Abstract
We report a 21-year-old woman with Turner’s syndrome, Graves’ disease and primary hyperparathyroidism. At 12 years of age, she was of short stature, and was diagnosed with Turner’s syndrome and treated with growth hormone. At the age of 17 years, she was diagnosed with Graves’ disease. On treatment with methimazole, her laboratory findings normalized. At the age of 20 years, her serum calcium and intact parathyroid hormone levels were high. The upper left parathyroid gland showed swelling and was resected, and adenoma was diagnosed pathologically. Then, primary hyperparathyroidism induced by the adenoma was diagnosed. After the parathyroidectomy, the patient’s serum calcium and intact parathyroid hormone levels normalized. Is likely that Turner’s syndrome and Graves’ disease were not associated with primary hyperparathyroidism. Multiple endocrine neoplasia type 1 was unlikely considering the clinical, laboratory, ultrasonographic, and scintigraphic findings.
Keywords: Short stature, Turner’s syndrome, Graves’ disease, primary hyperparathyroidism
Introduction
Turner’s syndrome (TS) is characterized by short stature, gonadal dysgenesis, and congenital heart disease. 1 In TS, the prevalence of Hashimoto’s thyroiditis (HT, 10%–24%) and Graves’ disease (GD, 1.7%–3.0%) is reportedly higher than that in the pediatric general population without TS. There is a continuum between HT and GD. Patients with chromosome disorder and coexisting HT may be at high risk of progression to GD. When HT and GD occur in association with TS, accurate screening and monitoring of thyroid function and autoimmunity should be performed to improve clinical practices and healthcare in children.2–6
Primary hyperparathyroidism (PHPT) is characterized by hypercalcemia and overproduction of parathyroid hormone (PTH). 7 The clinical findings include reduced bone mineral density, nephrolithiasis, and gastric ulcers. Parathyroidectomy of the abnormal gland is effective. PHPT associated with TS or Graves’ disease is rare.8,9
Case report
A 12-year-old girl visited our hospital due to her short stature. She was 130.7 cm tall (standard deviation (SD) = −3.6), and weighed 42.0 kg (SD = −0.24). She was born to non-consanguineous parents after a normal pregnancy and delivery.
She had cubitus valgus, a short neck, and breast budding (Tanner stage II) without a webbed neck or congenital heart anomaly. The laboratory results were as follows: luteinizing hormone (LH) 11.7 mIU/mL (reference range = 0.3–2.5 mIU/mL); follicle-stimulating hormone (FSH), 37.7 mIU/mL (reference range = 0.8–4.0 mIU/mL); estradiol = 18.6 pg/mL (reference range = 5–120 pg/mL). All other laboratory findings, including thyroid hormone, were normal. Chromosomal examination revealed the karyotype 46,X, del(X)(p11.1). The patient was diagnosed with TS.
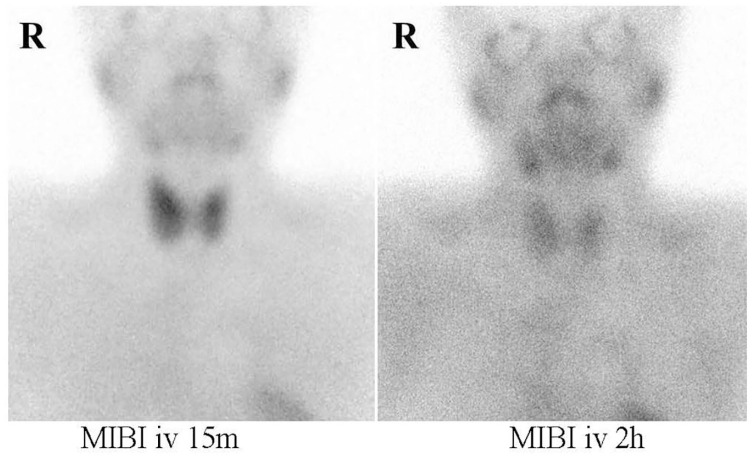
She began growth hormone (GH) therapy when she was 13 years and 4 months of age. Her thyroid hormone level was within the normal range at the time of therapy initiation. Her height reached 146.6 cm (SD = −2.2). Menarche began spontaneously at the age of 14 years and 2 months, but her cycle was irregular and gradually stopped. At the age of 17 years and 1 month, she underwent Kaufmann’s therapy. At that time, she showed a moderately sized goiter. The diagnosis of GD was established based on the following serum findings: thyroid-stimulating hormone (TSH), <0.005 μIU/mL (reference range = 0.38–4.3 μIU/dL); free thyroxine (fT4) = 3.49 ng/dL (reference range = 0.94–1.60 ng/dL); triiodothyronine (T3), 3.00 ng/mL (reference range = 0.94–1.70 ng/dL); thyroid-stimulating antibody = 624% (reference range = <180%). Increased accumulation of these markers was revealed by technetium (Tc) scintigraphy. At the age of 20 years, the laboratory data were as follows: serum calcium (Ca), 10.6 mg/dL (reference range 8.8–10.6 mg/dL); P, 3.3 mg/dL (reference range = 2.5–4.5 mg/dL); intact parathyroid hormone (iPTH) = 105 pg/mL (reference range = 16–65 pg/mL). The patient had no symptoms of hyperparathyroidism (Table 1). We noted the following after 7 months: serum Ca = 11.1 mg/dL; P = 3.5 mg/dL; iPTH = 103 pg/mL; 1,25(OH) vitamin D = 62.2 pg/mL (reference range = 25–45 pg/mL); calcitonin = 23 pg/mL (reference range = 15–86 pg/mL); PTH-related peptide = <1.0 pmol/L (reference range = <1.0 pmol/L); urine Ca/urine creatinine (Cr) ratio = 0.82 (reference range = <0.17); Ca/Cr clearance ratio = 0.022 (reference range = >0.01); tubular reabsorption of phosphate = 87% (reference range = 80%–92%). She had no fatigue, hypoglycemia, nausea, vomiting, or gastrointestinal bleeding. Her serum immunoreactive insulin, C-peptide immunoreactive, gastrin, glucagon, adrenocorticotropic hormone (ACTH), and prolactin levels were 4.6 μIU/mL (reference range = <13 μIU/mL), 1.7 ng/mL (reference range = 0.5–2.0 ng/mL), 10 pg/mL (reference range = <200 pg/mL), 9.6 pg/mL (reference range = 5.4–55.0 pg/mL), 20.6 pg/mL (reference range = 7.2–63.3 pg/mL), and 7.9 ng/mL (reference range = < 15 ng/mL), respectively. Abdominal ultrasonography showed no urolithiasis or pancreatic abnormalities. According to dual-energy X-ray absorptiometry, her lumbar (L2–4) bone mineral density was 0.912 g/cm2 (reference range = 0.74–1.33 g/cm2), with a Z-score of −0.9. Cervical echography showed hypoechoic masses of parathyroid glands in the upper left (15 mm × 7 mm × 6 mm), lower left (6 mm × 5 mm × 7 mm), and lower right (5 mm × 5 mm × 7 mm), and Tc-99 m methoxy isobutyl isonitrile (MIBI) scintigraphy showed accumulation in the same areas (Figure 1). During the operation, the upper and lower left parathyroid glands and lower right parathyroid gland were resected. Pathological analysis revealed an adenoma weighing 399 mg in the upper left parathyroid gland; the other resected glands were normal. There was no evidence of malignancy. She was diagnosed with PHPT. After parathyroidectomy, her serum Ca and iPTH levels normalized.
Table 1.
Laboratory results and treatment.
| 13Y4M at GH treatment | 17Y1M at diagnosis of Graves’ disease | 20Y8M at diagnosis of PHPT | 21Y7M after parathyroidectomy | |
|---|---|---|---|---|
| Ca (8.8–10.6 mg/dL) | 10.3 | 10.9 | 11.1 | 9.8 |
| P (2.5–4.3 mg/dL) | 4.2 | 3.1 | 3.5 | 3.7 |
| iPTH (16–65 pg/mL) | 103 | 51 | ||
| TSH (0.38–4.3 μIU/mL) | 1.2 | < 0.005 | 0.014 | 0.331 |
| T3 (0.94–1.70 ng/mL) | 3.00 | |||
| Free T3 (2.40–4.00 pg/mL) | 4.19 | 3.79 | 3.63 | |
| Free T4 (0.94–1.60 ng/dL) | 1.04 | 3.49 | 1.44 | 1.38 |
| LH (0.3–2.5 mIU/mL) | 11.7 | 1.6 | 9.0 | |
| FSH (0.8–4.0 mIU/mL) | 37.7 | 3.2 | 30.5 | |
| Estrogen (5–120 pg/mL) | 18.6 | 34.3 | 24.5 | |
| IGF-1 (188–654 ng/mL) | 304 | 498 |
Treatment
GH 
Methimazole 
Kaufmann’s treatment 
Y: year; M: month; GH: growth hormone; PHPT: primary hyperparathyroidism; iPTH: intact parathyroid hormone; TSH: thyroid stimulating hormone; T3: triiodothyronine; T4: thyroxine; LH: luteinizing hormone; FSH: follicle-stimulating hormone; IGF: insulin-like growth factor.
Values in brackets indicate the normal range.
Figure 1.
Methoxy isobutyl isonitrile (MIBI) scintigraphy showed slight accumulation in the upper and lower left parathyroid glands and lower right parathyroid gland at 2 h compared to 15 min after administration of technetium 99 m MIBI.
Discussion
Associations between autoimmune diseases (ADs) such as thyroid AD and TS have been reported.1,2 The risk of AD is approximately twice as high in patients with TS than in the general female population. 10 The mechanism by which complications such as AD and TS develop remains unclear. 10
It has been hypothesized that the incidence and frequency of chromosomal abnormalities are higher in patients with HT than in those with GD; however, the reason for this difference is poorly understood.2,4,5 There have been several reports on patients with TS who developed GD during GH therapy, but a direct link is unlikely. 11
In our case, we treated the GD with methimazole and TS with Kaufmann’s therapy. During these therapies, the patient’s serum levels of Ca and PTH increased, and she was finally diagnosed with PHPT. A few cases of PHPT with TS have been reported. 8
There have been several reports of an association between PHPT and GD. 9 Patients with thyrotoxicosis frequently exhibit hypercalcemia, reflecting hormone-related increases in bone resorption and resulting in suppressed PTH levels. However, this is not the case in patients with PHPT or familial hypocalciuric hypercalcemia (FHH), an autosomal-dominant disorder caused by mutation of the gene encoding the Ca-sensing receptor.10,12 FHH was unlikely in our patient; the urine Ca/urine Cr ratio of 0.82, Ca/Cr clearance ratio of 0.22, and hypercalcemia resolved after surgical removal of the parathyroid gland. 13
Our patient had concomitant GD and PHPT, and her Ca and PTH levels were moderately increased during treatment for GD. Before treatment with methimazole, her Ca level remained within normal limits. Her Ca and PTH levels normalized after parathyroidectomy.
PHPT can sometimes present as multiple endocrine neoplasia type 1 (MEN1) and involves various combinations of tumors, including parathyroid, enteropancreatic, and anterior pituitary tumors, among others. The typical age of onset of PHPT in MEN1 patients is 20–25 years.14,15
If the PHPT had been caused by an autoimmune process, a link between TS, GD, and PHPT would be plausible, but this is unlikely since the PHPT was cured by excision of the adenoma. Also, if PHPT develops in association with MEN1, the development of all three diseases in relation to an autoimmune process is unlikely. Thus, PHPT likely developed independently.
Our patient was 21 years of age, and her normal endocrine laboratory examination and clinical, ultrasonographic, and scintigraphic findings indicated that MEN1 was unlikely. Moreover, there was no family history of PHPT or MEN1-related tumors. Genetic analysis to determine MEN1 status has been recommended for patients with PHPT aged <30 years,14,15 but our patient did not give consent for this.
Conclusion
We report a 21-year-old female TS patient with GD and PHPT. If the PHPT had been caused by an autoimmune process, a link between the three conditions would have been plausible, but the PHPT seems to have developed independently based on the clinical, endocrine laboratory examination, ultrasonographic, and scintigraphic findings.
Acknowledgments
Part of this study was reported at the European Society for Pediatric Endocrinology (ESPE) Meeting in 2018 (ESPE Abstract (2018) 89. P-P3-066).
Footnotes
Author contributions: Sh.N., E.T., H.K.,T.O., M.O. and Sa.N. evaluated the patient clinically. Sh.N. and E.T. wrote the manuscript. H.K. and T.O. were involved in the surgical resection. M.O. and Sa.N. provided the conceptual advice. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: We written informed consent was obtained from the patient and her parent for their anonymized information to be published in this article.
ORCID iD: Shigeru Nagaki  https://orcid.org/0000-0002-0627-1368
https://orcid.org/0000-0002-0627-1368
References
- 1. Sybert VP, McCauley E. Turner’s syndrome. N Engl J Med 2004; 351(12): 1227–1238. [DOI] [PubMed] [Google Scholar]
- 2. Livadas S, Xekouki P, Fouka F, et al. Prevalence of thyroid dysfunction in Turner’s syndrome: a long-term follow-up study and brief literature review. Thyroid 2005; 15(9): 1061–1066. [DOI] [PubMed] [Google Scholar]
- 3. Aversa T, Lombardo F, Corrias A, et al. In young patients with Turner or Down syndrome, Graves’ disease presentation is often preceded by Hashimoto’s thyroiditis. Thyroid 2014; 24(4): 744–745. [DOI] [PubMed] [Google Scholar]
- 4. Costo C, Pepe G, Pomi AL, et al. Hashimoto’s thyroiditis and Graves’ disease in genetic syndromes in pediatric age. Genes 2021; 12: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aversa T, Lombardo F, Valenzise M, et al. Peculiarities of autoimmune thyroid disease in children with Turner or Down syndrome: an overview. Ital J Pediatr 2015; 41: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valenzise M, Aversa T, Corrias A, et al. Epidemiology, presentation and long-term evolution of Graves’ disease in children, adolescents and young adults with Turner syndrome. Horm Res Paediatr 2014; 81(4): 245–250. [DOI] [PubMed] [Google Scholar]
- 7. Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol 2018; 14: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park J, Kim YM, Choi JH, et al. Turner syndrome with primary hyperparathyroidism. Ann Pediatr Endocrinol Metab 2013; 18(2): 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abboud B, Sleilaty G, Mansour E, et al. Prevalence and risk factors for primary hyperparathyroidism in hyperthyroid patients. Head Neck 2006; 28(5): 420–426. [DOI] [PubMed] [Google Scholar]
- 10. Jorgensen KT, Rostgaard K, Bache I, et al. Autoimmune diseases in women with Turner syndrome. Arthritis Rheum 2010; 62(3): 658–666. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa M, Inamo Y, Harada K. A case report of Turner syndrome with Graves’ disease during recombinant human GH-therapy and review of literature. Clin Pediatr Endocrinol 2006; 15(2): 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2: 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mizamtsidi M, Nastos C, Mastorakos G, et al. Diagnosis, management, histology and genetics of sporadic primary hyperparathyroidism: old knowledge with new tricks. Endocr Connect 2018; 7(2): R56–R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kihara M, Miyauchi A, Ito Y, et al. MEN1 gene analysis in patients with primary hyperparathyroidism: 10-year experience of a single institution for thyroid and parathyroid care in Japan. Endocr J 2009; 56(5): 649–656. [DOI] [PubMed] [Google Scholar]
- 15. Lassen T, Friis-Hansen L, Rasmussen AK, et al. Primary hyperparathyroidism in young people, when should we perform genetic testing for multiple endocrine neoplasia 1(MEN 1)? J Clin Endocrinol Metab 2014; 99(11): 3983–3987. [DOI] [PubMed] [Google Scholar]