Abstract
In recent years, there has been an emerging interest in the use of claims and electronic health record (EHR) data for evaluation of medical device safety and effectiveness. In Korea, national insurance electronic data interchange (EDI) code has been used as a medical device data source for common data model (CDM). This study performed a preliminary feasibility assessment of CDM-based vigilance. A cross-sectional study of target medical device data in EHR and CDM was conducted. A total of 155 medical devices were finally enrolled, with 58.7% of them having EDI codes. Femoral head prosthesis was selected as a focus group. It was registered in our institute with 11 EDI codes. However, only three EDI codes were converted to systematized nomenclature of medicine clinical terms concept. EDI code was matched in one-to-many (up to 104) with unique device identifier (UDI), including devices classified as different global medical device nomenclature. The use of UDI rather than EDI code as a medical device data source is recommended. We hope that this study will share the current state of medical device data recorded in the EHR and contribute to the introduction of CDM-based medical device vigilance by selecting appropriate medical device data sources.
Subject terms: Health care, Medical research, Computational biology and bioinformatics
Introduction
Medical devices play important roles in disease diagnosis, prevention, and treatment. However, they also carry potential risks of serious injuries and even fatality1,2. Therefore, postmarket medical device vigilance (MDV) is crucial for public health protection, and medical device adverse event (MDAE) information should be collected, evaluated, analyzed, and disseminated through a timely and reliable method3. Current MDV methods rely on passive reporting, which is a combination of mandatory and voluntary adverse event reporting systems used by patients, physicians, manufacturers, and healthcare organizations. For a long time, these reporting systems have been useful for identifying unexpected and unique adverse events4. However, passive surveillance is limited by the voluntary nature of reporting, the strong inherent bias associated with spontaneous reports, underreporting, and the lack of denominator data on comprehensive exposure4–7. To overcome these limitations, an active surveillance system via common data model (CDM) could be helpful. CDM is a logical and semantic data model that can be used to standardize multiple data sources into a common format. It has been effectively implemented with pharmaceutical products (including vaccines)8. In addition, applying passive and active surveillance simultaneously using electronic health records (EHR) could augment sample size, increase population heterogeneity, and cross-validate results9.
In recent years, there has been an emerging interest in the use of claims and EHR data for evaluating medical device safety and effectiveness10. Attempts have been made to implement MDV active surveillance through a web-based platform for sharing the experiences on medical device incidents11 and through a multicenter, prospective, observational research study12. However, both studies used a centralized method with patient data entered into the database as a common language agreed between network organizations. Studies of CDM-based MDV have not been reported yet. There is a difference between pharmacovigilance and medical device. Pharmacovigilance uses CDM to analyze adverse events associated with the use of a drug while medical device vigilance analyzes adverse events associated with the use of a medical device. This study was conducted to identify the current situation and derive improvements, assuming that medical device information deficiencies in EHR or CDM could become a hurdle for CDM-based MDVs.
Results
Soonchunhyang University Bucheon Hospital (SCHBC) in Bucheon, Gyeonggi Province, Republic of Korea is covered by Federated E-health Big Data for Evidence Renovation Network (FEEDER-NET)13,14. According to the user manual provided by FEEDER-NET15, institutional unique prescription codes were used as medical device data sources and converted into electronic data interchange (EDI) codes used by national insurance claims in Korea. The EDI code is registered in Athena. It can be entered into the CDM as it is. However, since it is a non-standard term, it is converted to the standard term, Systemized Nomenclature of Medicines—Clinical Terms (SNOMED-CT), and entered into the CDM if possible (Fig. 1). Global Medical Device Nomenclature (GMDN) terms are the basis of SNOMED CT medical device content16.
Figure 1.
Current CDM mapping process of medical device data in Korea and scopes of this study. Among medical device data recorded in each medical institution's EHR, the institutional unique code recorded in the OCS is converted into a national insurance electronic data interchange (EDI) code. EDI codes are mapped using an OMOP CDM standard vocabulary. The standard vocabulary for medical device data is SNOMED. EHR electronic health records, CDM common data model, SCHBC Soonchunhyang University Bucheon Hospital, OCS online charting system, EDI electronic data interchange, EMR electronic medical record, PACS picture archiving and communication system, LIS laboratory information system, MDV medical device vigilance.
Analysis of medical device data recorded in EHR
Among the 2112 medical devices posted by the Ministry of Food and Drug Safety (MFDS), 433 posts had MDAE occurrence. Fifty-five medical devices were reported to have health effects on patients. Of the remaining 379 medical devices whose health effects were not reported, 101 had moderate or high potential risks. A total of 155 medical devices were finally enrolled to investigate medical device data in EHR (Fig. 2a). Medical device information from SCHBC EHR was classified into four groups (Fig. 2b). Ninety-one medical devices (91/155, 58.7%) were prescribed by code (EDI code linked) on online charting system (OCS) when used in patients. Fifty devices without other information recorded other than OCS prescriptions were classified into group 1. Forty-one medical devices (41/155, 26.5%) recorded as barcode data in the surgical nursing record on EMR were classified into group 2. Ten medical devices (10/155, 6.5%) recorded model name on picture archiving and communication system (PACS) were classified into group 3. Nine of them had model names displayed separately from the image without serial number or lot number. The other one, a gastroscope, had model name and serial number of the device displayed as part of the image. Fifty-four medical devices without direct device data were classified into group 4. Twenty-two of them did not have any records. Nineteen of them did not have a direct record. They were assumed to be used medical devices needed for treatment or surgeries. Thirteen had a number assigned by the user in the electronic medical record (EMR). However, they were considered as group 4 because such user given number had been assigned to a new device without any comments when replacing the device.
Figure 2.
A total of 155 medical devices were finally enrolled for the investigation of medical device data on EHR (a). Medical device information EHR was classified into four groups (b). MDAE medical device adverse event, MD medical device, MFDS Ministry of Food and Drug Safety, EHR electronic health records, EDI electronic data interchange, PACS picture archiving and communication system, EMR electronic medical record.
Figure 3 shows changes of implanted medical device records in our institution. Prior to the introduction of EMR, OCS prescriptions using the National Insurance EDI code and scan images of barcode stickers for devices were recorded. Initially, the device name was interlinked with the OCS prescription and the barcode data was replaced by a keyboard entry lot number instead of a scan image. In 2019, barcode readers were introduced to improve barcode data recording. When the barcode is read, the entire barcode data and some distinguished data (lot or serial number and expiration date) are entered at a specified location.
Figure 3.
Changes of implanted medical device records in our institution.
Focus analysis of medical device data
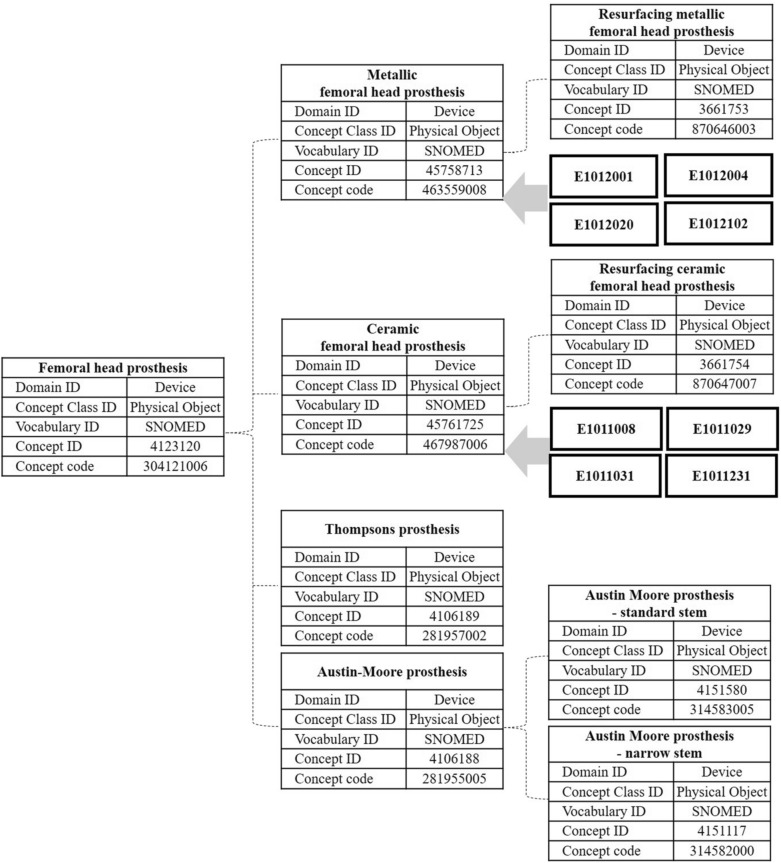
National insurance has 31 and 28 codes for metal and ceramic femoral heads, respectively. There were a total of 11 codes registered in our institute's OCS (Table 1). On the CDM of SCHBC, three of those 11 femoral head codes were mapped to the standard concept of SNOMED-CT (code mapping was provided as Supplementary Table S1), while the other eight EDI terms were mapped to the non-standard concept of SNOMED-CT as shown in Fig. 4.
Table 1.
List of 11 national insurance EDI codes registered in our institute and their concept ID used to map to the CDM.
| National insurance EDI code | SCHBC CDM | ||||
|---|---|---|---|---|---|
| Code | Code name | Concept_ID | Name | Vocabulary | Record count |
| E1012001 | Osteonics femoral head (Howmedica Osteonics Corp, Mahwah, NJ, US) | 42097997 | Osteonics femoral head | EDI | 0 |
| E1012004 | VerSys femoral head (Zimmer, Inc, Warsaw, IN) | 42103020 | VerSys femoral head | EDI | 6 |
| E1012020 | ic-head (Implantcast GmbH, Buxtehude, Germany) | 42094220 | ic-head | EDI | 10 |
| E1012102 | Exeter femoral head (Howmedica Osteonics Corp, Mahwah, NJ, US) | 42092029 | Exeter head | EDI | 177 |
| E1011008 | Ceramic head (DePuy Synthes Companies, Marwah, Indiana, US) | 42089833 | Ceramic head | EDI | 84 |
| E1011029 | Ceramic ball head (Plus Orthopedics AG, Rotkreuz, Switzerland) | 42089827 | Ceramic ball head | EDI | 38 |
| E1011031 | V40 Alumina femoral head (Howmedica Osteonics Corp, Mahwah, NJ, US) | 42102799 | V40 Alumina femoral head | EDI | 334 |
| E1011231 | Biolox Delta Ceramic V40 femoral head (Howmedica Osteonics Corp, Mahwah, NJ, US) | 42088730 | Biolox Delta ceramic V40 femoral head | EDI | 0 |
| E1011023 | Femoral head, ceramic (Lima Corporate Spa, Udine, Italy) | 45761725 | Ceramic femoral prosthesis | SNOMED-CT | 35 |
| E1011002 | Ceramic head (Howmedica Osteonics Corp, Mahwah, NJ, US) | ||||
| E1011204 | Ceramic femoral head (CeramTec AG, Plochingen, Germany) | ||||
CDM common data model, SCHBC Soonchunhyang University Bucheon Hospital, EDI electronic data interchange, SNOMED-CT systematized nomenclature of medicine clinical terms.
Among metal and ceramic heads, EDI codes E1012102 and E1011031 represent the largest record count (bold).
Figure 4.
Femoral head hierarchy of SNOMED-CT. Only three of 11 EDI codes registered on the SCHBC CDM are mapped to the concept ID 45761725, while the remaining eight are mapped to concept ID 4558713 or 45761725 depending on the material. CDM common data model, SCHBC Soonchunhyang University Bucheon Hospital, EDI electronic data interchange, SNOMED-CT systematized nomenclature of medicine clinical terms.
Both metal and ceramic femoral heads were used most frequently by Howmedica Osteonics Corp (Mahwah, NJ, USA). Thus, they were selected as focus groups: E1012001, E1012102, E1011031, and E1011231. E1011002 was not included as a focus group because it had a prescription record currently excluded from national insurance.
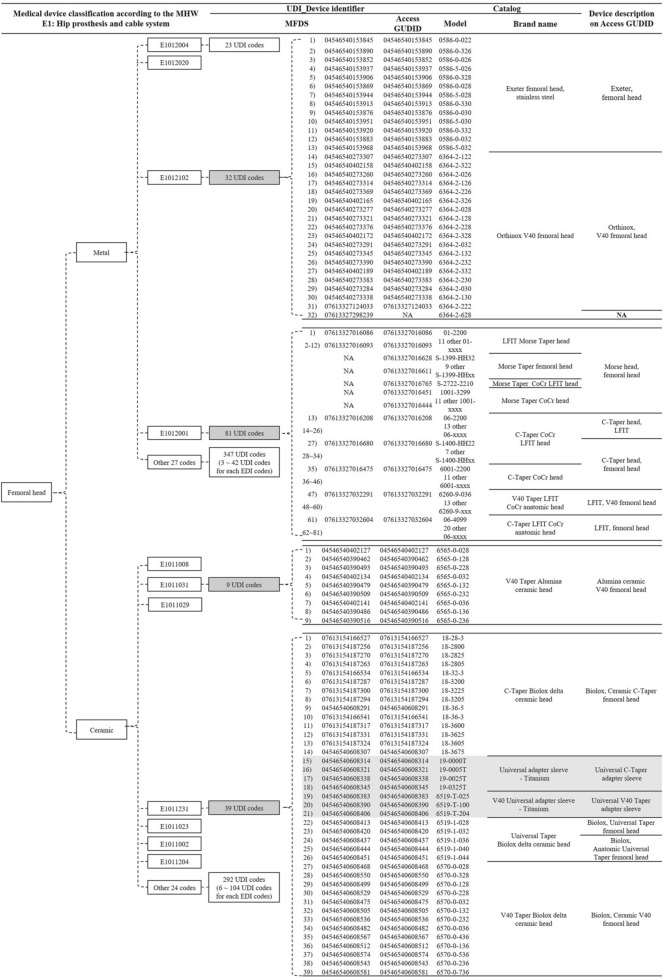
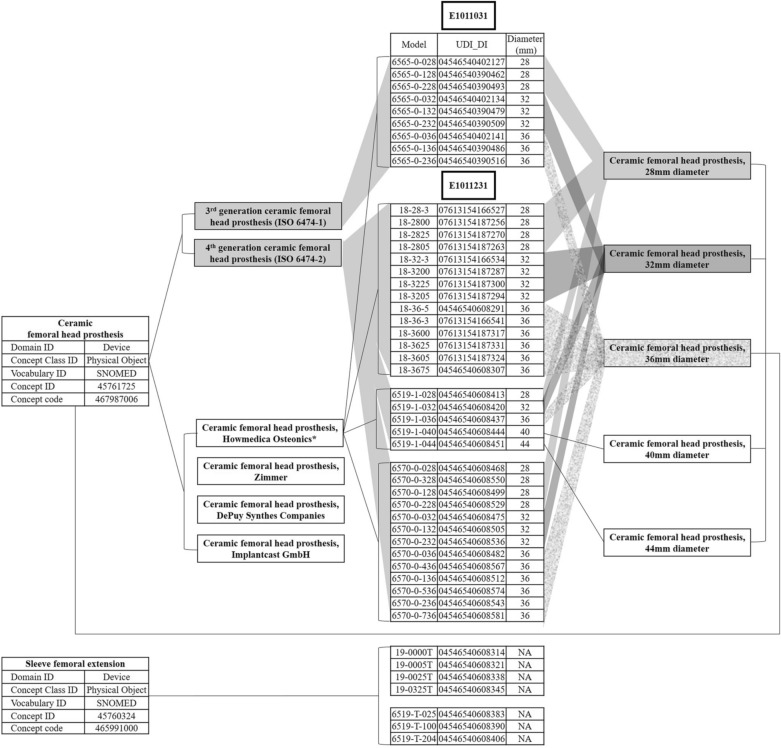
Various medical device data sources used in this study provided different medical device classifications and data (Table 2). The national insurance EDI code was commonly used by MOHW and MFDS data sources. Because EDI codes were applied without distinction to medical device specifications, the four EDI codes selected as focus groups were reclassified using the MFDS's medical device classification criteria. Models were available from the MFDS, the manufacturer’s catalog, and Access Global Unique Device Identification Database (GUDID). EDI data were found from the MFDS and Access GUDID. There were 31 EDI codes registered as metal femoral head in national insurance (Fig. 5). Each of 32 models registered under the E1012102 code was gaved a unique device identifier (UDI). The manufacturer's catalog categorized 32 models as Exter femoral head stainless steel and Orthinox V40 chemical head. The 32nd model (6364-2-628) of the E1012102 was registered with the MFDS as 07613327298239, but was not registered with the Access GUDID. The 81 models registered under the E1012001 code were categorized under five brand names depending on the type of taper (Morse, C and V40) and Low Friction Ion Treatment (LFIT) application. The first model to the 12th model were listed in the catalog as LFIT Morse taper head and Access GUDID as Morse head femoral head. By searching for Morse head femoral head in Access GUDID, 22 additional UDIs were identified that were not in the MFDS. They were classified under three brand names by the manufacturer. The 27th to 34th models were classified as C-Taper CoCr LFIT heads in the catalog, but were classified as C-Taper heads in Access GUDID. There were 30 EDI codes registered as ceramic femoral head in national insurance. The nine models registered with E1011031 code had a single brand name. Thirty-nine models were registered in E1011231, with six models (from 15th to 21st) being adapter sleeves, not femoral heads. The remaining 33 models were classified as three in the catalog and four brand names in the Access GUDID. Figure 6 presents ceramic femoral head hierarchies according to manufacturer, ceramic type, and head diameter. The 48 models registered in codes E1011031 and E1011231 were reclassified for the hierrarchies presented.
Table 2.
Various medical device data acquired from multiple sources.
| Ministry of Health and Welfare | Ministry of Food and Drug Safety | Manufacturer’s global catalog | Access GUDID | |
|---|---|---|---|---|
| Target device | National insured devices | Devices licensed for sale in Korea | Stryker’s devices on the market | Devices licensed for sale in USA |
| Category | O (own taxonomy) | O (own taxonomy) | NA | GMDN |
| Class (1–4) | X | O | NA | NA |
| License number | X | O | NA | NA |
| Bander/manufacturer | O | O | O | O |
| Brand name | O | O or X | O | O |
| EDI | O | O* | NA | NA |
| Model | X | O | O | O |
| UDI | X | O | X | O |
| Lot number | X | X | X | X |
GUDID global unique device identification database, GMDN global medical device nomenclature, NA not available, EDI electronic data interchange, UDI unique device identifier.
*In case of insurance coverage.
Figure 5.
Analysis of various medical device data for the chemical head selected as a focus group. MHW Ministry of Health and Welfare, UDI unique device identifier, MFDS Ministry of Food and Drug Safety, GUDID global unique device identification database.
Figure 6.
Ceramic femoral head hierrarchy was applied to Stryker's products registered with national insurance EDI codes E1011031 and E1011231. EDI electronic data interchange, SNOMED systematized nomenclature of medicine clinical terms, UDI unique device identifier, DI device identifier.
Discussion
MDV is currently performed through a variety of voluntary and mandatory reporting mechanisms to report events, not rates17. The calculation of the latter requires a denominator, that is, the total number of the used devices. For drugs, the situation is different. Sentinel Initiative monitors the safety and comparative effectiveness of drugs by leveraging national drug codes recorded at the point of care or the point of sale and transmitted to payers via insurance claims18. In Korea, the MFDS is required to record and preserve usage of some medical devices such as implanted medical devices for more than a year in order to track patients. However, medical institutions tend to write this as a dedicated document for medical management rather than recording it in EMR (personal communication). Mapping these data to an OMOP CDM is hard to expect. Therefore, national insurance EDI code has been used as a medical device data source in OMOP CDM (Fig. 1), similar to the Sentinel Initiative. However, there has been no approach or feasibility assessment on whether CDM established by Korean institutions is suitable for MDV. Based on EHR record and OMOP CDM conversion status of medical device data, this study performed preliminary feasibility assessment of CDM-based MDV.
Adequacy of medical device EDI code for MDV purpose
As of 2016, 97.1% of Korea's population are covered by national insurance. Health Insurance Review and Assessment Service (HIRA) has developed the EDI code system to classify and identify drugs, medical services, and devices. It also maintains the EDI code system. HIRA mandates the use of EDI vocabulary in the claim system. For this reason, every Korean EHR system uses the EDI vocabulary for most drugs, medical procedures, and devices. It has advantages as a data source in that it is structured and domestic standardized. However, EDI term has not been acknowledged as a standard vocabulary in the way that the Current Procedural Terminology, fourth edition has in the United States because its quality has never been audited19. Instead, there were several attempts to map the EDI term to SNOMED-CT20–22. Nevertheless, the EDI code as a medical device data source showed several limitations in the present study. First, only a few codes were mapped to standard terms (Table 1). Second, it excluded non-claimed medical devices (groups 3 and 4 in Fig. 2a,b). Third, medical devices might be unintentionally intermixed in a single EDI code even if all EDI codes are mapped to standard terms (Fig. 5).
Candidates for medical device data source other than EDI code
EHR data capture is critical to device-tracking efforts23. For this purpose, the structure of the data and the presence of records on EHR are both important. EHRs are recorded as either unstructured keyboard entry narrative data or structured coded data24. Structured data enable the reuse of data collected in the course of clinical care. However, structured data systems are typically difficult to use, time consuming, and often inflexible for expressing complex clinical concepts. Previously reports have indicated that 80% of medical data are unstructured data while only 20% of medical data are structured data used mostly for disease diagnosis25. As shown in Fig. 2b, structured medical device data include prescription code modified national insurance EDI code (50 devices), barcode entry (41 devices) including UDI, and model (9 devices) recorded in PACS. Other medical devices without charging patients have been recorded in an unstructured form for management or not recorded. These unstructured data cannot be processed by algorithm directly. Additional data mining, natural language processing, and text analytics would be needed. In the Veteran Administration’s Cardiovascular Assessment, Reporting, and Tracking (CART) initiative using free text and limited structured data entry, a dataset of questionable validity for surveillance efforts has been observed26. Tradeoffs between structured and unstructured data recording observed in Fig. 3 complicate the reuse of data. Which medical device data should be captured was discussed considering the existence of EHR records, the structure of the data, and the potential for standardization.
The first option was brand name. It has advantages of easy verbal communication. However, it is not recorded in EHR. Considering the discrepancy in brand name recorded in the manufacturer’s catalog and Access GUDID with only a few brand names registered in the MFDS (Table 2), it is considered difficult to standardize brand names and record them in EHR. It was predicted that CDM conversion of brand name level might increase the loss of data integrity. In pharmacovigilance, brand name level CDM conversion showed 6 to 7% information loss to drug exposure8,9. The second option was model (catalog number). The model is familiar to users as it is recorded in the catalog as well as in the MFDS. It gives specifications, enabling detailed classification of medical devices. The International Consortium of Orthopaedic Registries (ICOR)27 is using models of devices for their identification. However, there has been no worldwide consensus on the encoding of part numbers28. Different prostheses have been identified with the same model and different models have been used for the same prosthesis. In addition, variations in models within and between different companies’ products are possible. For example, in Fig. 5, the LFIT Morse taper head is named 01-xxx. However, Morse taper femoral head is named S-1399-HHxx, although the two have the same manufacturer. Additional EHR records would be required to use the model because only 10 medical devices’ model were recorded in our EHR (Fig. 2a, group 3). The last option was UDI. UDI includes device identifier (UDI-DI) and production identifier (UDI-PI). The UDI-DI should be globally unique29. It should identify the specific version or model of a device and its manufacturer. The UDI-PI identifies one or more of the following when present on the label of the device: the lot or batch within which a device was manufactured, the serial number, the expiration date, and the date on which a specific device was manufactured30. Manufacturers must label medical devices with UDI code in a machine-readable format, such as a barcode, radiofrequency identifier (RFID), and human-readable text. The United States Food and Drug Administration (FDA) released a proposed rule in July 2012 that medical devices distributed in the US should be fitted with UDI31. Manufacturers must submit key product to GUDID that can be accessed by both patients and providers32. The European Union (EU) medical device regulations also require fitting of UDI to all medical devices sold in the EU. This requirement came into effect from 2015 to 201933. European Database on Medical Devices (EUDAMED) is expected to have UDI coding34. In Korea, mandatory UDI attachment was implemented by the MFDS in July 2019. All medical devices should have UDI attachment by 2022. To efficiently manage them, an integrated information system for medical device is also in operation35. Although it is introduced after 2019, UDI is recorded in the EHR as a barcode entry in our institute (Fig. 3). It can be easily structured as it is generated in accordance with regulations. Therefore, the authors determine that UDI is the most appropriate medical device data source to be mapped to CDM.
Health systems may benefit from UDI adoption for several reasons other than postmarket surveillance. The UDI establishes a standard identifier to track the device that can be captured by barcode scanning already widely used for many purposes36. Scanning this single UDI barcode is easier than individually capturing several fields, such as manufacturer, product type, and serial number. It can improve inventory management systems to monitor inventory, enable automatic reordering, and ensure that devices are used before their shelf life expires37. Moreover, knowledge of a patient's implanted devices or those used previously can improve clinical care. If issues are identified or the device is recalled, they could be notified quickly38. As with barcode e-MAR (electronic drug administration records), which significantly reduces medication errors, barcode scanning in medical devices can improve patient safety39. For UDI adaption, hospitals need to upgrade their EHR and supply-chain management programs to capture UDIs and train staff. Although some large health care systems have successfully implemented UDI for implantable devices26, UDI has had limited effect because it is available in neither EHR nor claims38. The Office of the National Coordinator for Health Information Technology has ruled that EHRs must have the capability to record UDIs to receive certification. However, this capability is not mandatory18. Requiring the UDI in claims is expected to prompt EHR integration32. It has the potential to greatly enhance postmarket surveillance and provide essential data for performing research using real-world evidence. CMS has previously expressed concern about such a requirement because the cost and complexity of changing operating and technical systems to include the UDI in claims. The current proposal shortens claims requirement to include only UDI-DI, meaning that specific models could be identified if UDI-PI is not be available. A partial implementation that uses only UDI-DI could decrease the utility of the UDI because important information about a faulty device's production may be missing38. Additionally, even if all providers modify their EHR related to claims and if payers modify their claims-processing systems to accept UDIs, MDV system will not automatically emerge. In the MDV system, some unique characteristics of medical devices should be considered40. Medical devices consist of multiple components with complex interactions among devices. The effect of device-specific learning41 and differences in experience between providers can influence clinical outcomes42. Mechanical failure, even software error, could be a potential cause of failure. None of these issues is usually a problem with drugs7,43,44. A functioning medical device evaluation system would be needed.
Although UDI can make unique identification of medical device possible45, it is only useful for identifying an issue with a specific device from one manufacturer. Generic device grouping of UDI would enable systematic problem to be identified early. Global Medical Device Nomenclature (GMDN) code fulfils requirements of a generic device group46. The GMDN is a list of generic. It was specified as the naming convention for the device portion of the UDI by FDA. The Preferred Term in GMDN is the valid description of a group of devices. It does not differentiate between device models or those from different manufacturers with the same intended use and technology. Therefore, GMDN system enables early identification of a systematic problem not limited to one manufacturer, but shared by other products that use the same technology or materials. In SNOMED-CT system, the only medical device standard vocabulary considered in OMOP CDM, the concept of device is mapped to a subset of GMDN codes. However, various analyses were considered difficult with stratification of SNOMED-CT. Work to expand the GMDN content in SNOMED-CT is required to satisfy wider purposes of SNOMED-CT linked to the use of medical devices. To support its further development, developing medical device descriptions in clinical terminology, linking to additional clinical data, and adding more layers of more specific medical device terms would be needed16. For example, the authors identified two EDI codes from ceramic femoral heads as examples of UDI for medical devices and sub-grouped them into materials, manufacturers, and specifications based on opinions of orthopedic experts (Fig. 6).
This study has several limitations. First, since a single institution status was investigated, the situation might be different for other countries or institutions. Second, only representative devices in each medical device category were analyzed. Therefore, the EHR recording status for devices not directly identified might differ from this study, even if they are included in the target medical device category.
OMOP CDM, which is built in our institution, uses EDI code as a medical device data source. Despite its advantages of structural and standardization, EDI is matched in one-to-many with UDI, including devices classified as different GMDNs. Therefore, it is difficult to ensure the accuracy of surveillance results if all medical devices corresponding to a single EDI code are considered to have a single characteristic. To communicate safety information of medical devices, it is recommended to use internationally accepted UDI rather than EDI code as a data source. To introduce UDI, each institution needs to have a barcode reader, develop EHR, and train users. To compensate for the vulnerability of UDI to systematic problem identification, analysis of generic device groups using GMDN is necessary. In this respect, SNOMED-CT that reflects GMDN is appropriate for CDM-based MDVs. However, since analysis according to various device characteristics is difficult with current classification, adding more specific layers is essential. Finally, even if UDI is recorded in EHR and converted to CDM, additional MDV systems are needed to analyze it.
Methods
We conducted a cross-sectional study at Soonchunhyang University Bucheon Hospital (SCHBC) in Bucheon, Gyeonggi Province, Republic of Korea. This study was approved by the Institutional Review Board of SCHBC. Informed consent was waived (SCHBC 2020-08-028-001).
Analysis of medical device data recorded in EHR
To evaluate the adequacy of EDI for MDV purposes and to determine the existence of medical device data other than EDI, the target medical device was selected and medical device records of EHR were grouped.
Selection of target medical devices: According to the Ministry of Food and Drug Safety (MFDS) notice in May 2020, medical devices in Republic of Korea are classified into 2112 categories. The following three criteria were applied to select medical devices to evaluate the frequency of MDAE and their risk to patients:
Medical devices with MDAEs posted on the MFDS medical device information portal. MDAEs reported in the Republic of Korea have been posted on the MFDS medical device information portal since October 2016 (https://udiportal.mfds.go.kr/). Since posts before August 2018 did not include details of MDAE, this study was conducted using information posted after 2018.
Medical devices with patients’ health effect caused by MDAE posted on the portal.
Although MDAEs posted on the portal did not show their effects on patients’ health, medical devices were classified as moderate (class 3) or high (class 4) potential risk according to the classification of medical devices in the Republic of Korea.
According to data format, data were classified as unstructured narrative text and structured coded data47. The number and percentage of medical devices corresponding to each information level and data formet were obtained. Microsoft Excel 2019 (Microsoft, Redmond, WA, USA) was used for statistical processing.
Focus analysis of medical device data
To provide a detailed example of medical device data, focus group analysis was conducted using single item: femoral head. Medical device data related to the focus group were retrieved and collected from the MOHW's medical device price list notice48, the MFDS's Information Portal49, and the U.S. national library of medicine's Access GUDID50.
To analyze SNOMED-CT conversion status of medical device data, Observational Health Data Sciences and Informatics (OHDSI) open-source software and OMOP CDM version 5.3 database were used. EHR of SCHBC was converted to source name of cdmpv531_0920_bucheon. Central vocabulary service Athena (http://athena.ohdsi.org) was used to assess the hierarchy of SNOMED-CT.
Ethics approval
This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (SCHBC) (approval number: SCHBC 2020-08-028-001).
Consent to participate
Informed consent was waived by the IRB.
Supplementary Information
Author contributions
Conceptualization, Y.K.L. and J.H.K.; Data curation, S.C. and K.C.N.; Formal analysis, S.C. and S.J.C.; Methodology, S.C., K.C.N. and Y.K.L.; Writing-original draft, S.C.; Writing—review and editing, S.C., S.J.C., J.K.K., S.H.L., Y.K.L. and J.H.K.
Funding
This research was supported by Soonchunhyang University Research Fund and a 2020 research grant (Grant Number, 20172-MFDS-362) from the Ministry of Food and Drug Safety, Republic of Korea.
Data availability
Data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ju Han Kim, Email: juhan@snu.ac.kr.
You Kyoung Lee, Email: cecilia@schmc.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03332-6.
References
- 1.Hauser RG, Kallinen LM, Almquist AK, Gornick CC, Katsiyiannis WT. Early failure of a small-diameter high-voltage implantable cardioverter–defibrillator lead. Heart Rhythm. 2007;4:892–896. doi: 10.1016/j.hrthm.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH. Semper fidelis—Consumer protection for patients with implanted medical devices. N. Engl. J. Med. 2008;358:985–987. doi: 10.1056/NEJMp0800495. [DOI] [PubMed] [Google Scholar]
- 3.Yoon C, et al. Differences in perspectives of medical device adverse events: Observational results in training program using virtual cases. J. Korean Med. Sci. 2019;34:e255. doi: 10.3346/jkms.2019.34.e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Shea JC, Kramer JM, Califf RM, Peterson ED. Part I: Identifying holes in the safety net. Am. Heart J. 2004;147:977–984. doi: 10.1016/j.ahj.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Resnic FS. Postmarketing surveillance of medical devices—Filling in the gaps. N. Engl. J. Med. 2012;366:875. doi: 10.1056/NEJMp1114865. [DOI] [PubMed] [Google Scholar]
- 6.Vidi VD, Matheny ME, Resnic FS. Post-marketing device safety surveillance. Contemp. Clin. Trials. 2011;32:307–308. doi: 10.1016/j.cct.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Normand S-LT, Hatfield L, Drozda J, Resnic FS. Postmarket surveillance for medical devices: America’s new strategy. Br. Med. J. 2012;345:e8648. doi: 10.1136/bmj.e6848. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, et al. ADEpedia-on-OHDSI: A next generation pharmacovigilance signal detection platform using the OHDSI common data model. J. Biomed. Inform. 2019;91:103–119. doi: 10.1016/j.jbi.2019.103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, et al. An evaluation of the THIN database in the OMOP common data model for active drug safety surveillance. Drug Saf. 2013;36:119–134. doi: 10.1007/s40264-012-0009-3. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320:867–868. doi: 10.1001/jama.2018.10136. [DOI] [PubMed] [Google Scholar]
- 11.Chen M-F, et al. Web-based experience sharing platform on medical device incidents for clinical engineers in hospitals. J. Med. Biol. Eng. 2018;38:835–844. doi: 10.1007/s40846-018-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidi VD, Matheny ME, Donnelly S, Resnic FS. An evaluation of a distributed medical device safety surveillance system: The DELTA network study. Contemp. Clin. Trials. 2011;32:309–317. doi: 10.1016/j.cct.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burn E, et al. Deep phenotyping of 34,128 adult patients hospitalised with COVID-19 in an international network study. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-18849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park RW. The distributied research network, observational health data sciences and informatics, and the South Korea research network. Korean J. Med. 2019;94:309–114. [Google Scholar]
- 15.Evidnet, FeederNet, https://feedernet.com/resource (2021).
- 16.White J, Carolan-Rees G. Current state of medical device nomenclature and taxonomy systems in the UK: Spotlight on GMDN and SNOMED CT. JRSM Short Rep. 2013;4:1–7. doi: 10.1177/2042533313483719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig A, O’Meley P, Carter P. The need for greater reporting of medical device incidents. Innovations. 2019;3:56–63. [Google Scholar]
- 18.Krupka DC, et al. Transmitting device identifiers of implants from the point of care to insurers: A demonstration project. J. Patient Saf. 2021;17:223. doi: 10.1097/PTS.0000000000000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seong Y, et al. Incorporation of Korean Electronic data interchange vocabulary into observational medical outcomes partnership vocabulary. Healthc. Inform. Res. 2021;27:29–38. doi: 10.4258/hir.2021.27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang, E. J. et al. Mapping Korean EDI medical procedure code to SNOMED CT. In MEDINFO 2019: Health and Wellbeing e-Networks for All 178–182 (2019). [DOI] [PubMed]
- 21.You, S. C. et al. Conversion of national health insurance service-national sample cohort (NHIS-NSC) database into observational medical outcomes partnership-common data model (OMOP-CDM). In MEDINFO 2017:Precision Healthcare Through Informatics 467–470 (2017). [PubMed]
- 22.Korea Health Information Service. Healthcare information standard, https://hins.or.kr (2021).
- 23.Paxton EW, Inacio MCS, Khatod M, Yue EJ, Namba RS. Kaiser Permanente national total joint replacement registry: Aligning operations with information technology. Clin. Orthop. Relat. Res. 2010;468:2646–2663. doi: 10.1007/s11999-010-1463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbloom ST, et al. Data from clinical notes: A perspective on the tension between structure and flexible documentation. J. Am. Med. Inform. Assoc. 2011;18:181–186. doi: 10.1136/jamia.2010.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nancy AM, Maheswari R. A review on unstructured data in medical data. J. Crit. Rev. 2020;7:2202–2208. [Google Scholar]
- 26.Campion TR, Jr, Johnson SB, Paxton EW, Mushlin AI, Sedrakyan A. Implementing unique device identification in electronic health record systems: Organizational, workflow, and technological challenges. Med. Care. 2014;52:26–31. doi: 10.1097/MLR.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 27.Sedrakyan A, et al. The international consortium of orthopaedic registries: Overview and summary. JBJS. 2011;93:1–12. doi: 10.2106/JBJS.K.01125. [DOI] [PubMed] [Google Scholar]
- 28.Robertsson O, Mendenhall S, Paxton EW, Inacio MCS, Graves S. Challenges in prosthesis classification. JBJS. 2011;93:72–75. doi: 10.2106/JBJS.K.00990. [DOI] [PubMed] [Google Scholar]
- 29.IMDRF UDI Working Group. Unique Device Identification (UDI) of Medical Devices, http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-131209-udi-guidance (2013).
- 30.Barlas S. New FDA medical device rule imposes minimal burden on hospitals: Facilities able to scan unique device identifiers will benefit. Pharm. Ther. 2013;38:720. [PMC free article] [PubMed] [Google Scholar]
- 31.Gross TP, Crowley J. Unique device identification in the service of public health. N. Engl. J. Med. 2012;367:1583. doi: 10.1056/NEJMp1113608. [DOI] [PubMed] [Google Scholar]
- 32.Aston JW, Howarth AL, Wilson NA, Mahabir RC. The value of unique device identifiers in plastic surgery. Aesthet. Surg. J. 2018;38:1264–1266. doi: 10.1093/asj/sjy210. [DOI] [PubMed] [Google Scholar]
- 33.Camus D, et al. New European medical device regulation: How the French ecosystem should seize the opportunity of the EUDAMED and the UDI system, while overcoming the constraints thereof. Therapies. 2019;74:73–85. doi: 10.1016/j.therap.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Melvin T, Torre M. New medical device regulations: The regulator’s view. EFORT Open Rev. 2019;4:351–356. doi: 10.1302/2058-5241.4.180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Food and Drug Safety. Medical devices act, https://elaw.klri.re.kr/kor_service/lawView.do?hseq=50798&lang=ENG (2019).
- 36.Wilson NA, Drozda J. Value of unique device identification in the digital health infrastructure. JAMA. 2013;309:2107–2108. doi: 10.1001/jama.2013.5514. [DOI] [PubMed] [Google Scholar]
- 37.Drozda JP, Jr, Dudley C, Helmering P, Roach J, Hutchison L. The Mercy unique device identifier demonstration project: Implementing point of use product identification in the cardiac catheterization laboratories of a regional health system. Healthcare. 2016;4:116–119. doi: 10.1016/j.hjdsi.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Dhruva SS, Ross JS, Schulz WL, Krumholz HM. Fulfilling the promise of unique device identifiers. Ann. Intern. Med. 2018;7:183–185. doi: 10.7326/M18-0526. [DOI] [PubMed] [Google Scholar]
- 39.Poon EG, et al. Effect of bar-code technology on the safety of medication administration. N. Engl. J. Med. 2010;362:1698–1707. doi: 10.1056/NEJMsa0907115. [DOI] [PubMed] [Google Scholar]
- 40.Senders JW. On the complexity of medical devices and systems. BMJ Qual. Saf. 2006;15:i41–i43. doi: 10.1136/qshc.2005.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resnic FS, et al. Quantifying the learning curve in the use of a novel vascular closure device: an analysis of the NCDR (National Cardiovascular Data Registry) CathPCI registry. JACC Cardiovasc. Interv. 2012;5:82–89. doi: 10.1016/j.jcin.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A, et al. The data extraction and longitudinal trend analysis network study of distributed automated postmarket cardiovascular device safety surveillance. Circ. Cardiovasc. Qual. Outcomes. 2015;8:38–46. doi: 10.1161/CIRCOUTCOMES.114.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah JS, Maisel WH. Recalls and safety alerts affecting automated external defibrillators. JAMA. 2006;296:655–660. doi: 10.1001/jama.296.6.655. [DOI] [PubMed] [Google Scholar]
- 44.Rosen CJJ. The rosiglitazone story—Lessons from an FDA Advisory Committee meeting. N. Engl. J. Med. 2007;357:844–846. doi: 10.1056/NEJMp078167. [DOI] [PubMed] [Google Scholar]
- 45.Jiang G, Yu Y, Kingsbury PR, Shah N. Augmenting medical device evaluation using a reusable unique device identifier interoperability solution based on the OHDSI common data model. Stud. Health Technol. Inform. 2019;264:1502–1503. doi: 10.3233/SHTI190505. [DOI] [PubMed] [Google Scholar]
- 46.Anand K, Veermaram C, Saini SK, Singh BK. Global medical device nomenclature: The concept for reducing device-related medical errors. J. Young Pharm. 2010;2:403–409. doi: 10.4103/0975-1483.71637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Häyrinen K, Saranto K, Nykänen P. Definition, structure, content, use and impacts of electronic health records: A review of the research literature. Int. J. Med. Inform. 2008;77:291–304. doi: 10.1016/j.ijmedinf.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Ministry of Health and Welfare, Ministry of Health and Welfare notice 2021-174, Medical device price list, https://www.hira.or.kr/rd/insuadtcrtr/bbsView.do?pgmid=HIRAA030069000400&brdScnBltNo=4&brdBltNo=51884&isPopupYn=Y (2021).
- 49.Ministry of Food and Drug Safety. Medical device information portal https://udiportal.mfds.go.kr (2021).
- 50.U.S. National library of medicine, Access GUDID, https://accessgudid.nlm.nih.gov (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.