Take Home Message
This study shows no effect of enzalutamide on hospitalized COVID-19 patients or a decrease in the risk of COVID-19 severity from any androgen-inhibiting drug in the population. Collectively, there is evidence of no beneficial role of androgen inhibition in COVID-19.
Keywords: COVID-19, SARS-CoV-2, Antiandrogen, Randomized trial, Enzalutamide, Bicalutamide, Androgen deprivation therapy
Abstract
Background
Men are more severely affected by COVID-19. Testosterone may influence SARS-CoV-2 infection and the immune response.
Objective
To clinically, epidemiologically, and experimentally evaluate the effect of antiandrogens on SARS-CoV-2 infection.
Designs, settings, and participants
A randomized phase 2 clinical trial (COVIDENZA) enrolled 42 hospitalized COVID-19 patients before safety evaluation. We also conducted a population-based retrospective study of 7894 SARS-CoV-2–positive prostate cancer patients and an experimental study using an air-liquid interface three-dimensional culture model of primary lung cells.
Intervention
In COVIDENZA, patients were randomized 2:1 to 5 d of enzalutamide or standard of care.
Outcome measurements
The primary outcomes in COVIDENZA were the time to mechanical ventilation or discharge from hospital. The population-based study investigated risk of hospitalization, intensive care, and death from COVID-19 after androgen inhibition.
Results and limitations
Enzalutamide-treated patients required longer hospitalization (hazard ratio [HR] for discharge from hospital 0.43, 95% confidence interval [CI] 0.20–0.93) and the trial was terminated early. In the epidemiological study, no preventive effects were observed. The frail population of patients treated with androgen deprivation therapy (ADT) in combination with abiraterone acetate or enzalutamide had a higher risk of dying from COVID-19 (HR 2.51, 95% CI 1.52–4.16). In vitro data showed no effect of enzalutamide on virus replication. The epidemiological study has limitations that include residual confounders.
Conclusions
The results do not support a therapeutic effect of enzalutamide or preventive effects of bicalutamide or ADT in COVID-19. Thus, these antiandrogens should not be used for hospitalized COVID-19 patients or as prevention for COVID-19. Further research on these therapeutics in this setting are not warranted.
Patient summary
We studied whether inhibition of testosterone could diminish COVID-19 symptoms. We found no evidence of an effect in a clinical study or in epidemiological or experimental investigations. We conclude that androgen inhibition should not be used for prevention or treatment of COVID-19.
1. Introduction
During the COVID-19 pandemic, men have constituted the majority of patients requiring hospital care and intensive care [1]. Although some risk factors may be more prevalent among men in many countries, this does not explain the large gender-based difference in COVID-19 severity [2].
It has been hypothesized that the higher prevalence of severe COVID-19 among men is influenced by androgens, which may affect SARS-CoV-2 virulence by regulating the expression of proteins important for virus internalization. One of these, TMPRSS2, modulates viral spike proteins that enable SARS-CoV-2 internalization. In prostate cancer cells, the androgen receptor (AR) directly regulates TMPRSS2, and this mechanism has also been described in lung cells from both men and women [3], [4], [5]. Androgens may also regulate ACE2, another critical protein for virus internalization [3], [6].
In line with these proposed mechanisms, epidemiological studies have suggested that androgen inhibition may have a beneficial impact on infection rates [7] and COVID-19 symptoms [8], [9]. However, other reports have not found such correlations [10].
In the present three-pronged study, we aimed to elucidate the potentially beneficial role of androgen inhibition as a COVID-19 treatment using a translational approach. The multicenter randomized clinical trial COVIDENZA investigated the effect of the antiandrogen enzalutamide in hospitalized patients with COVID-19. In parallel, we used a three-dimensional (3D) model of primary human lung cells to explore the effect of antiandrogens on SARS-CoV-2 infection efficacy. Finally, a population-based epidemiologic study using Swedish register data investigated the association between the use of androgen-inhibiting drugs and the risk of severe COVID-19.
2. Patients and methods
2.1. Part 1: COVIDENZA clinical trial of enzalutamide as therapy for COVID-19
2.1.1. Design
COVIDENZA is a randomized, open, phase 2, multicenter clinical trial designed for superiority. Patients were randomized 2:1 to receive 5 d of oral enzalutamide (160 mg/d) plus standard of care, or standard of care alone. The study protocol specified an interim safety analysis after the inclusion of 45 patients, and a power calculation after inclusion of 100 patients.
The study protocol (Supplementary material) was approved by the Swedish Ethical Review Authority (Dnr 2020–02122) and the Swedish Medical Products Agency (Eudract number: 2020-002027-10) and registered at ClinicalTrial.gov (NCT04475601) before the first study enrollment. The trial was conducted in compliance with the study protocol, the Declaration of Helsinki, Good Clinical Practice guidelines, and current national and international regulations governing clinical trials. A list of consecutive randomization numbers was created before the start of inclusion and was stratified for site and gender. Allocation concealment was assured by using a central password-protected computer database. On inclusion of a new patient in an electronic case report form (eCRF), blinded randomization was enabled. An external data safety and monitoring board (DSMB) and external monitors were appointed. All data were collected in an eCRF in accordance with general data protection regulations. Adverse events were classified according to Common Terminology Criteria for Adverse Events v5.0.
2.1.2. Inclusion and exclusion criteria
Eligible patients were hospitalized men and women aged >50 yr with a positive SARS-CoV-2 polymerase chain reaction (PCR) test and not requiring mechanical ventilation who gave informed consent before any trial procedure. The main exclusion criteria were ongoing hormonal cancer therapy, other critical illness, and immunosuppressive disease (Supplementary material).
2.1.3. Outcomes
The primary outcome was time to mechanical ventilation (or death) and time to discharge from hospital, whichever came first. A 7-point ordinal scale was used (COVIDENZA protocol, Supplementary material). Secondary outcomes were duration of supplemental oxygen; need for mechanical ventilation; laboratory assessments, including viral load; duration of hospital stay; hospital readmission; and mortality (Supplementary material). The normalized viral load was defined as the difference between the cycle threshold (Ct) on day 0 and Ct. Undetectable signals in virus PCR analysis were included as Ct = 40.
2.1.4. Statistical analysis of COVIDENZA
All statistical analyses were based on two-sided tests, and p < 0.05 was considered statistically significant. Primary and secondary endpoints were analyzed following the protocol, considering all randomized participants for intention-to-treat analysis. Association between time to discharge (from day of inclusion) and study arm, adjusted for age and gender, was analyzed using Fine and Gray regression (with death as a competing event). The differences between the study arms in terms of number of days with supplemental oxygen or days in hospital were analyzed using the Mann-Whitney U test. The viral load at baseline (Ct0) was compared between study arms using a t test. The relative viral load (ΔCt = Ct0 − Ct = log2(VL/VL0)) was computed for days 2, 4, and 6 and compared between the study arms using a t test. Statistical analyses were performed using R v3.6.3. The full statistical analysis plan is available in the Supplementary material.
3. Results
3.1. Patients in COVIDENZA
The first patient was included on July 29, 2020, and trial inclusion was stopped on November 29, 2020 for safety analysis. On the basis of DSMB recommendations, after 42 patients were randomized, the sponsor stopped inclusion (November 29, 2020). The analysis data set was completed on March 24, 2021 after finalized external monitoring, and included 42 patients hospitalized for severe COVID-19 symptoms. Table 1 presents the baseline characteristics for the patients in the enzalutamide arm (n = 30) and control arm (n = 12). The only marked difference in baseline comorbidity between the arms was that the enzalutamide arm included eight patients (27%) with type 2 diabetes, compared to none in the control arm.
Table 1.
Baseline patient characteristics in COVIDENZA
| Variable | Control | Treatment |
|---|---|---|
| (n = 12) | (n = 30) | |
| Female, n (%) | 2 (16.7) | 9 (30.0) |
| Median age, yr (IQR) | 69.5 (58.5–73.3) | 63.0 (56.8–69.8) |
| Median body mass index, kg/m2 (IQR) | 29.4 (26.3–30.6) | 29.2 (25.0–31.6) |
| Smoking status, n (%) | ||
| Never smoked | 6 (54.5) | 16 (53.3) |
| Quit more than 6 mo ago | 5 (45.5) | 13 (43.3) |
| Smoking regularly | 0 (0.0) | 0 (0.0) |
| Not known | 0 (0.0) | 1 (3.3) |
| Nicotine habits, n (%) | ||
| No other nicotine habits | 8 (72.7) | 25 (83.3) |
| Snuff | 2 (18.2) | 2 (6.7) |
| Not known | 1 (9.1) | 3 (10.0) |
| Medical history, n (%) | 10 (90.9) | 23 (76.7) |
| Hyperlipidemia | 1 (8.3) | 5 (16.7) |
| Hypertension | 6 (50.0) | 13 (43.3) |
| Type 2 diabetes | 0 (0.0) | 8 (26.7) |
| Previous myocardial infarction | 2 (16.7) | 2 (6.7) |
| Asthma | 3 (25.0) | 3 (10.0) |
| Atrial fibrillation | 0 (0.0) | 4 (13.3) |
| Arthrosis | 0 (0.0) | 5 (16.7) |
| Cancer | 2 (16.7) | 3 (10.0) |
| Other e | 8 (66.7) | 15 (50.0) |
| COVID-19 related | ||
| Median symptomatic time before hospitalization, d (IQR) | 8.5 (7.0–10.0) | 8.5 (7.0–10.0) |
| Median time in hospital before inclusion, d (IQR) | 1.0 (1.0–1.3) | 1.0 (1.0–1.0) |
| Ordinal scale at baseline, n (%) | ||
| 3 | 1 (8.3) | 5 (16.7) |
| 4 | 10 (83.3) | 21 (70.0) |
| 5 | 1 (8.3) | 4 (13.3) |
IQR = interquartile range
a Fisher’s exact test.
b Student’s t test
c Mann Whitney U test.
d χ2 test.
e Other conditions registered for four or fewer patients.
3.2. Follow-up and outcomes
Three patients withdrew their consent, one in the enzalutamide arm (day 0) and two in the control arm (days 0 and 1). All other patients (29 in the enzalutamide arm and 10 in the control arm) were followed for 45 d. Remdesivir was given to eight patients (27%) in the enzalutamide arm and four (33%) in the control arm. Corticosteroids were administered to 25 patients (83%) in the enzalutamide arm and 11 (92%) in the control arm (all concomitant treatments are presented in Supplementary Table 1).
A total of three patients (two in the enzalutamide arm [4%], and one in the control arm [8%]) reached the primary endpoint of a need for mechanical ventilation (ordinal scale 6). The two patients in the enzalutamide arm were admitted to the intensive care unit (ICU) on days 5 and 9, respectively, after starting study treatment. The one patient in the control arm was admitted to the ICU on study day 0. The primary endpoint of time to mechanical ventilation was not evaluated because there were few events.
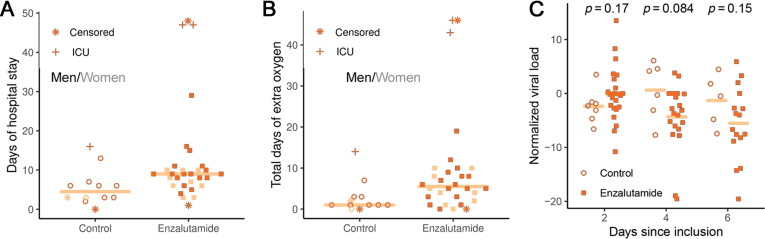
Among patients followed for at least 1 wk (n = 39), those in the enzalutamide arm stayed in the hospital for a median of 9 d (median absolute deviation [MAD] 3.0), while patients in the control arm stayed for a median of 6 d (MAD 4.4; Mann-Whitney U test p = 0.010; Fig. 1 A). In the enzalutamide arm, the hazard ratio (HR) for discharge from hospital, adjusted for age and gender, was 0.43 (95% confidence interval [CI] 0.20–0.93; p = 0.032). Among the same patients, those in the enzalutamide arm required supplemental oxygen for a longer time; the median difference in duration between the arms was 4 d (MAD 5.9; p = 0.022; Fig. 1B).
Fig. 1.
Results from the COVIDENZA trial. (A) Total days of hospitalization from inclusion to discharge and (B) days of oxygen consumption. The line indicates the median number of days. Controls and enzalutamide-treated patients are indicated by circles and filled squares, respectively (dark for men and light for women). Censored cases are indicated by an asterisk, and patients admitted to the ICU indicated by cross. (C) Viral load on days 2, 4, and 6 compared to baseline (Ct0-Ct) for all patients (n = 42). Controls are indicated by light circles, and enzalutamide patients by dark squares. ICU = intensive care unit.
The arms did not differ in baseline laboratory parameters (Supplementary Table 2). In addition, we observed no difference between the study arms in the change in laboratory parameters from day 0 to day 6 (Supplementary Fig. 1). There was no difference in the decrease in SARS-CoV-2 load between the two arms: the difference in ΔCt on day 4 was −5.6 (95% CI −10.7 to 0.8; p = 0.084; Fig. 1C).
3.3. Safety
A total of 22 adverse events (AEs) were reported in 16 patients, including 19 AEs in the enzalutamide arm and three AEs in the control arm (Supplementary Table 3). Of these, only five AE were grade 3 or higher, including four in the enzalutamide arm and one in the control arm. All of these patients recovered without sequelae (Table 2 ). The only death occurred in the control arm, on day 14.
Table 2.
Adverse event of grade 3 or higher among patients in COVIDENZA
| Event | Grade | Study arm | Days after randomization/last enzalutamide | Relation to enzalutamide a | Outcome |
|---|---|---|---|---|---|
| Respiratory failure | 5 | Control | 0/NA | NA | Death |
| Gastritis | 3 | Control | 8/NA | NA | Recovered |
| Pneumomediastinum | 3 | Enzalutamide | 4/ongoing | Unlikely related | Recovered |
| Pneumonia b | 3 | Enzalutamide | 20/15 | Unlikely related | Recovered |
| Pyelonephritis b | 3 | Enzalutamide | 20/15 | Not related | Recovered |
| Partial epilepsia | 3 | Enzalutamide | 38/33 | Not related | Recovered |
NA = not applicable.
Relatedness evaluated by the investigator.
The same patient.
3.4. Part 2: effect of enzalutamide on SARS-COV-2 infection in human lung cells
We investigated the mechanism and effect of enzalutamide on SARS-CoV-2 replication using an in vitro primary lung model in which human bronchial epithelial cells (HBECs) were isolated from 12 patients (6 men and 6 women) and cultured in trans-wells at an air-liquid interface (ALI), treated with 40 μM enzalutamide, and apically infected with SARS-CoV-2. For detailed methods see the Supplementary material.
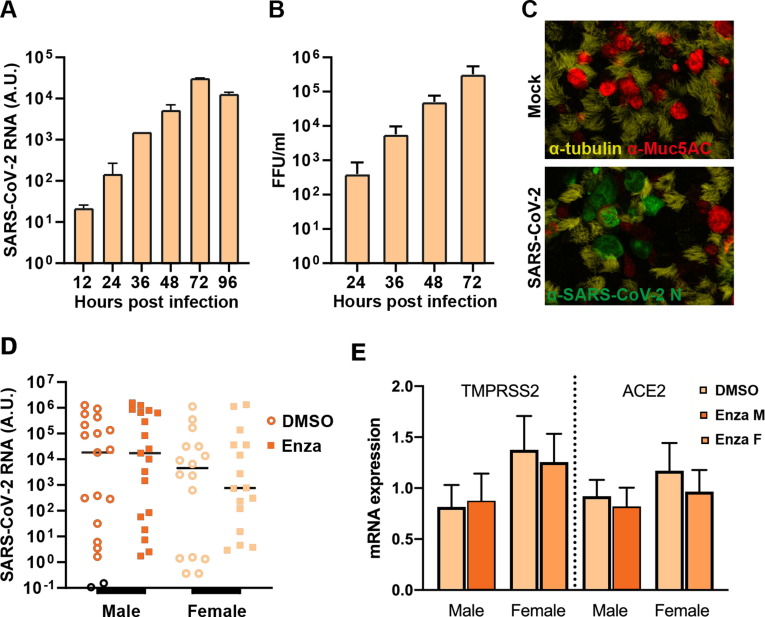
In an initial experiment, the levels of both viral RNA and infectious virions released from the HBEC ALI culture exponentially increased over the first 72 h in cells not pretreated with enzalutamide (Fig. 2 A,B). α-Tubulin-positive ciliated cells were the main cell type infected by SARS-CoV-2 (Fig. 2C). We observed high variability in virus susceptibility between the different HBEC ALI cultures; however, we did not detect any effect of enzalutamide treatment on virus replication in cells from male or female donors (Fig. 2D). In this model, enzalutamide treatment did not decrease the mRNA expression of TMPRSS2 or ACE2 (Fig. 2E).
Fig. 2.
Results from the in vitro study using HBEC ALI cultures. (A) Apical viral load from infected HBEC ALI cultures at the time points indicated, as quantified by qualitative polymerase chain reaction of SARS-CoV2 virus particles (mean + SEM). (B) Apical load of infectious SARS-CoV-2 particles, quantified via a focus-forming assay and presented as focus-forming units per ml (FFU/ml; mean + SEM). (C) Fluorescent imaging to visualize goblet cells (mucin; Muc5AC; red), ciliated cells (α-tubulin; yellow), and cells infected by SARS-CoV-2 (nucleocapsid [N] protein; green). (D) Quantification of SARS-CoV2 RNA released to the apical side of the infected HBEC ALI cultures treated with enzalutamide or carrier controls (DMSO). The line represents the median. (E) Cellular expression of TMPRSS2 and ACE2 mRNA in HBEC ALI cultures treated with enzalutamide (filled bars) or carrier control (DMSO; open bars) for male and female donors. HBEC = human bronchial epithelial cells; ALI = air-liquid interface; SEM = standard error of the mean; DMSO = dimethylsulfoxide; A.U. = absorbance units; Enza = enzalutamide; M = male; F = female.
3.5. Part 3: epidemiological analysis of the risk associated with androgen inhibition in terms of COVID-19 outcome
3.5.1. Epidemiological study population
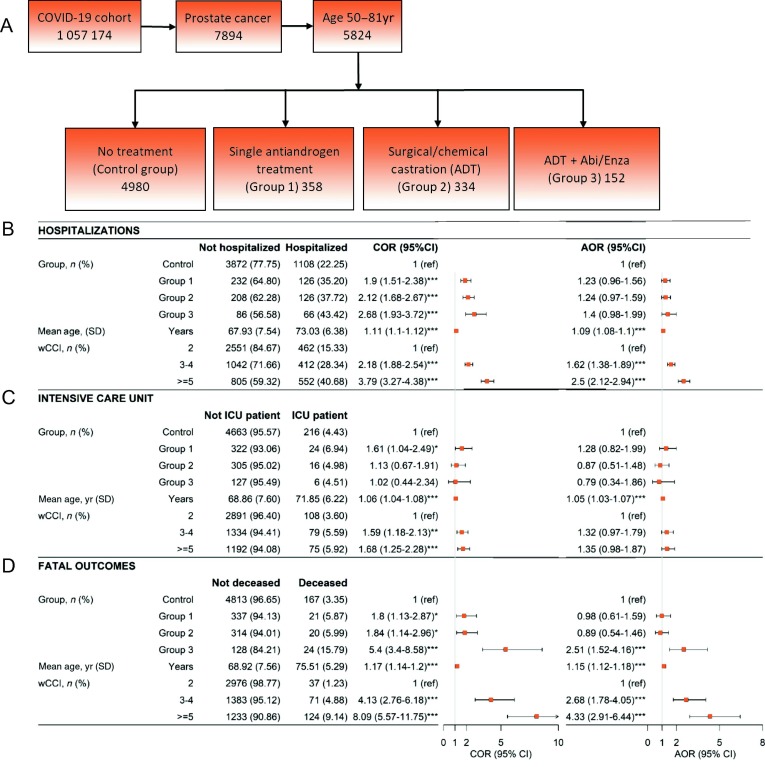
To evaluate the effect of androgen-inhibiting therapies on COVID-19 outcome, we identified prostate cancer patients from Swedish national registers. These were categorized into four groups (exact definitions in the Supplementary material): a single antiandrogen treatment (group 1), surgical or chemical castration (androgen deprivation therapy [ADT], group 2), ADT plus abiraterone acetate or enzalutamide (group 3), and prostate cancer patients with no ongoing or prior hormonal therapy (control group). The outcomes were defined as: (1) patients requiring hospitalization for either the main or contributing diagnoses; (2) patients requiring intensive care; and (3) death with COVID-19 as a main or contributing cause.
The individual burden of comorbidities was calculated using the weighted Charlson comorbidity index (wCCI) [11], [12], [13] based on entries up to 2 mo before the date of COVID-19 diagnosis. Full details on the methodology are provided in the Supplementary material.
The study population consisted of 7894 prostate cancer patients with confirmed COVID-19. Hormone-treated prostate cancer patients (groups 1, 2, and 3) were older and had higher comorbidity scores in comparison to the control group, which comprised prostate cancer patients without hormonal therapy. There was no difference between groups 1 and 2, while group 3 had significantly higher comorbidity scores compared to group 2 (p < 0.001, Pearson χ2 test).
Unadjusted analyses showed that patients on androgen inhibition treatment had higher risk for all negative outcomes (hospitalization, need for intensive care [only group 1], and death) due to COVID-19 (Fig. 3 ). After adjustment for age and comorbidities, none of these higher risks remained statistically significant for groups 1 and 2 (Fig. 3). However, the group receiving ADT plus abiraterone/enzalutamide (group 3) had greater mortality after adjustments (Fig. 3). There was no difference in outcome between patients in group 3 given abiraterone acetate or enzalutamide (Supplementary Table 4). This analysis was performed in age groups matching the COVIDENZA trial (50–81 yr, n = 5824), but the same results apply when including all age groups (Supplementary Fig. 2).
Fig. 3.
(A) Schematic illustration of the design of the epidemiological study. (B) Multivariable analysis of the risk of hospitalization. (C) Multivariable analysis of the risk of admission to the intensive care unit. (D) Multivariable analysis of the risk of death (fatal outcomes). The crude odds ratio (COR) and adjusted odds ratio (AOR) with 95% confidence interval (CI) are shown with the corresponding forest plots. Abi/Enza = abiraterone/enzalutamide; SD = standard deviation; ICU = intensive care unit; wCCI = weighted Charlson comorbidity index.
Every year of age was associated with increase in the risk of hospitalization and death from COVID-19. The odds ratio per year increment in age was 1.09 for hospitalization, 1.15 for the need for intensive care, and 1.05 for death. In addition, our analysis showed that greater comorbidity was associated with higher odds of needing hospitalization and dying from COVID-19.
4. Discussion
Three parallel studies with a translational approach were conducted to evaluate the hypothesis that androgen inhibition is beneficial in COVID-19. Together, these studies indicate that inhibition of AR signaling with enzalutamide does not appear to confer any beneficial effect in hospitalized patients and does not inhibit SARS-CoV-2 virus replication in lung cells in vitro. In addition, long-term androgen inhibition does not prevent severe COVID-19 disease in the male Swedish population. Contradicting the hypothesis, we observed a prolonged hospital stay among enzalutamide-treated patients in the COVIDENZA trial.
The lack of beneficial effect of enzalutamide on, and perhaps even worsening of, COVID-19 symptoms in the COVIDENZA trial may be explained by its documented induction of CYP3A4 [14], which affects corticosteroid efficacy. Swedish national guidelines as of September 2020 indicate that corticosteroids should be used for the treatment of hospitalized COVID-19 patients on the basis of results from the RECOVERY trial [15]. In the present trial, 86% of the patients received corticosteroids. It is possible that enzalutamide-induced CYP3A4 induction could have decreased the efficacy of the cortisone therapy administered, potentially resulting in a worse outcome in the enzalutamide compared to the control arm.
However, in the epidemiological part of our study, we observed a trend towards higher risk of hospitalization in all three treatment groups. The trend strongly suggests that androgen inhibition therapy is not beneficial for COVID-19 outcome, and may indicate that interaction with CYP3A4 was not the only reason for the poor results for enzalutamide treatment in COVIDENZA, since neither group 1 nor group 2 treatments affect CYP3A4.
We observed a higher risk of death from COVID-19 in the group of patients with metastatic prostate cancer treated with ADT in combination with abiraterone/enzalutamide. In Sweden, enzalutamide is almost exclusively used in combination with medical or surgical castration as second- or third-line therapy for metastatic castration-resistant prostate cancer. Since this population is very frail with late-stage metastatic prostate cancer that could be poorly covered by the comorbidity index, it is likely that the higher risk of death is associated with comorbidities and not with the added abiraterone/enzalutamide treatment. In contrast to the present study, a retrospective study of 58 patients with prostate cancer demonstrated that patients with long-term castration therapy exhibited lower risk for both hospitalization and need for oxygen [8]. The present epidemiological study comprised 7894 patients and investigated the effects of long-term use of androgen inhibition therapy on the risk of requiring intensive care and death, but not oxygen supplementation. Beyond these differences, it is unclear why these two studies had contradictory results. However, the COVIDENZA trial results support a poor outcome after antiandrogen therapy in severe COVID-19.
These results are in contrast to the initial hypotheses, and to the epidemiological data presented by Montopoli et al [7] early in the pandemic indicating that prostate cancer patients treated with ADT showed a lower risk of SARS-CoV-2 infection. In addition, a recent study showed a lower risk of SAR-CoV-2 infection after treatment with 5α-reductase inihibitors [16]. This may indicate that limiting androgen signaling could protect against getting infected, but, according to our data, not from being severely affected when already infected.
In COVIDENZA, SARS-CoV-2 levels in nasopharynx swabs decreased at the same rate among enzalutamide-treated patients and patients in the control group. By contrast, a Brazilian study tested the antiandrogen proxalutamide in nonhospitalized patients [17] and found that inhibition of androgen signaling appeared to affect viral internalization. Several experimental studies support a role of androgen inhibition in SARS-CoV-2 virulence [4], [18], although contrasting data also exist [19]. The mechanism may be context-dependent, since different species, cell lines, cell types, and model systems generate variable results. However, in line with the COVIDENZA results, the 3D model of primary human lung cells in our study exhibited no enzalutamide-induced decrease in TMPRSS2 expression or viral load. This finding supports the lack of beneficial clinical effect of enzalutamide in the COVIDENZA trial, and of continuous androgen inhibition in the Swedish population.
In addition to a decrease in viral load, the Brazilian researchers reported a decrease in the risk of hospitalization following proxalutamide treatment of patients with mild COVID-19 [20]. When viewed together with the COVIDENZA results, this indicates that antiandrogen therapy may have a beneficial effect at an early time point in COVID-19 infection, but may be unfavorable in later disease stages when the overactivated immune system contributes to severe symptoms [21]. By contrast, the epidemiological part of our study does not support a beneficial role of continuous treatment with either antiandrogens or medical castration. A recent meta-analysis reached the same conclusion [22]. If there is a specific window for antiandrogen treatment during the early symptomatic phase of COVID-19, as suggested by the Brazilian study [17], [20], this would depend on an as yet unknown mechanism for androgen regulation of TMPRSS2, discriminating between the effects of continuous versus short-term antiandrogen treatment.
The present clinical trial used a rather short-term treatment (5 d), which could be a limitation. However, the maximum enzalutamide concentration is reached within 2 h, ensuring a rapid effect on androgen signaling inhibition. Furthermore, we did not want to inflict long-term hormonal effects. The half-life of enzalutamide is approximately 6 d, so we estimate an effect during at least 12 d, which would cover the period within which any treatment effect should be demonstrated.
To the best of our knowledge, this is the first trial evaluating any antiandrogen treatment for female COVID-19 patients. Enzalutamide has been used in a few clinical trials in women with breast cancer (NCT01889238, NCT02007512), which showed that enzalutamide can be safely used in women. The testosterone levels in postmenopausal women are sufficient for activation of AR, which is why the mechanisms could also be valid in women. Therefore, we deemed it unethical to exclude women from a possible treatment effect. An additional rationale was that enzalutamide increases estrogen levels in women [23], and experimental studies in female mice have shown that estrogen decreases SARS-CoV-2 infection [24].
It has previously been shown that comorbidity increases the risk of severe COVID-19 outcomes [2]. This was also seen in the present study, since the risks of hospitalization and death due to COVID-19 increased with the comorbidity score, while the specific risk of being admitted to the ICU was not significantly affected by the comorbidity score. This may be explained by the clinical discrimination in admitting patients to the ICU only if they were expected to benefit from the treatment. It may also be explained by a registration bias due to the establishment of intermediate care units for COVID-19 patients in which high-flow oxygen therapy and intensive monitoring and care are provided, but which are not classified as ICUs in the medical records. Another weakness of the epidemiological study is that we cannot exclude residual confounding.
5. Conclusions
In summary, the present three-pronged study revealed that neither hospitalized COVID-19 patients treated with enzalutamide nor Swedish men with long-term androgen inhibition experienced any benefit in terms of COVID-19 severity. These results are supported by in vitro experiments showing no evidence of enzalutamide affecting SARS-CoV2 infection in human lung cells.
We conclude that enzalutamide should not be used as therapy for severe COVID-19 disease, and that bicalutamide and ADT do not prevent severe COVID-19. Further research on these therapeutics in this setting are not warranted.
Author contributions: Andreas Josefsson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Josefsson, Welén, Ahlm, Gisslén, Bjartell, Nilsson, Thellenberg Carlsson, Överby, Fors Connolly.
Acquisition of data: Stranne, Bremell, Styrke, Gisslén, Repo, Östholm Balkhed, Niward, Robinsson, Henningsson, Angelin, Lindquist, Rosendal, Lenman, Allard, Becker, Buckland, Rudolfsson, Fonseca-Rodríguez.
Analysis and interpretation of data: Josefsson, Welén, Freyhult, Fors Connolly, Fonseca-Rodríguez, Överby.
Drafting of the manuscript: Josefsson, Welén, Fors Connolly, Överby, Rosendal.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Freyhult, Fonseca-Rodríguez, Rosendal, Lenman.
Obtaining funding: Josefsson, Fors Connolly, Överby, Allard.
Administrative, technical, or material support: Freyhult, Buckland, Rudolfsson, Becker, Lenman.
Supervision: Josefsson, Welén, Fors Connolly, Överby.
Other: None.
Financial disclosures: Andreas Josefsson certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Anders Bjartell has received consultant fees and/or honoraria from Ferring, Janssen, AstraZeneca, Astellas, Bayer, Ferring, Ipsen, Sandoz, and Recordati; and participates in DSMB/advisory boards for Bayer, Janssen, AstraZeneca, and Merck. Johan Stranne has received honoraria from Astellas, Bayer, Janssen-Cilag, Ipsen, and Ferring. Magnus Gisslén participates in DSMBs for AstraZeneca and scientific advisory boards for Gilead, GSK/ViiV, and MSD; has received honoraria from Amgen, Biogen, BMS, Gilead, GSK/ViiV, Janssen-Cilag, MSD, Novocure, and Novo Nordic; and has received institutional grants or contracts from Gilead Sciences and Janssen-Cilag. Andreas Josefsson has received an unconditional research grant for COVIDENZA from Astellas Pharma and honoraria from Astellas, Ipsen, Sandoz, and Janssen-Cilag. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This investigator-initiated trial was supported by an unconditional research grant from Astellas Pharma Ltd. The sponsor had no role in the study design; in the data collection, analysis, or interpretation; or in writing the manuscript. AJ is supported by the Knut and Alice Wallenberg Foundation and Swedish Prostate Cancer Federation. KW is supported by the Swedish Cancer Society (CAN 2017/478 and 20 1055 PjF) and the Swedish Prostate Cancer Federation. AKÖ is supported by the Swedish Heart Lung foundation (no. 20200385), and the Knut and Alice Wallenberg Foundation (grants to Science for Life Laboratory, 2020.0182). AMFC is supported by Central ALF-funding, Region Västerbotten (RV-836351), Base unit ALF-funding (RV-939769); Strategic Funding during 2020 from the Department of Clinical Microbiology, Umeå University; and The Laboratory for Molecular Infection Medicine Sweden (MIMS).
Acknowledgments: Support, project management, and facilities to conduct the COVIDENZA trial were provided by the Clinical Research Center at Umeå University Hospital. We thank staff at the clinical trial unit in Umeå (Marja-Liisa Lammi Tavelin, Lisette Marjavaara, Emma Wede, Kristina Öjbrant, Ida Lundström, and Anna Ramnemark) for excellent ongoing help with the startup and continued work with the trial. We also thank the Centre for Clinical Cancer studies at Karolinska Institute for skillful help (Sanna Nyström and Susanne Wallberg). We thank Fredrik Granström at ICT Services and System Development at Umeå University for helping us create the electronic Clinical Report Form, and statistician Professor Marie Eriksson (Umeå University) for help with the randomization process. We also thank all the research personnel for helping with all the work needed to start the COVIDENZA trial at the different sites, and for feedback to help us improve the trial: Frida Samuelsson, Jennie Bobeck, Mia Mickelsson, Helena Gisslén, Jenny Holm Fernström, Ann Carlstrand, Elisabeth Bengtsson, Kristina From, Sofia Sjöberg, Jennifer Amidi, Annika Löfgren, Suzy Lindberg, Malin Karlström, Mathias Cortés Rico, Malin Lindell, Beatrice Backman Lönn, Maria Casserdahl, Kerstin Almroth, Britt-Inger Dahlin, Ester Fridenström, Malin Hellgren, Rebecka Sundén, Ann-Charlotte Borgefeldt, Yvonne Pantzar, and Cecilia Magnusson. We thank the Data Safety and Monitoring Board for external review of COVIDENZA: Associate Professor Martin Eklund, Professor Annika Bergquist, Associate Professor Jan Adolfsson, Professor Lars Hagberg, Professor Jan-Erik Damber, and Senior Medical Advisor Helén Seeman-Lodding. We also thank Sonja Huldén, Judge of Appeal, for legal support. We acknowledge the work of the biobanks in the different regions: Biobank West, Biobank North, Uppsala Biobank, and the Biobank facilities in Sundsvall, Linköping, and Jönköping. We also thank Wolfgang Lohr, Institute of Epidemiology, Umeå University, for data curation in the epidemiological study. We acknowledge the Biochemical Imaging Center at Umeå University and the National Microscopy Infrastructure (VR-RFI 2016-00968) for assistance with microscopy. We thank Anders Blomberg and Gregory Rankin at Umeå University for kindly providing us with lung tissue for HBEC isolation. The computations were performed using resources provided by the Swedish National Infrastructure for Computing through the Uppsala Multidisciplinary Center for Advanced Computational Science under project SNIC 2020/6-251.
Associate Editor: James Catto
Statistical Editor: Andrew Vickers
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eururo.2021.12.013.
Appendix A. Peer Review Summary
The following are the Supplementary data to this article:
References
- 1.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghazizadeh Z, Majd H, Richter M, et al. Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. BioRxiv preprint. https://doi.org/10.1101/2020.05.12.091082 [DOI] [PMC free article] [PubMed]
- 4.Leach D.A., Mohr A., Giotis E.S., et al. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nat Commun. 2021;12:4068. doi: 10.1038/s41467-021-24342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Janne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Dalpiaz P.L., Lamas A.Z., Caliman I.F., et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montopoli M., Zumerle S., Vettor R., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V.G., Zhong X., Liaw B., et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31:1419–1420. doi: 10.1016/j.annonc.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wambier C.G., Vano-Galvan S., McCoy J., et al. Androgenetic alopecia present in the majority of hospitalized COVID-19 patients: the “Gabrin sign”. J Am Acad Dermatol. 2020;83:680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koskinen M., Carpen O., Honkanen V., et al. Androgen deprivation and SARS-CoV-2 in men with prostate cancer. Ann Oncol. 2020;31:1417–1418. doi: 10.1016/j.annonc.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsson J.F., Appelros P., Askling J., et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. doi: 10.2147/CLEP.S282475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons J.A., de Vries M., Krauwinkel W., et al. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54:1057–1069. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RECOVERY Collaborative Group, Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyon M, Li J, Cullen J, et al. 5α-Reductase inhibitors are associated with reduced risk of SARS-CoV-2 infection: a matched-pair, registry-based analysis. J Urol. In press. 10.1097/ju.0000000000002180. [DOI] [PubMed]
- 17.Cadegiani F.A., McCoy J., Gustavo Wambier C., et al. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13 doi: 10.7759/cureus.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao Y., Wang X.M., Mannan R., et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci U S A. 2020;118 doi: 10.1073/pnas.2021450118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F., Han M., Dai P., et al. Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide. Nat Commun. 2021;12:866. doi: 10.1038/s41467-021-21171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy J., Goren A., Cadegiani F.A., et al. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front Med. 2021;8 doi: 10.3389/fmed.2021.668698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zhang X., Tan Y., Ling Y., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 22.Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J. In press. 10.22037/uj.v18i.6691. [DOI] [PubMed]
- 23.Schwartzberg L.S., Yardley D.A., Elias A.D., et al. A phase I/Ib Study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer. Clin Cancer Res. 2017;23:4046–4054. doi: 10.1158/1078-0432.CCR-16-2339. [DOI] [PubMed] [Google Scholar]
- 24.Chan J.F., Zhang A.J., Yuan S., et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71:2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.