Abstract
Resting state functional connectivity (rsFC) offers promise for individualizing stimulation targets for transcranial magnetic stimulation (TMS) treatments. However, current targeting approaches do not account for non-focal TMS effects or large-scale connectivity patterns. To overcome these limitations, we propose a novel targeting optimization approach that combines whole-brain rsFC and electric-field (e-field) modelling to identify single-subject, symptom-specific TMS targets. In this proof of concept study, we recruited 91 anxious misery (AM) patients and 25 controls. We measured depression symptoms (MADRS/HAMD) and recorded rsFC. We used a PCA regression to predict symptoms from rsFC and estimate the parameter vector, for input into our e-field augmented model. We modeled 17 left dlPFC and 7 M1 sites using 24 equally spaced coil orientations. We computed single-subject predicted ΔMADRS/HAMD scores for each site/orientation using the e-field augmented model, which comprises a linear combination of the following elementwise products (1) the estimated connectivity/symptom coefficients, (2) a vectorized e-field model for site/orientation, (3) rsFC matrix, scaled by a proportionality constant. In AM patients, our connectivity-based model predicted a significant decrease depression for sites near BA9, but not M1 for coil orientations perpendicular to the cortical gyrus. In control subjects, no site/orientation combination showed a significant predicted change. These results corroborate previous work suggesting the efficacy of left dlPFC stimulation for depression treatment, and predict better outcomes with individualized targeting. They also suggest that our novel connectivity-based e-field modelling approach may effectively identify potential TMS treatment responders and individualize TMS targeting to maximize the therapeutic impact.
Subject terms: Cognitive control, Depression
Introduction
Major depressive disorder (MDD) is characterized by depressed mood or loss of interest or pleasure [1]. Symptoms include feelings of worthlessness; indecisiveness or difficulty concentrating, change in weight, appetite, and sleep; psychomotor agitation; and suicidal ideation [1]. According to the World Health Organization (WHO) World Mental Health Surveys Initiative [2], lifetime prevalence rates within the United States average 16.9%, with women showing higher prevalence than men (20.2% vs. 13.2%) [2]. The most common treatments for MDD include antidepressant medication, psychotherapy/cognitive behavioral therapy, or a combination of the two (https://www.nimh.nih.gov/health/statistics/major-depression.shtml). Although antidepressant use is prevalent, there are reports of mixed efficacy [3]. Repetitive transcranial magnetic stimulation (rTMS) to the left dorsolateral prefrontal cortex (dlPFC) has been cleared by the US Food and Drug Administration, and has been shown to be effective as an antidepressant treatment in treatment resistant depression, a patient population not responsive to or tolerant of previous antidepressant medication trials [4–6]. Although many studies find a significant reduction in depressive symptoms following rTMS treatment [7–13], there have also been studies finding limited efficacy for major depression [14, 15], leaving room for improvement.
One possible explanation for the heterogeneity in TMS treatment response for major depression is that the standard scalp-based targeting approach (i.e., the 5 cm rule) does not account for individual variations in cortical anatomy, and often selects regions outside the dlPFC, which may explain some of the modest therapeutic effects [16]. Image-guided TMS incorporating MRI-neuronavigation, taking into account individual differences in brain anatomy, can target specific functional brain networks with greater accuracy [17, 18]. Using structural MRI to locate a specific site at the junction of BA 9 and 46 has also shown greater clinical efficacy in reducing depression than the traditional 5 cm rule [15]. More recent approaches have used functional MRI (fMRI) to target regions that are downstream of the dlPFC, like the subgenual anterior cingulate cortex (sgACC) [19–21] that are thought to be dysfunctional in MDD patients [22–25]. Key to this approach is to use functional connectivity of the downstream site to identify the dlPFC site with the strongest connectivity. Finally, using e-field modelling can improve accuracy even further. Indeed, a recent report estimates the difference between conventional neuronavigated targeting and e-field informed target to be up to 14 mm [26].
Connectivity-based approaches represent the current cutting-edge in the field and are an improvement on the standard scalp-based targeting approach used clinically. However as currently implemented, they typically suffer from two fundamental limitations, which we address in the current work using a novel computational model based on whole brain functional connectivity and electric-field modelling. The first limitation is that by focusing on a single downstream region to define the TMS target they do not account for off-target effects in other downstream regions. To address this limitation, we use electric-field modelling to estimate the changes in connection strength across the entire brain, thus accounting for changes in connectivity that would otherwise be considered “off-target”. The second limitation is that most connectivity-based targeting approaches do not typically quantify the link between functional connectivity and symptoms, making it difficult to make a quantitative prediction about symptom change following neuromodulation. To address the second limitation, we use a PCA regression approach to quantify the relationship between functional connectivity and depression symptoms, and use the resulting coefficients to estimate the net effect of TMS on connectivity and thus symptoms. The overall concept is to generate a predicted symptom change score following neuromodulatory TMS at a particular site and stimulation. We then iterate the model across sites and orientations to find the site/orientation combination that leads to the maximal reduction in MDD symptoms. Importantly, because this approach is data-driven it has an added benefit of being both site and symptom independent.
The current work is a proof of concept demonstration of this novel targeting approach using structural and fMRI collected from the Dimensional Connectomics of Anxious Misery dataset (See Seok et al., 2020 [27] for full characterization of cohort). Although we used a dimensional approach to recruit a diagnostically heterogeneous patient sample, it is important to note the corresponding DSM-5 diagnoses represented in the sample all have data supporting therapeutic use of TMS [4–13, 28–35]. The goal is to characterize the relationship between symptoms and connectivity, then model the effect of TMS on connectivity in order to predict the change in symptoms that would be likely to occur given stimulation at a particular site and orientation. In the current work, we apply this modeling approach to two targets, a positive control left dlPFC and a negative control motor cortex (M1), based on targeting guidelines from the literature (See Supplementary Table 1 and 2). We use bootstrapping to estimate the reliability of the targets identified using this approach at the single-subject level, and permutation testing to determine whether this approach makes group-level predictions that are consistent with the literature [36–38].
Materials and methods
Participants
One hundred eighteen participant (Age (years): M = 28.42, SD = 7.92) entries were pulled from the Dimensional Connectomics of Anxious Misery dataset [27], and included anxious misery (AM) participants (93 total, 64 were females) and healthy controls (25 total, 16 were females; See Supplementary Table 3). Mean (SD) age of the AM cohort was M = 28.29 (8.2) and of healthy controls M = 28.92 (6.91). Group assignment was based on the Neuroticism score on the NEO Five-Factor Inventory (NEO FFI) rather than on diagnosis in keeping with the RDoC concept of finding symptom domains across diagnoses. The population distribution of Neuroticism scores was estimated from a previously collected sample of 635 adults [39]. Different distributions were used for males (M = 19.1 ± 7.1) and females (M = 22.2 ± 7.9). Participants were assigned to the AM group if their Neuroticism scores were at least 1 SD above the mean (males ≥ 26.2 and females ≥ 30.1). The mean Neuroticism score for males and females in the AM group were as follows (Males = 33.04 ± 3.39; Females = 32.82 ± 4.65), while the mean Neuroticism score for males and females in the HC were as follows (Males = 23.33 ± 37.07; Females = 23 ± 3.58). Among the AM group, 24 were medicated, while 69 were unmedicated. In post-hoc analyses, the cross-diagnostic AM cohort included a range of different primary diagnoses, namely MDD (N = 41), persistent depressive disorder (N = 9), social anxiety disorder (N = 5), generalized anxiety (N = 21), and PTSD (N = 17).
Basic Inclusion/Exclusion criteria included MRI contraindications, histories of certain neurological or cognitive disorders and events, histories of exclusionary psychiatric conditions or any other factors that may have affected patient safety (See Seok et al [27]. for complete list). In addition, participants in the AM cohort needed to score a minimum of one (1) standard deviation above a point estimate of the general adult population mean (distributions for males and females were different) in the NEO FFI [40] in order to be included in the study [27]. Conversely, healthy controls needed to score one (1) standard deviation or more below the mean point estimates to be eligible to participate. Subjects were recruited by means of IRB-approved advertisement and phone calls. All participants signed an informed consent form, and the protocol was approved by the Institutional Review Board for human subject research at the University of Pennsylvania. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Procedure
On the initial visit, written consent was obtained from all eligible participants prior to enrolling in the study, followed by a baseline visit with study staff to ascertain their medical history and demographic information. In addition, a research version of the Structured Clinical Interview for DSM-5 (SCID-5-RV) [1] was conducted by a trained member of the staff to document their psychiatric diagnosis and history, and the remaining assessments (See Below) were conducted. In a follow-up visit, a 2 h fMRI scan was conducted, where structural, diffusion, resting state, and task-based fMRI data were collected as part of the larger project.
Assessments
Many clinical measures were used to identify the behavioral and cognitive features of AM. In this project, eligible participants completed 17 self-report and 3 clinician-administered measures. The clinician-administered measures included the Montgomery-Åsberg Depression Rating Scale (MADRS) [41] and the Hamilton Depression Rating Scale (HAMD) [42] in order to assess depression severity.
Model steps
Image acquisition and preprocessing
T1, T2, and resting state fMRI were acquired on a Siemens Prisma 3T using a 64-channel head coil. SimNIBS (Version 2.1) was used to generate 3D head and coil geometries and compute the e-field models [43]. fMRI data were preprocessed using the Human Connectome Project Pipelines v4.0.1 and the eXtensible Connectivity Pipeline (XCP Engine) [44]. For a full description of the image acquisition and preprocessing steps, see the Supplementary Methods.
PCA regression
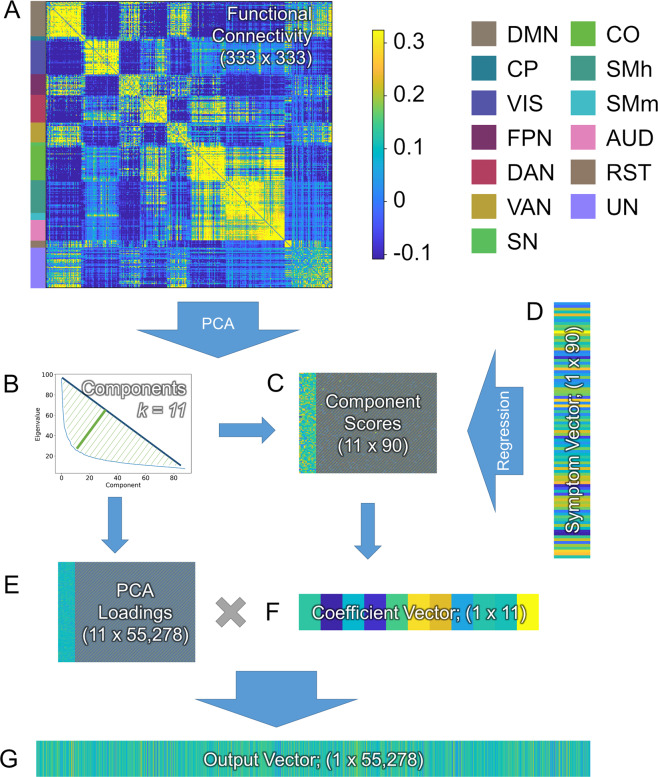
For the first step in our analysis (See Fig. 1 and Appendix for detailed equations), we used principal components analysis (PCA) regression to model the relationship between functional connectivity and symptoms [45, 46, Eq. A2]. We began by conducting a PCA on the functional connectivity data for each group (i.e., AM and control) to reduce noise and minimize collinearity [47]. We restricted attention to the subset of components that explained largest proportions of variability, with the assumption that these contain most of the relevant signal. For this we used a geometric approach that identified the eigenvalue at the elbow of the scree plot (i.e., the eigenvalue furthest from the hypotenuse connecting the first and last eigenvalues). The principal component scores for each of these components were then used to model symptom scores (MADRS and HAMD) in a multiple linear regression. The resulting coefficient estimates were then projected back into feature space (Eq. A3). The result was a vector where each element represents the coefficient for the corresponding functional connection in a linear model predicting symptom score.
Fig. 1. The PCA-Regression data reduction approach used to summarize the relationship between symptoms and connectivity in the current model.
A Resting state functional connectivity (rsFC) is calculated using Pearson’s correlation across all subjects for all regions in the Gordon atlas [49]. B A principal component analysis (PCA) is used to identify orthogonal components in the rsFC data, and a geometric approach is used to identify a minimal number of components that explain a maximal proportion of the variability. C Component scores for the selected components are extracted and entered into a multiple linear regression to predict symptoms (D). The PCA loadings from the selected components (E) are combined with the coefficient vector from the regression (F) using matrix multiplication to create the output vector (G), which is used to represent multiple regression coefficients projected into the rsFC feature space. Network Color key: DMN = Default Mode Network; CP = CinguloParietal; VIS = Visual; FPN = FrontoParietal Network; DAN = Dorsal Attention Network; VAN = Ventral Attention Network, SN = Salience Network, CO = CinguloOpercular, SMh = SomatoMotor (hand), SMm = SomatoMotor (mouth), AUD = Auditory, RST = RetrosplenialTemporal, UN = Unassigned nodes.
Selection of sites and orientations
To characterize the dlPFC, we defined a series of 17 sites along the anterior-to-posterior gradient of the L dlPFC, which was prioritized based on prior clinical efficacy findings (See Supplementary Table 1). This site vector was anchored by three therapeutic sites described in [38] ([−41, 16, 54] based on 5 cm rule, [−36, 39, 43] BA9, [−44, 40, 29] BA46), and extends 12 mm posterior to the 5 mm site and 12 mm anterior to the BA46 site. We also plotted two additional sites along the vector. First is the site corresponding to the Beam/F3 stimulation site typically identified using the EEG 10/20 system [48]. Second is the site along the vector with the maximal anti-correlation with the sgACC. Note that this second site was calculated from the rsFC data for each subject and plotted on the individual subject heatmaps.
To characterize the hand knob, which was used as a negative control region, we defined a series of seven sites along the medial to lateral axis of the primary motor cortex in the left hemisphere (See Supplementary Table 2). This anchor was centered on the hand knob, which was identified from Neurosynth using an association test with the search term “hand”. The center of mass for the primary motor cortex cluster was used as the hand knob coordinate ([−36, −24, 58]). At each site, we generated 24 different e-field models. For each model, the roll and pitch of the TMS coil was defined perpendicular to the scalp. The yaw vector was selected from 24 possible orientation vectors spaced at 15-degree increments.
E-field connectivity matrices
For each participant, the e-field map was downsampled (i.e., averaged across voxels in each parcel) to the Gordon atlas [49] and concatenated into a 1 × 333 vector. This vector was then sorted by e-field magnitude to resemble a scree plot, and thresholded at the value furthest from the hypotenuse connecting the first and last value in the vector (See Fig. 2). To estimate how stimulation at a specific site and orientation might affect connectivity, the downsampled e-field vector was used to form a 333 × 333 matrix where the values in the matrix represent the average current induced in the ROIs for each connection.
Fig. 2. Methods used to summarize electric (e)-field models in connectivity space.
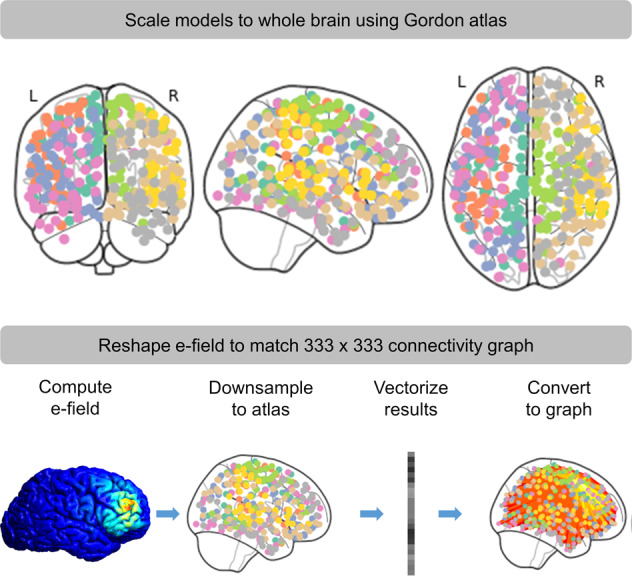
Normalized e-field models were first downsampled to the Gordon atlas [49]. The results were then converted to a 1 × 333 vector. This vector was then used to form a 333 × 333 matrix where the values in the matrix represent the average current induced in the ROIs for each connection.
E-field/connectivity symptom estimates
The predicted neuromodulatory effect of TMS on symptoms was modelled using each participant’s e-field model matrix [50], the single-subject connectivity matrix, and the estimated group-level coefficients derived from the symptom/connectivity regression, according to Eqs A2–3. These calculations were iterated across all sites and orientations and spatially smoothed using a Gaussian kernel (SD = size(heatmap)/10; See MATLAB function smoothdata) to create the heatmap shown in Fig. 3, where each value in the heatmap represents the predicted relative change in symptoms (sans application of an empirically-determined constant scaling factor C) for that subject given hypothetical TMS administered at that particular site and orientation.
Fig. 3. Iteration of model across site and orientation.
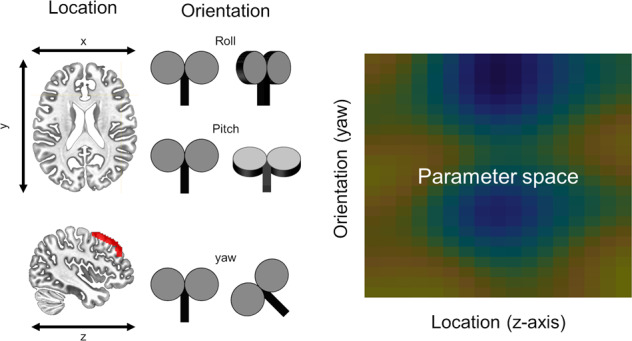
To understand how placement and orientation of the TMS coil might impact symptoms, we computed our model across multiple sites and orientations. Sites were defined at equally spaced points along the anterior to posterior axis of the middle frontal gyrus. Roll and pitch were defined orthogonal to the scalp at each stimulation site. Multiple equally spaced yaw vectors were defined at each stimulation site, representing all possible coil orientations. Electric (e)-field models were conducted at each site/orientation combination, entered into our symptom prediction model, and the results were plotted in this site × orientation heatmap.
Statistical analysis
Individual subject predictions
One goal of this project was to determine whether the modelling approach above could be used to make targeting decisions for the application of TMS to single subjects. Accordingly, to be effective our model would need to show a systematic effect of site and orientation on predicted symptom change in order to be used for single-subject targeting. To examine this possibility, we created single-subject heatmaps corresponding to the predicted relative change in symptoms as a function of site and orientation, and identified the point in these heatmaps that yielded the maximum predicted relative decrease in symptom scores, corresponding to the optimal site/orientation of stimulation for that subject.
We then used bootstrapping to estimate the reliability of this targeting approach. For each of the 10,000 iterations of the bootstrapping analysis, we sampled subjects with replacement from the original distribution, recalculated the PCA regression and the corresponding vector (See Appendix: EQ5). Next we recreated the individual subject heatmaps, and identified the optimal stimulation site. Across bootstrap iterations we calculated the Euclidean distance between the single-subject original optimal site/orientations and the bootstrap-calculated optimal site/orientations. Then for each subject, in order to form a confidence region, we identified the value corresponding to the 95th percentile from the distance metric bootstrap distribution, which was used to display the reliability of the single-subject target in the heatmaps.
Group-level predictions
To determine whether our novel modelling approach predicted significant changes at the group level, for each site/orientation we conducted one-sample t-test of the predicted change in symptoms (i.e., predicted post treatment symptom score—baseline symptom score) against zero. A significant negative t-score would indicate a predicted decrease in symptoms at the group level. Accordingly, the null hypothesis for each site/orientation combination is that the model predicts no significant group-level change in symptoms following a hypothetical treatment at that site/orientation. We repeated this process for all site/orientation combinations, and generated heatmaps from the resulting t-scores. We used cluster-based permutation testing to correct for multiple comparisons across site and orientation [51]. First we conducted 10,000 permutations where the sign of the predicted symptom change was randomly flipped across subjects and permutations. For each permutation, we calculated t-scores at each site/orientation combination within the heatmap. We thresholded these t-scores using a two-tailed alpha of 0.025. Next we identified clusters of t-scores surviving the threshold, summed the values across elements within the cluster, identified the cluster with the maximum summed t-score, and built a null distribution with these max summed t-score values. We then identified the critical values corresponding to the 1.25th and 98.75th percentiles (with alpha = 0.025 to correct for the number of regions of interest tested) of the max summed t-score distribution generated from the permutation procedure. Finally, a two-step thresholding process was implemented to threshold the group-level statistical maps. First, the individual t-scores were thresholded using an alpha of 0.025. Then, summed t-scores from the surviving clusters from these heatmaps were thresholded using the critical values from the max summed t-score null distribution.
Results
PCA regression
Separate PCA regression analyses were conducted for the AM group and the control subjects. For the AM group, 11 signal components were selected, and these components explained 31% of the variability in the functional connectivity signal, 16.8% of the variability in the MADRS scores, and 15.7% of the variability in the HAMD scores (See Table 1 for PCR component β-values for MADRS and HAMD scores and Supplementary Fig. 1 for component item loadings). For the control group, six components were selected, and these components explained 40% of the variability in the functional connectivity signal, 33.7% of the variability in the MADRS scores, and 44.3% of the variability in the HAMD scores (See Supplementary Fig. 2).
Table 1.
PCR beta coefficients predicting clinical measures.
| Predictor | MADRS | HAMD |
|---|---|---|
| Anxious misery group | ||
| Intercept | 21 | 13.22 |
| Component 1 | 0.09 | 0.08 |
| Component 2 | −0.19 | −0.05 |
| Component 3 | 0.04 | 0.08 |
| Component 4 | −0.17 | −0.06 |
| Component 5 | 0.12 | 0.01 |
| Component 6 | 0.28 | 0.25 |
| Component 7 | 0.21 | 0.15 |
| Component 8 | 0.01 | 0.01 |
| Component 9 | 0.08 | 0.04 |
| Component 10 | 0.07 | 0.04 |
| Component 11 | 0.32 | 0.21 |
| Healthy control group | ||
| Intercept | 0.68 | 1.28 |
| Component 1 | 0.02 | 0.07 |
| Component 2 | 0.05 | 0.04 |
| Component 3 | 0.02 | 0.04 |
| Component 4 | 0.02 | 0.05 |
| Component 5 | −0.02 | −0.05 |
| Component 6 | −0.01 | 0.04 |
The stability of the PCA was evaluated using results from the bootstrapping analysis described below. For each of the 10,000 iterations of the bootstrapping analysis, we sampled subjects with replacement from the original distribution, recalculated the PCA and calculated both the number of selected components as well as the total variability explained by the selected components. For the AM group, there was an average of 15.02 ± 2.89 components selected across the bootstrapping iterations and these components explained an average of 53.71 ± 6.63% of the variability in FC across the sample. For the HC group, there was an average of 8.99 ± 4.11 components selected across the bootstrapping iterations and these components explained an average of 75.14 ± 18.22% of the variability in FC across the sample.
Individual subject predictions
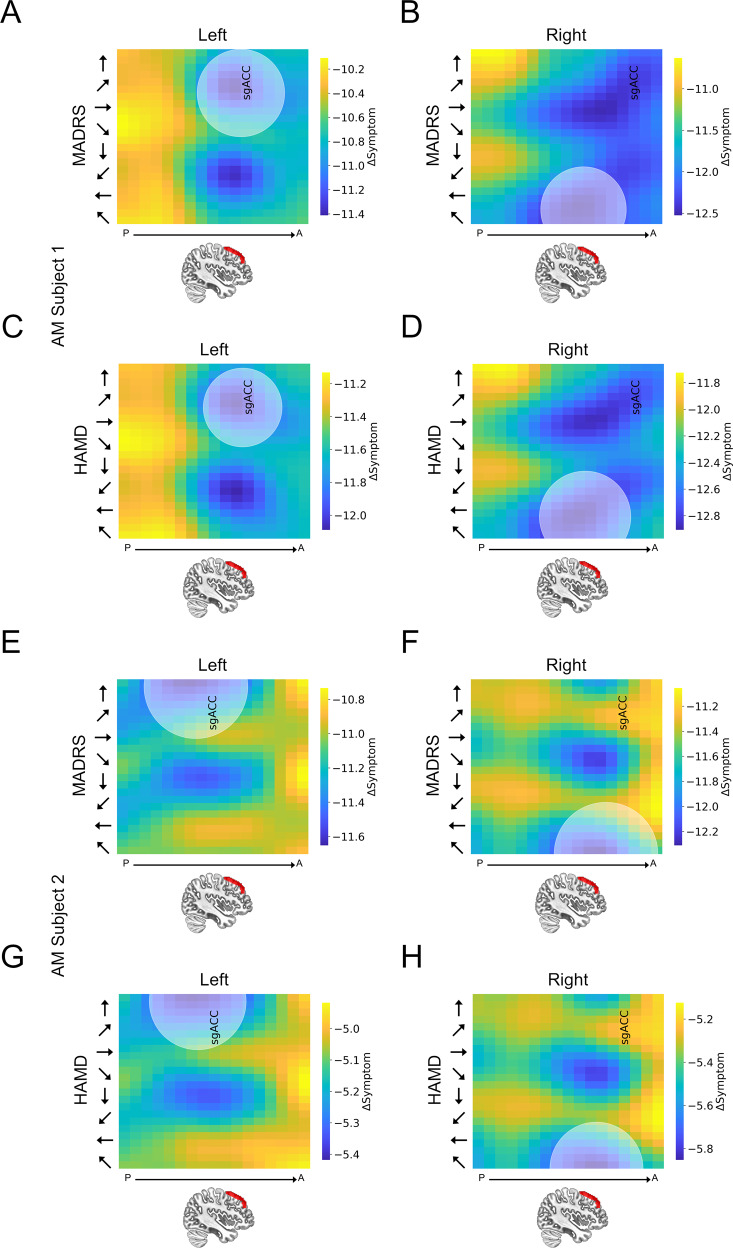
Single subject heatmaps from the AM group show clear systematic patterns of predicted symptom change as a function of both site and orientation (See Fig. 4), suggesting that this modelling approach can be used to simultaneously optimize these two parameters at the single subject level. These patterns are characterized by punctate “cool spots” in the heatmap that identify a clear site/orientation combination that yields maximal predicted symptom reduction. To characterize the variability in our targeting approach, we conducted 10,000 bootstrapping iterations and identified the optimal site/orientation combination on each. We then calculated the Euclidean distance between the bootstrap-derived values and the original values and plotted a circle with an area that corresponds to the 95th percentile in the bootstrapped Euclidean distance distribution on the single subject graphs shown in Fig. 4 and Supplementary Fig. 3. Importantly, this pattern seems to be specific to those in the AM group as control subjects do not show the characteristic “cool spots” in their heatmaps (See Supplementary Fig. 3).
Fig. 4. Individual subject heatmaps plotting dlPFC predictions for the anxious misery group.
A, C, E, G Heatmaps representing the predicted MADRS and HAMD scores following a hypothetical course of TMS treatment to the left dlPFC. B, D, F, H Heatmaps representing the predicted MADRS/HAMD scores following a hypothetical course of TMS treatment to the right dlPFC. Colors represent the predicted change in MADRS/HAMD scores. Y-axis represents coil orientation. X-axis represents location along the Z-axis of the middle frontal gyrus. The center point of the shaded circle on the heatmaps represents the site and orientation of stimulation predicted to have the maximal reduction in symptoms for each subject. The area of the shaded circle represents the variability (i.e., Euclidean distance [95% confidence interval]) in this optimal site assessed using bootstrapping. sGACC = site along vector with maximal anti-correlation with the subgenual anterior cingulate cortex.
Group-level predictions
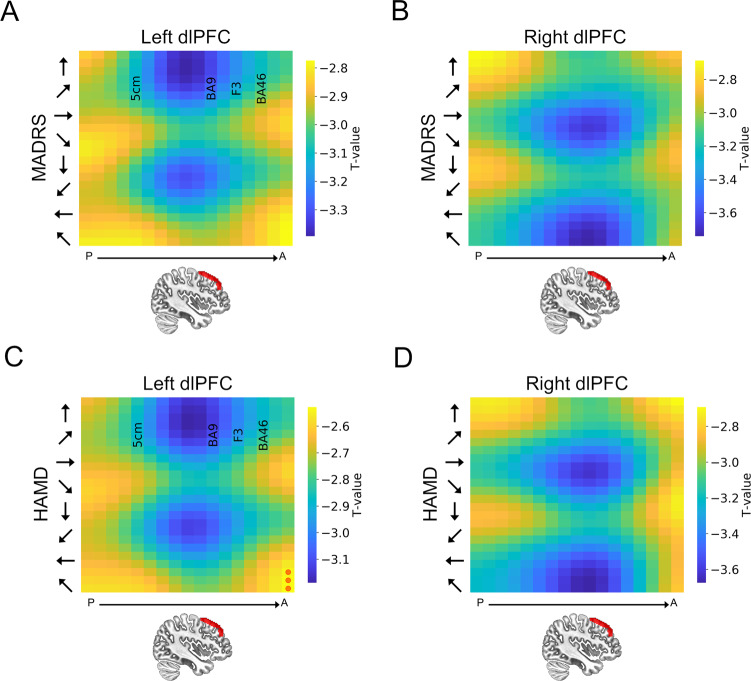
To determine whether the model made significant predictions at the group level, we calculated the predicted change in depression symptoms for each site/orientation combination by subtracting the observed pre-TMS MADRS/HAMD from the predicted post-TMS MADRS/HAMD scores. These difference scores represent the predicted change in depression symptoms, given a specific site/orientation of stimulation. We then compared these values to 0 (i.e., no effect of TMS) using a single sample t-test. We corrected for multiple comparisons across sites/orientations the permutation testing procedure described above. As shown in Fig. 5 our model predicts a significant decrease in MADRS and HAMD scores in AM subjects with both left and right dlPFC stimulation. In the left hemisphere, the cool spots are adjacent to BA9 at 30–45° orientation. In the right hemisphere, the cool spots are positioned slightly anterior to the cool spots in the left hemisphere, and strongest at 120 and 135° orientation. Consistent with the results at the single-subject level, these results suggest that there is a systematic relationship between both site and orientation and predicted symptom change. Importantly, these results are limited to the AM subjects, as the model predicts no significant changes in depression symptoms in the healthy control subjects (See Supplementary Fig. 4). In contrast to the data from the dlPFC, the model does not predict any significant changes in depression symptoms for site/orientation combinations centered on the hand region of M1 for either measures (MADRS/HAMD) in AM patients (Supplementary Fig. 5) or healthy controls (Supplementary Fig. 6).
Fig. 5. Group-level heatmaps plotting dlPFC predictions for the anxious misery group.
A Heatmap representing the predicted MADRS scores following a hypothetical course of TMS treatment to the left dlPFC. B Heatmap representing the predicted MADRS scores following a hypothetical course of TMS treatment to the right dlPFC. C Heatmap representing the predicted HAMD scores following a hypothetical course of TMS treatment to the left dlPFC. D Heatmap representing the predicted HAMD scores following a hypothetical course of TMS treatment to the right dlPFC. Y-axis represents coil orientation. Red circles represent sites where the change in MADRS/HAMD scores was not statistically different from 0. 5 cm = Site in vector corresponding to the 5 cm rule target commonly used in therapeutic applications for depression [38]. BA9 = Site in vector corresponding to Broadmann area 9 [38]. F3 = Site in vector closest to the BEAM/F3 target commonly used in therapeutic applications for depression [48]. BA46 = Site in vector corresponding to Broadmann area 46 [38].
It is important to assess the degree to which variability in image quality affects the predictions made by our model. Accordingly, we calculated tSNR and average motion (RMS across TRs) and correlated these values with the predicted symptom change scores for MADRS and HAMD for both the AM and HC groups (See Supplementary Table 4). For the AM group, image quality metrics were uncorrelated with symptom predictions generated by our model, suggesting that differences in image quality cannot account for our effects in this group. In contrast, for the HC group, average motion across TRs was marginally correlated with predicted changes in both MADRS and HAMD, suggesting that image quality may have played a part in the predictions for this group. Given that the predicted symptom scores reflect the variability in both the symptom and the imaging data, this group difference could be due to the substantially different levels of variability in MADRS/HAMD scores in the AM group compared to the HC group.
Discussion
Here we present a proof of concept methodological study where we propose a model that uses functional connectivity, symptom scores, and electric-field modelling to predict symptom change following a hypothetical course of neuromodulatory treatment with TMS. Our model calculates the relationship between symptoms and connectivity, as well as the effect of TMS on connectivity to yield a predicted change score in the symptom, assuming TMS is delivered at the site and orientation specified in the model. We then tested the model systematically across sites and coil orientations in two cortical locations serving as positive (dlPFC) and negative (M1) controls. For the dlPFC, our model suggests that stimulation at this site would lead to a significant reduction in depression symptoms (MADRS/HAMD) in AM patients but not controls, and the magnitude of this potential effect should vary systematically as a function of both site and orientation. Importantly, this observation is specific to the dlPFC, as our model suggests that stimulation at M1 would likely not result in a significant decrease in MADRS or HAMD, regardless of position or orientation. In addition, these observations seem to be reliable at both the single-subject and the group level, suggesting that this approach has sufficient ability to identify subject-specific TMS targets to maximize symptom reduction.
Consistent with previous work implicating the junction of BA9 and BA46 as a potentially optimal site of stimulation for depression symptoms [52–56], our model suggests that stimulation marginally posterior to this site would maximally reduce both MADRS and HAMD scores across subjects compared to the other sites tested [36]. Our model adds to the work suggesting that rsFC networks can inform TMS targeting [37], and our results are consistent with the findings that individualized rsFC maps may be most informative [38]. Previous research has shown that regions of the dlPFC that are more strongly anticorrelated with sgACC tend to show better clinical efficacy when targeted with therapeutic TMS [52, 57], and the closer the stimulation site is to this optimally anticorrelated dlPFC location, the better the clinical outcome [55, 56, 58]. Importantly, active rTMS to the l dlPFC has been shown to reduce anticorrelation between the dlPFC and sgACC [54, 59, 60], and prospective targeting based on individualized dlPFC—sgACC connectivity leads to a greater reduction in symptoms compared to traditional targeting [54, 55]. In addition, there is some research to suggest that individualized targeting of BA46 through a parcel guided approach can improve treatment response in patients who have previously failed standard TMS targeted at the dlPFC using the 5 cm rule [36]. Based on this work, it could be argued that the current state-of-the-art for individualized TMS targeting for depression is to target sites in the left dlPFC with maximal connectivity with the subgenual cingulate cortex.
However, a review of 33 studies with pre-post resting state shows that although TMS induces robust changes in rsFC, the majority of effects are outside of the stimulated functional network [61]. Indeed, the changes in functional connectivity following rTMS treatment for depression can be seen throughout the brain in regions that are important for affective responding (e.g., insula, amygdala, inferior parietal lobule, etc.), suggesting that the net effects on symptoms may be driven by these large-scale network connectivity changes [62]. Consistent with this idea, treatment response has also been linked to rsFC changes outside of the sgACC [63, 64]. Together these results suggest that a whole brain connectivity model may outperform single connection targeting approaches, like the ones described above.
In keeping with these results, our whole brain connectivity approach has several potential benefits over single connection targeting approaches [52–56]. First, our model uses e-field modelling to account for the variability in electric field spread due to individual differences in head and cortical anatomy, and uses this information to inform targeting. Second, our approach accounts for other connections in the brain that (1) may be impacted by TMS treatment, and (2) contribute to symptom reduction. Importantly, our model uses a data driven approach to weight these connections by the amount of variability they explain in the depression symptoms. Finally, our approach uses agnostic data-driven estimates between behavioral characteristics and functional connectivity to drive targeting. Therefore, this model can be extended to other symptoms/behavioral characteristics where reliable measures of behavioral markers are available (e.g., addiction, anxiety, etc.).
The main focus of the paper was depressive symptoms, which we captured using scores on the MADRS and HAMD. However, it is important to note that the model described here can be applied to other symptom measures as well. In previous work, we have used various symptom clustering approaches to extract distinct symptom measures from available clinical data [65], resulting in several distinct symptom clusters. Although out of the scope of the current work, future studies should determine the degree to which these other distinct symptom clusters could potentially be used to direct TMS targeting. Indeed, defining biotypes for depression and anxiety starting with neural circuit dysfunction [66], may yield additional symptom-specific targets. Accordingly, the model presented here could be used not only to individualize targeting at the single subject level, but it could potentially be extended to individualize targeting at the single session level, where each TMS session in a course of treatment is targeted at a specific symptom or set of symptoms, thereby maximizing the therapeutic effect.
Our approach differs from other connectivity-based targeting approaches, including that of similar paper by Siddiqi et al (2020) [67], in two fundamental ways. First, these studies typically calculate connectivity with the stimulation site using a voxelwise approach. This approach assumes a direct correspondence between the TMS effect and the functional connectivity. That is, it doesn’t take into consideration the focality of the TMS-induced electric field. Our approach weights the connectivity changes proportional to the e-field model, which should yield a more accurate estimate of the TMS effects. Second, despite data showing that the orientation of the coil with respect to the underlying cortical anatomy impacts the electric field at the target site [68], most targeting approaches do not control or account for coil orientation. With our approach, this is built in to the model at the e-field modelling step, and multiple iterations of the model are calculated at each target site with varying yaw orientations. Although real-world tests should be conducted to establish how much improvement is to be gained by controlling for coil orientation, our modelling data suggests that orientation accounts for as much variability in the simulated TMS response as does location.
Our approach is similar to other connectivity-based targeting approaches in that it assumes that pre-stimulation resting state functional connectivity (rsFC) will be predictive of treatment outcome. There are data suggesting that this may be the case. For instance, TBS induces changes in rsFC in networks that are functionally connected to stimulation site [69], and changes in connectivity are correlated with task performance related to the stimulation site [70]. Interleaved TMS/fMRI using resting state-based targeting shows that TMS induces network specific changes in activity in regions strongly vs. weakly connected to the stimulation site [21]. Similarly, baseline resting state connectivity predicts TMS-evoked BOLD responses in targets downstream from the stimulation site [71]. In terms of treatment outcome, baseline dlPFC/striatal connectivity predicts improvement in depression symptoms following a standard rTMS treatment protocol [64]. Similarly, sgACC connectivity at baseline predicted treatment outcome in a recent accelerated iTBS trial, and changes in sgACC connectivity were also associated with reduced depressive symptoms [72]. Finally, connectivity between the dlPFC and the anterior insula at baseline predicted treatment outcome following treatment with both 10 Hz and iTBS [73].
From a broader perspective (presented by Fitzqerald [2021] [74]), it is important to consider how data-driven targeting approaches can impact clinical practice. The majority of the studies evaluating the efficacy of rTMS for the treatment of depression used the 5 cm rule to target the dlPFC. Although there are issues with this approach, widespread adoption of individualized targeting in the clinic means that these patients are being given a treatment regimen that potentially differs from that which has been previously shown to be effective. In addition, it remains to be seen whether rsFC based targeting leads to substantially improved clinical outcomes. Importantly, to establish the superiority of such a method would require large samples, as the results would rely on the sub-population of patients whose individualized site is located substantially distal relative to the control site.
Strengths and limitations
The strength of the work described here stems from the generality of its applicability. The model described in this work is both site and symptom independent. Therefore, the methods described here can be used to optimize TMS targeting for a variety of disorders shown to be effectively treated with TMS [75]. This approach could be used prospectively to screen for potential TMS effects within new disorders or for novel symptom dimensions. The model is also able to make predictions at the single-subject level regarding the optimal stimulation site for a given symptom, allowing for single-subject optimization of TMS targeting. In all cases, the predictions of the model can be directly tested by applying TMS according to the model predictions. Another strength is that our results are consistent for MADRS and HAMD scores, suggesting that our model is capable of identifying stimulation sites to target depressive symptoms. However, it should be noted MADRS and HAMD scores are highly correlated across subjects (AM, r = 0.857; Control, r = 0.664), which means that the similarity in results across the two measures is likely driven by this shared variability. Accordingly, it is important not to characterize the results from these two scores as independent results, but rather different characterizations of the same underlying relationship between depression and rsFC.
The primary weakness of this study is that it is a proof of concept study, rather than a confirmatory study, meaning we do not actually administer TMS and measure the pre/post symptom changes in the current work. We understand validation of the current model is the necessary next step. Publication of the model in its initial state will allow multiple labs to test the predictions of the model, allowing for a more rapid validation/refinement of the work. To facilitate this process, we will make the code for our model publicly available.
Another potential weakness of the current work is that we used Pearson’s correlations, a nondirectional measure, to model functional connectivity. However, it is likely that TMS affects upstream and downstream connections differentially [76]. Accordingly, it may be more appropriate to model the functional connections using effective connectivity, and differentially weight the connections based on whether they are upstream or downstream of the stimulation site. Of course this becomes more complicated in our model, given that the stimulation “site” is modelled as a thresholded map of the current induced across the whole brain, given a TMS coil position and orientation. Accordingly, to factor in differential effects for upstream and downstream connections, it would be necessary to generate an effective connectivity map of the entire brain. While techniques like dynamic causal modelling (DCM) can be used to generate realistic effective connectivity models with a limited number of sites and connections [77–79], it remains to be seen whether this approach can be reliably used to generate whole brain effective connectivity models for use at the single-subject level [80].
It should also be noted that the current analysis was limited to a single vector of points along the anterior to posterior gradient of the dlPFC. This limited search space could help explain the small effects when comparing across site. In the future we plan to expand this method to the whole brain to identify potentially novel therapeutic stimulation sites for depression and other psychiatric disorders.
Another limitation is that the model assumes that changes in connectivity tend to scale linearly with the magnitude of the electric field induced, given a particular site/orientation of stimulation. This may be another explanation for the small effects when comparing across site. This mathematical assumption likely holds for little if any in vivo applications of TMS. Indeed, it is well known that the effects of TMS on outcome variables like cortical excitability [81, 82], functional connectivity [54, 60], and behavior [83, 84] are dependent on the stimulation parameters used [61, 85]. Indeed, amplitude [86], frequency [87], pattern [88], number of sessions [6], and intervals [88] between sessions are all known to be important determinants of observed TMS effects. We understand that the linear assumption put forth in our model is a massive oversimplification, but we see this as a starting point. Accordingly, we have included a proportionality constant in our model to accommodate parameter-dependent scaling in connectivity changes. Our goal is to state the initial model as parsimoniously as possible, to allow the model to be tested with a variety of experimental and clinical TMS protocols. As more information becomes known about the relationship between rTMS parameters and connectivity changes, researchers will be able to adjust the proportionality constant of the model to suit their protocol.
Conclusions
Individualized targeting is one key to maximizing the efficacy of clinical rTMS, and mapping TMS-induced changes in rsFC to changes in clinical symptoms is critically important for this goal. The model proposed is one potential approach to accomplish this goal. The primary strength of this model is the generality of our approach, as the model proposed is both site and symptom independent. In this initial work, we show that the model makes predictions at both positive and negative control sites that are consistent with observed symptom changes following therapeutic applications of rTMS for depression [52–56]. The next step for this work is to test the predictions of this model using a variety of therapeutic TMS protocols. Critically, it remains to be seen whether our approach, along with other data driven, yet expensive approaches are superior to less expensive empirical approaches. What is needed is a head-to-head comparison of outcomes using these approaches in a real-world clinical sample. Such comparisons are critically needed to justify large-scale adoption of these data-driven approaches in the clinic.
Supplementary information
Acknowledgements
This study utilized the high-performance computational capabilities of the Cubic computing cluster at the University of Pennsylvania. (https://www.med.upenn.edu/cbica/cubic.html). The authors would like to thank Maria Prociuk for her expertize and assistance in submitting the paper. We would also like to thank the participants for their time and effort.
Author contributions
NLB: Designed Study, Conducted analysis, wrote paper, provided supervision, edited paper. JCB: Conducted statistical analysis; edited paper. DS: Assisted with data collection and preprocessing. WM: Assisted with data collection and preprocessing. ZD: Assisted with statistical analysis and paper preparation. TG: Assisted with data collection and preprocessing,. MT: Assisted with data collection and preprocessing, assisted with paper preparation. NS: Assisted with data collection and preprocessing. MJ: Assisted with data collection and preprocessing. DJO: Assisted with study design; edited paper. YIS: Assisted with study design, wrote paper; provided supervision; edited paper.
Funding
This project was supported in part by a 2018 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (NLB); and by a K01 award K01MH121777 (NLB). Financial support for this study was provided by U01MH109991 (YIS) and by an endowment (YIS).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01110-6.
References
- 1.American Psychiatric Association. Anxiety disorders. In Diagnostic and statistical manual of mental disorders (5th ed.). Arlington VA (2013). 10.1176/appi.books.9780890425596.dsm05.
- 2.Scott KM, de Jonge, P, Stein DJ & Kessler RC Mental disorders around the world: Facts and figures from the WHO World Mental Health surveys. Mental Disorders Around the World: Facts and Figures from the WHO World Mental Health Surveys (2018). 10.1017/9781316336168
- 3.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. (2018). 10.4088/JCP.16cs10905 [DOI] [PMC free article] [PubMed]
- 5.Conelea CA, Philip NS, Yip AG, Barnes JL, Niedzwiecki MJ, Greenberg BD, et al. Transcranial magnetic stimulation for treatment-resistant depression: naturalistic treatment outcomes for younger versus older patients. J Affect Disord. (2017). 10.1016/j.jad.2017.03.063 [DOI] [PMC free article] [PubMed]
- 6.O'reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: a Multisite Randomized Controlled Trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Philip NS, Aiken EE, Kelley ME, Burch W, Waterman L, Holtzheimer PE. Synchronized transcranial magnetic stimulation for posttraumatic stress disorder and comorbid major depression. Brain Stimulation. 2019;12:1335–1337. doi: 10.1016/j.brs.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Clarke E, Clarke P, Gill S, Paterson T, Hahn L, Galletly C. Efficacy of repetitive transcranial magnetic stimulation in the treatment of depression with comorbid anxiety disorders. J Affect Disord. (2019). 10.1016/j.jad.2019.03.085 [DOI] [PubMed]
- 9.Kumar S, Singh S, Parmar A, Verma R & Kumar N. Effect of high-frequency repetitive transcranial magnetic stimulation (rTMS) in patients with comorbid panic disorder and major depression. Australas Psychiatry (2018). 10.1177/1039856218771517 [DOI] [PubMed]
- 10.Philip NS, Ridout SJ, Albright SE, Sanchez G & Carpenter LL. 5-Hz Transcranial Magnetic Stimulation for Comorbid Posttraumatic Stress Disorder and Major Depression. J Trauma Stress (2016). 10.1002/jts.22065 [DOI] [PMC free article] [PubMed]
- 11.Gwynette MF, Lowe DW, Henneberry EA, Sahlem GL, Wiley MG, Alsarraf H, et al. Treatment of Adults with Autism and Major Depressive Disorder Using Transcranial Magnetic Stimulation: an Open Label Pilot Study. Autism Res. (2020). 10.1002/aur.2266 [DOI] [PMC free article] [PubMed]
- 12.Mantovani A, Aly M, Dagan Y, Allart A, Lisanby SH. Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression. J Affect Disord. 2013;144:153–9. doi: 10.1016/j.jad.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 13.White D, Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann Clin Psychiatry. 2015;27:192–6. [PubMed] [Google Scholar]
- 14.Thompson L. Treating major depression and comorbid disorders with transcranial magnetic stimulation. J Affect Disord. 2020;276:453–60. doi: 10.1016/j.jad.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald PB, Maller JJ, Hoy KE, Thomson R & Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. (2009). 10.1016/j.brs.2009.03.002 [DOI] [PubMed]
- 16.Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. (2009). 10.1038/npp.2008.233 [DOI] [PubMed]
- 17.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110:19944–9. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sack AT, Cohen Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. (2009). 10.1162/jocn.2009.21126 [DOI] [PubMed]
- 19.Siddiqi SH, Trapp NT, Shahim P, Hacker CD, Laumann TO, Kandala S, et al. Individualized connectome-targeted transcranial magnetic stimulation for neuropsychiatric sequelae of repetitive traumatic brain injury in a retired NFL player. J Neuropsychiatry Clin Neurosci. 2019;31:254–63. doi: 10.1176/appi.neuropsych.18100230. [DOI] [PubMed] [Google Scholar]
- 20.Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- 21.Oathes DJ, Zimmerman JP, Duprat R, Japp SS, Scully M, Rosenberg BM, et al. Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Exp Brain Res. (2021). 10.1007/s00221-021-06036-5 [DOI] [PMC free article] [PubMed]
- 22.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry (2011). 10.1016/j.biopsych.2010.09.034 [DOI] [PubMed]
- 23.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005). 10.1016/j.neuron.2005.02.014 [DOI] [PubMed]
- 24.Mayberg, HS, et al. Cingulate function in depression. Neuroreport (1997). 10.1097/00001756-199703030-00048
- 25.Drevets WC, Savitz J & Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. (2008). 10.1017/S1092852900013754 [DOI] [PMC free article] [PubMed]
- 26.Gomez LJ, Dannhauer M, Peterchev AV. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage. 2021;228:117696. doi: 10.1016/j.neuroimage.2020.117696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seok D, Smyk N, Jaskir M, Cook P, Elliott M, Girelli T, et al. Dimensional Connectomics of Anxious Misery, a Human Connectome Study Related to Human Disease: overview of Protocol and Data Quality. NeuroImage Clin. 2020;28:102489. doi: 10.1016/j.nicl.2020.102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–96. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- 29.Dilkov D, Hawken ER, Kaludiev E, Milev R. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;78:61–5. doi: 10.1016/j.pnpbp.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin DF, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: A pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016;209:222–8. doi: 10.1192/bjp.bp.115.168203. [DOI] [PubMed] [Google Scholar]
- 31.Diefenbach GJ, Assaf M, Goethe JW, Gueorguieva R, Tolin DF. Improvements in emotion regulation following repetitive transcranial magnetic stimulation for generalized anxiety disorder. J Anxiety Disord. 2016;43:1–7. doi: 10.1016/j.janxdis.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Li Y, Bianchi MT, Zhan S, Jiang F, Li N, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11:1103–9. doi: 10.1016/j.brs.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Kozel FA, Motes MA, Didehbani N, DeLaRosa B, Bass C, Schraufnagel CD, et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: a randomized clinical trial. J Affect Disord. 2018;229:506–14. doi: 10.1016/j.jad.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter LL, Conelea C, Tyrka AR, Welch ES, Greenberg BD, Price LH, et al. 5Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J Affect Disord. 2018;235:414–20. doi: 10.1016/j.jad.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimul. 2013;6:377–83. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Ortega M, Kangarlu A, Lee S, Perera T, Kangarlu J, Palomo T, et al. Parcel-guided rTMS for depression. Transl Psychiatry. 2020;10:283. doi: 10.1038/s41398-020-00970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–43. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–60. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrae RR, Costa PT., Jr Brief Versions of the NEO-PI-3. J Individ Differ. 2007;28:116–28. [Google Scholar]
- 40.Costa PT & McCrae RR. Revised NEO Personality Inventory (NEO PI-R) and NEO Five Factor Inventory: Professional Manual. Odessa, FL: Psychological Assessment Resources (1992). 10.1037/0003-066X.52.5.509
- 41.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 42.HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry (1960). 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed]
- 43.Thielscher A, Antunes A, Saturnino GB. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2015;2015:222–5. doi: 10.1109/EMBC.2015.7318340. [DOI] [PubMed] [Google Scholar]
- 44.Ciric R, Rosen AFG, Erus G, Cieslak M, Adebimpe A, Cook PA, et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc. (2018). 10.1038/s41596-018-0065-y [DOI] [PMC free article] [PubMed]
- 45.Balderston NL, Liu J, Roberson-Nay R, Ernst M, Grillon C. The relationship between dlPFC activity during unpredictable threat and CO2-induced panic symptoms. Transl Psychiatry. 2017;7:1266. doi: 10.1038/s41398-017-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jolliffe IT. A Note on the Use of Principal Components in Regression. Appl Stat. (1982). 10.2307/2348005
- 47.Sripada C, Angstadt M, Rutherford S, Kessler D, Kim Y, Yee M, et al. Basic Units of Inter-Individual Variation in Resting State Connectomes. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-018-38406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trapp NT, Bruss J, King Johnson M, Uitermarkt BD, Garrett L, Heinzerling A, et al. Reliability of targeting methods in TMS for depression: Beam F3 vs. 5.5 cm. Brain Stimul. 2020;13:578–81. doi: 10.1016/j.brs.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laakso I, Murakami T, Hirata A, Ugawa Y. Where and what TMS activates: experiments and modeling. Brain Stimul. 2018;11:166–74. doi: 10.1016/j.brs.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao Q, Yang Y, Yu H, Fan L, Luan S, Zhang L, et al. Anatomical Connectivity-Based Strategy for Targeting Transcranial Magnetic Stimulation as Antidepressant Therapy. Front Psychiatry. 2020;11:1–8. doi: 10.3389/fpsyt.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siddiqi SH, Trapp NT, Hacker CD, Laumann TO, Kandala S, Hong X, et al. Repetitive Transcranial Magnetic Stimulation with Resting-State Network Targeting for Treatment-Resistant Depression in Traumatic Brain Injury: a Randomized, Controlled, Double-Blinded Pilot Study. J Neurotrauma. 2019;36:1361–74. doi: 10.1089/neu.2018.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol Psychiatry. 2018;84:28–37. doi: 10.1016/j.biopsych.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual Functional Connectivity Predicts Antidepressant Treatment Response to Transcranial Magnetic Stimulation: independent Validation and Evaluation of Personalization. Biol Psychiatry. 2019;86:2018–20. doi: 10.1016/j.biopsych.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Cash RFH, Weigand A, Zalesky A, Siddiqi SH, Downar J, Fitzgerald PB, et al. Using Brain Imaging to Improve Spatial Targeting of Transcranial Magnetic Stimulation for Depression. Biol Psychiatry. 1–12 (2020). 10.1016/j.biopsych.2020.05.033 [DOI] [PubMed]
- 58.Cash RFH, Cocchi L, Lv J, Fitzgerald PB & Zalesky A. Functional Magnetic Resonance Imaging-Guided Personalization of Transcranial Magnetic Stimulation Treatment for Depression. JAMA Psychiatry. 1–3 (2020). 10.1001/jamapsychiatry.2020.3794 [DOI] [PMC free article] [PubMed]
- 59.Singh A, Erwin-Grabner T, Sutcliffe G, Antal A, Paulus W, Goya-Maldonado R. Personalized repetitive transcranial magnetic stimulation temporarily alters default mode network in healthy subjects. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-42067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beynel L, Powers JP, Appelbaum LG. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: a systematic review. Neuroimage. 2020;211:116596. doi: 10.1016/j.neuroimage.2020.116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen F, Gu C, Zhai N, Duan H, Zhai A, Zhang X. Repetitive Transcranial Magnetic Stimulation Improves Amygdale Functional Connectivity in Major Depressive Disorder. Front Psychiatry. 2020;11:1–9. doi: 10.3389/fpsyt.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry. 2017;7:1–9. doi: 10.1038/s41398-017-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avissar M, Powell F, Ilieva I, Respino M, Gunning FM, Liston C, et al. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul. 2017;10:919–25. doi: 10.1016/j.brs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu M, Linn KA, Shinohara RT, Oathes DJ, Cook PA, Duprat R, et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci U S A. 2019;116:8582–90. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams, LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. (2017). 10.1002/da.22556 [DOI] [PMC free article] [PubMed]
- 67.Siddiqi SH, Taylor SF, Cooke D, Pascual-Leone A, George MS, Fox MD. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177:435–46. doi: 10.1176/appi.ajp.2019.19090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thielscher A, Opitz A, Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;54:234–43. doi: 10.1016/j.neuroimage.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 69.Handwerker DA, Ianni G, Gutierrez B, Roopchansingh V, Gonzalez-Castillo J, Chen G, et al. Thetaburst TMS to the posterior superior temporal sulcus decreases resting-state fMRI connectivity across the face processing network. bioRxiv (2019). 10.1101/794578 [DOI] [PMC free article] [PubMed]
- 70.Balan PF, Gerits A, Mantini D, Vanduffel W. Selective TMS-induced modulation of functional connectivity correlates with changes in behavior. Neuroimage. 2017;149:361–78. doi: 10.1016/j.neuroimage.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 71.Hawco C, Voineskos AN, Steeves J, Dickie EW, Viviano JD, Downar J, et al. Spread of activity following TMS is related to intrinsic resting connectivity to the salience network: a concurrent TMS-fMRI study. Cortex. 2018;108:160–72. doi: 10.1016/j.cortex.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Baeken C, Duprat R, Wu GR, De Raedt R, van Heeringen K. Subgenual Anterior Cingulate–Medial Orbitofrontal Functional Connectivity in Medication-Resistant Major Depression: a Neurobiological Marker for Accelerated Intermittent Theta Burst Stimulation Treatment? Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:556–65. doi: 10.1016/j.bpsc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Iwabuchi SJ, Auer DP, Lankappa ST, Palaniyappan L. Baseline effective connectivity predicts response to repetitive transcranial magnetic stimulation in patients with treatment-resistant depression. Eur Neuropsychopharmacol. 2019;29:681–90. doi: 10.1016/j.euroneuro.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgerald PB. Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimul. 2021;14:730–6. doi: 10.1016/j.brs.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 75.Slotema CW, Blom JD, Hoek HW, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–84. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 76.Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry (2001). 10.1016/S0006-3223(01)01199-4 [DOI] [PubMed]
- 77.Clarke R, Johnstone T. Prefrontal inhibition of threat processing reduces working memory interference. Front Hum Neurosci. 2013;7:228. doi: 10.3389/fnhum.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daunizeau J, David O & Stephan KE. Dynamic causal modelling: a critical review of the biophysical and statistical foundations. NeuroImage (2011). 10.1016/j.neuroimage.2009.11.062 [DOI] [PubMed]
- 79.Friston KJ, Li B, Daunizeau J & Stephan KE. Network discovery with DCM. Neuroimage (2011). 10.1016/j.neuroimage.2010.12.039 [DOI] [PMC free article] [PubMed]
- 80.Prando G, Zorzi M, Bertoldo A, Corbetta M, Zorzi M, Chiuso A. Sparse DCM for whole-brain effective connectivity from resting-state fMRI data. Neuroimage (2020). 10.1016/j.neuroimage.2019.116367 [DOI] [PubMed]
- 81.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá M. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. (1998). 10.1097/00004691-199807000-00005 [DOI] [PubMed]
- 82.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology (1997). 10.1212/WNL.48.5.1398 [DOI] [PubMed]
- 83.Balderston NL, Beydler EM, Roberts C, Deng ZD, Radman T, Lago T, et al. Mechanistic link between right prefrontal cortical activity and anxious arousal revealed using transcranial magnetic stimulation in healthy subjects. Neuropsychopharmacology. 2020;45:694–702. doi: 10.1038/s41386-019-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balderston NL, Beydler EM, Goodwin M, Deng ZD, Radman T, Luber B, et al. Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Transl Psychiatry. 2020;10:1–10. [DOI] [PMC free article] [PubMed]
- 85.Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–6. doi: 10.1152/jn.00781.2010. [DOI] [PubMed] [Google Scholar]
- 86.Komssi S, Kähkönen S & Ilmoniemi RJ. The Effect of Stimulus Intensity on Brain Responses Evoked by Transcranial Magnetic Stimulation. Hum Brain Mapp. (2004). 10.1002/hbm.10159 [DOI] [PMC free article] [PubMed]
- 87.Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210:1260–4. doi: 10.1016/j.psychres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Huang Y-ZZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.