Abstract
A new strategy to effectively lock the conformation of substituents at the 3,3’-positions of axial-chiral biisoquinoline N,N’-dioxides was developed based on the strong dipole–dipole interaction between 1,2,3-triazole and pyridine N-oxide rings. The crystal structure and the DFT calculations of 3,3’-bis(1-benzyl-1H-1,2,3-triazole-4-yl)-1,1’-biisoquinoline N,N’-dioxide (3a) provided strong support for this strategy. Furthermore, we successfully demonstrated that readily available 4-trimethylsilyl-1,2,3-triazoles are viable nucleophiles for Hiyama cross-coupling.
Keywords: axial-chiral Lewis bases; 1,2,3-triazoles; Hiyama cross-coupling; catalyst design; computational chemistry
Graphical Abstract
Introduction
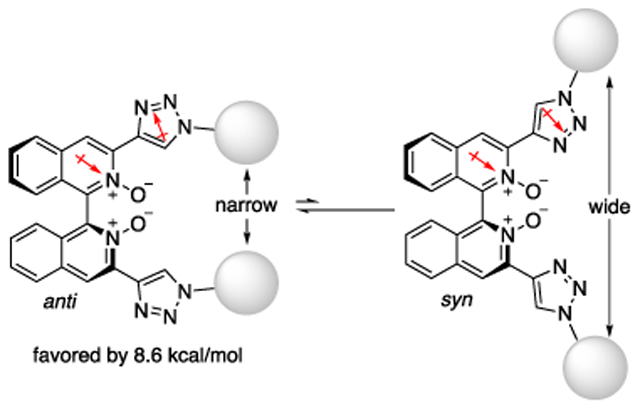
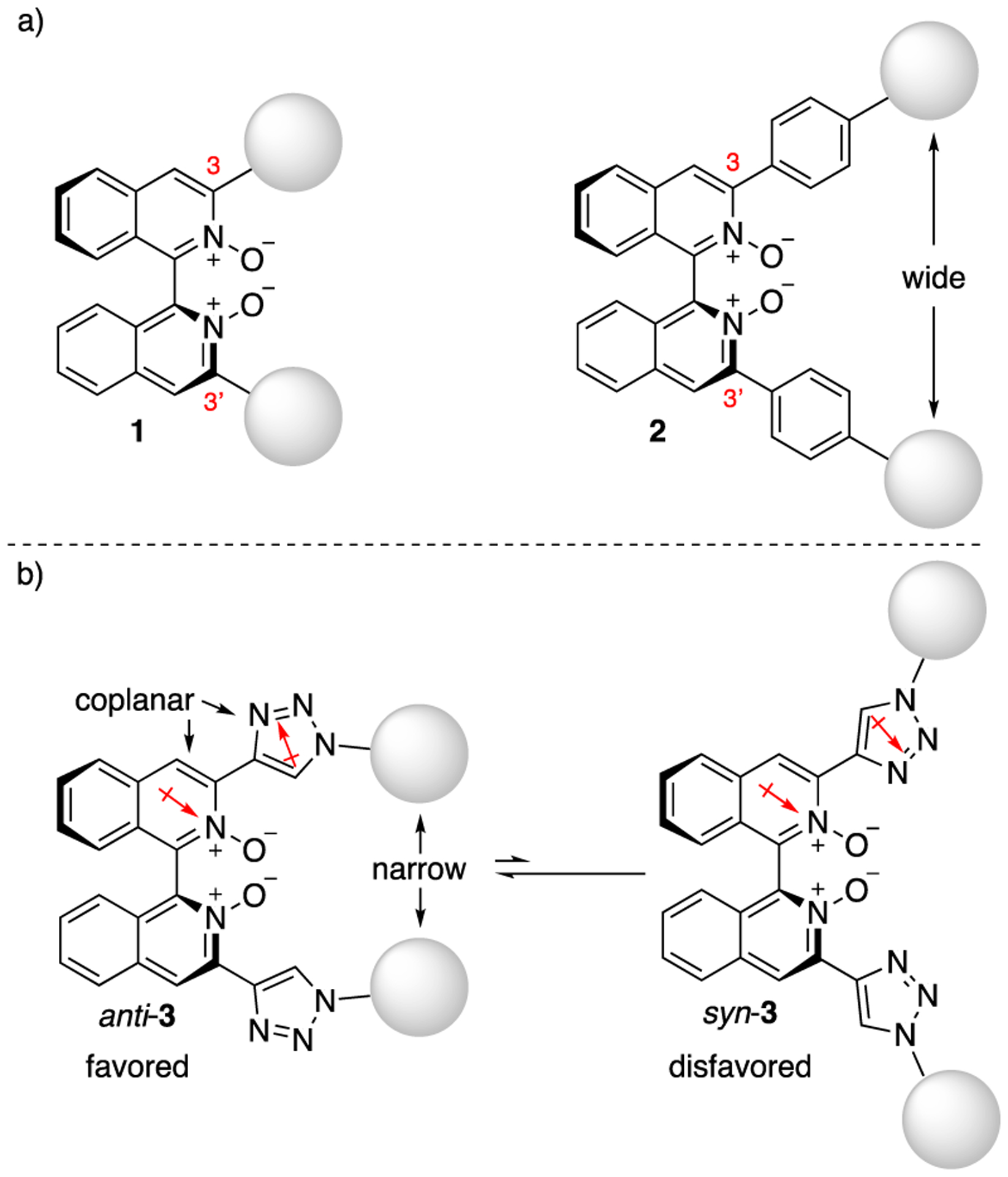
1,1’-Binaphth-2-ol (BINOL) is among the most successful chiral scaffolds ever developed.1 Its strength comes from the substituents at its 3,3’-positions that have proven particularly effective to increase asymmetry around the reaction site (i.e., space proximal to its hydroxyls). In much the same way, axial-chiral 3,3’-functionalized biisoquinoline (or bipyridine) N,N’-dioxides (1) have emerged as an important class of chiral Lewis base catalysts for the activation of chlorosilanes (e.g., SiCl4).2 Their 3,3’-substituents can be readily extended by adding a phenyl linker,3 but such a modification leads to a widemouthed cavity,3a projecting 3,3’-substituents away from the reaction space (2). Therefore, as part of our longstanding interests in the development of new chiral Lewis bases,2b,4 we conceived of biisoquinolines bearing a deep chiral cavity (3). We envisioned that we could control the conformation of 3,3’-substituents by taking advantage of large dipole moments of a pyridine N-oxide ring and a 1,2,3-triazole ring (4.24 D and 4.38 D, respectively).5,6 More specifically, the anti-conformation with respect to the directions of two dipole moments shown in Figure 1b is expected to be strongly favored over the syn-conformation.6 Herein, we report the synthesis of 3,3’-triazolyl biisoquinoline N,N’-dioxides via Hiyama cross-coupling with readily available 1-substituted-4-trimethylsilyl-1H-1,2,3-triazoles, the crystal structure of a triazolyl biisoquinoline, and the computational conformational analysis of itself and its SiCl4 complex.
Figure 1.
The design of new Lewis bases based on dipole–dipole interaction.
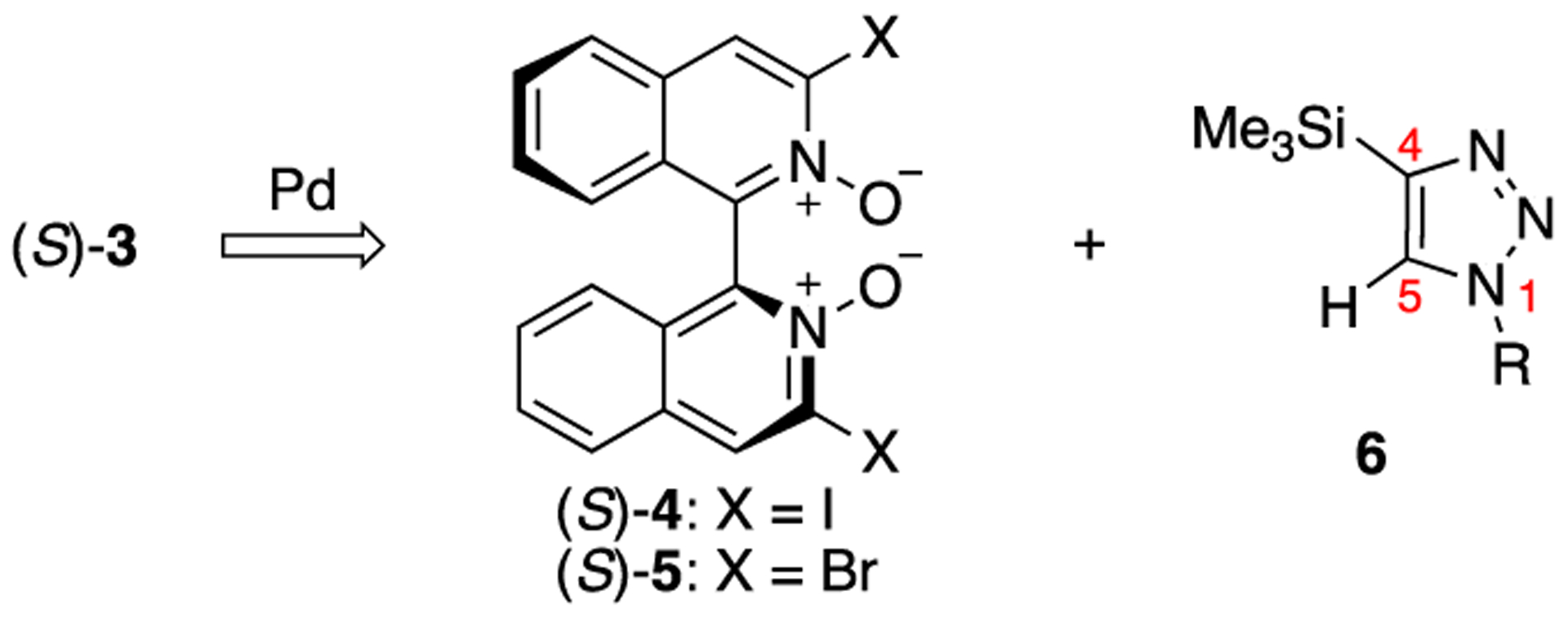
A sequence of Sonogashira coupling of 3,3’-diiodo-1,1’-biisoquinoline N,N’-dioxide (4, Scheme 1) followed by the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC)7,8 appeared a most reliable approach since we previously developed a method to halogenate the 3,3’-positions of biisoquinoline N,N’-dioxide.2b However, Sonogashira coupling of (S)-3,3’-diiodo-1,1’-biisoquinoline N,N’-dioxide and trimethylsilylacetylene turned out too elusive to move forward in our hands (see Supporting Information (SI) for details). As such, we considered a cross-coupling reaction of (S)-3,3’-dihalo-1,1’-biisoquinoline N,N’-dioxides and 4-metallo-1-substituted-1,2,3-triazoles. The Pd- or Cu-catalyzed direct arylations of 1-substituted-1,2,3-triazoles (i.e., 4, 5-unsubstituted) are exclusively C5-selective.9 4-Boronyl triazoles10 remain an elusive substrate for Suzuki-Miyaura cross-coupling just like 2-pyridyl boronates (heterocyclic boronic acids and esters in which boron is attached to a carbon center adjacent to the ring heteroatom are often unstable due to their susceptibility to hydrolytic protodeboronation).11 Although 4-stannyl triazoles12 were shown to undergo Stille cross-coupling, only thermal azide-alkyne cycloaddition reactions are known for commercially available tributylstannylacetylene that was reported unreactive for some azides.12f Negishi cross-coupling is reported only for 1,5-disubstituted-4-zincio-1,2,3-triazoles and 1-substituted-5-zincio-1,2,3-triazoles.13 Kumada cross-coupling of 4-magnesio-1,2,3-triazoles,14 and Hiyama cross-coupling of 4-silyl-1,2,3-triazoles15 are not known to the best of our knowledge. Underdevelopment of the methods to prepare 1,4-disubstituted-1,2,3-triazoles via C4 arylation is presumably because this motif can be readily prepared by CuAAC from the corresponding azides and acetylenes. In this context, we opted to investigate the feasibility of the corresponding Hiyama cross-coupling with readily available 1-substutited-4-trimethylsilyl-1H-1,2,3-triazoles (6) because of the clear benefits of organo(trialkyl)silanes that include low toxicity, high stability, good solubility and easy handling.16 It should be mentioned that in contrast to heteroatom substituted silanes, aryl(trialkyl)silanes have drawn little attention as cross-coupling nucleophiles and have remained a long-standing important challenge.16
Scheme 1.
A cross-coupling approach.
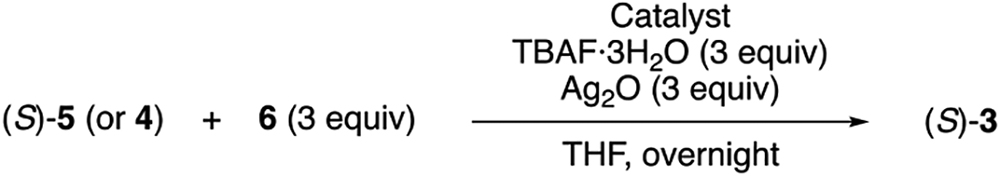
We began our investigation based on the Hiyama cross-coupling reactions of 2-trimethylsilylpyridines reported independently by the groups of Gros, Whittaker, and Marples.17 We chose 1-benzyl-4-trimethylsilyl-1H-1,2,3-triazole (6a) and (S)-4 or 5 as our model substrates and tested them with Pd(PPh3)4 in the presence of TBAF·3H2O and Ag2O in THF. In contrast to those previous reports, neither desired 3a nor the corresponding mono-coupled by-product formed in isolable quantities (Table 1, entries 1 & 2). Therefore, we screened several commercially available Pd catalysts and found that PdCl2(dppe), PdCl2(dppp), and PdCl2(BINAP) provided the desired product (3a) in 12%, 15% and 7% yields, respectively along with trace amount of the mono-coupled by-product (entries, 4, 5 & 8). We then evaluated different solvents, fluoride sources using PdCl2(dppp) and Ag2O, and in turn, metal additives with PdCl2(dppp) and TBAF·3H2O, and found that THF, TBAF·3H2O and Ag2O were best out of all tested (see SI for details). We next probed the reaction temperatures and catalyst loading, and found that 40 °C and 40 mol % catalyst loading were optimum (entry 12) which gave 3a in 41% yield. Since our main objective of this study was to develop a preparative method for catalyst 3, we proceeded to test the model substrates on a 1 mmol scale and obtained the desired product in 46% yield with only trace amount of the corresponding mono-coupled product. As we used 40 mol % of a Pd catalyst, 46% yield corresponds to the catalyst turnover of 2.3 times (two coupling sites in 5). We evaluated three more triazoles on a 1 mmol scale. 1-Mesityl-4-trimethylsilyl-triazole (6b) worked in a similar efficiency (54% yield). 1-Benzhydryl-4-trimethylsilyl-triazole (6c) provided the desired product in 31% yield with trace amount of the mono-coupled product and 1-(1-adamantyl)-4-trimethylsilyl-triazole (6d) afforded the desired product in 23% and the mono-coupled product in 19%. These results represent the first example of the Hiyama cross-coupling of 4-trimethylsilyl-1,2,3-triazoles, and demonstrated that the transformation was feasible. It should be mentioned that 5-unsubstituted 1,2,3-triazoles have proven useful as hydrogen bond donors and have been incorporated into organocatalysts, anion-binding receptors, etc.7,18,19 These molecules were made by a sequence of Sonogashira and CuAAC reactions. Since Sonogashira reactions are often limited to aryl iodides20 that are not always readily available, the present method could possibly complement the currently adapted synthetic sequence as demonstrated herein (vide supra).
Table 1.
Hiyama cross-coupling of 4-TMS-triazoles.
| ||||||
|---|---|---|---|---|---|---|
| Entry | ArX | 6 | Catalyst | mol % | Temp (°C) | Yield (%)a |
| 1 | 4 | 6a | Pd(PPh3)4 | 20 | rt | 3a, trace |
| 2 | 5 | 6a | Pd(PPh3)4 | 20 | rt | 3a, trace |
| 3 | 5 | 6a | Pd2(dba)3/P(o-tol)3 | 20 | rt | 3a, 0 |
| 4 | 5 | 6a | PdCl2(dppe) | 20 | rt | 3a, 12 |
| 5 | 5 | 6a | PdCl2(dppp) | 20 | rt | 3a, 15 |
| 6 | 5 | 6a | PdCl2(dppb) | 20 | rt | 3a, trace |
| 7 | 5 | 6a | PdCl2(dppf) | 20 | rt | 3a, 0 |
| 8 | 5 | 6a | PdCl2(BINAP) | 20 | rt | 3a, 7 |
| 9 | 5 | 6a | Pd2(dba)3/Xantphos | 20 | rt | 3a, trace |
| 10 | 5 | 6a | PdCl2(dppp) | 20 | 60 | 3a, 24 |
| 11b | 5 | 6a | PdCl2(dppp) | 40 | 60 | 3a, 42 |
| 12b | 5 | 6a | PdCl2(dppp) | 40 | 40 | 3a, 41 |
| 13b,c | 5 | 6a | PdCl2(dppp) | 40 | 40 | 38,46d |
| 14 b,c | 5 | 6b | PdCl2(dppp) | 40 | 40 | 3b, 54d |
| 15b,c | 5 | 6c | PdCl2(dppp) | 40 | 40 | 3c, 31d |
| 16b,c | 5 | 6d | PdCl2(dppp) | 40 | 40 | 3d, 23d |
0.1 mmol of 4 or 5 was used for entries 1 – 12. 6a(R = Bn), 6b (R = Mes), 6c (R = Bzh) & 6d (R = 1-ad).
1H NMR yield estimated with 1,1,2,2-tetrachloroethane as an internal standard unless otherwise noted.
4.8 equiv of 6, TBAF·3H2O, and Ag2O.
1 mmol scale reaction.
Isolated yield after flash chromatography on silica gel.
We were able to obtain crystals of 3a suitable for the X-ray structural analysis (Figure 2). In the solid state, 3a was found to adapt the anti-conformation in accordance with our hypothesis based on the dipole–dipole interaction (Figure 1). The dihedral angle between its triazole and pyridine N-oxide rings is 7.66 °. At least in the solid state, the 3,3’-substituents effectively embrace the reaction space, forming a deep chiral cavity. We also performed a computational conformational analysis of 3a using density functional theory. Calculations have been performed with the PBEh-3c method and the C-PCM solvation model with the dielectric constant of dichloromethane (DCM). The calculated most stable conformation of 3a turned out almost identical to its crystal structure (see Figure S1 in SI). The anti-conformation was found to be favored by 8.6 kcal/mol over the syn-conformation and the calculated average dihedral angle between its triazole and pyridine N-oxide rings is 7.75 °, providing further support for our hypothesis that the dipole-dipole interaction effectively locks the conformation of 3,3’-substituents. This energy difference roughly corresponds to the equilibrium ratio of 1,000,000:1 (= anti:syn).
Figure 2.
The crystal structure of 3a.
The binding geometry of 3a to SiCl4 was computationally investigated (Figure 3). To our delight, 3a was found to bind to SiCl4 through its two oxygen atoms with the binding energy of 30.8 kcal/mol in contrast to some 1,2,3-triazole bearing ligands that are known to coordinate to a metal via its triazole nitrogen atoms.18f,21 Furthermore, the dipole–dipole interaction between the triazole and pyridine N-oxide rings appeared to dominate their relative conformation even after 3a complexed with SiCl4 although those two rings slightly twisted out of the co-planarity (the dihedral angle in the complex is = 20.57 °). Furthermore, the anion–π-type interaction22 between chlorine atoms of a hypervalent chlorosilane and phenyl rings of the benzyl units was found in 3a–SiCl4 complex, which brought the benzyl groups to embrace the reaction space, leading to a structurally well-defined, deep chiral pocket. It should be mentioned that a pileup of electron density occurs at the peripheral chlorine atoms of a hypervalent silicon complex of this kind.2b,23, 24
Figure 3.
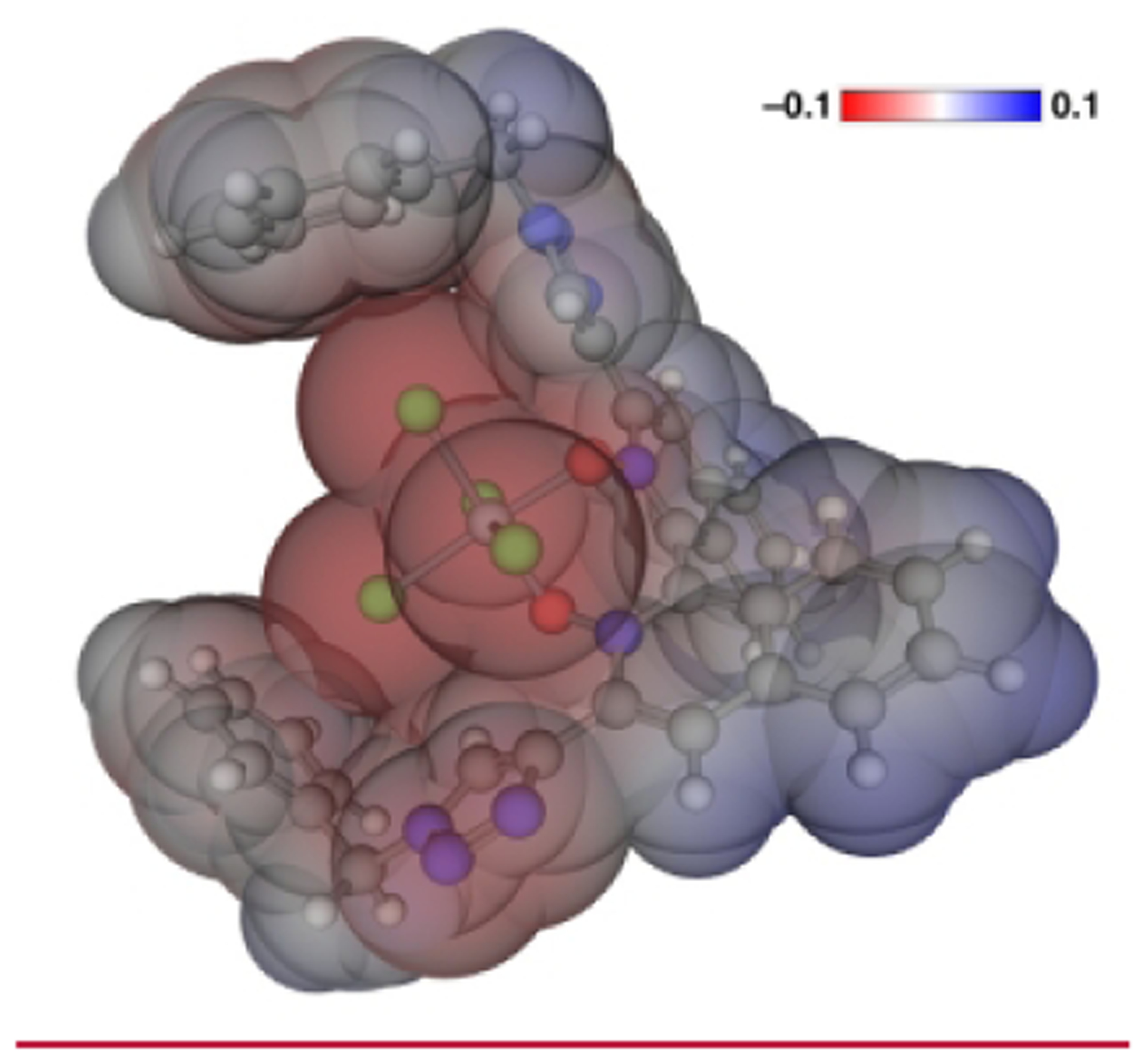
The most stable conformation and potential of 3a-SiCl4 complex calculated with PBEh-3c/C-PCM(DCM).
Next, we conducted preliminary tests to probe the catalytic performance of 3a–d in comparison to a conventional Lewis base catalyst (2a)2b,25 (Scheme 2). The asymmetric transfer hydrogenation of N-aryl ketimines with trichlorosilane is a proven testing ground for new chiral Lewis bases (eq. 1).26 To our delight, 3a–d were found more enantioselective than 2a (44–56% ee vs. 24% ee). Furthermore, 3a was substantially more reactive than the others (71% yield). As 3a–d provided promising reactivity and enantioselectivity for the testing ground reaction, we preliminarily evaluated them for the direct catalytic asymmetric synthesis of α-chiral primary amine that is among the long-standing important challenges in organic synthesis (eq. 2).27 Catalysts 3a, c and d enantioselectively catalyzed the reduction of amine salt 9 albeit with low yields while 2a and 3b were ineffective. Catalyst 3a was found distinctively more enantioselective than 3c or d. It should be mentioned that the Lewis base-catalyzed asymmetric transfer hydrogenation of ketimines with trichlorosilane currently remains limited to N-aryl and alkyl protected ones.26 These preliminary observations clearly demonstrated that 3,3’-triazolyl biisoquinoline N,N’-dioxides are complementary to existing Lewis base catalysts, and bode well for the development of their applications.
Scheme 2.
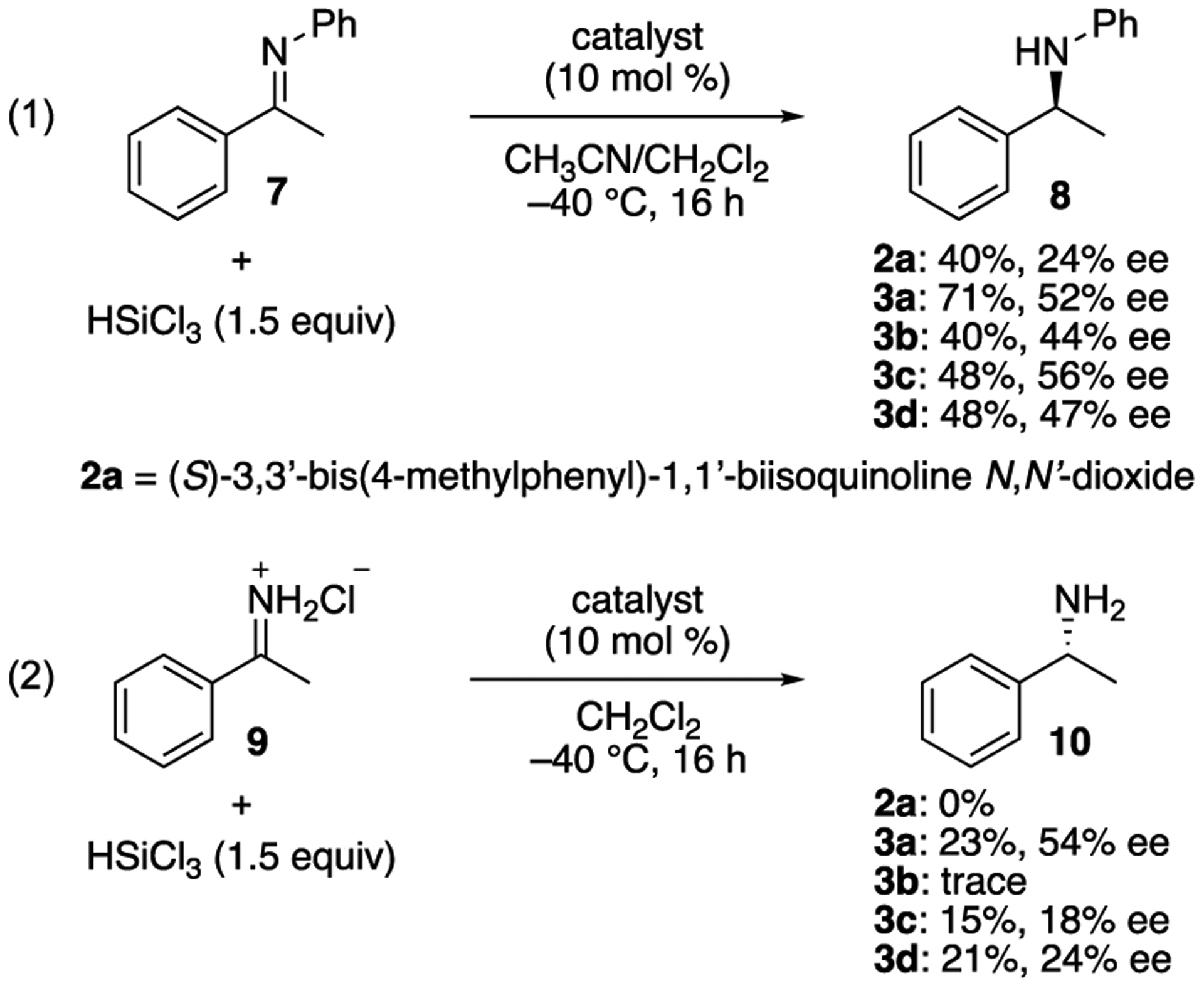
Preliminary evaluation of catalysts (reaction conditions were not fully optimized). Yields refer to 1H NMR yield determined with 1,1,2,2-tetrachloroethane as an internal standard (see SI for details).
In summary, we developed a new strategy to effectively control the conformation of substituents at the 3,3’-positons of axial-chiral biisoquinoline (or bipyridine) N,N’-dioxides on the basis of the strong dipole–dipole interaction between 1,2,3-triazole and pyridine N-oxide rings. The X-ray structure and the DFT calculations of 3a provided strong support for this strategy. Furthermore, we successfully demonstrated that readily available 4-trimethylsilyl-1,2,3-triazoles are viable nucleophiles for Hiyama cross-coupling reaction where aryl(trialkyl)silanes have drawn little attention as cross-coupling nucleophiles and have remained a long-standing important challenge.
Supplementary Material
Acknowledgments
We thank the National Institute of Health (1R15 GM139087-01) and the Florida Institute of Technology for financial support of this work. We thank Professor Christopher D. Chouinard for the initial HRMS analysis of the compounds made during this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Experimental procedures and detailed characterization data of all new compounds including the X-ray structures of 3a and 6d are provided as a PDF. Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC Nos. 2082619 – 2082620 for compounds 3a and 6d. Copies of this information may be obtained free of charge from, The Director, CCDC 12 Union Road, Cambridge CB2 1EZ, UK [fax: (int.code) +44 (1223) 336–033 or deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk]. The DFT-optimized geometries are available in a separate file (TXT).
References and notes
- 1.Selected reviews.; a) Parmar D; Sugiono E; Raja S; Rueping M; Chem. Rev 2014, 114, 9047–9153.; [DOI] [PubMed] [Google Scholar]; b) Chen Y; Yekta S; Yudin AK; Chem. Rev 2003, 103, 3155–3211. [DOI] [PubMed] [Google Scholar]
- 2.Selected references.; a) Fukazawa Y; Vaganov VY; Shipilovskikh SA; Rubtsov AE; Malkov AV Org. Lett 2019, 21, 4798–4802.; [DOI] [PubMed] [Google Scholar]; b) Reep C; Morgante P; Peverati R; Takenaka N Org. Lett 2018, 20, 5757–5761.; [DOI] [PubMed] [Google Scholar]; c) Bai B; Shen L; Ren J; Zhu HJ Adv. Synth. Catal 2012, 354, 354–358.; [Google Scholar]; d) Bai B; Zhu HJ; Pan W Tetrahedron, 2012, 68, 6829–6836.; [Google Scholar]; e) Kadlčíková A; Hrdina R; Valterová I; Kotora M Adv. Synth. Catal 2009, 351, 1279–1283.; [Google Scholar]; f) Hrdina R; Dračínský M; Valterová I; Hodačová J; Císařová I; Kotora M Adv. Synth. Catal 2008, 350, 1449–1456.; [Google Scholar]; g) Malkov AV; Westwater M-M; Gutnov A; Ramírez-López P; Friscourt F; Kadlčíková A; Hodačová J; Rankovic Z; Kotora M; Kočovský P Tetrahedron, 2008, 64, 11335–11348.; [Google Scholar]; h) Denmark SE; Fan Y; Eastgate MD J. Org. Chem 2005, 70, 5235–5248.; [DOI] [PubMed] [Google Scholar]; i) Kina A; Shimada T; Hayashi T Adv. Synth. Catal 2004, 346, 1169–1174.; [Google Scholar]; j) Shimada T; Kina A; Ikeda S; Hayashi T Org. Lett 2002, 4, 2799–2801.; [DOI] [PubMed] [Google Scholar]; k) Denmark SE; Fan YJ Am. Chem. Soc 2002, 124, 4233–4235. [DOI] [PubMed] [Google Scholar]
- 3.Selected references.; a) Jiao P; Nakashima D; Yamamoto H; Angew. Chem. Int. Ed 2008, 47, 2411–2413.; [DOI] [PubMed] [Google Scholar]; b) Cheng X; Vellalath S; Goddard R; List BJ Am. Chem. Soc 2008, 130, 15786–15787.; [DOI] [PubMed] [Google Scholar]; c) Terada M; Nakano M; Ube H; J. Am. Chem. Soc 2006, 128, 16044–16045.; [DOI] [PubMed] [Google Scholar]; d) Wipf P; Jung J-K; J. Org. Chem 2000, 65, 6319–6337. [DOI] [PubMed] [Google Scholar]
- 4.a) Morgante P; Captain B; Chouinard CD; Peverati R; Takenaka N Tetrahedron Letters, 2020, 61, 152143.; [Google Scholar]; b) Peng Z; Takenaka N The Chemical Record, 2013, 13, 28–42.; [DOI] [PubMed] [Google Scholar]; c) Chen J; Captain B; Takenaka N Org. Lett 2011, 13, 1654–1657; [DOI] [PubMed] [Google Scholar]; d) Takenaka N; Sarangthem R; Captain B Angew. Chem. Int. Ed 2008, 47, 9708–9710 [DOI] [PubMed] [Google Scholar]
- 5.a) Jug K; Chiodo SJ Phys. Chem. A 2003, 107, 4172–4183.; [Google Scholar]; b) Linton EP J. Am. Chem. Soc 1940, 62, 1945–1948 [Google Scholar]
- 6.Angelo NG; Arora PS J. Am. Chem. Soc 2005, 127, 17134–17135. [DOI] [PubMed] [Google Scholar]
- 7.Selected reference.; Neel AJ; Hehn JP; Tripet PF; Toste FD J. Am. Chem. Soc 2013, 135, 14044–14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selected reference.; Rostovtsev VV; Green L,G; Fokin VV; Sharpless KB Angew. Chem. Int. Ed 2002, 41, 2596–2599 [DOI] [PubMed] [Google Scholar]
- 9.a) Ackermann L; Potukuchi HK; Landsberg D; Vicente R Org. Lett 2008, 10, 3081–3084.; [DOI] [PubMed] [Google Scholar]; b) Chuprakov S; Chemyak N; Dudnik AS; Gevorgyan V Org. Lett 2007, 9, 2333–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Jung SH; Choik K; Pae AN; Lee JK; Choo H; Keum G; Cho YS; Min S-J Org. Biomol. Chem 2014, 12, 9674–9682.; [DOI] [PubMed] [Google Scholar]; b) Kim T; Song JH; Jeong KH; Lee S; Ham J Eur. J. Org. Chem 2013, 3992–3996.; [Google Scholar]; c) Grob JE; Nunez J; Dechantsreiter MA; Hamann LG J. Org. Chem 2011, 76, 10241–10248 [DOI] [PubMed] [Google Scholar]
- 11.Cook AF; Gombert A; McKnight J; Pantaine LRE; Willis MC Angew. Chem. Int. Ed 2021, 60, 11068–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Edem PE; Czorny S; Valliant JF J. Med. Chem 2014, 57, 9564–9577.; [DOI] [PubMed] [Google Scholar]; b) Darwish A; Blacker M; Janzen N; Rathmann SM; Czorny S; Hillier SM; Joyal JL; Babich JW; Valliant JF Med. Chem. Lett 2012, 3, 313–316.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Leclerc J-P; Falgueyret J-P; Girardin M; Guay J; Guiral S; Huang Z; Li CS; Oballa R; Ramtohul YK; Skorey K; Tawa P; Wang H; Zhang L Bioorg. Med. Chem. Lett 2011, 21, 6505–6509.; [DOI] [PubMed] [Google Scholar]; d) Cebrián C; de Cózar A; Prieto P; Díaz-Ortiz A; de la Hoz A; Carrillo JR; Rodriguez AM; Montilla F Synlett 2010, 55–60.; [Google Scholar]; e) Ito S; Satoh A; Nagatomi Y; Hirata Y; Suzuki G; Kimura T; Satow A; Maehara S; Hikichi H; Hata M; Kawamoto H; Ohta H Bioorg. Med. Chem 2008, 16, 9817–9829.; [DOI] [PubMed] [Google Scholar]; f) Chan DCM; Laughton CA; Queener SF; Stevens MFG Bioorg. Med. Chem 2002, 10, 3001–3010.; [DOI] [PubMed] [Google Scholar]; g) Sakamoto T; Uchiyama D; Kondo Y; Yamanaka H Heterocycles 1993, 35, 1273–1279. [Google Scholar]
- 13.a) Tüllmann CP; Chen Y-H; Schuster RJ; Knochel P Org. Lett 2018, 20, 4601–4605.; [DOI] [PubMed] [Google Scholar]; b) Smith CD; Greaney MF Org. Lett 2013, 15, 4826–4829.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tsuritani T; Mizuno H; Nonoyama N; Kii S; Akao A; Sato K; Yasuda N; Mase T Organic Process Research and Development 2009, 13, 1407–1412.; [Google Scholar]; d) Akao A; Tsuritani T; Kii S; Nonoyama N; Mase T; Yasuda N Synlett 2007, 31–36. [Google Scholar]
- 14.a) Karsakova IV; Smirnov AY; Baranov MS Chemistry of Heterocyclic Compounds 2018, 54(7), 755–757.; [Google Scholar]; b) Meng D; Andre P; Bateman TJ; Berger R; Chen Y-H; Desai K; Dewnani S; Ellsworth K; Feng D; Geissler WM; Guo L; Hruza A; Jian T; Li H; Metzer J; Parker DL; Reichert P; Sherer EC; Campeau L-C; Orr R; Poirier M; McCabe-Dunn J; Araki K; Nishimura T; Sakurada I; Hirabayashi T; Wood HB Bioorg. Med. Chem. Lett 2013, 25, 5437–5443.; [DOI] [PubMed] [Google Scholar]; c) Zink DM; Baumann T; Nieger M; Bräse S Eur. J. Org. Chem 2011, 1432–1437.; [Google Scholar]; d) Liu D; Gao W; Dai Q; Zhang X Org. Lett 2005, 7, 4907–4910.; [DOI] [PubMed] [Google Scholar]; e) Krasiński A; Fokin V; Sharpless B Org. Lett 2004, 6, 1237–1240. [DOI] [PubMed] [Google Scholar]
- 15.a) Panja C; Puttaramu JV; Chandran TK; Nimje RY; Kumar H; Gupta A; Arunachalam PN; Corte JR; Mathur A Journal of Fluorine Chemistry 2020, 236, 109516–109523.; [Google Scholar]; b) Fiandanese V; Maurantonio S; Punzi A; Rafaschieri GG Org. Biomol. Chem, 2012, 10, 1186–1195.; [DOI] [PubMed] [Google Scholar]; c) Kónya K; Fekete S; Ábrahám A; Patonay T Mol. Divers 2012, 16, 91–102.; [DOI] [PubMed] [Google Scholar]; d) White JR; Price GJ; Schiffers S; Raithby PR; Plucinski PK; Frost CG Tetrahedron Letters 2010, 51, 3913–3917.; [Google Scholar]; e) Demaray JA; Thuener JE; Dawson MN; Sucheck SJ Bioorg. Med. Chem. Lett 2008, 18, 4868–4871.; [DOI] [PubMed] [Google Scholar]; f) Melai V; Brillante A; Zanirato PJ Chem. Soc., Perkin Trans 2, 1998, 2447–2449.; [Google Scholar]; g) Zanirato P J. Chem. Soc., Perkin Trans 1, 1991, 2789–2796. [Google Scholar]
- 16.Selected reviews.; a) Minami Y; Hiyama T Chem. Eur. J 2019, 25, 391–399.; [DOI] [PubMed] [Google Scholar]; b) Komiyama T; Minami Y; Hiyama T ACS Catal 2017, 7, 631–651.; [Google Scholar]; c) Komiyama T; Minami Y; Hiyama T Synlett 2017, 28, 1873–1884. [Google Scholar]
- 17.a) Blakemore DC; Marples LA Tetrahedron Letters 2011, 52, 4192–4195.; [Google Scholar]; b) Louërat F; Tye H; Napier S; Garrigou M; Whittaker M; Gros PC Org. Biomol. Chem 2011, 9, 1768–1773.; [DOI] [PubMed] [Google Scholar]; c) Louërat F; Gros PC Tetrahedron Letters 2010, 51, 3558–3560.; [Google Scholar]; d) Napier S;Marcuccio SM; Tye H; Whittaker M Tetrahedron Letters 2008, 49, 6314–6315.; [Google Scholar]; e) Pierrat P; Gros P; Fort Y Org. Lett 2005, 7, 697–700. [DOI] [PubMed] [Google Scholar]
- 18.Selected; a) Gómez-Martínez M; Pérez-Aguilar M; Piekarski DG; Daniliuc CG; Mancheño OG Angew. Chem. Int. Ed 2021, 60, 5102–5107.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stockerl S; Gutiérrez D,; Mancheño OG Journal of Molecular Catalysis A: Chemical 426, 2017, 572–585.; [Google Scholar]; c) Zurro M; Asmus S; Beckendorf S; Mück-Lichtenfeld C; Mancheño OG J. Am. Chem. Soc 2014, 136, 13999–14002 [DOI] [PubMed] [Google Scholar]; d) Beckendorf S; Mancheño OG Synthesis 2012, 44, 2162–2172. [Google Scholar]; Selected reviews: [Google Scholar]; e) Reep C; Sun S; Takenaka N Asian J. Org. Chem 2019, 8, 1306–1316.; [Google Scholar]; f) Zurro M; Mancheño OG Chem. Rec 2017, 17, 485–498. [DOI] [PubMed] [Google Scholar]
- 19.Selected references:; a) Liu Y; Parks FC; Sheetz EG; Chen C-H; Flood AJ Am. Chem. Soc 2021, 143, 3191–3204.; [DOI] [PubMed] [Google Scholar]; b) Liu Y; Zhao W; Chen C-H; Flood A Science 2019, 365, 159–161.; [DOI] [PubMed] [Google Scholar]; c) Mullen KM; Mercurio J; Serpell CJ; Beer PD Angew. Chem. Int. Ed 2009, 48, 4781–4784.; [DOI] [PubMed] [Google Scholar]; d) Kumar A; Pandey PS Org. Lett 2008, 10, 165–168. [DOI] [PubMed] [Google Scholar]; Selected Reviews: [Google Scholar]; e) Cai J; Sessler JL Chem. Soc. Rev 2014, 43, 6198–6213.; [DOI] [PubMed] [Google Scholar]; f) Hua Y; Flood A Chem. Soc. Rev 2010, 39, 1262–1271. [DOI] [PubMed] [Google Scholar]
- 20.Selected reference.; Míšek J; Teplý F; Stará IG; Tichý M; Šaman I; Císařová I; Vojtíšek P; Starý I Angew. Chem. Int. Ed 2008, 47, 3188–3191. [DOI] [PubMed] [Google Scholar]
- 21.a) Leckie L; Mapolie SF; Catalysis Communications 2019, 131, 105803.; [Google Scholar]; b) Leckie L; Mapolie SF; Applied Catalysis A, General 2018, 565, 76–86. [Google Scholar]
- 22.Selected reviews.; a) Wheeler SE; Accounts of Chemical Research 2013, 46, 1029–1038.; [DOI] [PubMed] [Google Scholar]; b) Hay BP; Bryantsev VS Chem. Commun 2008, 2417–2428. [DOI] [PubMed] [Google Scholar]
- 23.Lu T; Porterfield MA; Wheeler SE Org. Lett 2012, 14, 5310–5313. [DOI] [PubMed] [Google Scholar]
- 24.Denmark SE; Beutner GL Angew. Chem. Int. Ed 2008, 47, 1560–1638. [DOI] [PubMed] [Google Scholar]
- 25.Catalyst 2a was found optimum for conjugate reduction of acrylamides with trichlorosilane.; Xu C; Reep C; Jarvis J; Naumann B; Captain B; Takenaka N Catalysts 2021, 11, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selected; a) Zhang Z; Rooshenas P; Hausmann H; Schreiner PR Synthesis 2009, 9, 1531–1544.; [Google Scholar]; b) Guizzetti S; Benaglia M; Rossi S Org. Lett 2009, 11, 2928–2931.; [DOI] [PubMed] [Google Scholar]; c) Wang C; Wu X; Zhou L; Sun J Chem. Eur. J 2008, 14, 8789–8792.; [DOI] [PubMed] [Google Scholar]; d) Zheng H; Deng J; Lin W; Zhang X Tetrahedron Letters 2007, 48, 7934–7937.; [Google Scholar]; e) Malkov AV; Mariani A; MacDougall KN; Kočovský P Org. Lett 2004, 6, 2253–2256.; [DOI] [PubMed] [Google Scholar]; f) Iwasaki F; Onomura O; Mishima K; Kanematsu T; Maki T; Matsumura Y Tetrahedron Letters 2001, 42, 2525–2527. [Google Scholar]; Selected reviews:; g) Kočovský P; Malkov AV in Lewis Base Catalysis in Organic Synthesis; Vedejs E; Denmark SE, Eds.; Wiley-VCH, 2016; Vol. 3, pp 1077–1112.; [Google Scholar]; h) Guizzetti S; Benaglia M Eur. J. Org. Chem 2010, 5529–5541 [Google Scholar]
- 27.Selected reference:; a) Hou G; Gosselin F; Li W; McWilliams JC; Sun Y; Weisel M; O’Shea PD; Chen C; Davies IW; Zhang XJ Am. Chem. Soc 2009, 131, 9882–9883. [DOI] [PubMed] [Google Scholar]; Selected review:; b) Yin Q; Shi Y; Wang J; Zhang X Chem. Soc. Rev 2020, 49, 6141–6153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


