Abstract
Deficits in social cognition and functioning are common in major depressive disorder (MDD). Still, no study into the pathophysiology of MDD has examined the social cognition-related neural pathways through which familial risk for MDD leads to depression and interpersonal impairments. Using resting-state fMRI, we applied a graph theoretical analysis to quantify the influence of nodes within the fronto-temporo-parietal cortical social cognition network in 108 generation 2 and generation 3 offspring at high and low-risk for MDD, defined by the presence or absence, respectively, of moderate to severe MDD in generation 1. New MDD episodes, future depressive symptoms, and interpersonal impairments were tested for associations with social cognition nodal influence, using regression analyses applied in a generalized estimating equations approach. Increased familial risk was associated with reduced nodal influence within the network, and this predicted new depressive episodes, worsening depressive symptomatology, and interpersonal impairments, 5–8 years later. Findings remained significant after controlling for current depressive/anxiety symptoms and current/lifetime MDD and anxiety disorders. Path-analysis models indicate that increased familial risk impacted offspring’s brain function in two ways. First, high familial risk was indirectly associated with future depression, both new MDD episodes and symptomatology, via reduced nodal influence of the right posterior superior temporal gyrus (pSTG). Second, high familial risk was indirectly associated with future interpersonal impairments via reduced nodal influence of right inferior frontal gyrus (IFG). Finally, reduced nodal influence was associated with high familial risk in (1) those who had never had MDD at the time of scanning and (2) a subsample (n = 52) rescanned 8 years later. Together, findings reveal a potential pathway for the intergenerational transmission of vulnerability via the aberrant social cognition network organization and suggest using the connectome of neural network related to social cognition to identify intervention and prevention targets for those particularly at risk.
Subject terms: Social neuroscience, Depression
Introduction
Humans are a gregarious species and successful navigation of the social environment is crucial for survival and mental and physical well-being [1]. The human brain matures in the context of early-life species-typical caregiving and is structurally and functionally shaped by social interactions across the life span [2, 3]. Our neocortex evolved and expanded disproportionately compared to other primates, and a large proportion of it is involved in sensory-driven as well as higher-order socio-affective processes to solve ecological problems by means of social bonding [4]. Collectively, these cortical regions are thought to form a core of the human social cognition network [5–7].
Deficits in many components of human social functioning (e.g., social perception, empathy, emotion regulation, theory-of-mind, social decision making, and social approach) are prominent features of major depression disorder (MDD) and operate in a bidirectional manner [8]. Yet, little research into the pathophysiology of MDD has investigated the neural network underlying social-cognitive processes. No study, to our knowledge, has identified ‘neural signatures’ of vulnerability for depression by investigating features of the neural network related to social cognition in individuals at high/increased familial risk for MDD and whether abnormalities related to the integrity of this network are mechanisms through which familial risk for MDD leads to future depressive episodes, severity of symptomatology, and interpersonal maladjustment.
Neuroimaging studies have identified a complex set of fronto-temporo-parietal brain regions that are recruited during carefully curated experimental tasks that engage diverse advanced neurocognitive processes [9–11], charting a social cognition network [12, 13]. This network includes a collection of cortical brain regions, such as the posterior superior temporal gyrus (pSTG), precuneus, and posterior cingulate cortex (PCC)—supporting higher-level top-down explicit social cognitive processes [14], as well as the inferior frontal gyrus (IFG) and supramarginal gyrus (SMG)—containing mirror neurons responsive to observed and executed actions, implicated in fast, implicit, and automatic identification of facial expressions, action-perception, empathy and affective mimicry [15]. In addition, recent meta-analyses [16, 17] have confirmed anatomical similarity between the social cognition network and the brain at rest, and numerous studies have found concordance between resting-state connectivity and brain connectivity during various social-based tasks [6, 18–20]. This has led researchers to speculate that during rest, when non-social external stimuli are absent, the human brain may revert back to a state in which social cognitions are predominant for a continuous cognitive model to evaluate and predict the social environment [19, 21].
Integrity of the social cognition network is hypothesized to foster a “mental protective system” [3, 22, 23] by forming and maintaining stable social relations throughout life [24–26] and supporting mental countermeasures [27–29]; which in turn protect vulnerable social members-functions, and thus mitigate the pernicious effects of social isolation, loss, depression, and adversity [30]. Since social functioning and skills emerge from the interaction between different neural processing levels [7, 9, 31], the social cognition brain structures, which support more higher-order social abilities, not only maintain close interconnectivity but are also connected via multiple ascending and descending projections to other brain sub-limbic and limbic networks [2, 9, 32].
While most of the empirical research on depression concentrates on the ventral affective system and the modulation role of prefrontal cortex, a limited number of studies have focused on the fronto-temporo-parietal social cognition regions and their (un)coordinated interactions. These studies reported reduced activity in the network’s regions [33], as well as reduced functional connectivity between these regions in association with the severity, duration, and the number of depressive episodes [34–36], which were also linked with diminished detection and orientation toward social-environmental cues in depressed patients [37, 38]. Still, most studies have been cross-sectional, identifying correlates of depression within the social cognition network and have not utilized prospective designs to test social cognition network’s predictive validity.
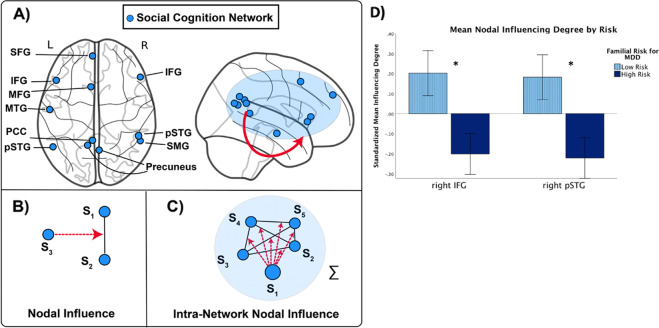
Moreover, functional neuroimaging studies of the social cognition network in depression have focused largely on brain activation or connectivity across discrete brain substrates or networks. This research has not focused on network organization—for instance, the influence that a given brain region has upon the connectivity of other brain regions or networks, and longitudinal studies have yet to test the importance of dynamics of the social cognition network in MDD. To index network organization, we applied Dependency Network Analysis (DEPNA) [39, 40], a newly developed graph theory method to quantify the influence that a given brain region has on the connectivity of all other nodes within a priori defined network of interest (i.e., ‘nodal influence’; see Table S1). This method measures the direction of influence among neural network nodes according to each node’s partial correlation influence (see Fig. 2A–C). The higher this measure, the greater the contribution (importance) that this node has upon the connections of all other nodes—that is, the more likely this node is influencing information flow across a neural network. The DEPNA reveals crucial information that cannot be provided by standard functional connectivity analysis of BOLD signal and other graph theory methods, including identifying important network hubs that may underlie specific processes, and making inferences about the influence hierarchy of a network without relying on the temporal resolution of the analyzed signal, which is an advantage given the poor temporal resolution of fMRI data.
Fig. 2. The social cognition network and dependency network analysis (DEPNA).
In the top panels, we depict the social cognition network: A Blue nodes within social cognition network; In the bottom panels, we explain the core metrics in the dependency network analysis [39]; B correlation influence which measures the relative effect of region S3 on the function correlation between S1 and S2; C node influence within the same network “S” (Intra-network nodal influence); D a repeated measure general linear model (GLM) revealed significant main effect of familial risk status (F = 9.18, p = 0.003). High familial risk compared with low familial risk for MDD was associated with reduced right IFG and pSTG nodal influence on connections within the social cognition network. Standardized means ± SEM is shown. ∗pFDR < 0.05. IFG inferior frontal gyrus, SFG superior frontal gyrus, SMG supramarginal gyrus, MTG middle temporal gyrus, pSTG posterior superior temporal gyrus, PCC posterior cingulate cortex, MFG middle frontal gyrus.
Relative to individuals at low familial risk for depression (i.e., the absence of a history of MDD in previous generations), those at high familial risk (i.e., offspring of individuals with moderate to severe MDD) are 2–4 times more likely to develop depression themselves [41]. The mechanisms through which high familial risk for MDD leads to depression and interpersonal impairments in offspring is unclear; it is a critical question to identify intervention and prevention targets for those particularly at risk. A few social-based studies which have examined the effects of maternal depression on young offspring’s brain function and structure reported abnormalities in a set of temporo-parietal brain regions [42], including the IFG [43, 44] and STS/STG [45, 46].
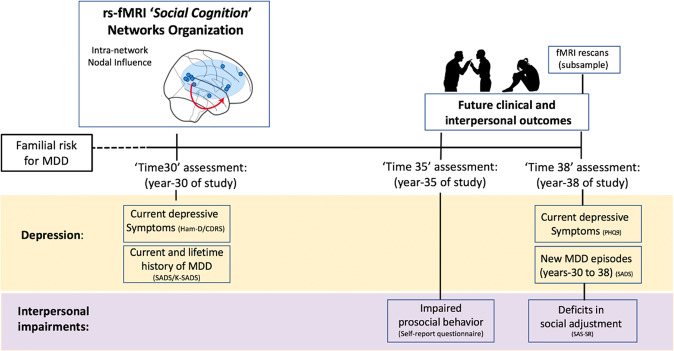
Based on findings to date, using a 3-generation, longitudinal study of familial depression, we sought to identify abnormalities in the organization of the social cognition network during resting-state fMRI (rs-fMRI) in 108 offspring at high familial risk for MDD relative to offspring at low familial risk (by virtue of the presence vs. absence of MDD in the first-generation probands, respectively). We also examined whether these network abnormalities would (1) prospectively predict new major depressive episodes, future depressive symptoms, and interpersonal impairments and (2) mediate the relation between familial risk and these prospective outcomes. Furthermore, we rescanned a subsample of 52 participants 8 years later, which allowed us to examine the extent to which differences in nodal influence between the high- and low-risk groups were conserved over-time, and whether social cognition network abnormalities were still present several years later (For study design and timeline, see Fig. 1).
Fig. 1. Study design and timeline.
Longitudinal study timeline. Familial risk for major depression disorders (MDD) was defined based on first generation’s (G1) history of MDD. In ‘Time 30’, year 30 of the study, participants (G2 and G3) underwent the first fMRI assessment, psychiatric diagnosis was conducted, and depressive/anxiety symptoms were assessed. In ‘Time 35’, year 35 of the study, participants reported prosocial behavior. In ‘Time 38’, year 38 of the study, psychiatric diagnosis was conducted, depressive symptoms were assessed, participants were assessed on an interpersonal measure, and a subsample underwent a second set of fMRI scans.
Three primary hypotheses were tested
Individuals at high/increased familial risk for MDD would manifest aberrant functional connectivity of the social cognition network, namely reduced nodal influence on connections within the network (Intra-network nodal influence).
Reduced social cognition nodal influence would predict new major depressive episodes (first onset or recurrent episodes), more severe depressive symptoms (assessed 8 years after MRI scanning), and interpersonal impairments (lower prosocial behavior and worse social adjustment, assessed 5 and 8 years after MRI scanning, respectively), adjusting for current depressive and anxiety symptoms and current/lifetime history of major depressive and anxiety disorders (Prospective associations).
Reduced nodal influence on connections within the network would mediate the association between high familial risk for MDD and an individual’s future depression and interpersonal impairment (Mediation).
We further explored whether similar mechanisms of reduced social cognition nodal influence would be found in connections between this network and two other neural networks involved in social processing, the cingulo-insular interoception and the limbic emotional processing networks (Inter-network nodal influence; Supplementary section; Fig. S1b, e).
Method
The Institutional Review Board of the New York State Psychiatric Institute approved the study procedures. Adult participants provided informed consent; minors provided informed assent, and a parent/guardian provided consent.
Participants
The study began in 1982, with recruitment of first-generation (G1) probands with moderate to severe MDD seeking treatment at outpatient facilities, and a comparison group of adults from the same community with no lifetime psychopathology, as determined by several interviews. Complete details on study design, sample selection, and assessments are reported elsewhere [41].
Briefly, risk status for depression was defined based on G1, such that offspring (second generation and third generation, G2 and G3) were defined as “high risk” if G1 had a history of MDD and were otherwise defined as “low risk”. The high- and low-risk families have been followed prospectively for up to 38 years and up to eight time points (‘Times’). The current study is based on year 30 of the study (‘Time 30’), at which time the first fMRI assessment was completed, and years 35 (‘Time 35’) and 38 (‘Time 38’), at which clinical and interpersonal outcomes data were collected, and a second fMRI assessment was collected in a subsample. The present study utilized data from the G2 (biological children of G1) and G3 (grandchildren of G1) participants who were fMRI-scanned at the ‘Time 30’ after the initial start of the study, from 43 original families (children and/or grandchildren of 43 G1 proband). As shown in Table 1, at Time 30, data were collected from 108 participants with mean age (standard deviation) of 31.8 [14] and 56 (52%) identifying as women. Of the 108 participants, 62 (57%) were subsequent family members of depressed probands (generation 1) (=high-risk families) and 46 of family members of non-depressed probands (generation 1) (=low-risk families). Fifty-eight percent drew from generation 3, with mean age of 21.4 (4.91), and 49% being women. Of the 108 participants in the initial ‘Time 30’ fMRI sample, 94, 93, 92, and 83 participants were assessed on a battery of clinical and interpersonal measures: social adjustment, new MDD episodes, depressive symptoms, and prosocial behavior, respectively, and 52 underwent a second set of fMRI scans, 8 years later [sex: 28 females, 53.8%; age: 21–62 years old, mean: 37.5 + 12.9; risk: 28 from high-risk families, 53.8%, generation: 20 G2, 38.5%]. For study design and timeline, see Fig. 1; for more demographic and clinical characteristics of participants, see (Table 1 and Table S2a, b). Also, we examined short-term (i.e., two resting fMRI runs within the same scanning session) and long-term reliability (i.e., ‘Time 30’ and ‘Time 38’ fMRI scans). All participants were Caucasian, and G1 participants were all drawn from the same community. Exclusion criteria consisted of psychotic symptoms, pregnancy, and MRI contraindications.
Table 1.
Demographic and clinical characteristics of study participants.
| High familial risk (n, %) | Low familial risk (n, %) | Total sample | Test statistic | p value | ||
|---|---|---|---|---|---|---|
| Number of participants (fMRI) | 62 (57%) | 46 (43%) | 108 | |||
| Second generation (G2) | 33 (73%) | 12 (27%) | 45 | |||
| Third generation (G3) | 29 (46%) | 34 (54%) | 63 | |||
| Age (years) | ||||||
| ‘Time 30’: Full sample | 34.1 + 14.3 (median = 33.6; range:13–59) | 28.75 + 12.2 (median = 23.6; range: 12–53) | Mean ± SD:31.83 + 13.6 (median = 27; range:12–59) | F = 4.17 | 0.05 | |
| Second generation (G2) | 45.8 + 7.7 | 47.5 + 5.2 | 46.3 + 7.22 (Range: 27.2–59.1) | F = 0.66 | 0.5 | |
| Third generation (G3) | 20.7 + 5.36 | 22.1 + 4.47 | Mean ± SD: 21.4 + 4.91 (Range: 12–31.5) | F = 1.15 | 0.2 | |
| ‘Time 38’: Full sample | 40.06 + 14.3 (median = 39.4; range: 20–64) | 35.76 + 12.06 (median = 31.73; range: 19–59) | 38 + 13.5 (median = 33.7; range: 19–64) | F = 2.35 | 0.12 | |
| Second generation (G2) | 51.40 + 7.98 | 53.12 + 5.86 | 51.86 + 7.45 (Range: 33.5–65) | F = 0.42 | 0.5 | |
| Third generation (G3) | 26.46 + 5.47 | 28.9 + 4.4 | Mean ± SD: 27.7 + 5.04 (Range: 18–38) | F = 3.28 | 0.1 | |
| Sex | ||||||
| Second generation (G2) | ||||||
| Male | 15 | 6 | 21 | X2 = 0.73 | 0.5 | |
| Female | 18 | 6 | 24 | |||
| Third generation (G3) | ||||||
| Male | 15 | 17 | 32 | X2 = 0.19 | 0.5 | |
| Female | 14 | 17 | 31 | |||
| Marital status (no)a | ||||||
| Single or never married | 24/50 | 26/41 | 50/91 | |||
| Married or remarried | 18/50 | 13/41 | 31/91 | |||
| Separated or divorced | 8/50 | 2/41 | 10/91 | |||
| Educational attainment (no)a | ||||||
| <High school | 2/49 | 0/41 | 2/90 | |||
| High school | 30/49 | 25/41 | 55/90 | |||
| >College | 18/49 | 15/41 | 33/90 | |||
| Annual household income | ||||||
| Mean (SD) | 64,918 (27,484) | 69,146 (24,313) | 66,617 (26,214) | F = 0.63 | 0.42 | |
| Current symptomatology (Time 30) | ||||||
| Current depressive symptoms (z score) (Time 30) | 0.18 (1.04) | −0.24 (0.87) | F = 4.78 | 0.03b | ||
| Current anxiety symptoms (z score) (Time 30) | 0.23 (1.13) | −0.29 (0.67) | F = 7.73 | 0.006b | ||
| Diagnosis | ||||||
| Current/lifetime depressive disorder (up to Time 30) (=Yes) | 34 (54%) | 9 (19%) | 43 (40%) | X2 = 13.71 | 0.0001b | |
| Current/lifetime anxiety disorder (up to Time 30) (=Yes) | 27 (43%) | 7 (15%) | 34 (31%) | X2 = 9.82 | 0.002b | |
| Current/lifetime disruptive disorder (up to Time 30) (=Yes) | 16 (25%) | 7 (15%) | 23 (21%) | X2 = 1.76 | 0.20 | |
| Current/lifetime substance use disorder (up to Time 30) (=Yes) | 18 (29%) | 11(23%) | 29 (26%) | X2 = 0.35 | 0.55 | |
| Current/lifetime suicidal attempts or gesture (up to Time 30) (=Yes) | 9 (14%) | 2 (3%) | 11 (10%) | X2 = 2.98 | 0.08 | |
| Psychotropic medications (ever used) (=Yes) | 27 (43%) | 9 (20%) | 36 (33%) | X2 = 6.83 | 0.009b | |
Depressive symptoms were determined by Hamilton Depression Rating Scale and the Children’s Depression Inventory for adults and children, respectively. Anxiety symptoms were determined by the Hamilton Anxiety Rating Scale and the Revised Children’s Manifest Anxiety Scale for adults and children, respectively. Because the assessment measures for depressive and anxiety symptoms differed in adults vs. children, symptom severity scores were transformed into z-scores. Values are mean ± SD unless specified.
aOnly for participants 18 and older.
bStatistical significance.
Assessments
Psychopathology was assessed at each time point using the semi-structured Schedule for Affective Disorders and Schizophrenia–Lifetime interview for adults SADS [47] and the children’s version (K-SADS) for participants up to 18 years of age [48] at up to eight time points over up to 38 years. Interviews were conducted by trained doctoral or master’s level mental health professionals who were blinded to the clinical status of the parents and other generations (see Supplementary Methods). At ‘Time 30’, depressive symptoms were assessed using the Hamilton Depression Rating Scale [49] or the Children’s Depression Rating Scale [50], and anxiety symptoms were determined by the Hamilton Anxiety Rating Scale [51] and the Revised Children’s Manifest Anxiety Scale [52] for adults and children, respectively. We selected measures a priori from a follow-up battery for depressive symptoms that included: (1) Depressive symptoms at ‘Time 38’, using the patient health questionnaire-9 [53], and (2) New MDD episodes (New episodes vs. No new episodes) after ‘Time 30’ and up to ‘Time 38’ of the study, from the SADS and K-SADS.
Interpersonal outcomes included: (1) Prosocial behavior at ‘Time 35’, using a 15 item ‘prosocial’ scale [used here as a synoptic term covering elements of compassion, social connectedness, and human engagement; prosocial scores were transformed into z-scores; see Supplementary Methods and [54]]; and (2) Interpersonal impairment at ‘Time 38’, assessed using the Social Adjustment Scale-Self Report (SAS-SR) Short version, a 32-item widely used, validated measure, that assesses overall social adjustment (e.g., work, social, and leisure activities, relationships with “nuclear” and extended family members) over the past 2 weeks. A higher score indicates greater social impairment. Complete details on the SAS-SR-Short are reported elsewhere [55, 56].
Imaging
Image preprocessing
Structural MRI scans were processed using FreeSurfer V6.0. rs-fMRI images were initially pre-processed with the following steps in FSL: (1) slice timing correction [57, 58], (2) motion correction using rigid body translation and rotation, (3) coregistration of structural and functional images using boundary-based registration [59]. Linear regression using multiple nuisance regressors was applied. Nuisance regressors consisted of global signal, six motion parameters, averaged white matter signal, and averaged cerebrospinal fluid signal, as well as their temporal derivatives (18 regressors in total). Mean Framewise displacement (FD) and voxel-wise differentiated signal variance (DVARS) were computed using fsl_motion_outliers [60]. We also used “scrubbing” by removing outlier volumes, defined as FD > 0.5 mm from the previous frame or DVARS > 50 [61]. If rs-fMRI runs had more than ~30% of the volumes flagged as outliers, the run was removed completely from subsequent analysis. Mean FD and DVARS, as well as the number of scrubbed volumes, are summarized across two groups—see Fig. S2. Finally, the fMRI was spatially smoothed by 8 mm FWHM Gaussian kernel, and temporally band-pass-filtered (0.009–0.08 Hz).
Definition of networks of interest
Relevant comprehensive and updated meta-analyses of neuroimaging studies were used to define the social cognition network [12]. Eleven regions were defined within the social cognition network (see Fig. 2A, blue nodes). Seeds from this network have been repeatedly shown in studies on the social brain and neural network for social cognition to underpin human sensory-driven as well as higher-level social-affective social processes, behavior, and bonding (for reviews and meta-analyses see: [7, 12, 62, 63]). In sum, we generated 11 spherical seeds (radius = 5 mm) from predefined coordinates (see Table S1). Definition of the two other networks of interest (interoception and emotional processing) is described in the Supplementary Methods and Materials.
Functional connectivity analysis
We first extracted the average time series from each seed region. We then adopted a newly developed graph-based analysis, DEPNA [39], to examine the directional information flow between different nodes or networks. The basic calculation unit of DEPNA is called correlation influence, or nodal influence, which measures the relative effect of a given node, S3, on the correlation between two other nodes, S1 and S2 (Fig. 2B). The mathematical definition of nodal influence of S3 is: Nodal Influence (S3) = Correlation (S1, S2) − Partial correlation (S1, S2 | S3). Extending this to the network level, we define intra-network nodal influence as the sum of the nodal influences of S3 upon all pairwise combinations of nodes within the network in which S3 is found (Fig. 2C). We utilized this method to evaluate the nodal influence of each of the 11 nodes of the social cognition network based on its intra-network (social cognition network). See [39] for a detailed description of this method.
Analytic strategy
Regressions analysis
All analyses applied a generalized estimating equations (GEE) [64]; approach by means of the GENMOD procedure in the SAS software package, so as to estimate parameters while adjusting for the correlation of within-subject data (longitudinal studies) and other studies in which data are clustered within subgroups (nonindependence resulting from family relatedness). GEE models can handle a variety of correlated measures that arise from family research or correlated data that arise from longitudinal studies [65–67]. To accurately reflect the original study design, all analyses adjusted for generation when analyzing the sample. In addition, age and sex were considered a priori confounding variables and were included in all statistical models. We first examined the associations between familial risk for MDD (high = 1; low = 0) and nodal influence on connections within the social cognition network (Intra-network nodal influence) at ‘Time 30’. Using separate linear regressions (for each social cognition predefined region), with familial risk status as the independent variable and the nodal influence on the network connections as the dependent variables (continuous variables), while adjusting for confounding variables as covariates, as well as current/lifetime history of MDD up to ‘Time 30’ (no MDD = 0, MDD = 1). Next, we examined the longitudinal associations between nodal influence on connections within the social cognition network at ‘Time 30’ and future depression [depressive symptoms at ‘Time 38’ (a continuous variable), and new depressive episodes between ‘Time 30–38’ (no new episodes = 0; new episodes = 1)] and interpersonal impairments [prosocial behavior at ‘Time 35’ (a continuous variable) and social impairments at ‘Time 38’ (a continuous variable)]. Linear regressions (for each social cognition region) were used for continuous outcomes (depressive symptoms and interpersonal impairments) and logistic regressions for binary outcomes (new depressive episodes). Social cognition network nodal influence was the independent variable and depressive and interpersonal outcomes as the dependent variables. We controlled for confounding variables and included current/lifetime history of MDD and Anxiety disorder up to ‘Time 30’ and current depressive and anxiety symptoms at ‘Time 30’ (a continuous variable) as covariates, so that directionality of the associations could be more clearly modeled.
We controlled the false discovery rate (FDR) using the Benjamini-Hochberg (B-H) procedure [68]. Under this procedure, the adjusted alpha level that yields an overall FDR of 5% is 0.01, so we report all p values equal or less than 0.01 as significant, under this adjustment.
Mediation analysis
To examine whether social cognition nodal influence mediated the associations between family risk for MDD and depressive and interpersonal outcomes, four mediation tests were performed using PROCESS Macros for SPSS 26, developed by Preacher and Hayes [69]. We first examined the total effects of the family risk for MDD (independent variable) on the outcomes (dependent variables) using linear or logistic regressions. Next, we ran the mediation analyses. All mediation analyses included family risk for MDD as the “A” factor, nodal influence as the “B” factor (mediator) and future outcomes as the “C” factor. Unstandardized indirect effects were computed for each of 5000 bootstrapped samples, and the 95% confidence interval was computed by determining the indirect effects at the 2.5th and 97.5th percentiles. Effects of other covariates (age, sex, generation, and current/or lifetime history of MDD) were also controlled for in the mediation analyses.
Results
Demographics and descriptive statistics
As shown in Table 1, significantly different distributions of current/lifetime diagnosis were observed across familial risk status (high vs. low) (p values between 0.002 and 0.0001), and significantly different current symptomatology mean scores were observed across familial risk status (p values between 0.03 and 0.006). As for the future clinical and interpersonal variables (outcomes), number and percentages of participants with new MDD episodes, and mean scores (standard deviation) of prosocial behaviors at ‘Time 35’, and depressive symptoms and social impairments at ‘Time 38’ are displayed in Table S2a. Significantly different distributions were observed across familial risk status (p values between 0.01 and 0.04), with no significant differences between generations (Tables S2b).
Hypothesis 1: Social cognition intra-nodal influence
Table 2 provides a list of findings with an asterisk denoting statistical significance (PFDR < 0.05). We found that high/increased familial risk for MDD was associated with reduced Intra-social cognition network nodal influence of the right IFG (ß = −0.26, p = 0.006, qFDR < 0.05) and right pSTG (ß = −0.22, p = 0.0001, qFDR < 0.05) (see Fig. 2D). Findings held when controlling for current/lifetime history of MDD (MDD up to ‘Time 30’), as well as current anxiety symptoms at ‘Time 30’ and current/lifetime anxiety disorder (Table S8). Also, these findings were similar in the restricted set of individuals (n = 65) who had no current/lifetime history of MDD at the time of the fMRI scan (Table S12), suggesting that lower Inter-social cognition network nodal influence was not due to higher rates of current or lifetime MDD. Interaction terms testing if generation moderated the associations observed in the full sample failed to reach significance (Table S7). Finally, we stratified the data by generation and age range: (1) under 18 years old; (2) 18–45 years old; (3) over 45 years old and ran additional linear regressions. Results showed that regardless of the age range, the high-risk participants showed decreased nodal influence of the right IFG and pSTG on connections within the social cognition network (Table S4). Separate analyses for G2 (Table S5) and G3 (Table S6) generation showed a similar pattern of results.
Table 2.
Social cognition intra-network nodal influence.
| Region (Social cognition) | Standardized beta | SE | 95% CI | Z | p value |
|---|---|---|---|---|---|
| Hypothesis 1—predictor: familial risk for MDD | |||||
| L IFG | −0.14 | 0.08 | −0.18–0.18 | −1.6 | 0.10 |
| R IFG | −0.26 | 0.09 | −0.45 to −0.07 | −2.71 | 0.006* |
| L SFG | 0.11 | 0.10 | −0.08–0.31 | 1.1 | 0.26 |
| R SMG | −0.12 | 0.08 | −0.3–0.04 | −1.4 | 0.15 |
| L Precuneus | 0.05 | 0.10 | −0.15–0.25 | 0.5 | 0.61 |
| R Precuneus | 0.12 | 0.08 | −0.04–0.29 | 1.4 | 0.16 |
| L MTG | 0.06 | 0.11 | −0.14–0.28 | 0.6 | 0.55 |
| R pSTG | −0.22 | 0.05 | −0.33 to −0.11 | −3.8 | 0.0001* |
| PCC | 0.08 | 0.07 | −0.05–0.22 | 1.2 | 0.22 |
| L MFG | −0.03 | 0.09 | −0.19–0.16 | −0.4 | 0.68 |
| Hypothesis 2—prospective associations | |||||
| Outcome: depressive symptomatology (‘Time 38’) | |||||
| L IFG | −0.14 | 0.06 | −0.25 to −0.01 | −2.28 | 0.02 |
| R IFG | −0.16 | 0.07 | −0.33 to −0.04 | −2.58 | 0.01* |
| L SFG | −0.14 | 0.05 | −0.24 to −0.03 | −2.61 | 0.009* |
| R SMG | −0.21 | 0.06 | −0.33 to −0.09 | −3.40 | 0.0007* |
| L Precuneus | 0.001 | 0.06 | −0.12–0.12 | 0.02 | 0.98 |
| R Precuneus | −0.002 | 0.08 | −0.16–0.16 | −0.02 | 0.98 |
| L MTG | −0.008 | 0.07 | −0.16–0.14 | −0.11 | 0.91 |
| R pSTG | −0.2 | 0.06 | −0.33 to −0.06 | −2.85 | 0.003* |
| PCC | −0.22 | 0.06 | −0.34 to −0.09 | −3.51 | 0.0005* |
| L MFG | −0.16 | 0.11 | −0.38–0.06 | −1.38 | 0.16 |
| Outcome: new MDD episodes (‘Time 30–38’) | |||||
| L IFG | 0.35 | 4.52 | 8.5 to −9.23 | 0.08 | 0.93 |
| R IFG | 3.61 | 8.93 | 13.8 to −21.12 | 0.4 | 0.68 |
| L SFG | 1.57 | 4.16 | −6.58–9.73 | 0.38 | 0.70 |
| R SMG | −2.51 | 3.19 | −8.7–3.7 | −0.79 | 0.43 |
| L Precuneus | −0.20 | 4.68 | −9.37–8.97 | −0.04 | 0.96 |
| R Precuneus | 2.13 | 3.35 | −4.44–8.72 | 0.64 | 0.52 |
| L MTG | 5.11 | 5.11 | −2.9–15.72 | 1.12 | 0.29 |
| R pSTG | −14.05 | 5.16 | −24.15 to −3.94 | −2.72 | 0.006* |
| PCC | −8.45 | 5.14 | −18.52–1.62 | 11.62 | 0.1 |
| L MFG | −2.30 | 4.94 | −11.98–7.38 | -0.47 | 0.64 |
| Outcome: prosocial behavior (‘Time 35’) | |||||
| L IFG | 0.19 | 0.07 | 0.01–0.31 | 2.18 | 0.02 |
| R IFG | 0.03 | 0.09 | −0.14–0.21 | 0.37 | 0.70 |
| L SFG | −0.07 | 0.07 | −0.21–0.06 | −1.03 | 0.30 |
| R SMG | 0.02 | 0.08 | −0.14–0.19 | 0.30 | 0.76 |
| L Precuneus | −0.05 | 0.07 | −0.20–0.10 | −0.66 | 0.50 |
| R Precuneus | −0.05 | 0.05 | −0.17–0.12 | −0.94 | 0.34 |
| L MTG | −0.04 | 0.10 | −0.24–0.16 | −0.38 | 0.70 |
| R pSTG | 0.10 | 0.08 | −0.07–0.25 | 1.12 | 0.25 |
| PCC | 0.08 | 0.07 | −0.06–0.23 | 1.06 | 0.28 |
| L MFG | −0.02 | 0.09 | −0.19–0.15 | −0.23 | 0.82 |
| Outcome: social impairments (‘Time 38’) | |||||
| L IFG | −0.19 | 0.08 | −0.35 to −0.02 | −2.22 | 0.02 |
| R IFG | −0.20 | 0.07 | −0.35 to −0.04 | −2.58 | 0.008* |
| L SFG | −0.06 | 0.07 | −0.21–0.08 | −0.90 | 0.36 |
| R SMG | −0.05 | 0.12 | −0.29–0.19 | −0.42 | 0.67 |
| L Precuneus | −0.16 | 0.07 | −0.31 to −0.02 | −2.24 | 0.02 |
| R Precuneus | 0.008 | 0.09 | −0.18–0.19 | 0.08 | 0.93 |
| L MTG | −0.16 | 0.07 | −0.3 to −0.008 | −2.08 | 0.02 |
| R pSTG | −0.07 | 0.09 | −0.2–0.12 | −0.75 | 0.45 |
| PCC | −0.23 | 0.09 | −0.41 to −0.05 | −2.51 | 0.01* |
| L MFG | 0.03 | 0.11 | −0.19–0.26 | 0.27 | 0.78 |
Controlled for age, sex, generation, history of MDD (up to Time 30), and current depressive symptoms at Time 30. Higher scores in PHQ9 denote more severe depressive symptoms. New episodes of MDD: 1 = Yes, 0 = No episode. Higher scores in “prosocial behavior” denote greater prosocial behavior; Higher scores in Social Adjustment Scale (SAS-SR) denote poorer social adjustment/higher interpersonal impairment.
IFG inferior frontal gyrus, SMG supramarginal gyrus, MTG middle temporal gyrus, pSTG posterior superior temporal gyrus, PCC posterior cingulate cortex, SFG superior frontal gyrus.
*P < 0.05 FDR corrected.
Hypothesis 2: Prospective associations
Associations between clinical and interpersonal outcomes are shown in Table S3. Table 2 provides a list of findings with an asterisk denoting statistical significance (PFDR < 0.05). In each of the following models, we added current/ lifetime history of MDD and current depressive symptoms (‘Time 30’) as covariates.
Depression—reduced Intra-social cognition network nodal influence of the left IFG (ß = −0.14, p = 0.02, did not withstand FDR correction), right IFG (ß = −0.16, p = 0.01, PFDR < 0.05), left superior frontal gyrus (SFG; ß = −0.14, p = 0.009, PFDR < 0.05), right SMG (ß = −0.21, p = 0.0007, PFDR < 0.05) as well the right pSTG (ß = −0.2, p = 0.003, PFDR < 0.05) and the posterior cingulate cortex (PCC; ß = −0.22, p = 0.0005, PFDR < 0.05) predicted more severe depressive symptoms, 8 years later. Reduced nodal influence of the right pSTG predicted new MDD episodes (B = −0.14, p = 0.006, PFDR < 0.05).
Interpersonal impairments—reduced Intra-social cognition network nodal influence of the left IFG (ß = 0.19, p = 0.02, did not withstand FDR correction) predicted worse prosocial behavior, 5 years later. Reduced social cognition Intra-network nodal influence of the left IFG (ß = −0.19, p = 0.02, did not withstand FDR correction), right IFG (ß = −0.2, p = 0.008, PFDR < 0.05), left middle temporal gyrus (MTG; ß = −0.16, p = 0.02, did not withstand FDR correction), and PCC (ß = −0.23, p = 0.01, PFDR < 0.05) predicted future deficits in interpersonal adjustments, 8 years later.
Results remained significant after controlling for current anxiety symptoms at ‘Time 30’ and current/lifetime anxiety disorder (Table S8). Interaction terms testing whether generation moderated the associations observed in the full sample failed to reach significance (Table S7). Further results of age and generation effects are shown in Table S4–6. Findings were similar in the restricted set of individuals (n = 65) who had no current/lifetime history of MDD at the time of the fMRI scan (up to ‘Time 30’) (Table S13, 14), indicating that a lower degree of intra-social cognition network nodal influence was also predictive of first onset of depression, depressive symptoms, and interpersonal impairments. Also, for individuals with a current/lifetime history of MDD at time of fMRI scan (n = 36), reduced nodal influence of the right pSTG predicted recurrent MDD episodes after ‘Time 30’ and until ‘Time 38’ (Table S15).
Hypothesis 3: Mediation
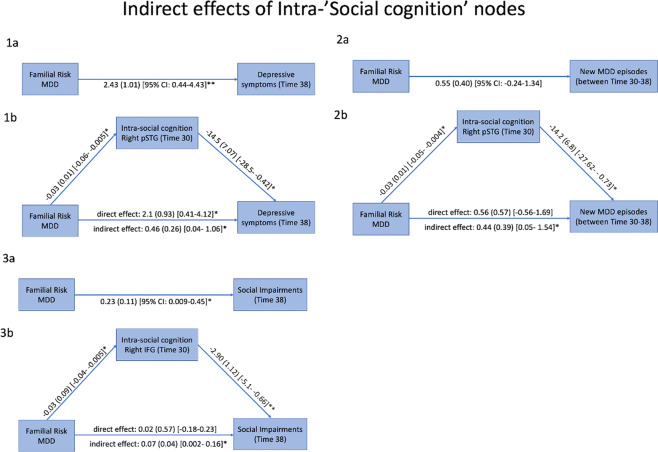
To examine whether reduced Intra-social cognition network nodal influence accounted for the association between familial risk for MDD and future depression and interpersonal outcomes, we ran four mediation analyses and included age, sex, generation, and current/lifetime history of MDD as covariates. We restricted these analyses to two key nodes within the social cognition network; the right IFG and right pSTG, in which increased familial risk for MDD was associated with reduced nodal influence, and reduced nodal influence predicted clinical and/or interpersonal outcomes. As shown in Fig. 3.1a, b, the relationship between familial risk for MDD and future depressive symptoms, 8 years later, was significant (total effect) and mediated by Intra-network nodal influence of right pSTG. The regression coefficient between increased familial risk for MDD and reduced Intra-network nodal influence of right pSTG was statistically significant, as was the regression coefficient between the reduced intra-network nodal influence of right pSTG and greater future depressive symptoms. The bootstrapped unstandardized indirect effect was 0.45, and the 95% confidence interval ranged from 0.04 to 1.06. Thus, the indirect effect was statistically significant. No significant indirect effect of familial risk on depressive symptoms through right IFG nodal influence was found [indirect effect: 0.225 (0.25), 95% CI: −0.219–0.795]. Next, as shown in Fig. 3.2a, b, the relationship between familial risk for MDD and new depressive episodes (no = 0, episode = 1) was not significant (total effect), but mediated by Intra-network nodal influence of right pSTG. The regression coefficient between high familial risk for MDD and reduced Intra-network nodal influence of right pSTG was statistically significant, as was the regression coefficient between the reduced intra-network nodal influence of right pSTG and new depressive episodes. The bootstrapped unstandardized indirect effect was 0.44 and the 95% confidence interval ranged from 0.05 to 1.54. Thus, the indirect effect was statistically significant. Finally, as shown in Fig. 3.3a, b, the relationship between familial risk for MDD and interpersonal impairments, 8 years later, was significant (total effect) and mediated by Intra-network nodal influence of right IFG. The regression coefficient between high familial risk for MDD and reduced Intra-network nodal influence of right IFG was statistically significant, as was the regression coefficient between reduced Intra-network nodal influence of right IFG and social impairments. The bootstrapped unstandardized indirect effect was 0.07 and the 95% confidence interval ranged from 0.002 to 0.16. Thus, the indirect effect was statistically significant.
Fig. 3. The indirect effects of intra-social cognition network nodal influence on future depression and interpersonal impairments.
1a There was a significant effect of family risk for MDD (0 = low; 1 = increase/high risk) on depressive symptoms at ‘Time 38’. 1b A significant indirect effect was detected, indicating that reduced influence of right pSTG on connections within the social cognition network mediated the effect of family risk on future depressive symptoms. 2a There was a non-significant effect of family risk for MDD on new major depressive episode between ‘Time 30’ and ‘Time 38’. 2b However, a significant indirect effect was detected, indicating that reduced influence of right pSTG on connections within the social cognition network mediated an effect of family risk on future depressive episodes (‘Time 30–38’). 3a There was a significant effect of family risk for MDD on social impairment ‘Time 30’. 3b A significant indirect effect was detected, indicating that reduced influence of right IFG on connections within the social cognition network mediated an effect of family risk on future interpersonal impairments. All covariates were held constant during these analyses but not presented for reasons of simplicity. Higher scores in PHQ9 denote greater severity of depressive symptoms. Higher scores in Social Adjustment Scale (SAS-SR) denote higher social impairments. *P < 0.05; **P < 0.01.
Control and inter-network nodal influence analyses
Control analyses
We explored if reduced nodal influence of the right IFG and pSTG in the high-risk group was specific to connections within the social cognition network or a general feature in the high-risk group. To do so, we examined associations between familial risk for MDD and nodal influence of the right IFG and pSTG on functional connections across the whole brain (defined from Power atlas; [70]), and whether these influencing degrees predicted future clinical and interpersonal impairments. Nodal influences of the right pSTG and IFG on connections across the whole brain were not associated with familial risk for MDD or linked with future outcomes (Table S16).
Inter-network nodal influence analyses (see Supplementary information)
We also conducted identical analyses (Hypotheses 1 and 2) for the inter-network nodal influence, namely, the influence of the social cognition nodes on connections within the interoception and emotional processing networks (Tables S9–11).
Reliability
We found similar associations between familial risk for MDD and social cognition nodal influence in a subsample of 52 participants who were rescanned at ‘Time 38’, 8 years after the initial scans (Table S17). We also assessed the short-term (across two runs within a scanning session) and long-term (between scanning sessions; ‘Times 30–38’) test-retest reliability of social cognition nodal influence based on intraclass correlation coefficient (ICC). Overall nodal influence within the social cognition network showed moderate to good short-term reliability (ICCs = 0.56 + 0.14 within ‘Time 30’, 0.51 + 0.21 within ‘Time 38’), and moderate long-term reliability from ‘Times 30–38’ (ICCs = 0.44 + 0.15). The short-term ICCs of the right IFG and pSTG were 0.49 and 0.47 at ‘Time 30’, 0.61 and 0.47 at ‘Time 38’, and long-term ‘Times 30–38’ ICCs of the two nodes were 0.42 and 0.54, respectively.
Discussion
To our knowledge, this is the first longitudinal study of a high-risk population to test prospective associations between the organization of the social cognition network and subsequent psychopathology and specific facets of social maladjustment. Following individuals at increased familial risk for depression (a high-risk design) over almost a decade and integrating scientific methodologies from diverse fields including psychiatry, psychology, and social neuroscience, we identified potential social cognition network-based biomarkers of vulnerability for developing depression and investigated potential mediational mechanisms for why high family risk for MDD prospectively predicts negative clinical and interpersonal outcomes.
Several key findings emerged from this longitudinal study. First, we applied a novel graph theory analysis to identify important network hubs that may drive socio-cognitive processes, and found that relative to individuals at low familial risk for MDD, those at increased risk showed reduced nodal influence of the right IFG and right pSTG on connections within the social cognition network. Second, we found that reduced nodal influence of various social cognition regions on connections within the network predicted greater severity of depressive symptoms at 8-year follow-up and recurrent and first onset of major depressive episodes across 8 years, in those with and without a current or lifetime history of MDD at Time 30, respectively. Third, we found that reduced nodal influence predicted deficits in interpersonal adjustment (i.e., difficulties in fulfilling various social roles in life) at 8-year follow-up. Findings remained significant after controlling for current depressive and anxiety symptoms as well as current/lifetime major depressive and anxiety disorders. Results suggest that reduced nodal influences of the right pSTG and IFG are network specific as their degree of influencing across the whole brain was not associated with either familial risk for MDD or future outcomes. Also, findings were similar in the full sample and participants with no current or lifetime history of MDD, suggesting that altered neural organization within the social cognition network may be a precursor to depression. Importantly, mediation analyses suggested that reduced nodal influence of right pSTG mediated the effect of familial MDD history on both future depressive symptoms and new MDD episodes, while reduced nodal influence of right IFG mediated the effect of familial MDD history on future deficits in interpersonal adjustment. Finally, we observed moderate to good short-term (within scanning session) and long-term (between scanning sessions) test-retest reliability of our nodal influence measures, higher than that of ROI-to-ROI functional connections reported in recent meta-analyses [71]. Also, associations between Intra-network nodal influence of the right IFG and right pSTG and familial risk for depression were similar over-years.
While previous neuroimaging studies of depression have primarily centered around the failure in the coordinated interaction between the mPFC and limbic areas [72], our findings extend previous findings by demonstrating the putative role of the fronto-temporo-parietal cortical social cognition network in depression development. Through a large-scale network perspective, the right IFG and right pSTG appear to be two major social cognition-related regions that play a less active role in shaping the network connectivity among individuals at increased familial risk for MDD and these regions may be critical mechanisms through which future depression and interpersonal impairments develop.
Our longitudinal findings provide the first evidence for potential pathways—namely, the social cognition network organization—that link familial risk for MDD and individuals’ depression and interpersonal impairment. Interestingly, while both reduced nodal influence of the right pSTG and right IFG were predicted by increased familial risk for MDD, path-analysis models charted two pathways of family risk for MDD—social cognition network organization: one mediated by the right pSTG and is associated with future depression, whereas the other by the right IFG and is linked with interpersonal impairment. Findings indicate that increased family risk for MDD may lead to individual’s aberrant social cognition network organization, namely reduced nodal influence of the right pSTG and right IFG, which in turn may lead to future depression (greater severity of symptoms and new MDD episodes), as well as worse future social adjustment, respectively.
These findings coincide with previous longitudinal magnetoencephalography studies showing that persistent maternal depression impairs the offspring’s ‘social brain’ functionality, namely in the right STS/STG, in response to attachment-specific, social-general cues [45], and others’ distress [46]. Also, studies reported that children at risk for MDD showed resting-state functional connectivity abnormalities in the right IFG, compared to a control group [73], and adolescent females at high familial risk for MDD who developed depression (the “non resilient” group) showed weaker functional connectivity between the right IFG, STG and prefrontal regions [74]. Finally, a study using diffusion tensor imaging tractography found diminished neural concordance in STG connectivity among dyads with a depressed parent [75].
The pSTG, the most integrative node of the social cognition network, is involved in fast-paced bottom-up sensorimotor coupling, such as perception of biological motion [76], and in slower higher-order socio-cognitive predictions, such as mentalizing and intentionality [77, 78]. Reduced functional connectivity between the right pSTG and other brain regions has been found in major depression [79–81], correlated with current episode duration and episode number [82], and linked with changes in depressive symptoms before and after electroconvulsive therapy [83].
The IFG, another core region in the social cognition network [84], plays a major role in emotional empathy [85] and is an important hub of the “mirror neuron system” [86–88]. Our results are in accordance with a plethora of studies highlighting the central role of IFG, and its connectivity with other brain regions in supporting social behavior and bonding [89]. Also, neuroimaging evidence shows that abnormal function and structure of IFG are highly implicated across a range of psychiatric disorders with significant impairments of social cognition and functioning, such as autism, schizophrenia, social anxiety disorder (e.g., [90–92]).
Furthermore, the findings regarding differences in nodal influence of the right IFG and right pSTG related to familial risk for depression were largely consistent over-time (i.e., regardless of when scanning was conducted). We found moderate to good short-term reliability (across two runs within scanning session) and a moderate long-term reliability (between scanning sessions, 8 years apart). Such reliability of nodal influence was much higher than recently published in a meta-analysis and systematic review investigating test-retest reliability of edge-level functional connectivity [71]. These findings allowed us to be more confident about findings and suggest that reduced nodal influence of these two key social cognition regions may reflect stable biomarkers rather than transient phenotypes or clinical states. Still, the moderate long-term reliability of the network integrity the social cognition network is in line with recent neuroscientific research emphasizing the structural and functional plasticity of the ‘situated’ social brain [2], which constantly updating information in response to changing contextual demands and expanding social abilities for the survival and thriving of the human species [26, 28, 32, 93–96].
Study limitations should be noted. We assessed social cognition network organization at rest and not during social task-based fMRI. Future studies are needed to generalize the findings to various multimodal, dynamic, and realistic social stimuli and task paradigms. Second, our sample was Caucasian and thus generalizability across racial and ethnic groups should not be assumed. Third, our findings are correlational in nature and do not imply causation, although the longitudinal design did allow for temporal separation of the predictor, mediator, and outcome in the mediation analysis. Fourth, due to the prospective cohort design, we only had access to the family members that could/would participate, and sample size was limited. This multigenerational study also resulted in abroad range of age of participants and in an unequal generation distribution across the two risk groups, which motivated the addition of age and generation covariates to analyses and further age-range and generation stratification analyses. Still, findings should be replicated on large population datasets with careful sampling to minimize differences between study groups (other than the risk status). Fifth, there were ~13.0% fewer participants at ‘Time 38’ than at ‘Time 30’, and both interpersonal outcomes were measured via self-report questionnaires, each measured once at a different time point, which can result in reporting biases. Sixth, we did not have sufficient data on personality disorder diagnosis and social isolation, which have shown to influence the functionality of the social cognition network [20, 97] and could not consider it as a covariate in the analyses. Finally, while controlling the FDR, it is still important to emphasize the lack of clear-cut a-prior hypotheses about specific brain regions within the social cognition network and their associations with subsequent depression and specific facets of social impairments.
By using our unique three-generation longitudinal design with richly characterized clinical and social functioning data over-time, we were able to identify, for the first time, that regions critical for adaptive social processing, particularly the right pSTG and right IFG, exert low nodal influence over social cognition network in those at high familial risk for MDD and predict future depression and interpersonal impairments. Our findings suggest that aberrant social cognition network organization may serve as a potential pathway for the intergenerational transmission of vulnerability.
Together, our findings provide a promising avenue to uncover the pathogenesis and sequelae of depression, and, hopefully, ignite future longitudinal research into identifying ‘neural signatures’ of vulnerability for depression, clarifying the nature of social functioning impairments in mood disorders, and deciphering connections within the social cognition network which might foster resilience to mental health problems [98]. Finally, our results may have future implications for research into the development of connectivity-based diagnosis, neuromodulation therapies, and early interventions aimed at improving social behavior and socioemotional regulation abilities, particularly among young children and adolescents from families at high risk, to reduce some of their vulnerability toward poor mental health.
Funding and disclosure
In the last 3 years, MMW has received research funding from the NIMH, Brain and Behavior Foundation, Templeton Foundation, and the Sackler Foundation, and has received book royalties from Perseus Press, Oxford Press, and APA Publishing. None of these are a conflict of interest. She received no royalties from the Social Adjustment Scale (SAS-SR) used in the study. JP’s research has been supported by Takeda (formerly Shire) and Aevi Genomics. None of these present any conflict with the present work, and no other authors report any disclosures.
Supplementary information
Acknowledgements
This work was supported in part by grant R01 MH-036197 from the National Institute of Mental Health (MMW, JP, Principal Investigators) and John Templeton Foundation (61330) (MMW, Principal Investigator).
Author contributions
EA and YW equally contributed to the present work. Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work (EA, YW, MMW, DS, AT, and JP); and drafting the work or revising it critically for important intellectual content (EA, YW, MMW, SAS, AT, and JP); and final approval of the version to be published (EA, YW, MMW, CS, MJG, AT, and JP); and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (EA, YW, MMW, CS, DS, MJG, SAS, AT, and JP).
Footnotes
The original online version of this article was revised due to missing affiliations.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/2/2021
A Correction to this paper has been published: 10.1038/s41386-021-01105-3
Contributor Information
Eyal Abraham, Email: eyal.abraham@nyspi.columbia.edu.
Jonathan Posner, Email: jep2111@cumc.columbia.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01065-8.
References
- 1.Wilson EO. The social conquest of earth. New york: WW Norton & Company; 2012.
- 2.Feldman R. The adaptive human parental brain: implications for children’s social development. Trends Neurosci. 2015;38:387–99. doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Feldman R. The neurobiology of human attachments. Trends Cogn Sci. 2017;21:80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar RI. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36:562–72. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- 5.Walker SC, McGlone FP. The social brain: neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides. 2013;47:379–93. doi: 10.1016/j.npep.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, et al. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. Plos One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcalá-López D, Smallwood J, Jefferies E, Van Overwalle F, Vogeley K, Mars RB, et al. Computing the social brain connectome across systems and states. Cereb Cortex. 2018;28:2207–32. doi: 10.1093/cercor/bhx121. [DOI] [PubMed] [Google Scholar]
- 8.Bora E, Berk M. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord. 2016;191:49–55. doi: 10.1016/j.jad.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Kiesow H, Spreng RN, Holmes AJ, Chakravarty MM, Marquand AF, Yeo BT, et al. Deep learning identifies partially overlapping subnetworks in the human social brain. Commun Biol. 2021;4:1–14. doi: 10.1038/s42003-020-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, et al. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull. 2021;147:293–327. [DOI] [PubMed]
- 11.Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–96. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, et al. Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex. 2017;88:124–42. doi: 10.1016/j.cortex.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–79. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 14.Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- 15.Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB. Definition and characterization of an extended social-affective default network. Brain Struct Funct. 2015;220:1031–49. doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer ML. Social by default: characterizing the social functions of the resting brain. Curr Dir Psychol Sci. 2019;28:380–6. [Google Scholar]
- 17.Hyatt CJ, Calhoun VD, Pearlson GD, Assaf M. Specific default mode subnetworks support mentalizing as revealed through opposing network recruitment by social and semantic FMRI tasks. Hum brain Mapp. 2015;36:3047–63. doi: 10.1002/hbm.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavor I, Jones OP, Mars RB, Smith SM, Behrens TE, Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352:216–20. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman MD. Social: why our brains are wired to connect. Oxford: OUP; 2013. [Google Scholar]
- 20.Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, et al. The default network of the human brain is associated with perceived social isolation. Nat Commun. 2020;11:1–11. doi: 10.1038/s41467-020-20039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohmatob E, Dumas G, Bzdok D. Dark control: the default mode network as a reinforcement learning agent. Hum Brain Mapp. 2020;41:3318–41. doi: 10.1002/hbm.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung D. The mental protection system for protective behaviors: the social brain and the mental immune system. J Behav Brain Sci. 2018;8:31. [Google Scholar]
- 23.Tops M, Koole SL, IJzerman H, Buisman-Pijlman FT. Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol Biochem Behav. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Abraham E, Hendler T, Zagoory-Sharon O, Feldman R. Network integrity of the parental brain in infancy supports the development of children’s social competencies. Soc Cogn Affect Neurosci. 2016;11:1707–18. doi: 10.1093/scan/nsw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo O, Gordon I. In and out of synchrony—behavioral and physiological dynamics of dyadic interpersonal coordination. Psychophysiology. 2020;57:e13574. doi: 10.1111/psyp.13574. [DOI] [PubMed] [Google Scholar]
- 26.Bzdok D, Dunbar RIM. The neurobiology of social distance. Trends Cogn Sci. 2020;24:717–33. 10.1016/j.tics.2020.05.016. [DOI] [PMC free article] [PubMed]
- 27.Okruszek Ł, Dolan K, Lawrence M, Cella M. The beat of social cognition: exploring the role of heart rate variability as marker of mentalizing abilities. Soc Neurosci. 2017;12:489–93. doi: 10.1080/17470919.2016.1244113. [DOI] [PubMed] [Google Scholar]
- 28.Yaniv AU, Salomon R, Waidergoren S, Shimon-Raz O, Djalovski A, Feldman R. Synchronous caregiving from birth to adulthood tunes humans’ social brain. Proc Natl Acad Sci. 2021;118. [DOI] [PMC free article] [PubMed]
- 29.Spreng RN, Bzdok D. Loneliness and neurocognitive aging. Adv Geriatr Med Res. 2021;3:2. doi: 10.20900/agmr20210009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman R. What is resilience: an affiliative neuroscience approach. World Psychiatry. 2020;19:132–50. doi: 10.1002/wps.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noonan MP, Mars RB, Sallet J, Dunbar RIM, Fellows LK. The structural and functional brain networks that support human social networks. Behavioural Brain Res. 2018;355:12–23. doi: 10.1016/j.bbr.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimon-Raz O, Salomon R, Bloch M, Romano GA, Yeshurun Y, Yaniv AU, et al. Mother brain is wired for social moments. Elife. 2021;10:e59436. doi: 10.7554/eLife.59436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cusi AM, Nazarov A, Holshausen K, MacQueen GM, McKinnon MC. Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci. 2012;37:154–69. 10.1503/jpn.100179. [DOI] [PMC free article] [PubMed]
- 34.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–72. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolls ET, Cheng W, Du J, Wei D, Qiu J, Dai D, et al. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cogn Affect Neurosci. 2020;15:75–86. doi: 10.1093/scan/nsaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheuer H, Alarcón G, Demeter DV, Earl E, Fair DA, Nagel BJ. Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Res. 2017;266:35–41. doi: 10.1016/j.pscychresns.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweerings J, Zvyagintsev M, Turetsky BI, Klasen M, König AA, Roecher E, et al. Fronto‐parietal and temporal brain dysfunction in depression: A fMRI investigation of auditory mismatch processing. Hum brain Mapp. 2019;40:3657–68. doi: 10.1002/hbm.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob Y, Winetraub Y, Raz G, Ben-Simon E, Okon-Singer H, Rosenberg-Katz K, et al. Dependency network analysis (DepNA) reveals context related influence of brain network nodes. Sci Rep. 2016;6:1–19. doi: 10.1038/srep27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob Y, Shany O, Goldin PR, Gross JJ, Hendler T. Reappraisal of interpersonal criticism in social anxiety disorder: A brain network hierarchy perspective. Cereb Cortex. 2019;29:3154–67. doi: 10.1093/cercor/bhy181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, et al. Offspring of depressed parents: 30 years later. Am J Psychiatry. 2016;173:1024–32. doi: 10.1176/appi.ajp.2016.15101327. [DOI] [PubMed] [Google Scholar]
- 42.Zou R, Tiemeier H, van der Ende J, Verhulst FC, Muetzel RL, White T, et al. Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. Am J Psychiatry. 2019;176:702–10. doi: 10.1176/appi.ajp.2019.18080970. [DOI] [PubMed] [Google Scholar]
- 43.Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry. 2016;80:859–68. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Porto JA, Bick J, Perdue KL, Richards JE, Nunes ML, Nelson CA. The influence of maternal anxiety and depression symptoms on fNIRS brain responses to emotional faces in 5-and 7-month-old infants. Infant Behav Dev. 2020;59:101447. doi: 10.1016/j.infbeh.2020.101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt M, Zeev-Wolf M, Goldstein A, Feldman R. Exposure to early and persistent maternal depression impairs the neural basis of attachment in preadolescence. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;93:21–30. doi: 10.1016/j.pnpbp.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Pratt M, Goldstein A, Levy J, Feldman R. Maternal depression across the first years of life impacts the neural basis of empathy in preadolescence. J Am Acad Child Adolesc Psychiatry. 2017;56:20–29. doi: 10.1016/j.jaac.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Mannuzza S, Fyer AJ, Klein DF, Endicott J. Schedule for affective disorders and schizophrenia—lifetime version modified for the study of anxiety disorders (SADS-LA): rationale and conceptual development. J Psychiatr Res. 1986;20:317–25. doi: 10.1016/0022-3956(86)90034-8. [DOI] [PubMed] [Google Scholar]
- 48.Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;1001:48109. [PubMed] [Google Scholar]
- 51.Hamilton M. The assessment of anxiety states by rating. Br J Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds CR, Richmond BO. Revised children’s manifest anxiety scale, second edition (RCMAS-2) manual. Torrance, CA: Western Psychological Services; 2008.
- 53.Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClintock CH, Lau E, Miller L. Phenotypic dimensions of spirituality: Implications for mental health in China, India, and the United States. Front Psychol. 2016;7:1600. doi: 10.3389/fpsyg.2016.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gameroff MJ, Wickramaratne P, Weissman MM. Testing the Short and Screener versions of the Social Adjustment Scale–Self‐report (SAS‐SR) Int J methods Psychiatr Res. 2012;21:52–65. doi: 10.1002/mpr.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33:1111–5. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- 57.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 58.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 59.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 61.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Happé F, Frith U. Annual research review: towards a developmental neuroscience of atypical social cognition. J Child Psychol Psychiatry. 2014;55:553–77. doi: 10.1111/jcpp.12162. [DOI] [PubMed] [Google Scholar]
- 63.Tso IF, Rutherford S, Fang Y, Angstadt M, Taylor SF. The “social brain” is highly sensitive to the mere presence of social information: An automated meta-analysis and an independent study. Plos one. 2018;13:e0196503. doi: 10.1371/journal.pone.0196503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 65.Cui J, Qian G. Selection of working correlation structure and best model in GEE analyses of longitudinal data. Commun Stat—Simul Comput. 2007;36:987–96. [Google Scholar]
- 66.Galbraith S, Daniel JA, Vissel B. A study of clustered data and approaches to its analysis. J Neurosci. 2010;30:10601–8. doi: 10.1523/JNEUROSCI.0362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Homish GG, Edwards EP, Eiden RD, Leonard KE. Analyzing family data: a GEE approach for substance use researchers. Addictive Behav. 2010;35:558–63. doi: 10.1016/j.addbeh.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 69.Preacher KJ, Hayes AF. Assessing mediation in communication research. London: The Sage sourcebook of advanced data analysis methods for communication research; 2008. p. 13–54.
- 70.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noble S, Scheinost D, Constable RT. A decade of test-retest reliability of functional connectivity: A systematic review and meta-analysis. Neuroimage. 2019;203:116157. doi: 10.1016/j.neuroimage.2019.116157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover CS, Whitfield-Gabrieli S, et al. Neural markers of depression risk predict the onset of depression. Psychiatry Res. 2019;285:31–39. doi: 10.1016/j.pscychresns.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. 2018;75:493–502. doi: 10.1001/jamapsychiatry.2017.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham E, Posner J, Wickramaratne PJ, Aw N, van Dijk MT, Cha J, et al. Concordance in parent and offspring cortico-basal ganglia white matter connectivity varies by parental history of major depressive disorder and early parental care. Soc Cogn Affect Neurosci. 2020;15:889–903. doi: 10.1093/scan/nsaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jastorff J, Popivanov ID, Vogels R, Vanduffel W, Orban GA. Integration of shape and motion cues in biological motion processing in the monkey STS. Neuroimage. 2012;60:911–21. doi: 10.1016/j.neuroimage.2011.12.087. [DOI] [PubMed] [Google Scholar]
- 77.Levy J, Goldstein A, Feldman R. Perception of social synchrony induces mother–child gamma coupling in the social brain. Soc Cogn Affect Neurosci. 2017;12:1036–46. doi: 10.1093/scan/nsx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hein G, Knight RT. Superior temporal sulcus—it’s my area: or is it? J Cogn Neurosci. 2008;20:2125–36. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- 79.Lai CH. Promising neuroimaging biomarkers in depression. Psychiatry Investig. 2019;16:662. doi: 10.30773/pi.2019.07.25.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pannekoek JN, Van Der Werff SJA, Meens PH, van den Bulk BG, Jolles DD, Veer IM, et al. Aberrant resting‐state functional connectivity in limbic and salience networks in treatment‐naive clinically depressed adolescents. J Child Psychol Psychiatry. 2014;55:1317–27. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- 81.Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo CC, Hyett MP, Nguyen VT, Parker GB, Breakspear MJ. Distinct neurobiological signatures of brain connectivity in depression subtypes during natural viewing of emotionally salient films. Psychological Med. 2016;46:1535. doi: 10.1017/S0033291716000179. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Wei Q, Wang L, Zhang H, Bai T, Cheng L, et al. Functional reorganization of intra‐and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp. 2018;39:1403–11. doi: 10.1002/hbm.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–70. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 85.Perry D, Hendler T, Shamay-Tsoory SG. Can we share the joy of others? Empathic neural responses to distress vs joy. Soc Cogn Affect Neurosci. 2012;7:909–16. doi: 10.1093/scan/nsr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 87.Gallese V, Rochat MJ, Berchio C. The mirror mechanism and its potential role in autism spectrum disorder. Developmental Med Child Neurol. 2013;55:15–22. doi: 10.1111/j.1469-8749.2012.04398.x. [DOI] [PubMed] [Google Scholar]
- 88.Abraham E, Hendler T, Shapira-Lichter I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R. Father’s brain is sensitive to childcare experiences. Proc Natl Acad Sci. 2014;111:9792–7. doi: 10.1073/pnas.1402569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kogler L, Müller VI, Werminghausen E, Eickhoff SB, Derntl B. Do I feel or do I know? Neuroimaging meta-analyses on the multiple facets of empathy. Cortex. 2020;129:341–55. doi: 10.1016/j.cortex.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon I, Wyk Vander, Bennett BC, Cordeaux RH, Lucas C, Eilbott MV, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci. 2013;110:20953–8. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacob Y, Shany O, Goldin PR, Gross JJ, Hendler T. Reappraisal of interpersonal criticism in social anxiety disorder: A brain network hierarchy perspective. Cereb Cortex. 2019;29:3154–67. doi: 10.1093/cercor/bhy181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takei Y, Suda M, Aoyama Y, Yamaguchi M, Sakurai N, Narita K, et al. Temporal lobe and inferior frontal gyrus dysfunction in patients with schizophrenia during face-to-face conversation: a near-infrared spectroscopy study. J Psychiatr Res. 2013;47:1581–9. doi: 10.1016/j.jpsychires.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 93.Djalovski A, Dumas G, Kinreich S, Feldman R. Human attachments shape interbrain synchrony toward efficient performance of social goals. Neuroimage. 2021;226:117600. doi: 10.1016/j.neuroimage.2020.117600. [DOI] [PubMed] [Google Scholar]
- 94.Abraham E, Hendler T, Shapira-Lichter I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R. Father’s brain is sensitive to childcare experiences. Proc Natl Acad Sci. 2014;111:9792–7. doi: 10.1073/pnas.1402569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martínez-García M, Paternina-Die M, Barba-Müller E, Martín de Blas D, Beumala L, Cortizo R, et al. Do pregnancy-induced brain changes reverse? The brain of a mother six years after parturition. Brain Sci. 2021;11:168. doi: 10.3390/brainsci11020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valk SL, Bernhardt BC, Trautwein FM, Böckler A, Kanske P, Guizard N, et al. Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Sci Adv. 2017;3:e1700489. doi: 10.1126/sciadv.1700489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mier D, Lis S, Esslinger C, Sauer C, Hagenhoff M, Ulferts J, et al. Neuronal correlates of social cognition in borderline personality disorder. Soc Cogn Affect Neurosci. 2013;8:531–7. doi: 10.1093/scan/nss028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feldman R. Social behavior as a transdiagnostic marker of resilience. Annu Rev Clin Psychol. 2021;17:5.1–5. doi: 10.1146/annurev-clinpsy-081219-102046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.