Summary
Electronic waste (e-waste) contains numerous chemicals harmful to human and ecological health. To update a 2013 review assessing adverse human health consequences of exposure to e-waste, we systematically reviewed studies reporting effects on humans related to e-waste exposure. We searched EMBASE, PsycNET, Web of Science, CINAHL, and PubMed for articles published between Dec 18, 2012, and Jan 28, 2020, restricting our search to publications in English. Of the 5645 records identified, we included 70 studies that met the preset criteria. People living in e-waste exposed regions had significantly elevated levels of heavy metals and persistent organic pollutants. Children and pregnant women were especially susceptible during the critical periods of exposure that detrimentally affect diverse biological systems and organs. Elevated toxic chemicals negatively impact on neonatal growth indices and hormone level alterations in e-waste exposed populations. We recorded possible connections between chronic exposure to e-waste and DNA lesions, telomere attrition, inhibited vaccine responsiveness, elevated oxidative stress, and altered immune function. The existence of various toxic chemicals in e-waste recycling areas impose plausible adverse health outcomes. Novel cost-effective methods for safe recycling operations need to be employed in e-waste sites to ensure the health and safety of vulnerable populations.
Introduction
Exponential growth in the electrical and electronic industries to meet customer demand has correspondingly generated large waste flows.1, 2 Electronic and electrical waste (e-waste) can be defined as any “electrical or electronic equipment, which is waste, including all components, subassemblies and consumables, which are part of the equipment at the time the equipment becomes waste”.3 The Global E-waste Monitor estimated that 53·6 million metric tons (Mt) of e-waste were produced globally in 2019. This figure is projected to grow to 74·7 Mt by 2030. Asia generated largest quantity of e-waste in 2019 (24·9 Mt), followed by the Americas (13·1 Mt), Europe (12·0 Mt), Africa (2·9 Mt), and Oceania (0·7 Mt).4 An estimated 80% of e-waste from developed countries is illegally exported to low-income and middle-income countries (LMICs) including China, India, Nigeria, Brazil, Ghana, and Pakistan, where labour costs and disposal are cheap and laws are less stringent or poorly enforced.5
E-waste contains numerous toxic chemicals including metals such as lead, cadmium, mercury, and nickel, and organic compounds such as flame retardants, chlorofluorocarbons, polycyclic aromatic hydrocarbons (PAHs), polybrominated diphenyl ethers (PBDEs), and polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs). E-waste recycling also recovers valuable materials including iron, aluminium, copper, silver, and rare earth metals but excessive exposure can be noxious.6, 7 These environmental contaminants pose severe threats to both the health of human beings and the environment.8
E-waste exposures occur in the informal and formal recycling sectors, and through contaminants that persist in the environment.9, 10 Most e-waste recycling occurs in the informal sector, often in unregulated work settings or as cottage industries in homes.11 Recovery of precious metals is inefficient, incomplete, and generally carried out without personal protective equipment or modern technology.12 Hazardous processes include open burning, manual dismantling, plastic chipping and melting, heating, and acid leaching, cyanide salt leaching, and mercury amalgamation.9, 13 Hazardous pollutants originating from such processes also contaminate ecosystems, leach into groundwater, contaminate food, and reduce air quality.14 Metal contaminants from e-waste are non-biodegradable, and can disturb the aquatic and terrestrial environment's ecological balance by persisting in the environment.15 Formal e-waste recycling, in which salvageable materials are safely removed with adequate worker and environmental protection, is expensive, limiting feasibility in LMICs. Although several LMICs have enacted legislation to restrain illegal import of e-waste into their countries, none of the legislation effectively regulates e-waste processing.16
E-waste exposures to people occur through multiple complex pathways. Type of exposure source, duration of exposure, and probable inhibitory, synergistic, or additive effects of multiple exposures are all factors that can influence health outcomes.17 It is difficult to ascertain the effect of exposure to a specific e-waste related compound or element in isolation. Inhabitants and workers living near e-waste recycling sites can be exposed through inhalation, ingestion, and dermal absorption when they come into physical contact with contaminated soil, dust, air, water, or food sources.6, 10 Residents living in the vicinity of e-waste recycling areas are at a particularly high risk of exposure. Exposure to contaminants associated with e-waste during gestation, infancy, or childhood can lead to obesity, asthma, or neurodevelopmental disorders.18 Adverse health outcomes associated with exposure to e-waste were reviewed in 2013 where 23 epidemiological studies were included from 2274 records published between Jan 1, 1965, and Dec 17, 2012.17 This Review updates evidence of the association between e-waste exposure and adverse human health consequences, following PRISMA guidelines.19
Methods
Search strategy and selection criteria
The complete review protocol, methods, and criteria were based on our previous systematic review.17 We searched Web of Science, EMBASE, PubMed, PsycNET, and CINAHL for articles published in English from Dec 18, 2012, to Jan 28, 2020 as previously described (full search terms are in the appendix).17 Our scope was limited to published epidemiological literatures that focused on exposure pathways in association with human health indicators (appendix). We excluded studies that reported outcomes in plants, animals, and in-vivo or in-vitro populations. We also excluded reviews, abstracts, editorials, correspondence, reports, book chapters, preface, commentary, and studies that did not report any human health outcome in relation to e-waste exposure. Our study was registered with PROSPERO, number CRD42021223833.
Data analysis
After preliminary title and abstract screening, relevant articles were retrieved based on predetermined criteria. Two independent reviewers (SMP and FJ) assessed eligibility, with disagreements resolved by consensus. The reviewers developed a data extraction sheet by piloting and revised accordingly. Each reviewer extracted data independently using a standardised protocol based on the following characteristics: publication details, study design, location, sampling population, sample size, exposure, health outcomes, and effect sizes of association between exposure and health outcomes (appendix). Risk of bias was determined by focusing on methodological criteria described elsewhere.17
Results
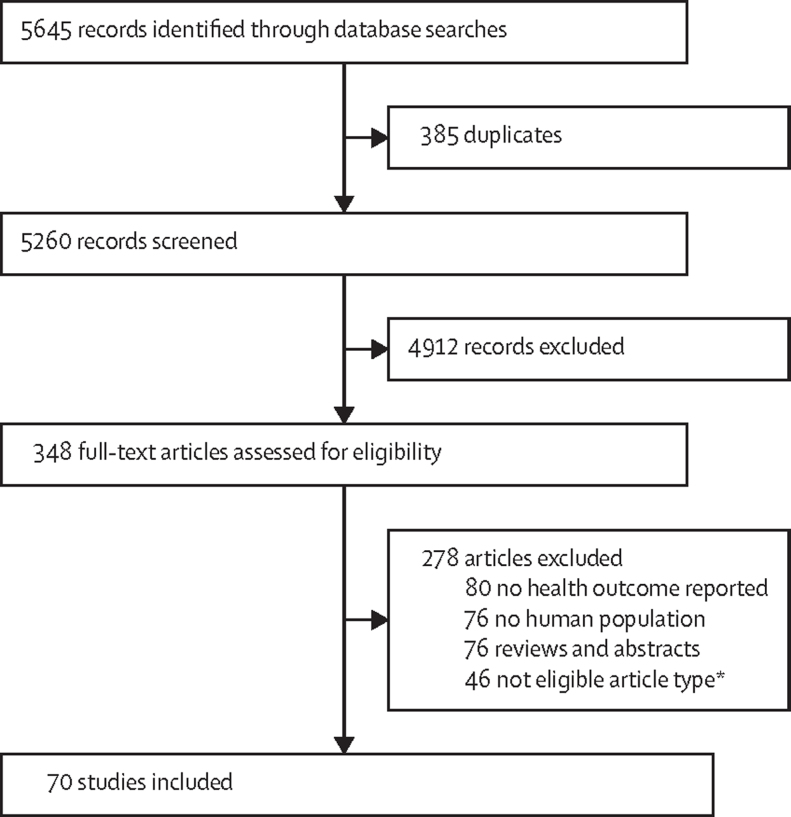
We identified 70 unique studies after full-text screening (figure). Most studies were done in China (n=66), followed by Vietnam (n=2), Ghana (n=1), and India (n=1). One study used a cohort design, and the rest were cross-sectional in nature. The most common reason for excluding studies was that health effects from e-waste exposures were not reported for the study population (figure). 11 Chinese articles with English abstracts were identified. During screening, one study was eligible for full-text review which we could not assess.
Figure.
Study profile
*Editorial, commentary, preface, news, correspondence, in-vitro experiments, case studies, reports, protocol articles, articles in Chinese, spotlights, chapters, and data articles.
Exposure to e-waste was associated with higher levels of many toxic chemicals and metals including: lead,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 cadmium,20, 22, 23, 26, 27, 28, 29, 31, 32, 33, 39, 45, 56, 57 mercury,32 manganese,26, 36, 39, 49 chromium,31, 39, 49, 58 nickel,49, 58, 59 PAHs,21, 48, 60, 61, 62, 63, 64 PBDEs,29, 35, 61, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 polychlorinated bisphenols (PCBs),35, 54, 67, 68, 69, 70, 72, 73, 74, 75, 77, 78 dechlorane plus (DP),77, 78, 79 PCDD/Fs,69 new flame retardants (NFR),72, 74 bromophenols,67 perchlorate and thiocyanate,80 polybrominated biphenyls,77 phthalate esters,81 bisphenols,82, 83 and organophosphates.84 The chemical classification of e-waste components, sources and potential routes of exposure have been reported in the previous systematic review.17
Nine cross-sectional studies from China estimated the effect of e-waste-derived toxic chemicals on physical growth indicators. In general, increased toxicant levels were associated with poor foetal development in early life (table 1). Levels of PBDEs, PAHs, lead, and cadmium were significantly higher in exposed populations than they were in non-exposed individuals.20, 21, 22, 23, 56, 60, 65, 66 In two studies, neonatal head circumference, body-mass index, and Apgar1 scores were negatively correlated with PBDE concentrations detected in placenta and umbilical cord.65, 66 During pregnancy, higher urinary PAHs were associated with reduced birthweight, head circumference, body-mass index, and Apgar1 score.60 One study reported that blood PAHs were negatively associated with height and chest circumference in children aged 3–7 years.21 In three studies, blood lead exposure was associated with decreased child growth and development.21, 22, 23 A 10 ng/g increase in placental cadmium concentration was associated with a decrease of 205 g in weight and 0·44 cm in body length.20 Maternal urinary cadmium was associated with reduced birthweight, length, head circumference and Apgar scores in females neonates.56 Conversely, two studies found no effect of blood cadmium on child growth parameters (table 1).22, 23
Table 1.
Growth and neurodevelopment effects from exposure to electronic waste
| Exposure setting | Exposed population | Control population | Toxic chemicals | Health outcomes | |
|---|---|---|---|---|---|
| Growth | |||||
| Huo et al (2019)60 | Cross-sectional: exposed area vs reference area, China | 155 pregnant women (mean age 26·63 years) | 102 pregnant women (mean age 27·68 years) | PAHs | Urinary ∑OHPAH=6·87 mg/g cre exposed vs 3·90 mg/g cre control (p<0·001); dominant metabolites=2-OHNap and 1-OHPyr. Elevated ∑OHPAHs associated with a decrease of 235 g in bodyweight (95% CI −452 to −17), decrease of 1·72 cm in head circumference (−2·96 to −0·48), decrease of 1·06 kg/m2 in BMI (−1·82 to −0·31), and decrease of 0·42 in Apgar1 score (−0·66 to −0·18; all p<0·05). |
| Li et al (2018)65 | Cross-sectional: exposed town vs reference town, China | 150 pregnant women (mean age 26·51 years) | 150 pregnant women (mean age 28·43 years) | PBDEs | ∑14PBDEs in umbilical cord=71·92 ng/g lw vs 15·52 ng/g lw (p<0·001) and negatively correlated with neonatal BMI (r=−0·20), Apgar1 score (r=−0·39), and head circumference (r=−0·37; all p<0·01). |
| Xu et al (2016)20 | Cross-sectional: exposed town vs reference town, China | 99 pregnant women (mean age 25·05 years) | 86 pregnant women (mean age 27·96 years) | Lead and cadmium | Placental lead=498 ng/g wt vs 27 ng/g wt (p<0·01); cadmium=96·19 ng/g wt vs 12·65 ng/g wt (p<0·01). Shorter neonatal length in exposed =49·78 vs 50·30 cm (p<0·01). Cadmium negatively correlated with neonatal weight (B=−0·20) and length (B–0·44; both p<0·05). Lead was not statistically associated with birth outcomes (p>0·05). 32 differentially expressed proteins identified from 54 protein spots, FUM expression lower in exposed placenta (605 pg/g wt vs 1019 pg/g wt; p<0·05). |
| Xu et al (2015)66 | Cross-sectional: exposed group vs reference group, China | 69 pregnant women (mean age 26·4 years) | 86 pregnant women (mean age 27·8 years) | PBDEs | Placental ∑PBDE=32·25 ng/g lw vs 5·13 ng/g lw; common congener=BDE −209, −28, −153, −183, −47, −99. Neonatal BMI=11·90 kg/m2vs 12·69 kg/m2, Apgar1 score=9·16 vs 10·0, and head circumference=33·52 cm vs 34·92 cm (all p<0·001). PBDE and BDE-47 negatively correlated with BMI, head circumference, and Apgar1 score, negative correlation between BDE-99 and BMI, BDE-28/153 and Apgar 1 score, and BDE-183 and BMI and Apgar1 score (all p<0·05). |
| Zhang et al (2018)56 | Cross sectional: exposed town vs reference town, China | 237 mother–neonate pairs (mean maternal age 26·29 years) | 212 mother–neonate pairs (mean maternal age 28·52 years) | Cadmium | Maternal urinary cadmium with male neonates=1·38 μg/g cre vs 0·75 μg/g cre, urinary cadmium with female neonates=1·59 μg/g cre vs 0·76 μg/g cre (both p<0·001). Urinary cadmium negatively associated with birthweight (β=−0·16), length (β=−0·17), head circumference (β=−0·38), Apgar1 and Apgar5 score (β=−0·26 and β=−0·43) in female neonates (all p<0·05), in male neonates urinary cadmium negatively associated with Apgar1 score (β=−0·21; p<0·01). |
| Xu et al (2015)21 | Cross-sectional: exposed town vs reference town, China | 95 children aged 3–7 years | 72 children aged 3–7 years | PAHs and lead | ∑16PAHs in blood=68·53 μg/L vs 26·92 μg/L, ∑7 carcinogenic PAHs=60·27 μg/L vs 21·30 μg/L, blood lead 13·89 μg/dL vs 8·55 μg/dL (all p<0·01). Blood lead negatively correlated with child height (Rs=−0·16; p<0·05); child height (β=−3·88) and chest circumference (β=−1·15) negatively associated with ∑16PAHs (p<0·05). |
| Yang et al (2013)22 | Cross-sectional: e-waste processing area, China | 246 kindergarten children aged 3–8 years | None | Lead and cadmium | Blood lead=7·30 μg/dL, blood cadmium=0·69 μg/L. Blood lead negatively associated with height (β=−0·10), weight (β=−0·14; both p<0·05), and positively associated with increase urinary excretion of DPD (mean 10·09 [SD 3·76 nmol/g]; p<0·01). No association between cadmium and bone, calcium metabolic biomarker (p>0·05). |
| Zeng et al (2019)23 | Cross sectional: exposed town vs reference town, China | 300 preschool children (mean age 4·66 years) | 170 preschool children (mean age 4·34 years) | Lead, cadmium, chromium, and manganese | Blood lead=6·81 μg/dL vs 4·98 μg/dL, blood cadmium=0·66 μg/L vs 0·54 μg/L, PM2·5=57·73 μg/m3vs 40·53 μg/m3, elevated lead and cadmium in PM2·5 (data not shown; all p<0·05). Lower birth length, weight, BMI in exposed (all p<0·05), blood lead negatively associated with height (β=−0·06), weight (β=−0·12), head circumference (β=−0·12), and chest circumference (β=−0·10; all p<0·05). No association between cadmium, chromium, manganese with growth parameters (p>0·05). |
| Neurodevelopment | |||||
| Cai et al (2019)24 | Cross-sectional: exposed town vs control town, China | 358 preschool children (aged 3–6 years) | 216 preschool children (aged 3–6 years) | Lead | Blood lead=4·88 μg/dL vs 3·47 μg/dL (p<0·001), serum cortisol=452 ng/mL vs 593 ng/mL (p<0·001), cortisol negatively associated with blood lead (B=−0·13, 95% CI −0·27 to −0·003; p<0·05). Elevated blood lead (>5 μg/dL) increased sensory integration difficulty scores (hearing, touch, body awareness, balance and motion, total sensory systems, r=0·10–0·18; p<0·05), scale for touch negatively correlated with serum cortisol levels (r=−0·16; p<0·05). |
| Liu et al (2015)25 | Cross-sectional: exposed town vs control town, China | 135 children (mean age 38 months) | 149 children (mean age 39 months) | Lead | Blood lead=11·30 μg/dL vs 5·77 μg/dL, lower cognitive scores (100 vs 120) and language scores (100 vs 111; both p<0·001), no differences of DRD2 genotypes among exposed (p>0·05). Blood lead related to reduced cognitive (β=−0·19) and language scores (β=−0·72; both p<0·001). No association between DRD2 polymorphism and cognitive or language scores (p>0·05). |
| Liu et al (2014)26 | Cross-sectional: e-waste disposal site, China | 240 kindergarten children aged 3–7 years | None | Lead, cadmium, and manganese | Blood lead=7·33 μg/dL, blood cadmium=0·69 μg/L, blood manganese=17·98 μg/L, serum S100β=0·12 μg/L. ADHD prevalence=18·6% (higher prevalence in males than in females). Blood lead, cadmium, and manganese correlated with conduct problems and antisocial behaviour (data not shown), serum S100β positively correlated with blood lead (≥10 μg/dL, r=0·47) and some behavioural abnormalities (p<0·05). |
| Zhang et al (2015)27 | Cross-sectional: e-waste recycling town, China | 243 preschool children (aged 3–7 years) | None | Lead and cadmium | Blood lead=7·9 μg/dL, blood cadmium=0·95 μg/L, ADHD=12·8%. Positive correlations between blood lead and ADHD scores (inattentive, hyperactive/impulsive, and total scores, β=0·22–0·28; p<0·001). No correlation with blood cadmium. Elevated blood lead increased risk of ADHD (odds ratio 2·4, 95% CI 1·1 to 5·2). |
| Liu et al (2018)28 | Cross-sectional: exposed group vs reference group, China | 120 children (mean age 37·49 months) | 138 children (mean age 38·80 months) | Lead and cadmium | Blood lead=11·30 μg/dL vs 5·77 μg/dL, blood cadmium=1·22 μg/L vs 0·72 μg/L (both p<0·001). Lower cognitive (100 vs 120) and language scores (99 vs 111), higher TSH, and higher FT4 (all p<0·01). Blood lead negatively correlated with cognitive scores (β=−1·57) and language scores (β=−0·80; both p<0·001) in mediation analysis, no correlation between blood cadmium and language or cognitive scores (p>0·05). FT3, FT4, and TSH did not mediate between lead and mental development. |
PAH=polycyclic aromatic hydrocarbons. cre=creatinine. ∑OHPAH=total hydroxylated PAH. 2-OHNap=2-OHnaphthalene. 1-OHPyr=1-hydroxypyrene. BMI=body-mass index. Apgar1=Apgar score at 1 min. Apgar1=Apgar score at 5 mins. PBDE=polybrominated diphenyl ether. lw=lipid weight. wt=weight. FUM=fumarate hydratase. DPD=deoxypyridinoline. S100β=S100 calcium-binding protein β. DRD2=dopamine receptor-2. ADHD=attention-deficit hyperactivity disorder. TSH=thyroid stimulating hormone. FT4=free thyroxine. FT3=free triiodothyronine.
Five cross-sectional studies investigated the relationship between lead, cadmium, and manganese and neurodevelopmental outcomes among young children in China (table 1).24, 25, 26, 27, 28 Higher blood lead levels were associated with poorer neurodevelopmental outcomes, while results were less consistent for other metals. In two studies, children had lower cognitive and language scores that were negatively correlated with blood lead;25, 28 by contrast, no association was found with cadmium levels.28 Cai and colleagues24 found that children with higher blood lead levels had more sensory processing difficulties than did children with low blood lead levels. Behavioural abnormalities were found in children with higher blood levels of lead, cadmium, and manganese26 while in another study, children with high blood lead (≥10 μg/dL) had 2·4-times higher odds of attention-deficit hyperactivity disorder than did those with low lead exposure (table 1).27
13 studies investigated thyroid29,67–73,79,80 and sex hormones30, 74, 85 (table 2). Overall, exposure to e-waste-induced toxic chemicals disrupted thyroid function and had endocrine-disrupting effects on sex hormones. In two studies, thyroid hormones (THs) were measured among children aged 4–8 years.29, 69 No hormonal differences were found between children in the exposed and control groups and the association was not significant between toxicants (PCBs, PBDEs, and PCDD/Fs) and THs.69 By contrast, PBDEs were negatively associated with free triiodothyronine in the adjusted regression model; however, no correlation was found between THs and blood lead or cadmium levels.29 Xu and colleagues69 measured adrenocorticotropic hormone in young children which was positively correlated with serum PBDEs (table 2). In pregnant women, serum PCBs and PBDEs levels were negatively associated with thyroid-stimulating hormone (TSH)71 and total thyroxine,71 respectively, while DP concentration was positively associated with total triiodothyronine levels in maternal sera reported by Ben and colleagues.79 In three studies, serum PCBs were negatively associated with TSH,67 free triiodothyronine,70 and free thyroxine70, 72 among adults. However, no association was observed between THs and PCBs/hydroxylated PCBs,73 or PBDEs70 in other studies. By contrast, serum PBDEs were negatively associated with thyroxine; similarly, NFR disrupted effects on thyroxine-binding globulin and TSH in Chinese adults.72 In Vietnamese females, lower concentration of iodine was found where serum perchlorate or thiocyanate yielded no correlations with THs (table 2).80
Table 2.
Hormonal and immunological function resulting from exposure to electronic waste
| Exposure setting | Exposed population | Control population | Toxic chemicals | Health outcomes | |
|---|---|---|---|---|---|
| Hormonal | |||||
| Lv et al (2015)68 | Cross-sectional: exposed villages vs reference village, China | 64 pregnant women | 10 pregnant women | PCBs and PBDEs | Serum ∑PCBs=26·2 ng/g lw vs 14·0 ng/g lw, ∑PBDE8=9·77 ng/g lw vs 4·80 ng/g lw, PCB-153=8·30 ng/g lw vs 3·33 ng/g lw (p=not shown). PCBs, PCB-153, and PCB-138 negatively associated with lower TSH (r=−0·34, r=−0·38, r=−0·45; all p<0·05); no association between PCBs/PBDEs and TT3, TT4, FT3, FT4 (p>0·05). |
| Ben et al (2014)79 | Cross-sectional: exposed group (>20 years of living) vs control (<3 years), China | 48 mother–infant pairs (mothers aged ≥18 years) | 24 mother–infant pairs (mothers aged ≥18 years) | DPs | DP in maternal sera=13·5 ng/g lw vs 3·68 ng/g lw, placenta=4·27 ng/g lw vs 1·25 ng/g lw, cord blood=4·02 ng/g lw vs 2·03 ng/g lw (all p<0·05), strong correlations between DP concentrations in maternal sera and cord sera, maternal sera and placentas, placentas and cord sera (r>0·7; p<0·001). Lower TSH=1·76 μIU/mL vs 2·25 μIU/mL (p<0·05), no difference in TT3, TT4, FT3, FT4. DP levels associated with TT3 in maternal sera (syn-DP: r=0·37; anti-DP: r=0·36; p<0·05). |
| Zheng et al (2017)71 | Cross-sectional: exposed group (>20 years of living) vs control (<3 years), China | 48 paired mother–fetus | 24 paired mother–fetus | PBDEs | PBDE in serum=19·3 ng/g lw vs 8·13 ng/g lw, umbilical cord=6·84 ng/g lw vs 4·47 ng/g lw, placental tissue=2·20 ng/g lw vs 1·06 ng/g lw (p<0·05), major congener=BDE-209 and BDE-153. Significant association between BDE-153 and TT4 in exposed group (β=−0·15, 95% CI −0·23 to −0·07, R2= 0·531; p<0·001). |
| Xu et al (2013)61 | Cross-sectional: exposed city vs control city, China | 101 pregnant women (mean age 26·20 years) | 53 pregnant women (mean age 26·72 years) | PAHs and PBDEs | UCB ∑16PAHs=14·43 ppb vs 10·05 ppb, ∑PBDE=57·55 ng/g vs 8·23 ng/g lipid (both p<0·001). Increased placental IGF-1 and IGFBP-3 expression of mRNA (IGF-1: 0·23 vs 0·19 and IGFBP-3: 1·91 vs 0·68 (both p<0·05). Lower birthweight and Apgar score in exposed group. ∑PBDEs, ∑4 ring-PAHs and ∑16PAHs positively correlated with IGFBP-3 (β=0·44, β=0·34, and β=0·26, respectively; all p<0·01). BDE-154, BDE-209, and ∑5 ring-PAHs correlated with IGF-1 mRNA (β=0·23, β=0·24, and β=0·29, respectively; all p<0·05). |
| Xu et al (2014)29 | Cross-sectional: e-waste area, China | 162 children aged 4–6 years | None | PBDEs, lead, and cadmium | Serum PBDE=189·99 ng/g lipid, blood lead=14·53 μg/dL, blood cadmium=0·77 μg/L. Mean FT3=6·28 pmol/L, FT4=17·78 pmol/L, TSH=2·85 μIU/mL, IGF-1=510·79 ng/mL, IGFBP-3=60·97 ng/mL. ∑PBDEs negatively associated with FT3 (β=−0·19) and positively associated with TSH (β=0·27; both p<0·005). BDE-153 correlated with blood lead (β=0·19; p<0·05), no correlation between THs and blood lead or cadmium (p>0·05). |
| Xu et al (2014)69 | Cross-sectional: exposed town vs control town, China | 21 children aged 8 years | 24 children aged 8 years | PCBs, PBDEs, and PCDD/Fs | Serum ∑PCBs=40·56 ng/g lipid vs 20·69 ng/g lipid, ∑PBDEs=32·09 ng/g lipid vs 8·43 ng/g lipid (both p<0·001), PCDD/F=206 pg/g lipid vs 160 pg/g lipid (p>0·05). Elevated mean of FT3, TT3, TT4, ACTH, cortisol, GH, and lower FT4, TSH, no difference among groups (p>0·05). ∑PBDEs positively associated with ACTH (r=0·61; p<0·05), cortisol positively associated with TSH (r=0·50) and GH levels (0·51; both p<0·05). |
| Eguchi et al (2014)80 | Cross-sectional: exposed town vs non exposed rural site, Vietnam | 83 local residents, aged 10–64 years | 48 local residents, aged 10–64 years | Perchlorate (ClO4−) and thiocyanate (SCN−) | Serum perchlorate=0·116 ng/mL vs 0·086 ng/mL (p<0·05). Thiocyanate=2020 ng/mL, iodine=3·11 ng/mL, PEC=2·28 μmol/L, greater concentration among males (p<0·05). TT3=1·2 ng/mL vs 1·3 ng/mL, FT3=3·3 pg/mL vs 3·4 pg/mL (p<0·05), no correlation between THs and perchlorate/thiocyanate (p>0·05). Iodine significant positive predictor of FT3 (β=0·16), TT3 (β=0·19), and negative predictor of TSH (β=−0·45; all p<0·01) in males. |
| Eguchi et al (2015)67 | Cross-sectional: exposed town vs non-exposed rural site, Vietnam | 77 adult workers and residents (mean age 33 years) | 34 adult workers and residents (mean age 37 years) | PCBs, OH-PCB, PBDEs, MeO-PBDE, OH-PBDE, and BPh | Serum PCBs=420 pg/g vs 290 pg/g, OH-PCBs=160 pg/g vs 82 pg/g, PBDEs=290 pg/g vs 230 pg/g, and BPhs=300 pg/g vs 200 pg/g (all p<0·05). FT3=3·3 pg/g vs 3·5 pg/g, TT3=1·2 pg/g vs 1·3 pg/g, TT4=78 pg/g vs 85 pg/g (all p<0·05), FT4=1·3 pg/g vs 1·2 pg/g, TSH=1·4 pg/g vs 1·5 pg/g (both p>0·05). Positive correlation between FT4, FT3, TT3, TT4 and PCBs/OH-PCB, and negative correlation between PCB and TSH in females (all p<0·05). |
| Xu et al (2015)70 | Cross-sectional: exposed town vs control town, China | 40 local residents, aged 15–65 years | 15 local residents, aged 15–65 years | PCBs and PBDEs | Serum ∑PCBs=964 ng/g vs 68 ng/g (p<0·001), ∑PBDEs=139 ng/g vs 75 ng/g (p>0·05). FT3=4·72 pmol/L vs 5·64 pmol/L, FT4=14·98 pmol/L vs 18·67 pmol/L (both p<0·001), TSH=2·51 μIU/mL vs 1·80 μIU/mL (p>0·05). ∑PCBs negatively correlated with FT3 (r=−0·41) and FT4 (r=−0·39), no correlation between PBDEs and THs (p>0·05). |
| Guo et al (2019)72 | Cross-sectional: exposed town vs control town, China | 54 adult residents aged 26–75 years | 58 adult residents aged 26–75 years | PCBs, PBDEs, and NFR | ∑PCB=310 ng/g lipid vs 42 ng/g lipid, ∑PBDE=190 ng/g lipid vs 74 ng/g lipid, ∑NFR=350 ng/g lipid vs 110 ng/g lipid (all p<0·05). No mean difference of T3, T4, FT3, FT4, TSH among groups (p>0·05), TBG=18 μmol/L vs 20 μmol/L (p<0·05). PCB-28, 52, 101, 138, 153 negatively associated with FT4 (p<0·05), PBDEs negatively associated with T4(p<0·05). ∑NFR negatively associated with TSH (p<0·05) and TBG (p<0·05). Positive association between PBDE congener and T3 (BDE-85, BDE-99) and FT3 (BDE-47; all p<0·05). |
| Zheng et al (2017)73 | Cross sectional: e-waste recycling workers, China | 79 adult workers, aged 22–59 years | None | PBDEs, PCBs, and OH-PCB | Serum PCBs=2251 ng/g lipid, PBDEs=724 ng/g lipid, and OH-PCBs 418 ng/g lipid, no association between THs and PCBs/OH-PCBs (p>0·05), elevated T3 and T4 associated with certain PBDEs congeners (β=0·11–0·17; p<0·05). TH-regulated gene expression associated with certain PCB, OH-PCB, and mostly PBDE congeners (p<0·05) |
| Yan et al (2013)30 | Cross-sectional: e-waste dismantling area, China | 187 men aged 18–60 years | None | Lead | Blood lead=100·08 μg/L (≤30 years=98·55 μg/L, 31–45 years=100·23 μg/L, and 46–60 years=101·45 μg/L). FSH (≤30 years=5·64 mIU/mL, 31–45 years=11·51 mIU/mL, 46–60 years=15·32 mIU/mL), LH (≤30 years=4·59 mIU/mL, 31–45 years=4·90 mIU/mL, 46–60 years=5·96 mIU/mL), Tr (≤30 years=4823 mIU/mL, 31–45 years=4157 mIU/mL, 46–60 years=3562 mIU/mL). Blood lead associated with FSH (r=0·96), LH (r=0·92), and Tr levels (r=0·89; all p<0·01). |
| Guo et al (2018)74 | Ecological study: exposed town vs control town, China | 54 local residents, aged 26–75 years | 58 local residents, aged 26–75 years | NFR, PCBs, and PBDEs | Serum ∑PCB=310 ng/g lipid vs 42 ng/g lipid, ∑PBDE=190 ng/g lipid vs 74 ng/g lipid, ∑NFR=350 ng/g lipid vs 110 ng/g lipid among exposed group (all p<0·05). Female FSH=12 mIU/mL vs 55 mIU/mL (p<0·05). NFR (TBB, DPa, DBDPE) and PBDE (BDE-153, 154, 183) negatively associated with female FSH, male Tr positively associated with NFR (TBECH, BTBPE, DPa) and PBDE congener (BDE-47, 100, 153, 183, 207; p<0·05). |
| Zhou et al (2013)85 | Cross-sectional: exposed town vs reference town, China | 46 parturient women (mean age 27·82 years) | 44 parturient women (mean age 24·89 years) | Not assessed | Serum E2=2137 pg/mL vs 1549 pg/mL, umbilical cord E2=2758 pg/mL vs 2211 pg/mL, serum PROG=100 ng/mL vs 61 ng/mL, umbilical cord PROG=156 ng/mL vs 146 ng/mL (all p<0·05). mRNA of ERalpha, ERbeta increased in placenta and umbilical cord among exposed, mRNA of PROG decreased in placenta and umbilical cord among exposed (all p<0·05). |
| Immunological | |||||
| Cao et al (2018)40 | Cross-sectional: exposed town vs reference town, China | 62 preschool children aged 3–7 years | 56 preschool children aged 3–7 years | Lead | Blood lead=5·06 μg/dL vs 3·60 μg/dL (p<0·001). Higher percentage of CD4+ Tcm and CD8+ Tcm cells among exposed (geometric mean=25·79% vs 21·43% and 0·89% vs 0·62%, respectively; p<0·001). No difference in serum cytokines (IL-2, IL-7, IL-15) among groups. Blood lead positively associated with CD4+ Tcm (β=0·49; p<0·05) and marginal change in CD8+ Tcm (p<0·05). |
| Huo et al (2019)41 | Cross-sectional: exposed group vs reference group, China | 132 preschool children aged 2–7 years | 135 preschool children aged 2–7 years | Lead | Blood lead=6·51 μg/dL vs 4·41 μg/dL, erythrocyte lead=16·60 μg/dL vs 11·77 μg/dL (p<0·001). Reduced erythrocyte CD44 and CD58 expression (68·03% vs 76·15% and 40·76% vs 46·22%, respectively; p<0·01). Elevated erythrocyte lead associated with lower CD44 (BQ4 −5·44% [95% CI −9·11 to −1·73]) and CD58 (BQ4 −4·27% [−6·90 to −1·68]). Higher cytokines (IL-1β, IL12p70, IFN-γ, except IL-2). Elevated blood lead correlated with higher IL-12p70 (rs=0·20), IFN-γ (rs=0·22), and lower IL-2 (rs=−0·15), leukocyte count (rs=−0·12), lymphocyte ratio (rs=−0·16), LMR (rs=−0·18; all p<0·05). |
| Zhang et al (2016)42 | Cross sectional: exposed town vs reference town, China | 285 preschool children aged 3–7 years | 126 preschool children aged 3–7 years | Lead | Blood lead=6·00 μg/dL vs 3·92 μg/dL, and lower NK cells (CD3−CD56+, CD3−CD56brightCD16low/−, and CD3CD56dimCD16+), increased platelets, IL-1β and lower IL-2, IL-27, MIP-1α, MIP-1β concentration in exposed (all p<0·05), negative association between CD3−CD56bright CD16low/− and blood lead (β=−0·182; p<0·05). Blood lead correlated with platelet, neutrophil, monocyte (Rs=0·11, 0·14, and 0·12, respectively; p<0·05). IL-1β positively and IL-27 negatively associated with blood lead (Rs=0·16 and −0·31; p<0·05). |
PCB=polychlorinated bisphenol. PBDE=polybrominated diphenyl ether. lw=lipid weight. ∑PCBs=total PCB. ∑PBDE8=sum of eight congeners. TSH=thyroid stimulating hormone. TT3=total triiodothyronine. TT4=total thyroxine. FT3=free triiodothyronine. FT4=free thyroxine. DP=dechlorane plus. syn-DP=syn (or endo)-dechlorane plus. anti-DP=anti (or exo) dechlorane plus. PAH=polycyclic aromatic hydrocarbons. UCB=umbilical cord blood. ppb=parts per billion. IGF-1=insulin-like growth factor. IGFBP-3=IGF binding protein 3. TH=thyroid hormone. PCDD/F=polychlorinated dibenzo-p-dioxins and dibenzofurans. ACTH=adrenocorticotropic hormone. GH=growth hormone. PEC=perchlorate-equivalent concentrations. OH-PCB=hydroxylated PCB. MeO-PBDE=methoxylated PBDE. OH-PBDE=hydroxylated PBDE. BPh=bromophenols. FSH=follicle-stimulating hormone. LH=luteinising hormone. Tr=testosterone. NFR=new flame retardants. TBG=thyroxine-binding globulin. TBB=2-ethylhexyl 2,3,4,5-tetrabromobenzoate. DPa=dechlorane plus anti. DBDPE=1,2-bis(2,3,4,5,6-pentabromophenyl)ethane. TBECH=tetrabromoethylcyclohexane. BTBPE=1,2-bis(tribromophenoxy)-ethane. E2=oestradiol. PROG=progesterone. ERalpha=oestrogen receptor alpha. ERbeta=oestrogen receptor beta. CD4+Tcm=CD4+ central memory T cells. CD8+Tcm=CD8+ central memory T cells. BQ4=beta coefficient in quartile 4. IL=interleukin. IFN=interferon. LMR=lymphocyte-to-monocyte ratio. NK=natural killer. MIP=macrophage inflammatory protein.
Three studies investigated sex hormones in Chinese adults.30, 74, 85 Elevated serum oestradiol and progesterone were found in exposed pregnant women.85 Guo and colleagues74 observed disrupting effects of NFR with PBDE congeners on female follicle-stimulating hormone and male testosterone. Moreover, correlation between male sex hormones and blood lead level was somewhat significant, depending on age group assessed.30 In two studies,29, 53 serum insulin-like growth factor (IGF)–IGF-binding protein (IGFBP) were measured in children aged 4–7 years where the investigators did not find an association between IGF-1 and PBDEs29 or lead.53 However, one PBDE congener (BDE-209) was positively associated with IGFBP-3.29 Xu and colleagues61 reported that placental IGF-1 and IGFBP-3 were significantly higher in women exposed to e-waste. Exposure to PBDEs and PAHs in utero affects IGF-1 and IGFBP-3 mRNA levels in the placenta, which might have adverse effects on foetal growth and development (table 2).
Alteration of proinflammatory cytokines was observed among preschool children41, 42, 64 and local adult residents75 living in an e-waste recycling area when compared with reference sites in China. However, proangiogenic cytokines (RANTES and GROα) did not differ significantly between the two groups.64 Multiple studies detected that elevated lead exposure stimulates cytokine secretion, including IL-1β,41, 42 IL-27,42 1L-12p70,41 and IFN-γ.41 Another study noted a potential association between IL-1β and urinary PAH exposure.64 Huo and colleagues41 estimated the effect of lead exposure on expression of erythrocyte adhesion molecules (CD44 and CD58) where reduced erythrocyte immunity was observed due to long-standing environmental lead contamination among preschool children. Cao and colleagues40 observed higher proportions of CD4+ central memory T cells in an e-waste recycling area where exposure to lead contributed to the increased percentages of peripheral CD4+ central memory T cells. Another study found that CR1 expression, which plays crucial roles in B-lymphocyte and T-lymphocyte immune responses, was depressed due to lead exposure.43 Two studies42, 45 identified lower natural killer cells among exposed children, in one study elevated lead levels resulted in lower percentages of natural killer cells42 while another study did not (table 2).45
Findings from two studies observed telomere aberration among e-waste-exposed pregnant women57 and local residents.75 The data suggested that telomere dysfunction is potentially induced through exposure to cadmium57 and persistent organic pollutants (POPs).75 Placental telomere attrition probably begins to occur at the cadmium concentration of 0·0294 mg/g.57 Upon proteomic analysis in the umbilical cord, the investigator found altered protective cell oxidative damage (CAT and GSTO1) and cell apoptosis (Cyt c) biomarkers associated following exposure to PBDEs.65 In four studies, the micronucleus rate was used to evaluate genotoxicity and was found to be significantly elevated among residents living near the e-waste disposal site.35, 54, 75, 78 However, He and colleagues78 revealed no correlation between POPs accumulation and micronucleus rate. RNA expression genes involved in ion binding, ion transport, immune regulation, apoptosis, and oxidoreductase activity were verified by quantitative fluorescence PCR where the number of genetic aberrations was higher in men than women.35 Multiple studies observed significantly decreased DNA methylation in populations living in e-waste exposed regions (table 3).36, 59, 75
Table 3.
Genetic and oxidative changes resulting from exposure to electronic waste
| Exposure setting | Exposed population | Control population | Toxic chemicals | Health outcomes | |
|---|---|---|---|---|---|
| Genetic | |||||
| Li et al (2018)65 | Cross-sectional: exposed town vs reference town, China | 150 pregnant women (mean age 26·51 years) | 150 pregnant women (mean age 28·43 years) | PBDEs | Umbilical cord ∑14PBDEs=71·92 ng/g lw vs 15·52 ng/g lw (p<0·001). Lower expression of CAT=902 pg/g wt vs 1305 pg/g wt, GSTO1=526 pg/g wt vs 562 pg/g wt, Cyt c=389 pg/g wt vs 268 pg/g wt (all p<0·01). ∑14PBDEs, BDE-17, BDE-99, BDE-183 associated with decreased CAT expression (β=−0·31 to −0·10), GSTO1 decrease with BDE-153, BDE-190 (β=−0·20 to −0·16), BDE-99, BDE-190 increased Cyt c expression (β=0·16 to 0·19; all p<0·05). |
| Lin et al (2013)57 | Cross-sectional: exposed town vs non-polluted town, China | 227 healthy puerperae (mean age 26·45 years) | 93 healthy puerperae (mean age 27·63 years) | Lead and cadmium | Placental cadmium=0·09 μg/g vs 0·02 μg/g (p<0·01), lead=1·25 μg/g vs 1·35 μg/g (p>0·05). Placental telomere length negatively correlated with cadmium (r=−0·14; p<0·05), no correlation between placental lead and telomere length (r=0·03; p>0·05). Positive correlation between mean TRF length and T/S ratio (R2=0·79; p<0·01). residence during pregnancy in exposed associated with telomere length (OR=2·0, 95% CI 0·07 to 0·60). |
| Zeng et al (2019)36 | Cross sectional: exposed town vs reference town, China | 101 pregnant women (mean age 27·3 years) | 103 pregnant women (mean age 28·0 years) | Lead, cadmium, manganese, and chromium | Umbilical cord blood lead=7·34 μg/dL vs 3·07 μg/dL (p<0·001), no difference of umbilical cord blood cadmium, manganese, and chromium among groups (p>0·05). Methylation of BAI1 (cg25614253; 8% vs 7%, hyper-regulated), CTNNA2 (cg20208879; 62% vs 64%, hypo-regulated; all p<0·05), both correlated with umbilical cord blood lead (r=0·16 and r=−0·19; p<0·05). In adjusted regression, umbilical cord blood lead negatively associated with CTNNA2 (β=−1·20, 95% CI −2·13 to −0·26). No correlation between umbilical cord blood cadmium, manganese, chromium levels, and the methylation levels of two CpGs. |
| Huo et al (2014)34 | Cross sectional: exposed town vs reference town, China | 189 neonates and 319 children | 84 neonatesand 185 children | Lead | Blood lead in neonates (2004–05: 10·50 μg/dL vs 7·79 μg/dL; 2006: 9·41 μg/dL vs 5·49 μg/dL), children (2004–05: 15·31 μg/dL vs 9·94 μg/dL; 2006: 13·17 μg/dL vs 10·04 μg/dL; all p<0·05). No difference of ALAD genotypes between groups (p>0·05), no significant differences between blood lead and ALAD-1/ALAD-1 or ALAD-1/ALAD-2 among newborns and children (all p>0·05). |
| Xu et al (2020)37 | Cross-sectional: exposed town vs reference town, China | 68 preschool children aged 3–7 years | 48 preschool children aged 3–7 years | Lead and cadmium | Blood lead=5·29 μg/dL vs 3·63 μg/dL (p<0·001), urinary cadmium 1·52 μg/g vs 1·21 μg/g cre (p>0·05). Higher promoter methylation levels at cg02978827, position +14, and lower methylation at position +4 of Rb1 (all p<0·05), no difference of methylation in CASP8, MeCP2 among groups. Strong positive trend of MeCP2 promoter methylation with increasing lead (R2=0·709) and cadmium (R2=0·687), minimal negative trend of Rb1 (R2=0·014 and R2=0·015) and CASP8 (R2=0·001 and R2=0·002). |
| Li et al (2014)35 | Cross-sectional: close proximity (≤5 km to e-waste recycling) vs remote group (<40 km), China | 30 adult residents (mean age 41 years) | 28 adult residents (mean age 33 years) | Calcium, copper, iron, lead, zinc, selenium, magnesium, and POPs | Lead=90·39 μg/L vs 68·40 μg/L, copper=17·34 μM vs 15·20 μM, MDA=1·29 vs 0·25 nmol/mL, PCBs=42·59 vs 10·14, PBDEs=23·05 vs 14·60, calcium=1·71 nM vs 1·82 nM, zinc=101 μM vs 127 μM (all p<0·05). Micronucleus=18·27% vs 7·32% (p<0·001). CD4+/CD8+ T cell ratios, CD4+CD25nt/hiCD127lo regulatory T cell percentage, and CD95 expression higher in close proximity group (p>0·05). RNA expression genes: men detrimentally affected (p<0·05). |
| Yuan et al (2018)75 | Cohort study: exposed town (e-waste disposal center) vs control town, China | 3349 local residents | 2606 local residents | PCBs, PBDEs, and lipid-standardised serum POP | Increased PCBs, PBDEs, ageing signal pathway (P53, Rb, P16INK4a, and P14ARF in plasma), IL-6 and IL-10 (p<0·05, data not shown), increased TNF-α (p>0·05, data not shown) among exposed. Micronucleus=20·62% vs 7·21% (p<0·01), telomere loss=1·24% vs 0·10%, fragile telomere=2·76% vs 0·69%, decreased LINE-1 DNA methylation in exposed. PBDE-184 correlated with telomere shortening (r=−0·27; p<0·05). POP exposures associated with type 2 diabetes, autoimmune disorders, abnormal pregnancy, and foetal growth. |
| Li et al (2020)59 | Experiment site (e-waste residents and former workers) vs reference site, China | 23 local residents and 23 former workers, aged 30–50 years | 45 residents aged 30–50 years | 25 metals | Arsenic=17·24 ng/mL vs 15·42 ng/mL vs 10·84 ng/mL, nickel=4·01 ng/mL vs 4·76 ng/mL vs 1·95 ng/mL, silver=0·16 ng/mL vs 0·22 ng/mL vs 0·03 ng/mL, lanthanum=0·30 ng/mL vs 0·47 ng/mL vs 0·03 ng/mL, cerium=2·43 ng/mL vs 4·08 ng/mL vs 0·06 ng/mL (all p<0·05 between controls vs e-waste residents and controls vs former workers). Blood cerium negatively correlated with global DNA methylation among former workers (r=−0·51; p<0·05). |
| He et al (2015)78 | Cross sectional: exposed town vs non-exposed town, China | 23 adult residents (mean age 35 years) | 25 adult residents (mean age 35 years) | PCBs, BDE, DP, HCB, HCH, and DDE | PCBs=149 ng/g lipid vs 35 ng/g lipid, DPs=8·14 ng/g lipid vs 1·96 ng/g lipid, BDE congeners=16·33 ng/g lipid vs 14·28 ng/g lipid (all p<0·05). Higher ROS activity (data not shown) and micronucleus rate (16·74% vs 7·8%) in exposed (both p<0·05), no correlation between POPs (PBDE/DP/PCB) and micronucleus rate (p<0·05). Expression of NEIL1/3, RPA3 downregulated, and E3 ligase RNF8 upregulated. Expression of CDC25A upregulated in males and downregulated in females among exposed (p<0·05). |
| Guo et al (2019)72 | Cross-sectional: exposed town vs control town, China | 54 local adult residents aged 26–75 years | 58 local adult residents aged 26–75 years | PCBs, PBDEs, and NFR | ∑PCB=310 ng/g lipid vs 42 ng/g lipid, ∑PBDE=190 ng/g lipid vs 74 ng/g lipid, ∑NFR=350 ng/g lipid vs 110 ng/g lipid; all p<0·05). Lower expression of TRα=14 × 10−3vs 29 × 10, TRβ=0·47 × 10−3vs 0·32 × 10−3, and higher expression of ID1=4·2 × 10−3vs 3·2 × 10−3 (all p<0·05). High PCBs, PBDEs and NFRs exposures decrease expression of TRα, and increase expression of ID1 (p<0·05). |
| Oxidative damage | |||||
| Ni et al (2014)31 | Cross-sectional: exposed town vs control town, China | 126 pregnant women (mean age 26·05) | 75 pregnant women (mean age 25·45) | Lead, cadmium, chromium, and nickel | Umbilical cord blood lead=110 ng/mL vs 57 ng/mL, cadmium=2·50 ng/mL vs 0·33 ng/mL (p<0·001), no difference of nickel and chromium among groups (p>0·05). Umbilical cord blood 8-OHdG=162 ng/mL vs 154 ng/mL (p>0·05). 8-OHdG positively associated with cadmium (β=0·13, 95% CI 0·05 to 0·20), chromium (β=0·09, 95% CI 0·01 to 0·16), and nickel (β=0·21, 0·11 to 0·32; all p<0·05). |
| Zhou et al (2013)85 | Cross-sectional: exposed town vs reference town, China | 46 parturient women (mean age 27·82) | 44 parturient women (mean age 24·89) | Not assessed | Increased MDA, suppressed SOD in maternal serum, umbilical cord serum, placentas, and umbilical cord among exposed (p<0·05). GPx decreased in placentas and umbilical cord in exposed (p<0·05). MDA, SOD, and GPx in maternal serum associated with umbilical cord serum (r=0·90, r=0·86, r=0·85; all p<0·01), MDA, SOD, GPx in placentas associated with umbilical cords (r=0·89, r=0·96, r=0·77; all p<0·01). |
| Xu et al (2018)32 | Cross-sectional: e-waste recycling area, China | 118 preschool children aged: 3–6 years | None | Lead, cadmium, and mercury | Blood lead=7·43 μg/dL, blood cadmium=0·72 μg/L, blood mercury=11·13 μg/L, median 8-OHdG=407·79 ng/g cre, median mRNA expression level of hOGG1=0·038. Elevated blood lead (quartiles 2–4) had higher 8-OHdG (βQ2–Q4=0·31–0·36; p<0·05) than low blood lead (quartile 1). No correlation between blood cadmium and 8-OHdG (p>0·05), elevated blood mercury (quartile 2) correlated with 8-OHdG than low blood mercury (βQ2=0·23; p<0·05). |
| Li et al (2013)77 | Cross-sectional: exposed region vs reference region, China | 23 rural residents (mean age 32·6 years) | 28 rural residents (mean age 33·2 years) | PCBs, PBDEs, PBB, DP, HCB, β-HCH, and p,pʹ-DDE | PCBs=60·4 ng/g lipid vs 28·4 ng/g lipid, DP=9·0 ng/g lipid vs 2·8 ng/g lipid, PBB-153=0·55 ng/g lipid vs 0·25 ng/g lipid (all p<0·01). Increased ROS levels in WBC and NG, lower ROS in respiratory burst of NG among exposed (data not shown; p<0·001). Positive correlation between PCBs and ROS in WBC, NG (R=0·30 and R=0·31; p<0·05), inverse correlation between ROS in respiratory burst and PCBs (R=−0·45; p<0·01), no relation between ROS and PBDEs, DP, PBB153 (p>0·05). |
| Lu et al (2016)62 | Cross-sectional: e-waste exposed town vs rural reference vs urban reference town, China | 130 local residents aged 0·4–87 years | 24 rural residents and 22 urban residents aged 0·4–87 years | PAH | Urinary ∑10OH-PAHs=25·4 μg/g cre vs 11·7 μg/g cre vs 10·9 μg/g cre, 8-OHdG=16·2 μg/g cre vs 12·3 μg/g cre vs 11·6 μg/g cre, MDA=47·9 μg/g cre vs 36·1 μg/g cre vs 31·3 μg/g cre (all p<0·05). 8-OHdG significantly increased with ∑10OH-PAHs (β=0·35, 95% CI 0·21 to 0·49) and individual OH-PAHs (β=0·10–0·35; p<0·05), urinary 1-PYR correlated with MDA (r=0·28; p<0·01) in exposed group. |
| Lu et al (2017)84 | Cross-sectional: e-waste exposed town vs rural vs urban reference town, China | 175 local residents aged 0·4–87 years | 29 rural residents and 17 urban residents aged 0·4–87 years | Cl-mOPs and NCl-mOP metabolites | Urinary ∑Cl-mOPs=1·7 ng/mL vs 0·93 ng/mL vs 0·56 ng/mL (p<0·05), ∑NCl-mOPs=1·5 ng/mL vs 0·60 ng/mL (p<0·05 for exposed vs rural) vs 0·96 ng/mL, most abundant mOPs=BCEP (Cl-mOP) and DPHP (NCl-mOP) increased among exposed than rural reference (p<0·05). Significant association between 8-OHdG and BCEP (r=0·50), BCIPP (r=0·48), DBP (r=0·21), and DPHP (r=0·44) in exposed site (all p<0·05). |
| Yang et al (2015)63 | Cross-sectional: e-waste recycling site, China | 116 rural residents (mean age 36·9 years) | None | PAHs | 1-HO-PYR=0·57 μg/g cre, HO-PHEs=2·2 μg/g cre, HO-FLU=5·0 μg/g cre, HO-BPs=7·0 μg/g cre, HO-NAPs=16·6 μg/g cre. Urinary MDA and 8-OHdG=74·7 μg/g cre and 185 μg/g cre. Positive association between MDA and hydroxy-PAH (1-HO-PYR [β=0·40], HO-PHEs [β=0·48], HO-FLUs [β=0·35], HO-BPs [β=0·48], HO-NAPs [β=0·28]; all p<0·001), no correlation between 8-OHdG and hydroxy-PAH (p>0·05). |
| Zhang et al (2019)81 | Cross sectional: exposed vs reference village, China | 124 local residents aged 0·4–87 years | 22 local residents aged 0·4–87 years | PAEs | Urinary ∑mPAE=248 ng/mL vs reference (data not shown; p<0·05), higher mCMHP, mEHHP, mEHP, mMP, mEP in exposed group (p<0·05). Positive correlation between mECPP, mCMHP, mEHHP, mEHP, mCPP, mBP, miBP, mMP (8 of 11 mPAEs) and 8-OHdG (r=0·18–0·36; p<0·05). |
| Zhang et al (2019)33 | Cross sectional: exposed town vs rural reference, China | 139 local residents aged 0·4–87 years | 26 local residents aged 0·4–87 years | Lead, cadmium, mercury, arsenic, cobalt, manganese, copper, zinc, thallium, tin, antimony, selenium, and aluminium | Urinary lead=4·98 ng/mL vs 1·23 ng/mL, cadmium=2·12 ng/mL vs 1·33 ng/mL, copper=22·2 ng/mL vs 16·9 ng/mL, antimony=0·20 ng/mL vs 0·11 ng/mL, arsenic=46·6 ng/mL vs 62·0 ng/mL (p<0·05). Urinary 8-OHdG positively correlated with all metals (except manganese and aluminium) in exposed group (r=0·324–0·710; p<0·01), high correlation coefficient between highly toxic arsenic, mercury, lead, cadmium and 8-OHdG (r=0·45–0·61; p<0·01). |
| Zhang et al (2016)82 | Cross sectional: exposed villages vs rural reference village vs urban reference village, China | 116 local residents aged 0·4–87 years | 22 rural residents and 20 urban residents aged 0·4–87 years | BPA and 7 BPs | Urinary BPA=2·99 ng/mL vs 0·59 ng/mL vs 0·95 ng/mL (p<0·01), BPS=0·36 ng/mL vs 0·39 ng/mL (p>0·05 for exposed vs rural) vs 0·65 ng/mL, BPF=0·35 vs 0·09 (p<0·01 for exposed vs rural) vs 0·56 ng/mL, urinary 8-OHdG=8·00 ng/mL vs 6·84 ng/mL vs 7·31 ng/mL (p value not shown). 8-OHdG positively correlated with BPA (r=0·41) and BPS (r=0·39) in exposed (both p<0·001), no relation with BPF (p>0·05). |
PBDE=polybrominated diphenyl ether. lw=lipid weight. wt=weight. CAT=catalase. GSTO1=glutathione S transferase omega-1. Cyt=cytochrome. BDE=brominated diphenyl ether. TRF=terminal restriction fragment. cre=creatinine. T/S ratio=telomere/single copy gene ratio. OR=odds ratio. BAI1=brain-specific angiogenesis inhibitor 1. CTNNA2=catenin cadherin-associated protein. ALAD=δ-aminolevulinic acid dehydratase. MDA=malondialdehyde. PCB=polychlorinated bisphenol. IL=interleukin. TNF=tumor necrosis factor. LINE-1=long interspersed nuclear element-1. POP=persistent organic pollutant. hOGG1=human repair enzyme 8-oxoguanine DNA glycosylase. HCB=hexachlorobenzene. HCH=hexachlorocyclohexane. ROS=reactive oxygen species. TR=TH receptor. IDI=iodothyronine deiodinase. 8-OHdG=8-hydroxy-2ʹ-deoxyguanosine. SOD=superoxide dismutase. GPx=glutathione peroxidase. WBC=white blood cell. NG=neutrophil granulocytes. PBB=polybrominated biphenyls. Cl-mOPs=chlorinated organophosphate metabolites. NCl-mOPs=non-chlorinated organophosphate metabolites. BCEP=bis(2-chloroethyl) phosphate. BCIPP=bis(1-chloro-2-propyl) phosphate. DBP=dibutyl phosphate. DPHP=diphenyl phosphate. PYR=pyrene. HO-PYR=hydroxypyrene. HO-PHEs=hydroxyphenanthrenes. HO-FLU=hydroxyfluorenes. HO-BPs=hydroxybiphenyls. HO-NAPs=hydroxynaphthalenes. PAH=polycyclic aromatic hydrocarbons. ∑OHPAH=total hydroxylated PAH. ∑mPAE=phthalate esters metabolites. mCMHP=mono-[(2-carboxymethyl)hexyl] phthalate. mEHHP=5mono-(2-ethyl-5-hydroxyhexyl) phthalate. mEHP=mono-2-ethylhexyl phthalate. mMP=mono-methyl phthalate. mEP=mono-ethyl phthalate. mECPP=mono-(2-ethyl-5-carboxypentyl) phthalate. mBP=mono-n-butyl phthalate. miBP=mono-(2-isobutyl) phthalate. mCPP=mono (3-carboxypropyl) phthalate. BP=bisphenol.
8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of DNA oxidative damage was measured as the outcome in eight studies.31, 32, 33, 62, 63, 81, 82, 84 Overall, the findings suggest that exposure to e-waste are associated with elevated oxidative stress. 8-OHdG concentrations were positively associated with elevated blood lead,32 hydroxylated PAHs,62 bis(2-chloro-isopropyl) phosphate, dibutyl phosphate, diphenyl phosphate,84 cadmium, chromium, nickel,31 phthalate esters,81 arsenic, mercury, lead, cadmium,33 and bisphenols-A (BPA)82 among e-waste exposed population. By contrast, some studies showed no statistical association between 8-OHdG and lead,31 cadmium,32 or hydroxy-PAHs.63 Li and colleagues77 found a possible linkage between PCBs and reactive oxygen species levels in immune cells which indicated higher oxidative stress in adults. Elevated malondialdehyde levels and decreases in both superoxide dismutase and glutathione peroxidase activities suggest that oxidative stress was higher among parturient women and their matching fetuses at an e-waste exposed site relative to referents (table 3).85
Respiratory outcomes were investigated in four studies in China where the data suggest that living in an e-waste exposed area might accelerate the respiratory symptoms of children aged 2–8 years.38, 39, 86, 87 In two studies, children exposed to e-waste had lower lung function levels including forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1).38, 87 Zeng and colleagues38 did not find a significant association between blood lead or cadmium, or the combined effects of blood lead and cadmium, and lung function, while each unit of haemoglobin (1 g/L) decline was associated with 5 mL decrease in FVC and 4 mL decrease in FEV1. However, elevated blood lead level (>5 μg/dL) was found as a risk factor for asthma (adjusted odds ratio 9·50, 95% CI 1·16–77·49).39 However, higher blood chromium and blood manganese in preschool children were associated with greater cough and wheeze, respectively.39 One study indicated that both birthweight and chest circumference might be good predictors for lung function levels which were positively associated.87 Zhang and colleagues86 found that severe PM2·5 pollution in an e-waste recycling area resulted in heavy individual PM2·5 chronic daily intake, and reduced Please add salivary agglutinin (SAG) level in exposed children. Furthermore, ambient PM2·5 pollution reduces airway antimicrobial activity by down-regulating saliva SAG levels, which might accelerate airway pathogen infection. However, no correlation between saliva SAG level and proinflammatory cytokines was found (table 4).86
Table 4.
Respiratory, cardiovascular, and haematological changes resulting from exposure to electronic waste
| Exposure setting | Exposed population | Control population | Toxic chemicals | Health outcomes | |
|---|---|---|---|---|---|
| Respiratory | |||||
| Zeng et al (2017)38 | Cross sectional: exposed town vs reference town, China | 100 preschool children aged 5–7 years | 106 preschool children aged 5–7 years | Lead and cadmium | Blood lead=5·53 μg/dL vs 3·57 μg/dL (p<0·001), blood cadmium=0·58 μg/L vs 0·57 μg/L (p>0·05), lower Hb, HCT, higher platelet, thrombocytosis in exposed (all p<0·05). FVC=1·23 L vs 1·33 L, FEV1 =1·16 L vs 1·24 L (both p<0·01), FVC/FEV1=0·95% vs 0·96% (p>0·05). No association between blood lead, cadmium, platelet, and FVC, FEV1 (p>0·05). 1 g/L Hb decline associated with 5 mL and 4 mL decrease in FVC and FEV1, respectively (p<0·05). |
| Zeng et al (2017)87 | Cross sectional: exposed town vs reference town, China | 100 preschool children aged 5–7 years | 106 preschool children aged 5–7 years | Not assessed | FVC=1·23 L vs 1·33 L, FEV1=1·16 L vs 1·24 L (both p<0·05). Birthweight=3·07 kg vs 3·25 kg, height=111·03 cm vs 112·56 cm, chest circumference=52·63 cm vs 53·42 cm (all p<0·05). Lung function associated with birthweight (FVC β=0·13, FEV1 β=0·15), chest circumference (FVC β=0·16, FEV1 β=0·15; all p<0·05). |
| Zeng et al (2016)39 | Cross sectional: exposed town vs reference town, China | 300 children aged 3–8 years | 170 children aged3–8 years | Lead, cadmium, chromium, and manganese | Blood lead=6·24 μg/dL vs 4·75 μg/dL, blood cadmium=0·57 μg/L vs 0·50 μg/L, lead in PM2·5=153 ng/m3vs 80 ng/m3, cadmium in PM2·5=5·58 ng/m3vs 3·48 ng/m3 (all p<0·05), no differences in blood chromium and manganese (p>0·05). Increased cough, dyspnoea, phlegm, wheeze in exposed (p<0·05). Blood chromium associated with cough (AOR=1·91, 95% CI 1·17 to 3·13), Blood manganese associated with wheeze (AOR 2·91, 95% CI 1·09 to 7·78), elevated blood lead (>5 μg/dL) associated with asthma (AOR 9·50, 95% CI 1·16 to 77·49; all p<0·05). |
| Zhang et al (2019)86 | Cross sectional: exposed town vs reference town, China | 110 preschool children aged 2–7 years | 112 preschool children aged 2–7 years | Not assessed | PM2·5=39·06 μg/m3vs 26·68 μg/m3, PM2·5 CDI=1·40 ng/kg per day vs 0·88 ng/kg per day (p<0·001). Saliva SAG=5·05 ng/mL vs 8·68 ng/mL, CDI negatively correlated with saliva SAG level (B=−1·21, 95% CI −2·29 to −0·13; p<0·05). Elevated white blood cells, neutrophils, monocytes, IL-8, and TNF-α in exposed (p<0·001), higher monocyte count associated with lower saliva SAG level (B=−6·25, 95% CI −11·76 to −0·75; p<0·05). |
| Cardiovascular | |||||
| Lu et al (2018)47 | Cross-sectional: exposed town vs reference town, China | 337 preschool children aged 3–7 years | 253 preschool children aged 3–7 years | Lead | Blood lead=7·14 vs 3·91 μg/dL; p<0·001, elevated SBP, PP, TG, LDL/HDL and Tc/HDL ratio, lower HDL (all p<0·05). Higher Lp-PLA2=93·29 ng/mL vs 79·65 ng/mL, IL-6=10·00 pg/mL vs 1·61 pg/mL, IL-8=2·38 pg/mL vs 1·59 pg/mL, and TNF-α=2·36 pg/mL vs 1·86 pg/mL (all p<0·05). Elevated blood lead associated with higher Lp-PLA2, IL-6 (rs=0·20 and rs=0·59), (TG B=0·08, 95% CI 0·02 to 0·14) and lower HDL (B=−0·07, 95% CI −0·12 to −0·01), PP (B=−3·10, 95% CI −1·37 to −1·82; all p<0·05). Lp-PLA2 negatively associated with PP and HDL (B=−2·09 and B=−0·05; p<0·01). |
| Zheng et al (2019)48 | Cross-sectional: exposed town vs reference town, China | 105 preschool children aged 3–7 years | 98 preschool children aged 3–7 years | Lead and PAHs | Blood lead=7·23 μg/dL vs 3·91 μg/dL and elevated urinary ∑OHPAHs, ∑OHNap and ∑OHFlu in exposed group (all p<0·05). Increased monocytes, neutrophils, leukocytes, serum S100A8/A9 and IL-6, IL12p70, IP-10, CD4+ T cell percentage in exposed. Elevated blood lead, urinary 2-OHNap and ∑OHFlu associated with higher levels of IL-6, IL12p70, IP-10, CD4+ T cell percentage, neutrophil and monocyte counts (all p<0·05). |
| Cong et al (2018)88 | Cross-sectional: exposed town vs reference town (and non-native), China | 228 preschool children aged 3–6 years | 104 native and 91 non-native preschool children aged 3–6 years | PM2·5, PM10, SO2, NO2, CO, and O3 | Higher concentrations of PM2·5, PM10, SO2, NO2, CO among exposed (data not shown, all p<0·001). Median heart rate=106 bpm vs 102 bpm and 100 bpm, plasma norepinephrine=4·42 nmol/L vs 3·88 nmol/L and 3·44 nmol/L (both p<0·01). Positive association between PM2·5, PM10, SO2, NO2and plasma norepinephrine, PM2·5, PM10, SO2, NO2,CO related to increase heart rate (p<0·05). |
| Gangwar et al (2019)58 | Cross-sectional: exposed vs residential, commercial, and vehicular vs residential, India | 28 local adult residents aged >18 years | 50 adults from residential, commercial, and vehicular sites and 54 adults from residential sites (both groups aged >18 years) | PM10, lead, copper, zinc, nickel, and chromium | PM10=243 μg/m3vs 233 μg/m3vs 193 μg/m3. Elevated zinc, lead, chromium, nickel, copper in blood, and PM10 among exposed, positive correlation between blood and air heavy metals (r=0·58–0·98; p<0·05). HTN=68% vs 44% vs 32% (p<0·05), positive correlation between ambient PM10with mean SBP and DBP (r=0·62 and 0·67; p<0·05), elevated PM10 related to low SpO2 (r=−0·78; p<0·05). BMI and HTN positively correlated (data not shown). |
| Burns et al (2016)89 | Cross-sectional: e-waste recycling activity, Ghana | 57 e-waste recyclers, aged 18–61 years | None | Not assessed | High exposures to noise=43·5%, moderate to high levels of stress: mean PSS score=25 of 40. Positive correlation between noise and heart rate (ρ=0·46; p<0·001), 1 dB increase in noise associated with a 0·17 increase in heart rate (p<0·01). |
| Haematological | |||||
| Dai et al (2017)43 | Cross-sectional: exposed town vs reference town, China | 332 preschool children aged 2–6 years | 152 preschool children aged 2–6 years | Lead | Blood lead=6·5 μg/dL vs 4·5 μg/dL, EPb=17·0 μg/dL vs 11·9 μg/dL (both p<0·001), lower median erythrocyte CR1 expression=6257 vs 8163 (p<0·01). Elevated erythrocyte lead and blood lead negatively associated with HCT, MCV, Hb, MCH, and MCHC (all p<0·05). High blood lead (>7·00) and erythrocyte lead (>18·6) associated with lower erythrocyte CR1 expression (βQ4=−0·16, 95% CI −0·32 to −0·008) and (βQ4=−0·19, 95% CI −0·35 to −0·03; both p<0·05). |
| Zeng et al (2018)44 | Cross sectional: exposed town vs reference town, China | 331 preschool children aged 3–7 years | 135 preschool children aged 3–7 years | Lead | Blood lead=5·64 μg/dL vs 3·68 μg/dL (p<0·01), higher median PLT, PCT, MPV, P-LCR level among exposed (p<0·01). Positive correlation between blood lead and PLT (r=0·10), PCT (r=0·12), MPV (r=0·11), P-LCR (r=0·09), child residence in exposed associated with PLT (R2=0·07), PCT (R2=0·11), MPV (R2=0·03; all p<0·05). |
| Zhang et al (2017)45 | Cross sectional: exposed group vs reference group, China | 153 preschool children aged 3–7 years | 141 preschool children aged 3–7 years | Lead and cadmium | Blood lead=10·34 μg/dL vs 8·30 μg/dL, blood cadmium=2·39 μg/L vs 1·79 μg/L (both p<0·001). Higher mean monocytes, eosinophils, neutrophils, basophils, and lower NK cells among exposed (all p<0·05). Blood lead escalated counts in monocytes (β=0·08), eosinophils (β=0·08), basophils (β=0·01), monocyte percentage (β=0·77) and decline neutrophils percentage (β=−4·15). Blood cadmium increase neutrophils percentage and counts (β=3·92 and 0·66; all p<0·05). |
| Dai et al (2019)64 | Cross-sectional: exposed area vs reference area China | 118 preschool children aged 2–7 years | 121 preschool children aged 2–7 years | PAHs | Urinary ∑OH-PAHs=3·05 μg/mmol cre vs 1·76 μg/mmol cre, ∑OHNa=1·48 μg/mmol cre vs 0·75 μg/mmol cre, ∑OHPh=0·94 μg/mmol cre vs 0·62 μg/mmol cre (all p<0·001). Increased cytokines (IL-1β=0·43 pg/mL vs 0·25 pg/mL, IP-10=28·5 pg/mL vs 25·5 pg/mL), lymphocyte ratio, platelet count, PCT and PLR in exposed (all p<0·01). ∑OH-PAHs negatively associated with MPV, PDW, P-LCR, MPVP and positively associated with platelet count, PLR, ∑OHNa positively associated with IL-1β mediated through MPV, PDW, P-LCR, PLR (all p<0·05). |
| Xu et al (2015)70 | Cross-sectional: exposed town vs control town, China | 40 local residents aged 15–65 years | 15 local residents aged 15–65 years | PCBs and PBDEs | ∑PCBs=964 ng/g vs 68 ng/g (p<0·0001), ∑PBDEs 139 ng/g vs 76 ng/g (p>0·05). Lower monocyte, lymphocyte and higher neutrophil, Hb, platelets among exposed group (all p<0·05). ∑PCBs negatively correlated with monocyte (r=−0·67), lymphocyte (r=−0·38) and positively correlated with neutrophils (r= 0·58), Hb (r=0·35), ∑PBDEs positively correlated with WBC (r=0·34), Hb (r=0·34), and platelets (r=0·37). |
| Chen et al (2019)46 | Experimental: exposed vs reference groups, China | 158 hospitalised patients aged 4–85 years | 109 hospitalised patients aged 4–85 years | Lead and cadmium | Blood lead=8·7 μg/dL vs 5·1 μg/dL (p<0·001), cadmium=2·1 μg/L vs 2·6 μg/L (p>0·05), RBC=4·5 × 103 cell per μL vs 4·2 × 103 cells per μL, Hb=137·0 g/dL vs 123·0 g/dL (both p<0·05), platelets (p>0·05). Blood lead positively correlated with blood cadmium (r=0·11; p<0·05). Positive correlation between blood lead and RBC (r=0·17), Hb (r=0·12, both p<0·05). |
Hb=haemoglobin. HCT=haematocrit. FVC=forced vital capacity. FEV1=forced expiratory volume in 1 s. AOR=adjusted odds ratio. SAG=salivary agglutinin. CDI=chronic daily intake. IL=interleukin. TNF=tumour necrosis factor. SBP=systolic blood pressure. PP=pulse pressure. Tc=total cholesterol. TG=triglyceride. Lp-PLA2=lipoprotein-associated phospholipase A2. PAH=polycyclic aromatic hydrocarbon. ∑OHPAH =total hydroxylated polycyclic aromatic hydrocarbon. ∑OHNap=total hydroxylated naphthalene. ∑OHFlu=total hydroxylated fluorene. Bpm=beats per min. HTN=hypertension. PSS=perceived stress scale. SpO2=blood oxygen level. dB=decibel. DBP=diastolic blood pressure. BMI=body-mass index. EPb=erythrocyte lead. CR1=complement receptor. MCV=mean corpuscular volume. MCH= mean corpuscular haemoglobin. MCHC=mean corpuscular haemoglobin concentration. PLT=platelet count. PCT=plateletcrit. cre=creatinine. MPV=mean platelet volume. P-LCR=platelet large cell ratio. NK=natural killer. PLR=platelet count to lymphocyte count. PDW=platelet distribution width. MPVP=mean platelet volume to platelet count. ∑PBDEs=polybrominated diphenyl ether. RBC=red blood cells.
Six studies investigated haematological function as an outcome where lead, cadmium, PCBs, PBDEs, and PAHs were the primary chemical exposures with high concentrations among exposed preschool children,43, 44, 45, 64 local residents,70 and hospitalised patients.46 Two studies revealed that lead and PAH exposure were risk factors related to platelet indices among preschool children.44, 64 Similarly, blood and erythrocyte lead exposure was related to disadvantageous changes in red blood cells indices and haemoglobin in children from an e-waste recycling area.43 Zhang and colleagues45 concluded that the alteration of the number and percentage of innate immune cells were linked to higher lead and cadmium levels among e-waste exposed children. One study enrolled hospitalised patients from exposed and reference area where lead levels were correlated with elevated haematological parameters (red blood cells and haemoglobin levels).46 Native residents in an e-waste dismantling environment had increased body burden of PCBs and specific PBDE congeners which contributed to abnormal changes in haematological markers (table 4).70
Findings from five cross-sectional studies47, 48, 58, 88, 89 reported cardiovascular-related outcomes from e-waste exposure. Exposure to e-waste increased toxic chemical levels and concomitant abnormal measures of cardiovascular physiology. Three studies investigated cardiovascular risk in preschool children where vascular inflammation and lipid disorder were exacerbated by lead and PAH exposure47, 48 and air pollutants resulted in increased heart rate and plasma norepinephrine in participants from e-waste recycling areas.88 In India, e-waste burning contributed to severe air pollution, potentially explaining alarming levels of heavy metals in adult residents, which were associated with increased prevalence of cardiovascular morbidity, specifically hypertension.58 Moreover, noise exposure was associated with increased heart rate in Ghanaian adults (table 4).89
We identified three studies49, 50, 51 that included the effect of heavy metals from e-waste on immune system responsiveness of young children after vaccination. Significantly elevated blood lead and lower antibody titres of vaccines were reported among exposed children than those from the reference group in all three studies.49, 50, 51 Children chronically exposed to lead had suppressed antibody titres, indicating reduced immune responsiveness against diphtheria, pertussis, tetanus, Japanese encephalitis, polio,49 and hepatitis B.49, 51 However, no significant correlation was found between blood lead and anti-measles, mumps, or rubella antibody titres (table 5).50
Table 5.
Vaccine, olfactory, reproductive, and other health effects from exposure to electronic waste
| Exposure setting | Exposure setting | Control population | Toxic chemicals | Health outcomes | |
|---|---|---|---|---|---|
| Vaccine | |||||
| Lin et al (2017)49 | Cross-sectional: exposed town vs reference town, China | 157 preschool children aged 3–7 years | 127 preschool children aged 3–7 years | Lead, zinc, arsenic, mercury, cadmium, chromium, copper, manganese, and selenium | Blood lead=9·43 μg/dL vs 6·79 μg/dL (p<0·001), elevated essential elements (manganese, copper, zinc, chromium; p<0·05) in exposed group. Lower antibody titres of diphtheria, pertussis, tetanus, Japanese encephalitis, polio, measles (all p<0·05), hepatitis B (p>0·05). Significant association between antibody titres and elevated lead (OR=0·31–0·45), copper (OR=0·47–0·60), and zinc (OR=0·48–0·56; all p<0·05). |
| Lin et al (2016)50 | Cross-sectional: exposed town vs reference town, China | 263 preschool children aged 2–7 years | 115 preschool children aged 2–7 years | Lead | Blood lead=5·61 μg/dL vs 3·57 μg/dL (p<0·001). Lower antibody titres (median measles Ab=669 mIU/mL vs 1047 mIU/mL, mumps Ab=272 U/mL vs 492 U/mL, rubella Ab=37·08 IU/mL vs 66·50 IU/mL; all p<0·001). Anti-measles Ab titre positively associated with anti-mumps and rubella (r=0·16 and 0·37; p<0·01), Positive correlation between anti-mumps and anti-rubella Ab titres (r=0·17; p<0·01). No correlation between blood lead and anti-MMR Ab titres (p>0·05). |
| Xu et al (2015)51 | Cross-sectional: exposed town vs reference town, China | 301 kindergarten children (mean age 4·77 years) | 289 kindergarten children (mean age 4·47 years) | Lead | Blood lead=6·76 μg/dL vs 6·05 μg/dL (p<0·01; 2011=8·76 μg/dL vs 7·89 μg/dL, 2012=5·83 μg/dL vs 4·61 μg/dL; both p<0·001), median HBsAb titres=1·04 s/co vs 4·06 s/co; p<0·001; 2011=0·83 s/co vs 4·64 s/co, 2012=1·31 s/co vs 3·80 s/co; p<0·001). HBsAb titres negatively associated with blood lead (β=−0·45 in 2011 and β=−0·37 in 2012; p<0·001). |
| Auditory and olfactory | |||||
| Liu et al (2018)52 | Cross-sectional: exposed town vs reference town, China | 146 preschool children aged 3–7 years | 88 preschool children aged 3–7 years | Lead and cadmium | Blood lead=4·94 μg/dL vs 3·85 μg/dL (p<0·001), urinary cadmium=2·49 μg/g cre vs 1·80 μg/g cre (p>0·05). Hearing loss=28·8% vs 13·6% (p<0·001). Hearing loss for lead exposure: AOR=1·24 (95% CI 1·03 to 1·49). |
| Xu et al (2020)37 | Cross-sectional: exposed town vs reference town, China | 68 preschool children aged 3–7 years | 48 preschool children aged 3–7 years | Lead and cadmium | Blood lead=5·29 vs 3·63 μg/dL; p<0·001, urinary cadmium=1·52 vs 1·21 μg/g cre; p>0·05, hearing loss (>25 dB)=50·0% vs 20·8%. AOR of lead for hearing loss=1·40 (95% CI 1·06 to 1·84). |
| Zhang et al (2017)53 | Cross sectional: exposed town vs reference town, China | 61 preschool children aged 4–7 years | 57 preschool children aged 4–7 years | Lead | Blood lead=9·40 mg/dL vs 5·04 mg/dL, serum BDNF=35·91 ng/mL vs 28·10 ng/mL (both p<0·001), IGF-1=170 vs 154 ng/mL (p>0·05), BDNF positively correlated with blood lead (β=0·68; p<0·01). Lower item and source olfactory memory scores (at 15 min, 5 h, and 24 h) among exposed (p<0·01), and negatively correlated with blood lead (β=−0·29 to −0·16; p<0·05), BDNF (−0·23 to −0·19; p<0·05). |
| Reproductive | |||||
| Yu et al (2018)76 | Exploratory: exposed town vs hospital bank, China | 32 local adult men (mean age 38·7 years) | 25 local adult men (mean age 36·0 years) | PBDE | BDE-28=5·02 pg/g vs 1·62 pg/g, BDE-47=6·75 pg/g vs 1·32 pg/g, BDE-153=7·36 pg/g vs 3·62 pg/g, (p<0·05) in semen, lower sperm count, sperm progressive motility among exposed, tail DNA (comet assay)=57·88% vs 33·55%, apoptosis rate (TUNEL assay)=32% vs 20% (all p<0·05). Inverse correlation between sperm concentration and count with BDE-47 (β=−0·29 and −0·40; p<0·05), sperm progressive motility ([A+B]%) and sperm viability negatively correlated with BDE-100 in dust (β=−0·36 and −0·11; p<0·05), positive correlation between BDE-28, BDE-47, BDE-153 and paired semen samples (rs=0·36–0·54; p<0·05). |
| Wang et al (2018)54 | Cross-sectional: exposed town vs reference town, China | 146 local male residents (mean age 35·8 years) | 121 local male residents (mean age 34·9 years) | Lead, copper, zinc, iron, calcium, magnesium, selenium, and PCBs | Higher blood lead, PCBs, MDA, and lower calcium, magnesium, SOD, GSH among exposed (p<0·05, data not shown), MDA, lead, calcium, magnesium and DNA damage associated with the duration of exposure (p<0·05, data not shown). DNA damage in lymphocytes and spermatozoa (TDNA%, TM, OTM by comet assay), DNA aberrations (CA=8·01 vs 1·80% and CBMN=26·30% vs 4·52%) greater in exposed (all p<0·01). Semen volume=1·39 mL vs 2·52 mL, motility rate=45·01% vs 58·48% and reduced sperm count among exposed (p<0·05). Exposure duration, PCBs, MDA, and lead revealed risk factors of semen quality (all p<0·05). 13 genes expression of mRNA upregulated and 7 genes downregulated. |
| Hepatic | |||||
| Chen et al (2019)46 | Cross-sectional: exposed vs reference groups, China | 158 hospitalised patients aged 4–85 years | 109 hospitalised patients aged 4–85 years | Lead and cadmium | Blood lead=8·7 μg/dL vs 5·1 μg/dL (p<0·001), cadmium=2·1 μg/L vs 2·6 μg/L (p>0·05), GGT=68·0 vs 26·0 (p<0·001), no difference of AST, ALT, AST/ALT, LDH among groups (p>0·05). Blood lead positively correlated with cadmium (0·117; p<0·05). Positive correlation between blood lead and ALT (r=0·111; p<0·05), Blood cadmium correlated with AST (r=0·22) and ALT (0·21; both p<0·001). Elevated blood lead (≥5 μg/dL) inducing abnormal liver function (AOR=1·94, 95% CI 1·00 to 3·73). |
| Renal | |||||
| Xu et al (2015)70 | Cross-sectional: exposed town vs control town, China | 40 local residents aged 15–65 years | 15 local residents aged 15–65 years | PCB and PBDE | ∑PCBs=964 ng/g vs 68 ng/g (p<0·001), ∑PBDEs 139 ng/g vs 75 ng/g (p>0·05). Serum creatinine=87·05 μmol/L vs 74·49 μmol/L, β2-MG=0·25 mg/L vs 0·18 mg/L (both p<0·001). ∑PCBs positively correlated with serum creatinine (r=0·40) and β2-MG (r=0·70; both p<0·01). |
| Oral | |||||
| Hou et al (2020)55 | Cross-sectional: exposed town vs control town, China | 357 preschool children aged 2·5–6 years | 217 preschool children aged 2·5–6 years | Lead | Blood lead=4·86 μg/dL vs 3·47 μg/dL, IL-6=6·96 pg/mL vs 2·76 pg/mL, and TNF-α=6·51 pg/mL vs 1·29 pg/mL (all p<0·05). Lower salivary sialic acids=9·58 mg/dL vs 17·57 mg/dL (p<0·05), dental caries=62·5% vs 53·9% (p<0·05). Negative association between blood lead and salivary sialic acid (B=−5·59, 95% CI −9·62 to −1·55), in mediation analysis, inverse correlation between blood lead and salivary sialic acid through IL-6 (B=−0·95, 95% CI −1·70 to −0·20). |
| Metabolic | |||||
| Song et al (2019)83 | Cross-sectional: exposed villages vs reference village, China | 119 elderly residents aged 56–93 years | 16 elderly residents aged 56–93 years | BPA and 6 alternatives | Serum BPA=3·2 ng/mL vs 2·8 ng/mL (p<0·05), dominant BPA alternatives=BPF (71%), BPAP (13%), BPAF (8%), BPS (7%). Abnormal FBG (<3·9 mmol/L or >6·1 mmol/L)=45% vs 31% (p<0·05), and associated with BPA (data not shown; p<0·05), high BPAF negatively correlated with low FBG (r=−0·30; p<0·001). |
OR=odds ratio. Ab=antibody. MMR=measles, mumps, and rubella. HBsAb=hepatitis B surface antibody. cre=creatinine. AOR=adjusted OR. BDNF=brain derived neurotrophic factor. IGF-1=insulin-like growth factor. PBDE=polybrominated diphenyl ether. BDE=brominated diphenyl ether. TUNEL=TdT-mediated dUTP Nick-End Labelling. PCB=polychlorinated bisphenol. MDA=malondialdehyde. SOD=superoxide dismutase. GSH=glutathione. TDNA=DNA in the comet tail. CA=chromosome aberrations. TM=tail moment. OTM=olive tail moment. CBMN=cytokinesis-block micronucleus. GGT=gamma glutamyl transpeptidase. AST=aspartate aminotransferase. ALT=alanine aminotransferase. β2-MG=β2-microglobulin. BPAP=bisphenol AP. BPAF=bisphenol AF. BPS=bisphenol S. FBG=fasting blood glucose.
In three studies,37, 52, 53 hearing function was estimated concerning lead and cadmium exposures among preschool children. Exposed children had a higher prevalence of hearing loss than did reference children.37, 52 Lead concentration was significantly higher among exposed children than the reference group37, 52, 53 while no difference was found in urinary cadmium.37, 52 Two studies found hearing loss was more likely due to lead exposure (adjusted odds ratio 1·24, 95% CI 1·03–1·49 and 1·40, 1·06–1·84).37, 52 Zhang and colleagues53 observed lower olfactory memory scores after odour exposure among children exposed to e-waste, which were negatively correlated with blood lead and serum brain-derived neurotrophic factor levels. Two studies identified that lower semen quality aggravated DNA damage (by comet assay) in e-waste exposed individuals compared with individuals from a reference site.54, 76 Semen quality parameters were negatively correlated with PBDE congeners in semen samples76 and exposure duration from e-waste has a strong association with genomic instability among adult men (table 5).54
Chen and colleagues46 showed elevated hepatic parameters in patients from the exposed group compared with reference groups. Both blood lead and cadmium were positively associated with alanine aminotransferase. Likewise, elevated blood lead (≥5 μg/dL) induced abnormal liver function (adjusted odds ratio 1·94, 95% CI 1·00–3·73). Elevated serum creatinine and urinary β2-MG, which are clinical renal function indicators, were significantly higher among the exposed adult residents than the control group and they were positively correlated with serum PCBs.70 Increased levels of dental caries in deciduous teeth (62·5% vs 53·9%) and concomitantly lower salivary sialic acids were found in exposed preschool children when compared with preschool children from a reference town. Higher blood lead was adversely associated with salivary sialic acid levels indicating weakened oral anti-inflammatory ability.55 Elderly individuals living in e-waste recycling areas had elevated BPA levels, correlating with e-waste dismantling activities indicating that BPA exposure is associated with abnormal fasting blood glucose (ie, hyperglycaemia and hypoglycaemia; table 5).83
Discussion
The current systematic review is an update of our previous systematic review conducted in 2013 and contains new evidence regarding the health effects from e-waste exposures. The 2013 review contained 23 articles over a span of 47 years. Since then, 70 studies were published in 7 years. Most studies followed an ecological or retrospective cohort design that collected cases from exposure and referral sites. Ten studies were conducted in recycling sites and did not contain reference sites.
The toxic chemicals in e-waste can have a significant adverse impact on health of people living in exposed areas, particularly during sensitive windows of development such as pregnancy and childhood. Endocrine-disrupting chemicals (EDCs) such as phenols, phthalates, parabens, flame retardants, and heavy metals have potentially played adverse health impacts on human reproduction and development.90 EDC exposures modulate various physiological processes in pregnant women, exposing the developing fetus to maternal nutritional, chemical, and environmental stressors. Such early life exposure could compromise the early developmental processes and predispose the fetus to adverse health risks later in life.91 Moreover, the cumulative effect of EDC exposure is a considerable concern while humans are exposed to a multitude of EDCs at varying doses that might have additive, synergistic, or adverse biological effects.92, 93 We provide evidence of adverse birth outcomes associated with heavy metals and organic chemicals.20, 56, 60, 65, 66 Previous studies have also shown that early exposure to heavy metals and organic pollutants in utero can lead to foetal growth retardation.94, 95 Significant associations between PCBs and PBDEs and maternal serum THs indicate links between maternal health and infant development. Since the chemical structure of PCBs and PBDEs is similar to that of THs, these chemicals can disrupt the thyroid endocrine system,73 altered TH homoeostasis and induce neurotoxicity.96 Across multiple studies, we found a consistent association between the effects of e-waste exposure and placental transfer of toxic chemicals, bioaccumulation of chemicals, DNA methylation, sex hormone homoeostasis, and oxidative damage in pregnant women. EDC exposures are associated with changes in the gestational endocrine milieu, including altered levels of sex steroids,97 altered developmental trajectory of developing fetuses via epigenetic modifications,98 modulation of immune system, altered inflammatory cytokine milieu to favour a proinflammatory state,99 and might also affect circulating inflammation markers (interleukin-6, interleukin-10, c-reactive protein, and tumour necrosis factor-α).100 A consistent theme in the literature more broadly is that exposures in early life can potentiate detrimental effects and resulting in acute infections, morbidity, or even death in infancy and childhood, plus chronic conditions might deleteriously influence health trajectories in later life.101 However, we have found some conflicting findings; for example the effect of cadmium exposure on child growth and development. As cadmium accumulates with age, a plausible explanation is that children might be too young to manifest bone problems resulting from cadmium exposure.22
Children are particularly vulnerable compared with adults to environmental exposures due to additional exposure routes (eg, breastfeeding, placental exposure, and frequent hand-to-mouth behaviours), their higher basal metabolic rate and immature systems that might be unable to handle and excrete some toxic materials efficiently.12 Compared with adults, children also have more time to develop diseases that could be triggered by toxic chemicals in childhood and can evolve through multiple stages and years.102 Some studies we reviewed suggest extensive toxicological effects in multiple organ systems among children, including growth and neurodevelopment, endocrine, respiratory, cardiovascular, haematological, immune, and genetic dysfunction related to e-waste exposures. However, consistency of association could not be assessed due to diverse variations in chemical exposure and associated outcomes.103 Also, several risk factors such as age, nutritional status, and predisposing conditions could influence the impact of toxic chemicals on health outcomes to some extent. However, the overall body of research suggests that children who are still growing and developing have a substantial risk of harm, from individual chemicals derived from e-waste.17, 104
Our review has identified adverse health outcomes in relation to e-waste exposure in addition to those identified in the previous review. A few studies have identified a link between chemical exposure from e-waste and suppressed immune response,49, 50, 51 hearing loss,37, 52 altered hepatic and renal function,46, 70 decline in oral anti-inflammatory ability,55 and abnormal FBG.83
One of the strengths of the current systematic review is that most studies relied on biomarkers, which provide an objective measure of chemical exposure (ie, the internal dose). However, other methodological issues were identified in the previous review that remain unchanged in the current review. First, the current review did not contain any longitudinal, prospective studies that establish any temporality of associations. Lack of long-term studies also affects data on diseases that have long latency periods. Second, we did not include any studies exploring dose–response relationships. Third, the sample sizes in the studies included in the current review were only 45–590 people; these low sample sizes are a source of continuing concern. However, given that so many studies identified an association between toxicants and adverse health outcomes within such small sample sizes, the effect sizes of the associations are probably substantial. Fourth, many studies did not adjust for confounders, which reduces our ability to draw inferences between exposure with health outcome.105 For example, BPA exposure is associated with obesity but BPA is found in less nutritious and calorie-dense packaged foods that can also increase the risk of obesity. If the consumption of packaged foods is not adjusted, BPA exposure could be assumed to be a risk factor for oxidative damage or adverse metabolic outcomes where consumption packaged foods might likely be driving these adverse health outcomes.106 Another example is socioeconomic background as a confounding factor. Children from lower socioeconomic backgrounds are more vulnerable to adverse cognitive health outcomes than are those from higher socioeconomic backgrounds.107 The current review contains multiple studies where the exposed and control groups were chosen from non-random populations. It is possible that socioeconomic background factors are driving some of the adverse health consequences and not the exposure to harmful toxicants. Fifth, there are periods of heightened vulnerability for the chemically exposed populations that can overestimate the health effects.105 Sixth, most of the studies in the current review explored health outcomes related to isolated exposures. We do not know the cumulative and interactive effects of exposure to chemical mixtures, possibly owing to a high cost for multiple measurements.105 Also, no studies in this Review measure the attributable risk from different exposures which could mask the contribution of potentially predominant route of toxicant exposure such as dietary intake. Finally, more than 90% of the studies in this Review were undertaken in China, which limits the generalisability of findings. Future studies should therefore include longitudinal designs, methodologies that allow for exploration of exposure and dose-response relationships, larger sample sizes, adjustments for confounders, random sampling of study populations, and an exploration of health effects in relation to increasing and decreasing levels of vulnerability and chemical mixtures in addition to isolated chemical exposures. Furthermore, future studies can also sample toxicants from multiple sources to gauge the extent to which e-waste contributes to toxicity levels relative to other sources. Most importantly, future studies need to be conducted among more diverse populations in different countries across the world.
Addressing e-waste is in alignment with multiple UN Sustainable Development Goals pertaining to environmental and human health protection (targets 3.9, 6.1, and 6.3), reducing adverse environment impacts of cities (target 11.6), sound management of e-waste in accordance with agreed international frameworks (target 12.4), and protection of labour rights along with economic growth for vulnerable populations (targets 8.3 and 8.8). Alongside the Sustainable Development Goals, other initiatives that continue to address e-waste at an international policy level. For example, the Basel Convention regulates the transboundary movement of hazardous wastes, including e-waste, and obliges countries to ensure safe management and disposal of e-waste and step focuses on scientifically developing a globally accepted standard for e-waste refurbishment and recycling. WHO's Initiative on E-waste and Child Health aims to increase access to evidence, knowledge, and awareness of the health impacts of e-waste, improve health sector capacity to identify risks and track progress of good e-waste policies and interventions that protect public health. Furthermore, initiatives within the context of developed countries are moving towards circular economy strategies that are increasingly focusing on aspects of upscale design and production aspects rather than curative aspects of e-waste management.4 These initiatives include emphasising consumer and producer-end responsibilities such as improving recycling habits and designing eco-friendly products.
Although 78 countries have identified policies, legislation, or regulation governing e-waste, these are not usually legally binding, and—where they are legally binding—enforcement is often a challenge.4 Ultimately, creating and enforcing policies to prevent the proliferation of e-waste is not nearly enough. In LMICs countries, policies and intervention focusing on curative strategies are imperative for tackling the proliferation of e-waste, both domestic and imported. Further initiatives need to explore cost-effective methods and appropriate technologies based on chemical toxicity for safe recycling operations, including metal recovery and improvement of disposal systems. Such approach should consider the economic benefits of value recovery processes while ensuring the health and safety of populations that depend on informal e-waste recycling for their livelihoods and survival.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We gratefully acknowledge the contribution from the libraries of icddr,b and the University of Queensland for this Review. M-NB is a staff member of WHO. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of the WHO. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support. The publication of this article was made possible with funding from Sida, Sweden. Data included in the tables were used to inform Table 2.1 in WHO's 2021 report on children and digital dumpsites: e-waste exposure and child health.108
Contributors
SMP and PDS conceived the work and did the analysis. SMP and ZI did the initial search and collected the articles. SMP and FJ independently reviewed articles and determined included articles. SMP was in charge of project administration. SMP drafted the original manuscript. All authors provided critical revision for important intellectual content and approved the final version to be published.
Supplementary Material
References
- 1.Kiddee P, Naidu R, Wong MH. Electronic waste management approaches: an overview. Waste Manag. 2013;33:1237–1250. doi: 10.1016/j.wasman.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zeng X, Chen M, Ogunseitan OA, Stevels A. “Control-alt-delete”: rebooting solutions for the e-waste problem. Environ Sci Technol. 2015;49:7095–7108. doi: 10.1021/acs.est.5b00449. [DOI] [PubMed] [Google Scholar]
- 3.UNEP . Basel Convention; Geneva, Switzerland: 2019. Technical guidelines on transboundary movements of electrical and electronic waste and used electrical and electronic equipment, in particular regarding the distinction between waste and non-waste under the Basel Convention. [Google Scholar]
- 4.Forti V, Balde CP, Kuehr R, Bel G. ISWA; Rotterdam: 2020. The Global E-waste Monitor 2020: quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)–co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association. [Google Scholar]
- 5.Sthiannopkao S, Wong MH. Handling e-waste in developed and developing countries: initiatives, practices, and consequences. Sci Total Environ. 2013;463–464:1147–1153. doi: 10.1016/j.scitotenv.2012.06.088. [DOI] [PubMed] [Google Scholar]
- 6.Tsydenova O, Bengtsson M. Chemical hazards associated with treatment of waste electrical and electronic equipment. Waste Manag. 2011;31:45–58. doi: 10.1016/j.wasman.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Matsukami H, Tue NM, Suzuki G, et al. Flame retardant emission from e-waste recycling operation in northern Vietnam: environmental occurrence of emerging organophosphorus esters used as alternatives for PBDEs. Sci Total Environ. 2015;514:492–499. doi: 10.1016/j.scitotenv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Heacock M, Kelly CB, Suk WA. E-waste: the growing global problem and next steps. Rev Environ Health. 2016;31:131–135. doi: 10.1515/reveh-2015-0045. [DOI] [PubMed] [Google Scholar]
- 9.Wong CS, Duzgoren-Aydin NS, Aydin A, Wong MH. Evidence of excessive releases of metals from primitive e-waste processing in Guiyu, China. Environ Pollut. 2007;148:62–72. doi: 10.1016/j.envpol.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Perkins DN, Brune Drisse MN, Nxele T, Sly PD. E-waste: a global hazard. Ann Glob Health. 2014;80:286–295. doi: 10.1016/j.aogh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Chan JKY, Wong MH. A review of environmental fate, body burdens, and human health risk assessment of PCDD/Fs at two typical electronic waste recycling sites in China. Sci Total Environ. 2013;463–464:1111–1123. doi: 10.1016/j.scitotenv.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 12.Song Q, Li J. A systematic review of the human body burden of e-waste exposure in China. Environ Int. 2014;68:82–93. doi: 10.1016/j.envint.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Hicks C, Dietmar R, Eugster M. The recycling and disposal of electrical and electronic waste in China—legislative and market responses. Environ Impact Assess Rev. 2005;25:459–471. [Google Scholar]
- 14.Huang K, Guo J, Xu Z. Recycling of waste printed circuit boards: a review of current technologies and treatment status in China. J Hazard Mater. 2009;164:399–408. doi: 10.1016/j.jhazmat.2008.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Li J, Liu L, Zhao N. Polybrominated diphenyl ethers fate in China: a review with an emphasis on environmental contamination levels, human exposure and regulation. J Environ Manage. 2012;113:22–30. doi: 10.1016/j.jenvman.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Schnoor JL, Zeng EY. E-waste recycling: where does it go from here? Environ Sci Technol. 2012;46:10861–10867. doi: 10.1021/es303166s. [DOI] [PubMed] [Google Scholar]
- 17.Grant K, Goldizen FC, Sly PD, et al. Health consequences of exposure to e-waste: a systematic review. Lancet Glob Health. 2013;1:e350–e361. doi: 10.1016/S2214-109X(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 18.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Ge J, Huo X, Zhang Y, Lau ATY, Xu X. Differential proteomic expression of human placenta and fetal development following e-waste lead and cadmium exposure in utero. Sci Total Environ. 2016;550:1163–1170. doi: 10.1016/j.scitotenv.2015.11.084. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Liu J, Huang C, Lu F, Chiung YM, Huo X. Association of polycyclic aromatic hydrocarbons (PAHs) and lead co-exposure with child physical growth and development in an e-waste recycling town. Chemosphere. 2015;139:295–302. doi: 10.1016/j.chemosphere.2015.05.080. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Huo X, Yekeen TA, Zheng Q, Zheng M, Xu X. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ Sci Pollut Res Int. 2013;20:4441–4447. doi: 10.1007/s11356-012-1366-2. [DOI] [PubMed] [Google Scholar]
- 23.Zeng X, Xu X, Qin Q, Ye K, Wu W, Huo X. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ Geochem Health. 2019;41:309–321. doi: 10.1007/s10653-018-0114-z. [DOI] [PubMed] [Google Scholar]
- 24.Cai H, Xu X, Zhang Y, Cong X, Lu X, Huo X. Elevated lead levels from e-waste exposure are linked to sensory integration difficulties in preschool children. Neurotoxicology. 2019;71:150–158. doi: 10.1016/j.neuro.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Xu X, Yekeen TA, Lin K, Li W, Huo X. Assessment of association between the dopamine D2 receptor (DRD2) polymorphism and neurodevelopment of children exposed to lead. Environ Sci Pollut Res Int. 2015;22:1786–1793. doi: 10.1007/s11356-014-2565-9. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Huo X, Liu D, Zeng X, Zhang Y, Xu X. S100β in heavy metal-related child attention-deficit hyperactivity disorder in an informal e-waste recycling area. Neurotoxicology. 2014;45:185–191. doi: 10.1016/j.neuro.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Huo X, Ho G, et al. Attention-deficit/hyperactivity symptoms in preschool children from an e-waste recycling town: assessment by the parent report derived from DSM-IV. BMC Pediatr. 2015;15:51. doi: 10.1186/s12887-015-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Zhang B, Lin K, Zhang Y, Xu X, Huo X. Thyroid disruption and reduced mental development in children from an informal e-waste recycling area: a mediation analysis. Chemosphere. 2018;193:498–505. doi: 10.1016/j.chemosphere.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Liu J, Zeng X, Lu F, Chen A, Huo X. Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from Guiyu, China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Lu XS, Li DL, Yu YJ. Effects of environmental lead pollution on blood lead and sex hormone levels among occupationally exposed group in an e-waste dismantling area. Biomed Environ Sci. 2013;26:474–484. doi: 10.3967/0895-3988.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Ni W, Huang Y, Wang X, Zhang J, Wu K. Associations of neonatal lead, cadmium, chromium and nickel co-exposure with DNA oxidative damage in an electronic waste recycling town. Sci Total Environ. 2014;472:354–362. doi: 10.1016/j.scitotenv.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Liao W, Lin Y, Dai Y, Shi Z, Huo X. Blood concentrations of lead, cadmium, mercury and their association with biomarkers of DNA oxidative damage in preschool children living in an e-waste recycling area. Environ Geochem Health. 2018;40:1481–1494. doi: 10.1007/s10653-017-9997-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Ruan J, Zhang B, et al. Heavy metals in human urine, foods and drinking water from an e-waste dismantling area: identification of exposure sources and metal-induced health risk. Ecotoxicol Environ Saf. 2019;169:707–713. doi: 10.1016/j.ecoenv.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Huo X, Peng L, Qiu B, Zheng L, Yekeen TA, Xu X. ALAD genotypes and blood lead levels of neonates and children from e-waste exposure in Guiyu, China. Environ Sci Pollut Res Int. 2014;21:6744–6750. doi: 10.1007/s11356-014-2596-2. [DOI] [PubMed] [Google Scholar]
- 35.Li K, Liu S, Yang Q, et al. Genotoxic effects and serum abnormalities in residents of regions proximal to e-waste disposal facilities in Jinghai, China. Ecotoxicol Environ Saf. 2014;105:51–58. doi: 10.1016/j.ecoenv.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Z, Huo X, Zhang Y, Hylkema MN, Wu Y, Xu X. Differential DNA methylation in newborns with maternal exposure to heavy metals from an e-waste recycling area. Environ Res. 2019;171:536–545. doi: 10.1016/j.envres.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Huo X, Liu Y, Zhang Y, Qin Q, Xu X. Hearing loss risk and DNA methylation signatures in preschool children following lead and cadmium exposure from an electronic waste recycling area. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2020.125829. [DOI] [PubMed] [Google Scholar]
- 38.Zeng X, Xu X, Boezen HM, Vonk JM, Wu W, Huo X. Decreased lung function with mediation of blood parameters linked to e-waste lead and cadmium exposure in preschool children. Environ Pollut. 2017;230:838–848. doi: 10.1016/j.envpol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Zeng X, Xu X, Zheng X, Reponen T, Chen A, Huo X. Heavy metals in PM2.5 and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environ Pollut. 2016;210:346–353. doi: 10.1016/j.envpol.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Cao J, Xu X, Zhang Y, Zeng Z, Hylkema MN, Huo X. Increased memory T cell populations in Pb-exposed children from an e-waste-recycling area. Sci Total Environ. 2018;616–617:988–995. doi: 10.1016/j.scitotenv.2017.10.220. [DOI] [PubMed] [Google Scholar]
- 41.Huo X, Dai Y, Yang T, Zhang Y, Li M, Xu X. Decreased erythrocyte CD44 and CD58 expression link e-waste Pb toxicity to changes in erythrocyte immunity in preschool children. Sci Total Environ. 2019;664:690–697. doi: 10.1016/j.scitotenv.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Huo X, Cao J, Yang T, Xu L, Xu X. Elevated lead levels and adverse effects on natural killer cells in children from an electronic waste recycling area. Environ Pollut. 2016;213:143–150. doi: 10.1016/j.envpol.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Dai Y, Huo X, Zhang Y, Yang T, Li M, Xu X. Elevated lead levels and changes in blood morphology and erythrocyte CR1 in preschool children from an e-waste area. Sci Total Environ. 2017;592:51–59. doi: 10.1016/j.scitotenv.2017.03.080. [DOI] [PubMed] [Google Scholar]
- 44.Zeng Z, Huo X, Zhang Y, Xiao Z, Zhang Y, Xu X. Lead exposure is associated with risk of impaired coagulation in preschool children from an e-waste recycling area. Environ Sci Pollut Res Int. 2018;25:20670–20679. doi: 10.1007/s11356-018-2206-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Xu X, Sun D, Cao J, Zhang Y, Huo X. Alteration of the number and percentage of innate immune cells in preschool children from an e-waste recycling area. Ecotoxicol Environ Saf. 2017;145:615–622. doi: 10.1016/j.ecoenv.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Xu X, Zeng Z, Lin X, Qin Q, Huo X. Blood lead and cadmium levels associated with hematological and hepatic functions in patients from an e-waste-polluted area. Chemosphere. 2019;220:531–538. doi: 10.1016/j.chemosphere.2018.12.129. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Xu X, Zhang Y, Zhang Y, Wang C, Huo X. Elevated inflammatory Lp-PLA2 and IL-6 link e-waste Pb toxicity to cardiovascular risk factors in preschool children. Environ Pollut. 2018;234:601–609. doi: 10.1016/j.envpol.2017.11.094. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, Huo X, Zhang Y, Wang Q, Zhang Y, Xu X. Cardiovascular endothelial inflammation by chronic coexposure to lead (Pb) and polycyclic aromatic hydrocarbons from preschool children in an e-waste recycling area. Environ Pollut. 2019;246:587–596. doi: 10.1016/j.envpol.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 49.Lin X, Xu X, Zeng X, Xu L, Zeng Z, Huo X. Decreased vaccine antibody titers following exposure to multiple metals and metalloids in e-waste-exposed preschool children. Environ Pollut. 2017;220:354–363. doi: 10.1016/j.envpol.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Xu X, Dai Y, Zhang Y, Li W, Huo X. Considerable decrease of antibody titers against measles, mumps, and rubella in preschool children from an e-waste recycling area. Sci Total Environ. 2016;573:760–766. doi: 10.1016/j.scitotenv.2016.08.182. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Chen X, Zhang J, et al. Decreased blood hepatitis B surface antibody levels linked to e-waste lead exposure in preschool children. J Hazard Mater. 2015;298:122–128. doi: 10.1016/j.jhazmat.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Huo X, Xu L, et al. Hearing loss in children with e-waste lead and cadmium exposure. Sci Total Environ. 2018;624:621–627. doi: 10.1016/j.scitotenv.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B, Huo X, Xu L, et al. Elevated lead levels from e-waste exposure are linked to decreased olfactory memory in children. Environ Pollut. 2017;231:1112–1121. doi: 10.1016/j.envpol.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Sun X, Fang L, et al. Genomic instability in adult men involved in processing electronic waste in Northern China. Environ Int. 2018;117:69–81. doi: 10.1016/j.envint.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Hou R, Huo X, Zhang S, Xu C, Huang Y, Xu X. Elevated levels of lead exposure and impact on the anti-inflammatory ability of oral sialic acids among preschool children in e-waste areas. Sci Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134380. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Xu X, Chen A, et al. Maternal urinary cadmium levels during pregnancy associated with risk of sex-dependent birth outcomes from an e-waste pollution site in China. Reprod Toxicol. 2018;75:49–55. doi: 10.1016/j.reprotox.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Lin S, Huo X, Zhang Q, et al. Short placental telomere was associated with cadmium pollution in an electronic waste recycling town in China. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gangwar C, Choudhari R, Chauhan A, Kumar A, Singh A, Tripathi A. Assessment of air pollution caused by illegal e-waste burning to evaluate the human health risk. Environ Int. 2019;125:191–199. doi: 10.1016/j.envint.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Guo C, Li X, et al. Associations between metal exposure and global DNA methylation in potentially affected people in E-Waste recycling sites in Taizhou City, China. Sci Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.135100. [DOI] [PubMed] [Google Scholar]
- 60.Huo X, Wu Y, Xu L, Zeng X, Qin Q, Xu X. Maternal urinary metabolites of PAHs and its association with adverse birth outcomes in an intensive e-waste recycling area. Environ Pollut. 2019;245:453–461. doi: 10.1016/j.envpol.2018.10.098. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Yekeen TA, Xiao Q, Wang Y, Lu F, Huo X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ Pollut. 2013;182:63–69. doi: 10.1016/j.envpol.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Lu SY, Li YX, Zhang JQ, et al. Associations between polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress in people living near e-waste recycling facilities in China. Environ Int. 2016;94:161–169. doi: 10.1016/j.envint.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Yang Q, Qiu X, Li R, Ma J, Li K, Li G. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environ Sci Pollut Res Int. 2015;22:1760–1769. doi: 10.1007/s11356-014-3284-y. [DOI] [PubMed] [Google Scholar]
- 64.Dai Y, Huo X, Cheng Z, Wang Q, Zhang Y, Xu X. Alterations in platelet indices link polycyclic aromatic hydrocarbons toxicity to low-grade inflammation in preschool children. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.105043. [DOI] [PubMed] [Google Scholar]
- 65.Li M, Huo X, Pan Y, Cai H, Dai Y, Xu X. Proteomic evaluation of human umbilical cord tissue exposed to polybrominated diphenyl ethers in an e-waste recycling area. Environ Int. 2018;111:362–371. doi: 10.1016/j.envint.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Huo X, Zhang Y, Li W, Zhang J, Xu X. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ Pollut. 2015;196:414–422. doi: 10.1016/j.envpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Eguchi A, Nomiyama K, Minh Tue N, et al. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: association with thyroid hormone levels. Environ Res. 2015;137:440–449. doi: 10.1016/j.envres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Lv QX, Wang W, Li XH, Yu L, Zhang Y, Tian Y. Polychlorinated biphenyls and polybrominated biphenyl ethers in adipose tissue and matched serum from an e-waste recycling area (Wenling, China) Environ Pollut. 2015;199:219–226. doi: 10.1016/j.envpol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Xu P, Lou X, Ding G, et al. Association of PCB, PBDE and PCDD/F body burdens with hormone levels for children in an e-waste dismantling area of Zhejiang Province, China. Sci Total Environ. 2014;499:55–61. doi: 10.1016/j.scitotenv.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 70.Xu P, Lou X, Ding G, et al. Effects of PCBs and PBDEs on thyroid hormone, lymphocyte proliferation, hematology and kidney injury markers in residents of an e-waste dismantling area in Zhejiang, China. Sci Total Environ. 2015;536:215–222. doi: 10.1016/j.scitotenv.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 71.Zheng MY, Li XH, Zhang Y, Yang YL, Wang WY, Tian Y. Partitioning of polybrominated biphenyl ethers from mother to fetus and potential health-related implications. Chemosphere. 2017;170:207–215. doi: 10.1016/j.chemosphere.2016.11.136. [DOI] [PubMed] [Google Scholar]
- 72.Guo LC, Yu S, Wu D, et al. Disruption of thyroid hormone regulated proteins and gene expression by polychlorinated biphenyls, polybrominated diphenyl ethers and new flame retardants in residents of an e-waste region. Environ Pollut. 2019;254 doi: 10.1016/j.envpol.2019.07.093. [DOI] [PubMed] [Google Scholar]
- 73.Zheng J, He CT, Chen SJ, et al. Disruption of thyroid hormone (TH) levels and TH-regulated gene expression by polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and hydroxylated PCBs in e-waste recycling workers. Environ Int. 2017;102:138–144. doi: 10.1016/j.envint.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Guo LC, Pan S, Yu S, et al. Human sex hormone disrupting effects of new flame retardants and their interactions with polychlorinated biphenyls, polybrominated diphenyl ethers, a case study in south China. Environ Sci Technol. 2018;52:13935–13941. doi: 10.1021/acs.est.8b01540. [DOI] [PubMed] [Google Scholar]
- 75.Yuan J, Liu Y, Wang J, et al. Long-term persistent organic pollutants exposure induced telomere dysfunction and senescence-associated secretary phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:1027–1035. doi: 10.1093/gerona/gly002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu YJ, Lin BG, Liang WB, et al. Associations between PBDEs exposure from house dust and human semen quality at an e-waste areas in South China-A pilot study. Chemosphere. 2018;198:266–273. doi: 10.1016/j.chemosphere.2018.01.150. [DOI] [PubMed] [Google Scholar]
- 77.Li R, Yang Q, Qiu X, et al. Reactive oxygen species alteration of immune cells in local residents at an electronic waste recycling site in northern China. Environ Sci Technol. 2013;47:3344–3352. doi: 10.1021/es400027v. [DOI] [PubMed] [Google Scholar]
- 78.He X, Jing Y, Wang J, et al. Significant accumulation of persistent organic pollutants and dysregulation in multiple DNA damage repair pathways in the electronic-waste-exposed populations. Environ Res. 2015;137:458–466. doi: 10.1016/j.envres.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Ben YJ, Li XH, Yang YL, et al. Placental transfer of dechlorane plus in mother-infant pairs in an e-waste recycling area (Wenling, China) Environ Sci Technol. 2014;48:5187–5193. doi: 10.1021/es404106b. [DOI] [PubMed] [Google Scholar]
- 80.Eguchi A, Kunisue T, Wu Q, et al. Occurrence of perchlorate and thiocyanate in human serum from e-waste recycling and reference sites in Vietnam: association with thyroid hormone and iodide levels. Arch Environ Contam Toxicol. 2014;67:29–41. doi: 10.1007/s00244-014-0021-y. [DOI] [PubMed] [Google Scholar]
- 81.Zhang B, Zhang T, Duan Y, et al. Human exposure to phthalate esters associated with e-waste dismantling: exposure levels, sources, and risk assessment. Environ Int. 2019;124:1–9. doi: 10.1016/j.envint.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 82.Zhang T, Xue J, Gao CZ, et al. Urinary concentrations of bisphenols and their association with biomarkers of oxidative stress in people living near e-waste recycling facilities in China. Environ Sci Technol. 2016;50:4045–4053. doi: 10.1021/acs.est.6b00032. [DOI] [PubMed] [Google Scholar]
- 83.Song S, Duan Y, Zhang T, et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: associations with fasting blood glucose. Ecotoxicol Environ Saf. 2019;169:822–828. doi: 10.1016/j.ecoenv.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 84.Lu SY, Li YX, Zhang T, et al. Effect of e-waste Recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress. Environ Sci Technol. 2017;51:2427–2437. doi: 10.1021/acs.est.6b05462. [DOI] [PubMed] [Google Scholar]
- 85.Zhou X, Ju Y, Wu Z, Yang K. Disruption of sex hormones and oxidative homeostasis in parturient women and their matching fetuses at an e-waste recycling site in China. Int J Occup Environ Health. 2013;19:22–28. doi: 10.1179/2049396712Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 86.Zhang S, Huo X, Zhang Y, Huang Y, Zheng X, Xu X. Ambient fine particulate matter inhibits innate airway antimicrobial activity in preschool children in e-waste areas. Environ Int. 2019;123:535–542. doi: 10.1016/j.envint.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 87.Zeng X, Xu X, Zhang Y, Li W, Huo X. Chest circumference and birthweight are good predictors of lung function in preschool children from an e-waste recycling area. Environ Sci Pollut Res Int. 2017;24:22613–22621. doi: 10.1007/s11356-017-9885-5. [DOI] [PubMed] [Google Scholar]
- 88.Cong X, Xu X, Xu L, et al. Elevated biomarkers of sympatho-adrenomedullary activity linked to e-waste air pollutant exposure in preschool children. Environ Int. 2018;115:117–126. doi: 10.1016/j.envint.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Burns KN, Sun K, Fobil JN, Neitzel RL. Heart rate, stress, and occupational noise exposure among electronic waste recycling workers. Int J Environ Res Public Health. 2016;13:e140. doi: 10.3390/ijerph13010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rouillon S, Deshayes-Morgand C, Enjalbert L, et al. Endocrine disruptors and pregnancy: knowledge, attitudes and prevention behaviors of French women. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grandjean P, Barouki R, Bellinger DC, et al. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology. 2015;156:3408–3415. doi: 10.1210/EN.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribeiro E, Ladeira C, Viegas S. EDCs mixtures: a stealthy hazard for human health? Toxics. 2017;5:5. doi: 10.3390/toxics5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fowler PA, Bellingham M, Sinclair KD, et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 94.Choi H, Jedrychowski W, Spengler J, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu X, Yang H, Chen A, et al. Birth outcomes related to informal e-waste recycling in Guiyu, China. Reprod Toxicol. 2012;33:94–98. doi: 10.1016/j.reprotox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Chen Z-J, Liu H-Y, Cheng Z, et al. Polybrominated diphenyl ethers (PBDEs) in human samples of mother-newborn pairs in South China and their placental transfer characteristics. Environ Int. 2014;73:77–84. doi: 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Johns LE, Ferguson KK, Soldin OP, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walker CL. Minireview: epigenomic plasticity and vulnerability to EDC exposures. Mol Endocrinol. 2016;30:848–855. doi: 10.1210/me.2016-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dietert RR. Misregulated inflammation as an outcome of early-life exposure to endocrine-disrupting chemicals. Rev Environ Health. 2012;27:117–131. doi: 10.1515/reveh-2012-0020. [DOI] [PubMed] [Google Scholar]
- 100.Zota AR, Geller RJ, Romano LE, et al. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ Int. 2018;115:9–20. doi: 10.1016/j.envint.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sly PD, Carpenter DO, Van den Berg M, et al. Health consequences of environmental exposures: causal thinking in global environmental epidemiology. Ann Glob Health. 2016;82:3–9. doi: 10.1016/j.aogh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song Q, Li J. A review on human health consequences of metals exposure to e-waste in China. Environ Pollut. 2015;196:450–461. doi: 10.1016/j.envpol.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Zeng X, Xu X, Boezen HM, Huo X. Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere. 2016;148:408–415. doi: 10.1016/j.chemosphere.2015.10.078. [DOI] [PubMed] [Google Scholar]
- 105.Braun JM, Gray K. Challenges to studying the health effects of early life environmental chemical exposures on children's health. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jurewicz J, Polańska K, Hanke W. Chemical exposure early in life and the neurodevelopment of children—an overview of current epidemiological evidence. Ann Agric Environ Med. 2013;20:465–486. [PubMed] [Google Scholar]
- 107.Dietrich KN, Krafft KM, Bornschein RL, et al. Low-level fetal lead exposure effect on neurobehavioral development in early infancy. Pediatrics. 1987;80:721–730. [PubMed] [Google Scholar]
- 108.WHO . World Health Organization; Geneva: 2021. Children and digital dumpsites: e-waste exposure and child health. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.