Abstract
Background
Despite nationwide efforts to address the diabetes epidemic and reduce prevalence disparities, higher rates persist among the poor, especially those with limited literacy. Currently, individuals with abnormal glycemia who have pre-diabetes and diabetes qualify for different programs. However, evidence suggests that, for low-income Hispanic/Latinos, offering a single intervention to all those with abnormal glycemia may provide a more culturally acceptable and effective approach. Our objective was to explore the feasibility of such an intervention led by community health workers (CHWs) among low-income Hispanic/Latinos with diabetes and at risk for diabetes.
Methods
Using a quasi-experimental mixed method design, we assessed weight, glycosylated hemoglobin, diabetes knowledge, and behavior changes of Hispanic/Latinos participants with pre-diabetes and diabetes living in Southern California. Biometric measurements, blood tests, and surveys were collected at baseline and 3 months post-intervention. Interviews and focus group discussions provided qualitative data.
Results
Although the program was less costly, results exceeded those reported for low-income H/L attending the National Diabetes Prevention Program and did not differ between pre-diabetes and diabetes groups. Instead, including individuals at different stages of the dysglycemic spectrum seemed to have enhanced the intervention. Physician referral and attendance of family/friends were associated with better outcomes.
Conclusion
Our findings indicate that a joint prevention/self-management intervention led by CHWs for low-income Hispanic/Latinos with diabetes and with pre-diabetes is feasible and cost-effective, providing results that could help reduce the success gap. Incorporating suggestions and replicating this study on a larger scale could help determine whether or not results are reproducible.
Keywords: mixed method design, prediabetic state, diabetes mellitus type 2, self-management, hyperglycemia, physicians, hispanic Americans, community health workers, literacy, feasibility studies
What do We Already Know About This Topic?
The currently available and widely promoted National Diabetes Prevention program and Diabetes Self-management Education and Support interventions, which accept individuals based on their diagnosis (pre-diabetes or diabetes, respectively), are less successful among low-income Latinos, and therefore may be contributing to an increase in the diabetes disparity gap in this population where basic survival and family support are paramount.
How Does Your Research Contribute to the Field?
It provides evidence that a diabetes prevention and self-management intervention led by CHWs which promotes the same lifestyle changes among all family members at various levels of the dysglycemic spectrum is feasible and could provide better results and reduce diabetes disparities in low-income underserved Latinos communities.
What Are Your Research’s Implications Towards Theory, Practice, or Policy?
Policies that support offering the same community health worker-led lifestyle intervention to all family members with abnormal glycemic levels, while addressing policy-driven social determinants of health (access to walk-able neighborhoods, affordable healthy food, and transportation), may result in a better return on investment among low-income Latinos communities since they have the potential of reducing the diabetes burden among those most affected by this condition.
The poor experience a disproportionate burden of diseases worldwide. 1 In the USA, where chronic diseases such as diabetes are inversely correlated with poverty and limited literacy,2,3 Hispanics/Latinos (H/L) earn less and have less formal education on average than non-Hispanic Whites (nHWs). 4 Not only are they more likely to develop diabetes they also experience worse complications.5-7
To reduce the health burden associated with type 2 diabetes,8,9 the Centers for Disease Control and Prevention 10 promote two different programs: one for individuals with pre-diabetes, and a separate one for those with diabetes. 11
The Diabetes Self-Management Education and Support (DSMES) program, for those with diabetes, consists of six to ten weekly meetings lasting two hours or less and is typically offered in clinical settings. Participants’ goals are customized. As for the National Diabetes Prevention Program (NDPP), a program offered to individuals with pre-diabetes, it is delivered in 12 months and usually held in community settings. 12 The latter is an adaptation of the Diabetes Prevention Program, a landmark intervention where 15.7% of the participants were H/L, mostly of high socio-economic status and literacy level.13,14 NDPP participants attend 16 one-hour-long weekly classes followed by monthly meetings and aim to lose 5% of their initial weight. Taught by diabetes educators or healthcare professionals, both programs promote healthy eating, exercise, self-monitoring, stress management, and problem-solving skills. Additionally, the DSMES promotes diabetes self-management (glucose monitoring, foot checking, regular physician visits, and proper medication use).
Evidence suggests that participants’ socio-economic status determines how much they will benefit from health promotion interventions.15,16 Some authors have even concluded that interventions focusing on individual behaviors increase inequality gaps proposing comprehensive and population-based policy interventions instead. 17
Both the NDPP and the DSMES programs, while evidence-based and reportedly successful overall, seem to have increased the inequality gap. 18 Not only do low-income H/L participants report challenges across both programs, 19 when compared to nHWs, they are less likely to enroll, 20 more likely to drop out if they enroll, and less likely to succeed despite accommodations (eg, eliminating cost and culturally adapting the material).21,22
A decade after the national launch of the NDPP, another challenge that threatens to further increase the diabetes health disparity gap 23 is selective lack of program funding: reimbursement to entities offering NDPP programs depends upon participants reaching the 5% weight loss threshold, a threshold rarely reached by low-income H/L programs. In our current crisis of ever-increasing diabetes and COVID-19 health disparities, this vulnerable population is in critical need of effective sustainable interventions.
It is generally accepted that social support improves adoption and retention of healthy behaviors regardless of cultural background, and there is clear evidence of the critical nature of family support for lifestyle changes in both diabetes prevention and self-management programs.24,25 In patients with diabetes, adopting a healthier lifestyle is a greater challenge than adhering to a medication regimen, and the impact of social support on specific behaviors varies.26,27 This is even more critical in collectivistic cultures such as the H/L culture, 28 with family members’ support, or lack of it, often cited as an important source of motivation or a barrier to making lifestyle changes.29-31
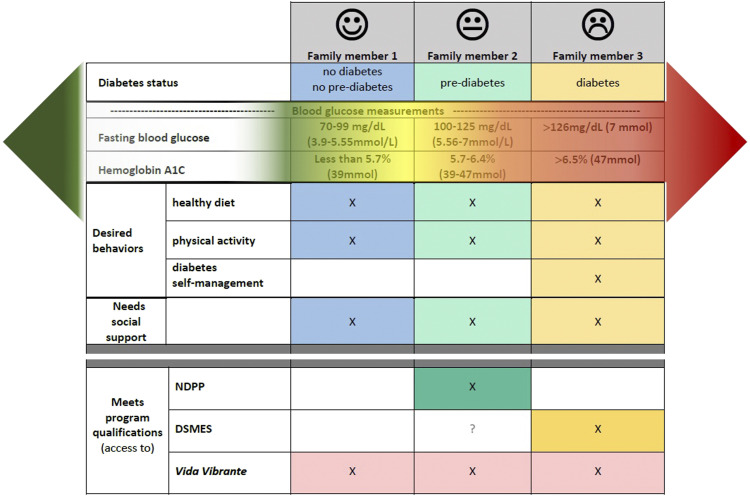
Lifestyle changes benefit individuals at all levels of the glycemic spectrum (blood glucose range from normal to diabetes levels) and family members of individuals with diabetes (those in either of the above-mentioned programs and those who do not qualify for either) often struggle with similar lifestyle-related risk factors and barriers. Thus, regardless of their location on the glycemic spectrum all could benefit from an intervention that facilitates lifestyle changes. A “family inclusive” approach is supported by the American Diabetes Association 32 but the reality is that individuals with diabetes and pre-diabetes rarely qualify to enroll in the same program: for example, the NDPP program only accepts those with pre-diabetes 1 and, although some DSMES programs accept individuals with pre-diabetes, not all do. 33 Therefore, the following could happen to an average family (as illustrated in Figure 1)
- individuals with diabetes would attend the six- to eight-week diabetes self-management program,
- family members with pre-diabetes would enroll in the NDPP and attend for one year,
- Others—who may well be at risk of diabetes since having a first-degree relative (sibling or parent) with diabetes is a known predictor of diabetes development—would not qualify at all for either of these programs. 34
Figure 1.
Potential scenario in a family with a family member diagnosed with diabetes.
Even if they all qualified for either of these programs, differences in sessions content and timing could exacerbate transportation and coordination challenges within the family unit.
It would therefore seem desirable and cost-effective to have one program delivered to all family members so they could benefit from mutual support for lifestyle changes. The concept of a “joint” program has been suggested and considered in the past, but concerns have been raised: fear of barriers between physicians and family members of patients with diabetes, the cost of involving family members in programs, and the challenge of keeping participants with pre-diabetes engaged.35-37 However, in view of recent healthcare system trends, there is reason to believe that these obstacles may be surmountable, if still present.
Physicians are using a more patient-centered approach, which is believed to enhance patient/caretaker-physician communication. 38 Community health workers (CHWs) are increasingly given important roles in both diabetes prevention and self-management interventions. They are being integrated at several levels of clinical care, 39 reducing costs, and improving results, especially among low-income populations. 40 Because CHWs capture low-income participants’ perspectives and barriers and specialize in addressing social determinants of health, they may be more effective at helping them surmount challenges. Furthermore, their ability—often through the use of a popular education approach—to keep individuals engaged in programs that would otherwise experience attrition, makes them a perfect fit for interventions focused on underserved populations.
In reviewing the literature, while there are multiple diabetes prevention or self-management interventions led by CHWs among low-income Latinos, the authors found a paucity of programs available and accessible to both H/L at high risk of diabetes and those with diabetes. 41 The few interventions that allowed family members of individuals with diabetes to join showed promising results but did not report weight changes nor diabetes status of family members.42,43 Thus, little is known about the feasibility and potential impact of a joint diabetes prevention program/intervention (ie, intervention for individuals at various levels of the dysglycemic spectrum) for low-income Hispanics/Latinos.
The purpose of this study was to explore the feasibility of implementing a CHW-led culturally sensitive lifestyle intervention for low-income H/L with diabetes (DM group) and H/L at risk of diabetes (NDM group). Besides weight and glycosylated hemoglobin (A1C), participants’ engagement, program cost, and impact of physician-patient/family interaction were assessed. In addition, participants were encouraged to share perceived barriers to attendance and behavior modification.
Methods
Setting and sampling procedures: This pilot mixed-method study was conducted among low-income H/L residing in the Inland Empire Region of Southern California, an area with one of the largest H/L populations in the US, many of whom lack health insurance coverage. It also has one of the highest rates of diabetes-related mortality in California.44,45
Program recruitment: Participants were contacted via flyers posted at community centers, through personal invitations from CHWs and at a clinic. Patients at risk of diabetes or with diabetes were encouraged by a culturally homologous Hispanic/Latino physician to enroll by adding their names to a sign-up sheet.
Program inclusion/exclusion criteria: For quantitative analyses, the inclusion criterion was “having attended at least 80% of the Vida Vibrante (Vibrante Life) Diabetes Prevention and Self-Management Intervention (VV).” Participants were excluded if they were unable/unwilling to answer the surveys.
Eligibility criteria for the qualitative portion included (1) having attended VV—to qualify for focus group discussions (FGDs); or (2) being a CHW who taught VV—to qualify for a key informant interview (KII). Focus group participants (N=33) were recruited from all cohorts immediately following program completion. CHWs (n=3) completed KIIs for triangulation reasons within 10 days of course completion.
Vida Vibrante program description: The program content is a cultural adaptation of the Group Lifestyle Balance (GLB) curriculum (accessible online). 46 The core content of the GLB was consolidated to limit participant burden and program cost with the main content of the classes consisting of basic information about healthy eating, moderate physical activity, and diabetes. Key concepts were associated with Latino sayings and pictures adapted from “loteria” cards (used by permission of the New Mexico Department of Health Diabetes Control Program) 47 to facilitate understanding by a wider range of literacy levels.
For 12 weeks, participants met weekly in groups taught by CHWs with the goal of weighing 5% less by the time they reached 12 months. However, participants were not required to keep track of their caloric intake, weight, or physical activity. Instead, CHWs recorded their weight at each session. Participants were asked to continue with their pharmaceutical treatments while attending the program. (See Table 1 for more program details).
Table 1.
Description of the Vida Vibrante Prevention and Self-Management Intervention.
| Category | Description |
|---|---|
| Program development | Community-academic partnership: Loma Linda University School of Public Health and El Sol Neighborhood Educational Center, a non-for-profit organization based in Southern California |
| Content development | The Group Lifestyle Balance (GLB) curriculum (accessible online) was adapted and shortened to 12 sessions. Throughout the development of the curriculum and intervention community health workers (CHWs) met with university faculty and students and provided feedback on content and formatting |
| Community health worker training | A minimum of 200 hours of basic community health worker training AND 60 hours of additional training specifically in preparation for teaching the Vida Vibrante Diabetes Prevention and Self-Management Intervention. |
| Overview of community health worker training | Training instructors modeled the desired teaching behavior and content that the CHWs were expected to emulate or share, and then required the CHWs to teach back portions of the program to fellow training participants before implementing it in the community |
| Program cost | Cost per participant <$200 (material, laboratory tests, and CHW salaries) paying full price for materials Expenses were incurred by the program leadership and the intervention was free to participants |
| Instructional material | • Handouts with minimal writing and with emphasis on key messages in the context of cultural values • Real food samples • Power point presentation slides • Dry erase board (optional) |
| Participants’ inclusion criteria | Overweight or obese (body mass index ≥ 25 kg/m2) AND present with pre-diabetes—fasting blood glucose between 100 and 125 mg/dL (5.55 and 6.94 mmol/L), or glycosylated hemoglobin (A1C) between 5.7% and 6.4% (39 and 46 mmol/mol) OR with diabetes—fasting blood glucose above 125 mg/dl (6.94 mmol/L) or A1C above 6.4% (46 mmol/mol) OR have a family history of diabetes. |
| Participants’ exclusion criteria | • Pregnant or breastfeeding • Random blood glucose level above 300 mg/dl (16.65 mmol/L) • Unable to perform physical activity due to a medical condition • Unable to read/speak Spanish or English |
| Sessions setting/format | • Teams of two CHWs teaching sessions together • Maximum group limit: 20 • Duration and frequency: Two-hours per week for 12 weeks |
| CHW tasks | • Prepare and teach sessions • Call participants weekly encouraging them to join the next session and to connect with one another for emotional support • Provide guidance and resources and a listening ear to participants struggling with barriers • Collect difficult and challenging questions and send these to physician assigned to the program • Promote the creation of small support groups that can be self-sufficient after course completion |
| Overview of each session | • Community health workers weigh participants • Time for debunking diabetes-related myths • Discussion of two or three main concepts adapted from “Loteria cards” using a participatory approach and popular education techniques • Opportunities for role-play and interactive games related to food and diabetes concepts |
| Weekly topics | •
Session 0 (Invitation to Vida Vibrante).
Introducing the program (this session is optional). • Session 1. Welcome to Vida Vibrante. How to begin a weight loss plan and monitor physical activity. Reviewing your A1C, weight and blood pressure. • Session 2. What is diabetes? The process (biology) of diabetes. Factors that contribute to diabetes. Diabetes complications. • Session 3. C.A.M.B.I.O (C.H.A.N.G.E.). Conozca su condición, Acepte su problema, fijese Metas, Buscar el reto, Iniciar la acción y afrontar los Obstáculos ( Translation: Know your condition, Accept your problem, Establish goals, Look for the challenge, Start and confront the obstacles). • Session 4. General nutrition. Portion-control, calories, reading labels, and planning healthy (low-fat, low calories) meals. • Session 5. Nutrition to control diabetes. What we eat and how it relates to diabetes, healthy alternatives, special circumstances. • Session 6. Physical activity. Benefits of physical activity, physical activity goals (150 minutes per week). Precautions. • Session 7. Diabetes self-management. Personal care, how to avoid diabetes complications, how to check your blood sugar, what to do in case of hypoglycemia, hyperglycemia and diabetic coma; pills and insulin injections. • Session 8. Stress management. How to avoid and manage stress, how to handle and create a plan to overcome specific stressful circumstances. • Session 9. Lifestyle habits and diabetes. Heart health and diabetes, consequences of smoking tobacco and drinking alcohol. • Session 10. Connecting with your health care team. Medical services in your community, regular check-ups to avoid diabetes, treatments for problems caused by diabetes. • Session 11. Reflection time. Applying learned concepts. • Session 12. Culmination of Vida Vibrante. Review. |
Study procedures, measures, and data collection: The quantitative portion of the study used a non-equivalent comparison group design with dependent pre-test and three months’ post-test samples. CHWs recorded participants’ height and weight, and participants completed self-report surveys at baseline and immediately following the intervention. Average blood glucose level over the past 3 months (A1C) was determined from a finger prick. Questionnaires, which included basic demographic information, diabetes status, recruitment type (clinic or community), diabetes knowledge questions, and whether or not family or friends attended, were available in English and Spanish.
A $10 gift certificate to a local grocery store was given as an incentive for program evaluation/surveys. Table 2 describes each variable in detail.
Table 2.
Variables used to assess average blood glucose, biometric measurements and self-reported data.
| Variable Name | Level of Measurement | Description | Unit/Answer Choices | |
|---|---|---|---|---|
| A1C (glycosylated hemoglobin) | Interval/ratio | Average blood glucose as measured through blood samples collected from a finger prick using Alere Afinion AS100 Analyzer and Alere Afinion HbA1c (Axis-Shield, Oslo, Norway) assay tests. | Percentage (%) | |
| Weight | Interval/ratio | Measured with participant standing without shoes or heavy clothing using Seca 700 mechanical beam scale and stadiometer (Itin Scale Co., Inc., Brooklyn, NY) scale, with the scale measuring up to 10th of a kg. | Pounds (lbs) | |
| Percentage of weight change | Interval/ratio | Calculated based on the following formula: (Weight at baseline minus weight immediately after intervention) /baseline weight. | Percentage (%) | |
| Height | Interval/ratio | Measured with participant standing straight and tall and without shoes using Seca 700 mechanical beam scale and stadiometer (Itin Scale Co., Inc., Brooklyn, NY). | Inches | |
| Body mass index (BMI) | Interval/ratio | Calculated based on the following formula: 703xweight in lbs /(height in inches). 2 | kg/m2 | |
| Body mass index (BMI) categories | Ordinal | Participants were categorized as being overweight if BMI was between 25kg/m2 and 29.9 kg/m2 and obese if BMI was 30 kg/m2 or more. | Overweight, obese | |
| Diabetes status | Dichotomous | Self-reported response to the following question: “Do you have diabetes?” | Yes/no | |
| Marital status | Nominal | Self-reported response to the following question: “Which of the following is your current marital status?” | Single, married, living with a partner, divorced/separated or widow(er) | |
| Education level | Ordinal | Self-reported response to the following question: “What was the last grade that you completed at school?” | no formal schooling, elementary (kindergarten through 8th grade), some high school but no diploma (secondary school), high school diploma, some college or vocational school, college diploma and graduate school (Master’s or Doctorate degree)* | |
| Family members and friends’ attendance | Dichotomous | Self-reported response to the following question: “Did you attend the Vida Vibrante course with a friend or relative/family member?” | Yes/no | |
| Language | Nominal | Self-reported response to the following question: “What language do you speak at home?” | English, Spanish, English and Spanish equally, other language | |
| Diabetes knowledge | Interval/ratio | Whether or not the response to the following 5 questions was correct: 1. The best way to check whether or not you have diabetes or high sugar levels is to have a urine test (answer: No). 2. To control diabetes it’s more important to take medications than to exercise and eat healthy (answer: No). 3. Diabetes can cause someone to lose the feeling in one’s hands and feet (answer: Yes). 4. Having uncontrolled diabetes can lead to blindness (answer: Yes). 5. Diabetes can lead to a person losing his/her foot or leg (answer: Yes). Correct answers were assigned a value of 2 and were summed for a knowledge score. Wrong answers or no answers were given a value of 0. Maximum points: 10 |
Yes/No | |
| Recruitment type | Dichotomous | Noted by community health workers and based on whether or not the person registered through a physician sign-up sheet. | Clinician-referred or community-recruited |
*Note: for educational level data analyses “some high school but no diploma” and “high school diploma” were merged, as well as “some College” and “College diploma”.
For the qualitative data collection, nine FGDs, each consisting of three to seven program participants, and three KIIs (with CHWs who taught), all lasting between 15 and 55 minutes, were conducted in Spanish by three trained bilingual interviewers (researchers) at two community centers upon program completion. Non-completers were also invited to provide feedback but did not. The semi-structured interview guides for the KIIs and FGDs were created based on Charmaz’s (2006) grounded theory approach 48 using a social determinants of health perspective. Questions encouraged participants to share perceived barriers to healthy behaviors and attendance. Participants were also asked to share changes they experienced since program enrollment, perceived impact of fellow attendees with a different diagnosis on their attendance and success, and about interaction with their physician (or the physician of a family member with diabetes). Probes were used to expand the exploration and allow new issues to be identified.
Data analyses: Quantitative data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 22. The primary outcome was change in weight. Chi-square tests were used to assess baseline differences between gender, diabetes status, recruitment setting (physician-referred or community-recruited), and family and friends’ (FF) attendance. An independent t (or Mann–Whitney) test was performed to evaluate weight, A1C and knowledge differences between groups, and sample paired t (or Wilcoxon) tests assessed pre- and post-changes. Data were inspected for outliers and inconsistencies before analyses.
FGDs and KIIs were audio-taped with the participants’ permission and transcribed verbatim by bilingual individuals. The transcripts were then analyzed for emergent themes supported by critical quotes using the 2017 computer software program MaxQDA® (v.12, Berlin, GDR) 49 to code the transcripts, using an a priori code book but allowing for new codes to emerge. A team of three research assistants developed the codebook which was later expanded as emergent themes were identified and organized. Analyses were conducted in Spanish, and relevant quotes were translated prior to inclusion in this manuscript.
Results
Table 3 displays participants’ baseline characteristics. Average participant age was 51.57 (±9.62) and most participants were females on limited income, spoke Spanish at home and lived with a spouse/partner. Only two participants had attended school beyond high school: 41% had either no formal education or only elementary school education, while 52% had at least partially attended high school. Slightly over half were overweight and the remainder were obese while 18 (55%) were at risk for diabetes and 15 (45%) had diabetes. When comparing the NDM and DM groups, there were no baseline differences except an expected difference in glycosylated hemoglobin. Table 4 displays intervention effects.
Table 3.
Participants’ baseline characteristics.
| Overall | No Diabetes | Diabetes | p | ||||
|---|---|---|---|---|---|---|---|
| n | %/mean (±SD) | N | %/mean (±SD) | n | %/mean (±SD) | ||
| Gender (%) | 33 | 18 | 15 | .85 | |||
| Male | 4 | 12.1 | 2 | 11.1 | 2 | 13.3 | |
| Female | 29 | 87.9 | 16 | 88.9 | 13 | 86.7 | |
| Age (yrs) | 30 | 51.57 (±9.62) | 17 | 50.53 (±9.04) | 13 | 52.92 (±10.55) | .51 |
| Marital status | 32 | 17 | 15 | .42 | |||
| Single | 1 | 3.1 | 0 | 0 | 1 | 6.7 | |
| Married/living with partner | 28 | 87.5 | 16 | 94.1 | 12 | 60 | |
| Separated or divorced | 2 | 6.1 | 0 | 0 | 2 | 20 | |
| Widow | 1 | 3.1 | 1 | 5.9 | 0 | 13.3 | |
| Education | 29 | 17 | 12 | .60 | |||
| No formal education | 1 | 3.4 | 0 | 0 | 1 | 8.3 | |
| Elementary | 11 | 38.0 | 4 | 23.5 | 7 | 58.3 | |
| High school | 15 | 51.7 | 13 | 76.5 | 2 | 16.7 | |
| College | 2 | 6.9 | 0 | 0 | 2 | 16.7 | |
|
A1C (%)
A1C (%) a |
27
12 |
6.15 (±1.51) 7.20(±1.80) |
14 2 |
5.42 (±.22) 5.85 (±.21) |
13 10 |
6.94 (±1.90) 7.47(±1.86) |
.01* .26 |
| Weight (lbs) | 33 | 173.48 (±34.19) | 8 | 174.39 (±32.24) | 5 | 172.4 (±37.52) | .87 |
| BMI b categories | 33 | 18 | 15 | .90 | |||
| Overweight | 18 | 54.5 | 10 | 55.6 | 8 | 53.3 | |
| Obese | 15 | 45.5 | 8 | 44.4 | 7 | 46.7 | |
| Language spoken home | 31 | 17 | 14 | .84 | |||
| Spanish | 27 | 15 | 88.2 | 12 | 85.7 | ||
| Both (English and Spanish) | 4 | 2 | 11.8 | 2 | 14.3 | ||
| Diabetes knowledge | 33 | 7.67 (±1.29) | 18 | 7.72 (±1.27) | 15 | 7.60 (±1.35) | .79 |
| MD c referral | 33 | 18 | 15 | .07 | |||
| No | 25 | 75.8 | 16 | 88.9 | 9 | 60 | |
| Yes | 8 | 24.2 | 2 | 11.1 | 6 | 40 | |
| Family and friends attend | 24 | 13 | 11 | .65 | |||
| No | 10 | 41.7 | 6 | 46.2 | 4 | 36.4 | |
| Yes | 14 | 58.3 | 7 | 53.8 | 7 | 63.6 | |
*Statistically significant differences between groups at baseline, p<.05.
a. Sensitivity analyses (only those with baseline A1C³5.7%).
b. BMI = Body mass index.
c. MD = Physician.
Table 4.
Between-and within-group differences at baseline and at 3-months follow-up.
| Measure | Baseline mean (SD) |
3 months mean (SD) |
Independent samples t test (between subjects)a | Cohen’s d | Paired samples t test (within subjects) | Cohen’s db |
|---|---|---|---|---|---|---|
| Changes in overall sample | ||||||
| Weight (lbs)** | 173.48(34.19) | 170.20(32.68) | ______ | _____ | .<001** | .77 |
| Knowledge score* | 7.67(1.29) | 8.36(.82) | ______ | _____ | .01* | .74 |
| Wtc by diabetes status | ||||||
| No diabetes (n=18)** | 174.39(32.24) | 170.75 (29.31) | .92 | .04 | .<001** | .76 |
| Diabetes (n=15)** | 172.4 (37.51) | 169.53(37.38) | .<001* | .78 | ||
| Knowledge by diabetes status | ||||||
| No diabetes (n=18) | 7.72 (1.27) | 8.39 (.85) | .79 | .08 | .44 | |
| Diabetes (n=15) | 7.80 (1.35) | 8.33 (.82) | .08 | .49 | ||
| Wt c by FF d attendance (all) | ||||||
| Yes FFd (n=14)** | 167.1(33.52) | 163.32(33.07) | .47 | .03 | .001** | 1.22 |
| No FFd (n=10) | 177.1 (40.44) | 173.80 (36.67) | .16 | .48 | ||
| Wt c by FF d attendance (only No-diabetes group) † | ||||||
| Yes FFd (n=7)** | 159.43 (22.27) | 154.5 (20.93) | .06† | 1.43 | .009** | 1.44 |
| No FFd (n=6) | 191 (43.88) | 188 (36.50) | .37 | .40 | ||
| Wtc by FFd attendance (only Diabetes group) | ||||||
| Yes FFd(n=7)* | 174.71(42.46) | 172.14(41.83) | .43 | .55 | .03* | 1.08 |
| No FFd (n=4) | 156.25 (27.02) | 152.50 (28.38) | .35 | .55 | ||
| Wtc by recruitment type (all) | ||||||
| MDRe(n=8)* | 170.25(39.05) | 165.06(39.53) | .62 | .19 | .02* | .66 |
| CRf (n=25)** | 174.52(33.31) | 171.84(30.93) | .003** | 1.14 | ||
| Wtc by recruitment type (only 2No-diabetes group) | ||||||
| MDRe (n=2)† | 176(33.94) | 168.25(32.88) | .94 | .05 | .06† | 7.31 |
| CRf (n=16)** | 174.19(33.18) | 171.06(.30) | .02** | .65 | ||
| Wtc by recruitment type (only Diabetes group) | ||||||
| MDRe (n=6) | 163.33 (43.44) | 164 (44.34) | .66 | .23 | .087 | .86 |
| CRf (n = 9)* | 175.11 (35.54) | 173.22 (34.32) | .04* | .84 |
a. Difference between groups at end of the intervention.
b. Effect size for within groups at 3-month post-test (Mpost - Mpre/pooled SD).
c. Wt = weight.
d. FF = family and friends.
e. MDR = physician-referred.
f. CR = community recruited.
*Statistically significant differences between pre-test and post-test within groups, p <.05.
**Statistically significant differences between pre-test and post-test within group, p <.01.
† = approximating statistical significance.
Quantitative results. Attendance/retention: Study participants attended on average 10 of the 12 sessions and attrition analyses did not indicate differences between the NDM and DM groups. The DM group attended 9.89 (±2.54) sessions and the NDM group 10.4 (±1.88) sessions (p=.52). However, there was a statistically significant higher retention rate for physician-referred participants compared to community-recruited participants (p=.0001).
Intervention effects—overall study sample: Weight loss—which ranged from 1 to 18 lbs—and improvement in diabetes knowledge were statistically significant (p<.001 and p=.01, respectively). Average weight loss was 3.29 (±4.26) lbs and had a large effect size (Cohen’s d=0.77). Among those with an abnormal baseline A1C (≥ 5.7%), there was a non-statistically significant trend towards improvement (p=.28) from 7.2% (±1.8) to 6.95% (±.48).
Intervention effects based on diabetes diagnosis: Weight loss average for the NDM group was 2.87 (±3.66) lbs or 1.69% (±2.24) of initial weight, whereas DM group participants lost on average 3.65 (±4.78) lbs or 1.91% (±2.31) of their initial weight. But group differences were not statistically significant (p =.92). Besides statistical significance (p<.001), weight loss effect sizes were large (Cohen’s d=.77 and .78). Figure 2 displays relative weight loss among participants based on certain characteristics. Improvements in diabetes knowledge and A1C within the NDM or the DM groups were non-statistically significant and did not differ between groups.
Figure 2.
Percentage of weight loss at program completion based on certain characteristics.
After determining pre-post changes within the DM and the NDM group, the authors explored the impact of family/friends’ attendance (also social support) and recruitment type.
Intervention effects based on family and friends’ attendance: Overall, individuals who attended with friends and family had a statistically significant weight loss of 3.75(±3.08) lbs (p=.001) with a large effect size (Cohen’s d = 1.22), while participants who attended with no friends or family members experienced a non-statistically significant weight loss (p=.16). This translates to losing 2.26% (±1.85) and 1.60% (±3.40) of their initial weight, respectively. Group differences were statistically significant (P =.06). Even within each group (NDM or DM) weight loss was only statistically significant among those whose family and friends attended. Effect sizes were large (Cohen’s d= 1.44 and 1.08, respectively).
Intervention effects based on recruitment type: Both the physician-referred group and the community-recruited group experienced a statistically significant weight loss. Although the former lost nearly twice the weight as the latter (5.19±4.54 lbs, p=.01 vs 2.68 ±4.88 lbs, p=.003)—a weight loss which translates to 3.17% (±2.88) and 1.38% (±1.86) of the initial weight, respectively—effect size was larger for community-recruited participants (Cohen’s d=1.14 vs .66). When analyzing weight loss by recruitment type within each group (NDM or DM), weight loss within the DM group was only statistically significant for the community-recruited group. As for the NDM group, weight loss was also statistically significant for community-recruited participants but only marginally statistically significant for physician-referred participants.
Qualitative results. FGDs were conducted with 33 participants, and the KIIs with three bilingual CHWs who led the program. As detailed in Table 5, five themes emerged: 1) changes among participants included acquiring more diabetes knowledge, engaging in more physical activity, consuming healthier food, and experiencing A1C improvements; 2) attending a “joint” program was deemed beneficial for participants with and without diabetes; 3) support and attendance of family members and friends was instrumental to participants’ success; 4) despite expressed reluctance to visit physicians, physician engagement and referral resulted in better outcomes; 5) perceived barriers to behavioral changes were being male, neighborhood characteristics, and limited family support, time, energy, money and transportation.
Table 5.
Themes derived from key informant interviews and focus group discussions.
| Themes | Summaries and Supporting Quotes |
|---|---|
| Theme 1. Changes among participants with and without diabetes included acquiring diabetes knowledge, engaging in more physical activity, consuming healthier food and A1C improvements |
Theme 1. Participants in both groups shared that some myths—such as the Mexican belief that one gets diabetes from experiencing a “susto”—were dispelled as they learned about diabetes and related lifestyle factors. They also pointed to the importance of trusted CHWs and the role of their physician: “I used to think that when one gets upset (“susto”) or has a strong emotion, one becomes diabetic automatically… but one should not blame [it] on getting upset (“susto”). It’s best to take care of one’s health…” (NDM) “It [diabetes] is serious because people can die, become blind, be amputated… wounds do not heal well…” (NDM) “[Nutrition and exercise] help control [blood sugar] levels just as much as medication”. (DM) “But with them [the CHWs], I learned to check the food labels, ‘cause I never knew about these;portions. That’s what I learned from [the CHWs].’" (DM) Participants stated that they modified their behaviors—engaged in more physical activity and ate healthier: “Those of us who are sedentary, who do nothing, are prone to diabetes. However, now we are more active, eating healthy.” (NDM) “I used to eat what we call ‘junk food’, potato chips, hamburgers.. and now I rarely eat those. Neither soda…“(DM) Some who had abnormal levels at baseline confirmed improvement in their hemoglobin A1C: “They found out that I had 7[%] or 6.9[%]…. I have improved quite a bit. Now I’m at 6.1[%]” (enrolled as NDM) |
| Theme 2. Attending a “joint” program was beneficial for individuals in both NDM and DM groups |
Theme 2. Having individuals at risk of diabetes interact with others who had diabetes was an opportunity to learn from those with diabetes about the consequences of uncontrolled diabetes and of lifestyle changes: “Your friend opened our eyes”(NDM) “I would accompany my mother in her process... so... she would drink juice with no sugar...I was with her when she exercised; when she was more active, she would lower her blood sugar.” (NDM attending with DM) “So, hey... I too learned that it can be controlled.” (NDM) “Seeing that someone had been amputated or lost her sight... I think besides the fear, the fear of consequences they see in others gives them the desire to get informed.” KII The enthusiasm of the more highly motivated individuals was contagious to those less motivated: “I admired [name] because she exercised a lot.” (NDM) Also, those with diabetes opened up and shared their experiences, their practical perspective with those at risk: “It’s my first time attending a diabetes course since I’ve had it[diabetes]; I had not been to any.” (DM) “Some had had diabetes for years and I made the most out of it so they could share their experiences.” KII |
| Theme 3. Family support and the attendance of family members and friends was considered instrumental in participant’s success |
Theme 3. Participants shared that having relatives and friends involved motivated them to make changes, sometimes because they were examples to others: “My friend invited me” “I enrolled in the course because of my mother-in-law... Here I am because I want to help her.” Also seeing loved ones making changes encouraged them: “Just from seeing my mom all motivated… I’d say'it can’t be that I’m not' [laughs] They felt that having everyone going through the same process of changing their lifestyle together would allow all to better understand what others were going through. In general, the message conveyed was that any close supportive social network was helpful: “When we go out together, we no longer feel lazy – once we are 3 or 4...” “Because you are living the same reality. If someone is not living my reality, I feel that they can’t help me out.” “We’re in the same boat." “We would all get together, laugh, chat.” “[support from family] very important because if you lose your motivation, for example, to go walk, you can tell your daughter 'let's go walk' and it will motivate you more.” Community health workers felt that it was vital for family members to be involved in the courses for any change to happen: “If nobody is living their reality, I feel that they cannot help because they don’t feel… KII |
| Theme 4. There was a reluctance to see physicians but, when involved and positively connected, physicians had a positive impact |
Theme 4. Several participants expressed a preference for alternative medicine and a reluctance to see physicians—a behavior they attributed to the Latino culture—especially in the absence of symptoms. Homemade teas are the preferred treatment and there is some degree of doubt about traditional medicine as practiced in the US: “We Mexicans, Latinos, all I know is we don’t go to the doctor. I tell you from experience. Just because nothing is hurting. And when it starts hurting, we think a little tea will get rid of it” “Our culture is very different; we don’t go to the physicians because we don’t like to have to wait...it’s a cultural thing which affects us a lot" “Chamomille tea for me is a miracle. It cures everything." When a physician they connected with was involved, patients took seriously the physician’s instructions about following the program recommendations and felt that it influenced their behavior: “She’s the one who told me: ‘we’re going to have a diabetes course.’ And then she added:‘I would like you to attend; this is very important.’ Then she said: ‘should I put your name down?’ I told her ‚‘Yes. Sign me up.’” “I exercise. My cholesterol came out high. My doctor told me I have to walk 1000 steps. So, I get up and take my little device...” However, there was an agreement that—unless they were engaged in the decision-making process—no positive change would happen: “If the doctor says it, if you don’t want to do it, you’re not going to do it because you don’t see the reason why. “When they go because they chose to, it’s different. When they are forced...”. KII |
| Theme 5. Perceived barriers included being a male, neighborhood characteristics, lack of family support, lack of time and energy, money or transportation |
Theme 5. Perceived barriers: a. Being a male. Being a male seemed to be associated with less motivation and less attendance. (These comments were made by women, as the men didn’t participate and share their input): “It doesn’t mean that men are not interested in their health but I think, based on their schedule, the woman is usally more frequently at home and the man always has to go out.” KII b. Neighborhood characteristics. Lacking a safe space for physical activity: “Some had places where they could walk while others did not have a place where they felt safe walking, so that too was a barrier.” KII c. Lack of family support. Several participants whose family members were not enrolled in the course expressed that they wanted to keep their families united but that they were experiencing family conflicts as a consequence of adopting healthy behaviors. Most of the problems stemmed from change in eating habits, with spouses rebelling or upset at them, children complaining, and mounting tension: “Yes, it has a lot to do. Me too: with my husband, because we don’t have the same condition “Sometimes they help you, but other times, they give you…. But yes, sometimes it’s a negative influence.” “Sometimes their influence is negative and it’s not helpful” d. Lack of time and energy to prepare food. Several felt that they didn’t have enough time nor energy to prepare healthy meals: “You feel very little energy to prepare your meals" “Time; because they work, get home tired, then work again. Always lack of time” e. Financial constraints. According to promotores, lack of money was the most common barrier. It made it difficult to purchase healthy food: “We emphasized a lot the community garden...because they kept saying ‘I don’t have...’” KII “Sometimes it’s cheaper to eat a hamburger than to make one. And olive oil... it’s so expensive!” Others didn’t agree. They did not think food cost was an issue since it could be cultivated “I agree. Well... to eat vegetables, if one doesn’t have the money one can cultivate the food and it’s much better.” f. Lack of transportation. Lack of transportation options was also a barrier either due to lack of money to pay for public transportation, inability to drive, or not having access to a car: “Simply put some do not have a car, others do not drive. That’s it.” KII |
NDM = participant with no diabetes. DM = participant with diabetes. All quotes were transcribed in Spanish and then translated. The three CHWs who taught the intervention were interviewed for the key informant interviews (KIIs). Focus group discussions (FGDs) were conducted with program participants (n=33). Unless followed by “KII”, quotes are all taken from FGDs statements.
Discussion
This study sought to explore whether a joint diabetes prevention and self-management intervention—for individuals with diabetes and those at risk for diabetes—could benefit low-income H/L with limited education and reduce the success gap between them and other populations known to respond better to lifestyle interventions.
Because of previously expressed concerns about such an endeavor being too costly, lack of interest among those with no diabetes and strained physician/family members relationships, the authors also assessed the impact of physician involvement and cost of implementing such program. We also sought to assess if the presence of participants with diabetes discouraged engagement from others without diabetes.
While enrollment of H/L in interventions has historically been low (a number that varies depending on source and setting)27,50 most classes were filled to capacity in our study. Trust has been cited as the most important factor in enrollment and we believe that recruitment by a trusted physician and/or community health worker in this study was a key factor. 51
According to our findings, H/L of low to medium educational level could indeed benefit from a “joint” program, regardless of their location on the dysglycemic spectrum, especially if attending with family or friends and when there is physician engagement. Overall, our study sample benefited from the intervention in that participants lost weight, increased their diabetes knowledge, had a trend towards A1C improvement, and adopted healthy behaviors. Approximately half of all participants lost at least 2.2 lbs at 12 weeks (program completion), a clinically significant weight loss.
Social support is known to play an important role in promoting healthy behavior. In our study sample, a higher proportion (57%) of those attending with family or friends reached clinically significant weight loss compared to those who attended with neither (30%), suggesting that having a family or friend was helpful. Due to survey limitations, it was not possible to differentiate those who attended with family from those attending with friends in the quantitative analyses; however, during the FGDs, several individuals with diabetes identified relatives who attended the course with them, and participants from both categories stated that the presence of friends and/or family members helped motivate them to engage in healthy behaviors. Weight loss differences were even greater when comparing clinician-referred and community-recruited participants (75% vs 40%, respectively), suggesting a positive correlation with physician engagement.
A1C sensitivity analyses showed a trend towards improvement but these changes were not statistically significant in the overall group nor within each of the groups. We believe this is because changes in nutrition and physical activity were not started until the fifth session, therefore allowing less than 8 weeks for A1C to reflect glucose improvement. 52 Furthermore, the relatively low group baseline average A1C level and the small sample size may have played a role.
When comparing groups with and without diabetes both groups lost weight and no statistically significant group differences were detected. Among the DM group, 53.3% had a clinically significant weight loss, thus reducing their risk of cardiovascular disease by at least 4 to 8%.53,54 Weight reduction in this group (2.87±3.66 lbs) was comparable to that reported in a 12 week study implemented among H/L adults with diabetes (n=34) living in the same geographical area as this study’s participants (2.07 lbs). 55
Among the NDM group, 44.4% lost clinically significant weight which translates to at least a 16% diabetes risk reduction. 56 Diabetes knowledge in this group increased as much as that of the DM group, suggesting similar engagement, since knowledge increase is a good reflection of engagement. 57 Indeed, judging from their diabetes knowledge, attendance and qualitative statements at program completion, the NDM group seems to have benefited from exposure to the DM groups. Higher attendance rates in the NDPP and other interventions have been associated with improved results. 58 Our results also indicate that family and friends attending improve success rates within both NDM and DM groups.
While most diabetes-related reports describe either diabetes prevention or self-management, two studies reported the results of interventions delivered to H/L individuals at varying levels of the glycemic spectrum. In one study where most participants were from Mexico, each person with diabetes enrolled with a family member with no diabetes. The eight week intervention was taught by nurses. 42 Attrition and knowledge changes were similar among participants with and without diabetes and there was a statistically significant decrease in A1C 10 weeks after baseline. However, weight changes were not reported. Another study conducted among Mexican American dyads (one participant with diabetes and a family member without diabetes) used a 12 week DSME/S intervention led by a nurse diabetes educator and a CHW (only for the social aspect and to make phone calls). Only changes among participants with diabetes were reported and A1C improvement was not statistically significant. 43
The VV intervention was unique in that it encouraged participants to develop a new social network (support group) within the larger group and was intended to promote weight loss and behavior change equally in both participants groups, rather than having family members focus on supporting another “sick” family member. It also differed from the above-mentioned studies in that it was CHW-led. We also assessed and reported weight changes in different subgroups.
Although concerns have been raised about family members-physician interaction, we were unable to identify—quantitatively or qualitatively—any ongoing communication barriers between physicians and patients’ family members. An article on the NDPP reported less physician referrals of monolingual Spanish-speaking to prevention programs (compared to those who spoke English).18,21 However, upon examining retention, engagement, and results of physician-referred participants in VV, they seem to have benefited more: not only was the percentage of weight loss among those recruited by a physician close to double that of the community-recruited group, the weight loss (3.17% ±2.88 at 12 weeks) was somewhat comparable to the average for non-Latinos NDPP participants (a dose response of .30% per session attended and 3.4±3.61% of initial weight lost at 16 weeks). VV participants’ results were also better than results from a recent NDPP report among mostly Latinas in Los Angeles, California (2.15% at 16 weeks).18,59,60 Whether or not the difference is due to characteristics of providers, of those who visit their physicians, or of patients’ family members is beyond the scope of this study. Certainly, the role of clinicians has been noted as a powerful motivator for healthy behavior in H/L culture, 61 especially among monolingual Spanish-speaking Latinos. 28
While quantitative data showed some advantage among physician-referred participants qualitative data revealed that physician-patient interactions are more complex. It was obvious that participants preferred using alternative medication and that some felt the need to justify seeking medical help. There was skepticism about traditional medicine as practiced in the USA, and a reluctance to spend time caring for oneself. Some went as far as to state that they may not pay attention to their physician unless he/she engages them actively in the decision-making process. Thus, referral from a physician would not, in and of itself, motivate these participants to adopt new lifestyle habits in the absence of a positive physician-patient interaction. Nevertheless, our study suggests that healthcare providers can have a positive influence on low-income at-risk H/L individuals and may promote more permanent results if they make referrals to diabetes prevention and self-management programs a priority.
In addition to their hesitation to seek medical care, H/L have the highest uninsured rates (32%), 62 making early diagnosis of asymptomatic conditions such as pre-diabetes a challenge. Moreover, despite recent interest in Family DSMES programs, 35 these interventions target individuals with diabetes, and requirements are more stringent than those in our study. Allowing for a mixed program approach would facilitate the process of addressing the needs of two important and potentially costly groups. Clearly, the benefits of interventions for low-income H/L would provide a wider net to “catch” individuals who would otherwise never be screened or served before they develop the disease.
A program such as VV could offer an option for many more individuals desperately needing the support of an intervention, especially in view of the program’s relatively low cost. The cost incurred for each participant of this 12 week intervention was a little under $200, which seems competitive when compared with the cost of the NDPP intensive first 16 weeks (between $500 and $800 per person).63,64 We posit that engaging CHWs to implement a shorter “hybrid” diabetes intervention, similar to “family therapy,” may be financially viable and a worthwhile return on investment, especially considering that a minimum of $14,000 can be saved per person for each year of delay in diabetes onset. 65
The focus group and KII participants corroborated the existence of several policy-related “barriers” to enrollment and behavior modification previously identified by other studies: lack of transportation, limited access to healthy food options, and lack of safe environments for physical activity. Less access to healthy food and safe places to exercise, and fewer transportation options are all known to be associated with poor neighborhoods. Other factors associated with less attendance and success were male gender, financial constraints, little time and energy due to long work hours, and family resistance to change. Men were a minority in this study and did not participate in the FGDs; however, according to available literature, men tend to generally attend lifestyle programs less frequently. 66
Strengths and limitations: Several limitations of this study need to be acknowledged. Since this was a pilot feasibility study, our sample size was small. Participants were mostly women of Mexican descent; furthermore, although it was obvious from the focus group statements that the great majority of participants had financial challenges, we could not confirm participants’ income. In addition, whether referral from a non-Hispanic/Latino physician would produce similar effects as that of a cultural homologous physician could not be ascertained. Thus, applicability to other low-income H/L may be limited. This program allowed family and friends to attend, with no differentiation between categories. Also, because our design did not allow us to dismantle our data to gather that information, we were unable to determine whether or not individuals who did not attend with friends and family lived alone or had any family members at all. The subcategories and differences in circumstances could have affected social support and should be explored in future studies.
One strength of this study is that we were able to compare two groups attending the same program taught by CHWs. Other strengths included having qualitative data confirm and further contextualize quantitative data (e.g., participants describing behavior changes and attendance of friends) and CHWs corroborating participants’ statements. Lastly, access to physician-referred participants allowed the authors to assess the impact of collaboration between clinicians, CHWs, and program development specialists.
Conclusions and Implications for Practice
There is an urgent need for effective, sustainable diabetes prevention, and self-management programs that are well attended by, and effective for, low-income H/L. Unless the current trends reverse, the continued lack of success of nationally promoted interventions among low-income H/L threatens to increase the diabetes health disparities in this population and in the country. Our findings show that both groups were engaged to the same degree and benefited equally from a joint program led by community health workers. Furthermore, the presence of family and friends, and referral from a physician improved results, showing the promise of closing the H/L success gap.
Rather than accommodating the shorter DSMES program for low-income H/L at risk of diabetes, or “forcing” participants to commit to a 1 year diabetes prevention program—when most H/L attend less than eight times even when they do not have to pay for the program—our results indicate that a “hybrid” shorter program offered to both groups may be worth considering, especially if it includes members of the same social network. A combined program may have the advantage of providing everyone within the dysglycemic spectrum with social support, a critical component in promoting success. Moreover, this would avoid duplication and help create a more unified message regarding the behaviors required to achieve glycemic control.
Finally, the results indicated that CHWs can successfully lead a “diabetes prevention and self-management intervention,” even in the presence of barriers associated with policies. Furthermore, as an integral part of programming, they may even compensate for the often less-than-ideal physician-patient interactions inherent in our healthcare system.
On a broader policy-making level, reducing barriers should be a high priority: providing incentives to physicians and reimbursement for CHW-led, culturally relevant lifestyle-based interventions, allowing family members and friends to attend, increasing access to healthy food and reliable transportation, and creating safer “walkable” communities in H/L neighborhoods. Programs should also be available year-round to facilitate enrollment shortly after referral. 66
Community/healthcare system partnerships which combine “hybrid” culturally sensitive CHW-led lifestyle-based diabetes prevention programs with supportive healthcare entities and progressive policies have the potential of dramatically reducing the risk of diabetes and diabetes complications, among those most afflicted in the Hispanic/Latino communities.
This study expands the literature by providing viable cost-effective options to health educators, healthcare professionals, and policymakers seeking to address the US health disparities and the diabetes burden among the most vulnerable. Replication of this study on a larger scale and extended for a longer time period (a year) may help confirm our findings and provide guidance to policymakers as our nation struggles to reduce health disparities among those bearing the heaviest burden of chronic diseases.
Acknowledgments
First of all, many thanks to community health workers Monica Acevedo, Beatriz Castro, Vanessa Rivera, Miriam Valero, Lidia Benitez, Juandretta Hern and Erika Marroquin who implemented the intervention, and to our community partner and Executive Director at El Sol Neighborhood Educational Center, Alex Fajardo. We are also grateful to Dr Toni Fernandez of Hesperia Clínica Médica Familiar, Dr Marino De Leon, Director of the Loma Linda University Center for Health Disparities and Molecular Medicine and Dr Eddy Jara from Loma Linda University School of Public Health. Lastly, we’d like to thank Noemi Avalos, Jessica Camacho, Suhail Hashim, Maria Anaya, Kelsy Escalante and Lily Lee who assisted with curriculum editing, training, recruiting, data analyses and interviews.
Note
To qualify for the NDPP, participants must be 18 years old or more, overweight, and with clinical evidence of pre-diabetes such as glycosylated hemoglobin between 5.7 and 6.4% or fasting plasma glucose of 100–125 mg/dL or 2-hour plasma glucose after a 75g glucose load between 140 and 199 mg/dL within the past year or have a previous clinical diagnosis of gestational diabetes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was partially funded by El Sol Neighborhood Educational Center, Loma Linda University Behavioral Health Institute and the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number P20MD006988.
Ethical Approval: All program participants provided written informed consent in Spanish or English, as approved by the Loma Linda University Institutional Review Board (#5150145), the same board that approved this study.
ORCID iD
Maud Joachim-Célestin https://orcid.org/0000-0001-5424-4666
References
- 1.World Health Organization . Noncommunicable diseases key facts. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases Published April 11, 2021, Accessed April 24, 2021.
- 2.American Diabetes Association . Economic costs of diabetes in the US. in 2017. Diabetes Care. 2018;41(5):917-928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . National Diabetes Prevention Statistics ReportEstimates of Diabetes and its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention, US. Department of Health and Human Services; 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 15, 2021. [Google Scholar]
- 4.Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metabol Res Rev. 2019;35(2):e3097. doi: 10.1002/dmrr.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Li M, Dong Y, et al. , 36. Diabetes Metab Res Rev; 2020. 10.1002/dmrr.3319. Diabetes is a risk factor for the progression and prognosis of COVID 19 Diabetes Metabol Res Reve3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karter AJ, Laiteerapong N, Chin MH, et al. Ethnic differences in geriatric conditions and diabetes complications among older, insured adults with diabetes. J Aging Health. 2015;27(5):894-918. doi: 10.1177/0898264315569455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquez I, Calman N, Crump C. A framework for addressing diabetes-related disparities in US latino populations. J Community Health. 2019;44(2):412-422. doi: 10.1007/s10900-018-0574-1. [DOI] [PubMed] [Google Scholar]
- 8.Khan T, Tsipas S, Wozniak G. Medical care expenditures for individuals with prediabetes: The potential cost savings in reducing the risk of developing diabetes. Popul Health Manag. 2017;20(5):389-396. doi: 10.1089/pop.2016.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care. 2013;19(5):421-430. [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . National Diabetes Program. Atlanta, GA: Centers for Disease Control and Prevention, US. Department of Health and Human Services; 2020. https://www.cdc.gov/diabetes/prevention/index.html. Published August 10, 2019. Accessed March 18, 2021. [Google Scholar]
- 11.U.S. Health and Human ServicesNational Diabetes Prevention Program . Employers and Worksites. Washington, DC: U.S. Dept. of Health and Human Services. https://www.cdc.gov/diabetes/professional-info/employers.html. Published January 27, 2020. Accessed April 1, 2021. [Google Scholar]
- 12.Mensa-Wilmot Y, Bowen S-A, Rutledge S, et al. Early results of states’ efforts to support, scale, and sustain the national diabetes prevention program. Prev Chronic Dis. 2017;14:E130. doi: 10.5888/pcd14.170478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahanty LM, Trief PM, Cibula DA, Weinstock RS. Barriers to weight loss and physical activity, and coach approaches to addressing barriers, in a real-world adaptation of the DPP lifestyle intervention: A process Analysis. Diabetes Educat. 2019;45(6):596-606. doi: 10.1177/0145721719883615. [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp A, Backholer K, Magliano D, Peeters A. The effect of obesity prevention interventions according to socioeconomic position: A systematic review. Obes Rev. 2014;15(7):541-554. doi: 10.1111/obr.12161. [DOI] [PubMed] [Google Scholar]
- 16.McGill R, Anwar E, Orton L, et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Publ Health. 2015;1515:894457. doi: 10.1186/s12889-015-1781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyseni L, Atkinson M, Bromley H, et al. The effects of policy actions to improve population dietary patterns and prevent diet-related non-communicable diseases: Scoping review. Eur J Clin Nutr. 2017;71(6):694-711. doi: 10.1038/ejcn.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie ND, Sauder KA, Phimphasone-Brady P, Amura CR. Rethinking the national diabetes prevention program for low-income whites. Diabetes Care. 2018;41(4):e56-e57. doi: 10.2337/dc17-2230. [DOI] [PubMed] [Google Scholar]
- 19.Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP). Diabetes Care. 2013;36(1):34-40. doi: 10.2337/dc12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22(2):226-230. [PubMed] [Google Scholar]
- 21.Chambers EC, Rehm CD, Correra J, et al. Factors in placement and enrollment of primary care patients in YMCA’s diabetes prevention program, Bronx, New York, 2010-2015. Prev Chronic Dis. 2010-2015;14:E28. doi: 10.5888/pcd14.160486. Published 2017 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin AL, Warren JP, Lipman RD. The landscape for diabetes education. Diabetes Educat. 2013;39(5):614-622. doi: 10.1177/0145721713499412. [DOI] [PubMed] [Google Scholar]
- 23.Testerman J, Chase D. Influences on diabetes self-management education participation in a low-income, Spanish-speaking, Latino population. Diabetes Spectr. 2018;31(1):47-57. doi: 10.2337/ds16-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiernan M, Moore SD, Schoffman DE, et al. Social support for healthy behaviors: Scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity. 2012;20(4):756-764. doi: 10.1038/oby.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamungkas R, Chamroonsawasdi K, Vatanasomboon P. A systematic review: Family support integrated with diabetes self-management among uncontrolled type II diabetes mellitus patients. Behav Sci. 2017;7(3):62. doi: 10.3390/bs7030062. Published 2017 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Standards of Medical Care in Diabetes-2016: Summary of Revisions . Diabetes Care. 2016;39 Suppl 1 Suppl 1:S4-S5. doi: 10.2337/dc16-S003 [DOI] [PubMed] [Google Scholar]
- 27.McCurley JL, Gutierrez AP, Gallo LC. Diabetes prevention in US. hispanic adults: A systematic review of culturally tailored interventions. Am J Prev Med. 2017;52(4):519-529. doi: 10.1016/j.amepre.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RE, Peterson KE, Rothschild SK, Resnicow K. Pushing the envelope for cultural appropriateness. Diabetes Educat. 2011;37(2):227-238. doi: 10.1177/0145721710395329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivers P, Hingle M, Ruiz-Braun G, Blew R, Mockbee J, Marrero D. Adapting a family-focused diabetes prevention program for a federally qualified health center: A qualitative report. Diabetes Educat. 2020;46(2):161-168. doi: 10.1177/0145721719897587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown SA, Perkison WB, García AA, et al. The starr county border health initiative: Focus groups on diabetes prevention in Mexican Americans. Diabetes Educat. 2018;44(3):293-306. doi: 10.1177/0145721718770143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joachim-Célestin M, Gamboa-Maldonado T, Dos Santos H, Montgomery SB. A qualitative study on the perspectives of latinas enrolled in a diabetes prevention program: Is the cost of prevention too high? Journal of Primary Care & Community Health. 2020;11:. doi: 10.1177/2150132720945423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck J, Greenwood DA, Blanton L, et al. 2017 National standards for diabetes Sslf-management education and support. Diabetes Educat. 2019;45(1):34-49. doi: 10.1177/0145721718820941. [DOI] [PubMed] [Google Scholar]
- 33.Klein HA, Jackson SM, Street K, Whitacre JC, Klein G. Diabetes self-management education: miles to go. Nursing Research and Practice. 2013;25:1-15. doi: 10.1155/2013/581012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention . Lifestyle Change Program Details. Atlanta. GA: US Department of Health and Human Services. https://www.cdc.gov/diabetes/prevention/lcp-details.html. PublishedApril 27, 2021 Accessed May 12 2021. [Google Scholar]
- 35.Felix HC, Narcisse MR, Long CR, McElfish PA. Effects of a family diabetes self-management education intervention on the patients’ supporters. Fam Syst Health. 2020;38(2):121-129. doi: 10.1037/fsh0000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29(7):1675-1688. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 37.Rosland A-M, Piette JD, Trivedi R, et al. Engaging family supporters of adult patients with diabetes to improve clinical and patient-centered outcomes: Study protocol for a randomized controlled trial. Trials. 2018;19(1):394. doi: 10.1186/s13063-018-2785-2. Published 2018 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powers BW, Jain SH. Patient-centered physician selection: A necessary first step for accountable care. Am J Manag Care. 2014;20(11 Spec):E16. [PubMed] [Google Scholar]
- 39.Trump LJ, Mendenhall TJ. Community health workers in diabetes care: A systematic review of randomized controlled trials. Fam Syst Health. 2017;35(3):320-340. doi: 10.1037/fsh0000283. [DOI] [PubMed] [Google Scholar]
- 40.Mirambeau AM, Wang G, Ruggles L, Dunet DO. A cost analysis of a community health worker program in rural Vermont. J Community Health. 2013;38(6):1050-1057. doi: 10.1007/s10900-013-9713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uusitupa M, Khan TA, Viguiliouk E, et al. Prevention of type 2 diabetes by lifestyle changes: A systematic review and meta-analysis. Nutrients. 2019;11(11):2611. doi: 10.3390/nu11112611. Published 2019 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Amirehsani KA, Wallace DC, McCoy TP, Silva Z. A family-based, culturally tailored diabetes intervention for hispanics and their family members. Diabetes Educat. 2016;42(3):299-314. doi: 10.1177/0145721716636961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen MM, Pasvogel A, Murdaugh C, Hepworth J. Effects of a family-based diabetes intervention on behavioral and biological outcomes for mexican american adults. Diabetes Educat. 2017;43(3):272-285. doi: 10.1177/0145721717706031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Census Bureau . Quick Facts, San Bernardino County, California; Riverside County, California. Washington DC: CaliforniaU.S. Department of Commerce. https://www.census.gov/quickfacts/fact/table/sanbernardinocountycalifornia,riversidecountycalifornia,CA/PST045219. Accessed May 2, 2021. [Google Scholar]
- 45.San Bernardino County . Community Indicators. Chronic Disease. San Bernardino, CA; 2021. https://indicators.sbcounty.gov/wellness/chronic-disease/. Accessed June 1, 2021. [Google Scholar]
- 46.Diabetes Prevention Support Center . DPP Group Lifestyle Balance Curriculum. Pittsburgh, PA: University of Pittsburgh; 2021. https://www.diabetesprevention.pitt.edu/group-lifestyle-balance-materials/. Accessed May 25, 2021. [Google Scholar]
- 47.New Mexico Department of Health . Diabetes Prevention and Control Program. Santa Fe: NM. Population and Community Health Bureau. Public Health Division. https://www.nmhealth.org/about/phd/pchb/dpcp/. Accessed May 2, 2021. [Google Scholar]
- 48.Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage Publications; 2006. [Google Scholar]
- 49.Verbi Software Consult Sozialforschung GmbH . MAXQDA Plus [Computer Software]. Berlin, Germany: Version 12; 2015. [Google Scholar]
- 50.Hanza MM, Goodson M, Osman A, et al. Lessons learned from community-led recruitment of immigrants and refugee participants for a randomized, community-based participatory research study. J Immigr Minority Health. 2016 Oct2016;1818(55):1241-1245. doi: 10.1007/s10903-016-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fortmann AL, Savin KL, Clark TL, Philis-Tsimikas A, Gallo LC. Innovative diabetes interventions in the US. hispanic population. Diabetes Spectr. 2019;32(4):295-301. doi: 10.2337/ds19-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yau JW, Thor SM, Ramadas A. Nutritional strategies in prediabetes: A scoping review of recent evidence. Nutrients. 2020;12(10):2990. doi: 10.3390/nu12102990. Published 2020 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Froom P, Goldbourt U. Secular decrease in blood pressure and reduction in mortality from cardiovascular disease in Israeli workers. J Hum Hypertens. 2004;18(2):113-118. doi: 10.1038/sj.jhh.1001645. [DOI] [PubMed] [Google Scholar]
- 54.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure. Hypertension. 2003;42(5):878-884. 10.1161/01.HYP.0000094221.86888AE. [DOI] [PubMed] [Google Scholar]
- 55.Metghalchi S, Rivera M, Beeson L, et al. Improved clinical outcomes using a culturally sensitive diabetes education program in a hispanic population. Diabetes Educat. 2008;34(4):698-706. doi: 10.1177/0145721708320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102-2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard B, Uno F, Tedre M. Can student engagement in online courses predict performance on online knowledge surveys. Int.J.Learn.Teach.Educ.Res. 2017;16(3):73-87. [Google Scholar]
- 58.Ritchie ND, Baucom KJW, Sauder KA. Benefits of participating with a partner in the national diabetes prevention program. Diabetes Care. 2020;43(2):e20-e21. doi: 10.2337/dc19-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bozack A, Millstein S, Garcel JM, Kelly K, Ruberto R, Weiss L. Implementation and outcomes of the New York state YMCA diabetes prevention program: A multisite community-based translation, 2010-2012. Prev Chronic Dis. 2014;11:E115. doi: 10.5888/pcd11.140006. Published 2014 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeFosset AR, Sivashanmugam M, Mosst J, Kuo T. Clinic- and community-based national diabetes prevention programs in Los Angeles. Health Educ Behav. 2021. doi: 10.1177/10901981211016759. [DOI] [PubMed] [Google Scholar]
- 61.Ragsdale C, Wright J, Shokar G, Salaiz R, Shokar NK. Hispanic patient perspectives of the physician’s role in obesity management. J Clin Med Res. 2017;9(2):170-175. doi: 10.14740/jocmr2868w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Health and Human Services Office of Minority Health . Profile: Hispanic/Latino Americans. Atlanta, GA: US Department of Health and Human Services. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=64. Accessed May 15, 2021. Published April 5, 2021. [Google Scholar]
- 63.Centers for Disease Control and Prevention . National Diabetes Prevention Program Coverage Toolkit. Cost & Value. Atlanta, GA: US Department of Health and Human Services. https://coveragetoolkit.org/cost-value-elements/. AccessedMay 12, 2021. PublishedFebruary 24, 2021. [Google Scholar]
- 64.Ritchie ND, Gritz RM. New medicare diabetes prevention coverage may limit beneficiary access and widen health disparities. Medical Care. 2018;56(11):908-911. doi: 10.1097/MLR.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 65.Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37(9):2557-2564. doi: 10.2337/dc13-2484. [DOI] [PubMed] [Google Scholar]
- 66.Walker EA, Weiss L, Gary-Webb TL, et al. Power up for health: Pilot study outcomes of a diabetes prevention program for men from disadvantaged neighborhoods. Am J Men’s Health. 2018;12(4):989-997. doi: 10.1177/1557988318758787. [DOI] [PMC free article] [PubMed] [Google Scholar]