Abstract
Background and objective
Hemodynamic forces may play a role in symptomatic delayed ipsilateral parenchymal hemorrhage (DIPH) of intracranial aneurysm (IA) after flow diverter placement. We aimed to investigate the hemodynamic risk factors in the postsurgical DIPH process.
Methods
Six patients with internal carotid artery (ICA) aneurysm developed to DIPH and 12 patients without DIPH (1:2 matched controls) after flow diverter were included between January 2015 to January 2019. Postsurgical hemodynamics of distal arteries (terminal ICA, middle cerebral artery (MCA), anterior cerebral artery (ACA)) were investigated using computational fluid dynamics, as well as the hemodynamic alteration between pre- and post-treatment. The DIPH related and unrelated distal arteries (either MCA or ACA) were discriminated and compared. Definition of imbalance index is the difference in increased velocity post-flow diverter between MCA and ACA and was used to evaluate the blood flow distribution of distal arteries.
Results
The mean and maximum flow velocities in the terminal ICA increased significantly after treatment in both groups. In DIPH group, the increase rate of mean velocity in the DIPH-related artery was significantly higher than that in DIPH-unrelated artery after the treatment (20.98 ± 15.38% vs −6.40 ± 7.74%; p = 0.028). Between the DIPH and control group, the baseline characteristics were well matched. However, a higher imbalance index of mean velocity was found in DIPH group (27.38 ± 13.03% vs 10.85 ± 14.12%; p = 0.031).
Conclusion
The mean velocity of DIPH related artery increased more, and the imbalance in increased blood flow distribution of distal arteries might play an important role in DIPH after flow diverter of IAs.
Keywords: Intracranial aneurysms, hemodynamics, flow diverter, delayed ipsilateral parenchymal hemorrhage, endovascular treatment
Introduction
Flow diversion is becoming a new strategy for treating intracranial aneurysms (IAs), which results in parent vessel reconstruction and intra-aneurysm thrombosis to cure the aneurysm.1,2 Several studies have demonstrated the safety and efficacy of the Pipeline embolization device (PED; ev3 Neurovascular, Irvine, CA, USA) for flow diversion for treating complex aneurysms such as large and giant intracranial aneurysms.3–5 However, some unexpected complications have also been reported, with high morbidity and mortality rates.6–9 Symptomatic delayed ipsilateral parenchymal hemorrhage (DIPH) is a severe complication of PED treatment, with a reported incidence of 3%. However, the etiology of this devastating complication remains unclear and its occurrence is unpredictable. 10
Several studies have suggested that hemodynamic changes in the distal artery after PED deployment might play an important role in the occurrence of DIPH.11–13 However, information on hemodynamics in relation to DIPH after PED treatment is limited. In this study, computational fluid dynamics (CFD) and advanced virtual stenting technique were used to explore the hemodynamics of the distal branch associated with DIPH. The hemodynamic changes at the terminal segment of the parent artery before and after PED placement were compared. Then, we identified the location of DIPH by computed tomography (CT) and determined the hemorrhage related-distal branch, either middle cerebral artery (MCA) or anterior cerebral artery (ACA). The hemodynamic changes in the distal branches related and unrelated to DIPH were compared after PED deployment. In addition, the patients without DIPH were matched as control, the blood flow distribution of the distal branches was analyzed between DIPH and control patients. The results of this study may be helpful to prevent such fatal complication after flow diversion.
Materials and methods
Patient selection
This retrospective study was approved by the ethics committee of our hospital. We reviewed the medical records and imaging data in our aneurysm database for patients diagnosed with unruptured IAs treated with PED from January 2015 to January 2019. Patients were screened based on the following inclusion criteria: (1) DIPH after PED treatment and DIPH region identified by CT; (2) all procedures technically successful with PED; and (3) sufficient resolution of three-dimensional (3 D) digital subtraction angiography images for CFD simulation. Cases with PED malposition or DIPH related to interventional procedures, such as intraprocedural microwire perforations were excluded. We also excluded cases where the DIPH could not clearly be localized to the MCA or ACA territory (occurred in a watershed region). We screened 352 patients with 382 unruptured IAs treated with PEDs in our database. Eight patients experienced unexpected DIPH complication with an incidence of 2.3% in our center. Two of them were excluded because of inadequate image quality for the computational simulation, and the remaining six patients, with six internal carotid artery (ICA) aneurysms and treated with PED and coils, were ultimately included in the DIPH group. Based on the larger sample size of patients without DIPH, for each case in the DIPH group, we enrolled two matched controls without DIPH as the control group. Patients in the control group should had at least 1-year angiographic follow-up results with the evidence to exclude DIPH. The primary basis for the matched controls was aneurysm size, aneurysm location and treatment method (PED alone or PED with coils). Additional matching was performed with age and sex. Twelve controls treated with PED and coils were matched successfully and included as the control group. Clinical and morphological factors for all patients (age, sex, smoking history, hypertension, presentation, aneurysm size, neck, modified Rankin Scale (mRS) score, platelet inhibition, CYP2C19 genotype and post-operative blood pressure) were collected. Information on the initial angiographic results, time of hemorrhage, treatment method, location of hemorrhage, and whether craniotomy was also recorded. The procedure of PED deployment was showed in Supplemental 1.
Computational modeling and hemodynamic simulations
Patient-specific aneurysm morphologies were reconstructed and obtained from 3 D rotational angiography images. The 3 D geometry surface was displayed, segmented, and smoothed using Geomagic Studio version 12.0 software (Geomagic, Research Triangle Park, NC, USA), and the geometries were saved in standard tessellation language format.
We simulated the hemodynamics of the aneurysms using previously developed computational modeling methods. 14 A novel virtual stenting technique 15 and porous media method 16 were used to simulate the in vivo stent and coil mass in the aneurysm dome region. The virtual stenting technique was validated in our previous studies, the results of the computational hemodynamics indicate that the virtual stenting technique is suitable for evaluating hemodynamic factors that affect treatment outcomes.17,18 Finite-volume elements for CFD simulation were created by merging the virtual stent with the aneurysm geometry using mesh generation software (ICEM CFD, V.14.0; ANSYS Inc., Canonsburg, PA, USA), to generate >1 million finite-volume tetrahedral elements. The largest element size was set at 0.2 mm, and the element size on the stent was set to one third the width of the strut of the stent. ANSYS CFX 14.0 software (ANSYS Inc.) was used to solve the flow-governing Navier-Stokes equations. Some assumptions were applied, including laminar, incompressible, Newtonian blood flow, and rigid vessel wall with no-slip boundary conditions. The blood density and dynamic viscosity were specified as 1060 kg/m3 and 0.004 Pa/s, respectively. A representative pulsatile period velocity profile was obtained using transcranial Doppler imaging and set as the inflow boundary condition. The outlet pressure conditions at outlet arteries in our study were imposed to 0. The flow waveforms were scaled to achieve a mean inlet wall shear stress of 15 dyne/cm under pulsatile conditions. Three cardiac cycle simulations were performed to establish numeric stability. To confirm stability, the results from the third cardiac cycle were collected as output for the final analyses.
Hemodynamic measurement and analysis of distal arteries
Measurement locations
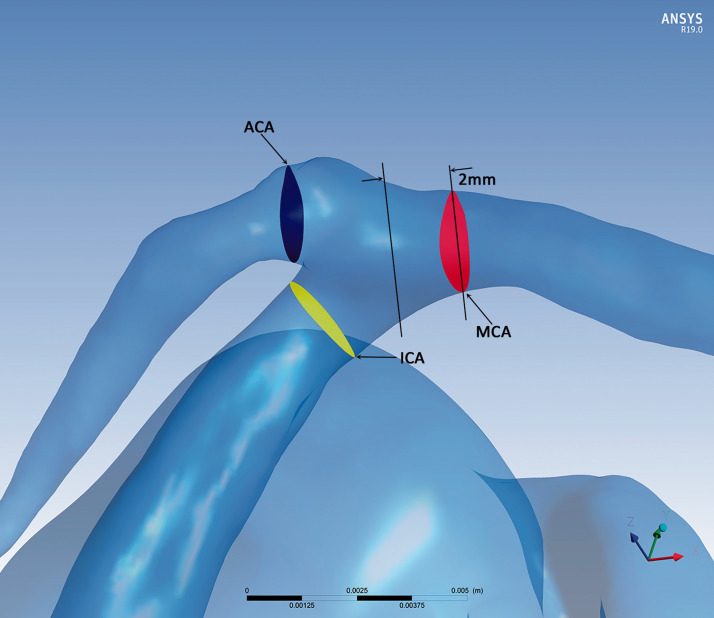
Three regions of interest in the artery distal to the aneurysm were selected to do the hemodynamic measurements and further analysis in both DIPH group and control group: the test planes of the terminal ICA, the initial MCA and the initial ACA, respectively (Figure 1). For the test plane of ICA, it was the plane that 2 mm proximal to the terminal of ICA. The test planes of the MCA and ACA were parallel 2 mm distal to the planes of which the MCA and ACA arose from the ICA. Parameters on these three regions represented ICA, MCA and ACA will be analyzed.
Figure 1.
The measurement section in patient-specific aneurysm model: the measurement section was the test plane of ICA (yellow), the MCA (red) and ACA (blue).
Then, in the DIPH group, we found out the DIPH-related artery, either MCA or ACA, according to the CT imaging following the method reported by Savoiardo. 19 The ACA supplies the medial part of the frontal and the parietal lobe, and the anterior portion of the corpus callosum, basal ganglia and internal capsule. The MCA supplies the lateral surface of the hemisphere, except for the medial part of the frontal and the parietal lobe (ACA), and the inferior part of the temporal lobe (posterior cerebral artery). Briefly, the ACA will be defined as the DIPH-related artery if the DIPH located in the ACA territory, and therefore the MCA of this patient will be defined as DIPH-unrelated artery. In contrast, if the DIPH located in the MCA territory, the MCA was the DIPH-related artery, and the ACA was the DIPH-unrelated artery. The cases in the control group will not follow such definition as there was no DIPH event. All the angiographic assessments and results were determined by two radiologists (with 5 years of experience in vascular neuroimaging) who were blinded to evaluate the slides. Disagreements were resolved by a third radiologist (10 years of experience in vascular neuroimaging).
The calculation and analysis of hemodynamic parameters
The minimum, mean, and maximum flow velocities on the test plane of terminal ICA at peak systole before and after PED placement in both groups were calculated using commercial software (ANSYS CFX 14.0; ANSYS, Inc.). The increase rates of the flow velocities on all test planes (ICA, MCA and ACA) at peak systole after the treatment were also calculated to allow comparison among different arteries as follows: (post-treatment parameter – pre-treatment parameter)/pre-treatment parameter. For each case in the DIPH group, in order to find out if there was hemodynamic changes difference between DIPH-related and -unrelated arteries after the PED treatment, the difference of the increase rate of the velocities between the DIPH-related and -unrelated arteries were further compared.
As there was no definition of DIPH-related artery in the controls, we would like to assess if there were the same flow changes between the two distal arteries (MCA and ACA) in the control group. Therefore, in order to quantitatively evaluate whether there was balanced distribution of increased blood flow between the two distal arteries (MCA and ACA) after the PED treatment between the two groups, a new metric termed the imbalance index was introduced. This metric was the absolute difference value of the increase rates in velocity between MCA and ACA. The higher value of imbalance index represents the imbalanced blood flow distribution between MCA and ACA, which means one of the distal arteries takes a much higher flow impact than the other after the flow diversion treatment. It was calculated as follows:
where VPOST-MCA represents the velocity of the test plane at the MCA after treatment, VPRE-MCA represents the velocity of the test plane at the MCA before treatment, VPOST-ACA and VPRE-MCA represents those at the ACA. “ ” represents the symbol of “Absolute Value”.
Statistical analysis
Propensity score matching (PSM) was used to match patients with DIPH to those patients without DIPH. PSM is a recognized method of balancing covariates in two groups to reduce selection bias. 13 All quantitative hemodynamic parameters were presented as means and standard deviations. For qualitative data, the χ2 test or the Fisher’s exact test was used to compare the differences between two groups. For quantitative data, the Mann–Whitney U-test was used. Hemodynamic changes in the terminal ICA after the PED treatment were assessed by Wilcoxon’s matched-pairs signed-rank test. And the increase rate of the velocities between the DIPH-related and -unrelated arteries in the cases of DIPH group were also assessed by Wilcoxon’s matched-pairs signed-rank test. Analyses were performed with SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). The level of statistical significance was established at p < 0.05.
Results
Clinical and aneurysm characteristics in DIPH group
In DIPH group, all 6 patients were treated by PED with coils, including two men and four women (mean age, 54.2 years). Four patients were incidental findings, 1 patient presented with headache, and 1 patient with facial numbness. One was giant aneurysms (>25 mm), 4 were large (10–25 mm), and 1 was small (<10 mm). DIPH occurred in 6 cases at 12 hours, 20 hours, 2 days, 12 hours, 4 days and 7 hours after the procedure, respectively (Table 1). The hematoma located in the temporal lobe (MCA was DIPH-related artery) in 4 patients and the other 2 were in the frontal lobe (ACA was DIPH-related artery). Two patients received craniotomy after DIPH happened. The outcome of these patients was as follows: Patient 1 died after DIPH, patient 2 showed a vegetative state. The other four patients were treated conservatively and recovered from the symptoms of DIPH at the last follow-up (mRS = 1 for each case).
Table 1.
Comparison of clinical, aneurysm characteristics between DIPH group and control group.
| DIPH group (n = 6) | Control group (n = 12) | P value | |
|---|---|---|---|
| Age, y | 54.17 ± 8.70 | 56.50 ± 9.74 | 0.51 |
| Female sex, n (%) | 4 (66.7) | 8 (66.7) | 1.00 |
| Hypertension (HTN), n (%) | 4 (66.7) | 4 (33.3) | 0.18 |
| Cigarette smoking, n (%) | 2 (33.3) | 6 (50) | 0.50 |
| Symptoms | 2 (33.3) | 8 (66.7) | 0.18 |
| Aneurysm size, mm | 17.81 ± 9.71 | 16.29 ± 8.37 | 0.85 |
| Aneurysm neck, mm | 12.84 ± 4.58 | 11.91 ± 7.16 | 0.57 |
| Aspect ratio (AR) | 1.35 ± 0.33 | 1.56 ± 0.72 | 0.93 |
| Size ratio (SR) | 2.90 ± 0.92 | 2.80 ± 1.62 | 1.00 |
| Pre-mRS | 0.33 ± 0.52 | 0.67 ± 0.49 | 0.19 |
| Procedure time, h | 2.04 ± 0.25 | 1.97 ± 0.23 | 0.78 |
| Volumes of iodinated contrast | 104.67 ± 12.11 | 103.08 ± 11.21 | 0.82 |
| Post-Diastolic pressure, mmHg | 77.17 ± 9.97 | 77.50 ± 8.22 | 1.00 |
| Post-Systolic pressure, mmHg | 134.67 ± 9.85 | 136.00 ± 7.25 | 0.93 |
| Platelet inhibition | |||
| AA% inhibition | 97.50 ± 2.35 | 94.32 ± 10.81 | 0.85 |
| MA-AA | 13.53 ± 4.08 | 14.93 ± 14.85 | 0.22 |
| ADP% inhibition | 44.68 ± 25.00 | 37.61 ± 23.45 | 0.57 |
| MA-ADP | 48.30 ± 7.55 | 43.71 ± 11.63 | 0.43 |
| CYP2C19 genotype | 0.57 | ||
| 1*/1*, n (%) | 2 (33.3) | 2 (16.7) | |
| 1*/2*,1*/3*, n (%) | 3 (50) | 9 (75) | |
| 2*/2*,2*/3*,3*/3*, n (%) | 1 (16.7) | 1 (8.3) | |
| First generation PED, n (%) | 6 (100) | 12 (100) | 1.00 |
| PED diameter, mm | 4.33 ± 0.30 | 4.21 ± 0.41 | 0.60 |
| PED length, mm | 23.00 ± 4.47 | 22.58 ± 3.73 | 0.88 |
| Packing density, % | 8.18 ± 1.70 | 9.22 ± 1.91 | 0.35 |
| Angiographic result | 0.50 | ||
| Complete obliteration, n (%) | 0 (0) | 0 (0) | |
| Residual neck, n (%) | 4 (66.7) | 6 (50) | |
| Residual aneurysm, n (%) | 2 (33.3) | 6 (50) |
HTN: hypertension; Pre-: pre-operative; mRS: modified Rankin scale.
Hemodynamic characteristics of distal arteries in DIPH group
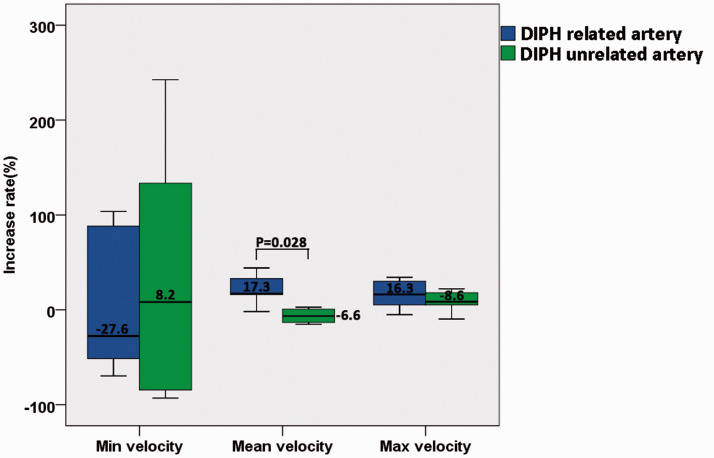
In the DIPH group, the mean and maximum velocities of the terminal ICA increased significantly after PED treatment (0.49 ± 0.24 m/s vs 0.53 ± 0.25 m/s, p = 0.046; 0.77 ± 0.37 m/s vs 0.82 ± 0.42 m/s, p = 0.028; respectively, Table 2). After the DIPH-related arteries was found out, the increase rates of velocity between the DIPH-related and DIPH-unrelated arteries of the patients in the DIPH group was compared (Figure 2). Following PED treatment, the increase rate of mean velocity in the DIPH-related artery was significantly higher than that in the DIPH-unrelated artery (20.98 ± 15.38% vs −6.40 ± 7.74%; p = 0.028).
Table 2.
Comparison of flow velocity at terminal ICA between pre- and post- treatment in two groups.
| Group | Min velocity |
Mean velocity |
Max velocity |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre (m/s) | Post (m/s) | P value | Pre (m/s) | Post (m/s) | P value | Pre (m/s) | Post (m/s) | P value | |
| DIPH group | 0.11 ± 0.17 | 0.10 ± 0.11 | 0.917 | 0.49 ± 0.24 | 0.53 ± 0.25 | 0.046* | 0.77 ± 0.37 | 0.82 ± 0.42 | 0.028* |
| Control group | 0.08 ± 0.09 | 0.15 ± 0.27 | 0.099 | 0.58 ± 0.60 | 0.62 ± 0.60 | 0.023* | 0.89 ± 0.90 | 0.98 ± 0.86 | 0.019* |
ICA: internal carotid artery; Pre: pre-operative; Post: post-operative; *p < 0.05.
Figure 2.
The comparison of increase rate of velocity between DIPH related artery and unrelated artery.
Blood flow distribution of distal arteries after treatment between DIPH and control group
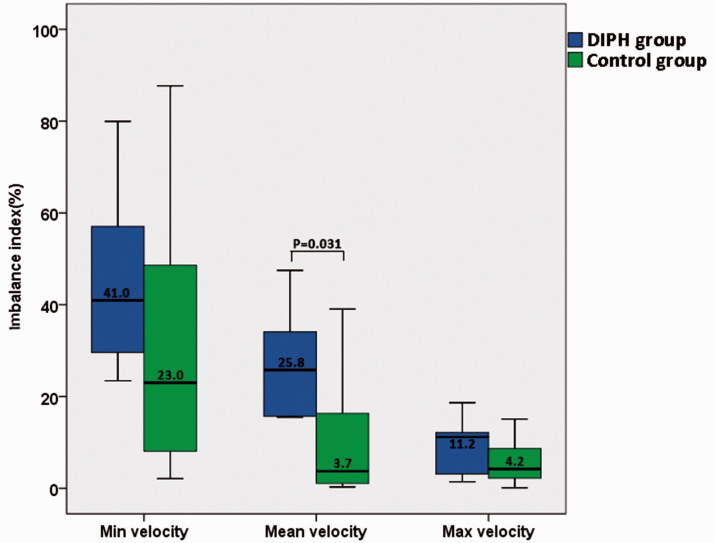
The baseline characteristics (including the clinical and aneurysmal characteristics, the hemodynamics before treatment, the increase rate of terminal ICA, MCA and ACA) between two groups were well matched, and have no statistical significance between two groups. (Tables 3 and 4) the blood flow distribution of the distal arteries (MCA and ACA) was further analyzed. The imbalance indexes of the velocity were calculated in both groups and compared the parameters between the groups. A significantly higher imbalance index of mean velocity in the DIPH group compared with the control group was found (27.38 ± 13.03% vs 10.85 ± 14.12%; p = 0.031). Comparison of the imbalance index of the velocity between DIPH group and Control group was showed in Figure 3. A DIPH examples are shown in Figure 4. The DIPH-related artery (MCA) had a significant increasing of flow velocity, while the velocity of the DIPH-unrelated artery (ACA) was unchanged (Figure 4(f)). However, the velocity in all arteries (terminal ICA, MCA, and ACA) increased balanced after PED treatment in a control example (Figure 5).
Table 3.
Wilcoxon signed-rank test for the velocity before treatment between DIPH group and control group.
| Terminal ICA |
MCA |
ACA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DIPH group | Control group | P value | DIPH group | Control group | P value | DIPH group | Control group | P value | |
| Min velocity (m/s) | 0.11 ± 0.17 | 0.08 ± 0.09 | 0.925 | 0.06 ± 0.06 | 0.15 ± 0.18 | 0.349 | 0.04 ± 0.04 | 0.04 ± 0.17 | 0.454 |
| Mean velocity (m/s) | 0.49 ± 0.24 | 0.58 ± 0.60 | 0.708 | 0.57 ± 0.36 | 0.67 ± 0.64 | 0.779 | 0.46 ± 0.25 | 0.49 ± 0.31 | 0.841 |
| Max velocity (m/s) | 0.77 ± 0.37 | 0.89 ± 0.90 | 0.454 | 0.91 ± 0.55 | 0.84 ± 0.74 | 0.841 | 0.67 ± 0.40 | 0.67 ± 0.49 | 1.000 |
ICA: internal carotid artery; MCA: middle cerebral artery; ACA: anterior cerebral artery; Pre: pre-operative; Post: post-operative.
Table 4.
Wilcoxon signed-rank test for increase rate of velocity between DIPH group and control group.
| Terminal ICA |
MCA |
ACA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DIPH group | Control group | P value | DIPH group | Control group | P value | DIPH group | Control group | P value | |
| Min velocity (%) | 1.57 ± 58.12 | 24.51 ± 33.29 | 0.385 | –16.59 ± 41.79 | 8.65 ± 49.84 | 0.250 | –11.29 ± 91.36 | 18.41 ± 25.15 | 0.892 |
| Mean velocity (%) | 42.89 ± 74.21 | 48.85 ± 63.44 | 1.000 | 28.94 ± 96.97 | 6.23 ± 79.32 | 0.213 | 4.00 ± 88.16 | 6.02 ± 83.58 | 0.750 |
| Max velocity (%) | 20.22 ± 16.16 | 35.89 ± 34.03 | 0.682 | 19.79 ± 24.57 | 16.79 ± 25.42 | 0.682 | 27.02 ± 43.98 | 23.12 ± 27.94 | 1.000 |
ICA: internal carotid artery; MCA: middle cerebral artery; ACA: anterior cerebral artery; Pre: pre-operative; Post: post-operative.
Figure 3.
Comparison of the imbalance index of distal arteries in velocity between DIPH group and control group. The imbalance index of mean velocity between the DIPH and the controls showed statistically significant difference, and the median value was 25.8 and 3.7.
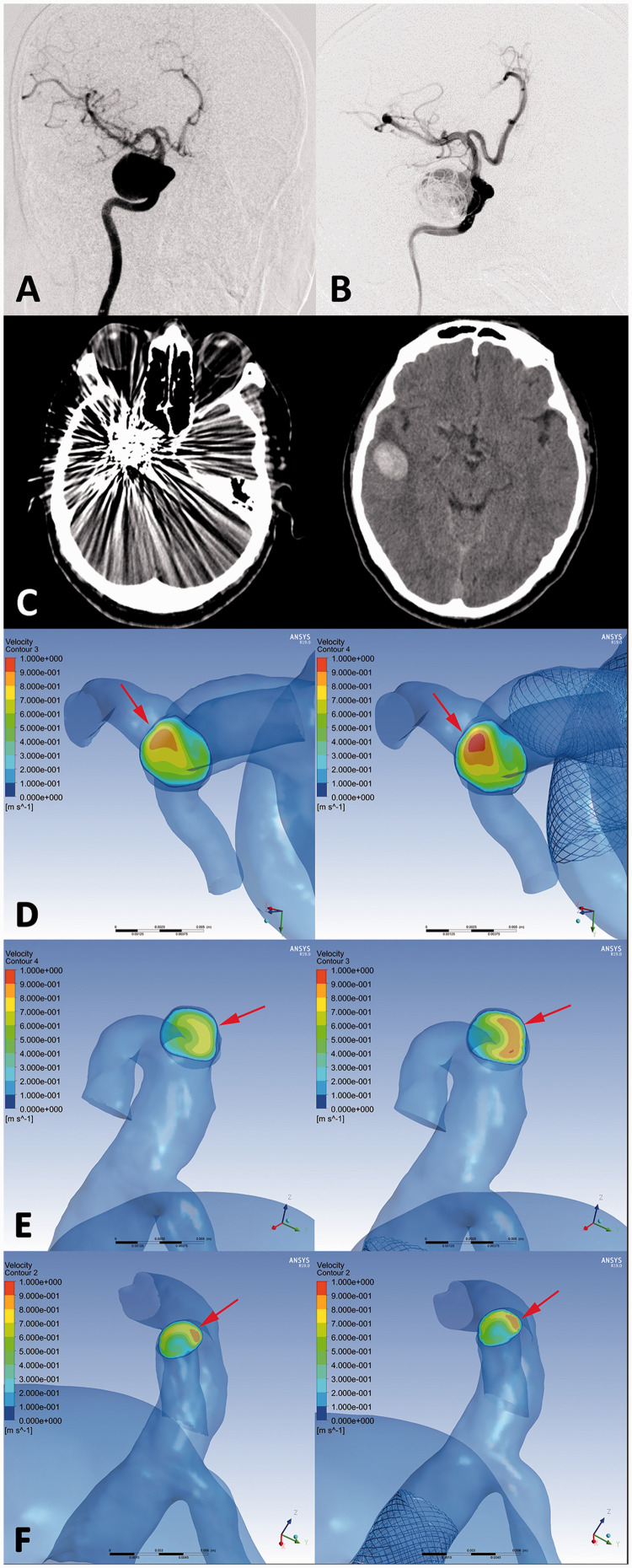
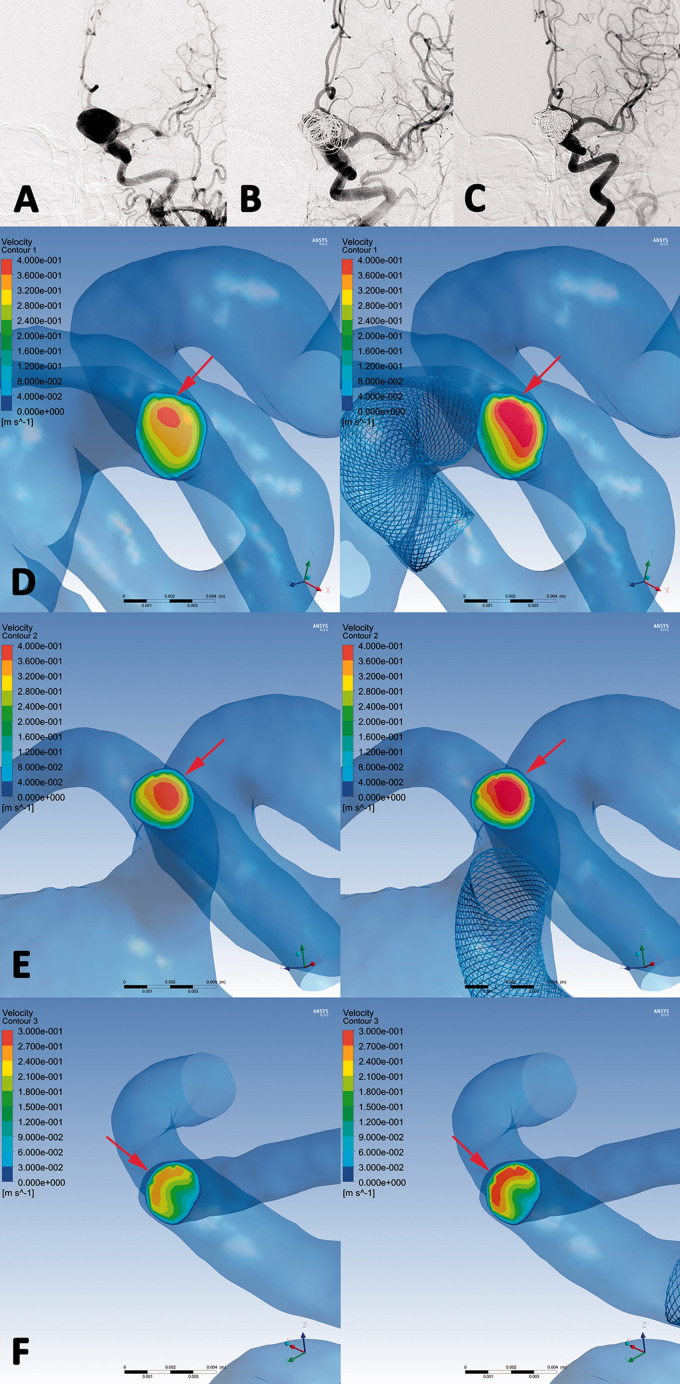
Figure 4.
An ophthalmic segment aneurysm (DIPH 3 in Supplemental Digital Content 1) of right internal carotid artery was treated with the first-generation PED assisted coiling. (a, b) The aneurysm showed residual sac filling at postprocedural immediate angiography(B). CT images showed the coiled aneurysm and DIPH at ipsilateral temporal lobe was found at 2 days later after the procedure (c). In hemodynamic simulation, the velocity on the cross section of terminal internal carotid artery was increased significantly compared with preprocedural results. (Increase rate =10.3%) (d, arrows) Furthermore, the flow velocity of DIPH related artery (middle cerebral artery) have significant increasing after PED treatment (Increase rate =19.6%) (e, arrows), while the velocity of DIPH unrelated artery (anterior cerebral artery) showed no significant change (Increase rate =0.9%) (f, arrows). The imbalance index of distal arteries was 18.7%.
Figure 5.
An ophthalmic segment aneurysm without DIPH (Control 5 in Supplemental Digital Content 1) of left internal carotid artery was treated with the first-generation PED assisted coiling. (a, b) The aneurysm showed residual sac filling at postprocedural immediate angiography (b), and complete occlusion at 6 months follow-up (c). In hemodynamic simulation, the velocity on the cross section of terminal internal carotid artery was increased significantly compared with preprocedural results. (Increase rate =11.1%) (D, arrows) Furthermore, the flow velocity of distal arteries (middle cerebral artery and anterior cerebral artery) have also significant increasing after PED treatment (Increase rate =13.5% and 12.8% respectively) (e, f, arrows) The imbalance index of distal arteries was 0.7.
Discussion
All aneurysms in this study were located in the ICA and were treated by PED coiling. In DIPH group, the mean velocity increased significantly more in the DIPH-related artery compared with the DIPH-unrelated artery in DIPH group. In DIPH and control groups, the mean and maximum flow velocities in the terminal ICA increased significantly after treatment. No baseline characteristics (including clinical, aneurysm and hemodynamic factors) showed significant between the DIPH and control group. However, we found that the imbalance in flow distribution was greater in the DIPH group compared with control group. These results suggest that an imbalance in increasing mean velocity in the two distal branches might be a hemodynamic risk factor in DIPH process, which may predispose patients to DIPH after the PED treatment.
Potential mechanism of DIPH after flow diversion
The incidence of delayed parenchymal hemorrhages after flow diversion is about 3%, over 80% occurred in the ipsilateral vascular territory of the flow diverter, and approximately 20% occurred in contralateral vascular territories.6,20 For the contralateral parenchymal hemorrhages, antiplatelet therapy might play an important role, for which contralateral vascular territories rarely cause deleterious hemodynamics or ischemic complications. However, no studies have demonstrated an association between the risk of hemorrhagic complications and dual antiplatelet therapy in platelet tests.21,22 Similar results showed in the study, there was no statistical significance in platelet function factors between DIPH and control group. The ipsilateral hemorrhages maybe support that the device and/or the procedure itself was the deleterious causal role. Some studies reported that DIPH might be related to the interventional materials or procedure used, such as perforation with the distal wire. 6 One histopathologic study reported that three patients died after PED-associated intracranial hemorrhage, and found foreign material in the distal vasculature of the hemorrhagic lesion in each case. 9 The foreign material might further cause thrombosis and vascular inflammation resulting in vascular injuries. However, Hu et al. 9 found no evidence of such inflammation in association with these emboli following PED treatment. Infarctions with subsequent hemorrhagic transformation may be another potential explanation for DIPH.7,8,23 Barnwell et al. 24 reported that foreign bodies were found in the small arteries after neurointerventional procedures in four patients, but only one patient suffered thalamic hemorrhage and the other three patients presented with infarctions. Another study reported many ischemic events in the respective organs during interventional procedures, while hemorrhagic events were rarely encountered on autopsy. 25 In our study, postprocedural MRI to detect silent ischemic strokes was not routinely performed in our center, for which a routine MRI evaluation after endovascular treatment is not required and clinical significance of positive diffusion weighing imaging lesions is not clear. 26 Li et al. 13 found that hemodynamics played an important role in postoperative aneurysm rupture after flow diversion, and thus we speculated that hemodynamic factors might also play an important role in the process of DIPH occurrence.
Hemodynamic factors related to DIPH after flow diversion
Flow diverters are approved for blood flow diversion for the endovascular treatment of large or giant wide-necked intracranial aneurysms, by diverting blood flow from the aneurysm to the parent artery and distal branches. Murakami et al. 27 reported a successful clip operation for a giant ICA aneurysm and found that increased perfusion of the ipsilateral cerebral cortex, exceeding cerebral autoregulatory abilities, led to cerebral hyperperfusion. This ‘reperfusion syndrome’ is related to a sudden increase in regional cerebral blood flow secondary to loss of cerebrovascular autoregulation. A similar phenomenon was found in aneurysms treated by flow diverters, for which the stent redirects most of the blood flow of a giant aneurysm into the parent artery. Chiu et al. 28 reported a case of ‘reperfusion syndrome’ following flow-diverter treatment of a large aneurysm, supported by the clinical presentation and hyperperfusion on CT perfusion. Such flow changes of distal arteries after flow diversion might be an important hemodynamic risk factor potentially resulting in DIPH. Similar results also showed in our study, which had significant increasing of flow velocity in distal parent artery after PED treatment.
Several studies have explored the hemodynamics of the parent vessel and distal intracranial artery in patients with DIPH following PED. Higher blood flow velocity and pressure in the distal arteries were treated as two important hemodynamic factors illustrating the mechanism of DIPH after flow-diverter placement. However, Mitha et al. 29 described a novel electrical analog model to evaluate the likely hemodynamic effect of stent placement, and found that minor increases in distal pressure were unlikely to be the main cause of the DIPH. In contrast, Cruz et al. 8 found an increase in blood flow in the parent artery after PED placement, and hypothesized that the delayed intraparenchymal hemorrhage was due to hemodynamic modifications of flow diversion. Velat et al. 7 reported on a patient in whom a complex intracranial aneurysm was treated successfully with PED but who developed DIPH 5 days later, and suggested that blood flow redirection by PED might have caused a spontaneous intraparenchymal hemorrhage. However, these were clinical studies with no direct evidence to support such a hypothesis.
Chen et al. 12 evaluated arterial flow after PED placement using quantitative flow measurements based on digital subtraction angiography, and found improved flow in the terminal ICA, MCA, and ACA. These results might support the occurrence of ‘reperfusion syndrome’ with quantitative flow measurements after PED treatment. However, the relationship between hemodynamic alterations and the occurrence of DIPH is unclear. Brunozzi et al. 11 assessed changes in MCA flow velocity after PED deployment by transcranial Doppler in two patients with DIPH and eight patients without DIPH. Ipsilateral MCA velocity increased more in patients with DIPH, suggesting a possible role of distal intracranial hemodynamic alterations in the occurrence of DIPH after PED treatment of cerebral aneurysms. However, the number of patients with DIPH was small and the control group was unmatched in terms of location of aneurysm and patient age, and bias could therefore not be ruled out. Moreover, blood in the DIPH region might originate from a different distal artery, and should be analyzed separately. In our study, we found out the DIPH-related and -unrelated arteries (MCA or ACA) and compared the hemodynamic alterations between DIPH-related and -unrelated arteries, to provide an internal control for the analysis of possible factors linked to DIPH. We found that the mean flow velocity increased more in the DIPH-related compared with the DIPH-unrelated artery. We also compared the blood flow distribution between the DIPH and control groups, and found a significantly higher imbalance index in the DIPH group. An imbalance in increased blood flow distribution might be an important hemodynamic factor associated with DIPH. The imbalance distribution of increased flow velocity at distal arteries would increase more burden of the artery related to hemorrhage. The artery with more cerebral blood flow might result to blood brain barrier disruption due to sudden hemodynamic change. 30 Blood brain barrier disruption may be associated with DIPH after flow diversion.
Limitations
There were several limitations related to the design of the present study. The first was its retrospective design and small sample size, since DIPH is a rare complication after flow diversion. Secondly, the mechanism of DIPH following flow diversion is probably multifactorial, and may include factors such as thrombus formation and inflammation. Post-procedural MRI results might be helpful to clarify the hemorrhagic transformation from the small infarct, which was not performed routinely in this study. A larger prospective study included MRI results after the procedure from a multicenter database is needed in the future. Thirdly, the post-procedure CT at follow up might be important to exclude the micro-hemorrhage in patients at control group, and should be pay more attention in future study. Fourthly, no patients with contralateral intraparenchymal hemorrhage were included in our study, this mechanism might differ from that of DIPH and needs to be studied separately. Lastly, several limitations of CFD hemodynamic analysis should be considered, and assumptions such as boundary conditions, a rigid wall, laminar flow and Newtonian blood, may affect the hemodynamic results. Moreover, the computational modeling of the stent and coils might not fully reflect the actual situation of endovascular treatment. However, these assumptions were previously reported to be useful for simulating and analyzing flow hemodynamics in aneurysms. 14 There are limitations with the use of the assumptions in CFD hemodynamic analysis as extrapolating findings. Therefore, the findings are exploratory, and further validation with directly measured hemodynamics methods (eg. TCD or MRA CFD) is necessary.
Conclusion
The flow velocities in distal parent artery to the aneurysm might be increase after flow diversion treatment. The mean velocity in DIPH-related artery increased much higher and the imbalanced flow distribution between MCA and ACA might be the risk of DIPH, and might provide a possible indicator for predicting such complications.
Supplemental Material
Supplemental material, sj-pdf-1-ine-10.1177_15910199211009120 for Imbalanced flow changes of distal arteries: An important factor in process of delayed ipsilateral parenchymal hemorrhage after flow diversion in patients with cerebral aneurysms by Wenqiang Li, Wei Zhu, Jian Liu and Xinjian Yang in Interventional Neuroradiology
Supplemental material, sj-pdf-2-ine-10.1177_15910199211009120 for Imbalanced flow changes of distal arteries: An important factor in process of delayed ipsilateral parenchymal hemorrhage after flow diversion in patients with cerebral aneurysms by Wenqiang Li, Wei Zhu, Jian Liu and Xinjian Yang in Interventional Neuroradiology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Key Research and Development Plan of China (grant number: 2016YFC1300800), the National Natural Science Foundation of China (grant numbers: 81220108007, 81801156, 81801158, 81471167 and 81671139) and the Capital's Funds for Health Improvement and Research (grant number: 2018-4-1077). Beijing Hospitals Authority Youth Programme (code: QML20190503).
Ethics approval: This study was approved by the ethics committee of Beijing Tiantan Hospital. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
ORCID iDs: Jian Liu https://orcid.org/0000-0002-5454-2847
Xinjian Yang https://orcid.org/0000-0001-7306-0125
Supplemental material: Supplemental material for this article is available online.
References
- 1.Murthy SB, Shah J, Mangat HS, et al. Treatment of intracranial aneurysms with pipeline embolization device: newer applications and technical advances. Curr Treat Options Neurol 2016; 18: 16. [DOI] [PubMed] [Google Scholar]
- 2.Leung GK, Tsang AC, Lui WM. Pipeline embolization device for intracranial aneurysm: a systematic review. Clin Neuroradiol 2012; 22: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallmes DF, Brinjikji W, Cekirge S, et al. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 2017; 127: 775–780. [DOI] [PubMed] [Google Scholar]
- 4.Yu SC, Kwok CK, Cheng PW, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device – a prospective study in 143 patients with 178 aneurysms. Radiology 2012; 265: 893–901. [DOI] [PubMed] [Google Scholar]
- 5.Chalouhi N, Starke RM, Yang S, et al. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke 2014; 45: 54–58. [DOI] [PubMed] [Google Scholar]
- 6.Rouchaud A, Brinjikji W, Lanzino G, et al. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview. Neuroradiology 2016; 58: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velat GJ, Fargen KM, Lawson MF, et al. Delayed intraparenchymal hemorrhage following pipeline embolization device treatment for a giant recanalized ophthalmic aneurysm. J Neurointerv Surg 2012; 4: e24. [DOI] [PubMed] [Google Scholar]
- 8.Cruz JP, Chow M, O'Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 2012; 33: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the pipeline embolization device. J Neurosurg 2014; 120: 365–374. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, Cloft HJ, Fiorella D, et al. Estimating the proportion of intracranial aneurysms likely to be amenable to treatment with the pipeline embolization device. J NeuroIntervent Surg 2013; 5: 45–48. [DOI] [PubMed] [Google Scholar]
- 11.Brunozzi D, Shakur SF, Hussein AE, et al. Middle cerebral artery flow velocity increases more in patients with delayed intraparenchymal hemorrhage after pipeline. J Neurointerv Surg 2018; 10: 249–251. [DOI] [PubMed] [Google Scholar]
- 12.Chen CW, Wong HF, Ye YL, et al. Quantitative flow measurement after placing a flow diverter for a distal internal carotid artery aneurysm. J Neurointerv Surg 2017; 9: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Tian Z, Zhu W, et al. Hemodynamic analysis of postoperative rupture of unruptured intracranial aneurysms after placement of flow-diverting stents: a matched case-control study. AJNR Am J Neuroradiol 2019; 40: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Jing L, Liu J, et al. Predisposing factors for recanalization of cerebral aneurysms after endovascular embolization: a multivariate study. J Neurointerv Surg 2018; 10: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Tian Z, Liu J, et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with enterprise stents and the pipeline device. J Transl Med 2016; 14: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsos AP, Kakalis NM, Ventikos YP, et al. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model: technical note. Neuroradiology 2008; 50: 341–347. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Meng Z, Zhang Y, et al. Phantom-based experimental validation of fast virtual deployment of self-expandable stents for cerebral aneurysms. Biomed Eng Online 2016; 15: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Liu J, Zhang Y, et al. Efficient simulation of a low-profile visualized intraluminal support device: a novel fast virtual stenting technique. Chin Neurosurg J 2018; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savoiardo M. The vascular territories of the carotid and vertebrobasilar systems. Diagrams based on CT studies of infarcts. Ital J Neurol Sci 1986; 7: 405–409. [DOI] [PubMed] [Google Scholar]
- 20.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 21.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364: 331–337. [DOI] [PubMed] [Google Scholar]
- 22.Brinjikji W, Lanzino G, Cloft HJ, et al. Risk factors for hemorrhagic complications following pipeline embolization device treatment of intracranial aneurysms: Results from the international retrospective study of the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 2308–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer S, Vajda Z, Aguilar PM, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012; 54: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnwell SL, D’Agostino AN, Shapiro SL, et al. Foreign bodies in small arteries after use of an infusion microcatheter. AJNR Am J Neuroradiol 1997; 18: 1886–1889. [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RI, Mehta RI, Solis OE, et al. Hydrophilic polymer emboli: an under-recognized iatrogenic cause of ischemia and infarct. Mod Pathol 2010; 23: 921–930. [DOI] [PubMed] [Google Scholar]
- 26.Meyers PM, Schumacher HC, Higashida RT, et al. Reporting standards for endovascular repair of saccular intracranial cerebral aneurysms. Stroke 2009; 40: e366–e379. [DOI] [PubMed] [Google Scholar]
- 27.Murakami H, Inaba M, Nakamura A, et al. Ipsilateral hyperperfusion after neck clipping of a giant internal carotid artery aneurysm. Case report. J Neurosurg 2002; 97: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 28.Chiu AH, Wenderoth J. Cerebral hyperperfusion after flow diversion of large intracranial aneurysms. J Neurointerv Surg 2013; 5: e48. [DOI] [PubMed] [Google Scholar]
- 29.Mitha AP, Mynard JP, Storwick JA, et al. Can the windkessel hypothesis explain delayed intraparenchymal haemorrhage after flow diversion? A case report and model-based analysis of possible mechanisms. Heart Lung Circ 2015; 24: 824–830. [DOI] [PubMed] [Google Scholar]
- 30.van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005; 4: 877–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ine-10.1177_15910199211009120 for Imbalanced flow changes of distal arteries: An important factor in process of delayed ipsilateral parenchymal hemorrhage after flow diversion in patients with cerebral aneurysms by Wenqiang Li, Wei Zhu, Jian Liu and Xinjian Yang in Interventional Neuroradiology
Supplemental material, sj-pdf-2-ine-10.1177_15910199211009120 for Imbalanced flow changes of distal arteries: An important factor in process of delayed ipsilateral parenchymal hemorrhage after flow diversion in patients with cerebral aneurysms by Wenqiang Li, Wei Zhu, Jian Liu and Xinjian Yang in Interventional Neuroradiology