Dear Editor,
SARS-CoV-2 vaccination has demonstrated efficacy in large clinical trials but immunocompromised patients including patients with rheumatic diseases are often excluded. A recent letter in Journal of Infection identified an anti-receptor binding domain (RBD) antibody (Ab) threshold below which protection against SARS-CoV-2 infection is inferior to 90% [1].
We aimed to explore the Ab response to SARS-CoV-2 following complete vaccination in 231 patients suffering from immune-mediated or inflammatory rheumatic diseases (IMID) followed at Cliniques Universitaires Saint-Luc and to assess factors predicting a suboptimal humoral response to vaccination in at-risk patients treated with disease modifying anti-rheumatic drugs (DMARD).
Two different electrochemiluminescent immunoassays were used to detect total antibodies in human serum: Elecsys anti-SARS-CoV-2 qualitative immunoassay using recombinant nucleocapsid antigen (anti-N) and Elecsys anti-SARS-CoV-2 S quantitative immunoassay using spike protein RBD (anti-RBD) (Roche Diagnostics GmbH, Mannheim, Germany). The two tests were carried out simultaneously, the presence of anti-N antibodies indicating post infection immunity. According to the manufacturer, a result was considered positive if the cut-off index was ≥1.0 for anti-N or ≥0.8 U/mL for anti-RBD. Values of anti-RBD Ab were converted to BAU/mL.
All patients completed the 2-doses series of vaccination (AZD1222/ Oxford–AstraZeneca ChAdOx1nCoV-19; BNT162b2/ Pfizer-BioNTech COVID-19 mRNA or mRNA-1273, Moderna) or a single-dose vaccination (JNJ-78436735, Johnson) against SARS-CoV-2 following the national vaccination program. Humoral immune response was defined by development of anti-RBD Ab >1 week following completion of 2-dose series of vaccination or >2 weeks after a single dose vaccination. Optimal response was defined as anti-RBD Ab>141 BAU/ml, threshold below which protection against SARS-CoV-2 infection is inferior to 90% as previously reported [1]. Patients were defined as responders if anti-RBD Ab titers were >141 BAU/ml and non-responders if anti-RBD Ab titers were ≤141 BAU/ml.
Two-hundred thirty one IMID patients (71.6%, rheumatoid arthritis; 23.4% spondyloarthropathies and 5% other inflammatory diseases) were included between 20th April and 20th September 2021. Mean age at inclusion was 56.4 ± 13.4 years [range, 26–91], 57% were women, median time since diagnosis was 7 years (IQR:11) and median time of Ab dosage after full vaccination was 1.6 months (IQR: 2). Fifty-nine percent of patients (n = 136) were treated with methotrexate, 7% (n = 17) with glucocorticoids, 49% (n = 113) with biologics and 7% (n = 4) with Rituximab. Eighty-four percent (n = 195) received an mRNA vaccine and 16% of patients (n = 36) a viral vector vaccine. Ab titers were not significantly different in patients tested less than 3 months after the last dose (225.0 ± 63.1 BAU/ml, mRNA vaccines; 188.2 ± 92.4 BAU/ml, viral vector vaccines) compared to patients tested >3 months after the last dose (205.7 ± 71.9 BAU/ml, mRNA vaccines; 188.5 ± 84.8 BAU/ml, viral vector vaccines).
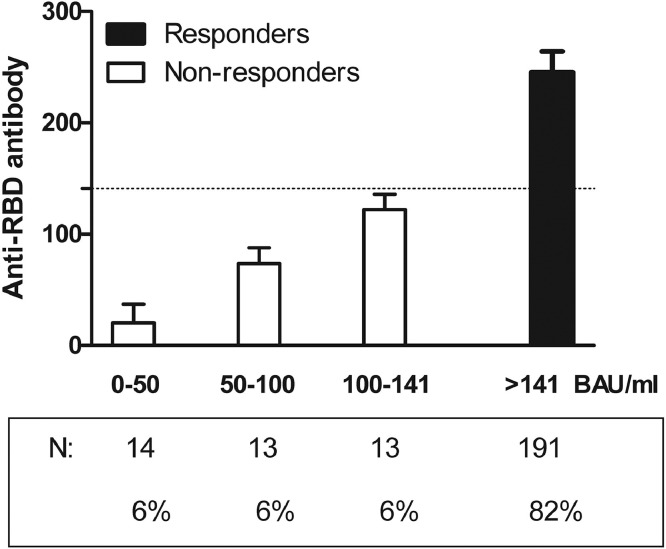
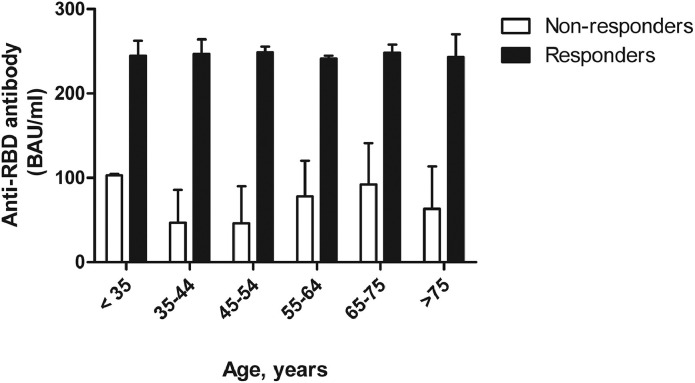
At the time of anti-RBD Ab dosage, 191 patients (82%, responders) mounted an optimal humoral response (anti-RBD Ab: 245.4 ± 18.8 BAU/ml) vs. 40 patients (18%, non-responders) who failed to respond optimally (anti-RBD Ab: 70.7 ± 44.9 BAU/ml) after complete vaccination (Fig. 1 ). Non-responders were equally distributed across all age categories (Fig. 2 ) and according to quintiles of anti-RBD Ab titers (Fig. 1). Age, gender, type of rheumatic disease, disease duration, comorbidities, previous COVID-19 infection and treatment (glucocorticoids, biotherapy, synthetic DMARD) were not significantly associated with optimal humoral response after vaccination. In contrast, in patients receiving viral vector vaccines, the percentage of non-responders (30.6%) was twice higher than in patients receiving mRNA vaccine (15.2%).
Fig. 1.
Anti-RBD antibody concentrations among responders and non-responders.
Fig. 2.
Distribution of patients according to anti-RBD antibody concentrations across different age categories.
In multivariate logistic analysis, humoral response remained significantly associated with vaccine type (15% non-responders, mRNA vaccines; 31% non-responders, viral vector vaccines, p = 0.02) when controlled for age, treatment and time between vaccination and Ab dosage.
In our cohort, 5 patients were treated by anti-CD20/anti-CTLA4 biotherapy associated with methotrexate (n = 4) or with mycophenolate mofetil (n = 1). Three out of them developed a good humoral response (anti-RBD Ab: >250 BAU/ml, n = 2 and 145 BAU/ml, n = 1, respectively) while a poor response was observed in the two others (anti-RBD Ab: 58.8 BAU/ml, and 0 BAU/ml, respectively).
Patients treated with methotrexate developed a humoral response similar to those without methotrexate (anti-RBD Ab: 215.6 ± 66.2 BAU/ml, 136 patients treated with methotrexate vs. 214.5 ± 77.4 BAU/ml, 95 patients without methotrexate).
In conclusion, 82% of patients with rheumatic disease developed an optimal humoral immune response following completion of anti-SARS-CoV-2 vaccine series. We confirm that the seroconversion rates are lower in IMID patients than previously reported in healthy controls or in health care workers in Belgium [3] but higher than those in kidney transplant recipients [4].
The mRNA vaccines were associated with optimal humoral response in 85% of patients and viral vector vaccines in only 69% of patients. Therefore, the dosage of anti-RBD Ab helps to identify patients with a poorer response in whom an additional dose of vaccine or monoclonal antibodies administration may be needed in order to ensure optimal protection against severe COVID-19.
In recent studies, certain therapies (anti-TNF, anti-IL17, anti-IL6, anti-IL12/23) seem not to impact seroconversion rates while others (anti-CD20, anti-CTLA-4) result in poorer responses in patients treated with immunosupressors for different rheumatic and non-rheumatic diseases [2,5]. Furthermore, we confirm a good response to vaccination in patients treated with TNF blockers, anti-IL 17, anti-IL6, anti-12/23 therapies and observed a poor response in patients treated with anti-CD20/anti-CTLA-4 although numbers are low (n = 5). Conflicting results were reported in patients using methotrexate [5,6]. We did not found that methotrexate hampers humoral response to vaccine in our cohort.
The current study has limitations. First, no data on cellular immunity are available. However, antibody response correlates well with T-cell mediated immunity [7]. Second, all patients were followed in a tertiary care center. Third, the results cannot be necessary extrapolated in the context of different strategies, access, timing of SARS-CoV-2 vaccination throughout the world. Besides age, treatment, other unknown factors may influence the response to vaccination. However, our data suggest that monitoring the anti-RBD antibody response is feasible [1] and may identify patients with poor response to vaccination and therefore potentially at risk of severe COVID-19 outcomes. Fourth, the threshold of protection reported by Dimeglio et al. [1] should be confirmed in other studies including both immunocompetent and immunosuppressed patients, particularly in the context of new emerging variants. Additional studies are needed to identify strategies to improve seroconversion rates in IMID patients.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Dimeglio C., Herin F., Martin-Blondel G., Miedougé M., Izopet J. Antibody titers and protection against a SARS-CoV-2 infection. J Infect. 2021 doi: 10.1016/j.jinf.2021.09.013. Sep 21 S0163-4453(21)00483-7 Epub ahead of print PMID:34560135 PMCID: PMC8452591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammitzbøll C., Bartels L.E., Bøgh Andersen J., Risbøl Vils S., Elbaek Mistegård C., Dahl Johannsen A., From Hermansen M.L., Kragh Thomsen M., Erikstrup C., Hauge E.M., Troldborg A. Impaired antibody response to the BNT162b2 messenger RNA Coronavirus Disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. Sep 2021;3(9):622–628. doi: 10.1002/acr2.11299. Epub 2021 Jul 17 PMID:34273260 PMCID: PMC8426741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021 Oct 19;326(15):1533–1535. doi: 10.1001/jama.2021.15125. PMID:34459863 PMCID: PMC8406205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devresse A., Saad Albichr I., Georgery H., Yombi J.C., De Greef J., Belkhir L., Mzougui S., Scohy A., Darius T., Buemi A., Goffin E., Kabamba B., Kanaan N. T-cell and antibody response after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation. 2021;105(10):e142–e143. doi: 10.1097/TP.0000000000003892. Oct 1 PMID:34310103 PMCID: PMC8487701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jena A., Mishra S., Deepak P., Kumar-M P., Sharma A., Patel Y.I., Kennedy N.A., Kim A.H.J., Sharma V., Sebastian S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2021 Aug 30 doi: 10.1016/j.autrev.2021.102927. Epub ahead of print PMID:34474172 PMCID: PMC8404391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberman R.H., Herati R.S., Simon D., Samanovic M., Blank R.B., Tuen M., Koralov S.B., Atreya R., Tascilar K., Allen J.R., Castillo R., Cornelius A.R., Rackoff P., Solomon G., Adhikari S., Azar N., Rosenthal P., Izmirly P., Samuels J., Golden B., Reddy S., Neurath M., Abramson S.B., Schett G., Mulligan M.J., Scher J.U. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. medRxiv. 2021 May 12 doi: 10.1101/2021.05.11.21256917. [Preprint] 2021.05.11.21256917 Update in: Ann Rheum Dis. 2021 May 25 PMID:34013285 PMCID: PMC8132259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K.E., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grützner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kühnle M.C., Poran A., Dong J.Z., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Palanche T., Schultz A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabó G.T., Karikó K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Türeci Ö. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. Jul 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. Epub 2021 May 27 PMID:34044428. [DOI] [PubMed] [Google Scholar]