Abstract
Background
Spontaneous spinal epidural hematoma (SSEH) is rare in children. Vascular malformation including arteriovenous fistulas and venous malformation is a rare cause of SSEH.
Case description
A 5-year-old girl presented with 2 episodes of SSEH at the upper thoracic spine and non-hemorrhagic episodes with spontaneous neurological recovery. Diagnostic study with MRI and spinal angiography demonstrated an unusual epidural arteriovenous fistula (AVF) with venous ectasia similar to venous malformation. She underwent embolization of the AVF with NBCA with mild transient neurological deterioration. Follow up angiography showed persistent occlusion of the embolized fistula and inconsistent visualization of another AVF to the patent venous ectasia.
Conclusions
This type of epidural AVF seems to be more common in children and tends to cause multiple neurologic episodes due to SSEH, venous expansion or thrombosis. Endovascular embolization with NBCA should be the first choice of treatment for this disease, unless emergent hematoma evacuation is necessary. Embolization should target at only the fistula site without significant penetration into the venous ectasia. Follow up is necessary for potential reappearance of AVF, even if AVF is occluded at the time of treatment. Time resolved MRI is useful to detect AVFs, thus for diagnosis and follow up of this disease.
Keywords: Spinal epidural hematoma, arteriovenous fistula, venous ectasia, embolization, pediatric
Introduction
The spontaneous spinal epidural hematoma (SSEH) rarely occurs mostly dorsal to the spinal cord. The cause of the SSEH is found in only 50–60% of cases, 1 which includes inherited or acquired coagulopathy, vascular malformation, infection, pregnancy, and hypertension. The vascular malformation as a cause of SSEH is mostly diagnosed by the intraoperative findings and pathology of the evacuated specimen. They are described by various names including arteriovenous fistula, venous malformation, and unclassified abnormal vein, but the details are not well understood.
We report a 5-year-old girl who presented with SSEH due to an unusual epidural arteriovenous fistula (AVF) with venous ectasia, similar to venous malformation.
Case description
History
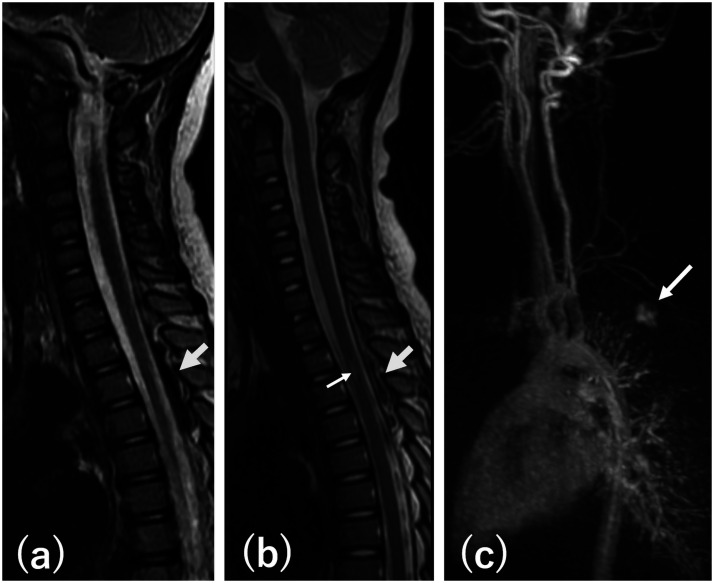
A 5-year-old girl initially presented with sudden onset of back pain and significant bilateral lower extremity weakness 16 months ago. There was no sensory or bowel/bladder disturbance. The symptoms completely disappeared within 24 hours but recurred next day. MRI obtained 3 days after the initial onset was interpreted as negative but retrospectively noticed showing a dorsal epidural mass at the T2-T4 levels suggesting a hematoma. The mass was low signal intensity on T2 (Figure 1(a)) and iso-intensity with an area of high intensity on T1 weighted images without contrast enhancement. She was admitted to an outside institution and treated with steroid pulse under the diagnosis of transverse myelitis. On admission, her leg weakness was 4/5 bilaterally without sensory or bowel/bladder disturbance. She completely recovered over 7 days and was discharged. She had been asymptomatic until 16 months later when she developed 4 episodes of sudden onset of severe back pain between the scapulas in one day, each one lasting for 10–40 minutes. No neurological deficits were noted. Two weeks later, she developed similar back pain associated with 2/5 weakness of both legs, numbness below the abdomen, and difficulty in urination. She was admitted to an outside institution where MRI of the spine showed a non-enhancing dorsal epidural mass at the T2-T4 vertebral levels with mixed density on T1 and T2 weighted images, suggesting a recurrent hematoma. This was associated with an area of T2 high signal abnormality within the spinal cord indicating edema (Figure 1(b)). She was treated with steroid pulse with complete neurological recovery over 7 days. Repeat MRI study 7 days after initiation of the steroid treatment showed decreasing size of the epidural mass and disappearance of T2 high signal abnormality in the spinal cord. Time Resolved Imaging of Contrast KineticS (TRICKS) showed a suspicious upper thoracic AVF (Figure 1(c)). She was then referred to us for further work up and treatment for the suspected spinal epidural arteriovenous fistula (SEDAVF).
Figure 1.
(a) T2 weighted sagittal MRI at the first presentation showing a dorsal epidural mass with low signal intensity extending from T2 to T4 (arrow) indicating epidural hematoma. (b) T2 weighted sagittal MRI at the second presentation showing a dorsal epidural mass with mixed signal intensity extending from T2 to T4 (large arrow) indicating epidural hematoma. There is an area of increased signal inside the spinal cord (small arrow) indicating edema. (c) TRICKS image showing early visualization of a dilated dorsal epidural vein (arrow) indicating existence of AVF.
Angiography, treatment, and follow up
On admission to our hospital, she was neurologically intact. Angiographic study showed an AVF supplied by the bilateral T3 pre-laminar arteries (Figure 2(a) to (c)). The draining vein was an enlarged dorsal epidural vein extending from T2 to T4 vertebral levels with protrusion posteriorly between the T3 and T4 spinous processes with stagnation of contrast material with further slow superior drainage through the paraspinal vein. No intradural venous reflux was noted. There was demonstration of the posterior spinal arteries (PSAs) from both T3 segmental arteries and the anterior spinal artery (ASA) from the left T4 segmental artery. This AVF was thought to be the cause of hemorrhage, for which she underwent endovascular treatment (Figure 2(d) to (f)). A Magic 1.2 FM microcatheter (Balt Montmorency France) was advanced to the pre-laminar artery beyond the origin of the left PSA thorough the left T3 segmental artery, where 25% n butyl cyanoacrylate (NBCA) mixed with lipiodol was injected, resulting in occlusion of the AVF. Postoperative angiography showed disappearance of the AVF with preservation of the ASA and PSAs. Stagnation of contrast material was noted in the venous ectasia (Figure 3(a) to (d)). Postoperatively, the patient developed 4/5 weakness of the left leg and urinary urgency. The patient was treated with steroid considering the etiology to be inflammation due to venous thrombosis related to embolization or NBCA itself, although repeat MRI showed no abnormal signal in the spinal cord. Her motor weakness improved within 3 days but urinary urgency persisted with slow improvement with frequent urinary tract infection. Eighteen months after the treatment, she was neurologically intact except for mild urinary frequency with no more frequent urinary tract infection. Follow up MRI showed resolution of the epidural hematoma and large enhancing dorsal epidural vein extending from T1 to T4 vertebral levels (Figure 4(a)), which was visualized at the normal venous phase on 4 D time-resolved angiography using keyhole (4 D-TRAK). Follow up angiography at that time showed persistent occlusion of the embolized AVF (Figure 4(b)). Right T3 intercostal angiogram, however, showed another AVF to the same epidural venous ectasia, supplied by a T2 or T3 segmental artery branch (Figure 4(c)). Further angiographic study to identify the feeder to the AVF using a microcatheter failed to demonstrate the AVF. Two repeat global injections of the right T3 intercostal artery (Figure 4(d)) and one injection of the right supreme intercostal artery supplying the T1 and T2 segmental arteries also failed to demonstrate the AVF. CT like image obtained after angiogram showed stagnation of contrast material within the venous ectasia (Figure 4(e)).
Figure 2.
Spinal angiography and embolization (a) PA view of the right T3 intercostal artery angiogram showing AVF draining to the dilated epidural vein (large arrow). The small arrow indicates the right PSA. (b) and (c) PA view of the left T3 intercostal artery angiogram in early (b) and late (c) phases showing AVF draining to the dilated epidural vein (large arrow in b). The small arrow indicates the left PSA. Angled arrow indicates the pre-laminar artery feeder to the AVF. Progressive expansion and stagnation of contrast material in the large irregular epidural vein with slow further venous drainage (arrow in c) is similar to venous malformation. (d) PA view of superselective angiogram of the left T3 pre-laminar artery showing the AVF and the left PSA. Arrow head indicates the tip of the microcatheter. (e) PA view of superselective angiogram of the left T3 pre-laminar artery from the further advanced microcatheter showing the AVF and the right PSA. The arrow head indicates the tip of the microcatheter. (f) Cast of NBCA occluding the fistula site with minimal penetration into the venous side. The tip of the microcatheter (arrow head) is slightly proximal to the one in (e) and at the point indicated by the arrow in (d).
Figure 3.
Spinal angiography after embolization. (a) PA oblique radiogram obtained just before embolization showing irregular stagnation of contrast material showing the entire extension of the venous ectasia. There is filling defect (arrow heads) indicating hematoma, corresponding with the filling defect seen in (d). The arrow indicates the tip of the microcatheter. (b) and (c) PA views of the post embolization angiogram of the right (a) and left (b) T3 intercostal arteries showing occlusion of the AVF. Angled arrow in (c) indicates the embolized pre-laminar artery. (d) Sagittal reconstruction of CT like image obtained after embolization showing stagnation of contrast material in the dilated dorsal epidural vein containing areas of filling defect, which looks exactly the same as the area of low signal intensity associated with the epidural mass seen in Figure 1(b).
Figure 4.
(a) Contrast enhanced T1 weighted sagittal MRI before the follow up angiogram, showing enhancement of the dilated dorsal epidural vein extending from T1 to T4 vertebral levels. (b–d) PA views of follow-up angiogram obtained 18 months after the embolization. (b) Left T3 intercostal artery angiogram showing no visualization of the AVF. Embolized prelaminar artery is decreased in size (angled arrow). The left PSA is preserved. (c) The first right T3 intercostal artery angiogram showing an AVF (arrow) not seen on the previous post embolization angiogram, supplied either by a T2 or T3 intercostal artery branch. (d) Repeat right T3 intercostal artery angiogram showing no visualization of the AVF. (e) Sagittal reconstruction of CT like image obtained after angiogram showing stagnation of contrast material in the dilated dorsal epidural vein.
At 20 months after the treatment, she was neurologically intact with mild urinary frequency without recurrence of symptoms.
Discussion
Spinal epidural hematoma typically presents with acute onset of severe back pain followed by development of clinical signs and symptoms of spinal cord dysfunction. The symptoms, however, can be subacute and nonspecific, especially in children, resulting in initial misdiagnosis such as transverse myelitis as in this case. 2 Most of SSEH arises dorsal to the spinal dura and commonly at the cervico-thoracic region, especially in children. 2 The source of hemorrhage seems to be the epidural vein which is valveless plexus unprotected from sudden fluctuation of intrathoracic or intra-abdominal pressure. 2 Vascular malformation is a rare cause of SSEH and two types, arteriovenous and venous, are described. Presence of AVF seems to be underestimated because spinal angiography is not frequently performed before and even after emergency evacuation of hematoma. Intraoperative findings or pathology of the surgically removed specimen sometimes identify a vascular malformation including venous malformation, but often fail to specify its type.
Yu et al. 3 reported 55 cases of SSEH. Among them, 29 patients were treated with microsurgery and a dilated vascular structure was detected in 13 cases (44.8%) during microsurgery and by pathology. Of these 13 cases, 12 underwent preoperative angiography and 3 showed a SEDAVF. The rest of 9 cases might have a venous malformation or a small slow flow SEDAVF difficult to detect by standard angiography. Discovery of a vascular malformation by surgery with negative angiography is also reported by others.1,4,5 Pathologically confirmed venous malformation as a cause of SSEH is also reported. 6
In Yu et al.’s series, forty patients received preoperative spinal angiography, and SEDAVF was detected in 6 patients (15%). Among them, 5 were a small slow-flow SEDAVF fed by a small branche of the segment artery and exclusively drained by the spinal cord epidural venous plexus without a venous pouch.3,7 These 5 cases are consistent with the commonly discovered SEDAVF as a cause of dorsal SSEDH, because the dorsal epidural vein is normally small.8,9 He described another case of SEDAVF which drained to a dilated epidural venous pouch. Another similar SEDAVF draining to a dilated epidural vein was described by Zhang, et al, which presented with mass effect without SSEH. 10
Venous malformation with AVF is well known to exist and to cause more swelling thus more symptoms than simple venous malformation in the field of body intervention. 11 Although it has never been reported in the spinal epidural space, we would like to point the similarity of our case to venous malformation with AVF. First, the AVF drained to an exceptionally large irregular venous lake where flow was very stagnant with long lasting stasis of contrast material, as demonstrated in two angiographic studies, typical for venous malformation. Secondly, the second angiographic study after occlusion of the main AVF demonstrated inconsistent visualization of an AVF, which is not impossible but unusual for general AVFs and quite possible for venous malformation depending on the venous pressure and angiographic parameters. The reason for inconsistent visualization of AVF in our case is not likely due to the difference in the venous pressure, because the airway pressure was controlled by general anesthesia and unchanged during the study. Angiographic injection rate and pressure were the same among the repeated studies. One possibility is that the catheter was in a semi-wedged position for the first injection, causing a high pressure to the feeder, thus opacifying the arteriovenous connection. Inconsistent angiographic demonstration of vascular malformation was also reported by Abram et al. 12 for a 10-year-old girl with multiple neurological episodes including one documented SSEH, but without detailed angiographic description.
Mass effect due to venous hypertension, thrombosis of the vein, or inflammation may cause non-hemorrhagic acute episodes10,13–15 which tend to be repetitive and seem to be more common in children and with vascular malformation. 3 Our patient experienced multiple episodes including 2 SSEHs demonstrated by MRI. Other minor episodes seemed to be non-hemorrhagic and probably due to venous expansion or inflammation secondary to venous thrombosis, although minor hemorrhages cannot to be ruled out.
In the literature, two cases similar to ours were reported by Chuang et al. 13 They were both 4 years old (a boy and a girl) and the AVF was at the dorsal epidural space at the cervico-thoracic junction. The AVF was slow flow with pooling of blood within a large epidural venous pouch. The first case presented with SSEH and had multiple AVFs. Embolization of the main AVF with significant penetration of NBCA into the venous pouch resulted in acute deterioration, probably due to acute venous expansion or hemorrhage secondary to compromised venous outflow. The second case presented with only pain due to mass effect, which improved by conservative management with decrease in the size of the venous pouch. Chuang et al. thought these epidural pouches may be acquired venous pseudoaneurysms due to a small rupture of an epidural vein. It makes, however, more sense to consider that these cases represent epidural AVF with venous ectasia.
For treatment, endovascular embolization should be the first choice rather than an extensive surgery to remove the entire venous pouch, unless emergent hematoma evacuation is necessary. Embolization for this kind of AVF should be focused on closing only the fistula point even without visualization of the other AVFs at the time of treatment. Our case also had mild deterioration of neurological function after embolization despite closing only the fistula point, probably due to inflammation caused by thrombosis or NBCA itself. Significant venous occlusion could have resulted in much more significant deterioration because of outflow restriction of the potential additional AVF, which was inconsistently demonstrated on the second angiography.
Based on the reports of the similar cases in the literature, this disease seems to be more common in children and tends to cause multiple neurologic episodes due to SSEH, venous expansion or thrombosis. Although epidural AVF with venous ectasia may be more widely accepted as the diagnosis for this disease rather than venous malformation with AVF, the ectatic vein needs special attention for treatment and follow up. Embolic material should not penetrate too much into the venous ectasia as mentioned above. After embolization of the AVF, continuous follow up is necessary even if AVF is occluded, because of potential development of another AVF and recurrent hemorrhage or non-hemorrhagic symptoms. The lesion should not be considered cured unless the venous ectasia completely thromboses. We do not regard this case to be cured and plan to follow the patient clinically as well as with periodical MRI. Time resolved MRI such as TRICKS or 4 D-TRAK is non-invasive and sensitive to detect AVFs as well as enhancing venous ectasia, therefore, useful for diagnosis and follow up for this disease.
Footnotes
Ethical approval statement: All procedures performed in this case report involving human participant were in accordance with the ethical standards of the institutional and/or national research committee (Saint Luke’s International Hospital 19-200) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions: YN and SS initiated the project in this manuscript. YN wrote the manuscript. All authors participated the treatment and discussed the results and commented on the paper. This manuscript has not been published or presented elsewhere in part or in its entirety, and is not under consideration by another journal. All the authors have approved the manuscript and agree with submission to this esteemed journal.
Informed consent: The patient provided written informed consent and consented to the submission of the case report to the journal.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Yasunari Niimi https://orcid.org/0000-0003-2239-6432
Shogo Shima https://orcid.org/0000-0002-2128-5602
References
- 1.Zhong W, Chen H, You C, et al. Spontaneous spinal epidural hematoma. J Clin Neurosci 2011; 18: 1490–1494. [DOI] [PubMed] [Google Scholar]
- 2.Patel H, Boaz JC, Phillips JP, et al. Spontaneous spinal epidural hematoma in children. Pediatr Neurol 1998; 19: 302–307. [DOI] [PubMed] [Google Scholar]
- 3.Yu JX, Liu J, He C, et al. Spontaneous spinal epidural hematoma: a study of 55 cases focused on the etiology and treatment strategy. World Neurosurg 2017; 98: 546–554. [DOI] [PubMed] [Google Scholar]
- 4.Akutsu H, Sugita K, Sonobe M, et al. A case of nontraumatic spinal epidural hematoma caused by extradural varix: consideration of etiology. Spine J 2003; 3: 534–538. [DOI] [PubMed] [Google Scholar]
- 5.Müller H, Schramm J, Roggendorf W, et al. Vascular malformations as a cause of spontaneous spinal epidural haematoma. Acta Neurochir (Wien) 1982; 62: 297–305. [DOI] [PubMed] [Google Scholar]
- 6.Solero CL, Fornari M, Savoiardo M. Spontaneous spinal epidural haematoma arising from ruptured vascular malformation. Case report. Acta Neurochir (Wien) 1980; 53: 169–174. [DOI] [PubMed] [Google Scholar]
- 7.Yu JX, Hong T, Ma YJ, et al. A new type of spinal epidural arteriovenous fistulas causes spinal epidural hemorrhage: an analysis of five cases and natural history consideration. World Neurosurg 2017; 103: 371–379. [DOI] [PubMed] [Google Scholar]
- 8.Miyagi Y, Miyazono M, Kamikaseda K. Spinal epidural vascular malformation presenting in association with a spontaneously resolved acute epidural hematoma. Case report. J Neurosurg 1998; 88: 909–911. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto N, Naito I, Takatama S, et al. Spinal epidural arteriovenous fistulas with unusual manifestation of sudden onset of severe neurological deficits: case report. Neurol Med Chir (Tokyo) 2013; 53: 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, He M, Mao B. Thoracic spine extradural arteriovenous fistula: case report and review of the literature. Surg Neurol 2006; 66 Suppl 1: S18–S23; discussion S23–S24. [DOI] [PubMed] [Google Scholar]
- 11.Burrows PE. Percutaneous treatment of slow-flow vascular malformations. In: Mulliken JB, Burrows PE, Fishman SJ. (eds) Vascular anomalies: hemangiomas and malformations. 2nd ed. New York: Oxford University Press, 2013, pp. 661–709. [Google Scholar]
- 12.Abram HS, DeLaHunt MJ, Merinbaum DJ, et al. Recurrent spontaneous spinal epidural hematoma in a child: first case report. Pediatr Neurol 2007; 36: 177–180. [DOI] [PubMed] [Google Scholar]
- 13.Chuang N, Shroff M, Willinsky R, et al. Slow-flow spinal epidrual AVF with venous ectasias: two pediatric case reports. AJNR Am J Neuroradiol 2003; 24: 1901–1905. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HF, Lu J, Wang LJ, et al. Spinal cord ischemia and spontaneous epidural hematoma caused by spinal epidural arteriovenous malformation: a warning. Am J Emerg Med 2017; 35: 519.e5–519.e9. [DOI] [PubMed] [Google Scholar]
- 15.Soltani S, Nogaro MC, Rougelot C, et al. Spontaneous spinal epidural haematomas in children. Eur Spine J 2019; 28: 2229–2236. [DOI] [PubMed] [Google Scholar]