Introduction
Alopecia areata (AA) is a T lymphocyte-mediated autoimmune condition characterized by hair loss due to an inflammatory response targeting the hair follicle. Recent reports have suggested that COVID-19 may trigger a variety of autoimmune conditions, including AA.1,2 Herein, we report 9 cases of AA following SARS-CoV-2 vaccination (Table I; Fig 1).
Table I.
Patient demographics and history, SARS-CoV-2 vaccine administered, approximate time between dose and hair loss, previously attempted treatments, and treatment prescribed at our institution
| Case | Sex and age | SARS-CoV-2 vaccine administered | Significant medical history | Significant family history | Approximate length of time to flare after vaccine | Severity of hair loss | Previous treatments | Treatment prescribed at our institution |
|---|---|---|---|---|---|---|---|---|
| 1 | Woman, 33 | Moderna | Hepatic steatosis, chronic hepatitis B virus | Brother with AA | 2 months after 2nd dose | Large patches of nonscarring alopecia of the scalp with foci of hair regrowth | ILTAC, pimecrolimus 1% cream, clobetasol 0.05% foam | Tofacitinib citrate 5 mg twice a day |
| 2 | Woman, 57 | Pfizer | Remote history of AA | NA | 4 months after 2nd dose | Widespread nonscarring alopecia of the scalp with foci of hair regrowth | Compounded tofacitinib 2%, clobetasol 0.05% ointment, clobetasol solution | Tofacitinib citrate 5 mg twice a day |
| 3 | Woman, 62 | Moderna | Remote history of AA | NA | 2 months after 2nd dose | Alopecia universalis | NA | Tofacitinib citrate 10 mg twice a day, bimatoprost 0.03% eye drops |
| 4 | Woman, 28 | Pfizer | AA, Hashimoto thyroiditis | NA | Within 1 week after 2nd dose | Alopecia universalis | ILTAC and PRP | Tofacitinib citrate 10 mg twice a day |
| 5 | Woman, 29 | Pfizer | Elevated levels of thyroglobulin antibody and thyroid peroxidase antibody | NA | Within 1 week after 2nd dose | Two patches of nonscarring alopecia of the scalp with areas of regrowth | NA | ILTAC |
| 6 | Man, 22 | Moderna | Elevated thyroid antibody, normal thyroid function tests | NA | 1 month after 2nd dose | Patches of nonscarring alopecia; 30% hair loss from scalp, 80% hair loss from beard | ILTAC | Tofacitinib citrate 10 mg twice a day |
| 7 | Man, 15 | Pfizer | NA | Grandmother with Hashimoto thyroiditis, sister with elevated thyroid antibody | Within 1 week after 2nd dose | Two patches of nonscarring alopecia of the scalp | NA | ILTAC |
| 8 | Man, 61 | Pfizer | Joint pain, on hydroxychloroquine | NA | 2 weeks after 1st dose | Alopecia totalis | NA | Pending possible trial of oral tofacitinib citrate |
| 9 | Man, 16 | Pfizer | NA | NA | Within 1-2 weeks after 1st dose | Patches of nonscarring alopecia with 70% loss of scalp hair; sparse eyebrows and eyelashes | ILTAC | Tofacitinib citrate 10 mg twice a day |
AA, Alopecia areata; ILTAC, intralesional triamcinolone; NA, not applicable; PRP, platelet-rich plasma.
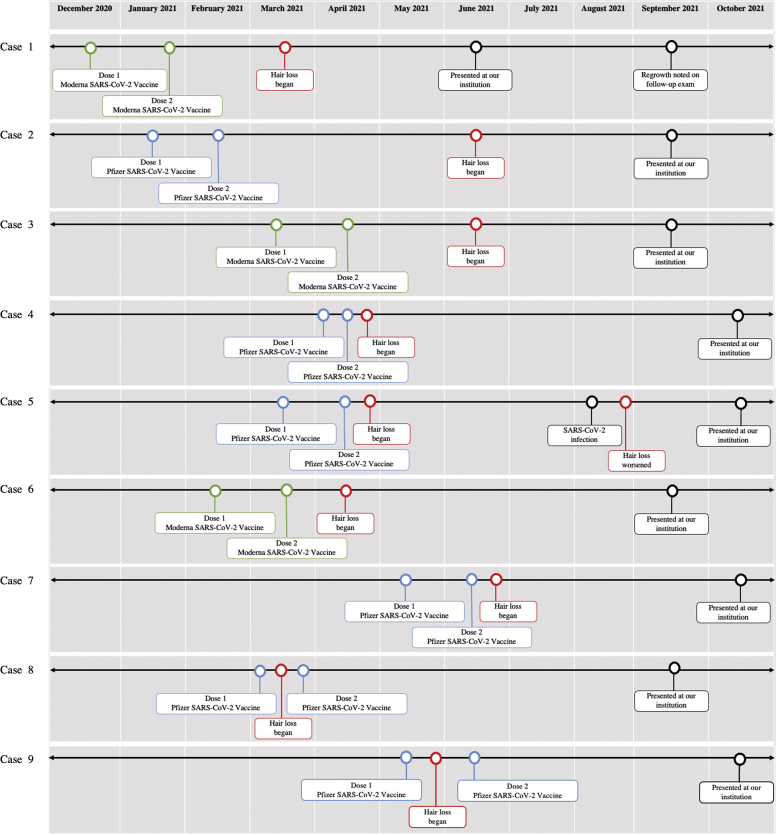
Fig 1.
Patient timelines showing dates of initial incidence and progression of hair loss, SARS-CoV-2 vaccine series, and presentation at our institution.
Case series
Case 1
A 33-year-old woman with hepatic steatosis in the setting of chronic hepatitis B virus presented for hair loss since March 2021. The patient's brother had a history of AA. She completed the Moderna SARS-CoV-2 vaccine series in January 2021. She presented with large patches of nonscarring alopecia with foci of hair regrowth. The patient received 4 treatments of intralesional triamcinolone (ILTAC), pimecrolimus 1% cream, and clobetasol 0.05% foam. On follow-up examination, decreased hair loss and increased regrowth were noted. The patient elected to begin tofacitinib citrate 5 mg twice a day for further improvement.
Case 2
A 57-year-old woman with a remote history of AA completed the Pfizer SARS-CoV-2 vaccine series in February 2021. Four months later, she experienced substantial hair loss. She attempted trials of compounded tofacitinib 2%, clobetasol 0.05% ointment, and clobetasol solution from an outside dermatologist with little improvement. On examination, widespread nonscarring alopecia of the scalp with foci of hair regrowth was observed. The patient was subsequently started on tofacitinib citrate 5 mg twice a day.
Case 3
A 62-year-old woman with a remote history of AA presented for hair loss since June 2021. She completed the Moderna SARS-CoV-2 vaccine series in April 2021. On examination, the patient had alopecia universalis with loss of scalp, facial, and body hair. The patient was subsequently treated with tofacitinib citrate 10 mg twice a day and bimatoprost 0.03% eye drops.
Case 4
A 28-year-old woman with a history of AA and Hashimoto thyroiditis well-controlled on levothyroxine noted increased hair loss following completion of the Pfizer SARS-CoV-2 vaccine series in April 2021. The patient reported loss of all scalp and body hair by June 2021. The patient received ILTAC and platelet-rich plasma therapy from an outside dermatologist with little improvement. Her examination was notable for alopecia universalis with loss of scalp, facial, and body hair. The patient was subsequently prescribed tofacitinib citrate 10 mg twice a day.
Case 5
A 29-year-old female began to experience hair loss following completion of the Pfizer SARS-CoV-2 vaccine series in April 2021. Her hair loss subsequently worsened following SARS-CoV-2 infection in August 2021. On presentation, the scalp had 2 patches of nonscarring alopecia with areas of regrowth. Laboratory results were notable for elevated levels of thyroglobulin antibody and thyroid peroxidase antibody. The patient was subsequently treated with ILTAC.
Case 6
A 22-year-old man with a history of elevated thyroid antibody but normal thyroid function tests presented for hair loss starting in April 2021, 1 month after completing the Moderna SARS-CoV-2 vaccine series. The patient received ILTAC from an outside dermatologist with limited improvement. On examination, there were patches of nonscarring alopecia with approximately 30% loss of scalp hair and 80% loss of beard hair. The patient was treated with tofacitinib citrate 10 mg twice a day.
Case 7
A 15-year-old man with no significant medical history presented with hair loss following completion of the Pfizer SARS-CoV-2 vaccine series in June 2021. The patient has a grandmother with Hashimoto thyroiditis and a sister with elevated thyroid antibody now on levothyroxine. On examination, the patient had 2 patches of nonscarring alopecia on the vertex of the scalp. His thyroid laboratory tests were within the normal ranges. The patient was subsequently treated with ILTAC.
Case 8
A 61-year-old man with a history of joint pain, treated with hydroxychloroquine 200 mg daily by rheumatology, presented for hair loss since March 2021, 2 weeks after his first dose of the Pfizer SARS-CoV-2 vaccine. On physical examination, the patient had alopecia totalis with loss of eyebrow, eyelash, and beard hair. The patient is pending a possible trial of oral tofacitinib citrate.
Case 9
A 16-year-old man with no significant medical history presented with hair loss following completion of the Pfizer SARS-CoV-2 vaccine series in June 2021. The hair loss began after the first dose and worsened following the second dose. He received ILTAC from an outside dermatologist with limited improvement. His examination was notable for patches of nonscarring alopecia with 70% hair loss from the scalp and sparse eyebrows and eyelashes. The patient was treated with tofacitinib citrate 10 mg twice a day.
Discussion
Vaccines have been implicated as triggers of autoimmune disease in genetically predisposed individuals. An antibody-mediated response prompted by vaccination may cross-react with self-antigen, leading to autoimmunity. It is possible that the messenger RNA SARS-CoV-2 Moderna and Pfizer vaccines can trigger a T cell-mediated immune response with the downstream effects of alopecia.
Hair loss following SARS-CoV-2 vaccination is an increasingly reported phenomenon in the United States and globally. A search of the Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System database revealed 915 cases of alopecia, 67 of AA, 1 of alopecia totalis, and 8 of alopecia universalis to date associated with the Pfizer or Moderna vaccines.3 A report from Italy describes 1 case of AA recurrence following Pfizer messenger RNA vaccine and 2 cases of AA recurrence following AZD1222/ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca).4 Essam et al5 also reported recurrent AA after a long period of disease quiescence in a middle-aged woman following immunization with the ChAdOx1 nCoV-19 vaccine. Furthermore, there are reports of AA in children and adults after other routine vaccinations. Chu et al6 reported 2 episodes of AA with subsequent regrowth in a child following the third dose of the Japanese encephalitis vaccine at the age of 27 months and the third dose of influenza vaccine at the age of 36 months. Sánchez-Ramón et al7 described AA in a patient following tetanus toxoid vaccination, and Wise et al8 reported 60 cases of AA after various routine childhood vaccinations.
This case series highlights that patients with personal or family histories of AA and other autoimmune diseases, particularly thyroid dysfunction, may be at higher risk of hair loss following SARS-CoV-2 vaccination. One patient had a family history of AA, and 3 patients had previous histories of AA with vaccination triggering disease progression to alopecia universalis in 2 of these 3 cases. Three of the patients had personal histories of thyroid disease or abnormal thyroid antibody levels, and 1 patient had a family history of thyroid disease. Of the remaining patients, 1 with joint pain treated with hydroxychloroquine may also have an undiagnosed autoimmune disorder. Patients with family histories of autoimmune conditions may be genetically predisposed to AA, and immune dysregulation in patients with coexisting autoimmune conditions may exacerbate disease progression.
The onset of AA varied considerably in our patient group: as early as 2 weeks after the first dose up to 4 months after completing both doses. Six of our 9 patients experienced hair loss within 1 month of completing their vaccine series, and 2 more experienced hair loss within 2 months. Chu et al6 and Essam et al5 reported hair loss between a few days to 1 week following vaccination, and Rossi et al4 described hair loss occurring between 2 to 3 weeks after. Wise et al8 found that 84% of hair loss in 50 reports of AA occurred within 1 month of vaccination, although they did report 1 patient with hair loss 10 weeks later. Further studies are needed to draw any conclusions regarding the timing between hair loss and vaccination.
This report is limited by the fact that cause and effect cannot be clearly identified in any of the cases. Extensive patient histories were taken to rule out other triggers of AA, such as illnesses or psychological stress, but given the patients' already increased risk of AA from comorbidities and/or family histories, hair loss following vaccination could be coincidental. Further studies are needed to explore the possible role of SARS-CoV-2 vaccination in activation of AA. This report suggests that physicians should have heightened clinical suspicion of SARS-CoV-2 vaccine-induced AA 1 to 2 months following vaccination after exclusion of other possible triggers. Of note, many medical treatments exist for alopecia, and spontaneous hair regrowth is estimated to occur in approximately 80% of patients within a year after the first incidence of hair loss.9 Indeed, 3 of our patients had foci of hair regrowth on examination. Overall, this report should not discourage from the overwhelming benefits of SARS-CoV-2 vaccination.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Sgubbi P., Savoia F., Calderoni O., Longo R., Stinchi C., Tabanelli M. Alopecia areata in a patient with SARS-Cov-2 infection. Dermatol Ther. 2020;33(6):e14295. doi: 10.1111/dth.14295. [DOI] [PubMed] [Google Scholar]
- 2.Fivenson D. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2021;60(1):127. doi: 10.1111/ijd.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services (DHHS) Public Health Service (PHS) Centers for Disease Control (CDC)/Food and Drug Administration (FDA) The Vaccine Adverse Event Reporting System (VAERS) 1990 - 11/05/2021, CDC WONDER online database. http://wonder.cdc.gov/vaers.html
- 4.Rossi A., Magri F., Michelini S., et al. Recurrence of alopecia areata after covid-19 vaccination: a report of three cases in Italy. J Cosmet Dermatol. 2021;20(12):3753–3757. doi: 10.1111/jocd.14581. [DOI] [PubMed] [Google Scholar]
- 5.Essam R., Ehab R., Al-Razzaz R., Khater M.W., Moustafa E.A. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol. 2021;20(12):3727–3729. doi: 10.1111/jocd.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu C.H., Cheng Y.P., Chan J.Y.L. Alopecia areata after vaccination: recurrence with rechallenge. Pediatr Dermatol. 2016;33(3):e218–e219. doi: 10.1111/pde.12849. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Ramón S., Gil J., Cianchetta-Sívori M., Fernández-Cruz E. Alopecia universal en un adulto tras la vacunación de rutina con toxoide tetánico. Med Clin (Barc) 2011;136(7):318. doi: 10.1016/j.medcli.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Wise R.P., Kiminyo K.P., Salive M.E. Hair loss after routine immunizations. JAMA. 1997;278(14):1176–1178. doi: 10.1001/jama.1997.03550140068042. [DOI] [PubMed] [Google Scholar]
- 9.Maclean K.J., Tidman M.J. Alopecia areata: more than skin deep. Practitioner. 2013;257(1764):29–32. 3. [PubMed] [Google Scholar]