Abstract
The WHO-named Coronavirus Disease 2019 (COVID-19) infection had become a pandemic within a short time period since it was detected in Wuhan. The outbreak required the screening of millions of samples daily and overwhelmed diagnostic laboratories worldwide. During this pandemic, the handling of patient specimens according to the universal guidelines was extremely difficult as the WHO, CDC and ECDC required cold chain compliance during transport and storage of the swab samples. The aim of this study was to compare the effects of two different storage conditions on the COVID-19 real-time PCR assay on 30 positive nasopharyngeal and/or oropharyngeal samples stored at both ambient temperature (22 ± 2 °C) and +4 °C. The results revealed that all the samples stored at ambient temperature remain PCR positive for at least six days without any false-negative result. In conclusion, transporting and storing these types of swab samples at ambient temperature for six days under resource-limited conditions during the COVID-19 pandemics are acceptable.
Keywords: COVID-19, Real-time PCR, Sample storage
1. Introduction
The Coronavirus Disease 2019 (COVID-19), which stems from the new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), has rapidly become a global pandemic since it first appeared in Wuhan, China, in November 2019 (WHO). With over two million cases of infections detected each day and tens of millions more samples being tested per day at the peak of the pandemic (Ref), the pandemic is causing an enormous burden to the world’s healthcare system, including diagnostic laboratories (Helmy et al., 2020). Real-time polymerase chain reaction (real-time PCR) is considered as the gold-standard confirmatory diagnostic laboratory test for COVID-19 (Lippi et al., 2020). Samples for this method are obtained from the upper and lower respiratory tract (oropharyngeal or nasopharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash or aspirate or nasal aspirate) and transferred immediately into a virus transport medium (VTM) (Loeffelholz and Tang, 2020). The VTM consists of serum albumin and some antibiotics against possible bacterial contamination and can be obtained commercially or prepared in the laboratory. It is an appropriate medium for the collection, transport, maintenance and freeze-storage of clinical specimens including viruses, mycoplasma or ureaplasma organisms. In general, it conserves the viability of the organism at room or cooled temperature for 48 h. The World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), European Centre for Disease Prevention and Control (ECDC), and several national health authorities announced several real-time PCR protocols and sample storage guidelines, and all emphasized that the accuracy of the real-time PCR tests mostly relies on proper specimen collection and storage (CDC, 2020; ECDC, 2020; WHO, 2020). All guidelines agreed that samples should be transferred and kept in conditions maintaining a cold chain. However, processing an extreme number of samples during the pandemic may impede transport and storing conditions according to the guidelines. Previous studies detected viral load reduction in samples kept at 20−25 °C after 48, 72 h of storage. This decrease varies as 1–4 CT. In addition, the longest period in which viral positivity was detected was 14 days in a study conducted with 5 samples (Basso, 2021; Rogers, 2020). We detected this test with the 3-channel kit, which allows detecting 2 different sequences of the virus (N Gene and Orf1ab), as opposed to the two-channel kit. In this study, we aimed to compare the effect of 4+ °C and room temperature (22 ± 2 °C) different conditions on the qPCR results of nasopharyngeal and/or oropharyngeal swab samples.
2. Materials & methods
2.1. Samples
The study was performed at GLAB-Corona which works as a Covid-19 routine diagnostic laboratory (Doganay et al., 2020). To determine the minimum number of samples, a GPower analysis with 80 % power, one-tailed t-test with alpha at 0.05 was performed and the total sample size was calculated as 27 (HHU, 2021). The study samples (30 positive and 5 negative swab samples) were randomly selected out of 171 positive and 1603 negative cases that were admitted to laboratory on 29.04.2020. Since the effect of storage conditions on SARS CoV-2 viral RNA integrity is unknown, here we focused on SARS CoV-2 positive samples. The study of five negative samples was considered sufficient to show the presence of human RNA throughout the study period. A single nasopharyngeal and/or oropharyngeal swab sample was obtained from each patient and immediately taken into a sterile tube with 3 mL of the Viral Transport Media (VTM, Innomed VTM001) by a trained healthcare worker at infectious disease clinic in Umraniye Teaching and Research Hospital. Real-time PCR tests were performed at the GLAB diagnostic laboratory. Following the diagnostic report, the study samples were randomly selected out of 171 positive and 1603 negative samples which were accepted and reported at the laboratory on the day of study.
For each positive and negative study sample, 2 mL of VTM were added to the samples for having enough sample quantity during the study, then evenly aliquoted into two tubes (2.5 mL of solution for each tube) for further analysis. One tube was stored in the refrigerator (+4 °C) and the other copy of the same sample at room temperature which was set to 22 °C by an automated air conditioning system for nine days. Deviation for room temperature was accepted as ±2 °C. The room temperature and refrigerator temperature were monitored continuously and recorded using data loggers.
2.2. Real-time PCR tests
The SARS-Cov-2 detection was performed by the SARS-CoV-2 qPCR test (DirectDetect SARS-Cov-2 qPCR Kit by Coyote Bioscience Co., Ltd) on BioRad CFX 96 platform (California, USA) according to the protocols provided by the manufacturer. The qPCR kit targets ORF1ab and the N gene of SARS-CoV-2 and the human RNaseP gene.
The commercial kit in use includes the reverse real-time PCR method combined with fluorescent probes to detect the conserved region of ORF1ab and the N gene in SARS-CoV-2. A preliminary nucleic acid extraction was not performed while using this kit, since the samples were treated with the lysis buffer, which is thought to provide RNA extraction, released the target nucleotide in the samples, and tolerated the inhibitor. The samples were taken directly from the swab tubes and then mixed with the lysis buffer, which is included in the kit, in a separate sterile tube. The prepared mixture was added directly into the real-time PCR reaction reagent after the vortex-spin process; and then real-time PCR was started directly. The PCR protocol included both cDNA synthesis (42 °C for 5 min and 15 cycles of 95 °C for 10 s and 50 °C for 15 s) and real-time PCR (95 ° C for 1 min, 30 cycles of 95 °C for 10 s and 55 ° C for 30 s with FAM/HEX/ROX reads). The entire process, from the sample's arrival to the laboratory to the final medical report, took place within 4 h. real-time PCR tests for all the samples were repeated every 24 h for nine days, for both conditions. The day when the samples were first collected was accepted as day-0. Similar with 4 °C refrigerator, the room temperature based automated air conditioning system (22 °C) was checked every hour. The cut-off Ct value was ≤29 for positive samples as indicated by the manufacturer and the LOD value 3.75 copies/reaction for both ORF1ab and N gene. The sample was reported as positive if both the ORF1ab and N gene showed positive amplification curves. If both targets were negative, the result is reported as negative. In the case of one positive target amplification, the test was repeated and evaluated as “test repeat” (TR). If repeated sample do not reveal two positive target amplification again, these samples were included in the "sample failure" group (SF). When there is not sufficient material left in swab tube for real-time PCR reaction, these samples were evaluated as "insufficient material" (IM).
2.3. Statistical analysis
Ct values of samples in daily study runs were compared by paired t-test and p values less than 0.05 were considered as significant. All p values were calculated two sided. All analysis was performed by Graphpad Prism V8.
3. Results
A total of 30 SARS-CoV-2 positive and 5 negative samples (all stored in +4 °C and room temperature) were enrolled in this study. All positive samples were tested with real-time PCR every day for nine days; however, negative samples were tested as control on the fifth and ninth days.
All of the 30 positive (100 %) samples that were kept at room temperature and 29 (96.7 %) of the refrigerated ones were continually detected as positive for the first five days (Table 1 ). All positive samples stored at room temperature were detected positive until day 9 without any false negativity and diagnostic accuracy remained 100 % throughout the study. For the positive samples stored at +4 °C, 1 sample was tested negative on day 2, day 3 and day 5 and 2 samples were tested negative on day 9. All negative samples were tested negative on day 5 and day 9 for both groups. Until day 5 we did not have sample insufficiency for real-time PCR reaction. Available number of samples on day 6 was 28 for +4 °C group and dropped to 8 on day 9. Sample size for room temperature group was 23 on day 7 and dropped to 13 on day 9. Study was terminated on day 9 due to insufficient material in swab tubes for further real-time PCR reaction (Table 1).
Table 1.
COVID-19 Real-time PCR results of daily testing of the study samples.
| + 4 °C |
Room Temperature |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testing Day | Available Sample (n) |
Positive (n) |
Negative (n) |
TR (n) |
SF (n) |
IM (n) |
Available Sample (n) |
Positive (n) |
Negative (n) |
TR (n) |
SF (n) |
IM (n) |
| Day 0 | 30 | 30 | 0 | 0 | 0 | 0 | 30 | 30 | 0 | 0 | 0 | 0 |
| Day 1 | 30 | 30 | 0 | 3 | 0 | 0 | 30 | 30 | 0 | 4 | 0 | 0 |
| Day 2 | 30 | 29 | 1 | 1 | 0 | 0 | 30 | 30 | 0 | 1 | 0 | 0 |
| Day 3 | 30 | 29 | 1 | 3 | 0 | 0 | 30 | 30 | 0 | 0 | 0 | 0 |
| Day 4 | 30 | 30 | 0 | 1 | 0 | 0 | 30 | 30 | 0 | 0 | 0 | 0 |
| Day 5 | 30 | 29 | 1 | 2 | 0 | 0 | 30 | 30 | 0 | 0 | 0 | 0 |
| Day 6 | 28 | 28 | 0 | 1 | 0 | 2 | 30 | 30 | 0 | 0 | 0 | 0 |
| Day 7 | 22 | 22 | 0 | 0 | 0 | 8 | 23 | 23 | 0 | 0 | 0 | 7 |
| Day 8 | 15 | 15 | 0 | 0 | 2 | 13 | 23 | 23 | 0 | 0 | 0 | 7 |
| Day 9 | 8 | 6 | 2 | 0 | 0 | 22 | 13 | 13 | 0 | 0 | 0 | 17 |
TR: Test repeat, SF: Sample failure, IM: Insufficient material.
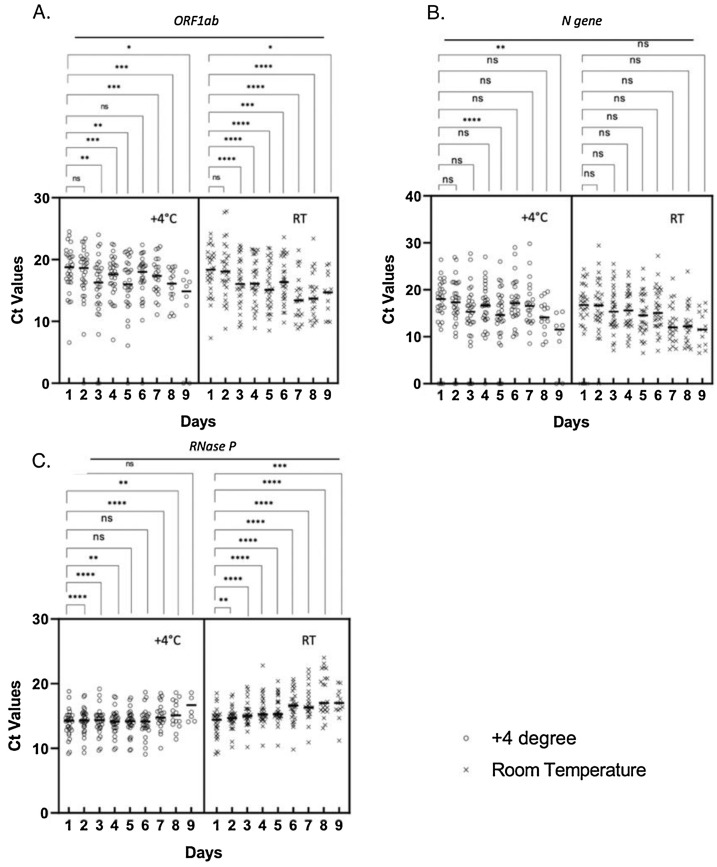
We excluded the negative samples and compared the individual Ct values at +4 °C and room temperature of ORF1ab, N and RNase P genes for nine days. At the beginning (on Day 0), the highest Ct value of the study samples was 25.02 and the mean Ct values were as follows: 16.08 for ORF1ab, 17.9 for N gene and 14.6 for internal control (Table S1). The Ct values of N gene showed no significant difference for five days at room temperature, but samples stored at +4 °C condition had Ct values that significantly decreased on day 5 and day 9 (Fig. 1 b). Ct values of ORF1ab for both conditions significantly decreased (Fig. 1a). We have also evaluated the Ct values of human RNase P gene and detected a significant increase on day 9, at +4 degrees (Fig. 1c). Ct values for all samples under both conditions are given on Supplementary Table 2 (Table S2).
Fig. 1.
The distribution of the Ct values of (A) ORF1ab, (B) N and (C) RNase P genes at +4 °C and at room temperature. The negative values are excluded and the difference between different days are compared by paired t-test, the significance is marked with * symbol. ns: not significant. (*: P ≤ 0.05; **:P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001) (RT: Room Temperature).
4. Discussion
real-time PCR is the gold standard diagnostic test for detection of SARS-CoV-2. False results of the real-time PCR test may be due to the inaccuracies in sampling, transport conditions, failures in the different analytic stages or data analysis. According to health authorities, swab samples should be stored at +4 °C (CDC, 2020; ECDC, 2020; WHO, 2020). Here we analyzed the real-time PCR diagnostic performance for SARS-CoV-2 on nasopharyngeal/oropharyngeal swab samples stored at ambient temperature. Recently many studies have investigated the effects of various factors on virus survival but, less is known on the impact of temperature on SARS-CoV-2 RNA detection by RT- PCR (Eslami and Jalili, 2020; Kim et al., 2021; Li et al., 2020; Xie and Zhu, 2020; Xu et al., 2020). In a limited number of studies related to other viruses (enterovirus, HSV-2, HHV-8, adenovirus, influenza), it has been reported that the storage of the samples at ambient temperature did not affect the test results (Druce et al., 2012; Zaniello et al., 2016). Our results showed that oropharyngeal/nasopharyngeal samples kept at room temperature remain positive in SARS-CoV-2 real-time PCR studies for at least six days.
A previous study comparing different commercial diagnostics kits showed that the ORF1ab gene was less stable than the other viral target genes (Zhou et al., 2020). Our results on individual Ct values also showed that the Ct values for ORF1ab gene were more ambiguous than the N gene at +4 °C and a significant difference was detected even on day 3. On the other hand, the Ct values for N gene remained positive at least for 5 days at room temperature. The human RNase P gene remained stable at +4 °C whereas at room temperature the amplification was detected at later cycles. RNA is already known to be easily degraded and this finding is not surprising especially at room temperature.
Previous studies on SARS-CoV-2 revealed that the Ct values were increased by time at different storage temperatures (Erster et al., 2021; Kim et al., 2021; Engelmann et al., 2021). Although it was not statistically significant, in our study this was the case for human RNase P gene Ct values; on contrary the Ct values for viral genes ORF and N showed a decreasing trend. During this study, samples were stored in an undisclosed commercial VTM solution and the commercial qPCR kit in use includes a lysis buffer step for viral RNA extraction instead of conventional RNA extraction methods, and we can speculate that the VTM content may create the environment to facilitate lysis of the viral envelope or nucleocapsid further increasing the effect of the lysis buffer. Studies with larger numbers of samples might enlighten the issue.
There are some limitations of this study, we used a commercial kit which had a threshold for positivity, so we could not define weak positivity. Thus, we could not evaluate the performance in weak positive samples. Temperatures higher than 24 °C may be common in some tropical research and/or storage settings, so the time period that samples remain positive may be variable in such conditions.
5. Conclusion
In the light of the data obtained from this study, we suggest that in a resource-limited setting like pandemics, transferring and storing nasopharyngeal/oropharyngeal samples at ambient temperature (20−24 ℃) might be acceptable and these samples can be analyzed and reported. Such a change in daily practice will result in considerable time and cost savings and reduce the number of sample rejection.
Data availability
Data will be made available on request.
Author statement
Nihat Buğra Agaoglu: Formal analysis, Investigation, Methodology, Writing - original draft. Jale Yıldız: Formal analysis, Investigation, Methodology, Writing - original draft.
Ozlem Akgun Dogan: Formal analysis, Investigation, Methodology, Supervision, Writing - review & editing.
Betsi Kose: Investigation.
Gizem Alkurt: Investigation.
Yasemin Kendir Demirkol: Investigation.
Arzu Irvem: Investigation.
Levent Doganay: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Gizem Dinler Doğanay: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank to sample collection team members of our institution for technical assistance. Authors would like to thank Sharon Lynn Pugh, Associate Professor Emeritus of Education at Indiana University Bloomington for language editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2021.114404.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Basso, et al. SARS-CoV-2 RNA identification in nasopharyngeal swabs: issues in pre-analytics. Clin. Chem. Lab Med. 2021;58:1579–1586. doi: 10.1515/cclm-2020-0749. [DOI] [PubMed] [Google Scholar]
- CDC . 2020. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- Doganay L., Agaoglu N.B., Irvem A., Alkurt G., Yildiz J., Kose B., Demirkol Y.K., Dogan O.A., Doganay G.D. Responding to COVID-19 in Istanbul: perspective from genomic laboratory. North. Clin. Istanb. 2020;7:311–312. doi: 10.14744/nci.2020.30075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce J., Garcia K., Tran T., Papadakis G., Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J. Clin. Microbiol. 2012;50:1064–1065. doi: 10.1128/JCM.06551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . 2020. Methodology for Estimating Point Prevalence of SARS-CoV-2 Infection by Pooled Real-Time PCR Testing ECDC TECHNICAL REPORT European Centre for Disease Prevention and Control. [Google Scholar]
- Engelmann I., Alidjinou E.K., Ogiez J., Pagneux Q., Miloudi S., Benhalima I., Ouafi M., Sane F., Hober D., Roussel A., Cambillau C., Devos D., Boukherroub R., Szunerits S. Preanalytical issues and cycle threshold values in SARS-CoV-2 real-time real-time PCR testing: should test results include these? ACS Omega. 2021;6:6528–6536. doi: 10.1021/acsomega.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster O., Shkedi O., Benedek G., Zilber E., Varkovitzky I., Shirazi R., Oriya Shorka D., Cohen Y., Bar T., Yechieli R., Tepperberg Oikawa M., Venkert D., Linial M., Oiknine-Djian E., Mandelboim M., Livneh Z., Shenhav-Saltzman G., Mendelson E., Wolf D., Szwarcwort-Cohen M., Mor O., Lewis Y., Zeevi D. Improved sensitivity, safety, and rapidity of COVID-19 tests by replacing viral storage solution with lysis buffer. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami H., Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10:92. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020:9. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HHU . 2021. G*Power 3.1. [Google Scholar]
- Kim N., Kwon A., Roh E.Y., Yoon J.H., Han M.S., Park S.W., Park H., Shin S. Effects of storage temperature and Media/Buffer for SARS-CoV-2 nucleic acid detection. Am. J. Clin. Pathol. 2021;155:280–285. doi: 10.1093/ajcp/aqaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li X., Guo Z., Wang Z., Zhang K., Li C., Wang C., Zhang S. Influence of storage conditions on SARS-CoV-2 nucleic acid detection in throat swabs. J. Infect. Dis. 2020;222:203–205. doi: 10.1093/infdis/jiaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020;58:1070–1076. doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg. Microbes Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, et al. Evaluation of Transport Media and Specimen Transport Conditions for the Detection of SARS-CoV-2 by Use of Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2020;58:e00708–20. doi: 10.1128/JCM.00708-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases. [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Yan C., Fu Q., Xiao K., Yu Y., Han D., Wang W., Cheng J. Possible environmental effects on the spread of COVID-19 in China. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniello B., Huang M.L., Cheng A., Selke S., Wald A., Jerome K.R., Magaret A.S. Consistent viral DNA quantification after prolonged storage at ambient temperature. J. Virol. Methods. 2016;228:91–94. doi: 10.1016/j.jviromet.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Pei F., Ji M., Wang L., Zhao H., Li H., Yang W., Wang Q., Zhao Q., Wang Y. Sensitivity evaluation of 2019 novel coronavirus (SARS-CoV-2) real-time PCR detection kits and strategy to reduce false negative. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.