Abstract
Quantitative systems pharmacology (QSP) has been proposed as a scientific domain that can enable efficient and informative drug development. During the past several years, there has been a notable increase in the number of regulatory submissions that contain QSP, including Investigational New Drug Applications (INDs), New Drug Applications (NDAs), and Biologics License Applications (BLAs) to the US Food and Drug Administration. However, there has been no comprehensive characterization of the nature of these regulatory submissions regarding model details and intended applications. To address this gap, a landscape analysis of all the QSP submissions as of December 2020 was conducted. This report summarizes the (1) yearly trend of submissions, (2) proportion of submissions between INDs and NDAs/BLAs, (3) percentage distribution along the stages of drug development, (4) percentage distribution across various therapeutic areas, and (5) nature of QSP applications. In brief, QSP is increasingly applied to model and simulate both drug effectiveness and safety throughout the drug development process across disease areas.
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The landscape of quantitative systems pharmacology (QSP) in regulatory submissions has never been analyzed in detail or published.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study addresses the question of how QSP modeling and simulation has been applied to various stages of drug development across different disease areas in regulatory submissions.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study informs us of the following with respect to the current landscape of applications of QSP modeling and simulation in regulatory submissions:
QSP applications in regulatory submissions are mainly for dose selection and optimization of dosing regimen for small molecular drugs and biological products. In these submissions, the majority of QSP applications model and simulate responses.
QSP is used by the sponsors to play a supportive role across the drug development stages.
QSP is applied to model and simulate the responses and safety of proposed small molecular drugs and biological products in humans for the goal of optimizing their dosing regimen, therapeutic window, and length of use.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
It is hopeful that this report informs the QSP community of the ranges and types of QSP applications in drug development across disease areas in regulatory submissions, thus stimulating the QSP community to further advance QSP applications in clinical drug development.
INTRODUCTION
Advances in systems biology, omics, big data, knowledge bases, and informatics have facilitated the creation of a new scientific domain, quantitative systems pharmacology (QSP). QSP mechanistically connects the pharmacological mechanism(s) of a proposed product, across the hierarchy of human biology, to the quantitative changes of its pharmacodynamic (PD) biomarkers/clinical end points following various dosing regimens in healthy participants or patients, through multiscale spatial and temporal modeling. 1 Applications of QSP to pharmaceutical research and development (R&D) has been on the rise, according to a recent survey of preclinical scientists in pharmaceutical companies. 2 The 2019 American Society of Clinical Pharmacology and Therapeutics Annual Meeting Preconference on QSP identified opportunities for QSP to impact pharmaceutical R&D. 3
Since 2013, there has been a notable increase in the number of regulatory QSP submissions received by the US Food and Drug Administration (FDA). 4 However, there has not been a comprehensive characterization of the nature of these submissions in terms of intended application. To address this gap, the FDA’s submission and review databases were searched for regulatory submissions that mentioned or contained mechanistic models, systems pharmacology, systems toxicology, quantitative systems toxicology, and QSP approaches, which were collectively named QSP submissions. This report summarizes the landscape analysis of QSP submissions as of December 31, 2020, and highlights the nature of these submissions.
METHODS
Mining the FDA’s regulatory submission database
The FDA’s internal regulatory submission and disciplinary review databases were text mined using a list of key words. The keywords included “mechanistic model,” “in silico model,” “systems biology,” “systems model,” “systems pharmacology,” “quantitative systems pharmacology,” “systems toxicology,” and “quantitative systems toxicology” and the name of a commercial modeling and simulation tool for predicting drug toxicity. The name of a commercial tool was included following discussion with colleagues and earlier observations of mining results. Other tools being developed for commercial use were found in one or two submissions with the key words mentioned previously. The word “modeling” was also used in our text mining in places where the word “model” is used in the aforementioned list. All identified documents were carefully reviewed. Any mechanistic or in silico models that did not include pharmacological mechanisms and effects were excluded, including physiologically based pharmacokinetic (PK) models or in silico models for quality control of drug products or for cheminformatics‐based prediction of carcinogenicity or genotoxicity. A few article submissions in 2013 and 2014 were identified through mining the FDA’s disciplinary reviews and were retrieved from the FDA’s Document Room. Confirmed QSP submissions were reviewed and included in our analysis.
Compilation and curation of QSP submissions
The submitted documents containing QSP were retrieved, reviewed, and curated to compile all relevant information. The information compiled included (1) Investigational New Drug (IND) application number, New Drug Application (NDA) number, or Biologics Licensed Application (BLA) number; (2) supporting document number (SDN) assigned to the document in an IND, NDA, or BLA submission; (3) sponsor name; (4) medical entity (entities) and its pharmacological target; (5) proposed indication; (6) date of submission; (7) objective(s) and model details; (8) sponsor’s proposed application(s); and (9) stage of development of the proposed product to which QSP was applied. An SDN number is sequentially assigned to each submission in an IND (including the Pre‐Investigational New Drug), NDA, or BLA submission in a chronological order for tracking and easy access in the FDA’s submission database. The document in which QSP was submitted along with the information listed previously were recorded and organized into the Office of Clinical Pharmacology's QSP Database so that any model updates and progresses made in subsequent submissions associated with an IND, NDA, or BLA were captured and documented.
Criteria for inclusion in the QSP Database
If a QSP modeling and simulation approach was submitted in more than one SDN in an IND, NDA, or BLA for a proposed product, the QSP elements in individual SDN submissions were carefully reviewed and compared to establish the basis for what submissions in IND, NDA, or BLA to include in the database. If a QSP approach in an IND was substantially updated in a later SDN submission compared with an earlier SDN submission, both SDN submissions were counted. See the following examples with respect to how substantial updates were identified. If an earlier SDN submission in an IND contained a brief description of a concept or plan and goal while a later SDN submission included detailed descriptions of a structural model, model output, and calibr
ation and validation plan, then both SDN submissions were counted for the IND. That is, there were two QSP submissions in that IND. If a modeling approach was originally used to model and simulate a drug response (PD or clinical) of a proposed product in an earlier SDN submission and was subsequently expanded to model and predict the quantitative changes of a safety biomarker in a later SDN submission, both SDN submissions were counted for the IND.
If significant changes were made to the QSP model or additional details pertaining to modeling and simulation along with detailed results were included in the NDA/BLA submissions compared with what was submitted in the IND, then submissions in both the IND and NDA/BLA were counted. If the same QSP was described in both the IND and NDA/BLA submissions for a proposed product/proposed indication pair without showing substantial differences in its IND and NDA/BLA submissions, then only the earliest submission was counted.
A proposed single‐agent product (small molecule or biologic) or a combination product can be developed for treating various indications such as different solid cancers (oncological diseases) with an IND assigned to each proposed indication. That is, there can be multiple INDs associated with a proposed product. When a same QSP approach was submitted in multiple IND submissions for a proposed product, all individual IND submissions were counted. The same principle of counting was also applied to NDA and BLA submissions. For the case that a same QSP approach was submitted in multiple INDs for a proposed product being studied in various combinations with other approved or investigational drug products for treating different cancers, each individual IND was counted. If a medical entity and its chemical derivative were both developed for the same proposed indication, their individual INDs or NDAs/BLAs were all counted even though a similar QSP model was submitted. That is, the medical entity and its chemical derivative are different chemical entities, justifying for their inclusions. For the scenario where the same QSP was submitted in an Emergency Use Authorization (EUA) for a proposed product before its NDA/BLA submission, both its EUA and NDA/BLA submissions were counted (only two submissions in total).
If the submissions used the term quantitative systems pharmacology but only included sequential, mechanistic kinetic release of the active drug from its prodrug/probody or double prodrug or double probody without inclusion of target engagement, mechanism of action, and pharmacological pathway(s), they were excluded. Biochemical kinetic models in which a proposed drug product’s pharmacological mechanism and pathway(s) were not included were also excluded. Simple PK and PD models, indirect response models, target‐mediated drug disposition models, physiologically based PK modeling and simulation, and any kinetic models without inclusion of mechanistic pharmacological effects/pathways or key consequential biological events were excluded, even though the term QSP was used in the submission to describe the proposed modeling approaches.
Landscape analysis
Following curation, all confirmed submissions were organized to compile information for analysis. The landscape analysis determined yearly submissions since 2013 and percentage distributions of submission across therapeutic areas, across development phases, and between INDs and NDAs/BLAs. The nature of QSP submissions and their proposed applications in individual submissions were also analyzed.
RESULTS
Heterogeneity in model details across submissions
No submission was found before 2013 in the FDA’s electronic regulatory submission and disciplinary review database. Therefore, the landscape analysis of regulatory QSP submissions in this report covered the period from January 1, 2013, to December 31, 2020. QSP submissions were found in IND submissions (meeting packages, information requests, investigator brochures, safety reports, annual reports, initial pediatric study plan, and postmarket requirement as well as postmarket commitment submissions) and NDA and BLA submissions (original or supplemental submissions [efficacy or safety]).
Submissions varied greatly in the level of detail, ranging from only brief descriptions of a proposed QSP or systems pharmacology approach/concept to all the details of modeling and simulation including assumptions, equations, code, calibration, and prediction as well as discussion/conclusion. Submitted models also varied in model scope and complexity, with some describing very complex networks of systems pharmacology, whereas others presented simpler models. Many submissions were excluded and not counted as QSP submissions, although the term quantitative systems pharmacology was used (see Methods). Some submissions used the terms, or followed the high‐level framework outlined, in the American Society of Mechanical Engineers Verification and Validation 40. 5
Drug development applications
QSP was applied to both small molecule drugs and biological products as well as to proposed single‐agent products and combination products. Per the FDA’s guidance, combination products are therapeutic products that combine drugs and/or biological products. QSP submissions continuously increased, with a total of 157 submissions between 2013 and 2020 (Figure 1). Therapeutic areas were classified by referencing the FDA’s list of Spectrum of diseases and conditions 6 and recent reorganization of review divisions within the Center for Drug Evaluation and Research. QSP applications were found across various therapeutic areas (Figure 2), with major applications in oncological diseases, hematologic malignancies, neurology, cardiology and nephrology, and infectious diseases. Diseases in the oncology therapeutic area, which contributed the largest percentage of submissions, include gastric cancer, non‐small cell lung cancer, solid cancers, and so on. Among applications for oncological diseases, QSP was applied to various stages of drug development; phase II submissions and phase III and NDA/BLA submissions contributed 35% and 34%, respectively (Figure 3a). In the preclinical‐to‐phase I category, there was only one preclinical application predicting for non‐human primates. Although QSP applications were found across all stages of drug development, QSP was mainly applied to IND stages (Figure 3b).
FIGURE 1.
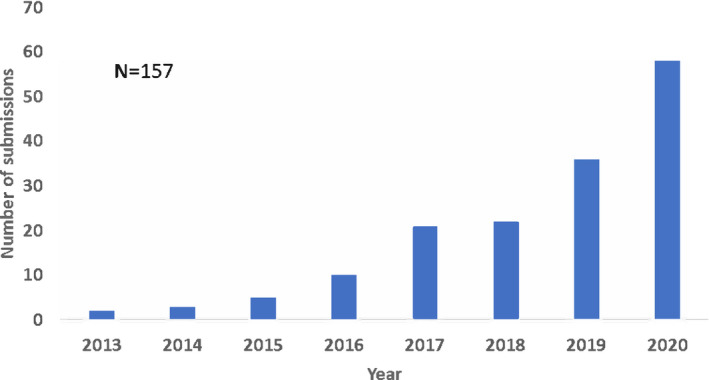
The yearly trend of quantitative systems pharmacology submissions since 2013
FIGURE 2.
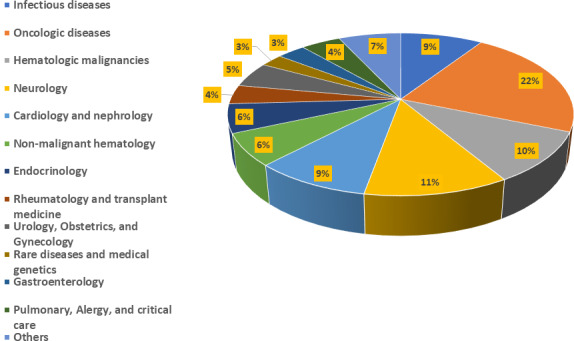
Applications of quantitative systems pharmacology across various therapeutic areas. Submissions in disease areas of anesthesiology, addiction medicine, pain medicine, hepatology and nutrition, dermatology and dentistry, and medical imaging and radiation medicine were grouped together into the “Others” category
FIGURE 3.
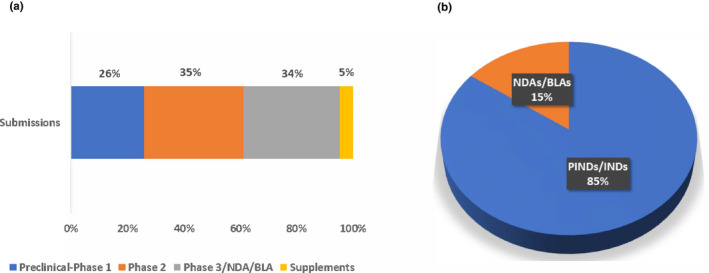
(a) Percentage distribution of quantitative systems pharmacology submissions along the drug development stages (the “Supplements” category includes efficacy and safety submissions following NDA/BLA approval for marketing, postmarket requirement, and postmarket commitment submissions). In the preclinical‐to‐phase I category, there was only one preclinical submission. (b) Proportion of quantitative systems pharmacology submissions between INDs and NDAs/BLAs. BLA, Biologics License Application; IND, Investigational New Drug Application; NDA, New Drug Application; PIND, Pre‐Investigational New Drug
From the application perspective, efficacy‐related applications accounted for 66.2% of the submissions, whereas the remaining were safety‐related applications. Among the efficacy‐related 103 submissions for phase I and beyond, approximately 97% of these submissions were devoted to dosing, including designing phase I dose‐finding and dose‐escalating studies (27.2%), designing phase II dose range studies (38.8%), supporting phase III dose selection and pediatric study plans (18.4%), and supporting the dosing regimens in NDA/BLA submissions (7.8%) as well as supporting NDA/BLA supplements (4.9%) (including a new dosing regimen for the same indication or the same dosing regimen for a new proposed indication in related diseases). Safety applications accounted for 33.8% of submissions and included predictions of liver toxicity, bone mineral density, cardiotoxicity, and plasma ion concentrations. Less than one third of QSP submissions (28.7%) were applied to predict hepatotoxicity, with a trend of reaching a peak in 2019 (12 submissions).
In seven submissions, QSP was applied to play a central role (addressing regulatory requirements or regulatory safety concerns), including three submissions requesting clinical waivers where QSP simulations were proposed to replace a clinical study required and four submissions predicting short‐term or long‐term nonhepatic safety issues, such as bone mineral density, in lieu of conducting clinical studies. The three clinical waiver submissions included two QSP applications for treating in‐born errors and one QSP application for drug repurposing. For the in‐born errors applications, one proposed to waive a clinical study comparing clinical and commercial batches in pediatric patients, whereas the other proposed to use QSP simulation to replace the PD study required in a postmarket commitment study.
CONCLUSION
Regulatory QSP submissions have steadily increased since 2013. QSP has been applied to both efficacy and safety aspects of drug development across various therapeutic areas and all stages of the drug development process. Considering the wide range of diverse applications, it is expected that QSP will play an increasingly important role in drug development.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
J.P.F.B., R.M., D.G.S., and Y.W. wrote the manuscript. J.P.F.B. and J.C.E. designed the research. J.P.F.B. and J.F. performed the research. J.P.F.B. analyzed the data. H.Z. contributed new reagents/analytical tools.
DISCLAIMER
This article only reflects the views of the authors and should not be construed to represent the views or policies of the US Food and Drug Administration.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Issam Zineh, Director, Office of Clinical Pharmacology, for his kind support with the quantitative systems pharmacology database and with drafting this manuscript as well as for his comments on the consistent criteria for inclusion of submissions in the database. Mr. Kirk Roy’s assistance to design and implement the searchable database for the quantitative systems pharmacology submission database at a US Food and Drug Administration’s SharePoint site is greatly appreciated. The authors also acknowledge the Office of Clinical Pharmacology review staff who forwarded to us their reviews of quantitative systems pharmacology submissions.
Bai JPF, Earp JC, Florian J, et al. Quantitative systems pharmacology: Landscape analysis of regulatory submissions to the US Food and Drug Administration. CPT Pharmacometrics Syst Pharmacol. 2021;10:1479–1484. 10.1002/psp4.12709
Funding information
No funding was received for this work.
REFERENCES
- 1. Bai JPF, Earp JC, Pillai VC. Translational quantitative systems pharmacology in drug development: from current landscape to good practices. AAPS J. 2019;21:72. [DOI] [PubMed] [Google Scholar]
- 2. Nijsen M, Wu F, Bansal L, et al. Preclinical QSP modeling in the pharmaceutical industry: an IQ consortium survey examining the current landscape. CPT Pharmacometrics Syst Pharmacol. 2018;7(3):135‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai JPF, Musante CJ, Petanceska S, Zhang L, Zhao L, Zhao P. American Society for clinical pharmacology and therapeutics 2019 annual meeting pre‐conferences. CPT Pharmacometrics Syst Pharmacol. 2019;8:333‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zineh I. Quantitative systems pharmacology: a regulatory perspective on translation. CPT Pharmacometrics Syst Pharmacol. 2019;8(6):336‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Society of Mechanical Engineers Assessing credibility of computational modeling through verification and validation: application to medical devices V V 40. https://wwwasmeorg/codes‐standards/find‐codes‐standards/v‐v‐40‐assessing‐credibility‐computational‐modeling‐verification‐validation‐application‐medical‐devices. Accessed July 2020.
- 6. US Food and Drug Administration Spectrum of diseases and conditions. https://www.fda.gov/drugs/development‐resources/spectrum‐diseasesconditions. Accessed July 2020.


