
| Caucasian | Random | 81.42 | ≤ 0.001 | 0.188 | 0.154-0.227 | -11.949 | ≤ 0.001 | 0.536 | 0.559 |
| Asian | Random | 94.02 | ≤ 0.001 | 0.124 | 0.057-0.250 | -4.481 | ≤ 0.001 | 0.452 | 0.191 |
| Country of origin | |||||||||
| UK | Random | 71.26 | 0.031 | 0.225 | 0.175-0.285 | -7.690 | ≤ 0.001 | 1.000 | 0.872 |
| France | Fixed | 0.00 | 0.877 | 0.171 | 0.134-0.217 | -10.537 | ≤ 0.001 | NA | NA |
| China | Fixed | 35.95 | 0.195 | 0.082 | 0.062-0.109 | -15.278 | ≤ 0.001 | 0.734 | 0.603 |
NA: Not Applicable.
Figure 2.
Forest plot for proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic in overall population.
Figure 3.
Forest plot for proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic in by ethnicity. A: Caucasian; and B: Asian.
Figure 4.
Forest plot for proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic in by country of origin. A: UK; B: France; and C: China.
Heterogeneity test
In the current meta-analysis, there was statistically significant heterogeneity (I2 = 89.33%, p ≤ 0.001) in the global population (Table 2). Therefore, subgroup analyses by ethnicity (Asian and Caucasian) and country of origin (UK, France and China) were performed to evaluate the source of heterogeneity. The subgroup analyses revealed that the heterogeneity was not reduced, or did not disappear, by ethnicity. However, the heterogeneity was reduced in the Chinese (I2 = 35.95 and PH = 0.195) and was also dispersed in the French (I2 = 0.00 and PH = 0.877) hematological cancer patients with the SARS-CoV-2 infection, which indicated that the country of origin might be the source of heterogeneity in our pooled data.
Sensitivity analysis
It is defined that the sensitivity analysis is a repeat of a meta-analysis to substitute alternative ranges of values for results that were arbitrary or unclear. Thus, in the current meta-analysis, a sensitivity analysis, by omitting each individual study at any time, was performed to assess the influence of a single study on the pooled ORs by repeating the meta-analysis. The sensitivity analysis results revealed that our pooled data did not change via omitting each single study. The results were also achieved by excluding a study 34 that focused on European, North American (USA) and Asian (mixed population) hematological cancer patients with the SARS-CoV-2 infection, indicating that our combined data were statistically reliable.
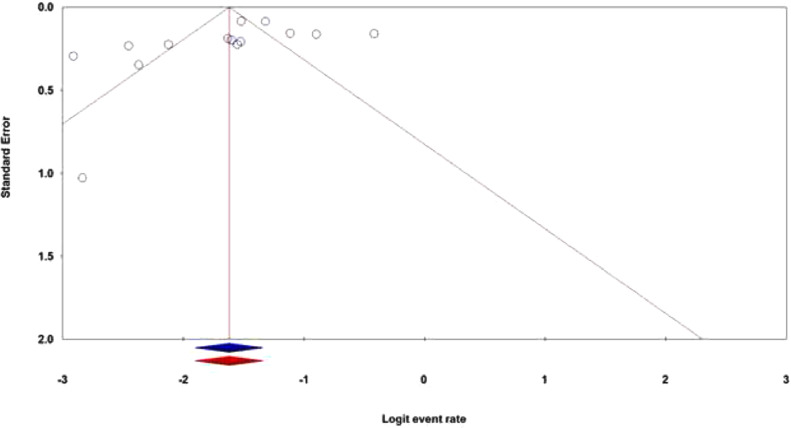
Publication bias
The publication bias is the well-known major problem in a meta-analysis, which exists when the selected studies differ systematically from all studies that should have been selected. In this meta-analysis, both the Egger's test and Begg's test were performed to assess the potential publication bias in the literature. The shapes of the Begg's funnel plot and the Egger's test revealed that there was an evidence of publication bias in the overall population (PBegg's = 0.028; PEgger's = 0.141). Thus, the Duval and Tweedie ‘‘trim and fill’’ method was utilized to adjust the publication bias in the overall population in the hematological cancer patients with the SARS-CoV-2 infection. As shown in Figure 5, the funnel plot of the trim and fill method did not change visually, indicating that the combined pooled ORs are reliable.
Figure 5.
The funnel plots of publication bias for proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic in overall population. ‘’Blue’’ without and ‘’Red’’ with the Duval and Tweedie ‘‘trim and fill’’ method.
Discussion
Cancer is one of the main leading causes of death worldwide, representing an estimated 9.6 million deaths in 2018. 40, 41, 42, 43 Cancer patients with a weak or suppressed immune system have a higher risk of experiencing infection with COVID-19, increased complications and higher mortality. 21 In the current meta-analysis, we assessed the proportion of hematological cancer patients with the SARS-CoV-2 infection during the COVID-19 pandemic. This meta-analysis was the first meta-analysis to date on the proportion of hematological cancer patients with the SARS-CoV-2 infection, incorporating data from 685 hematological cancer patients on three continents. To gain the true proportion of hematological cancer patients with the SARS-CoV-2 infection, it will be important for studies to collect data from consecutive case series and case-control studies on all cancer patients. Our pooled analysis showed that the proportion of hematological cancer patients with the SARS-CoV-2 infection during the COVID-19 pandemic was 16.5% in the overall population. Stratified analysis by ethnicity revealed that the proportions of Caucasian and Asian hematological cancer patients with the SARS-CoV-2 infection were 18.8% and 12.4%, respectively. Moreover, a subgroup analysis by country of origin indicated that the proportion of hematological cancer patients with the SARS-CoV-2 infection was highest in the United Kingdom (22.5%), followed by France (17.1%) and China (8.2%), respectively.
A retrospective study at a referral hematologic center in Rome, Italy reported that the prevalence of the SARS-CoV-2 infection in hematological cancer patients was 0.24% (95% CI 0.23 – 0.25), compared to 0.12% in the general population. The study revealed that the prevalence of the SARS-CoV-2 infection in hematological cancer patients was not significantly higher than that of the general population. 44 Desai et al., in a meta-analysis based on eleven studies, found that the cancer prevalence in people with the SARS-CoV-2 infection was 2.0%. 18 Liang et al., in a nationwide analysis in China based on 1,590 infected cases, found that the prevalence of cancer patients with the SARS-CoV-2 infection was 1% (95% CI, 0.61% - 1.65%), which was higher than the overall cancer incidence of 0.29% in China. Moreover, their report showed a higher risk of clinically severe events for patients who had undergone chemotherapy or surgery in the past month. 15 García-Suárez et al., in a population-based registry study based on 697 hematological cancer patients with the SARS-CoV-2 infection, showed those patients had threefold–fourfold higher rates of severe/critical disease and mortality rate, compared to infected patients in the general population. 19 They reported that was the highest mortality rates were in patients with AML (44%), followed by myelodysplastic syndrome (42%), Ph-negative myeloproliferative neoplasms (MPNs) (19%) and CML (13%). Moreover, their results indicated that the rates of severe/critical COVID-19 and mortality in hematological cancer patients with the SARS-CoV-2 infection were higher than those reported for infected solid cancer patients. 19,20 An Italian study showed that the clinical course of the SARS-CoV-2 infection in pediatric oncology patients was milder than that in adults with cancer and that pediatric patients did not need unnecessary treatments for SARS-CoV-2. 45 Vijenthira et al., in a meta-analysis based on 38 studies with 3,240 patients, reported that the risk of death among adult patients with hematologic malignancies with the SARS-CoV-2 infection was 34% (95% CI 28 - 39), while in pediatric patients, it was 4% (95% CI 1-9). 22
The results of the current meta-analysis should be evaluated with caution in view of the following considerations. First, in the current study, only studies published in English or Chinese languages were included, which might cause potential selection bias. Second, the limited numbers of hematological cancer patients with the SARS-CoV-2 infection by ethnicity might cause insufficient statistical power to estimate the proportion. Third, in this current meta-analysis, only studies among Asian and Caucasian patients were included, which may have caused an ethnicity bias and a difficulty in evaluating the proportion of hematological cancer patients with the SARS-CoV-2 infection in other ethnicities, such as Africans and mixed populations (Latin-American). Finally, the proportion of the assessment of the SARS-CoV-2 infection in hematological cancer patients was based on unadjusted estimates, whereas several important confounding factors, such as gender, age, type of hematological cancer and treatment regimen, were not considered in the stratified analysis due to the lack of original data.
Conclusion
This meta-analysis result indicated that the proportion of hematological cancer patients with the SARS-CoV-2 infection during the COVID-19 pandemic was 16.5%. Stratified analysis by ethnicity showed that the proportion was 18.8% and 12.4% in Caucasian and Asian hematological cancer patients with COVID-19, respectively. Moreover, the subgroup analysis by country of origin showed that the proportion was the highest in the United Kingdom (22.5%), followed by France (17.1%) and China (8.2%). Further larger sample sizes and multicenter studies among different ethnic groups are necessary to obtain a better assessment of the proportion.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
I would like to express my sincere gratitude to Dr. Fatemeh Asadian for his motivation, knowledge, and support during the course of this research.
References
- 1.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noorishadkam M, Lookzadeh MH, Mazaheri M, Mirjalili SR, Bahrami R, Asadian F, et al. Coronavirus disease 2019 (COVID-19) and late pregnancy loss in infected pregnant women: a mini review. World J Peri Neonatol. 2020;2(2):67–70. [Google Scholar]
- 3.Moradi F, Enjezab B, Ghadiri-Anari A. The role of androgens in COVID-19. Diabetes Metab Syndr. 2020;14(6):2003–2006. doi: 10.1016/j.dsx.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan A, Siglin J, Khan A. Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020;9(24):9205–9218. doi: 10.1002/cam4.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astuti I, Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global COVID-19 cases surpass 100 million as nations tackle vaccine shortages | Reuters. [Available from: 2021 February 1] https://www.reuters.com/article/us-health-coronavirus-global-cases-idUSKBN29W0L6.
- 8.Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10(40):40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ra SH, Lim JS, Kim GU, Kim MJ, Jung J, Kim SH. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76(1):61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkatout I, Karimi-Zarchi M, Allahqoli L. Gynecological cancers and the global COVID-19 pandemic. J Turk Ger Gynecol Assoc. 2020;21(4):272–278. doi: 10.4274/jtgga.galenos.2020.2020.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albiges L, Foulon S, Bayle A, Gachot B, Pommeret F, Willekens C, et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the gustave roussy cohort. Nat Med. 2020;1(10):965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali J, Sajjad K, Farooqi AR, Aziz MT, Rahat A, Khan S. COVID-19-positive cancer patients undergoing active anticancer treatment: an analysis of clinical features and outcomes. Hematology/Oncology Stem Cell Ther. 2021 doi: 10.1016/j.hemonc.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antikchi MH, Neamatzadeh H, Ghelmani Y, Jafari-Nedooshan J, Dastgheib SA, Kargar S, et al. The risk and prevalence of COVID-19 infection in colorectal cancer patients: a systematic review and meta-analysis. J Gastrointest Cancer. 2021;52(1):73–79. doi: 10.1007/s12029-020-00528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarahzadeh HM, Asadian F, Farbod M, Meibodi B, Abbasi H, Jafari M, et al. Cancer and coronavirus disease (COVID-19): comorbidity, mechanical ventilation, and death risk. JCO Glob Oncol. 2021;52(1):80–84. doi: 10.1007/s12029-020-00529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Global Oncol. 2020;(6):557–559. doi: 10.1200/go.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garciá-Suárez J, De La Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13(1):133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathania AS, Prathipati P, Abdul BAA, Chava S, Katta SS, Gupta SC, et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics. 2020;11(2):731–753. doi: 10.7150/thno.51471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starke KR, Petereit-Haack G, Schubert M, Kämpf D, Schliebner A, Hegewald J, et al. The age-related risk of severe outcomes due to covid-19 infection: a rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020;17(16):1–24. doi: 10.3390/ijerph17165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Liu L, Jiao J, Yang L, Zhu B, Li X. Characterisation of clinical, laboratory and imaging factors related to mild vs. severe covid-19 infection: a systematic review and meta-analysis. Ann Med. 2020;52(7):334–344. doi: 10.1080/07853890.2020.1802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marušić MF, Fidahić M, Cepeha CM, Farcas LG, Tseke A, Puljak L. Methodological tools and sensitivity analysis for assessing quality or risk of bias used in systematic reviews published in the high-impact anesthesiology journals. BMC Med Res Methodol. 2020;20(1):121. doi: 10.1186/s12874-020-00966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardona-Ospina JA, Henao-SanMartin V, Acevedo-Mendoza WF, Nasner-Posso KM, Martínez-Pulgarín DF, Restrepo-López A, et al. Fatal Zika virus infection in the Americas: a systematic review. Int J Infect Dis. 2019;88:49–59. doi: 10.1016/j.ijid.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. 2021;39(4):667–677. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlesi F, Foulon S, Bayle A, Gachot B, Pommeret F, Willekens C, et al. Cancer Research. Vol 80. American Association for Cancer Research (AACR); 2020. Abstract CT403: outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. CT403-CT403. [DOI] [Google Scholar]
- 34.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkrief A, Desilets A, Papneja N, Cvetkovic L, Groleau C, Lakehal YA, et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: a multicentre observational cohort study. Eur J Cancer. 2020;139:181–187. doi: 10.1016/j.ejca.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhogal T, Khan UT, Lee R, Stockdale A, Hesford C, Potti-Dhananjaya V, et al. Haematological malignancy and nosocomial transmission are associated with an increased risk of death from COVID-19: results of a multi-center UK cohort. Leuk Lymphoma. 2021 Jul;62(7):1682–1691. doi: 10.1080/10428194.2021.1876865. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Aznab M. Evaluation of COVID 19 infection in 279 cancer patients treated during a 90-day period in 2020 pandemic. Int J Clin Oncol. 2020;25(9):1581–1586. doi: 10.1007/s10147-020-01734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell B, Moss C, Papa S, Irshad S, Ross P, Spicer J, et al. Factors affecting COVID-19 outcomes in cancer patients: a first report from guy's cancer center in London. Front Oncol. 2020;10:1279. doi: 10.3389/fonc.2020. Front Oncol. 2020;10:127901279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaemmaghami F, Zarchi MK, Gilani MM, Mousavi A, Behtash N, Ghasemi M. Uterine sarcoma: clinicopathological characteristics, treatment and outcome in Iran. Asian Pac J Cancer Prev. 2008;9(3):421–426. [PubMed] [Google Scholar]
- 41.Behtash N, Zarchi MK, Deldar M. Preoperative prognostic factors and effects of adjuvant therapy on outcomes of early stage cervical cancer in Iran. Asian Pac J Cancer Prev. 2009;10(4):613–618. [PubMed] [Google Scholar]
- 42.Zarchi MK, Akhavan A, Gholami H, Dehghani A, Naghshi M, Mohseni F. Evaluation of cervical cancer risk-factors in women referred to Yazd-Iran hospitals from 2002 to 2009. Asian Pac J Cancer Prev. 2010;11(2):537–538. [PubMed] [Google Scholar]
- 43.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 44.Girmenia C, Gentile G, Micozzi A, Petrucci L, Malaspina F, DI Prima A, et al. COVID-19 in patients with hematologic disorders undergoing therapy: perspective of a large referral hematology center in Rome. Acta Haematol. 2020;143(6):574–582. doi: 10.1159/000510769. [DOI] [PubMed] [Google Scholar]
- 45.Bisogno G, Provenzi M, Zama D, Tondo A, Meazza C, Colombini A, et al. Clinical characteristics and outcome of severe acute respiratory syndrome coronavirus 2 infection in Italian pediatric oncology patients: a study from the infectious diseases working group of the associazione Italiana di oncologia e ematologia pediatrica. J Pediatr Infect Dis Soc. 2020;9(5):530–534. doi: 10.1093/jpids/piaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]