Abstract
Obesity is a serious medical condition that often co-occurs with stress-related psychiatric disorders. It is recognized that the brain plays a key role in the (patho)physiology of obesity and that there is a bidirectional relationship between obesity and psychopathology, yet molecular mechanisms altered in obesity have not been fully elucidated. Thus, we investigated relationships between obesity and synaptic density in vivo using the radioligand [11C]UCB-J (which binds to synaptic glycoprotein SV2A) and positron emission tomography in individuals with obesity, and with or without stress-related psychiatric disorders. Regions of interest were the dorsolateral prefrontal cortex, orbitofrontal cortex, ventromedial, amygdala, hippocampus, and cerebellum. Forty individuals with a body mass index (BMI) ≥ 25 kg/m2 (overweight/obese), with (n = 28) or without (n = 12) psychiatric diagnosis, were compared to 30 age- and sex-matched normal weight individuals (BMI < 25), with (n = 14) or without (n = 16) psychiatric diagnosis. Overall, significantly lower synaptic density was observed in overweight/obese relative to normal weight participants (ηp2 = 0.193, F = 2.35, p = 0.042). Importantly, in participants with stress-related psychiatric diagnoses, we found BMI to be negatively correlated with synaptic density in all regions of interest (p ≤ 0.03), but no such relationship observed for mentally healthy controls (p ≥ 0.68). In the stress-related psychiatric groups, dorsolateral prefrontal cortex synaptic density was negatively associated with measures of worry (r = −0.46, p = 0.01), tension/anxiety (r = −0.38, p = 0.04), fatigue (r = −0.44, p = 0.02), and attentional difficulties (r = −0.44, p = 0.02). In summary, the findings of this novel in vivo experiment suggest compounding effects of obesity and stress-related psychopathology on the brain and the associated symptomatology that may impact functioning. This offers a novel biological mechanism for the relationship between overweight/obesity and stress-related psychiatric disorders that may guide future intervention development efforts.
Subject terms: Psychiatric disorders, Obesity
Introduction
Obesity is a chronic disease that affects 13% of adults worldwide [1], including >42% of adult Americans [2]. By the year 2030, it is estimated that one in two adults in the United States will be classified as having obesity, defined as a body mass index (BMI; kg/m2) ≥30 [3]. Obesity is associated with physical comorbidities, including cardiovascular disease, type 2 diabetes, and certain cancers [4–6]. In addition to physical health comorbidities, obesity has also been associated with the co-occurrence with mood- and stress-related psychiatric disorders such as major depressive disorder (MDD), bipolar disorder (BP), generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD) [7–9].
Alterations in brain health have long been documented in stress disorders. A growing body of research consistently reports lower gray matter (GM) volumes and density in the prefrontal cortex (PFC) and hippocampus (HIP), as well as amygdala (AMY) and cerebellum (CB) among individuals with mood- and stress-related psychiatric disorders [10–14]. More recently, GM decrements have correlated with impaired cognitive performance, particularly executive function, and psychiatric symptom severity [12–14]. Based on preclinical findings, it has been hypothesized that lower synaptic density may contribute to lower GM volumes and symptom severity in individuals with psychiatric disorders. For example, dendritic atrophy and synaptic loss have been implicated in stress-induced GM volume reductions in mice [15]. Furthermore, chronic stress rodent models of depression implicate lower synaptic protein expression and reduced dendritic spine density in the PFC and HIP, corresponding with depression-like behaviors, whereas chronic treatment with typical antidepressants (e.g., serotonin-reuptake inhibitors) and acute administration of rapid-acting antidepressants (e.g., ketamine) are associated with synaptogenesis and reversal of depressive behaviors [16, 17]. Human postmortem research has demonstrated lower synaptic density in the brains of individuals with MDD, BP, or PTSD, with lower densities associated with lower synaptic gene and protein expression [18–20].
However, past studies in psychiatric disorders have either statistically corrected for, or all together ignored, potential main effects of body weight and obesity or its interactions with psychopathology on brain atrophy and synaptic dysfunction, despite the well-documented bidirectional association between obesity and mood- and stress-related psychiatric disorders [7–9]. Importantly, changes in GM volume and density similar to those observed in participants with psychiatric disorders, particularly corticolimbic areas important for executive function and mood regulation, have also been demonstrated among individuals with obesity relative to non-obese controls [21–23]. Furthermore, one recent study found a significant negative correlation between BMI and global GM volume in a healthy adult population [22]. On the other hand, converging preclinical evidence suggests that diet-induced obesity in rats is associated with synaptic deficits, which correlated with cognitive deficits [24], as well as depression- and anxiety-like behaviors [25]. In these preclinical models of obesity, changes in neuroimmune activity, elevated proinflammatory signaling, oxidative stress, and reductions in brain-derived neurotrophic factor (BDNF) were identified as possible mechanisms contributing to reduced synaptic density and plasticity [24–27]. Inflammation/neuroimmune activation is a proposed mediator of synaptic pathology in psychiatric disorders [25, 28, 29]. Furthermore, inflammation is a recognized component of the pathophysiology of obesity [30] and is a proposed link between obesity and risk for cognitive difficulties [31]. However, the relationship between alterations in synaptic density, cognitive performance, mood disturbance, particularly within the context of obesity, still remains unclear in human subjects. Thus, a need exists for the systematic analysis of how obesity may independently be correlated with synaptic density, as well as how this association may be differentially modulated by (or interact with) pathophysiology in psychiatric disorders.
Historically, the study of synaptic structure and physiology was restricted to preclinical or postmortem analyses. However, this is no longer the case, with multiple investigations capitalizing on advances in molecular imaging, particularly the recently developed radioligand [11C]UCB-J that binds to the synaptic vesicle protein 2A (SV2A). SV2A is a transmembrane glycoprotein ubiquitously and homogeneously expressed in presynaptic vesicles throughout the brain [32]. Using positron emission tomography (PET) to measure [11C]UCB-J binding to SV2A provides a method for estimating synaptic vesicle density and can serve as a surrogate for presynaptic terminal density in vivo [33]. Using this approach, our lab recently demonstrated that synaptic density was negatively correlated with depressive symptom severity among subjects with MDD, PTSD, or comorbid MDD/PTSD [34]. Here, we applied the same method and investigated the influence of BMI on synaptic density, as estimated by [11C]UCB-J PET, and the additive burden of psychopathology. Our primary hypothesis was that BMI would be negatively associated with synaptic density. Furthermore, we hypothesized that synaptic density alterations would be greater in individuals with overweight/obesity who presented with one or more stress-related psychiatric condition; for this study we included individuals with diagnoses of BP, GAD, MDD, and PTSD. Secondarily, we predicted a negative correlation between synaptic density and measures of mood and cognitive symptom severity in individuals with psychiatric diagnosis and overweight/obesity. We focused our analyses on dorsolateral, ventromedial, and orbitofrontal cortices (dlPFC, vmPFC, and OFC, respectively), AMY, HIP, and CB. These regions of interest (ROIs) were selected based on previous evidence implicating them in the structural and functional pathology of mood- and stress-related psychiatric disorders [10–14, 34], as well as overweight/obesity [35, 36].
To our knowledge, this is the first in vivo investigation of synaptic density within the context of overweight/obesity. Furthermore, this analysis is novel in its examination of the potential interaction between overweight/obesity and psychiatric illness on synaptic density, and the subsequent association with cognitive performance and mood symptom severity. We show a negative correlation between synaptic density and BMI, an association that was restricted to participants with one or more psychiatric diagnoses. Furthermore, we demonstrate that among participants with any psychiatric comorbidity, regardless of BMI status, regional synaptic density was related to symptom severity, particularly worry, fatigue, and attentional difficulties. Our findings suggest that higher BMI may be a risk factor for greater synaptic pathology within the context of psychiatric disorders. Furthermore, these data suggest that synaptic targets may be relevant for the treatment and improved outcomes for both physical and psychiatric conditions, and particularly their co-occurrences.
Methods
Participants
Seventy individuals were studied, and BMI was used to define overweight (BMI ≥ 25 kg/m2) or obesity (BMI ≥ 30) [2]. Participants were classified into four groups: (1) normal weight (NW) psychiatrically healthy controls (NWHC; n = 16; BMI = 23.29 ± 0.32) and had no current or history of psychiatric diagnosis; (2) normal weight individuals with one or more psychiatric diagnoses (NWPsy; n = 14; BMI = 22.16 ± 0.62); (3) overweight/obese, but psychiatrically healthy controls (OWHC; n = 12; BMI = 29.51 ± 0.84); and (4) overweight/obese with one or more psychiatric diagnosis (OWPsy; n = 28; BMI = 30.76 ± 0.88). Demographics and clinical characteristics are presented in Table 1.
Table 1.
Summary of subject demographics, clinical characteristics, and PET parameters.
| Normal weight (NW) | Overweight/obese (OW) | |||
|---|---|---|---|---|
| NWHC (n = 16) | NWPsy (n = 14) | OWHC (n = 12) | OWPsy (n = 28) | |
| Demographics | ||||
| Age (years), mean (SD) | 41.94 (4.00) | 40.77 (2.68) | 46.50 (5.03) | 37.46 (2.27) |
| Sex (female), n (%) | 10 (62.5) | 8 (57.1) | 5 (45.5) | 15 (51.7) |
| Race/ethnicity (non-white), n (%) | 7 (43.8) | 9 (64.3) | 7 (63.6) | 13 (44.8) |
| Clinical characteristics | ||||
| BMI (kg/m2), mean (SD) | 23.29 (0.32) | 22.16 (0.62) | 29.51 (0.84)a,*,** | 30.76 (0.88)a,*,** |
| Obese (BMI ≥ 30 kg/m2), n (%) | 0 (0.0) | 0 (0.0) | 6 (54.5) | 12 (41.4) |
| Smoking status (smoker), n (%) | 3 (18.8) | 4 (28.6) | 0 (0.0) | 7 (24.1) |
| Diagnosis, n (%) | ||||
| Bipolar disorder (BP) | – | 2 (14.3) | – | 3 (10.3) |
| Major depressive disorder (MDD) | – | 7 (50.0) | – | 6 (20.7) |
| Post-traumatic stress disorder (PTSD) | – | 1 (7.1) | – | 6 (21.43) |
| BP/PTSD | – | 0 (0.0) | – | 1 (3.4) |
| MDD/PTSD | – | 4 (28.6) | – | 11 (39.3) |
| MDD/PTSD/generalized anxiety disorder | – | 0 (0.0) | – | 1 (3.4) |
| Age of onset (years), mean (SD) | – | 23.11 (2.70) | – | 19.07 (2.15) |
| Psychiatric medications (any current), n (%) | – | 7 (50.0) | – | 8 (28.6) |
| HAMD-17 (total score), mean (SD) | 1.58 (1.70) | 16.16 (1.55)a,*,*** | 0.70 (1.87) | 14.53 (1.11)a,*,*** |
| PSWQ, mean (SD) | 35.92 (3.71) | 59.16 (3.39)a,*,*** | 31.61 (4.09) | 61.24 (2.42)a,*,*** |
| POMS total, mean (SD) | −2.24 (6.30) | 28.05 (5.69)a,*,*** | 0.14 (6.64) | 29.42 (4.12)a,*,*** |
| PET parameters | ||||
| Injected dose (MBq), mean (SD) | 538.95 (44.76) | 532.30 (47.70) | 662.66 (52.39) | 510.87 (34.04) |
| Injected mass (μg/kg), mean (SD) | 0.024 (0.003) | 0.022 (0.003) | 0.021 (0.003) | 0.015 (0.002) |
| Plasma-free fraction (fp), mean (SD) | 0.28 (0.007) | 0.29 (0.007) | 0.28 (0.008) | 0.27 (0.005) |
| Mean input function (SUV), mean (SD) | 0.221 (0.012) | 0.192 (0.013) | 0.275 (0.014)a,*,** | 0.259 (0.009)a,*,** |
NWHC normal weight mentally healthy control, NWPsy normal weight with any psychiatric morbidity, OWHC overweight/obese mentally healthy control, OWPsy overweight/obese with any psychiatric morbidity.
*p < 0.05 vs. NWHC.
**p < 0.05 vs. NWPsy.
***p < 0.05 vs. OWHC.
aUnivariate analysis correcting for age and sex, with post hoc tests correcting for multiple comparisons (Bonferroni).
For psychiatric comorbidities, diagnosis was confirmed at screening using the Structured Clinical Interview for DSM-5 [37]. All participants with MDD were in a major depressive episode. Depressive symptoms were additionally assessed using the Hamilton Depression Rating Scale (HAMD-17) [38], trait “worry” was measured using the Penn State Worry Questionnaire (PSWQ) [39], and general mood disturbance was measured using the Profile of Mood States (POMS) [40] questionnaire.
Exclusion criteria for psychiatric groups were diagnosis of substance use disorder (except nicotine use disorder) in the past 12 months; positive urine toxicology or pregnancy tests; history of loss of consciousness for more than 5 min; significant medical condition; and contraindications to MRI or PET. Exclusion criteria were the same for the psychiatrically healthy control groups, with the addition of no current, history of or first-degree family history of any DSM-5 diagnosis, not including nicotine use disorder. The Yale University Human Investigation Committee and the Radioactive Drug Research Committee approved the study. All participants provided written informed consent before inclusion in the study.
Participants underwent physical and neurological examination to exclude presence of active medical or neurological illness. Screening involved electrocardiography, hematology, blood chemistries, urinalysis and urine toxicology screening, and plasma pregnancy tests. Participants completed a brief computerized cognitive testing battery (Cogstate: https://Cogstate.com/computerized-tests), consisting of the Identification Test (IDN; visual attention), Detection Test (DET; psychomotor processing speed), One-Back Test (OBT; working memory), and International Shopping List Test (ISL; verbal short-term and long-term memory).
[11C]UCB-J PET imaging
T1-weighted MRI scans were acquired on 3-Tesla Siemens Prisma scanner, as previously described [34] (Supplementary Methods).
[11C]UCB-J was synthesized onsite, as previously reported [34, 41], and administered intravenously as a bolus over 1 min. using an automated infusion pump (Harvard PHD 22/2000, Harvard Apparatus). Subjects were scanned on a high-resolution human brain PET camera, the high-resolution research tomograph. All PET imaging and measurement of the metabolite-corrected arterial input function was performed according to previously described procedures [33, 42] (Supplementary Methods).
The injected radioactivity was within a dose range that produced good image quality (mean: 547.59 ± 21.62 MBq). Based on the in vivo affinity of UCB-J being previously determined as 3.4 nM (20 mg/kg) in non-human primates [41], the mass dose was expected to produce <1% occupancy in all groups, such that the radioligand was injected at tracer dose levels (mean: 19.59 ± 1.58 ng/kg).
PET image analysis
The primary outcome measure was total volume of distribution (VT), computed parametrically using the 1 tissue (1T) compartment model and a metabolite-corrected arterial input function, as validated previously [42]. VT is the tissue-to-plasma concentration ratio at equilibrium and reflects total uptake (specific plus nonspecific binding) of the radioligand. We have previously shown that the test-retest reproducibility of [11C]UCB-J VT is exceptionally good and that correcting for plasma-free fraction (fp) worsened absolute test-retest variability and intraclass correlation coefficient. Therefore, we used [11C]UCB-J VT as the primary outcome measure but have provided results for VT/fp for completeness (Supplementary Table S1).
ROIs were selected a priori based on previous structural and functional MRI findings in obese and psychiatric populations [10–14, 34–36, 43]. ROIs were derived from the Automated Anatomical Labeling atlas and applied to the parametric images using the combined transformations from template to PET space, additionally using a GM mask to calculate GM-specific tracer concentration [44].
Statistical analysis
Statistical analysis was performed in SPSS v22 (IBM). Group differences in demographics, clinical measures, cognitive test performance, and radiotracer characteristics were assessed using either analysis of variance with weight group (overweight/obesity vs. NW) and clinical group (any psychiatric diagnosis vs. HC) as fixed factors, or χ2 tests for categorical outcomes. A multivariate analysis of covariance (MANCOVA) with weight group (overweight/obesity vs. NW) and clinical group (any psychiatric diagnosis vs. HC) as fixed factors was used to evaluate regional differences in [11C]UCB-J VT, followed by post hoc tests with Bonferroni correction for multiple comparisons. Associations between regional VT and BMI were examined using Pearson’s partial correlations, as well as to assess relationships between VT or BMI and measures of mood symptoms and cognitive function symptoms. Given previous observations that age and sex can influence [11C]UCB-J VT [45], age and sex were included as co-variates for all analyses. All statistical tests were two-tailed, and findings were considered significant at p < 0.05.
Results
Sample demographics
A summary of sample demographics, clinical features, and PET parameters is presented in Table 1. Notably, the four groups were well matched for age, sex, race, and ethnicity and smoking status. A univariate analysis of covariance, correcting for age and sex, revealed that OWHC and OWPsy groups had on average a significantly higher BMI than the NWHC and NWPsy groups (F = 71.37, p < 0.001), with no effect of having a psychiatric diagnosis on BMI (F = 0.001, p = 0.969) or on the proportion of individuals with obesity within the overweight/obesity groups (χ2 = 0.173, p = 0.677).
NWPsy + OWPsy participants, regardless of weight-group designation, scored higher on the HAMD-17 (F = 77.87, p < 0.001), the PSWQ (F = 56.33, p < 0.001), and the POMS total scale (F = 25.49, p < 0.001) relative to NWHC + OWHCs. No significant main effects of weight group, or weight-group-by-psychiatric-diagnosis interactions were observed for any of these clinical measures. Between the NWPsy + OWPsy groups, there were no differences in terms of number of psychiatric diagnoses (1, 2, or 3 psychiatric conditions; χ2 = 1.5, p = 0.472), reported age of symptom onset (range: age 2–45 years; mean: 20.64 ± 1.88 years), or current treatment with any psychiatric medication (χ2 = 1.9, p = 0.172) (see Supplementary Table S2 for full medication list).
There were no significant differences between groups in total injected radioactivity (547.59 ± 180.91 MBq), injected mass (0.020 ± 0.013 ug/kg), or fp (0.28 ± 0.027). The average metabolite-corrected input function was significantly higher in OW relative to NW participants (F = 25.68, p < 0.0001), with no significant main effect or interaction with psychiatric diagnosis (see Supplementary text and Supplementary Fig. S1 for details).
Relationship between body mass index and SV2A density
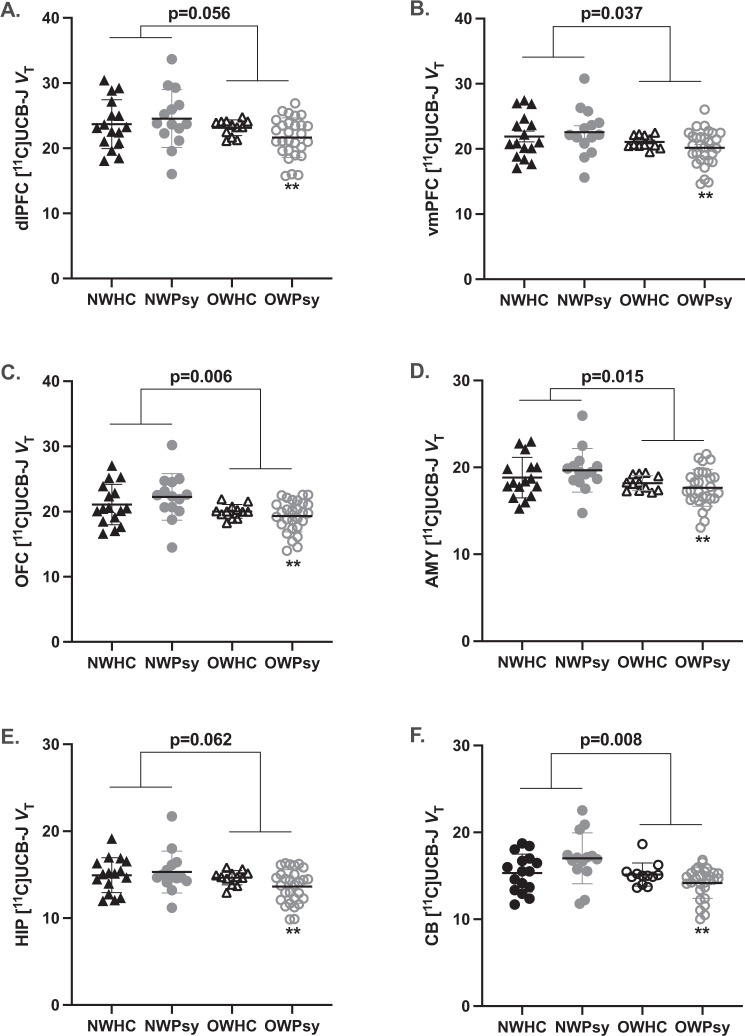
Using a MANCOVA, correcting for age and sex, we observed a main effect of weight group, with SV2A density ([11C]UCB-J VT) significantly lower in OW (OWHC + OWPsy) relative to NW (NWHC + NWPsy) participants (ηp2 = 0.193, F = 2.35, p = 0.042) (Fig. 1). The main effect of psychiatric diagnosis (ηp2 = 0.180, F = 2.16, p = 0.059), and weight-by-psychiatric diagnosis interaction (ηp2 = 0.165, F = 1.94, p = 0.165) were not significant. The main effect of weight group was statistically significant in the vmPFC (−8.0%, F = 4.53, p = 0.037), OFC (9.7%, F = 8.09, p = 0.006), AMY (−7.3%, F = 6.25, p = 0.015), and CB (−10.2%, F = 7.39, p = 0.008). The difference between OW and NW reached statistical significance in the dlPFC (−8.3%, F = 3.79, p = 0.056) and HIP (−7.7%, F = 3.61, p = 0.062).
Fig. 1. Effect of overweight/obesity status on synaptic density.
Synaptic density is shown as estimated by [11C]UCB-J volume of distribution (VT) as it binds to synaptic vesicle protein 2A (SV2A) in the A dlPFC, B vmPFC, C OFC, D AMY, E HIP, and F CB. A MANCOVA revealed significantly reduced SV2A density in overweight or obese (OW; n = 40) subjects relative to normal weight (NW; n = 30) subjects, overall (ηp2 = 0.193, F = 2.35, p = 0.042). Individual values are shown with the group mean ± SEM and p value associated with the main effect of weight group displayed above. **p ≤ 0.005 (Bonferroni) relative to NWPsy.
To test our a priori hypothesis that overweight/obesity would have a greater effect on VT among individuals with psychiatric morbidities, we performed post hoc tests looking specifically at NWHC vs. OWHC and NWPsy vs. OWPsy. We observed no significant differences between the NWHC and OWHC groups; however, the OWPsy group had significantly lower VT relative to the NWHC group (Bonferroni p ≤ 0.005) for all six ROIs (Fig. 1).
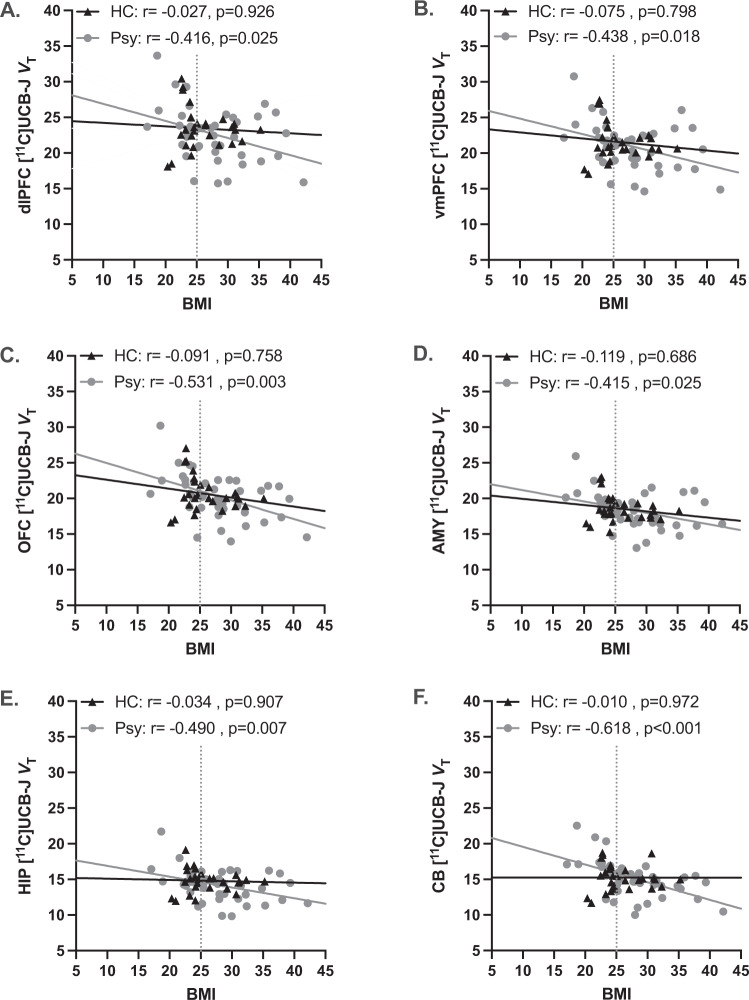
We further tested whether there was a relationship between BMI and regional VT. In the full sample (n = 70), there was a significant negative correlation such that a higher BMI was associated with lower VT for all ROIs (Supplementary Table S3). To investigate how psychopathology might influence the relationship between BMI and VT, we performed separate correlation analyses for HC (NWHC + OWHC; n = 28) and for subjects with any psychiatric diagnosis (NWPsy + OWPsy; n = 42) (Fig. 2). There were no significant correlations for the HCs, but correlations between BMI and SV2A density remained significant among the NWPsy + OWPsy groups in all ROIs (Supplementary Table S3). Specifically within the CB, the strength of the correlation between BMI and VT (i.e., the difference between the regression line slopes) for the NWPsy + OWPsy groups was significantly greater than that of HC (F = 5.55, p = 0.022).
Fig. 2. Relationship between BMI and synaptic density and the influence of psychiatric morbidity.
No relationship between BMI and SV2A density ([11C]UCB-J VT) was observed among the mentally healthy control subjects (HC; n = 28), but there was a significant negative correlation among subjects with psychiatric disorders (Psy; n = 42) in the A dlPFC, B vmPFC, C OFC, D AMY, E HIP, and F CB. The best-fit line for each group is shown with the associated partial correlation (r; adjusting for age and sex) and p value. The BMI cutoff value for overweight/obesity (BMI = 25) is illustrated by the gray dashed vertical line.
We also observed significantly lower tissue influx values (K1) for the OW relative to the NW groups (Supplementary Fig. S2) in the HIP (OW = 0.242 ± 0.006 vs. NW = 0.260 ± 0.006; −6.9%, p = 0.041), AMY OW = 0.237 ± 0.006 vs. NW = 0.262 ± 0.006; −8.7%; p = 0.005), and CB (OW = 0.331 ± 0.009 vs. NW = 0.363 ± 0.009; −8.9%; p = 0.014). Importantly, although there are reports of lower cerebral blood flow with elevated BMI [46], changes in blood flow and K1 do not affect VT for [11C]UCB-J [47]. However, when we included regional K1 as a covariate and reconducted the correlation analyses, the relationship between BMI and VT remained significant in the total sample, for NWPsy + OWPsy, and with no relationship between BMI and VT among the HC subjects (Supplementary Table S4). We also provide results for VT/fp as the outcome measure for completeness (Supplementary Table S1).
Symptom severity and SV2A density in subjects with psychiatric diagnoses
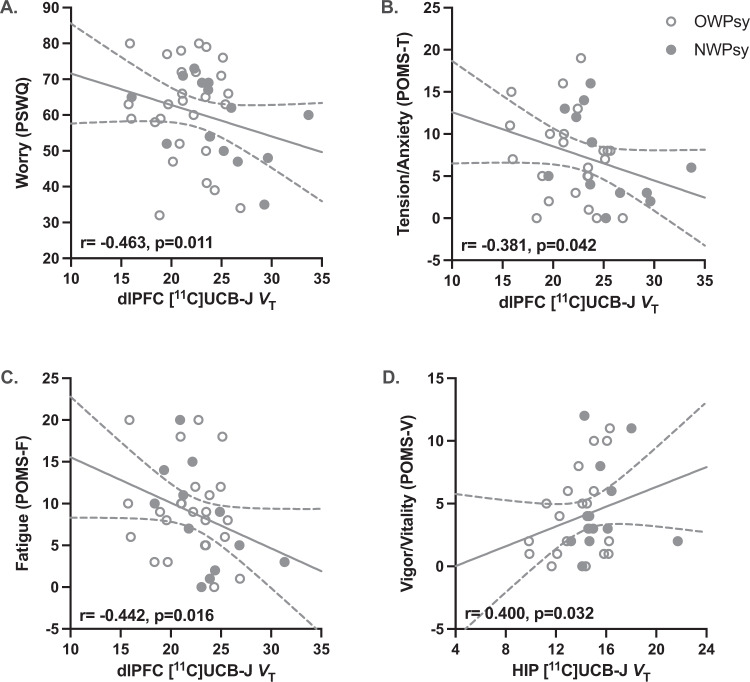
To investigate whether lower VT observed with increasing BMI had clinically relevant implications, we tested for correlations between regional VT and various measures of symptom severity among NWPsy + OWPsy subjects (Fig. 3). Tendencies to worry, characterized by intrusive and repetitive negative thinking, are commonly observed across psychiatric diagnoses [39], and were measured by the PSWQ [48]. We observed negative correlations between PSWQ scores and VT in the OFC (r = −0.410, p = 0.027), vmPFC (r = −0.461, p = 0.012), and dlPFC (r = −0.463, p = 0.011; Fig. 3A). There were negative correlations between scores on the POMS tension/anxiety (POMS-T) and fatigue (POMS-F) subscales and vmPFC (POMS-T: r = −0.367, p = 0.050; POMS-F: r = −0.428, p = 0.021) and dlPFC (POMS-T: r = −0.381, p = 0.042; Fig. 3B; POMS-F: r = −0.442, p = 0.016; Fig. 3B) SV2A density. In contrast, higher VT in the CB (r = 0.389, p = 0.037) and HIP (r = 0.400, p = 0.032; Fig. 3D) were associated with higher scores on the POMS vigor/vitality (POMS-V) subscale. There were no significant correlations for the three remaining POMS subscales (depression, anger/hostility, and confusion/bewilderment) or POMS total mood disturbance for any ROI (Supplementary Table S5).
Fig. 3. Relationship between synaptic density and mood symptoms in subjects with psychiatric disorders.
A Among clinical subjects (NWPsy + OWPsy), tendencies to worry, as measured by the Penn State Worry Questionnaire (PSWQ), were associated with lower dlPFC SV2A density ([11C]UCB-J VT). B Scores on the tension/anxiety and C fatigue subscales of the Profile of Mood States (POMS) scale were negatively correlated with dlPFC SV2A density, while D HIP SV2A density was positively correlated with scores on the vigor/vitality POMS subscale. The raw (uncorrected) individual values are shown with the best-fit line with the 95% confidence interval, but the correlation (r) and p values displayed represent the results of partial correlation analyses correcting for age and sex. Normal weight subjects with any psychiatric diagnosis (NWPsy) are represented by solid circles. Subjects with overweight/obesity with any psychiatric diagnosis (OWPsy) are represented by open circles.
Relationship between SV2A density, cognitive function, and body mass index
Performance on tests of working memory was worse in NWPsy + OWPsy relative to NWHC + OWHCs (F = 5.46; p = 0.023), as was short-term verbal memory performance (F = 5.28; p = 0.025). No significant main effects of weight group, or weight-group-by-psychiatric-diagnosis interactions were observed. There were no significant main effects of weight group or psychiatric diagnosis associated with visual attention or psychomotor processing speed measures.
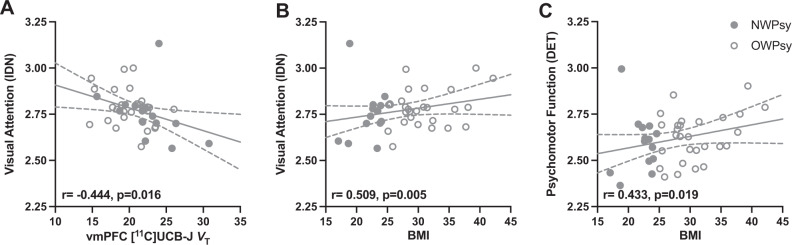
Examining the relationships between SV2A density and cognitive functioning in NWPsy + OWPsy participants (Fig. 4), we observed a negative correlation with response time on visual attention, which was significant for the dlPFC (r = −0.439, p = 0.017), vmPFC (r = −0.444, p = 0.016; Fig. 4A), OFC (r = −0.420, p = 0.023), HIP (r = −0.422, p = 0.022), and CB (r = −0.501, p = 0.006).
Fig. 4. Relationships between synaptic density, BMI, and measures of cognitive function in clinical subjects.
A Among clinical subjects (NWPsy + OWPsy), worse visual attention (reaction time; IDN) was associated with lower SV2A density ([11C]UCB-J VT). B BMI was positively correlated with visual attention (reaction time; IDN) and C psychomotor function (response speed; DET). For both the IDN and DET, higher scores reflect poorer performance. The raw (uncorrected) individual values are shown with the best-fit line with the 95% confidence interval, but the correlation (r) and p values displayed represent the results of partial correlation analyses correcting for age and sex. Subjects with overweight/obesity with any psychiatric diagnosis (OWPsy) are represented by open circles.
Correlations were observed between BMI and visual attention (r = 0.509, p = 0.005; Fig. 4B), where higher BMI was associated with longer response times, and between BMI and psychomotor processing speed (r = 0.433, p = 0.019; Fig. 4C), where higher BMI was associated with longer processing speeds. Correlations between SV2A density for all ROIs and performance on each of the four cognitive tests are provided in Supplementary Table S6.
Discussion
To our knowledge, this is the first experiment investigating in vivo synaptic-density levels, estimated by the PET quantification of [11C]UCB-J VT, as a function of BMI. We provide novel evidence that decrements in synaptic density are associated with higher BMI in individuals with coexisting psychiatric diagnoses but not in psychiatrically healthy controls. Furthermore, we show that overweight/obesity status and lower synaptic density in individuals with psychiatric conditions were associated with mood symptomatology, particularly anxiety (i.e., worry and tension) and cognitive alterations (attention, psychomotor processing).
It is noteworthy that participants of this study were relatively medically healthy and free of medical complications, including conditions typically associated with obesity, such as type 2 diabetes and cardiovascular disease. While this design enabled us to isolate correlates of overweight/obesity independent of medical comorbidities, it could also in part explain why we did not see stronger relationships between overweight/obesity and mood and cognitive symptoms, as these physiological disturbances have been cited as potential mediators of relationships between obesity, cognitive dysfunction, and mood disturbances [49]. This could also contribute to why a relationship between BMI and synaptic density was not observed among the mentally healthy controls in this study. We believe further investigation of potential interactions between overweight/obesity, synaptic density, and appetitive behaviors in individuals without psychiatric morbidities are still warranted.
Our findings of lower in vivo synaptic density associated with overweight/obesity (albeit restricted to the psychiatric morbidity group) are consistent with the preclinical literature. For example, rodent models of diet-induced obesity are associated with lower dendritic spine density and synaptic protein expression [24, 50]. Furthermore, lower synaptic density is congruent with observed lower GM volume and density, particularly in brain regions important for executive function and learning, in humans with obesity relative to lean individuals [35, 36]. Possible mechanisms contributing to lower synaptic density as a function of BMI include elevated systemic inflammation and neuroimmune activation [25, 51]. In addition, obesity or consumption of a high-fat diet is associated with increase in leptin levels and alterations in glucocorticoid signaling, of which both can impact neurotrophic factors such as BDNF/TrkB and mTor signaling in ways that could contribute to dendritic atrophy and synaptic loss [25, 52–56]. Consistent with this potential mechanism, in leptin-receptor-deficient mouse model of obesity, plasma corticosterone was elevated and associated with reduced BDNF protein expression and hippocampal synaptic deficits, along with impaired spatial memory, with these phenotypes being “rescued” by treatment with the glucocorticoid synthesis inhibitor metyrapone [57]. In human studies of obesity and type 2 diabetes, reducing glucocorticoid signaling, either by glucocorticoid receptor antagonism (e.g., mifepristone) or blocking cortisol synthesis (e.g., selective inhibition of the 1β-hydroxysteroid dehydrogenase type 1 enzyme), resulted in improved glucose metabolism and insulin sensitivity [58, 59]; testing the effect on mood symptoms and cognitive function would be an extension of this work.
Since correlations between BMI and SV2A densities were significant only within the psychiatric group, this could suggest that individuals with psychiatric conditions, particularly stress- and trauma-related disorders, are more vulnerable to brain alterations that may increase vulnerability to influences of higher BMI or obesity. This interpretation would be consistent with results of translational and clinical research that implicate synaptic dysfunction in the etiology and symptomology of psychiatric disorders [16, 18, 34, 60]. Furthermore, our findings suggest synaptic pathology as a potential point of convergence and interaction of biological mechanisms for overweight/obesity and stress-related psychiatric disorders. Indeed, preclinical findings support the notion that diet-induced obesity augments physiological, endocrine, and behavioral consequences of stress in rodents [61, 62]; in turn, chronic stress is associated with reduced synaptic density and plasticity and behavioral and cognitive deficits [16, 17].
We anticipated that lower SV2A estimates of synaptic density would have functional correlates, and thus predicted negative associations between SV2A density and better mood and cognitive functioning. Lower SV2A density has been associated with greater depression symptom severity in individuals with MDD, PTSD, or comorbid MDD/PTSD [34], and lower synaptic density in subjects with mild cognitive impairment and Alzheimer’s disease has correlated with poorer performance on cognitive tests [63, 64]. Congruent with these findings, here we observed that among participants with psychiatric morbidities, in brain regions important for emotional regulation and cognitive function including dlPFC, vmPFC, HIP, and CB, lower synaptic density was associated with severity of mood symptoms, particularly, tension/anxiety and fatigue as well as worse attentional skills. Furthermore, higher BMI was related to worse performance on cognitive assessments among subjects with psychiatric disorders. These data add to translational and clinical evidence linking lower synaptic density to chronic stress and psychiatric disorders [15, 18, 19, 34], and now overweight/obesity in clinically relevant manners. Importantly, we presently cannot say whether pre-existing lower synaptic density is a risk factor for the subsequent development of obesity and psychopathology, or rather a consequence of these conditions or experiences. Both possibilities warrant further investigation with longitudinal studies, as relationships between obesity, psychiatric comorbidity, and synaptic biology are complex.
Study limitations warrant consideration. First, VT was used as the PET outcome (as opposed to binding potential—BPND); however, a valid reference brain region, void of SV2A expression, has not been identified in individuals with mood disorders. We previously validated the use of VT as the PET outcome measure for [11C]UCB-J, calculated using a metabolite-corrected arterial input function [42]. Furthermore, Holmes et al. [34] previously observed between-group differences in centrum semioval—a small region of white matter that some (e.g., [45]) have suggested as a reference region—as a function of depression. Presently, group differences in centrum semioval VT were observed (Supplementary Fig. S3), as well as correlations between VT, BMI and clinical measures (Supplementary Table S8), providing additional evidence that the centrum semiovale is not a valid reference region to use in the current study population.
Second, SV2A has been reported to be ubiquitously expressed by synaptic vesicles; however, recent evidence suggests that SV2A may be differentially expressed by inhibitory vs. excitatory neuronal subtypes, which could contribute to differences in vesicle-trafficking and neurotransmitter-release dynamics [65]. With the currently available PET methods, we cannot remark on cell-type specificity of SV2A expression or exclude that differences in SV2A expression reflect a shift in synaptic vesicle function rather than synaptic density, per se. Third, we used BMI (per CDC guidelines) to define overweight/obesity. However, BMI does not account for body composition, nor is it a direct measurement of any specific health outcome, with the cutoff designating “healthy” vs. “unhealthy” being relatively arbitrary [66, 67].
Despite these limitations, this work is novel in its study of relationships between overweight/obesity and in vivo measurements of synaptic density and suggests synaptic pathology as a potential point of convergence between biological mechanisms for overweight/obesity and stress-related psychiatric disorders. In addition, these results promote that weight management (and addressing associated medical comorbidities) should be considered as an important component of a comprehensive treatment plan for individuals with psychiatric disorders and concurrent overweight/obesity. For example, in addition to supporting a healthy weight, exercise has been shown to enhance μ-opioid receptor activation, BDNF production, and mTor signaling, and can reduce cortisol, factors that in turn are associated with increased positive affect, reduction of depression and anxiety symptoms, and improved cognitive performance [68–71]. Furthermore, these data build on the growing recognition that specifically addressing brain dysfunction underpinning mood and cognitive symptoms is paramount in the treatment of obesity [72, 73].
In summary, here we demonstrate a negative relationship between BMI and synaptic density among individuals with psychiatric disorders. We believe these data suggest synaptic dysfunction as a possible point of intersection for the pathophysiology of overweight/obesity and stress-related psychiatric disorders. Thus, we provide a novel biological mechanism for the high prevalence of these two co-occurring diseases and add to the growing evidence base implicating synaptic loss in the pathophysiology of stress-related conditions and their role in contributing to overweight/obesity.
Supplementary information
Supplementary Material for Lower synaptic density is associated with psychiatric and cognitive alterations in obesity
Author contributions
RHA was responsible for data analysis and interpretation, in addition to drafting and revising the manuscript. SEH made substantial contributions to data acquisition and analysis, as well as manuscript revisions. AMJ, MNP, and SRB provided critically important intellectual content and manuscript revisions. REC and RHP advised data analysis and interpretation. IE was responsible for the study conception and design and oversaw all aspects of the work.
Funding and disclosures
This work was funded in part by the National Institute of Mental Health, the U.S. Department of Veterans Affairs National Center for PTSD, and the Nancy Taylor Foundation. RHA receives support from the NIMH (T32 MH014276). AMJ is consultant for Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and receives research support from the American Diabetes Association, Eli Lilly, Novo Nordisk, and NIH/NIDDK. SEH, MNP, SRB, REC, RHP, and IE have no further conflicts of interest to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01111-5.
References
- 1.World Health Organization. Obesity and overweight. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 Feb 2021.
- 2.Centers for Disease Control and Prevention. Defining adult overweight and obesity. 2020. https://www.cdc.gov/obesity/adult/defining.html. Accessed 11 Nov 2020.
- 3.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–50. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 4.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 5.García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, García-Martinez JM, Castaño A, De la Vieja A. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer. 2016;114:716–22. doi: 10.1038/bjc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJO. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34:4249–55. doi: 10.1200/JCO.2016.69.6187. [DOI] [PubMed] [Google Scholar]
- 7.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanovics EA, Potenza MN, Pietrzak RH. PTSD and obesity in US military veterans: prevalence, health burden, and suicidality. Psychiatry Res. 2020;291:113242. doi: 10.1016/j.psychres.2020.113242. [DOI] [PubMed] [Google Scholar]
- 9.Rajan TM, Menon V. Psychiatric disorders and obesity: a review of association studies. J Postgrad Med. 2017;63:182–90. doi: 10.4103/jpgm.JPGM_712_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo C, Zhu J, Wang C, Qu H, Ma X, Qin W. Different spatial patterns of brain atrophy and global functional connectivity impairments in major depressive disorder. Brain Imaging Behav. 2017;11:1678–89. doi: 10.1007/s11682-016-9645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Doherty DCM, Tickell A, Ryder W, Chan C, Hermens DF, Bennett MR, et al. Frontal and subcortical grey matter reductions in PTSD. Psychiatry Res: Neuroimaging. 2017;266:1–9. doi: 10.1016/j.pscychresns.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen M-H, Kao Z-K, Chang W-C, Tu P-C, Hsu J-W, Huang K-L, et al. Increased proinflammatory cytokines, executive dysfunction, and reduced gray matter volumes in first-episode bipolar disorder and major depressive disorder. J Affect Disord. 2020;274:825–31. doi: 10.1016/j.jad.2020.05.158. [DOI] [PubMed] [Google Scholar]
- 13.Moorhead TWJ, McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res: Neuroimaging. 2012;203:139–45. doi: 10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–61. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 16.Hare BD, Ghosal S, Duman RS. Rapid acting antidepressants in chronic stress models: molecular and cellular mechanisms. Chronic Stress. 2017;1:2470547017697317. doi: 10.1177/2470547017697317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–49. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young KA, Thompson PM, Cruz DA, Williamson DE, Selemon LD. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol Stress. 2015;2:67–72. doi: 10.1016/j.ynstr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann MJ, Tesar AK, Beier J, Berg M, Warrings B. Grey matter alterations in obesity: a meta-analysis of whole-brain studies. Obes Rev. 2019;20:464–71. doi: 10.1111/obr.12799. [DOI] [PubMed] [Google Scholar]
- 22.Hamer M, Batty GD. Association of body mass index and waist-to-hip ratio with brain structure. UK Biobank Study. 2019;92:e594–600. doi: 10.1212/WNL.0000000000006879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology. 2019;291:763–71. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 24.Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci USA. 2015;112:15731–6. doi: 10.1073/pnas.1511593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2016;41:1874–87. doi: 10.1038/npp.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, et al. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontology: Ser A. 2018;74:290–98. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HR, Park M, Choi J, Park K-Y, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–39. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 29.Wohleb ES. Neuron–Microglia interactions in mental health disorders: “for better, and for worse”. Front Immunol. 2016;7:544. [DOI] [PMC free article] [PubMed]
- 30.Karczewski J, Śledzińska E, Baturo A, Jończyk I, Maleszko A, Samborski P, et al. Obesity and inflammation. Eur Cytokine Netw. 2018;29:83–94. doi: 10.1684/ecn.2018.0415. [DOI] [PubMed] [Google Scholar]
- 31.Yeomans MR. Adverse effects of consuming high fat–sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc. 2017;76:455–65. doi: 10.1017/S0029665117000805. [DOI] [PubMed] [Google Scholar]
- 32.Stout KA, Dunn AR, Hoffman C, Miller GW. The synaptic vesicle glycoprotein 2: structure, function, and disease relevance. ACS Chem Neurosci. 2019;10:3927–38. doi: 10.1021/acschemneuro.9b00351. [DOI] [PubMed] [Google Scholar]
- 33.Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin S-F, Chen M-K, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96–48ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 34.Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529. doi: 10.1038/s41467-019-09562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannacciulli N, Del Parigi A, Chen K, Le DSN, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC, USA: American Psychiatric Publication; 2013.
- 38.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 39.Gustavson DE, du Pont A, Whisman MA, Miyake A. Evidence for transdiagnostic repetitive negative thinking and its association with rumination, worry, and depression and anxiety symptoms: a commonality analysis. Collabra Psychology. 2018;4:13. doi: 10.1525/collabra.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollock V, Cho DW, Reker D, Volavka J. Profile of Mood States: the factors and their physiological correlates. J Nerv Ment Dis. 1979;167:612–14. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Nabulsi NB, Mercier J, Holden D, Carré S, Najafzadeh S, Vandergeten M-C, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84. doi: 10.2967/jnumed.115.168179. [DOI] [PubMed] [Google Scholar]
- 42.Finnema SJ, Nabulsi NB, Mercier J, Lin S-F, Chen M-K, Matuskey D, et al. Kinetic evaluation and test–retest reproducibility of [11C] UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041–52. doi: 10.1177/0271678X17724947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park B-Y, Seo J, Yi J, Park H. Structural and functional brain connectivity of people with obesity and prediction of body mass index using connectivity. PloS One. 2015;10:e0141376. doi: 10.1371/journal.pone.0141376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller-Gärtner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–83. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 45.Carson R, Naganawa M, Matuskey D, Mecca A, Pittman B, Toyonaga T, et al. Age and sex effects on synaptic density in healthy humans as assessed with SV2A PET. J Nucl Med. 2018;59:541–41. [Google Scholar]
- 46.Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 2011;19:1095–7. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smart K, Liu H, Matuskey D, Chen M-K, Torres K, Nabulsi N, et al. Binding of the synaptic vesicle radiotracer [11C] UCB-J is unchanged during functional brain activation using a visual stimulation task. J Cereb Blood Flow Metab. 2021;41:1067–79. [DOI] [PMC free article] [PubMed]
- 48.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 49.Mansur RB, Brietzke E, McIntyre RS. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. 2015;52:89–104. doi: 10.1016/j.neubiorev.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. 2018;38:8889–904. doi: 10.1523/JNEUROSCI.0789-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beyer F, Kharabian Masouleh S, Kratzsch J, Schroeter ML, Röhr S, Riedel-Heller SG, et al. A metabolic obesity profile is associated with decreased gray matter volume in cognitively healthy older adults. Front Aging Neurosci. 2019;11:202. [DOI] [PMC free article] [PubMed]
- 52.Zonneveld MH, Noordam R, van der Grond J, van Heemst D, Mooijaart SP, Sabayan B, et al. Interplay of circulating leptin and obesity in cognition and cerebral volumes in older adults. Peptides. 2021;135:170424. doi: 10.1016/j.peptides.2020.170424. [DOI] [PubMed] [Google Scholar]
- 53.Khazen T, Hatoum OA, Ferreira G, Maroun M. Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids. Sci Rep. 2019;9:12270. doi: 10.1038/s41598-019-48800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karanian T, Campbell C, Sloan A, Dupree L, Walker B, Andersen C. Variability in salivary cortisol is associated with body mass index and diet quality. Curr Dev Nutr. 2020;4:532–32. [Google Scholar]
- 55.Motamedi S, Karimi I, Jafari F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): kill two birds with one stone. Metab Brain Dis. 2017;32:651–65. doi: 10.1007/s11011-017-9997-0. [DOI] [PubMed] [Google Scholar]
- 56.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–08. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology. 2014;42:165–77. doi: 10.1016/j.psyneuen.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubbi S, Muniyappa R, Sharma ST, Grewal S, McGlotten R, Nieman LK. Mifepristone improves adipose tissue insulin sensitivity in insulin resistant individuals. J Clin Endocrinol Metab. 2021;106:1501–15. [DOI] [PMC free article] [PubMed]
- 59.Almeida C, Monteiro C, Silvestre S. Inhibitors of 11β-hydroxysteroid dehydrogenase type 1 as potential drugs for type 2 diabetes mellitus—a systematic review of clinical and in vivo preclinical studies. Scientia Pharmaceutica. 2021;89:5. [Google Scholar]
- 60.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agrimi J, Spalletti C, Baroni C, Keceli G, Zhu G, Caragnano A, et al. Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion. EBioMedicine. 2019;47:384–401. doi: 10.1016/j.ebiom.2019.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santana JMS, Vega-Torres JD, Ontiveros-Angel P, Lee JB, Torres YA, Gonzalez AYC, et al. Oxidative stress and neuroinflammation in a rat model of co-morbid obesity and psychogenic stress. Behav Brain Res. 2021;400:112995. [DOI] [PMC free article] [PubMed]
- 63.Vanhaute H, Ceccarini J, Michiels L, Koole M, Sunaert S, Lemmens R, et al. In vivo synaptic density loss is related to tau deposition in amnestic mild cognitive impairment. Neurology. 2020;95:e545. doi: 10.1212/WNL.0000000000009818. [DOI] [PubMed] [Google Scholar]
- 64.Fang XT, Mecca AP, Naganawa M, O’Dell RS, Chen M-K, van Dyck CH, et al. ICA-derived sources of synaptic density PET ([11C]UCB-J) relate to cognitive impairment severity in Alzheimer’s disease. Alzheimer’s Dement. 2020;16:e041197. [Google Scholar]
- 65.Bae JR, Lee W, Jo YO, Han S, Koh S, Song WK, et al. Distinct synaptic vesicle recycling in inhibitory nerve terminals is coordinated by SV2A. Prog Neurobiol. 2020;194:101879. doi: 10.1016/j.pneurobio.2020.101879. [DOI] [PubMed] [Google Scholar]
- 66.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. 2008;32:S56–9. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 67.Gutin I. In BMI we trust: reframing the body mass index as a measure of health. Soc Theory Health. 2018;16:256–71. doi: 10.1057/s41285-017-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiura M, Sakata M, Ishii K, Toyohara J, Oda K, Nariai T, et al. Central μ-opioidergic system activation evoked by heavy and severe-intensity cycling exercise in humans: a pilot study using positron emission tomography with 11C-carfentanil. Int J Sports Med. 2017;38:19–26. doi: 10.1055/s-0042-114779. [DOI] [PubMed] [Google Scholar]
- 69.Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife. 2016;5:e15092. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, et al. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behavioural Brain Res. 2017;323:56–67. doi: 10.1016/j.bbr.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beserra AHN, Kameda P, Deslandes AC, Schuch FB, Laks J, Moraes HSD. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother. 2018;40:360–68. doi: 10.1590/2237-6089-2017-0155. [DOI] [PubMed] [Google Scholar]
- 72.Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neurosci Biobehav Rev. 2013;37:2489–503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 73.Volkow ND, O’Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry. 2007;164:708–10. doi: 10.1176/ajp.2007.164.5.708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material for Lower synaptic density is associated with psychiatric and cognitive alterations in obesity