Abstract
Objective
Dysregulation of local nitric oxide (NO) synthetases occurs during ischemia and reperfusion associated with cardiopulmonary bypass, deep hypothermic circulatory arrest (DHCA), and reperfusion. Rapid fluctuations in local NO occurring in neonates and infants probably contribute to inflammation-induced microglial activation, and neuronal degeneration after these procedures, eventually impairing neurodevelopment. We evaluated the anti-inflammatory efficacy of inhaled NO (iNO) in a piglet model emulating conditions during pediatric open-heart surgery with DHCA.
Methods
Infant Yorkshire piglets underwent DHCA (18°C) for 30 minutes, followed by reperfusion and rewarming either with or without iNO (20 ppm) in the ventilator at the onset of reperfusion for 3 hours (n = 5 per group, DHCA-iNO and DHCA). Through craniotomy, brains were extracted after perfusion fixation for histology.
Results
Plasma NO metabolites were elevated 2.5 times baseline data before DHCA by iNO. Fluoro-Jade C staining identified significantly lower number of degenerating neurons in the hippocampus of the DHCA-iNO group (p = 0.02) compared with DHCA group. Morphologic analyses of ionized calcium-binding adapter molecule-1 stained microglia, evaluating cell body and dendritic process geometry with Imaris imaging software, revealed subjectively less microglial activation in the hippocampus of pigs receiving iNO.
Conclusions
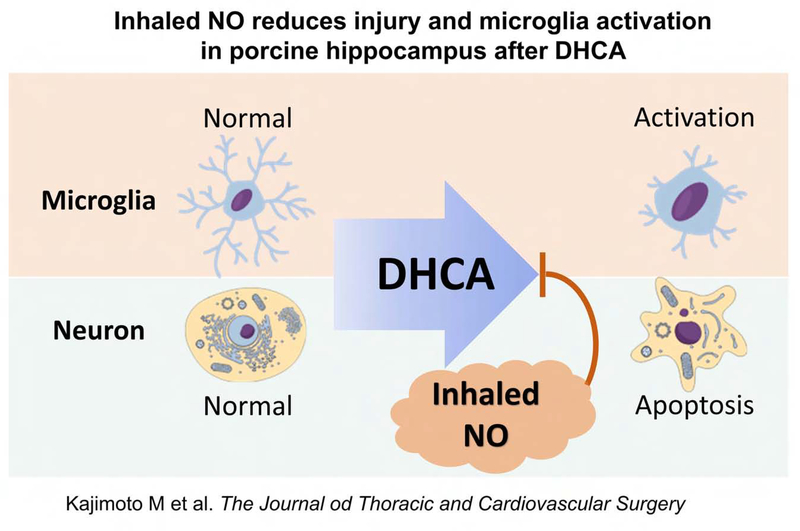
Using DHCA for 30 minutes, consistent with clinical exposure, we noted that iNO reduces neuronal degeneration in the hippocampus. Additionally, iNO reduces microglial activation in the hippocampus after DHCA. The data suggest that iNO reduces neuronal degeneration by ameliorating inflammation and may be a practical mode of neuroprotection for infants undergoing DHCA.
Introduction
Neurodevelopmental deficits in children with congenital heart disease are significant medical, social, and financial issues for patients and their families.1 Multiple plausible hypotheses exist for defining the etiology of congenital heart disease associated neurodevelopmental impairments.2, 3 One of the primary hypotheses is that cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest (DHCA), required for repair of many complex cardiovascular defects, induces immediate and late neurodevelopmental morbidities and neuronal cell death.4–6 Selective antegrade cerebral perfusion (SCP) during DHCA theoretically minimizes ischemic brain injury by supplying oxygen and nutrition.7, 8 However, some studies in animal models show that SCP does not totally ameliorate neuronal death,9 while clinical investigations using magnetic resonance imaging revealed that new cerebral pathology becomes apparent in the immediate postoperative period.10, 11 Thus, more strategies for neuroprotection against the deleterious effects of DHCA are needed.
Multiple factors associated with DHCA contribute to cerebral injury.5, 12, 13 These include ischemia/reperfusion, disruption of cerebral blood flow autoregulation, and inflammatory microglial activation. Nitric oxide (NO) is a key regulator for many of these processes and serves as a signal for normal neuronal development.14 Disruption in NO homeostasis has been implicated as a cause of neurodevelopmental deficits in vulnerable infants. More recent studies have demonstrated that global delivery of NO by inhalation prevents hippocampal damage and preserves cerebral autoregulation after traumatic injury to the neonatal and juvenile pig brain.15, 16 Furthermore, inhaled NO (iNO) reduced concentration of inflammatory cytokines within the brain and cerebral spinal fluid.17
Accordingly, we tested the hypothesis that iNO reduced inflammation and neuronal injury after DHCA in the immature brain. Our first experiments served to validate the histological methods used to evaluate microglial activation in pig brain exposed to 60 minutes DHCA. Microglia reside in the brain parenchyma and function as phagocytes, participating in brain homeostasis.18 They respond rapidly to stimuli within their environment by undergoing a morphological transformation and functional activation. With their transition from a ramified-to amoeboid-activated state, microglial release cytokines that amplify the inflammatory response by recruiting other immune modulating cells to the site of brain injury. In subsequent series of experiments, we then determined if iNO ameliorated neuronal injury and microglial activation, an index of inflammatory response, after a clinically relevant duration of DHCA for 30 minutes.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. Detailed methods are provided in the Supplemental Methods. All experimental procedures were conducted according to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and approved by Seattle Children’s Institutional Animal Care and Use Committee. Sixteen infant male mixed breed Yorkshire piglets (S&S farms, Ramona, CA) were used in these studies.
Study Design
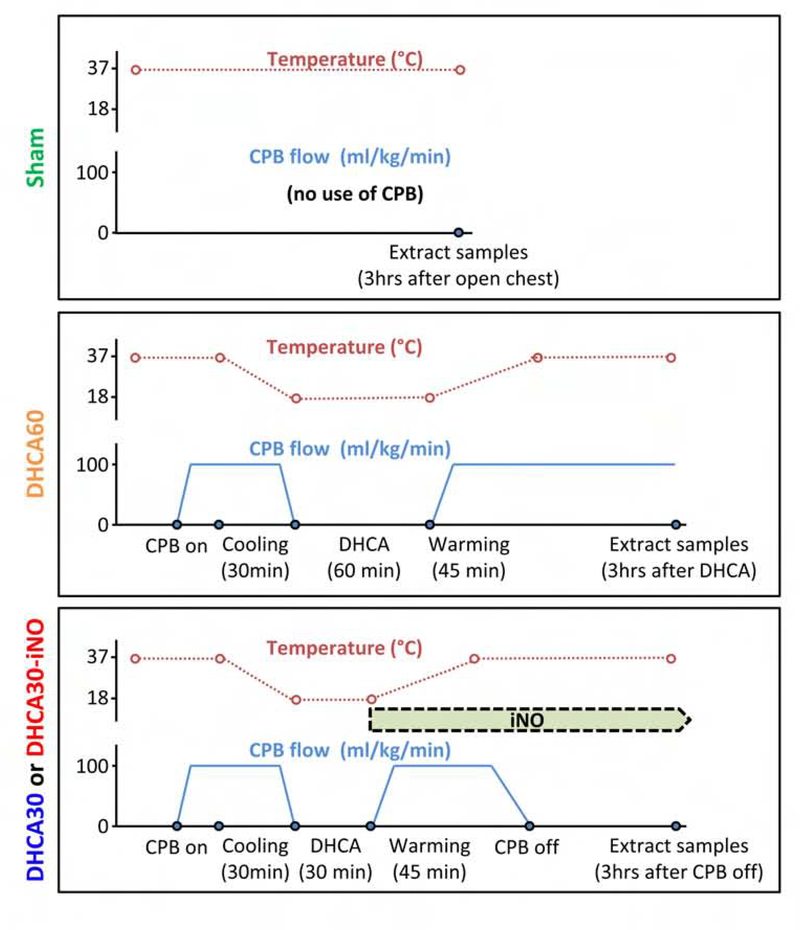
We performed the initial set of experiments to refine methods for identification of microglial activation in pigs undergoing DHCA and reperfusion. In these preliminary experiments, we subjected pigs (n =3) to DHCA at 18°C for 60 minutes followed by rewarming and reperfusion (DHCA60 group). Brains were fixed and extracted after 3 hours of reperfusion without weaning from the CPB circuit; histological sections were compared to piglets in Sham group (n = 3), just received median sternotomy without CPB. In the second group of experiments, we exposed piglets to more clinically relevant DHCA for 30 minutes, followed by reperfusion and rewarming, then weaned from CPB after 45 minutes. These pigs were randomized (5 per group) to receive or not iNO (DHCA30-iNO or DHCA30) at the onset of reperfusion for 4 hours, and then brain extracted. Diagram of experimental protocols are shown in Figure 1.
Figure 1. Diagrams of the experimental protocol, showing the time course of esophageal temperature and CPB flow.
Pigs in Sham group received a median sternotomy without CPB as a Control group. Pigs in DHCA60 group had 60 minutes of DHCA at 18°C and followed by 3 hours continuous CPB after DHCA completion. Pigs in DHCA30 and DHCA30-iNO groups had 30 minutes of DHCA and weaning from CPB consistent with clinical protocols. Pigs in DHCA30-iNO groups received inhalation of NO at a dose of 20 ppm from the start rewarming to protocol completion.
Perioperative management and operative technique
Anesthesia and CPB were performed as described previously.5, 19 After heparinization (350 IU/kg), CPB was initiated with a roller pump console (Sarns 8000) and a membrane oxygenator (CX-RX05RW, Terumo, Tokyo, Japan) and connected to cannulas via the ascending aorta (12–14 Fr) and right atrium (20–24 Fr). A CPB flow of 100–125 ml/kg/min was maintained, and study animals were cooled to an esophageal temperature of 18°C by a heat exchanger (Sarns/3M 11160). Before induction of circulatory arrest, the ascending aorta was clamped, and cold del Nido cardioplegia was infused for myocardial arrest. After completion of DHCA, CPB flow was increased gradually to 125–150 ml/kg/min until an esophageal temperature of 37°C was reached for over 45 minutes to rewarm. Whole brains were fixed by perfusion of 4% paraformaldehyde from the brachiocephalic artery at the end of protocol (Figure 1) and removed via a craniotomy.
Inhaled NO administration
NO was provided through inhalation at a dose of 20 parts per million (ppm) from the NO delivery system (INOmax, Mallinckrodt Pharmaceuticals, Bedminster, NJ) through the ventilator after the completion of DHCA. The duration of iNO treatment was for ~4 hours, from the start of rewarming to protocol completion (Figure 1). The inspired NO and NO2 concentrations were monitored with INOmax. Blood methemoglobin level was measured at regular intervals by a Radiometer ABL 800.
Histology
After fixation in 4% paraformaldehyde, brains were sliced into 1cm coronal sections and then followed by 10, 20 and 30% sucrose cryoprotection. The frontal cortex and the hippocampus of the brain (Figure S1) were sectioned using a cryostat (Leica CM3050 S) into 5-μm-thick slices and mounted onto slides for the assessment of brain injury. While 50-μm-thick slices were used for the morphological assessment of microglia.
TUNEL and Fluoro-Jade C staining
Apoptosis via DNA fragmentation was detected by using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique with a kit (In Situ Cell Death Kit; Roche, Indianapolis, IN). Fluoro-Jade C, which is highly specific in labeling degenerative neurons,20, 21 was purchased from Sigma Aldrich (St. Louis, MO). These stains were performed according to the manufacturer’s recommendations.
Immunohistochemistry for microglia
For the assessment of microglial morphology, 50-μm-thick sections were labeled with primary antibody against the ionized calcium binding adaptor molecule 1 (Iba-1; microglia marker, 1:500, Wako, Osaka, Japan) for 24 hours at 4°C and then Alexa Fluor 488-conjugated secondary antibody (1:200, Invitrogen) was applied for 2 hours at room temperature. Additionally, at the time of secondary antibody application, sections were stained with Hoechst (1:1000, Invitrogen) for counterstaining to visualize nuclei.
Imaging
Cerebral injury
TUNEL and Fluoro-Jade C stained sections were used for the assessment of cerebral injury and apoptosis. The images were obtained using a fluorescence microscope and were analyzed by image software, Fiji. Quantitative analysis represented counting of 10 fields from 4 independent samples (total 40 fields) per animal. Quantification was performed at 200x magnification, and cell counts were averaged for the 40 200x fields in each animal. A reproducibility assessment was carried out by randomly selecting and recounting one to four sections from each animal.
Morphological analysis of microglia
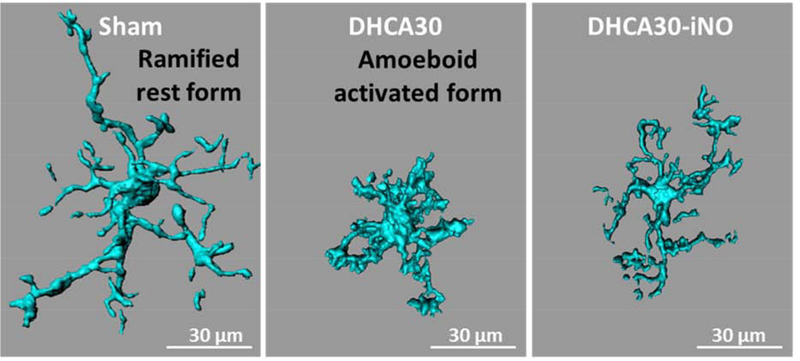
Imaging was performed on a Zeiss LSM 710 laser scanning confocal microscope (Zeiss, Jena, Germany). Z stacks were performed with 0.3-μm steps in the z-direction, and recorded with 1,024 × 1,024 pixel resolution. Three-dimensional reconstruction of microglia was analyzed using Imaris software (Bitplane, Concord, MA). At least 9 microglia were reconstructed per slide. Morphological parameters were measured (total dendrite process length, number of terminal branch points, number of branch segment and cell body volume). It is known that microglia morphology and function of microglia are closely related. Resting microglia exhibited a ramified morphology, and the activated state of them is characterized by swollen ramified cells with shorter and thicker processes and a larger cell body.
Statistical analyses
Reported values are the means ± standard error (SE) or median (the upper and lower quartiles or interquartile range) in the text, figures, and tables. Statistical analysis of the differences between 2 groups (Sham and DHCA60, or DHCA30 and DHCA30-iNO) was carried out with two tailed unpaired t-test or Mann-Whitney U test using PRISM 5.0 (Graph Pad Software Inc., San Diego, CA). On morphological analysis of microglial, each symbol represents one pig with at least nine measured cells per pig. The criterion for significance was P < 0.05 for all comparisons. To obtain unbiased data, cell quantifications were performed blinded.
RESULTS
Microglia activation after 60 minutes DHCA
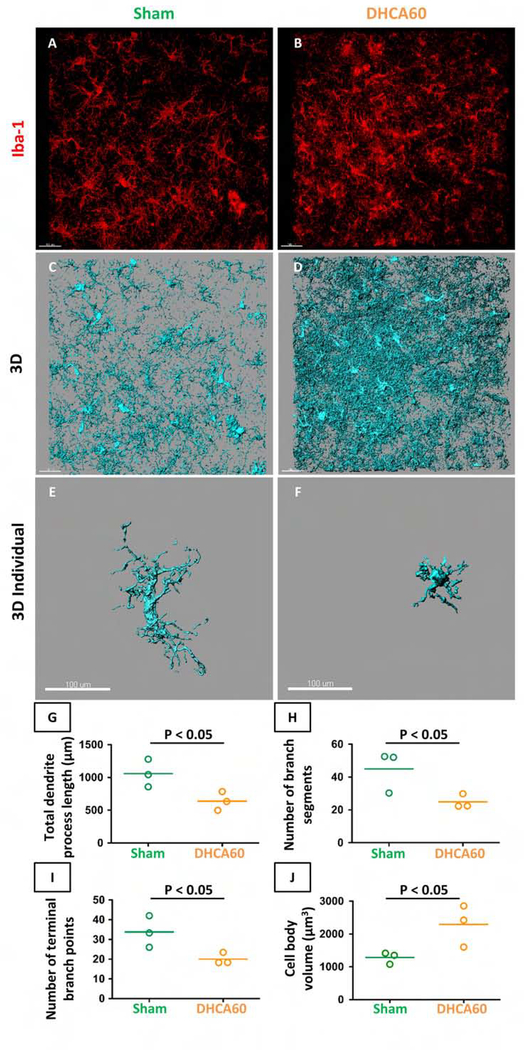
Our previous data showed that 60 minutes DHCA induced greater neuronal injury in the cerebral cortex compared to the Sham procedure.5 However, microglia activation was not evaluated in those experiments. Therefore, for proof of principle and to validate our methodology, we initially assessed microglial activation using DHCA exposure for 60 minutes compared with Sham.
A comparison of preoperative animal age (37.3 ± 2 days in Sham group, vs. 37.7 ± 2 days in DHCA60 group) and weights (11.0 ± 0.4 kg in Sham group, vs. 9.1 ± 0.8 kg in DHCA60 group) showed no differences. The morphological characteristics of microglia were analyzed from the 3-dimensional confocal image (Figure 2). Cerebral cortex exposed to 60 minutes DHCA demonstrated strongly activated microglia with larger cell body volume and shorter branch length compared with Sham. These results provided quantitative confirmation that 60 minutes DHCA activated microglia in the cerebral cortex.
Figure 2. Microglia activation under 60 minutes DHCA in cerebral cortex sections.
Pictures showed staining of Iba-1 (A, B), the 3-dimensional (3D) reconstructed microglia (C, D) and representative microglia (E, F). Staining of Iba-1 showed a dramatical change of microglial morphology in DHCA60 (activated microglia) compared with Sham (ramified microglia). Morphological parameters showed activated microglia in DHCA60 (G-J). Iba-1, ionized calcium-binding adapter molecule-1. Scale bar = 100 μm. The horizontal line in the graph G-J represents the mean value. Data are from n = 3 animals per group.
Response to Inhaled Nitric Oxide
In this protocol, we used DHCA for 30 minutes, consistent with clinical exposure during cardiovascular surgeries in neonates and infants. A comparison of preoperative animal age and weights showed no differences between the groups (Table 1). There were no operative or technical complications in any groups.
Table 1.
Hemodynamic data.
| Baseline |
P | Completion of DHCA |
P | Endpoint |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| DHCA30 | DHCA30-iNO | DHCA30 | DHCA30-iNO | DHCA30 | DHCA30-iNO | ||||
| Age, day | 61 (18) | 51 (22.5) | 0.93 | ||||||
| BW, kg | 15.2 (7.6) | 12.8 (6.2) | 0.55 | ||||||
| NO, ppm | - | - | - | - | 0 | 20 | |||
| NO2 (ex), ppm | - | - | - | - | 0 | 0.4 (0.3) | |||
| Hgb, g/dl | 9.0 (0.7) | 8.5 (2.0) | 0.6 | 7.3 (1.5) | 6.7 (1.4) | 0.39 | 8.5 (1.6) | 7.7 (2.3) | 0.54 |
| MetHb, % | 2.4 (0.3) | 2.4 (1.5) | 0.34 | 2.5 (0.4) | 2.7 (2) | 0.67 | 1.2 (1.6) | 1.9 (1.0) | 0.33 |
| Temperature, °C | 36.7 (0.7) | 37.1 (1.8) | 0.9 | 19.1 (5.0) | 19.8 (3.5) | 0.72 | 36.5 (1.4) | 36.7 (0.9) | 0.88 |
| HR, bpm | 110 (21) | 97 (22) | 0.17 | - | - | 127 (12) | 123 (21) | 0.74 | |
| SBP, mmHg | 74 (7) | 77 (10) | 0.11 | - | - | 55 (14) | 57 (16) | 0.97 | |
| mBP, mmHg | 55 (9)) | 59 (12) | 0.29 | - | - | 39 (15) | 47 (15) | 0.43 | |
| PAP, mmHg | 17 (7) | 15 (12) | 0.68 | - | - | 27 (7) | 19 (11) | 0.11 | |
| CO, L/min | 1.11 (0.33) | 1.04 (0.72) | 0.92 | - | - | 0.80 (0.33) | 0.58 (0.30) | 0.56 | |
BW, body weight; NO2 (ex), exhaled nitrogen dioxide; Hgb, hemoglobin; MetHb, methemoglobin; HR, heart rate; SBP, systemic systolic blood pressure; mBP, mean systemic systolic blood pressure; PAP, systolic pulmonary arterial pressure; CO, cardiac output. Endpoint, just before sacrifice at 3 hours after CPB completion. Values are the median (interquartile range); n = 5 per group. P value, DHCA30 vs DHCA30-iNO.
Blood parameters and hemodynamics
Arterial blood gas data including hemoglobin and glucose did not differ at baseline before starting CPB and the end of experiments between 2 groups. Hemoglobin levels in all pigs were maintained greater than 6.0 g/dL without blood transfusion. Arterial pH, CO2, and O2 were normally maintained within a range of 7.35–7.48, 35–50 mmHg and >100 mmHg respectively. Blood methemoglobin level was maintained less than 3% in all pigs. Table 1 demonstrated hemodynamic data at baseline, 30 minutes of DHCA and endpoint of experiments. Perfusion parameters including cardiac output directly measured by aortic flow were not significantly different at baseline and endpoint of experiments between 2 groups.
Plasma NOx level
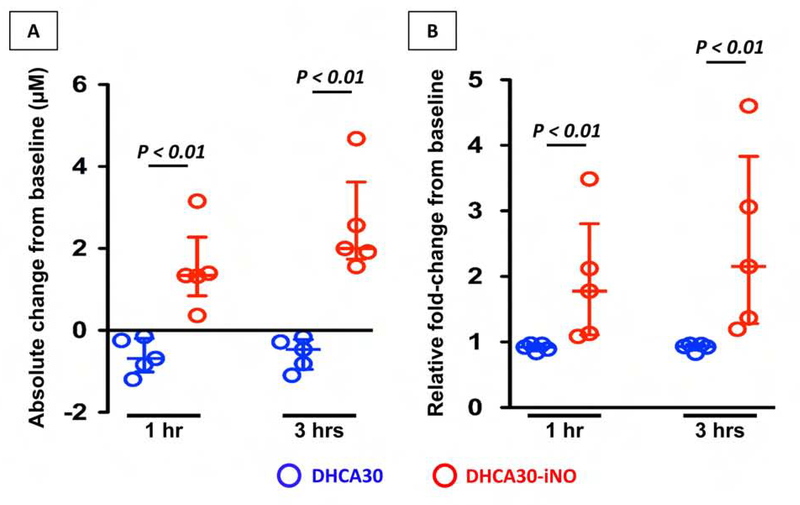
NO level in plasma was estimated as the total NOx; nitrate (NO3−) + nitrite (NO2−) concentration,22 frequently used to assess NO bioavailability in vivo, after the reduction of nitrate to nitrite using colorimetric assay kit (Cayman Chemical, Ann Arbor, MI). Baseline total NOx were highly variable among animals. However, iNO treatment achieved a significant increase in plasma NOx. Absolute change in total NOx and relative change from baseline are shown in Figure 3 at 1 and 3 hours for both treatment groups.
Figure 3. Plasma NOx level.
Absolute change (A) and relative fold-changes (B) from baseline (before CPB) for plasma NOx level at 1 hour and 3 hours after CPB off. Inhalation of NO after DHCA significantly increased plasma NOx level at both 1 hour and 3 hours. The upper and lower horizontal lines represent the upper and lower quartiles, and the middle horizontal line represents the median. Data are from n = 5 animals per group.
Cerebral injury
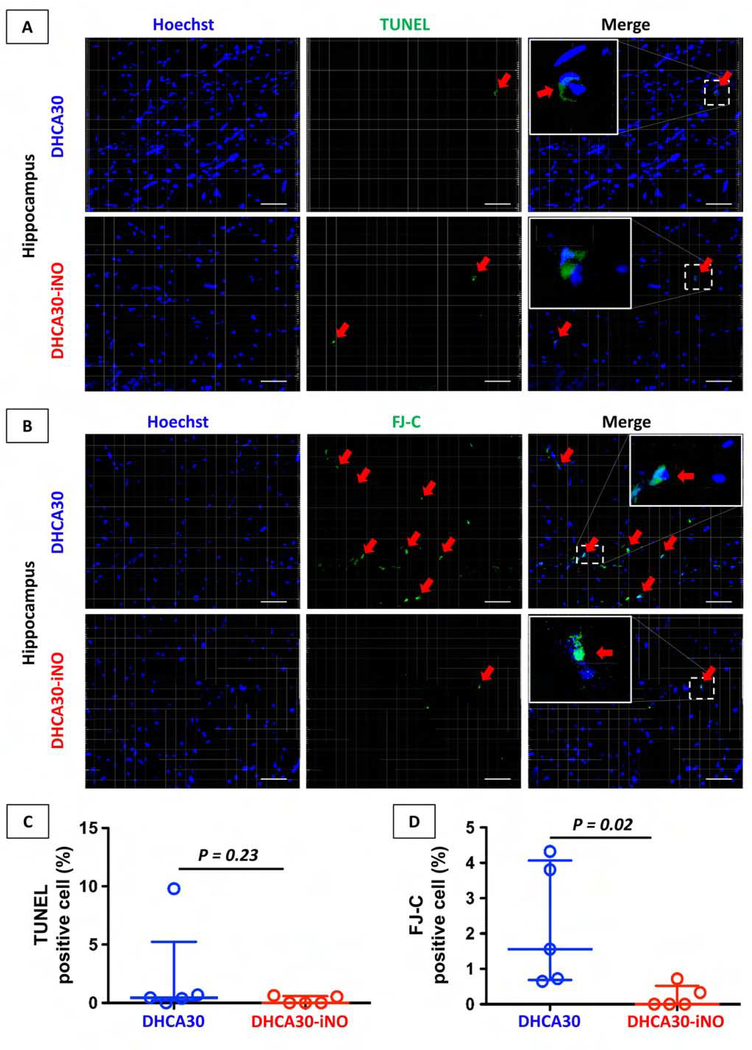
TUNEL-positive or Fluoro-Jade C-positive cells in the cerebral cortex were rare in both DHCA30 and DHCA30-iNO groups (Figure S2). Therefore, we focused on hippocampus which is especially vulnerable to the ischemic insult among the various brain regions and relates to neurocognitive abnormalities in children undergoing DHCA.23, 24 The hippocampus in DHCA30-iNO showed no significant difference in TUNEL staining compared to DHCA30 (Figure 4A and C). However, Fluoro-Jade C staining in the hippocampus showed significantly less neuronal degeneration in DHCA30-iNO group than those in DHCA30 group (Figure 4B and D).
Figure 4. Cerebral injury after 30 minutes DHCA in the hippocampus.
(A, C) Apoptosis cells identified by TUNEL staining in representative hippocampus sections. The proportion of apoptotic cells was not significantly different between DHCA30 and DHCA30-iNO. (B, D) Degenerative neurons identified by FJ-C showed very minimal staining in DHCA30-iNO compared to DHCA30. Red arrows show TUNEL or FJ-C positive cells. TUNEL, the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; FJ-C, Fluoro-Jade C. Scale bar = 50 μm. The upper and lower horizontal lines represent the upper and lower quartiles, and the middle horizontal line represents the median in the graph C and D. Data are from n = 5 animals per group.
Cerebral Neuro-inflammatory reaction
Microglial activity was measured by the assessment of microglial morphology on cerebral cortex and hippocampus in Figure 5A and B respectively. Microglia in DHCA30-iNO showed longer total dendrite process length and more significant number of branch segments and terminal branch points than in DHCA30 in both cerebral cortex and hippocampus. Microglial cell body volume was also significantly smaller in DHCA30-iNO group than in DHCA30 group on the hippocampus, though not cerebral cortex. Thus, NO inhalation reduced microglial activation in the brain, especially hippocampus, after DHCA.
Figure 5. Cerebral Neuro-inflammatory reaction after 30 minutes DHCA.
The representative 3-dimensional reconstructed microglia stained with Iba-1 in sections of the frontal cerebral cortex (A) and hippocampus (B) from the fixed brain (Figure S1). Microglial morphological change to active form was less in DHCA30-iNO than DHCA30, especially in the hippocampus. Iba-1, ionized calcium binding adaptor molecule 1. Scale bar = 30 μm. The upper and lower horizontal lines represent the upper and lower quartiles, and the middle horizontal line represents the median in the graphs. Data are from n = 5 animals per group.
Discussion
NO has been used frequently for the treatment of pulmonary hypertension in infants and children after surgery for congenital heart disease. Additionally, results from two randomized clinical trials suggest that NO delivered directly into the CPB circuit improves clinical outcomes and protects the heart from reperfusion injury.25, 26 Inhibition of the inflammatory processes stimulated by CPB has been implicated as the mechanism for this benefit. In this study, we focused on evaluation of iNO for neural protection. We used an established animal model, which emulates DHCA in a human infant. We found selective and localized inflammation occurring in the brain shortly after insult (Figure 2). Our prior studies identified neuronal apoptosis within the cerebral cortex following prolonged DHCA (60 minutes).5 However, apoptosis occurred minimally after a briefer and more clinically relevant 30 minute DHCA exposure. Using Fluoro-Jade C as a more sensitive histological marker for cellular injury than TUNEL,20, 21 we identified a significant number of cells with neuronal degeneration after DHCA30 (Figure 4). This neuronal degeneration occurred in association with microglial activation identified morphologically within a few hours of insult, and predominantly within the hippocampus (Figure 5) a region closely linked to neurocognitive abnormalities in children with congenital heart disease.24
Global delivery of NO during the reperfusion period after DHCA significantly ameliorated early microglial activation in association with a reduction in neuronal degeneration (Figure 6). The data suggest that iNO decreases neuronal degeneration by modifying the intrinsic inflammatory response. NO elicits many actions, which could be responsible for the cerebral protective effect. Like traumatic brain injury and stroke, clinically relevant models show that iNO reduction in systemic inflammation preserves the brain-blood barrier integrity, and impairs the passage of cytokines, which activate the microglia.15, 17, 27, 28 Studies in immature pigs with traumatic brain injury show that iNO reduces hippocampal necrosis after fluid percussion brain injury.15, 17 This benefit occurs in association with a reduction in cerebrospinal fluid cytokines including MARK (mitogen-activated protein kinase)-ERK(extracellular regulated kinase) signaling and a pro-inflammatory cytokine, IL-6. Inhalation of NO also prevented fluid percussion brain injury induced impairments in immature pig cerebral blood flow and autoregulation through either direct action on capillary beds, or through inhibition of endothelin-1 release within the brain. CPB also impairs cerebral autoregulation.29 Links between impaired cerebral autoregulation and neurological dysfunction in children after CPB have not been clearly established. However, other experimental porcine models have shown that iNO prevents cerebral flow fluctuations due to extreme variations in systemic blood pressure, such as would occur after DHCA and reperfusion.15, 16 NO potentially preserves cerebral autoregulation and brain-blood barrier through multiple mechanisms,30 although these still require elucidation in intact animal models.
Figure 6.
DHCA induced neuronal injury and inflammation. Inhaled nitric oxide reduced injury and microglia activation in porcine hippocampus after DHCA. NO, nitric oxide; DHCA, deep hypothermic circulatory arrest.
Local NO is synthesized by one of 3 NO synthases (NOS): neuronal NOS, inducible NOS, and endothelial NOS. Expression and activity for these isoforms vary according to site and lead to selective and localized functions for NO, which may serve cross-purposes with regards to neuroprotection. Some studies show that interventions, which should decrease local NO production, prevent neuronal apoptosis after DHCA in fully mature brains.31 But, other investigators demonstrated that strategies, aimed at increasing local NO synthesis, prevented metabolic changes associated with DHCA induced neuronal injury in developing brain.32, 33 These studies all modulated NO through pharmacologic inhibition or stimulation of NOS rather than directly delivering NO. Thus, contradictory results among these studies could be explained by differences in drug delivery, and regional variations in NOS isoform distribution within the brain, or by developmental differences between the experimental models. In this study, we assume that we saturated all NO receptor sites therefore removing any local regulation by NOS isoforms. Our data suggest that global NO delivery through inhalation might be more beneficial than NOS specific strategies. However, further studies will be required to determine the validity of this contention.
Study Limitations
The major limitation in this study relates to the time-course of our experimental protocol. In this first experimental stage, we evaluated histopathology after 3 hours of reperfusion. As noted, our preliminary studies showed that microglial activation could be detected in this model with only 3 hours of reperfusion. However, microglial activation and apoptosis both peak much later in other models.34, 35 Conceivably we might have detected a greater impact by iNO if we had waited longer to terminate experiments and fix the brain. Alternatively, NO might affect only early microglial activation with no prolonged benefit. Secondly, we delivered iNO only with reperfusion after DHCA completion and not during ischemia. Studies in rat neonatal stroke models have investigated cerebral infarct size response varied according to a dose and timing of iNO delivery.27 They showed that iNO during ischemia reduced infarct lesions by increasing collateral blood flow. However, there is no effective method to deliver iNO during ischemia with DHCA and no studies have examined iNO delivery prior to insult as brain protection. We chose the practical route and delivered iNO with ventilation after reperfusion in this study. Future studies can define whether further benefit exists for earlier iNO delivery, and should include experimental groups on SCP during moderate hypothermia and with iNO administration through the CPB oxygenator.25, 26 Finally, pigs studied in the second set of experiments were slightly older than those in the first experiment for logistic reasons. We have yet to determine if this relatively minor age difference influences the impact of iNO. We chose not to include a Sham group for analyses in our second set experiment to reduce animal usage because we found minimal neuronal apoptosis, neural degeneration or microglial activation in Sham animals in our first set experiment. Therefore, we do not know the full extent of neuroprotection by iNO.
Conclusions
We performed a proof of principle study, which showed that iNO inhibited microglial activation and associated neuronal degeneration within the immature pig brain after DHCA and reperfusion. The strategy employed in this translational study redirects iNO, frequently used to treat reactive pulmonary hypertension after surgery for congenital heart disease, towards use as a prophylactic against brain injury in the same patient population. With high resolution provided by Fluoro-Jade C staining and individual microglial morphological analyses, we found that iNO showed greater protective impact in the hippocampus than in the cortex. Our data supports results from other studies23, 24 showing increased vulnerability of the developing hippocampus compared to the cortex. As noted prior studies employing other neurological injury models,15, 16 show that the hippocampus exhibits selective sensitivity to iNO protective action. Importantly, the hippocampus is a region closely linked to development of neurocognition, often impaired in children exposed to DHCA as infants. Thus, overall the data support the performance of future studies to evaluate iNO delivery as a relatively straightforward and easy to administer mode of protection against neuro-developmental disorders for children undergoing CPB procedures.
Supplementary Material
Central Picture.
Inhaled NO reduced microglial activation induced by DHCA in hippocampus.
Central Message: DHCA induces microglial activation and neuronal degeneration. Inhaled NO after DHCA may reduce neuronal degeneration by ameliorating neuroinflammation in the hippocampus.
Perspective Statement.
DHCA for 30 minutes, consistent with clinical exposure, induces neuroinflammation and neuronal degeneration. Inhaled NO after DHCA reduces neuronal degeneration by ameliorating early phase inflammation. The anti-inflammatory action by inhaled NO may protect against neuro-developmental disorders in children undergoing DHCA procedures.
Acknowledgment
The authors thank a perfusion student (Erica Frana) at Seattle Children’s for helping us manage CPB.
Disclosure/conflict of interest: Research reported in this publication was supported by the National Heart Lung and Blood Institute (HL-R01–60666) awarded to M.A.P. Mallinckrodt Pharmaceuticals (Bedminster, NJ) provided the NO delivery system (INOmax) for research use and supplementary funding.
Abbreviations and Acronyms
- CPB
cardiopulmonary bypass
- DHCA
deep hypothermic circulatory arrest
- Iba-1
ionized calcium binding adaptor molecule 1
- iNO
inhaled nitric oxide
- NO
nitric oxide
- NOS
nitric oxide synthases
- SCP
selective antegrade cerebral perfusion
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marino BS, Cassedy A, Drotar D, Wray J. The impact of neurodevelopmental and psychosocial outcomes on health-related quality of life in survivors of congenital heart disease. J Pediatr. 2016;174:11–22 e2 [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Biarge M, Jowett VC, Cowan FM, Wusthoff CJ. Neurodevelopmental outcome in children with congenital heart disease. Semin Fetal Neonatal Med. 2013;18:279–85 [DOI] [PubMed] [Google Scholar]
- 3.Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. 2008;30:437–46 [DOI] [PubMed] [Google Scholar]
- 4.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL Jr., Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajimoto M, Ledee DR, Olson AK, Isern NG, Robillard-Frayne I, Des Rosiers C, et al. Selective cerebral perfusion prevents abnormalities in glutamate cycling and neuronal apoptosis in a model of infant deep hypothermic circulatory arrest and reperfusion. Journal of cerebral blood flow and metabolism. 2016;36:1992–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavroudis CD, Karlsson M, Ko T, Hefti M, Gentile JI, Morgan RW, et al. Cerebral mitochondrial dysfunction associated with deep hypothermic circulatory arrest in neonatal swine. Eur J Cardiothorac Surg. 2018;54:162–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar JD, Coleman RD, Griffith S, McNeil JD, Steigelman M, Young H, et al. Selective cerebral perfusion: Real-time evidence of brain oxygen and energy metabolism preservation. Ann Thorac Surg. 2009;88:162–9 [DOI] [PubMed] [Google Scholar]
- 8.Cavus E, Hoffmann G, Bein B, Scheewe J, Meybohm P, Renner J, et al. Cerebral metabolism during deep hypothermic circulatory arrest vs moderate hypothermic selective cerebral perfusion in a piglet model: A microdialysis study. Paediatr Anaesth. 2009;19:770–8 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H, Guleserian KJ, Rose R, Fotiadis C, Boyer PJ, Forbess JM. Hypothermic extracorporeal circulation in immature swine: A comparison of continuous cardiopulmonary bypass, selective antegrade cerebral perfusion and circulatory arrest. Eur J Cardiothorac Surg. 2009;36:992–7 [DOI] [PubMed] [Google Scholar]
- 10.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–9 [DOI] [PubMed] [Google Scholar]
- 11.Algra SO, Jansen NJ, van der Tweel I, Schouten AN, Groenendaal F, Toet M, et al. Neurological injury after neonatal cardiac surgery: A randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–33 [DOI] [PubMed] [Google Scholar]
- 12.Redmond JM, Gillinov AM, Zehr KJ, Blue ME, Troncoso JC, Reitz BA, et al. Glutamate excitotoxicity: A mechanism of neurologic injury associated with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1994;107:776–86; discussion 86–7 [PubMed] [Google Scholar]
- 13.Grimm JC, Magruder JT, Wilson MA, Blue ME, Crawford TC, Troncoso JC, et al. Nanotechnology approaches to targeting inflammation and excitotoxicity in a canine model of hypothermic circulatory arrest-induced brain injury. Ann Thorac Surg. 2016;102:743–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ergenekon E, Gucuyener K. Nitric oxide in developing brain. Eur J Paediatr Neurol. 1998;2:297–301 [DOI] [PubMed] [Google Scholar]
- 15.Pastor P, Curvello V, Hekierski H, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation through prevention of impairment of atp and calcium sensitive k channel mediated cerebrovasodilation after traumatic brain injury. Brain research. 2019;1711:1–6 [DOI] [PubMed] [Google Scholar]
- 16.Hekierski H, Pastor P, Curvello V, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal neuronal cell necrosis after traumatic brain injury in newborn and juvenile pigs. J Neurotrauma. 2019;36:630–8 [DOI] [PubMed] [Google Scholar]
- 17.Curvello V, Pastor P, Hekierski H, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury through inhibition of et-1, erk mapk and il-6 upregulation in pigs. Neurocritical care. 2019;30:467–77 [DOI] [PubMed] [Google Scholar]
- 18.Morrison H, Young K, Qureshi M, Rowe RK, Lifshitz J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep. 2017;7:13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajimoto M, Atkinson DB, Ledee DR, Kayser EB, Morgan PG, Sedensky MM, et al. Propofol compared with isoflurane inhibits mitochondrial metabolism in immature swine cerebral cortex. Journal of cerebral blood flow and metabolism. 2014;34:514–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-jade c results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31 [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Ma WG, Gao GD, Mao QX, Zheng J, Sun LZ, et al. Fluoro jade-c staining in the assessment of brain injury after deep hypothermia circulatory arrest. Brain Res. 2011;1372:127–32 [DOI] [PubMed] [Google Scholar]
- 22.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastuszko P, Schears GJ, Pirzadeh A, Kubin J, Greeley WJ, Wilson DF, et al. Effect of granulocyte-colony stimulating factor on expression of selected proteins involved in regulation of apoptosis in the brain of newborn piglets after cardiopulmonary bypass and deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2012;143:1436–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latal B, Patel P, Liamlahi R, Knirsch W, O’Gorman Tuura R, von Rhein M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr Res. 2016;80:531–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Checchia PA, Bronicki RA, Muenzer JT, Dixon D, Raithel S, Gandhi SK, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children--a randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–6 [DOI] [PubMed] [Google Scholar]
- 26.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: A randomised controlled trial. Intensive Care Med. 2016;42:1744–52 [DOI] [PubMed] [Google Scholar]
- 27.Charriaut-Marlangue C, Bonnin P, Gharib A, Leger PL, Villapol S, Pocard M, et al. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke. 2012;43:3078–84 [DOI] [PubMed] [Google Scholar]
- 28.Terpolilli NA, Feiler S, Dienel A, Muller F, Heumos N, Friedrich B, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab. 2016;36:2096–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldas JR, Haunton VJ, Panerai RB, Hajjar LA, Robinson TG. Cerebral autoregulation in cardiopulmonary bypass surgery: A systematic review. Interact Cardiovasc Thorac Surg. 2018;26:494–503 [DOI] [PubMed] [Google Scholar]
- 30.Armstead WM, Vavilala MS. Translational approach towards determining the role of cerebral autoregulation in outcome after traumatic brain injury. Experimental neurology. 2019;317:291–7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 31.Tseng EE, Brock MV, Lange MS, Troncoso JC, Blue ME, Lowenstein CJ, et al. Glutamate excitotoxicity mediates neuronal apoptosis after hypothermic circulatory arrest. Ann Thorac Surg. 2010;89:440–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsui SS, Kirshbom PM, Davies MJ, Jacobs MT, Greeley WJ, Kern FH, et al. Nitric oxide production affects cerebral perfusion and metabolism after deep hypothermic circulatory arrest. Ann Thorac Surg. 1996;61:1699–707 [DOI] [PubMed] [Google Scholar]
- 33.Hiramatsu T, Jonas RA, Miura T, duPlessis A, Tanji M, Forbess JM, et al. Cerebral metabolic recovery from deep hypothermic circulatory arrest after treatment with arginine and nitro-arginine methyl ester. J Thorac Cardiovasc Surg. 1996;112:698–707 [DOI] [PubMed] [Google Scholar]
- 34.Barakat R, Redzic Z. The role of activated microglia and resident macrophages in the neurovascular unit during cerebral ischemia: Is the jury still out? Med Princ Pract. 2016;25 Suppl 1:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupalla K, Allegrini PR, Sauer D, Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol. 1998;96:172–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.