To the Editor,
Pathogenesis of auto-immune hepatitis (AIH) is still unknown but both genetic and environmental are probably involved. In patient with high genetic susceptibility, molecular mimicry can induce AIH.
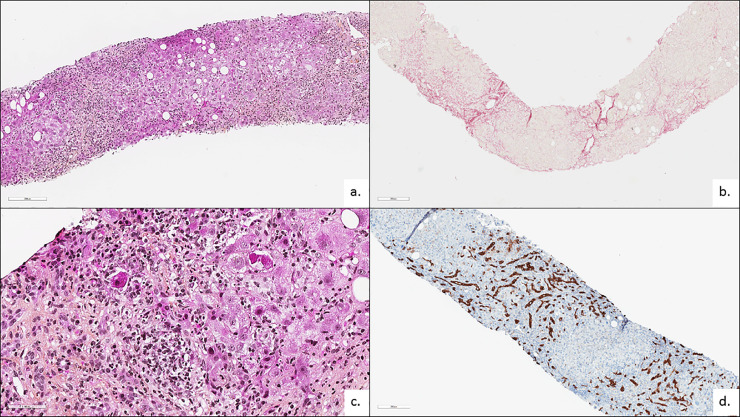
We report here three cases of 80, 73 and 68 years old women who developed severe AIH after COVID 19 vaccination with no history of auto-immune disease. The first case received two doses of Pfizer-BioNTech, the second one dose of Moderna and the third one dose of AstraZeneca Covid 19 vaccine. AIH onset was rapid, with diagnosis of hepatitis, 10, 21 and 20 days after vaccination, respectively. Initial clinical symptoms were similar: asthenia, pruritus and jaundice and physical exam was normal. Initial blood tests showed severe acute hepatitis with total bilirubin of 78, 334 and 752 µmol/L; AST of 583, 1163 and 2314 UI/L; ALT of 541, 1027 and 2029 UI/L respectively for the three patients. INR was normal in the two first cases, but of 2.37 for the last case. Screening for viral hepatitis was negative (hepatitis A, B, C, E virus, CMV, HSV, EBV). Ceruloplasmin, serum copper, alpha-1-antitrypsine, TSH were all normal. None of the patient drunk alcohol or had travelled or used intravenous drugs or herbal supplements. Anti-nuclear antibodies were positive in all cases. Specific liver autoantibodies were initially negative for the three patients (anti-smooth muscle, anti-mitochondrial, anti-SLA, anti-Sp100, anti-gp210) but one of them developed anti-smooth muscle antibodies at one month of follow up. Total IgG were increased in all cases (20.9 g/L, 18 g/l and 18.5 g/l). Abdominal ultra sound Doppler showed normal liver with no steatosis, no biliary dilation. All three patients had liver biopsy with similar findings: diffuse acute hepatitis with lobular and portal intense lymphoplasmacytic infiltrate, with interface hepatitis and hepatocyte necrosis. (Fig. 1 ) .Clinical and biological evolution was slightly different: the first two patients were treated with steroids for 4 weeks (1 mg/kg) with a rapid good evolution. The third patient had a poor clinical and biological course with hepatic encephalopathy and liver failure (Total bilirubin: 820 µmol/L; INR of 3) requiring urgent listing for liver transplantation; she died 3 days after of liver failure and sepsis.
Fig. 1.
Histological description of AIH
Figure 1: Histological and immunochemistry findings. Diffuse hepatitis (a: HES X100), lobular and portal (black arrows). Portal and lobular intense lymphoplasmacytic infiltrate (c: HES X400) with interface hepatitis and hepatocyte necrosis (asterisk = acidophilic bodies). Ductular reaction (CK-7 immunostaining X100).
Bril et al. reported the first case of AIH, only 6 days after a first dose of Pfizer-BioNTech SARS-CoV-2 vaccination [1]. In our patients, delay between vaccination and AIH onset was also short but one of the patient had two doses of vaccine and this is consistent with reported case of AIH after influenza vaccination [2]. In addition, age of our patients are older than in previous reported severe AIH, strongly suggesting that vaccination was the trigger for AIH development [3]. Two other cases of AIH post COVID 19 vaccination were reported suggesting that the association of COVID 19 vaccination and AIH is potentially not coincidental [4,5].
We report here three case of severe AIH after SARS-CoV-2 vaccination whatever the type of vaccine. This should not discourage vaccination but may raise some awareness to diagnose and rapidly treat AIH since it can lead to liver failure.
Authors’ contribution
DE and JD wrote the paper
PML were involved in patient care and liver biopsy analysis and images.
DE and FV were involved in patient care and inclusion.
All co-authors approved the final version of the paper
Declaration of Competing Interest
This study did not receive any funding. Authors have no conflict of interest to declare.
References
- 1.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.008. S0168827821002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muratori P., Serio I., Lalanne C., Lenzi M. Development of autoimmune hepatitis after influenza vaccination; trigger or killer? Clin Res Hepatol Gastroenterol. 2019;43:e95–e96. doi: 10.1016/j.clinre.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 3.De Martin E., Coilly A., Chazouillères O., Roux O., Peron J.-.M., Houssel-Debry P., et al. Early liver transplantation for corticosteroid non-responders with acute severe autoimmune hepatitis: the SURFASA score. J Hepatol. 2021 doi: 10.1016/j.jhep.2020.12.033. S0168827821000416. [DOI] [PubMed] [Google Scholar]
- 4.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following sars-cov-2 vaccine: may not be a casualty. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.05.038. S0168-8278(21)00412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.009. S0168-8278(21)00424–4. [DOI] [PMC free article] [PubMed] [Google Scholar]