Abstract
Background
Patients hospitalised with COVID-19 are at risk for thrombotic events after discharge; the role of extended thromboprophylaxis in this population is unknown.
Methods
In this open-label, multicentre, randomised trial conducted at 14 centres in Brazil, patients hospitalised with COVID-19 at increased risk for venous thromboembolism (International Medical Prevention Registry on Venous Thromboembolism [IMPROVE] venous thromboembolism [VTE] score of ≥4 or 2–3 with a D-dimer >500 ng/mL) were randomly assigned (1:1) to receive, at hospital discharge, rivaroxaban 10 mg/day or no anticoagulation for 35 days. The primary efficacy outcome in an intention-to-treat analysis was a composite of symptomatic or fatal venous thromboembolism, asymptomatic venous thromboembolism on bilateral lower-limb venous ultrasound and CT pulmonary angiogram, symptomatic arterial thromboembolism, and cardiovascular death at day 35. Adjudication was blinded. The primary safety outcome was major bleeding. The primary and safety analyses were carried out in the intention-to-treat population. This trial is registered at ClinicalTrials.gov, NCT04662684.
Findings
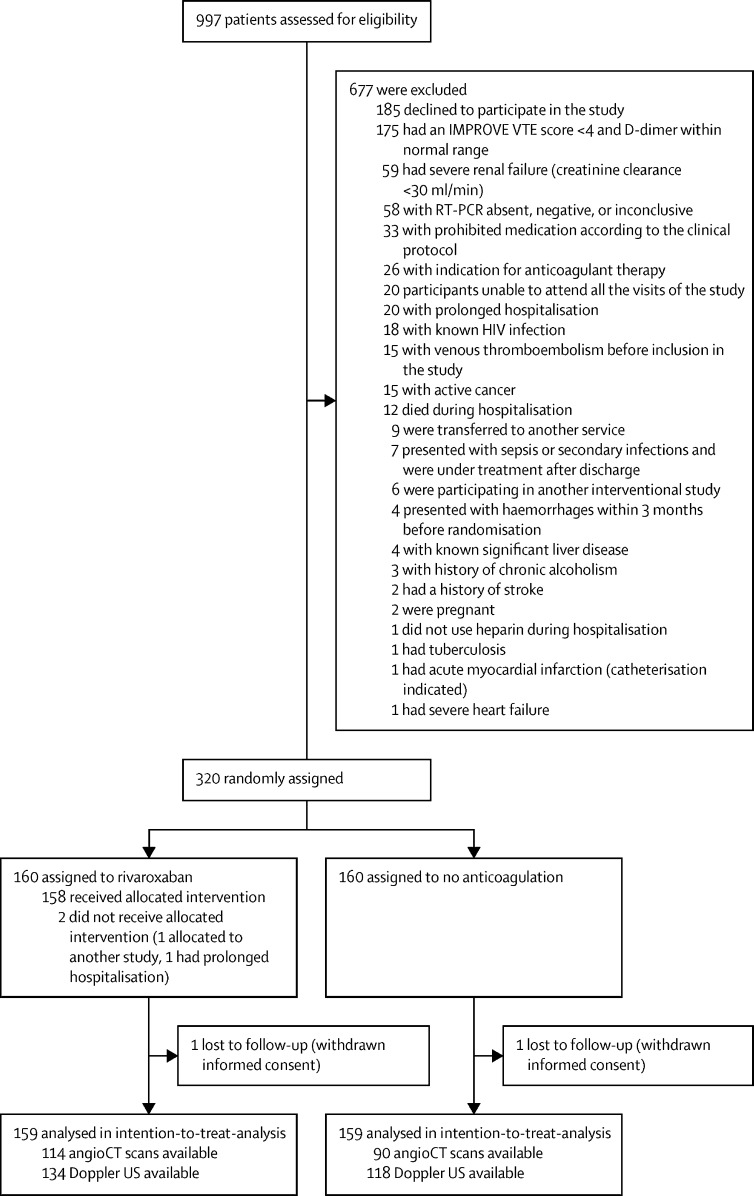
From Oct 8, 2020, to June 29, 2021, 997 patients were screened. Of these patients, 677 did not meet eligibility criteria; the remaining 320 patients were enrolled and randomly assigned to receive rivaroxaban (n=160 [50%]) or no anticoagulation (n=160 [50%]). All patients received thromboprophylaxis with standard doses of heparin during hospitalisation. 165 (52%) patients were in the intensive care unit while hospitalised. 197 (62%) patients had an IMPROVE score of 2–3 and elevated D-dimer levels and 121 (38%) had a score of 4 or more. Two patients (one in each group) were lost to follow-up due to withdrawal of consent and not included in the intention-to-treat primary analysis. The primary efficacy outcome occurred in five (3%) of 159 patients assigned to rivaroxaban and 15 (9%) of 159 patients assigned to no anticoagulation (relative risk 0·33, 95% CI 0·12–0·90; p=0·0293). No major bleeding occurred in either study group. Allergic reactions occurred in two (1%) patients in the rivaroxaban group.
Interpretation
In patients at high risk discharged after hospitalisation due to COVID-19, thromboprophylaxis with rivaroxaban 10 mg/day for 35 days improved clinical outcomes compared with no extended thromboprophylaxis.
Funding
Bayer.
Introduction
Thrombotic events complicate COVID-19 at higher rates than previously observed in other comparable clinical situations, such as acute distress respiratory syndrome not related to SARS-CoV-2.1 Prophylactic use of parenteral anticoagulants during hospitalisation is recommended,2 and there is emerging consensus about the role of in-hospital heparin as primary thromboprophylaxis.3, 4 There is no consensus on the use of extended thromboprophylaxis beyond the hospital stay. Multiple studies of post-discharge patients with COVID-19 show incidences of symptomatic venous thromboembolism ranging from below 1%5 to 2·5%.6, 7 In the largest prospective registry, which included 4906 post-discharge patients with COVID-19, the incidence of the primary endpoint of venous thromboembolism, arterial thromboembolism, or all-cause death was 7·13%, and was 46% lower in patients prescribed post-discharge prophylactic anticoagulation.6
Extended venous thromboembolism prophylaxis after hospitalisation for medically ill (non-COVID-19) patients was previously assessed. The MARINER trial8 evaluated rivaroxaban 10 mg or 7·5 mg (if creatinine clearance <50mL/min) once per day versus placebo for 45 days after hospital discharge in 12 019 medically ill patients. Although the trial did not achieve superiority on the primary endpoint of symptomatic or fatal venous thromboembolism, there was a statistically significant 56% reduction in the relative risk of the prespecified outcome of isolated symptomatic venous thromboembolism, a 27% relative risk reduction of symptomatic venous thromboembolism and all-cause death, and a 28% relative risk reduction in major and fatal thromboembolic events.8 There was no statistically significant increase in the rate of major bleeding.9
Research in context.
Evidence before this study
COVID-19 leads to higher rates of thrombotic events than previously observed in other comparable clinical situations. Prophylactic dosage of parenteral anticoagulants during hospitalisation is mandatory, and there is emerging consensus about optimal dose of in-hospital heparin as primary thromboprophylaxis. However, there is no consensus on extended thromboprophylaxis after hospitalisation. We searched MEDLINE, the Cochrane Central register of Controlled Trials, Web of Science, and Scopus using the terms (“rivaroxaban” OR “apixaban” OR “dabigatran” OR “edoxaban” OR “heparin” OR “enoxaparin”) AND (“extended thromboprophylaxis” OR out-of-hospital thromboprophylaxis”) AND (“SARS-CoV-2” OR “COVID” OR “coronavirus” OR “COVID-19”) AND (“randomised” OR “clinical trials”), with no date or language restrictions. We did not find any published randomised trial assessing the effects of extended thromboprophylaxis after hospitalisation due to COVID-19.
Added value of this study
The MICHELLE trial is the first study, to our knowledge, to randomly assign patients hospitalised because of COVID-19, with high IMPROVE scores at hospital discharge, to receive either prophylactic doses of rivaroxaban (10 mg/day) or no anticoagulation for 35 days. Our study is also the first to use systematically CT pulmonary angiogram for the primary efficacy outcome evaluation. The results of the MICHELLE trial show that in patients at high risk discharged after COVID-19 hospitalisation, thromboprophylaxis with rivaroxaban 10 mg/day for up to 35 days improved clinical outcomes, reducing thrombotic events compared with no post-discharge anticoagulation. The MICHELLE trial provides high-quality evidence to guide medical decisions.
Implications of all the available evidence
To our knowledge, this study is the first positive direct oral anticoagulant randomised controlled trial in thromboprophylaxis for medically ill patients. In the era of the COVID-19 pandemic, in which most medical decisions regarding anticoagulation strategies have been made based on low-quality evidence, our study provides high-quality data to help clinicians in the decision making process when managing and treating patients with COVID-19.
There are conflicting recommendations about the role of post-hospital discharge extended antithrombotic prophylaxis in patients hospitalised due to COVID-19.10, 11, 12 We hypothesised that in patients hospitalised with COVID-19, prophylaxis with rivaroxaban 10 mg/day for 35 days after discharge would improve clinical outcomes, including major and fatal thromboembolic events.
Methods
Study design
The MICHELLE trial was a pragmatic, open-label (with blinded adjudication), multicentre, randomised, controlled trial in patients discharged after hospitalisation for COVID-19. The methods have been previously described.13 The changes in the protocol during the study period are described in the appendix (p 24). The study was conducted at 14 hospitals in Brazil. Details around the centres are described in the appendix (p 5). The objective was to assess whether rivaroxaban 10 mg/day for 35 days, initiated at hospital discharge, reduced the combination of symptomatic and asymptomatic venous and arterial thromboembolism, including cardiovascular death.
The study was led by an academic steering committee (appendix p 3) whose members designed the trial and coordinated the scientific and medical aspects of the study. Science Valley Research Institute (São Paulo, Brazil) was responsible for data and site management, as well as all statistical analysis. All events were evaluated by an independent clinical events adjudication committee, including a core laboratory for image analysis, whose members were unaware of the study treatment assignment. The study also had an independent data safety monitoring board for safety surveillance during the trial.
The Brazilian National Commission for Research Ethics and ethics committees at all the participating sites approved the trial protocol. The MICHELLE trial institutional review board number is CAAE 35432520.3.1001.5485. The last version of the protocol is included in the appendix (p 42).
Participants
We included patients at discharge who were hospitalised with COVID-19 (confirmed by RT-PCR, antigen, or IgM tests) for a minimum of 3 days (intensive care unit [ICU] stay was allowed). Patients received standard prophylactic doses of parenteral enoxaparin (40 mg subcutaneously once per day), unfractionated heparin (5000 IU two or three times per day), or fondaparinux (2·5 mg once per day) during hospitalisation. Patients also needed to have an increased risk for venous thromboembolism, defined as an elevated modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) venous thromboembolism (VTE) score of 2–3 with a D-dimer level of more than 500 ng/mL using local laboratory criteria or a score of 4 or more independent of the D-dimer level at hospital discharge. It was recommended that an elevated D-dimer level close to hospital discharge was to be used by sites when available, but any high D-dimer level (above 500 ng/mL) during any time of hospitalisation was considered for calculating the IMPROVE VTE score during randomisation. This approach was also used in the MARINER trial.9
No patient was scanned with Doppler ultrasound or pulmonary angiogram CT in hospital before being recruited. Suspicion or confirmation of a thrombotic event was an exclusion criterion, so patients with previously positive scans were not eligible for the MICHELLE trial. Vasopressor support was allowed for patients in the ICU and no limit to the level of oxygen support was a limitation to enrol patients in this pragmatic clinical trial. Full eligibility criteria are provided in the appendix (p 7).
Following local regulations, all participants provided written or electronically signed informed consent.
Randomisation and masking
Patients were randomly allocated in a 1:1 ratio to receive either thromboprophylaxis with rivaroxaban 10 mg/day or regular follow-up (no anticoagulation) for 35 days (SD 4). Randomisation was done in permuted blocks of variable size, using a central, concealed, web-based, automated randomisation system (RedCap, version 11.0.3). The MICHELLE trial was an open-label study, with no masking of investigators or patients to group allocation.
Procedures
Patients were screened for the eligibility criteria during hospitalisation. Baseline assessment included demographic characteristics, risk factors, medical history, intrahospital anticoagulation use, ICU stay, and laboratory data (D-dimer and creatinine clearance). Study medication was provided at randomisation and started within the first 24 h after hospital discharge and maintained for 35 days, irrespective of the second evaluation day. Patients allocated to the no anticoagulation group received no intervention. At randomisation, patients were asked to report in the protocol evaluations or by assessment any symptom suggestive of venous or arterial thromboembolism or bleeding. In every consultation, the investigators performed a detailed assessment of chest pain, dyspnoea, peripheral oedema, pain in the lower limbs, pulse evaluation, and signs of bleeding.
The first visit was on day 7 after randomisation and was done either by telephone or at an outpatient clinic. The second visit was done on day 35 (SD 4) at an outpatient clinic or hospital. On the same day, bilateral lower limb venous Doppler ultrasound and CT pulmonary angiograms were performed.
Outcomes
The primary efficacy outcome was a composite of symptomatic or fatal venous thromboembolism, asymptomatic venous thromboembolism detected by bilateral lower limb venous Doppler ultrasound and CT pulmonary angiogram, symptomatic arterial thromboembolism (myocardial infarction, non-haemorrhagic stroke, and major adverse limb event), and cardiovascular death at day 35.
The primary safety outcome was major bleeding, defined according to the International Society on Thrombosis and Haemostasis (ISTH) criteria.14 During the study, we added an amendment including arterial events for the primary outcome. We included myocardial infarction, non-haemorrhagic stroke, and major adverse limb events, primarily because of the prespecified MARINER subanalysis,8 published in 2020, that showed a relative risk reduction of 28% on arterial events.
The secondary efficacy outcomes were a combination of symptomatic or fatal venous thromboembolism; a composite of symptomatic venous thromboembolism or all-cause mortality; and a composite of symptomatic venous thromboembolism, myocardial infarction, non-haemorrhagic stroke, or cardiovascular death (death from known cardiovascular disease or death in which cardiovascular disease cause cannot be excluded).
The secondary safety outcomes were a combination of major, clinically relevant non-major, and other bleeding, according to ISTH criteria.14 Prespecified subgroup analyses were age (≤60 years or >60 years); body-mass index (≤30 kg/m2 or >30 kg/m2); creatinine clearance (30 to <50 mL/min or ≥50 mL/min); modified IMPROVE VTE score (2–3 or ≥4); D-dimer concentration (≤500 ng/mL or >500 ng/mL), and antiplatelet use.
An independent clinical events classification committee, whose members were unaware of the study treatment assignment, adjudicated all venous and arterial thromboembolic and bleeding events, and causes of death. All presumed or suspected thromboembolic events were reported for adjudication, regardless of the availability of imaging tests. If an imaging test was positive, the event was classified as a confirmed pulmonary embolism, venous thromboembolism, or arterial thrombosis. If imaging results were not available, but there was a high clinical suspicion of deep venous thrombosis or pulmonary embolism, the case was classified as probable deep venous thrombosis or pulmonary embolism. An independent core laboratory adjudicated all CT pulmonary angiograms, and pulmonary embolism was considered if three observers detected a defect in the intraluminal filling in the CT. The clinical event adjudication committee had access to necropsy data. According to the clinical events classification charter, patients who died with an unknown cause of death were classified as a possible pulmonary embolism. Outcome definitions are provided in the appendix (p 6).
Statistical analysis
The sample size was calculated assuming 80% power, at a significance level of 0·05, of a primary efficacy endpoint occurrence of 15% in the standard of care group (control group) and 5% in the treatment group, with a relative risk reduction of 67%.
If there was a true absolute difference in favour of the proposed treatment of 10% (15% vs 5%), then 282 patients were required with a power of 80% that the upper limit of a 95% CI would exclude a difference in favour of the standard group of more than 50%. With a projected drop-out rate of 10%, 320 patients were enrolled (n=160 per group).
This 56% relative risk reduction was observed in symptomatic events in the MARINER trial, which included more than 12 000 patients.9 For the MICHELLE trial, we projected a relative risk reduction of 67% on the anticoagulation group because we incorporated both mandatory bilateral lower limb Doppler ultrasound and pulmonary angiogram CT scans for all patients as part of the primary outcome. We believed that an increase of 11% in the relative risk reduction driven by asymptomatic events (both deep-venous thrombosis and pulmonary embolism) was realistic. The importance of asymptomatic thrombosis is not dismissible and earlier data showed that all-cause mortality is two times higher among patients with asymptomatic proximal deep-venous thrombosis.15
Efficacy analyses were done using the intention-to-treat principle.
The efficacy analysis tests were one-sided, with a type I error rate of 2·5%, assuming a two-sided 95% CI. The cumulative incidence of the composite events was compared between the rivaroxaban and control groups, and the relative risk (RR) or risk ratio was estimated. The same analysis was carried out for secondary efficacy outcomes. For the safety analysis, statistical tests were two-sided, with a type I error rate of 5% and a two-sided 95% CI. Superiority of the treatment group was claimed if the upper limit of 95% CI was less than 1.
Sensitivity analyses were performed for the primary and secondary outcomes using the per-protocol population (patients who received the proposed treatment [anticoagulation or no anticoagulation] with at least 80% of intervention compliance (definitions in the appendix p 10). Additional sensitivity analyses were performed for the risk difference using a 95% Wilson CI. For the risk difference, superiority of the treatment group was claimed if the upper limit of the 95% Wilson CI was less than 0. Details around the Wilson CI for a risk difference are described in the appendix (p 26).
Subgroup analyses for the primary outcome were performed between treatment and control groups according to predefined groups of age, body-mass index, creatinine clearance, modified IMPROVE VTE score, D-dimer levels, and use of antiplatelets. Interaction tests were performed to assess the homogeneity of the treatment effect between rivaroxaban versus control for the primary outcome.
The statistical analysis plan was completed before the end of the study and unblinding of the study results and is available in the appendix (p 27). All analyses were done with SPSS PASW Statistics software, version 17.0 (IBM, Armonk, NY, USA) and Excel.
The data safety monitoring board met every 2–3 months during the trial. The data safety monitoring board was tasked with looking at general efficacy and safety signals. There were no formal stopping rules. An interim analysis was performed by a statistician, who was aware of the study treatment assignment, in approximately 200 patients with complete 35-day follow-up. At that point, 287 patients had been randomly assigned into the study. These results were not shared with the trial leadership or with anyone outside of the data safety monitoring board. Details around the interim analysis and statistical analysis plan are described in the appendix (p 23).
The MICHELLE trial is registered at ClinicalTrials.gov, NCT04662684.
Role of the funding source
The study funder had no role in the planning and design of the study, data collection, analysis, and interpretation, nor writing of the manuscript.
Results
From Oct 8, 2020, to June 29, 2021, 997 patients were screened. Of these patients, 677 did not meet eligibility criteria; the remaining 320 patients were enrolled and randomly assigned to receive rivaroxaban (n=160 [50%]) or no anticoagulation (n=160 [50%]; figure 1 ). Two patients in the rivaroxaban group did not receive the intervention (one patient was assigned to another study, the other patient had a prolonged hospitalisation and needed prolonged parenteral anticoagulation). Two patients (one from each group) withdrew informed consent and were excluded from the primary analysis. Thus, 159 patients per group were included in the intention-to-treat analysis. Of the rivaroxaban group, 116 (73%) patients had CT pulmonary angiogram performed and 135 (85%) had bilateral lower limb venous Doppler ultrasound on day 35. Of the control group, 90 (57%) patients had CT pulmonary angiogram and 119 (75%) had ultrasound (figure 1).
Figure 1.
Trial profile
IMPROVE VTE=International Medical Prevention Registry on Venous Thromboembolism venous thromboembolism
Baseline characteristics were balanced between groups (table 1 ). The mean age was 57·1 years (SD 15·2), 127 (40%) were women, 191 (60%) were men, and the mean body-mass index was 29·7 kg/m2 (SD 5·6). At hospital discharge, 304 (97%) of 315 patients had a creatinine clearance of 50 mL/min or more, median hospitalisation time was 8 days (IQR 6–12), and 165 (52%) patients were in the ICU. 273 (86%) patients received enoxaparin 40 mg once per day as in-hospital thromboprophylaxis, 197 (62%) had an IMPROVE VTE score of 2–3 with increased D-dimer levels, and 214 (92%) of 233 had increased D-dimer levels (above the upper limit of normal of 500 ng/mL). 16 (5%) patients received antiplatelet therapy. 157 (99%) of 159 patients took the study medication up to day 35 without a discontinuation greater than 7 days (appendix p 10).
Table 1.
Baseline characteristics (intention-to-treat analysis)
| Rivaroxaban (n=159) | Control (n=159) | ||
|---|---|---|---|
| Age, years | 57·8 (14·8) | 56·4 (15·6) | |
| Age ≥75 years | 18 (11%) | 15 (9%) | |
| Sex | |||
| Female | 62 (39%) | 65 (41%) | |
| Male | 97 (61%) | 94 (59%) | |
| Body-mass index, kg/m2 | 29·6 (5·6) | 29·9 (6·0) | |
| Creatinine clearance | |||
| 30 to <50 mL/min | 6/158 (4%) | 5/157 (3%) | |
| ≥50 mL/min | 152/158 (96%) | 152/157 (97%) | |
| Duration of index hospitalisation, days | 8 (5·5; 12) | 8 (6; 12) | |
| ICU or CCU stay | 86 (54%) | 79 (50%) | |
| In-hospital enoxaparin 40 mg use | 136 (86%) | 137 (86%) | |
| In-hospital unfractionated heparin use | 23 (14%) | 22 (14%) | |
| IMPROVE VTE score | |||
| 2–3 | 98 (62%) | 99 (62%) | |
| ≥4 | 61 (38%) | 60 (38%) | |
| D-dimer level above ULN during index hospitalisation | 106/115 (92%) | 108/118 (92%) | |
| Antiplatelet use | 8 (5%) | 8 (5%) | |
Data are mean (SD), n (%), median (IQR), or n/N (%). CCU=cardiac care unit. ICU=intensive care unit. IMPROVE VTE=International Medical Prevention Registry on Venous Thromboembolism venous thromboembolism. ULN=upper limit of normal.
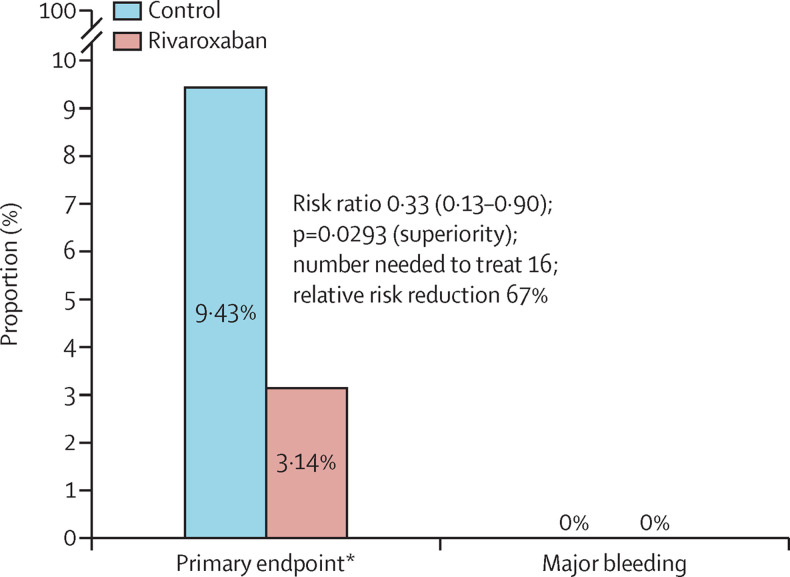
For the primary efficacy outcome at day 35, five (3·14%) of 159 patients allocated to the rivaroxaban group and 15 (9·43%) of 159 patients allocated to the control group had a primary efficacy outcome event (RR 0·33, 95% CI 0·13–0·90; p=0·0293) yielding a relative risk reduction of 67% (figure 2 ). The primary efficacy outcome was driven mainly by pulmonary embolism in the control group (table 2 ). Most asymptomatic pulmonary emboli were segmental or subsegmental, but there were proximal pulmonary emboli as well (n=3 deaths due to pulmonary embolism in the control group). There were no ISTH-defined major bleeding events in either group. Similar results were seen in the per-protocol and other sensitivity analysis (appendix pp 10–20).
Figure 2.
Primary efficacy and safety outcomes
The primary endpoint was a composite of symptomatic or fatal venous thromboembolism, asymptomatic venous thromboembolism detected by bilateral lower limb venous Doppler ultrasound and CT pulmonary angiogram, symptomatic arterial thromboembolism (myocardial infarction, non-haemorrhagic stroke, and major adverse limb event), and cardiovascular death at day 35.
Table 2.
Efficacy and safety outcomes (intention-to-treat analysis)
| Rivaroxaban (n=159) | Control (n=159) | Relative risk (95% CI) | p values (two-sided) | ||
|---|---|---|---|---|---|
| Primary efficacy outcome | 5/159 (3·14%) | 15/159 (9·43%) | 0·33 (0·13–0·90) | 0·0293 | |
| Secondary efficacy outcomes | |||||
| Symptomatic and fatal VTE | 1/159 (0·63%) | 8/159 (5·03%) | 0·13 (0·02–0·99) | 0·0487 | |
| Symptomatic VTE and all-cause mortality | 4/159 (2·52%) | 9/159 (5·66%) | 0·44 (0·14–1·41) | 0·1696 | |
| Composite of symptomatic VTE, myocardial infarction, stroke, and cardiovascular death | 1/159 (0·63%) | 9/159 (5·66%) | 0·11 (0·01–0·87) | 0·0360 | |
| Components of the primary outcome | |||||
| Symptomatic DVT | 0 | 3 (1·89%) | 0·14 (0·01–2·74) | 0·1968 | |
| Symptomatic pulmonary embolism | 1 (0·63%) | 2 (1·26%) | 0·50 (0·05–5·46) | 0·5698 | |
| Fatal pulmonary embolism | 0 | 3 (1·89%) | 0·14 (0·01–2·74) | 0·1968 | |
| Asymptomatic DVT on duplex scan | 3 (1·89%) | 1 (0·63%) | 3·00 (0·32–28·53) | 0·3391 | |
| Asymptomatic pulmonary embolism on CT pulmonary angiogram | 1 (0·63%) | 4 (2·52%) | 0·25 (0·03–2·21) | 0·2127 | |
| Symptomatic arterial thrombosis | 0 | 1 (0·63%) | 0·33 (0·01–8·12) | 0·5001 | |
| Myocardial infarction | 0 | 0 | NA | NA | |
| Non-haemorrhagic stroke | 0 | 0 | NA | NA | |
| Major adverse limb event | 0 | 0 | NA | NA | |
| Cardiovascular death | 0 | 1 (0·63%) | 0·33 (0·01–8·12) | 0·5001 | |
| Primary safety outcome | |||||
| Major bleeding | 0 | 0 | NA | NA | |
| Secondary safety outcomes | |||||
| CRNM | 2/159 (1·26%) | 2/159 (1·26%) | 1·00 (0·14–7·01) | 1·0000 | |
| Other bleeding | 2/159 (1·26%) | 1/159 (0·63%) | 2·00 (0·18–21·84) | 0·5698 | |
| Combination of major, CRNM, and other bleeding | 4/159 (2·51%) | 3/159 (1·89%) | 1·33 (0·30–5·86) | 0·7034 | |
Data are n/N (%), or n (%), unless otherwise specified. CRNM=clinically relevant non-major. DVT=deep vein thrombosis. NA=not applicable. VTE=venous thromboembolism.
For the prespecified secondary efficacy outcomes, symptomatic and fatal venous thromboembolism occurred in one (0·63%) of 159 patients in the rivaroxaban group compared with eight (5·03%) of 159 patients in the control group (RR 0·13, 95% CI 0·02–0·99; p=0·0487); symptomatic venous thromboembolism and all-cause mortality occurred in four (2·52%) of 159 patients in the rivaroxaban group and nine (5·66%) of 159 patients in the control group (RR 0·44, 95% CI 0·14–1·41; p=0·1696); and the composite of symptomatic venous thromboembolism, myocardial infarction, stroke, or cardiovascular death occurred in one (0·63%) of 159 patients in the rivaroxaban group and nine (5·66%) of 159 patients patients in the control group (RR 0·11, 95% CI 0·01–0·87; p=0·0360). Similar results were seen in the per-protocol and other sensitivity analysis (appendix pp 10–20).
For the secondary safety analysis, clinically relevant non-major bleeding occurred in two patients treated with rivaroxaban (one nose and one urinary bleed) and two in the control group. The prespecified combination of major, clinically relevant non-major, and other bleeding occurred in four (2·52%) of 159 patients receiving rivaroxaban and three (1·89%) of 159 patients allocated to no anticoagulation. The primary and secondary efficacy and safety outcomes are presented in table 2. Similar results were seen in the per protocol and other sensitivity analysis (appendix pp 11–20). Allergic reactions to the study medication occurred in two (1·3%) patients assigned to the rivaroxaban group.
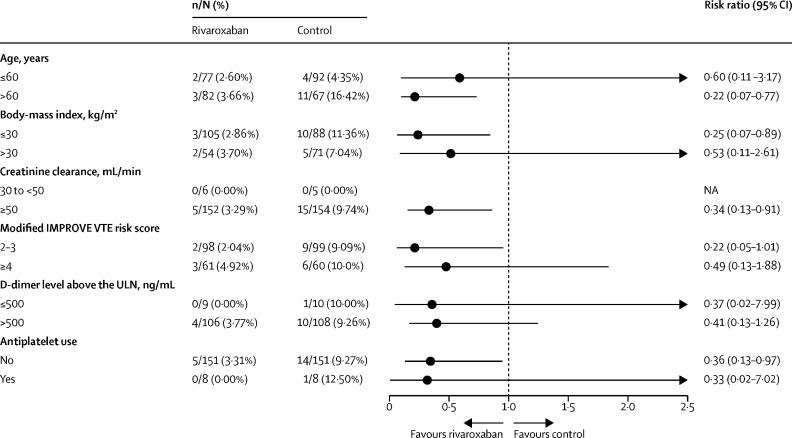
Results for the primary outcome were consistent across prespecified subgroups with no signs of heterogeneity. The subgroups that passed the interaction test were patients with advanced age, patients with obesity, patients with moderate renal failure (creatinine clearance <50 mL/min), patients with an elevated IMPROVE VTE score (≥4), patients with increased D-dimer, and patients under antiplatelet therapy (figure 3 ).
Figure 3.
Subgroup analysis
IMPROVE VTE=International Medical Prevention Registry on Venous Thromboembolism venous thromboembolism. ULN=upper limit of normal.
Discussion
In this open-label, multicentre, randomised, controlled trial of patients at high risk hospitalised with confirmed COVID-19, a treatment regimen of standard in-hospital parenteral thromboprophylaxis and extended post-discharge thromboprophylaxis with rivaroxaban 10 mg/day for 35 days (SD 4), when compared with no anticoagulation, resulted in better clinical outcomes, including a reduction in major and fatal thromboembolic events without increasing major bleeding, after standard in-hospital parenteral thromboprophylaxis. The results were consistent across all prespecified subgroups. These results provide high-quality evidence and will inform clinical practice guidelines about the role of extended thromboprophylaxis in hospitalised patients with COVID-19.
To our knowledge, the MICHELLE trial is the first randomised study in the field of extended post-discharge thromboprophylaxis for patients with COVID-19 that has shown clinical benefit. The difference was driven mainly by a lower incidence of pulmonary embolism (one symptomatic and one asymptomatic pulmonary embolism detected by CT pulmonary angiogram) in the treatment group compared with the control group (four asymptomatic, three symptomatic, and three fatal). Two of the fatal pulmonary emboli in the control group were confirmed by autopsy and the other one was considered a pulmonary embolism because the investigator reported to the clinical events classification committee that the patient died from acute right heart failure and his clinical suspicion was death due to pulmonary embolism. Following the prespecified criteria, the clinical events classification committee considered this death as due to pulmonary embolism where pulmonary embolism could not be ruled out, with high clinical suspicion.
In the active group, not only the absolute number of events was lower than in the control group, but the magnitude of events was also deceased (more asymptomatic events in the anticoagulant group than in the control group). In addition, distal deep-venous thrombosis was detected in the active group. The impact of distal venous thrombosis, however, is not to be minimised. As measured in an earlier clinical trial16 on extended prophylaxis for the medically ill, the effect of extended prophylaxis is prolonged past the treatment period. This legacy effect is proposed to be precisely a consequence of the asymptomatic distal thrombosis. As our population was treated, there is no certainty that these events would not to become clinically symptomatic later. In addition, both groups had the same imaging protocol and distal thromboses were equally likely to be detected.
The lowering of the risk of the primary outcome was consistent across predefined subgroups of patients hospitalised with COVID-19, suggesting consistent homogeneity in the study. Previous studies in patients hospitalised with COVID-19 have shown that both an elevated IMPROVE VTE score and an elevated D-dimer level, a relatively new biomarker for patients hospitalised with COVID-19, are able to predict an increased risk of thrombotic events in the post-discharge period.17, 18
In earlier trials involving medically ill patients that compared extended prophylaxis strategies with standard prophylaxis with low molecular weight heparin for 10 days (SD 4), extended prophylaxis significantly decreased venous thromboembolism rates, but at the cost of rates of major bleeding events at least twice that observed in the comparator group.19 We observed a higher number of thrombotic events in the MICHELLE trial, mainly driven by pulmonary embolism, as compared with previous observational studies.5, 7, 17 The reasons behind these findings include increased IMPROVE VTE scores at randomisation, long hospital length of stay (12·6–16·4 days), and the fact that around 165 (50%) patients were in the ICU. The mean hospital stay for the MARINER trial was 6·7 days, which was about two times shorter than observed in MICHELLE. Finally, the systematic use of imaging at day 35 to detect potential asymptomatic deep vein thrombosis and pulmonary embolism might have also played a role in our findings.
Bleeding events were low in the MICHELLE trial and consistent with the safety profile observed in the MARINER trial. This goal was achieved by excluding patients who had active cancer or gastrointestinal ulcer, bronchiectasis, bleeding in the previous 3 months, recent surgery, use of dual antiplatelet therapy, creatinine clearance below 30 mL/min or were receiving dual antiplatelet therapy.
Extended venous thromboembolism prophylaxis after hospitalisation for medically ill patients remains controversial. Despite the results of the MARINER trial that did not show an overall statistically significant difference between rivaroxaban and placebo in reducing venous thromboembolism-related death after 45 days post discharge, this extended thromboprophylaxis strategy reduced the rate of symptomatic venous thromboembolism by 56% with no increase in major bleeding. Given the highly prothrombotic nature of COVID-19, in addition to the substantial number of patients leaving the hospital with high IMPROVE VTE scores and D-dimer levels, it was reasonable to assess the role of a prolonged venous thromboembolism prophylaxis strategy with rivaroxaban in reducing thrombotic events without statistically significantly increasing major bleeding in patients hospitalised with COVID-19. In contrast to the MARINER trial, we opted for a shorter course of out-of-hospital thromboprophylaxis (35 days vs 45 days), which aligned with previous trials.16, 20 We decided to use a 35-day follow-up period following the MAGELLAN study, which was a positive trial from an efficacy endpoint perspective.20 Given that we included pulmonary angiogram CT for outcome evaluation and given the previous experience of losing follow-up exams after hospital discharge in venous thromboembolism prophylaxis trials in medically ill patients (the Doppler ultrasound loss in the ADOPT trial was 35%, as an example),21 the choice of 35 days seemed reasonable from both a clinical perspective as well as clinical trial design.
Other clinical studies are actively assessing extended thromboprophylaxis in patients with COVID-19. A study in Mexico enrolling 130 patients will evaluate prophylactic and full-dose heparin administered in-hospital followed by rivaroxaban 10 mg/day or no intervention (NCT04508439). The out-of-hospital phase will assess adverse events and biomarkers. Another study, the XACT trial (NCT04640181), is evaluating 150 patients randomised to in-hospital enoxaparin or oral rivaroxaban (10 mg/day, 15 mg/day, or 20 mg/day) through discharge for 28 days; the primary outcome is a combination of death or 30-day all-cause mortality, mechanical ventilation, intubation, or transfer to an ICU. A larger trial, ACTIV-4c (NCT04650087), plans to enrol 4000 patients in the USA to assess the effectiveness and safety of anticoagulants and antiplatelets (apixaban, aspirin, or placebo) administered to patients who have been discharged from the hospital. The primary objective is reducing myocardial infarction, stroke, arterial and venous thrombosis, and death within 30 days after hospital discharge for moderate and severe COVID-19. Finally, the HEAL-COVID (NCT04801940) trial plans to enrol patients at hospital discharge after a first admission due to COVID-19. Patients will be randomly assigned in a three-group trial to apixaban, atorvastatin, or standard of care. The primary efficacy outcome is hospital-free survival over 12 months.
The MICHELLE trial is part of a comprehensive programme of anticoagulation strategies for Brazil's COVID-19 pandemic. Four other randomised clinical trials are evaluating the role of antithrombotic strategies at different times around COVID-19. The COALIZAO-ACTION trial4 showed that in patients hospitalised with COVID-19 and elevated D-dimer concentrations, in-hospital therapeutic anticoagulation with rivaroxaban 20 mg once per day followed by rivaroxaban 20 mg once per day for up to 30 days did not improve in-hospital clinical outcomes and increased bleeding compared with prophylactic anticoagulation. The trial results suggest that the rivaroxaban 20 mg once-per-day dose should be avoided as a routine anticoagulation strategy for in-hospital thromboprophylaxis in patients hospitalised with COVID-19.4 The MICHELLE trial, on the other hand, demonstrated that a lower dose of rivaroxaban at the time of hospital discharge and extended for 35 days in the right patient population improves clinical outcomes, highlighting the importance finding the most favorable antithrombotic regimen for these patients. The CARE trial (NCT0475785) is enrolling 1000 COVID-19 outpatients with moderate symptoms to either rivaroxaban 10 mg versus placebo to reduce the need for hospitalisation and mortality. The APOLLO trial (NCT04746339) is evaluating apixaban 2·5 mg twice per day versus placebo in the out-of-hospital setting to reduce mortality. All four trials together will provide high-quality evidence and inform practice around the management of patients with COVID-19 in the pre-hospital, in-hospital, and post-discharge settings and should help physicians in the decision-making process for anticoagulation in patients with COVID-19.
The MICHELLE trial has strengths and limitations. The open-label design has a potential risk of bias, especially concerning clinical event ascertainment. We projected the trial based on the statistically significant 56% relative risk reduction observed in MARINER for symptomatic venous thromboembolism and have used, for the first time, a traditional antithrombotic clinical trial design, CT pulmonary angiograms to count clinical events.
Such a strategy seems to have been appropriate given that the superiority achieved for the primary efficacy outcome was driven mainly by pulmonary embolism. We also projected the trial to detect asymptomatic proximal venous thromboembolism events because of the correlation to increased mortality in hospitalised medically ill patients;15 however, few events were asymptomatic deep vein thrombosis. A potential limitation was the lack of a placebo, which would have made the trial not feasible. Not every patient received CT pulmonary angiogram (45 [28%] in the active group and 69 [43%] in the control group) or Doppler ultrasound (25 [16%] in the active group and 41 [26%] in the control group). These rates are consistent with previous studies that have also reported the loss of image evaluation (Doppler ultrasound) in this patient population.20, 21 Reasons for lack of imaging included fear of returning to the hospital in the middle of the pandemic, increased creatinine levels, and reported allergy to media contrast. Notably, a higher number of imaging evaluations occurred in those patients receiving anticoagulation, decreasing the risk of reporting bias and making the results of MICHELLE even more conservative. The primary efficacy outcome of the MICHELLE trial was driven by pulmonary embolism and we could not differentiate pulmonary embolism or immunomediated primary pulmonary arterial thrombosis. Finally, the MICHELLE trial had a small sample size of 320 patients. Future trials with larger study populations (such as ACTIV-4c) are warranted to confirm our findings.
In conclusion, in patients at high risk discharged after hospitalisation due to COVID-19, evidence suggests that thromboprophylaxis with rivaroxaban 10 mg/day through 35 days improved clinical outcomes, reducing thrombotic events, compared with no post-discharge anticoagulation. The use of extended prophylactic-dose rivaroxaban should be considered at hospital discharge as an attractive strategy to improve clinical outcomes in patients with creatinine clearance of more than 30 mL/min who were hospitalised with COVID-19 and an IMPROVE VTE score 2–3 plus increased D-dimer levels or an IMPROVE VTE score of 4 or more.
Data sharing
Anonymised participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Declaration of interests
ER reports grants and consulting fees from Bayer and Pfizer; grants from the Brazilian Ministry of Science and Technology; and personal fees from Aspen Pharma, Biomm Pharma, and Daiichi Sankyo, outside of the submitted work. LBA reports grants from Bayer, Pfizer, and the Brazilian Ministry of Science and Technology. DC reports personal fees from Bayer, Janssen, Daiichi Sankyo, and Pfizer; and grants from Stago. ACS reports consulting fees from Janssen Research & Development, Bayer, Portola, Boehringer Ingelheim, Bristol Myers Squibb, and ATLAS group; and grants from Janssen and Boehringer Ingelheim. MLS reports personal fees from Bayer, Pfizer, and Sanofi. EEJ reports consulting and personal fees form Bayer. CD reports consulting and personal fees from Bayer, Novartis, and Daiichi Sankyo. SMVS reports personal fees from Bayer. RCC reports personal fees from Boehringer Ingelheim and AstraZeneca. ATaf reports personal fees from Janssen and Recovery Force and grants from Bio Tap, Idorsia, Bristol Myers Squibb, Novo Nordisk, Janssen, and Doasense. RDL reports grants and personal fees from Bristol Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This trial was an investigator-initiated study with financial support from Science Valley Research Institute Brazil and Bayer, who provided the study drug and partial financial support. We are grateful to all staff, particularly the data safety monitoring board, clinical events committee, and core laboratory members for their thorough work, to all site personnel who helped to enrol participants, and to all health-care professionals who took care of our patients during this study protocol. Finally, we thank all of the participants, without whom this research would not have been possible.
Contributors
ER, LBA, and RDL conceived of the trial and wrote the initial proposal. All other authors contributed intellectually to the protocol development. CC estimated the sample size and drafted the statistical analysis. ER, LBA, DC, VCRA, CCCdO, GGV, MLS, MSBJ, EEJ, BALdF, MSR, CD, KI, SMVS, KdAAR, NFdM, PFGMMT, ALMLdO, BBdMC, and RCRM actively enrolled patients for the trial. RCC and MVBS performed the imaging exams. ATac and RCC did the laboratory work. CC performed all statistical analysis. The initial draft of the Article was written by ER, DC, and RDL, who had full access to and verified all the data underlying the study. All authors had access to the data, significantly contributed to the Article, agreed to submit for publication, and vouch for the integrity, accuracy, and completeness of the data and for the fidelity of the trial to the protocol. ACS, ATaf, and JF were active members of the steering committee and contributed intellectually to the protocol development and data analysis. JLdS was the lead for the logistics and data collection for the trial.
Contributor Information
MICHELLE investigators:
Tania Benevenuto Caltabiano, Breno Hattori, Marcello da Silva Jardim, Igor Marinho, Ivan Silva Marinho, Liane Mara Melo Batista, Lucas Rivabem, Carlos Alberto Kenji Nakashima, Ana Carla Gois Franco, Renata Fernanda de Oliveira Pereira, Giana Caroline Strack Neves, Izara de Castro e Souza, Bruno Moraes Ribas, Flavia Ramos Tristão, and Marcus Vinicius Barbosa Santos
Supplementary Material
References
- 1.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes RD, de Barros E, Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannis D, Allen SL, Davidson A, et al. Thromboembolic outcomes of hospitalized COVID-19 patients in the 90-day post-discharge period: early data from the Northwell CORE-19 Registry. Blood. 2020;136:33–34. [Google Scholar]
- 7.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spyropoulos AC, Ageno W, Albers GW, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75:3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 10.Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost. 2020;120:1597–1628. doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tritschler T, Mathieu ME, Skeith L, et al. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020;18:2958–2967. doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramacciotti E, Agati LB, Calderaro D, et al. Medically ill hospitalized patients for COVID-19 thrombosis extended prophylaxis with rivaroxaban therapy: rationale and design of the MICHELLE trial. Am Heart J. 2021;242:115–122. doi: 10.1016/j.ahj.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 15.Raskob GE, Spyropoulos AC, Cohen AT, et al. Association between asymptomatic proximal deep vein thrombosis and mortality in acutely ill medical patients. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]
- 17.Giannis D, Allen SL, Tsang J, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SL, Gianos E, Barish MA, et al. Prevalence and predictors of venous thromboembolism or mortality in hospitalized COVID-19 patients. Thromb Haemost. 2021;121:1043–1053. doi: 10.1055/a-1366-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 21.Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.