Abstract
Background
COVID-19 pandemic continues to be a priority in public health worldwide, and factors inherent to SARS-CoV-2 pathogenesis and genomic characteristics are under study. Investigations that evaluate possible risk factors for infection, clinical manifestations, and viral shedding in different specimens also need to clarify possible associations with COVID-19 prognosis and disease outcomes.
Study design
In this study, we evaluated SARS-CoV-2 positivity and estimated viral loads by real-time RT-PCR in stool, sera, and urine samples from 35 patients, with a positive SARS-CoV-2 RNA molecular test in respiratory sample, attended at a University COVID-19 referral hospital in Goiania, Goias, Brazil. Whole-genome sequencing was also performed in samples with higher viral load.
Results
The positivity index was 51.43%, 14.28%, and 5.71% in stool, sera, and urine specimens, respectively. The median viral load was 8.01 × 106 GC/g, 2.03 × 106 GC/mL, and 1.36 × 105 GC/mL in stool, sera, and urine, respectivelly. Of all patients, 88.57% had previous comorbidities, and 48.39% of them had detectable SARS-CoV-2 RNA in at least one type of clinical specimen evaluated by this study (stool, sera or urine). A higher viral load was observed in patients with more than two previous comorbidities and that were classified as severe or critical conditions. Samples with the highest viral loads were sequenced and characterized as B.1.1.33 variant.
Conclusion
We conclude that SARS-CoV-2 RNA is present in more than one type of clinical specimen during the infection, and that the most critical patients had detectable viral RNA in more than one clinical specimen at the same time point.
Keywords: SARS-CoV-2 RNA, viral shedding, comorbidity, B.1.1.33 variant, whole-genome sequencing
1. Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has, to date, affected over 216,000,000 people worldwide, causing more than 4490,000 deaths [1]. The SARS-CoV-2 was classified in the family Coronaviridae together with MERS-CoV and SARS-CoV, and it was immediately associated with severe respiratory syndrome [2,3]. Clinical manifestations of SARS-CoV-2 infection ranges from asymptomatic presentation to severe, usually associated with pneumonia, coagulopathy, and sepsis, which can culminate with death [4]. Main symptoms or signs of this illness include fever, dyspnea, dry cough, and fatigue. Other reported symptoms are headache, coryza, diarrhea, vomiting, and abdominal pain [4,5].
Even though the virus can spread to various sites in the infected body [6], not many studies have assessed viral loads in clinical specimens, other than respiratory tract samples [6], [7], [8], [9]. Therefore, in the present study, we evaluated the positivity and estimated loads of SARS-CoV-2 in different clinical specimens obtained from patients that were hospitalized in a COVID-19 referral University Hospital in Goiás, Brazil, in association with clinical aspects of the infection and comorbidities presented by the patients.
2. Materials and methods
2.1. Experimental design and sample collection
This is a single-center study conducted at a University Hospital (Hospital das Clínicas-HC/Universidade Federal de Goiás-UFG) located in Goiânia, Goiás, Brazil. The hospital is a regional reference for COVID-19 patients with underlying chronic conditions and pregnancy. The study was approved by the Ethics Committee of the Hospital das Clínicas/Brazil (CAEE 30,804,220.2.0000.5078). Positive SARS-CoV-2 RNA molecular test in respiratory samples was considered as inclusion criteria. Therefore, patients that met these criteria treated at the HC/UFG from May to October 2020 were enrolled in the study.
Clinical specimens (stool, urine, and serum) were collected after the participant signed the Informed Consent Form or by their responsible relative. After samples were collected, they were immediately transported to the laboratory (LABCOVID/UFG), under proper packaging, where they were processed and stored at −80 °C until further testing.
2.2. Sample processing and RNA extraction
Stool, urine, and serum samples were processed according to the previously described protocol [8]. After processing, all samples were subjected to RNA extraction using the commercial Qiagen viral RNA mini kit (Qiagen, Hilden, Germany).
2.3. SARS-CoV-2 RNA detection
RNA extracts were tested by real-time polymerase chain reaction post reverse transcription (real-time RT-PCR) using the Promega GoTaq® Probe 1- Step RT-qPCR System, according to the manufacturer's instructions. Primers and probes were manufactured by IDT (Integrated DNA Technologies, Iowa, USA), following the protocol approved by the Centers of Disease Control (CDC, USA), and targeted two regions of the N gene (N1 and N2) and the human RNAse P (RP) gene, as an endogenous control. Samples with cycle threshold (Ct) lower than 40 (considering all targets) were positive for SARS-CoV-2 RNA. The viral loads in genomic copies (GC) per mL/g of clinical specimens were estimated based on a standard curve of serial dilutions (106 to 10° GC/µL) of the synthetic positive control nCoVPC from IDT (Integrated DNA Technologies, Iowa, USA).
2.4. Whole-genome MinION sequencing and mutations analyses
Stool samples with the highest viral loads were sequenced following Midnight protocol as previously described [10] using MinION (Oxford, UK). After sequencing, a quality threshold was set and only sequences with high coverage of at least 200x per base pair, coverage length of >98% of the genome, were accepted. The FASTA file with consensus sequences was uploaded in the Nextclade Beta (https://clades.nextstrain.org/) [11] to identify mutations profile, considering PANGO linages. The alignment was performed by MAFFT v.7.477 [12] and checked manually. The data set of sequences was constituted of sequences available at the GISAID EpiCoV database [13], collected between August 1st and October 31st 2020, in Goias, Brazil. The Maximum-likelihood phylogenetic tree was built by IQ-tree web [14] server and visualized in FigTree v1.4.4 [15].
2.5. Clinical data and statistical analyses
The patients' clinical profiles were classified according to the National Institute of Health categories [5]: mild, moderate, severe, or critical COVID-19, according to clinical observations and laboratory results, obtained from medical records, and were validated by two infectious diseases physicians of the collaborative group. The statistical analysis of the data was performed by the software R version 4.0.5 [16], using descriptive analysis.
3. Results
Following the inclusion criteria, 35 patients were enrolled in the study, and one stool, serum, and urine sample each were obtained from each patient. The samples were collected over 4 to 43 days after they had presented the first symptom. Considering the period of sample collection, 51.43% (18/35) of patients had samples collected into 4 to 10 days after the first symptom; 28.57% (10/35) from 11 to 15 days after the first symptom, and 20% (7/35) from 16 to 43 days after the first symptom. Clinical and demographic characteristics of the population are displayed in Table 1 . From the total number of patients, 51.43% (18/35) were male. Patients' age ranged from 25 to 86 years old, with a median of 45 years old. Considering clinical disease profile, 17.14% (6/35) of patients were classified as mild, 28.57% (10/35) as moderate, 31.43% (11/35) as severe, and 22.85% (8/35) as critical.
Table 1.
Clinical, laboratory and demographic characteristics of patients.
| Characteristics | Percentile (n/total) | Median |
|---|---|---|
| Sex | ||
| Male | 51.43% (18/35) | – |
| Female | 48.57% (17/35) | – |
| Age | ||
| 25–86 years old | – | 45 years old |
| Clinical disease profile | ||
| Mild | 17.14% (6/35) | – |
| moderate | 28.57% (10/35) | – |
| severe | 31.43% (11/35) | – |
| critical | 22.85% (8/35) | – |
| SARS-CoV-2 RNA positivity index | ||
| Stool | 51.43% (18/35) | 8.01 × 106 GC/g1 |
| Serum | 14.28% (5/35) | 2.03 × 106 GC/mL1 |
| Urine | 5.71% (2/35) | 1.36 × 105 GC/mL1 |
| Risk factors/Comorbidities | ||
| Pregnancy | 58.82% (10/17) | – |
| Obesity | 25.71% (9/35) | – |
| Hypertension | 22.86% (8/35) | – |
| Diabetes mellitus | 17.14% (6/35) | – |
| Cardiopathy | 17.14% (6/35) | – |
| Chronic kidney disease | 14.28% (5/35) | – |
| Age>65 years old | 11.42% (4/35) | – |
| Previous respiratory disease | 11.42% (4/35) | – |
| Cancer | 2.85% (1/35) | – |
Viral loads were expressed in genomic copies per milliliter (stool and urine) or gram (stool).
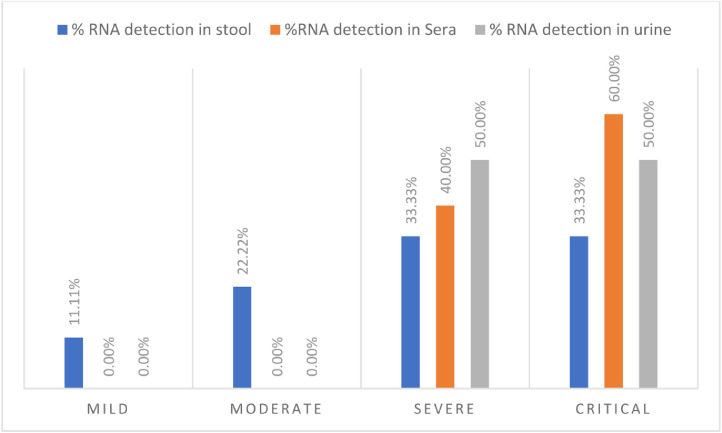
The SARS-CoV-2 RNA positivity index in stool samples was 51.43% (18/35), with viral loads ranging from 2.0 × 104 GC/g to 6.53 × 1010 GC/g, with a median of 8.01 × 106 GC/g. In urine samples, the positivity index was 5.71% (2/35), with viral loads ranging from 2.22 × 104 G.C./mL to 2.49 × 105 G.C./mL, and a median of 1.36 × 105 G.C./mL. In sera, the positivity was 14.28% (5/35) with viral loads ranging from 7.86 × 104 GC/mL to 3.98 × 106 GC/mL (median of 2.03 × 106 GC/mL). As shown in Fig. 1 , considering the patient's clinical profile, the highest positivity index was detected in samples from severe and critical patients and only 33,33% (6/18) of patients classified as mild and moderate presented positivity in stool samples.
Fig. 1.
SARS-CoV-2 RNA positivity indexes in different specimens, according to the patients’ clinical profile. SARS-CoV-2 RNA was detected in urine or sera samples only in patients who presented severe or critical COVID-19.
Positivity indexes for SARS-CoV-2 RNA in stool were 50% (9/18) in samples collected from day 4 to day 10 of symptoms, 38,89% (7/18) in samples collected from day 11 to day 15, and 11,12% (2/18) in samples obtained from day 16 to day 43 of symptoms. All samples collected after 18 days of symptoms did not have detectable SARS-CoV-2 RNA. Regarding urine samples, 100% (2/2) of positivity was detected 4 to 10 days after the first symptom. Considering sera samples, 60% (3/5) presented positivity from day 11 to 15 days of symptoms and 20% (1/5) 4 to 10 and 16 to 43 days after the first symptom, respectively.
Patients with the highest viral loads in stool samples also had concomitant positivity in serum samples, and the two patients that were positive in urine were also positive in stool samples. All patients from whom it was possible to detect concomitant positivity in more than one sample (stool and serum or stool and urine samples) had severe or critical COVID-19. Eighty percent (4/5) of patients that had SARS-CoV-2 RNA-positive serum samples had a fatal outcome.
The primary reported symptoms at admission were dyspnea (74.28%), cough (71.42%), fever (68.57%), myalgia (37.14%), asthenia (37.14%), headache (37.14%), and indisposition (28.57%). The least commonly reported symptoms were seizure, skin rash, and bleeding (2.86%). Gastrointestinal symptoms were also recorded, 20.0% of the patients had diarrhea and abdominal pain, and 8.57% had nausea and vomiting. Among patients positive for SARS-CoV-2 in stool samples, 44.44% had at least one gastrointestinal symptom.
During the hospitalization period, 82.85% (28/35) of the patients presented pneumonia, of whom 64.28% (18/28) required oxygen support. Regarding the need for oxygen support, 55.55% (10/18) used a nasal catheter and/or mask with the reservoir; 38,88% (7/18) used a mechanical ventilator, and 5.55% (1/18) non-invasive ventilation.
Considering the administration of medications during hospitalization, 74.28% (26/35) of the patients received antibiotic therapy, 68.57% received corticosteroid therapy, 42.86% (15/35) received therapy with anticoagulants, and 20.0% (7/35) received treatment with bronchodilators. These four combined therapies were administered to 17.14% of the patients, classified as severe or critical COVID-19, from which 66.66% died.
Of all 35 patients, 88.57% (31/35) had at least one comorbidity, with 45.72% (16/35) having two or more. Among the most frequent underlying conditions considered as COVID-19 risk factors, the most frequent were obesity 25.71% (9/35), hypertension 22.86% (8/35), diabetes mellitus 17.14% (6/35), and cardiopathy 17.14% (6/35). Considering the total number of women included in the study (17), 58.82% were pregnant, with a gestational age of 19 to 39 weeks, but only two had detectable SARS-CoV-2 RNA in stool samples, and one of them also had in concomitance a positive serum sample.
Among patients with comorbidities and presented positivity in stool samples, 20.0% were classified as mild, 13.33% as moderate, 40.0% as severe, and 26.66% as critical. Viral loads in stool were higher in samples from patients with comorbidities. The overall death rate was 22.86% (8/35), and 87.5% (7/8) of the patients that died had comorbidities associated. All patients who died were classified as severe COVID-19 cases and their hospitalization period ranged from 13 to 78 days.
The three stool samples with the highest viral loads were submitted to whole-genome sequencing, one collected in August 2020 and two collected in October 2020. The genomes were deposited in GISAID EPICoV database: EPI_ISL_3,769,334, EPI_ISL_3,769,414, EPI_ISL_3,769,304. According to the Pango Linagens platform, all samples were classified as B.1.1.33 variant. By submitting the nucleotide sequences on the NextClade platform, it was possible to observe the profile of mutations found in these genomes. All mutations found were described in Table 2 . Of notice, the three genomes presented the D614G mutation in the Spike protein (Gene S).
Table 2.
Synapomorphyc mutations* of SARS-CoV-2 lineage B 1.1.33.
| Genomic region | Nucleotide changes | Amino Acid changes |
|---|---|---|
| ORF1a | C8481T | P2739L |
| C9866T | L3201F | |
| ORF1b | C14408T | P314L |
| T17011C | S1182P | |
| A21311C | Q2615P | |
| ORF3a | G25793T | R134L |
| ORF6 | T27299C | I33T |
| ORF8 | G28179T | G96C |
| N | G28881A | R203K |
| G28882A | G204R | |
| G28883C | I292T | |
| S | A23403G | D614G |
The profile of mutations was obtained by NextClade database platform.
The Maximum-likelihood phylogenetic tree was built on data set that contained the three sequences of this study plus 23 sequences deposited in GISAID between August 1st and October 31st 2020, collected in Goias, Brazil (Fig. 2 ). Following the phylogenetic analyses, the sample collected in August 2020 was similar to others samples collected in the same period in Goias. Furthermore, the same pattern was observed in the two samples collected in October 2020.
Fig. 2.
SARS-CoV-2 lineages circulating in Goiás between August to October 2020. In blue are the sequences obtained in this study, classified as B.1.1.33 linage. In red are represented the outgroup (Gamma variant). The analysis involved 26 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 27,873 positions in the final dataset.
4. Discussion
This study detected the RNA and estimated SARS-CoV-2 loads in different specimens of COVID-19 patients. Although it was not possible to obtain periodically serially collected samples from each patient during infection, the data found here regarding positivity is in accordance with data from other studies that also reported SARS-CoV-2 RNA positivity in stool in more than 50% of the patients [7,17,18], and lower positivity rates in urine samples [9,17,19,20].
The SARS-CoV-2 RNA detection in other specimens than respiratory tract samples (urine, sera and stool) has been reported by other studies [8,9,[20], [21], [22], [23], [24]]. However a defined period of shedding in those clinical specimens remains unclear. In this study, detection of SARS-CoV-2 RNA was more frequent up to 15 days after the first symptom, considering stool and urine samples. This data, coupled with the concomitant positivity observed in more than one clinical specimen, suggest the importance of exploring clinical specimens other than nasopharyngeal swabs for the diagnosis of COVID-19, since they may remain positive after SARS-CoV-2 RNA is no longer detected in the respiratory tract [25]. Futhermore, the viral sheeding in stool and urine bring on the possibility of new routes of transmission that need to be better elucidated.
The two patients who had detectable SARS-CoV-2 RNA in urine did not present renal dysfunctions during the hospitalization. However, they were classified into severe or critical COVID-19. Few other studies have been able to report viral shedding in urine, and the possible correlation between viral shedding in the urine and disease severity is reinforced by our data [22,23,26]. The presence of SARS-CoV-2 RNAemia has been already linked to disease severity [17,24,27,28], as we could also observed in this study. Also, RNAmia may suggest systemic infection and more studies should be conducted to clarify this matter.
The cohort followed by this study presented, in its majority, previous comorbidities, and more than half of the patients developed severe disease. We could also notice a pattern that the presence of previous comorbidities constitutes a risk factor [27], [28], [29] for the development of severe COVID-19, which has also been shown by other studies. Furthermore, we could observe that the patients included in this study who had more than one comorbidity had the highest viral loads, when compared to those patients that had only one type of comorbidity.
The main symptoms recorded at admission were dyspnea, cough, and fever, similar to what has been already reported [17,26,30,31]. A small number of patients who presented positivity in stool, had also diarrhea, nausea or vomiting. However, shedding in the stool is not directly related to gastrointestinal symptoms, as it has been reported [8,17,23,25]. As for comorbidities as predictors of disease severity, some studies [17,[30], [31], [32], [33]] have reported obesity, hypertension, and diabetes mellitus as common comorbidities among COVID-19 hospitalized patients, as also indicated by our results.
The three samples sequenced in this study were characterized asthe same variant, B.1.1.33, predominant in the state of Goiás, according to the sequences in GISAID collected during the same period. Furthermore, this variant was the predominant one circulating in Brazil at the beginning of the pandemic [34,35]. The D614G mutation was present in all three of our samples. This mutation is characteristic of the B.1.1.33 lineage and has previously been associated with a greater affinity for the ACE-2 receptor and, therefore, higher transmissibility and infectivity [36].
This study has some limitations, starting with the fact that we could only obtain one sample of each clinical specimen per patient during their hospitalization period. Also, the number of enrolled patients was low, and therefore it was not possible to acess statistical significance of the data, but still we could observe a trend of higher viral loads in stool and positivity in other specimens such as urine and sera as an indicator of illness severity. Also, that SARS-CoV-2 positivity is found in concomitance in stool and sera. We believe that our findings may contribute to the knowledge of SARS-CoV-2 RNA-positivity and viral loads in distinct clinical specimens and the clinical characteristics of COVID-19. The samples obtained during the study will be included in a biobank for future analysis on the molecular and antigenic characteristics of SARS-CoV-2.
Ethical approval
Ethical approval was obtained from the Research Ethics Committees of Clinical Hospital - Federal University of Goiás/protocol: 30,804,220.2.0000.5078.
CRediT author statement
Déborah Anjos: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Data curation, Writing – Original draft and review & editing, Visualization. Fabíola Souza Fiaccadori: Visualization, Supervision, Writing - Review & Editing. Carolina Servian do Prado: Investigation, Writing - Review & Editing. Simone Gonçalves da Fonseca: Resources, Visualization, Supervision, Writing - Review & Editing. Adriana Oliveira Guilarde: Resources, Writing - Review & Editing. Moara Alves Santa Bárbara Borges: Data Curation, Writing - Review & Editing. Fernanda Craveiro Franco: Data Curation, Formal Analysis, Writing - Review & Editing. Bergmann Morais Ribeiro: Writing - Review & Editing, Resources Menira Souza: Conceptualization, Funding acquisition, Project administration, Writing- Reviewing and Editing.
Declaration of Competing Interest
None.
Acknowledgements
Fundação de Apoio a Pesquisa em Goiás (FAPEG) for financial support (Grant number 202010267000279).
References
- 1.W.H.O. WHO; 2021. WHO Health Emergency Dashboard.https://covid19.who.int/ 2021. [Google Scholar]
- 2.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C.S.G. of the I.C. on T. of V. ICTV The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.W.H. Organization . World Health Organization; 25 January 2021. COVID-19 Clinical management: Living Guidance.https://apps.who.int/iris/bitstream/handle/10665/333912/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1-eng.pdf?sequence=1&isAllowed= No. WHO/2, World Health Organization, https://app.magicapp.org/#/guideline/j1WBYn/rec/EalkPn, 2021. (accessed May 3, 2021) [Google Scholar]
- 5.N.I. of NIH, Health. 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; pp. 1–354.https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 6.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brunink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/jmv.25825?download=true [DOI] [PubMed] [Google Scholar]
- 8.Kim J.-.M., Kim H.M., Lee E.J., Jo H.J., Yoon Y., Lee N.-.J., Son J., Lee Y.-.J., Kim M.S., Lee Y.-.P. Detection and isolation of SARS-CoV-2 in serum, urine, and stool specimens of COVID-19 patients from the Republic of Korea. Osong Public Health Res. Perspect. 2020;11:112. doi: 10.24171/j.phrp.2020.11.3.02. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7282421/pdf/ophrp-2020-11-3-112.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng L., Liu J., Xu W., Luo Q., Chen D., Lei Z., Huang Z., Li X., Deng K., Lin B. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.N. Freed, O. Silander, SARS-CoV2 genome sequencing protocol (1200 bp amplicon “midnight” primer set, using Nanopore Rapid kit) V.5, 2021. (n.d.). https://doi.org/10.17504/protocols.io.btsrnnd6.
- 11.Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., Sagulenko P., Bedford T., Neher R.A. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh K., Standley D.M. MAFFT Multiple Sequence Alignment Software Version 7: improvements in Performance and Usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data–from vision to reality. Eurosurveillance. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trifinopoulos J., Nguyen L.-.T., von Haeseler A., Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A. Rambaut, FigTree: tree Figure Drawing Tool, v.1.4.4, (2020). http://tree.bio.ed.ac.uk/software/figtree.
- 16.R.C.T. R, R: a language and environment for statistical computing, (2021).
- 17.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. Bmj. 2020:369. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N., Gong Y., Meng F., Shi Y., Wang J., Mao P., Chuai X., Bi Y., Yang P., Wang F. Comparative study on virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C., Wang H., Li K., Tang A., Dai Y., Wu B., Zhang H., Chen J., Liu J., Wu W. SARS-CoV-2 viral shedding characteristics and potential evidence for the priority for faecal specimen testing in diagnosis. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0247367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.S., Connor T.R., Gaze W.H., Moura I.B., Wilcox M.H., Farkas K. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshandel M.R., Nateqi M., Lak R., Aavani P., Motlagh R.S., Shariat S.F., Badr T.A., Sfakianos J., Kaplan S.A., Tewari A.K. Diagnostic and methodological evaluation of studies on the urinary shedding of SARS-CoV-2, compared to stool and serum: a systematic review and meta-analysis. Cell. Mol. Biol. 2020;66:148–156. [PubMed] [Google Scholar]
- 24.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kipkorir V., Cheruiyot I., Ngure B., Misiani M., Munguti J. Prolonged SARS-CoV-2 RNA detection in anal/rectal swabs and stool specimens in COVID-19 patients after negative conversion in nasopharyngeal RT-PCR test. J. Med. Virol. 2020;92:2328–2331. doi: 10.1002/jmv.26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L. Wang, X. Li, H. Chen, S. Yan, Y. Li, D. Li, Z. Gong, SARS-CoV-2 infection does not significantly cause acute renal injury: an analysis of 116 hospitalized patients with COVID-19 in a single hospital, Wuhan, China, Wuhan, China (2/17/2020). (2020).
- 27.C. for D.C. and P. CDC People with Certain Medical Conditions. People at Increased Risk. 2021;2021 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- 28.Yang J., Zheng Y.A., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maltezou H.C., Raftopoulos V., Vorou R., Papadima K., Mellou K., Spanakis N., Kossyvakis A., Gioula G., Exindari M., Froukala E. Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiaa804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X., Tian Y., Sin N.N. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16:1698. doi: 10.7150/ijbs.45357. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7098032/pdf/ijbsv16p1698.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang Z., Zhang Y., Hang C., Ai J., Li S., Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J. Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7151466/pdf/main.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang K., Wu L., Luo Y., Gong B. Quantitative assessment of SARS-CoV-2 RNAemia and outcome in patients with coronavirus disease 2019. J. Med. Virol. 2021;93:3165–3175. doi: 10.1002/jmv.26876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berastegui-Cabrera J., Salto-Alejandre S., Valerio M., Pérez-Palacios P., Arnaiz-De Las Revillas F., Abelenda-Alonso G., Oteo-Revuelta J.A., Carretero-Ledesma M., Muñoz P., Pascual Á. SARS-CoV-2 RNAemia is associated with severe chronic underlying diseases but not with nasopharyngeal viral load. J. Infect. 2021;82:e38–e41. doi: 10.1016/j.jinf.2020.11.024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7688428/pdf/main.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavov S.N., Giovanetti M., dos Santos Bezerra R., Fonseca V., Santos E.V., Rodrigues E.S., Adelino T., Xavier J., Borges J.S., Evaristo M., Lima M.T., de Carvalho Pereira G., Yamamoto A.Y., Clé D.V., Calado R.T., Covas D.T., Alcantara L.C.J., Kashima S. Molecular surveillance of the on-going SARS-COV-2 epidemic in Ribeirao Preto City, Brazil. Infect. Genet. Evolut. 2021;93 doi: 10.1016/j.meegid.2021.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candido D.S., Claro I.M., de Jesus J.G., Souza W.M., Moreira F.R.R., Dellicour S., Mellan T.A., du Plessis L., Pereira R.H.M., Sales F.C.S., Manuli E.R., Thézé J., Almeida L., Menezes M.T., Voloch C.M., Fumagalli M.J., Coletti T.M., da Silva C.A.M., Ramundo M.S., Amorim M.R., Hoeltgebaum H.H., Mishra S., Gill M.S., Carvalho L.M., Buss L.F., Prete C.A., Ashworth J., Nakaya H.I., Peixoto P.S., Brady O.J., Nicholls S.M., Tanuri A., Rossi Á.D., v. Braga C.K., Gerber A.L., de C. Guimarães A.P., Gaburo N., Alencar C.S., Ferreira A.C.S., Lima C.X., Levi J.E., Granato C., Ferreira G.M., Francisco R.S., Granja F., Garcia M.T., Moretti M.L., Perroud M.W., Castiñeiras T.M.P.P., Lazari C.S., Hill S.C., de Souza Santos A.A., Simeoni C.L., Forato J., Sposito A.C., Schreiber A.Z., Santos M.N.N., de Sá C.Z., Souza R.P., Resende-Moreira L.C., Teixeira M.M., Hubner J., Leme P.A.F., Moreira R.G., Nogueira M.L., Ferguson N.M., Costa S.F., Proenca-Modena J.L., Vasconcelos A.T.R., Bhatt S., Lemey P., Wu C.-.H., Rambaut A., Loman N.J., Aguiar R.S., Pybus O.G., Sabino E.C., Faria N.R. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., Pohlmann A., King J., Steiner S., Kelly J.N., Portmann J., Halwe N.J., Ulrich L., Trüeb B.S., Fan X., Hoffmann B., Wang L., Thomann L., Lin X., Stalder H., Pozzi B., de Brot S., Jiang N., Cui D., Hossain J., Wilson M.M., Keller M.W., Stark T.J., Barnes J.R., Dijkman R., Jores J., Benarafa C., Wentworth D.E., Thiel V., Beer M. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]