Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a serious, potentially life-threatening hypersensitivity syndrome that usually occurs after exposure to a drug. The clinical features include rash, edema, fever, lymphadenopathy, and involvement of other organs. The etiopathogenesis is still being investigated. DRESS syndrome tends to occur after ingestion of a drug by a genetically predisposed individual. Reactivation of viruses, including human herpesvirus 6, human herpesvirus 7, Epstein-Barr virus, and human cytomegalovirus, has been reported in patients with DRESS syndrome, but the exact contribution of reactivation of viruses to symptoms and severity has not yet been established.1 We report a case of DRESS syndrome following administration of the AstraZeneca COVID-19 vaccine (Vaxzevria).
Case report
A 45-year-old woman presented with an 8-day history of a gradually worsening rash consisting of red papules on the face, trunk, and extremities. She complained of chills, sore throat, and hoarseness. She had no preceding medical conditions and was taking no regular medication. She took levocetirizine and fexofenadine for the rash. She had received her first dose of AstraZeneca COVID-19 vaccine (Vaxzevria) 7 weeks before onset of the rash.
On examination, there was erythema and edema of the eyelids and lips (Fig 1) and multiple pink papules and plaques on the face, trunk, and extremities, some with superficial scale. There were small pustules on the upper lip. Edema of the arms and legs with coalescent erythema was observed. Violaceous patches on the feet (Fig 2) with some targetoid lesions and some central erosions were noted. There was conjunctivitis, erythema of the pharynx, and cervical lymphadenopathy.
Fig 1.

Erythema, edema, papules and plaques, and occasional pustules on the face.
Fig 2.
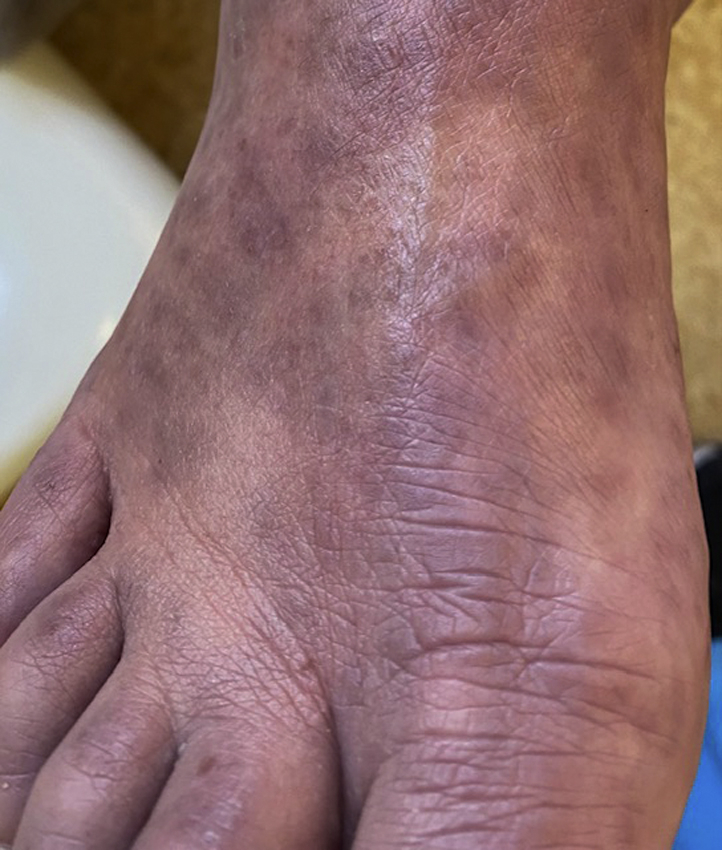
Violaceous patches on the feet with targetoid lesions.
Blood test results on admission showed an elevated eosinophil count of 1.48 × 109/L (normal range 0.02-0.5 × 109/L), C-reactive protein 32.3 (normal range <5.0), normal liver function tests, COVID-19 IgG weakly positive, COVID-19 polymerase chain reaction tests negative × 2, negative respiratory panel, and negative serology for Mycoplasma pneumoniae, cytomegalovirus, Epstein-Barr virus, and human herpesvirus 6. Chest computed tomography showed serositis with mild fluid in the pleural and peritoneal cavities.
Skin biopsy showed diffuse marked spongiosis with patchy vacuolar interface change and occasional dyskeratotic cells (Fig 3). There was a patchy lichenoid band of lymphocytes with perivascular lymphocytes and abundant eosinophils (Fig 4). These features were consistent with the clinical impression of DRESS syndrome.
Fig 3.

Low-power view of epidermal spongiosis and interface change with superficial dermal perivascular inflammatory infiltrate and vascular telangiectasia. (Hematoxylin-eosin stain; original magnification: ×100.)
Fig 4.

High-power view of dermal perivascular inflammatory infiltrate comprising lymphocytes and abundant eosinophils. (Hematoxylin-eosin stain; original magnification: ×400.)
The patient was started on intravenous hydrocortisone on admission, and her condition improved. However, 5 days after admission, on switching to oral prednisolone, she had a relapse with chills, malaise, conjunctivitis, generalized erythema, and edema. Her eosinophil count rose to 3.65 × 109/L. Her liver enzymes rose slightly to 40 IU/L for aspartate aminotransferase (normal range 5-34 IU/L), to 87 IU/L for alanine aminotransferase (normal range <55 IU/L), and to 39 IU/L for gamma-glutamyl transferase (normal range 9-36 IU/L). Intravenous methylprednisolone was administered for 3 days, with marked improvement in skin condition and reduction in eosinophil count and liver enzymes. Oral prednisolone 60 mg daily was commenced after the course of methylprednisolone and slowly weaned. At review 3 weeks later, she still had some edema of the face, arms, and legs, and at review 7 weeks later, while she was taking prednisolone 8 mg daily, there was mild edema remaining, with a dusky purple color of the skin on the hands and feet. Liver enzymes remained normal, with a very mild increase in eosinophil count to 0.6 × 109/L.
Discussion
DRESS is a drug-induced hypersensitivity reaction that usually occurs 2 to 8 weeks after exposure to the culprit drug. It has an associated mortality of around 10%.2 The most common drugs that cause DRESS are anticonvulsants, antibiotics, antiretrovirals, and allopurinol. DRESS has been reported rarely after influenza vaccination.3, 4, 5
Our patient was taking no other medications before the onset of her symptoms. Therefore, we believe that her DRESS syndrome was precipitated by her first dose of AstraZeneca COVID-19 vaccine (Vaxzevria). We are not aware of any other cases of DRESS syndrome occurring after the AstraZeneca vaccine. A case has been reported of a severe cutaneous adverse reaction to the Janssen Ad26.COV2.S COVID-19 vaccine, with a differential diagnosis of acute generalized exanthematous pustulosis, DRESS, or acute generalized exanthematous pustulosis-DRESS overlap.6
DRESS syndrome is treated by withdrawal of the offending agent, supportive care, monitoring for systemic involvement, and corticosteroids. Relapses can occur with a steroid taper, as occurred in our patient. Symptoms can persist for up to a year in some patients.7
Organ damage can be very severe in some patients, leading to permanent impairment of organ function. Several autoimmune diseases, including thyroiditis, type 1 diabetes mellitus, and systemic lupus erythematosus, have been reported from a few months to several years after recovery from DRESS syndrome.8 Patients with DRESS syndrome require careful follow-up after recovery, and clinicians need to be aware of the possibility of autoimmune sequelae.
The role of reactivation of Herpesviridae in the pathogenesis of DRESS remains uncertain. Drug-specific T cells producing tumor necrosis factor alpha and interferon gamma have been identified and may be important in the pathogenesis.9
We present this case to highlight the importance of a vaccination history in a patient presenting with clinical features suspicious for DRESS syndrome. This is particularly relevant in the case of vaccines requiring 2 doses, since the second dose should be withheld, as should boosters of the same vaccine. DRESS syndrome often has a longer latent period than other drug reactions, and thus the drug history should include exposures in the preceding 8 weeks. Some patients have a genetic predisposition to drug reactions and may be at higher risk of developing a severe reaction, including DRESS syndrome after the COVID-19 vaccine.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Roujeau J.C., Dupin N. Virus reactivation in drug reaction with eosinophilia and systemic symptoms (DRESS) results from a strong drug-specific immune response. J Allergy Clin Immunol Pract. 2017;5:811–812. doi: 10.1016/j.jaip.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y.C., Chiu H.C., Chu C.Y. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–1379. doi: 10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt N., Levinson M., Stephenson G. Drug reaction with eosinophilia and systemic symptoms associated with H1N1 vaccination. Intern Med J. 2012;42:1365–1366. doi: 10.1111/imj.12012. [DOI] [PubMed] [Google Scholar]
- 4.Solak B., Dikicier B.S., Kara R.O., Erdem T. DRESS syndrome potentially induced by allopurinol and triggered by influenza vaccine. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-214563. bcr206214563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin D.W.J., Martin G.E., McLean C., Cheng A.C., Giles M.L. A case of drug reaction with eosinophilia and systemic symptoms (DRESS) without a typical precipitant. Med J Aust. 2020;212(7):300–312. doi: 10.5694/mja2.50519. [DOI] [PubMed] [Google Scholar]
- 6.Lospinoso K., Nichols C.S., Malachowski S.J., Mochel M.C., Nutan F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26,COV2.S COVID-19 vaccine. JAAD Case Rep. 2021;13:134–137. doi: 10.1016/j.jdcr.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tetart F., Picard D., Janela B., Joly P., Musette P. Prolonged evolution of drug reaction with eosinophilia and systemic symptoms: clinical, virologic and biological features. JAMA Dermatol. 2014;150:206–207. doi: 10.1001/jamadermatol.2013.6698. [DOI] [PubMed] [Google Scholar]
- 8.Kano Y., Tohyama M., Aihara M., et al. Sequelae of 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on severe cutaneous adverse reactions (ASCAR) J Dermatol. 2015;42:276–282. doi: 10.1111/1346-8138.12770. [DOI] [PubMed] [Google Scholar]
- 9.Ye Y.M., Hur G.Y., Kim S.H., et al. Drug-specific CD4+ T-cell immune responses are responsible for antituberculous drug-induced maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms syndrome. Br J Dermatol. 2017;176(2):378–386. doi: 10.1111/bjd.14839. [DOI] [PubMed] [Google Scholar]


