Abstract
Introduction
The short- and long-term effects of coronavirus disease 2019 (COVID-19) on erectile function and penile vasculature remains poorly understood and is of particular importance as the virus has been found to be present within the penile tissue.
Aim
We determined the association of COVID-19 infection and subsequent diagnoses of erectile dysfunction.
Methods
We assessed the risk of ED in men with COVID-19 in the United States (US) using the TriNetX Research Network, a federated electronic medical records network of over 42 healthcare organizations and 66 million patients from the US. We identified adult men (≥ 18 years) with a recorded COVID-19 infection (ICD-10-CM B34.2, U07.1, U07.2, J12.81, J12.82, B97.29) since January 1, 2020, and compared them to an equivalent number of adult men who did not have COVID-19 over the same timeframe. Men with prior history or diagnosis of ED before January 1, 2020 were excluded. We accounted for confounding variables through propensity score matching for age, race, body mass index (BMI), and history of the following comorbid medical conditions: diabetes mellitus (E11), hypertension (I10), ischemic heart disease (I20-25), or hyperlipidemia (E78).
Outcomes
We assessed the association between COVID-19 and ED (N52) as a primary outcome through regression analysis with statistical significance assessed at P< .05.
Results
Prior to propensity score matching, men with COVID-19 were found to be older than men without COVID-19 (47.1 ± 21.4 vs 42.4 ± 24.3 years). Additionally, men with COVID-19 were noted to have increased prevalence of diabetes mellitus (DM) and hypertension (HTN) when compared to men without COVID-19 (13% DM and 27% HTN vs 7% DM and 22% HTN). After propensity score matching, we compared 230,517 men with COVID-19 to 232,645 men without COVID-19 and found that COVID-19 diagnosis was significantly associated with ED (odds ratio 1.20, 95% confidence interval 1.004–1.248, P= .04).
Clinical Implications
Our findings indicate that clinicians should consider evaluating erectile dysfunction among men with recent COVID-19 diagnoses and counsel them regarding the risk of developing erectile dysfunction.
Strengths and Limitations
Strengths include large sample size and adjustment for confounding variables. Limitations include reliance on a global federated dataset, retrospective study design, and lack of data regarding ED (mild vs moderate vs severe), COVID-19 infection severity, or history of prostate cancer and radiation,
Conclusion
There is an increased chance of new onset erectile dysfunction post-COVID-19 infection.
Chu KY, Nackeeran S, Horodyski L, et al. COVID-19 Infection Is Associated With New Onset Erectile Dysfunction: Insights From a National Registry. Sex Med 2022;10:100478.
Keywords: Erectile Dysfunction, COVID-19, Coronavirus, Endothelial Dysfunction
INTRODUCTION
With the coronavirus disease 2019 (COVID-19) pandemic ongoing since 2019, increasing attention is turning to sequelae of the virus. While primary infection occurs in the respiratory system, it is recognized that COVID-19 can impact other areas of the body, including vasculature, heart, and kidneys.1 It has been demonstrated that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to angiotensin-converting enzyme 2 (ACE2) to infect goblet secretory cells of the nasal mucosa and alveolar pneumocytes, and ACE2 is also expressed in high levels throughout vascular endothelial cells.2 End-organ damage seen with severe COVID-19 infections is thought to be a result of endothelial dysfunction, resulting both from direct infection and a systemic inflammatory response, and electron microscopy has demonstrated viral elements in the endothelial cells of affected organs including the penis.3
Additionally, anecdotal reports exist of patients developing erectile dysfunction (ED) following COVID-19 infection, and ED has been found to be higher in men with a history of COVID-19 infection compared to a matched cohort in a prior study.3,4 With both postulated mechanistic actions of COVID-19 causing endothelial dysfunction, and anecdotal reports of ED development, we hypothesize there exists an significant association between COVID-19 infection and new-onset diagnosis of erectile dysfunction in adult men.
METHODS
Data Source and Study Design
This is a retrospective cohort study using electronic health record (EHR) data from the TriNetX private database. TriNetX is a health research network giving access to electronic medical records from 42 healthcare organizations and approximately 66 million patients in the United States. TriNetX complies with the Health Insurance Portability and Accountability Act (HIPAA), and is certified to the ISO 27001:2013 standard. TriNetX ensures protection of the healthcare data it has access to by maintaining an Information Security Management System (ISMS) and meeting the requirements of the HIPAA Security Rule. All data accessed, including any patient level data, only contained de-identified information as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The de-identification process is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. This research utilized the data licensed by TriNetX and described above, does not require IRB or ethics review, as analyses with this data do not meet the definition of “research involving human subjects” as defined within 45 CFR 46.102(f), due to de-identification of all patient data.
Relevant information including demographics, body measurements, and medical diagnoses including comorbid medical conditions was collected for analysis. A patient was considered to have a diagnosis if a corresponding ICD code was linked within the TriNetX database as obtained through the patient's EHR. All included patients were adult males age ≥ 18 years. Men with a prior diagnosis of ED, prostatectomy, and pelvic radiation therapy were excluded. The first cohort included patients with a recorded COVID-19 infection (ICD-10-CM B34.2, U07.1, U07.2, J12.81, J12.82, B97.29) since January 1, 2020. This group was compared with the second cohort of adult males without prior COVID-19 infection.
Statistical Analysis
Odds ratios (ORs) were calculated to compare ED rates for COVID-19 (+) patients against COVID-19 (-) patients. Statistical significance was evaluated at P< .05. Potential confounding variables were accounted for via propensity score matching. These factors were age, race, body mass index (BMI), and a history of comorbid conditions including diabetes mellitus (ICD-10-CM E11), hypertension (ICD-10-CM I10), ischemic heart disease (ICD-10-CM I25) or hyperlipidemia (ICD-10-CM E78). The association between COVID-19 infection and ED was assessed as a primary outcome through regression analysis, with statistical significance at P< .05. All statistical analyses were conducted on the TriNetX platform, which utilizes a combination of JAVA, R, and Python programming languages.
Subgroup Analysis
In order to assess the effect that severity of respiratory disease and overall patient health had on COVID-associated erectile dysfunction, we conducted 2 additional subgroup analyses. We first compared COVID patients who had procedure codes for ventilators (CPT 94002, 94003) or severe respiratory disease (ICD-10 J80, J96.0, J96.2) to non-COVID patients. We then compared patients without histories of hypertension (I10), diabetes (E08-E11), or obesity (E66) to non-COVID patients.
RESULTS
A total of 11,083,653 male patients were identified through retrospective analysis of the TriNetX database. A total of 246,990 men were identified with COVID-19 diagnosis since January 1, 2020, while the rest did not have COVID-19. Prior to propensity score matching, men with COVID-19 were found to be older than men without COVID-19 (47.1 ± 21.4 vs 42.4 ± 24.3 years). The cohorts were similar in both racial and ethnic demographics. Additionally, men with COVID-19 were noted to have increased prevalence of diabetes mellitus (DM) and hypertension (HTN) when compared to men without COVID-19 (14% DM and 27% HTN vs 9% DM and 22% HTN) (Table 1). Following propensity score matching, we compared 230,517 men with prior COVID-19 infection to 232,645 men without history of COVID-19. It was found that COVID-19 diagnosis was significantly associated with ED (OR 1.20, 95% CI 1.004–1.248, P= .04; Table 2).
Table 1.
Baseline characteristics for men with and without COVID included in this study
| Characteristic | No COVID (N = 10,836,663) | COVID (N = 246,990) | P value |
|---|---|---|---|
| Age (years) | 42.4 ± 24.3 | 47.1 ± 21.4 | <.0001 |
| Race | <.0001 | ||
| White | 64% | 61% | |
| Black | 14% | 16% | |
| Asian | 3% | 3% | |
| Native American | 1% | <1% | |
| Unknown | 18% | 19% | |
| Ethnicity | <.0001 | ||
| Hispanic/Latino | 9% | 8% | |
| Not Hispanic/Latino | 59% | 58% | |
| Unknown | 32% | 33% | |
| Type 2 DM (E11) | 9% | 14% | <.0001 |
| HTN (I10) | 22% | 27% | <.0001 |
| HLD (E78) | 20% | 25% | <.0001 |
| Obesity (E66) | 9% | 14% | <.0001 |
| Alcohol use disorder (F10) | 3% | 3% | .7702 |
| Nicotine dependence (F17) | 8% | 8% | .068736 |
Table 2.
Measures of association between COVID-19 infection and subsequent development of new onset erectile dysfunction (ED)
| Outcome | Number male COVID-19 patients with outcome (%) | Unbalanced OR (95% CI) | Balanced OR |
|---|---|---|---|
| ED | 1,150 (0.48%) | 1.22 (1.15–1.30) P < .0001 | 1.20 (1.10–1.31) P < .0001 |
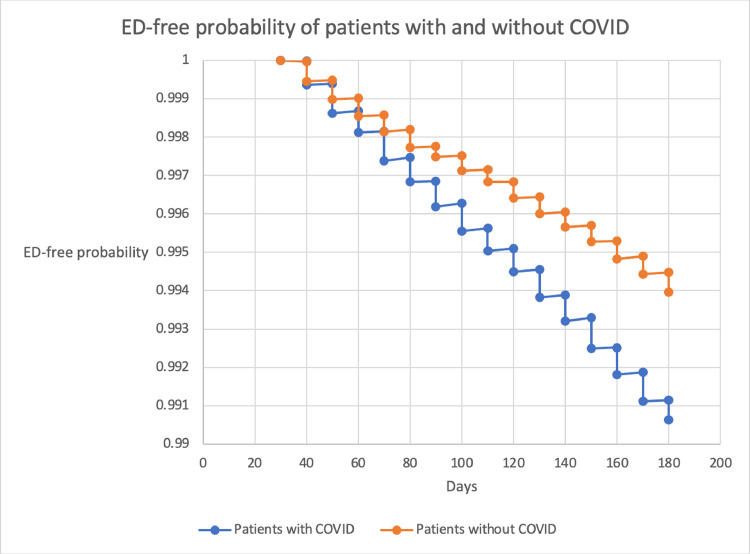
To assess the effect that severity of respiratory disease and overall patient health had on COVID-19 associated ED, we conducted 2 additional subgroup analyses. We first compared COVID-19 patients who had procedure codes for ventilators (CPT) or severe respiratory disease (ICD-10) to non-COVID-19 patients. We found that while COVID-19 patients in this sub-group had an overall higher proportion diagnosed with ED prior to propensity score matching (OR 1.36, 95% CI 1.15–1.60, P= .0002), there was no significant association with ED following propensity score matching (OR 1.02, 95% CI 0.82-1.28, P = .7097). We then compared patients without histories of hypertension, diabetes, or obesity to non-COVID-19 patients and found a significant association with ED in this sub-group both before and after controlling for potentially confounding variables (OR 1.32, 95% CI 1.08–1.60, P= 1.0053) (Figure 1).
Figure 1.
Ed-free probability of patients with and without COVID-19.
A limitation of the TriNetX database was the inability to isolate individual patient data for time to diagnosis, but we conducted a Kaplan-Meier analysis and used the log-rank test to evaluate freedom from ED. Covid-19 patients were found to have a higher probability of ED diagnosis versus those without Covid-19, as time progressed. Additionally, this overall trend increased in probability as time went on (Table 3).
Table 3.
Subgroup analyses of effect of respiratory disease and overall patient health on COVID-19 associated erectile dysfunction
| Cohort | Number in COVID cohort with outcome (%) | Number in non-COVID cohort with outcome (%) | Unbalanced OR (95% CI) | Balanced OR (95% CI) |
|---|---|---|---|---|
| All COVID patients | 998 (0.34%) | 27,287 (0.24%) | 1.43 (1.35–1.53) P < .0001 | 1.14 (1.04–1.25) P = .0039 |
| COVID patients with serious respiratory disease | 144 (0.33%) | 27,287 (0.24%) | 1.36 (1.15–1.60) P = .0002 | 1.02 (0.82–1.28) P = .7097 |
| COVID patients without hypertension, diabetes, or obesity | 235 (0.14%) | 27,287 (0.24%)* | 1.23 (1.08–1.41) P = .0014 | 1.32 (1.08–1.60) P = .0053 |
All outcomes assessed between 1 and 6 months after COVID-19 diagnosis.
Comparison made against cohort without hypertension, diabetes, or obesity.
DISCUSSION
Previously, COVID-19 has been shown to have harmful effects on various organs throughout the body, including the heart, kidneys, and vascular system.1 Our current understanding of COVID-19 and its effect on the penile tissue and subsequent erectile function remains unclear. The importance in identifying the virus's short and long-term effects on male reproductive organs are potentiated by a recent study observing COVID-19 particles in penile tissue through transmission electron microscopy and hemoxylin and eosin (H&E) staining.3 As COVID-19 causes widespread endothelial dysfunction, and erectile function is dependent on a functional endothelium and proper vascular flow,5 we hypothesize that a COVID-19 infection may be associated with new onset erectile dysfunction.
In this study, we analyzed a multinational electronic medical record registry to explore the association between males with COVID-19 infection and erectile dysfunction. We observed that the odds of having an erectile dysfunction diagnosis was 20% higher if the male patient had a prior COVID-19 diagnosis. Our findings are consistent with the results from a retrospective analysis by Sansone et al, where they compared Sexual Health Inventory for Men (SHIM) questionnaire scores between COVID-19 (+) and Covid-19 (-) patients. They observed 28% of COVID-19 (+) men with ED compared to 9.33% of COVID-19 (-) men with ED. Further statistical analysis with propensity score matching showed an association of a five-fold risk of contracting COVID-19 among subjects with ED.6 A recent study on National Sales Perspective data during the COVID-19 pandemic showed an increase in oral PDE5-inhibitors sales, particularly tadalafil.7 While this data could be interpreted in a few ways, such as increased sexual activities due to more time sequestered at home, it may also suggest increased erectile dysfunction.
While endothelial dysfunction is the hypothesized mechanistic action of erectile dysfunction development in patients with history of COVID-19, it could also be secondary to development of hypogonadism. It has been noted that the hyperinflammatory state typical of COVID-19 infection is characterized by cytokines which are also associated with worsening male sexual dysfunction, including TNF-α, IL-6 and IL-1β.8,9 These cytokines are also associated with male hypogonadism, and interestingly, an association has been found between COVID-19 infection and subsequent development of hypogonadism in a small review.10,11 Furthermore, Dhindsa et al found that low testosterone concentrations may play an integral role in development of worse outcomes in males with COVID-19.12
This study has several strengths, including large sample size and adjustment for confounding variables through propensity score matching. There are limitations with utilizing the TriNetX database, including lack of data regarding degree of ED (mild vs moderate vs severe), as well as the data of exact treatments these men were receiving. It is very important to consider that researchers are still trying to understand the long-term effects of COVID-19, and much of it may not reveal itself for some time as the pandemic is ongoing. The durability of new onset ED diagnosis in the context of COVID-19 will require further research in more longitudinal studies. There are risks of misclassification of medical diagnoses, incomplete data, and duplicated data for patients that receive care at multiple health care organizations. Additionally, as the database is constantly being updated with inclusion of new data points, our conclusion is drawn from the statistical analyses performed on only data included prior to that day. Because of the study design, only an association can be determined, but we cannot demonstrate causality. Although the main analysis of this study returned statistically significant results, it should be noted that the clinical significance of an odds ratio of 1.20 is questionable, and the absolute risk increase of approximately 0.1% should be considered when interpreting the results. Future directions would include exploring the effectiveness of ED therapy in men with COVID-19 versus non-COVID-19, differentiating psychogenic versus organic ED, as well as further tailored studies to better understand the causative effect and mechanism of COVID-19 on erectile dysfunction development.
CONCLUSIONS
There is an association of new onset of ED in men who had COVID-19 infection. This may be due to virus-induced endothelial cell dysfunction; however, an underlying mechanism and causation has not yet been clearly elucidated. While it appears that COVID-19 infection may be a risk factor for ED, additional research is needed to establish causality.
STATEMENT OF AUTHORSHIP
Kevin Y. Chu: Investigation, Writing – original draft, Writing – review & editing; Sirpi Nackeeran: Investigation, Writing – original draft, Writing – review & editing; Laura Horodyski: Investigation, Writing – original draft, Writing – review & editing; Thomas A. Masterson: Investigation, Writing – original draft, Writing – review & editing, Supervision; Ranjith Ramasamy: Writing – original draft, Writing – review & editing, Supervision.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: None.
References
- 1.Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kresch E, Achua J, Saltzman R, et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: Histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health. 2021;39:466–469. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sansone A, Mollaioli D, Ciocca G, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021;44:223–231. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardi Y, Dayan L, Apple B, Gruenwald I, Ofer Y, Jacob G. Penile and systemic endothelial function in men with and without erectile dysfunction. Eur Urol. 2009;55:979–985. doi: 10.1016/j.eururo.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Sansone A, Mollaioli D, Ciocca G, et al. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9:1053–1059. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez I, Gul Z, Gellad WF, Davies BJ. Marked increase in sales of erectile dysfunction medication during COVID-19. J Gen Intern Med. 2021;36:2912–2914. doi: 10.1007/s11606-021-06968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen SF, Ho Y-C. SARS-CoV-2: A storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiorino MI, Bellastella G, Giugliano D, Esposito K. From inflammation to sexual dysfunctions: A journey through diabetes, obesity, and metabolic syndrome. J Endocrinol Invest. 2018;41:1249–1258. doi: 10.1007/s40618-018-0872-6. [DOI] [PubMed] [Google Scholar]
- 10.Mohamad N-V, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 11.Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhindsa S, Zhang N, McPhaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]