Abstract
Background:
Many severe asthma (SA) patients fail to respond to Type 2 inflammation targeted therapies. We previously identified a cohort of SA subjects expressing Type 1 inflammation manifesting with IFN-γ expression with variable Type 2 responses.
Objective:
We investigated the role of the chemotactic receptors CXCR3 and CCR5 in establishing Type 1 inflammation in SA.
Methods:
Bronchoalveolar lavage microarray data from the Severe Asthma Research Program (SARP I/II) were analyzed for pathway expression and paired with clinical parameters. Wild Type, Cxcr3−/− and Ccr5−/− mice were exposed to a Type 1High SA model with analysis of whole lung gene expression and histology. Wild type and Cxcr3−/− mice were treated with an FDA-approved CCR5 inhibitor (maraviroc) with assessment of airway resistance, inflammatory cell recruitment by flow cytometry, whole lung gene expression and histology.
Results:
A cohort of subjects with increased IFN-γ expression showed higher asthma severity. IFN-γ expression correlated with CXCR3 and CCR5 expression but in Cxcr3−/− and Ccr5−/− mice Type 1 inflammation was preserved in a murine SA model, most likely due to compensation by the other pathway. Incorporation of maraviroc in the experimental model blunted airway hyperreactivity, despite only mild effects on lung inflammation.
Conclusions:
IFNG expression in asthmatic airways was strongly correlated with expression of both the chemokine receptors CXCR3 and CCR5. While these pathways provide redundancy for establishing Type 1 lung inflammation, inhibition of the CCL5/CCR5 pathway with maraviroc provided unique benefits in reducing airway hyperreactivity. Targeting this pathway may be a novel approach for improving lung function in Type 1high asthmatic individuals.
Clinical Implications:
In Type 1 high asthma, CCR5 and CXCR3 provide redundant pathways for lung inflammation, but CCR5 inhibition provides a potential novel target for reducing airway hyperreactivity.
Keywords: CXCR3, CXCL9, CXCL10, CCL5, CCR5, IFN-γ, severe asthma, maraviroc
Capsule Summary:
Type 1 inflammation in asthma is associated with more severe disease, but inhibition of the CCL5/CCR5 pathway in these patients may provide a novel approach to reduce airway hyperreactivity
Introduction
Asthma is a common disease affecting 5–10% of the population in developed countries1,2, and nearly 5% of patients have severe asthma (SA) with poor response to corticosteroid therapy. While great strides have been made in the management of Type 2 high asthma through the use of targeted biologic therapy3, nearly 50% of asthma patients lack evidence of this phenotype4. For these patients as well as for those with elevated Type 2 inflammation who fail to respond to targeted biologic therapy, improved understanding of the underlying pathophysiology is key to identifying novel pathways and therapeutic targets.
We have previously shown that approximately 30% of SA patients have evidence of Type 1 inflammation marked by elevated airway levels of interferon-γ (IFN-γ) 5 C-X-C Ligand 10 (CXCL10, IP-10) is also elevated in these patients and associated with worse disease as evidenced by higher need for steroids and more frequent exacerbations 6 CXCL10 (along with sister ligands CXCL9 and CXCL11) binds to C-X-C Receptor 3 (CXCR3) to promote chemotaxis of Th1 cells7, 8 as well as activation of eosinophils9 and mast cells10. As Th1 cells produce IFN-γ that promotes CXCL10 expression, this can lead to a positive feed forward loop to augment local Type 1 inflammation, an important component of the immune viral response11, but a driver of local pathology in autoimmunity7. Importantly, IFN-γ induction of CXCL10 is refractory to corticosteroids, making this a potential component of steroid resistance in severe asthma patients6, 12, 13.
The CXCL10-CXCR3 pathway is not the only pathway for Th1 chemotaxis. C-C motif ligand 5 (CCL5, RANTES) acts through the C-C Receptor 5 (CCR5) receptor to promote homing of cells to sites of inflammation. While co-expression of both receptors is uncommon in peripheral blood, it is highly prevalent at sites of inflammation in autoimmune disease14, and has been described in the lung 15.
In this study, we investigated the effect of chemotactic blockade in a previously described murine SA model characterized by elevated type 1 inflammation and minimal steroid response5. We demonstrate the important role that both chemotactic pathways play in establishing Type 1 inflammation in the lung. Finally, we show that inhibition of the CCL5/CCR5 pathway provides unique benefits in improving airway hyperreactivity despite only limited improvement in lung inflammation.
Methods
Human Subjects
Data previously obtained from subjects enrolled in the Severe Asthma Research Program (SARP) I/II cohorts was used. In brief, non-smoking subjects between ages 18–60 with asthma from racially/ethnically diverse backgrounds were recruited. All subjects had FEV1 >60% predicted. Subjects meeting American Thoracic Society (ATS) 2000 definition of severe asthma were classified as having severe asthma (SA) with the remaining subjects classified as having mild to moderate asthma (MMA). Additional non-smoking subjects without asthma were recruited as healthy controls (HC). Subjects underwent regularly scheduled visits that included spirometry, exhaled nitric oxide (FeNO), sputum sample, clinical questionnaire and medication use questionnaire. Subjects underwent bronchoscopy with bronchial epithelial brushings and bronchoalveolar lavage. Samples were processed by microarray as previously published6. Immunohistochemistry data was obtained from endobronchial biopsies as previously published.16 All data was previously obtained prior to the start of the current project.
Murine Models
C57BL/6 wild type (WT) mice (Cat#000664), mice deficient in CXCR3 (Cxcr3−/−, Cat # 005427) and mice deficient in CCR5 (Ccr5−/− Cat# 005796) were purchased from the Jackson Laboratory. All animals were cared for according to the NIH Policy on Humane Care and Use of Laboratory Animals with adherence to the Guide for the Care and Use of Laboratory Animals. Mice were housed under pathogen-free conditions and underwent treatments between 8–10 weeks of age. Age-matched mixed sex mice were used in this study. As described previously5, mice were exposed to a Type-1 dominant SA model. 17Mice were sensitized to 25 μg house dust mite antigen (HDM) (low-endotoxin, Greer Laboratories cat # XPB70D3A2.5) and combined with 5μg cyclic-di-GMP (Axxora cat # BLG-C057–01) on days 1, 3 and 5. Mice were rested for 5 days and then subjected to 3 challenge sets involving 3 consecutive daily challenges with HDM and cyclic-di-GMP with 4 days of rest between each set. Each challenge set included 0.5μg cyclic-di-GMP with 25 μg HDM on day 1, followed by 25 μg HDM only on the following 2 days. Mice were then sacrificed on day 28 and the lungs were used for histology and preparation of whole lung homogenate. For flow cytometry studies, an abbreviated model was used with the mice sacrificed after the first challenge was completed and lungs processed for single cell suspension. For maraviroc studies, maraviroc was dosed in drinking water (300mg/L) beginning 24 hours prior to the initial model event (sensitization or challenge) and continued until harvest (Fig E5, A, in this article’s Online Repository at www.jacionline.org).
Intracellular Staining and Flow Cytometry
Lungs of anesthetized mice were perfused with sterile PBS, removed and processed for single cell preparation as described previously.5 Briefly, the lung tissues were digested in a collagenase A/DNase suspension and then dissociated on a gentleMACS dissociator. A single cell suspension was prepared by passing the dissociated tissue through a 70 μm cell strainer, and then treated with RBC lysis buffer (BD Pharm Lyse™). Cells were then stimulated for 2.5 hours with PMA (50 ng/ml)/ionomycin (1 ug/ml) in the presence of brefeldin A and monensin. Cells were washed, resuspended in Hanks’ balanced salt solution and stained with fixable viability dye eFIuor 780 (eBioscience). Cells were fixed overnight using a Foxp3/Transcription Factor Staining Buffer Set (eBioscience) and stained for surface markers as well as intracellular cytokines for 45 minutes in permeabilization buffer. Data were acquired on a FACSAria flow cytometer (BD Immunocytometry Systems) and analyzed using FlowJo software (Tree Star, Inc).
Measurement of Airway Resistance
Airway hyperresponsiveness (AHR) in mice was measured as previously described with some modifications.5 Mice were anesthetized using Xylazine (12 mg/kg)/Sodium Pentobarbital (90 mg/kg) and Pancuronium (0.8 mg/kg) and subjected to the forced oscillation technique for measuring AHR using a Flexivent system (SCIREQ). Measurements of lung function were made following perturbation with increasing doses of methacholine (0–100mg/ml).
Statistical Analysis
Statistical Analysis was completed in GraphPad Prism 7.03 for all in vitro and murine studies. Human Data was analyzed using STATA SE 15. For parameters with normal distribution including murine studies, one-way ANOVA with Dunn’s post-hoc test or Student’s T-test (with Welch’s correction for unequal standard deviations as needed) were used. For all human data, non-parametric analyses were used with Wilcoxon rank-sum testing for two group comparisons, Kruskal-Wallis test with Sidak’s post-hoc testing for multigroup analysis of variance, Spearman’s Non-parametric Correlation for comparison of association between continuous variables and Fisher’s Exact testing for contingency groups. Exact p-values are reported throughout, analysis of variance for multigroup comparisons were considered significant at a p<0.05 (in these settings exact value for post-hoc testing is reported).
Results
IFN-γ is elevated in a sub-cohort of more severe asthma subjects
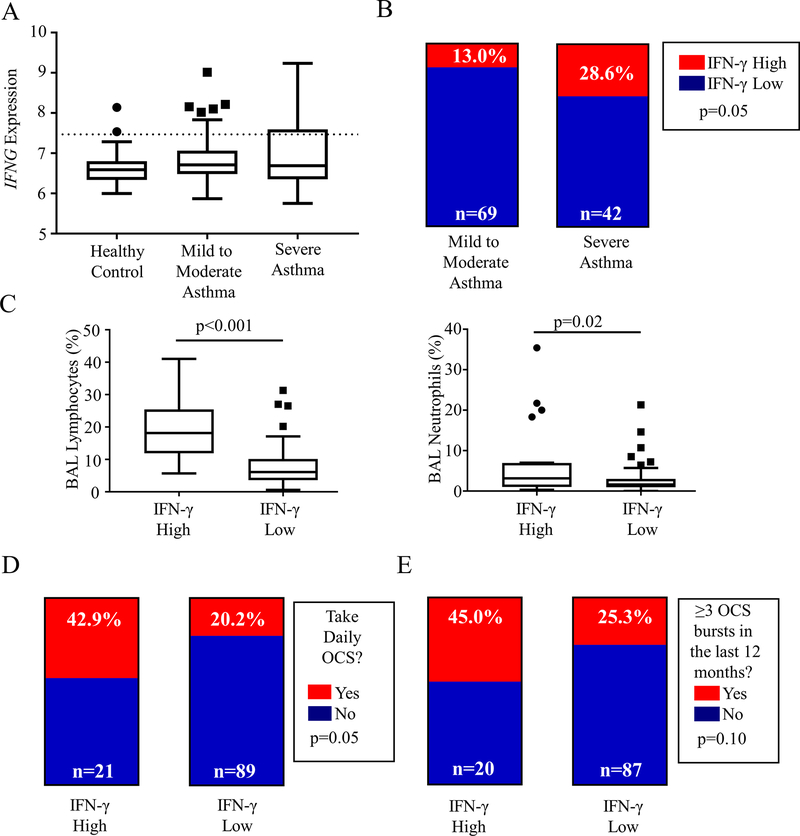
We examined previously obtained clinical and microarray data (obtained from bronchoalveolar lavage [BAL] cell pellets) from subjects enrolled in the Severe Asthma Research Program I/II (SARP I/II); 148 subjects had microarray data available for analysis. This included HC (n=38), subjects with MMA (n=69) and subjects with SA (n=42). As expected, significant differences were noted between asthma severity groups in age, BMI, Asthma Quality of Life Questionnaire (Juniper AQLQ, higher score indicates better quality), spirometry and markers of Type 2 inflammation (Table EI, online repository). Notably, there was no difference in race or sex between groups. While IFNG levels were not significantly different across groups (p=0.34), there was notably greater variance with increasing asthma severity (Fig 1, A, Fig E1, A, online repository). This suggested that a cohort of asthma subjects expressed notably elevated levels of IFN-γ compared to the healthy control group.
FIG 1:
IFN-γ is elevated in a sub-group of asthma patients with more severe disease. A Microarray data from SARP I/II bronchoalveolar lavage cell pellet RNA (BAL) was analyzed. IFNG expression in BAL is shown across asthma severity groups; while there was no statistical difference in median, a population of asthma patients with significantly elevated IFNG expression compared to healthy controls is noted; these patients were identified by a cutoff of two standard deviations above the healthy control median (y=7.467, dotted line). B After dividing the cohort into IFN-γ high and low, the prevalence of these groups was compared by asthma severity (Fisher’s Exact test). C BAL Cell differentials (% cell content) were compared for lymphocytes and neutrophils between IFN-γ High and Low groups; Mann-Whitney U test. D and E Daily use of oral corticosteroid (OCS) and the presence of frequent exacerbations (≥3 in the prior 12 months) were compared between IFN-γ High and Low Groups (Fisher’s Exact Test)
To characterize this IFN-γ high subset of subjects, we assigned a cutoff gene expression value of 7.467 based on two standard deviations above the healthy control (healthy control median 6.591, SD 0.438; Fig 1, A, dotted line). This divided the asthma cohort into IFN-γ high subjects (n = 21) and IFN-γ low (n = 90). Notably, a significantly higher proportion of subjects were IFN-γ high among those with SA than among those with MMA (28.6% vs 13.0%, p=0.05, Fig 1, B), a trend that held when subjects were divided into mild, moderate and severe categories (p=0.06, Fig E1, B, online repository). IFN-γ high subjects were significantly older, with a trend towards later age of onset of asthma (Table I). IFN-γ high subjects also showed a trend towards a lower forced expiratory volume at 1 second (FEV1) with a significant reduction in forced vital capacity (FVC) (Table I).
Table I:
Demographics and Clinical Parameters of IFN-γ high and low asthma patients.
| IFNG Low n=90 | IFNG High n=21 | p-value | ||
|---|---|---|---|---|
| Age at Enrollment (years) | 30.80(23.58–44.58) | 43.53(34.38–51.00) | 0.005 | |
| Age at diagnosis (years) | 7(3–12) | 10.5(4–33) | 0.16 | |
| Sex = 1 | 31 (34%) | 7 (21%) | 0.92 | |
| Race | 1 | 26(20.8%) | 6(27.3%) | 0.82 |
| 2 | 96(76.8%) | 16(72.7%) | ||
| 3 | 1(0.8%) | 0(0%) | ||
| 4 | 2(1.6%) | 0(0%) | ||
| BMI (kg/m2) | 29.04(25.14–34.35) | 31.41(26.47–36) | 0.23 | |
| Ever smoked | 18(20.4%) | 2(9.5%) | 0.24 | |
| Atopy | 85(75.2%) | 18(81.82%) | 0.51 | |
| Juniper AQLQ | 4.87(3.57–5.53) | 4.81(3.75–5.10) | 0.37 | |
| FeNO (ppb) | 32.5(20.0–62.3) | 38(18.6–48.7) | 0.34 | |
| IgE (IU/ml) | 95.5(44.5–351.5) | 166(43–280) | 0.48 | |
| Sputum Eosinophils (%) | 1.2(0.2–4.0) | 1.7(0.7–3.6) | 0.54 | |
| Blood Eosinophils (x103 cells/μL) | 0.300(0.100–0.400) | 0.150(0.100–0.300) | 0.13 | |
| FEV1 (% predicted) | 80.0(64.0–97.0) | 70.0(56.0–81.0) | 0.05 | |
| FVC (% predicted) | 92.0(77.5–103.0) | 84.0(67.5–93.0) | 0.02 | |
| FEV1/FVC (x100) | 72.7(64.2–80.4) | 70.3(59.0–78.3) | 0.19 | |
| ICS use | 54(61.4%) | 16(76.2%) | 0.31 | |
| High Dose ICS | 29(48.3%) | 10(62.5%) | 0.40 | |
| Anti-IgE Therapy | 7(8.0%) | 2(10.0%) | 0.67 | |
Continuous Variables are shown as median(25%–75%). Contingency Variables are shown as category(%). Continuous Variables analyzed by Mann-Whitney U-test, Contingency Variables with Fisher’s Exact Test. Exact p-values shown for all tests. BMI = Body Mass Index, AQLQ = Asthma Quality of Life Questionairre, FeNO = Fraction of Exhaled Nitric Oxide, FEV1 = Forced Expiratory Volume at 1 Second, FVC = Forced Vital Capacity, ICS = Inhaled Corticosteroids.
Comparing BAL cell differentials, IFN-γ high subjects had notably elevated lymphocyte and neutrophil content in their BAL samples (Fig 1, C) despite no difference in BAL total cell counts (Fig E1, C, online repository). There was no difference in eosinophil counts between groups (p=0.74, data not shown), although notably all subjects with an eosinophil percentage greater than 5% were in the IFN-γ low group. This supports a greater contribution of lymphocytes and neutrophils to the overall airway inflammation in IFN-γ high subjects. Some subjects also had endobronchial biopsy immunohistochemistry data that was also available for review (IFN-γ high n = 8, IFN-γ low n = 23). Reviewing these data showed a similar trend to elevation of CD4+ cells in the endobronchial biopsies of IFN-γ high subjects along with a significant increase in CD68+ cells consistent with increased number of macrophage/monocyte cells in this group. No differences were seen in these large airway samples in the presence of eosinophils, neutrophils or mast cells (Fig E2, online repository), suggesting that these differences are manifested primarily in the small airways.
To clinically characterize these subjects, we examined medication utilization and disease exacerbations. There was no difference noted in inhaled corticosteroid use between groups (Table I). There was an increased use of systemic oral corticosteroids in the IFN-γ high group (42.9% vs. 20.2%, p=0.05; Fig 1, D). There was also a trend towards more subjects having required multiple oral steroid bursts (45.0% vs. 25.3%, p=0.10; Fig 1, E) or having required an ED/Urgent Care evaluation for asthma in the prior 3 months (60% vs 41.9%, p=0.21; Fig E1, D, online repository) although neither reached statistical significance.
Limiting our analysis to only SA subjects, we observed significant differences in BAL cell differentials (increased lymphocyte and neutrophil percentages), between IFN-γ high and IFN-γ low groups, although clinical differences were no longer apparent (Table EII, online repository). However, as the significant majority of IFN-γ high subjects were moderate or severe (Fig E1, B, online repository), it follows that IFN-γ high subjects tended to be sicker with higher steroid requirements.
IFN-γ is correlated with the CXCL10/CXCR3 and CCL5/CCR5 chemotactic pathways.
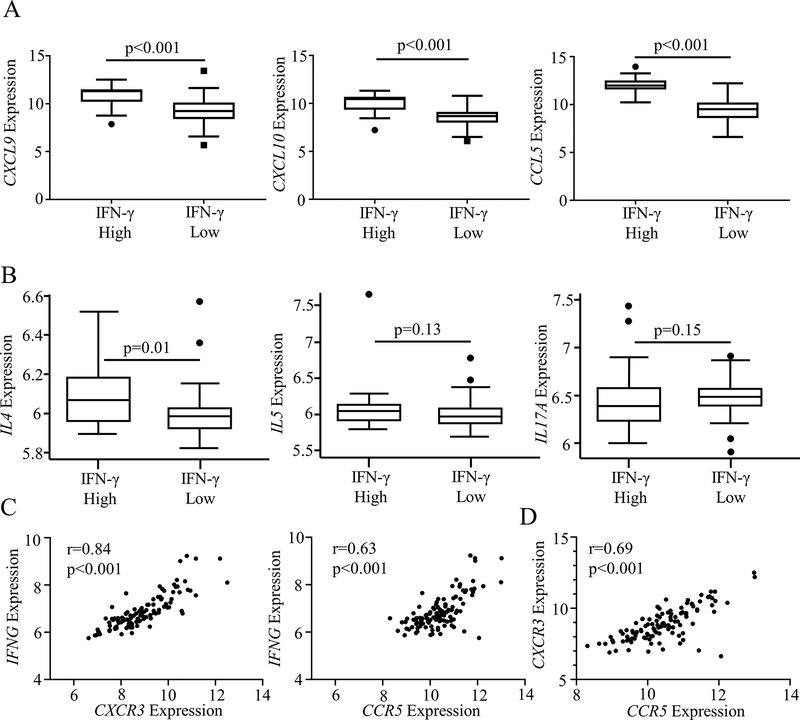
To understand the pathways involved in Type 1 signaling in these subjects, we examined expression and correlation of IFN-γ with known chemotactic signals. While comparison of CXCL9, CXCL10 and CCL5 expression across all asthma severity groups showed no significant difference, expression of each of these genes was elevated in IFN-γ high subjects compared to IFN-γ low subjects (Fig 2, A, Fig E3, A, online repository). We also observed strong correlation for each ligand with their cognate receptor (r = 0.75 and r = 0.69 for CXCL9 and CXCL10 with CXCR3 respectively, r = 0.65 for CCL5 with CCR5, p<0.001 for all measures; Fig E3, B, online repository). Examining co-expression with Type 2 and Type 3 cytokine pathways, IFN-γ high subjects showed no difference in IL5 or IL17A expression, but a significant elevation in IL4 was seen in the IFN-γ high subgroup, consistent with a prior description of elevated exhaled nitric oxide expression in SA subjects with high IFN-γ18 (Fig 2, B).
FIG 2:
There is a strong correlation between CXCR3 and CCR5 in asthma that suggests a dual chemotactic role. A Microarray data from SARP I/II bronchoalveolar lavage cell pellet RNA (BAL) was analyzed. CXCL10 and CCL5 expression in BAL was compared between IFN-γ High and Low groups (Mann-Whitney U test). B Expression of the Type 2 chemokine IL4 was elevated in IFNG high subjects, while no difference was observed in the eosinophilic cytokine IL5 or the Type 3 cytokine IL17A (Mann-Whitney U test). C Both CXCR3 and CCR5 correlate with IFNG suggesting a role in promoting Type 1 inflammation (Spearman Nonparametric Correlation). D There is strong correlation between both CCR5 and CXCR3 suggesting both pathways are upregulated in certain patients (Spearman Nonparametric Correlation).
When comparing chemotactic pathways with IFNG expression, we noted significant correlation with both receptors (r=0.78 and r = 0.57 for CXCR3 and CCR5 with IFNG respectively, p<0.001 for all measures; Fig 2, C). This suggested that both pathways might play a role in the recruitment of IFN-γ-expressing cells, as has been noted in other conditions 14, 15. To confirm this possibility, we examined association between the two receptors, and noted strong correlation (r=0.69, p<0.001; Fig 2, D). These correlations further strengthened when restricted to SA subjects (Fig E4, A–C, online repository). This supported the hypothesis that both the CXCL10/CXCR3 and CCL5/CCR5 pathways contribute to IFN-γ upregulation in a severe asthma phenotype.
Cxcr3−/− mice and Ccr5−/− mice have a preserved severe asthma phenotype
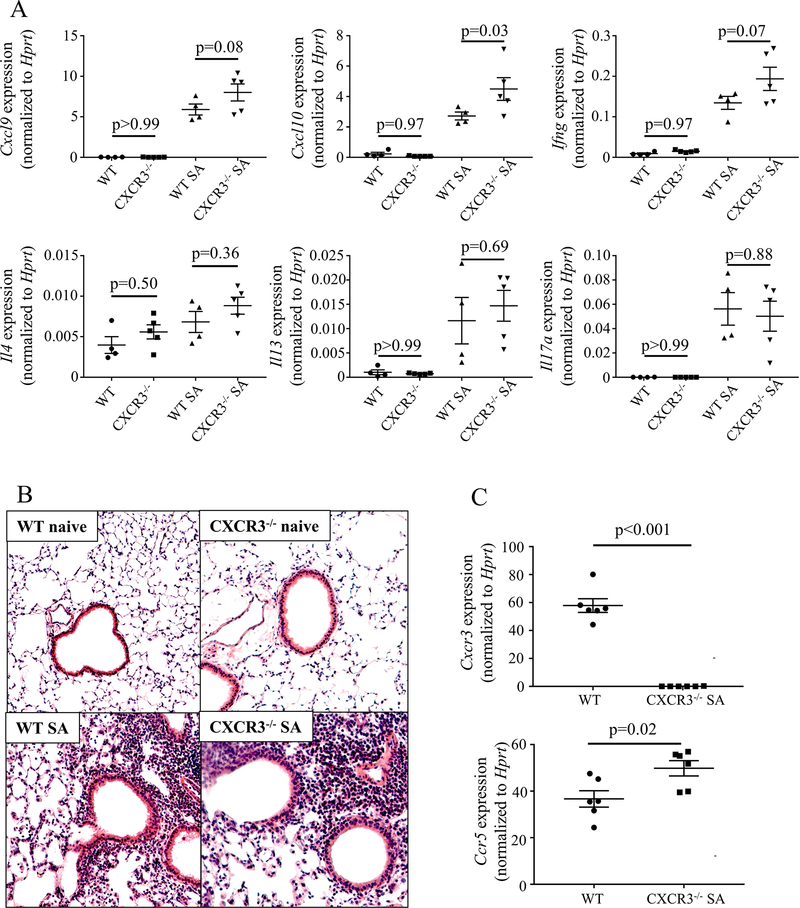
To better understand the importance of each chemotactic pathway in Type 1 inflammation, we exposed mice to a murine SA model (Fig E5, A, online repository) that induces IFN-γ dependent AHR which is resistant to corticosteroid treatment, parallel with the same phenomenon observed in human severe asthma5, 6, 12. This model utilizes a bacterial second messenger molecule (cyclic-di-GMP) to achieve a Type-1 dominant immune profile, consistent with reports in severe asthmatic children of a Type 1 inflammatory signature paired with recovery in BAL of pathogenic bacteria and viruses despite an absence of overt infectious disease in these subjects.17 WT and Cxcr3−/− mice were subjected to the SA model. Following sacrifice, whole lung RNA was isolated and assessed by qRT-PCR. Cxcr3−/− mice had higher lung expression of Cxcl10 (p = 0.03) and a trend towards higher expression of Cxcl9 (p = 0.08) and Ifng (p = 0.07) (Fig 3, A). No significant differences in Il4, Il13, or Il17a gene expression were detected between the two groups of mice (Fig 3, A). Histologic analysis of lung tissue sections showed similarity between Cxcr3−/− and WT mice at baseline (naïve) and also after being subjected to the SA model (Fig 3, B). To confirm our suspicion that the Ccl5/Ccr5 pathway was potentially responsible for the persistent Type 1 inflammation in Cxcr3−/− mice we analyzed expression of Cxcr3 and Ccr5 mRNA in whole lung tissue RNA from mice subjected to the SA model. Cxcr3−/− mice displayed increased Ccr5 expression compared to that detected in the WT mice (p = 0.02) (Fig 3, C). This suggested that increased chemotaxis via the CCL5/CCR5 pathway may functionally compensate for the loss of CXCL10/CXCR3 signaling.
FIG 3:
Cxcr3−/− mice have a similar inflammatory profile and phenotype to C57Bl/6 mice in a murine severe asthma model. A Whole lung PCR compared cytokine expression between naïve and severe asthma model (SA) exposed WT and Cxcr3−/− mice (one-way ANOVA with α<0.05 for each panel, Sidak’s post-hoc test for within treatment comparison shown; data representative of two independent experiments). B H&E staining of lung sections shows similar inflammation between treatment groups in both WT mice and Cxcr3−/− mice (20x magnification, representative sections) C Whole lung PCR compared expression of the Cxcr3 and Ccr5 genes in WT and Cxcr3−/− mice (Student’s T-test, data representative of 2 independent experiments).
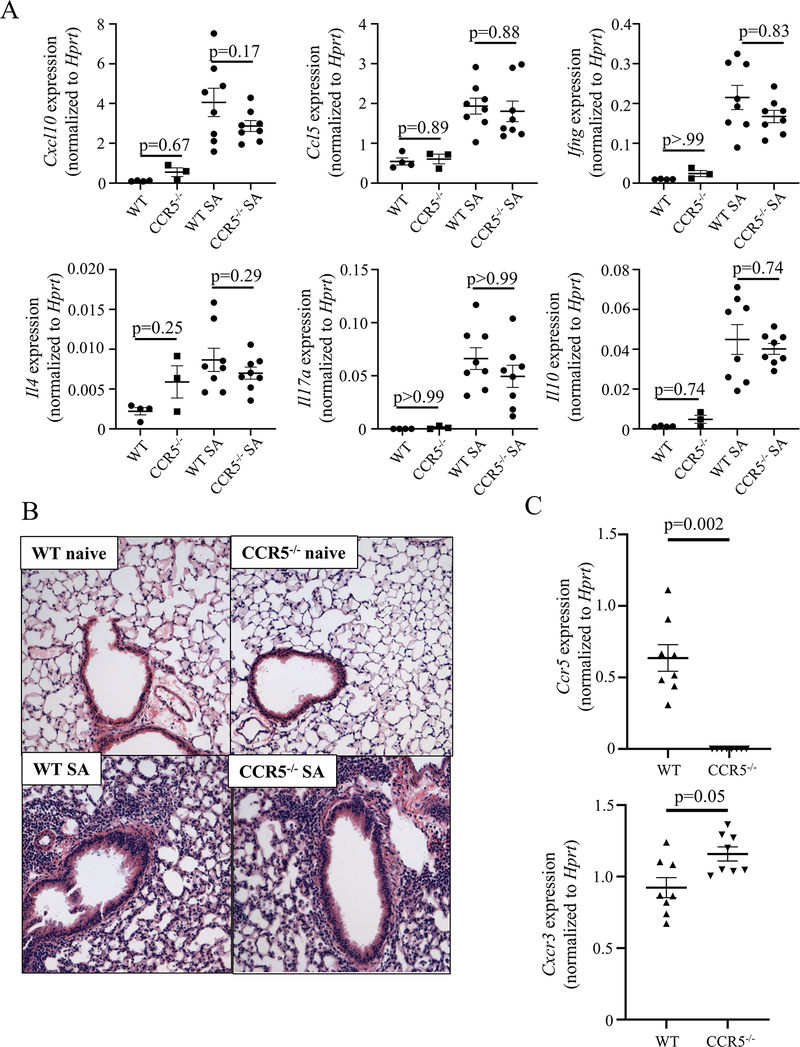
To assess the importance of the CCL5/CCR5 pathway, we subjected WT and Ccr5−/− mice to the SA model. Whole-lung RNA analysis by qRT-PCR again showed persistent elevations in Cxcl10, Ccl5, Ifng, Il4, Il17, and l10 without any significant difference between WT and Ccr5−/− mice (Fig 4, A). Histological analysis revealed a similar profile of increased inflammation in the two groups of mice (Fig 4, B). Finally, examination of receptor expression in Ccr5−/− mice revealed higher Cxcr3 expression compared to that in WT mice (p=0.05, Fig 4, C). These data combined with the results obtained using Cxcr3−/− mice strongly suggested redundancy in the ability of these pathways to mediate chemotaxis leading to Type 1 inflammation, and is consistent with previously described co-expression of CXCR3 and CCR5 protein on T-cells in human lungs.15
FIG 4:
Ccr5−/− mice also have a similar inflammatory profile and phenotype to WT mice in a murine severe asthma model. A Whole lung PCR compares cytokine expression between naïve and severe asthma model (SA) exposed WT mice and Ccr5−/− mice (One-way ANOVA with α<0.05 is significant for each panel, Sidak’s post-hoc test for within treatment comparison; data are pooled from 2 independent experiments). B H&E staining of lung sections shows similar inflammation between treatment groups in both WT mice and Ccr5−/− mice (20x magnification, representative sections). C Whole lung PCR compared expression of the Ccr5 and Cxcr3 genes in WT and Ccr5−/− mice (Student’s T-test, data pooled from 2 independent experiments).
CCR5 and CXCR3 blockade effectively decreases but does not eliminate Th1 lymphocyte recruitment
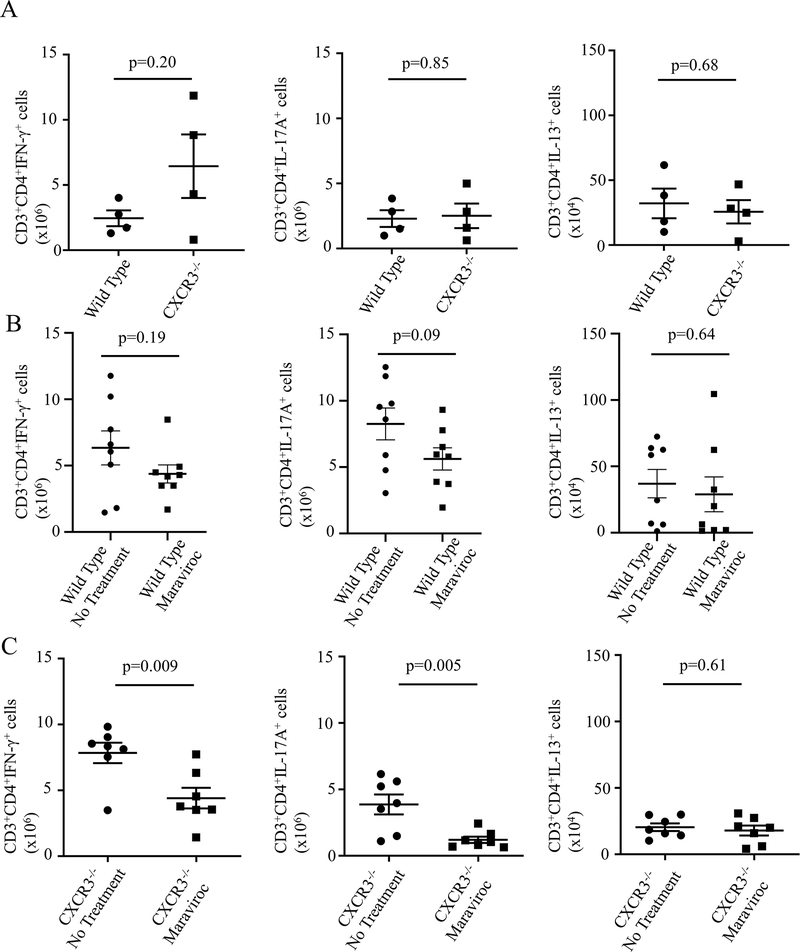
In order to address the apparent overlapping functions of the Cxcr3 and Ccr5 pathways in the SA model, we next examined the effect of chemokine receptor blockade on specific cell recruitment to the lung. We utilized an abbreviated mouse model for these studies, examining cell recruitment after the first challenge was completed (Fig E5, A, online repository). In both WT and Cxcr3−/− mice, we observed a similar degree of cellular recruitment with IFN-γ+ cells forming the predominant component of the pulmonary T-lymphocyte population (gating strategy in Fig E5, B, online repository). We did not observe any significant difference in IFN-γ+, IL-17A+, IL-13+ or IL-10+ T-lymphocytes between WT and Cxcr3−/− mice, (Fig 5, A).
FIG 5:
Dual Receptor inhibition significantly reduces IFN-γ+ cell recruitment to the lungs in a severe asthma model. A Assessment of lung T-lymphocyte (CD3+CD4+) cell populations by ICS in Cxcr3−/− mice shows no significant difference in T-cell subtype recruitment (Student’s T-test, data representative of 2 independent experiments). B WT mice were treated with maraviroc or no treatment with a non-significant trend to decrease in IFN-γ+ and IL-17a+ cells noted in maraviroc treated mice with no change in IL-13+ cells (Student’s T-test, data pooled from 2 independent experiments). C Cxcr3−/− mice were treated with maraviroc or no treatment with significant reductions in IFN-γ+ and IL-17a+ cells with maraviroc treatment and no change in IL-13+ cells (Student’s T-test, data pooled from 2 independent experiments).
Next, to assess the ability of CCR5 blockade to attenuate Th1 chemotaxis to the lung, we utilized maraviroc, a small molecule inhibitor of Ccr519. Mice received maraviroc via drinking water beginning prior to sensitization, and pulmonary cell recruitment was assessed after the first challenge. In WT mice, maraviroc had no significant effect on either total cell numbers in the lung or the percentage of IFN-γ+ or IL-13+ cells, although a trend towards reduction in both IFN-γ+ and IL-17A+ cell numbers was observed (Fig 5, B). These data suggested a limited efficacy for the drug in blocking T-lymphocyte lung chemotaxis when the CXCL10/CXCR3 pathway was intact. However, utilizing maraviroc in Cxcr3−/− mice to simulate a double knockout of the genes, we observed a substantial decrease in total cell numbers and in IFN-γ+ (p=0.009) and IL-17A+ (p=0.005) cells (Fig 5, C). These data support an essential component for both pathways in T cell recruitment to the lung and further supports the limited efficacy of single pathway blockade for reducing cellular inflammation.
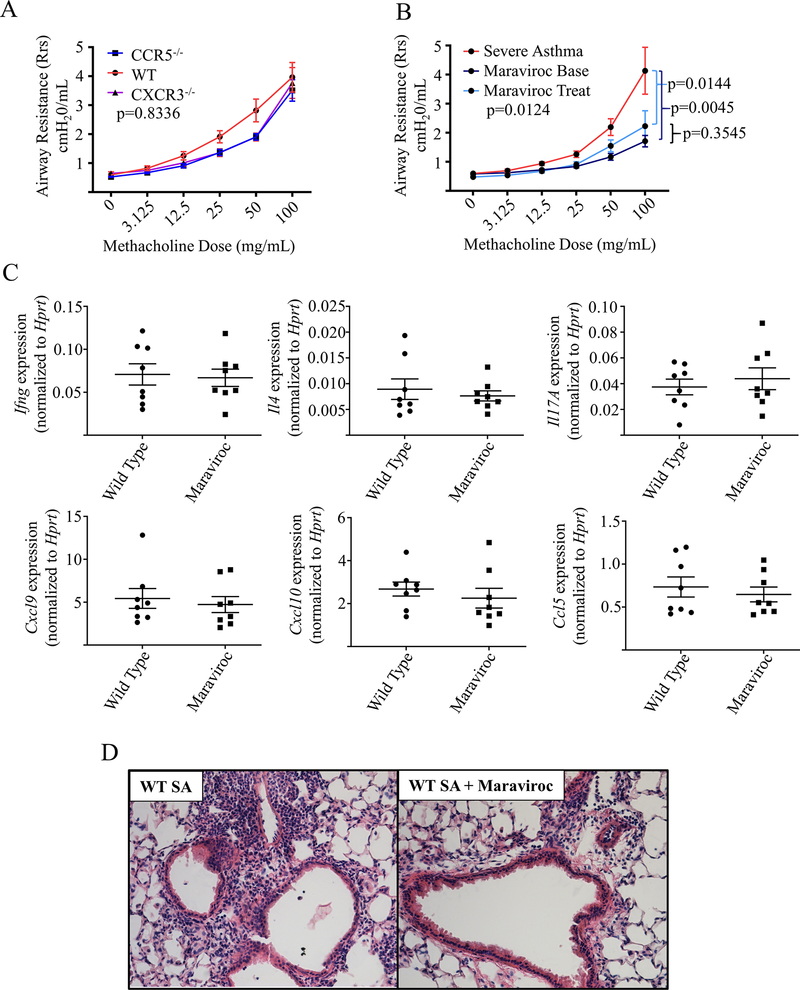
Efficacy of CCR5 inhibition in blocking airway hyperreactivity (AHR)
Although both Ccr5−/− and Cxcr3−/− mice failed to exhibit a reduction in lung inflammation individually, we investigated the effect of pathway blockade on AHR. Since dissociation between lung inflammation and AHR has been observed in asthma20, we had reason to suspect that single pathway blockade might still prove effective in this regard. AHR was assessed in our murine SA model utilizing a methacholine challenge protocol as previously described.5 We observed no effect of single pathway deletion on AHR in the Ccr5−/−and Cxcr3−/− mice (Fig 6, A). Given our data that single pathway deletion resulted in compensatory alternative pathway overexpression in genetic knockout mice (Fig 3, C, and Fig 4, C), we hypothesized that single pathway inhibition might be effective in WT mice without the problem of compensatory increase in the alternative pathway. To assess this, we again utilized the CCR5 inhibitor maraviroc in WT mice. Use of maraviroc in mice prior to sensitization with HDM (Maraviroc Base, Fig E5, online repository) resulted in a significant reduction in AHR (Fig 6, B) compared to that in the untreated control mice, suggesting that in WT mice single pathway blockade could attenuate symptoms. Given these findings, we also assessed the treatment potential of CCR5 in allergen-sensitized mice. In this treatment mode (Maraviroc Treat), we again observed significant reduction in AHR compared to that in the untreated mice (Fig 6, B). To confirm our hypothesis that this benefit in AHR was dissociated from lung inflammation, we assessed cytokine expression and inflammation in the lungs. While we found no difference in cytokine expression in whole lung with pre-challenge maraviroc (Fig 6, C), we did observe a modest improvement in the degree of airway inflammation by histology, consistent with the changes we noted in T-lymphocyte cell populations observed by flow cytometry (Fig 5, B), although some degree of inflammation did remain (Fig 6, D). Overall, these data suggest a role for CCR5 in promoting AHR in T1 dominant severe asthma, but that the CXCL10/CXCR3 pathway may provide an alternate mechanism for AHR when overexpressed, which is in agreement with prior data in T2 driven models.21
FIG 6:
Maraviroc effectively inhibits Airway hyperreactivity without modifying lung inflammation. A WT mice (WT, n = 7), Cxcr3−/− mice (n=5) and Ccr5−/− mice(n=6) were subjected to the SA model and underwent airway resistance testing with increasing concentrations of methacholine (data pooled from 2 separate experiments, Kruskall-Wallis testing). B WT mice were exposed to the SA model (Severe Asthma, n = 16) and a subset were treated with either maraviroc throughout the model beginning pre-sensitization (Maraviroc Base, n = 8) or post-sensitization but prior to challenges (Maraviroc Treat, n = 8) (data pooled from 4 experiments, Kruskall-Wallis testing with Dunn’s post hoc testing for intergroup comparisons). C WT and Maraviroc treated mice (Maraviroc) completed the full SA model and underwent assessment of whole lung cytokine expression by PCR with no significant differences observed. D Histology from WT and pre-challenge Maraviroc treated mice showed a modest improvement but residual inflammation in the maraviroc group (H&E staining, 20x magnification).
Discussion
While Type 2 inflammation is now well described in asthma with multiple therapeutic options available, the understanding of mechanisms underlying airway inflammation and hyperreactivity in asthma patients with a more complex IFN-γ high inflammatory phenotype remains poor. We previously demonstrated a role for IFN-γ high/Type 1 high inflammation in a subset of asthma patients that is generally steroid unresponsive5, 6 12 In this study, analysis of the SARP I/II microarray BAL data has revealed a Type-1 high group marked by increased expression of IFN-γ. These patients are marked by a more lymphocytic and neutrophilic sputum content and appear to have worse disease overall given the higher proportion of Type-1 high patients that are severe. Although markers of disease control (exacerbations, ED visits) did not reach statistical significance in our study, this is likely due to our study being underpowered for this outcome (only 21 subjects with Type-1 high asthma were identified); the overall trend, however, points towards greater exacerbation frequency and is consistent with our prior clinical data in CXCL10 high patients 6 as well as a recent publication implicating Th1 cells as the dominant source of worsening lung function in rhinovirus mediated asthma exacerbations.22 While patients with relatively low Type-2 markers are often less responsive to steroids 4,23, even some patients with elevated Type 2 markers can remain poorly controlled despite steroids or Type 2 targeted biologics due to a mixed inflammatory profile, indicating a need for novel asthma therapies.
Improved understanding of the pathways involved in establishing Type 1 inflammation in asthma will be important to identify targets for novel therapeutics. To that end, we have previously shown CXCL10 elevation in Type-1 high asthma and posited that the IFN-γ/CXCL10/CXCR3 axis represented an important chemotactic factor to drive Th1 recruitment to the lung in a possible forward feeding signaling loop11. Prior studies in Type 2 predominant murine asthma models have shown mixed effects of CXCR3 deficiency, with improvement in an ovalbumin (OVA)-driven model24 but worsening asthma in an HDM model 25 which may reflect variations in Type-1/Type-2 balance between these models. Here, we observed that Cxcr3−/− mice showed a trend towards greater overall and Type 1 inflammation in a SA model. While the endpoint responses in these studies are variable, all of them including our own show a consistent increase in IFN-γ in the Cxcr3−/− mice regardless of the model used. As CXCR3 also plays a significant role in eosinophil9, 26 and mast cell recruitment10, 27, it is possible that the effect in the overall model is determined by the altered immune balance resulting from Type-2 reduction and Type-1 increase that appears to be consistently caused by loss of CXCR3.
Given the role of CXCR3 in Th1 recruitment7, the consistent increase in IFN-γ levels in the lung suggested an alternative mechanism of establishing type 1 inflammation.CCR5, one of the principal chemotactic receptors for CCL5 (RANTES), is often co-expressed with CXCR3 on T-cells at sites of inflammation in autoimmune disorders 14 Although co-expression has been previously identified in the lung, this prior study had failed to show increased expression in asthma, which was likely due to the selection of only a small number of mild asthma subjects (n=4) 15 In our study we see strong correlation between CXCR3, CCR5 and IFNG at the RNA level, correlations which increased with asthma severity. Turning to murine models, Ccr5−/− mice have been shown to have diminished airway inflammation in a Type 2 dominant model induced with OVA 28, but when we examined Ccr5−/− in our SA model we observed minimal effect on Type 1 or Type 2 inflammation. As CCR5 is also an important recruiter of eosinophils 29, 30, it is again possible that this discrepancy in results rests in the variable Type 1 and Type 2 pathway induction between these models.
Prior studies have shown a dissociation between AHR and lung inflammation, suggesting that the molecular pathways for these components may be linked but separable.20 Here, we show that in mice deficient in one of these receptors, significant overexpression of the other receptor is able to maintain increased AHR. However, blockade of the CCR5 pathway alone in WT mice led to a significant reduction in AHR with both pre-sensitization and pre-challenge (treatment) dosing strategies, suggesting that inhibition of the CCL5/CCR5 pathway was effective in blunting AHR even after airway inflammation was already established. In a similar fashion, it is possible that blockade of CXCR3 alone in a WT mouse might also reduce AHR. Notably, our findings regarding CCR5 and AHR are in keeping with our recent study evaluating severe asthma immune phenotypes by deep immune profiling of BAL cells through cytometry time of flight (CyTOF). In this study, which identified a severe asthma subgroup with lymphocyte-predominant/Th1High asthma immune profile, CD161+CCR5+ were the most strongly associated cell type with airway obstruction assessed via FEV1.31 Since maraviroc is an FDA-approved drug for the treatment of patients with HIV, our findings raise the exciting possibility that it could be repurposed for the treatment of a subset of patients with SA.
The mechanism for this effect on AHR could be related to mast cell recruitment and activation. AHR has previously been linked to mast cell presence in an OVA model, with IFN-γ playing a significant role in mast cell function,27 and Th1, rather than Th2, cells are known to play a significant role in rhinovirus related worsening lung function in asthma, arguing for a link between Type 1 signaling and AHR.22, 32 We previously described airway mast cell presence in our murine SA model.6 In our recently published study, the subset of SA patients characterized by a Type 1High T-cell dominated immune response also displayed a signature of increased mast cell gene expression in their BAL cells.31 Mast cells can express both CCR533 and CXCR310 suggesting a role for epithelial and airway smooth muscle cells in the production of the cognate chemokines34 in recruitment of mast cells to the airways. In addition to inflammatory signaling via histamines, leukotrienes and prostaglandins, mast cells can exert direct influence on AHR via protease activity through the protease activated receptors (PAR-2),5, 35 a function which may be dependent on local inflammatory milieu.36 This suggests that the AHR mediating effects of CCR5 inhibition could also be related to alterations in this milieu rather than solely impaired chemotaxis given the post-sensitization efficacy that we observed. Further examination of the roles of CXCR3, CCR5, and their ligands in mast cell recruitment and function in asthma are needed to fully understand the therapeutic potential of these pathways.
One caveat with regard to our study is that the cross-sectional nature of the SARP I/II cohort makes conclusions regarding the natural history and persistence of the Type 1 high phenotype that we have proposed impossible to ascertain. Longitudinal cohort studies with repeated airway sampling will be critical to understanding the natural history and persistence of this phenotype over time. Furthermore, the cross-sectional assessment prevents any prospective assessment of asthma severity or exacerbations as they pertain to the Type 1 high phenotype. As shown in Fig 1, the variance in IFNG expression is wide in the SA group without a clear point of demarcation for IFNG high status. While our study relies on a cutoff point defined by expression levels in healthy controls, future work will investigate the continuum of IFNG expression as it relates to disease control and expression levels of other inflammatory pathways.
Additionally, the mechanisms underlying Type 1 inflammation mediated airway inflammation and hyperreactivity specifically also remain unclear. How CCR5 directly contributes to AHR and how CCR5 blockade affects other pathways involved in Type 1 high asthma remain unelucidated and an important focus of future work. Pairing BAL cell content/RNA signatures with airway level changes at both the molecular and cellular level will also provide new insights into potential mechanisms and serves as an exciting future research direction to address these issues.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (AI 106684 (AR and SEW), F32 HL137089 (MG), HL113956(AR), AI048927(AR)) and through the Parker B. Francis Foundation (MG); SARP I/II microarray data provided by the Severe Asthma Research Program.
Abbreviations
- AHR
Airway Hyperreactivity
- CCL5
C-C Ligand 5 (RANTES)
- CCR5
C-C chemokine receptor 5
- CXCL9
C-X-C Ligand 9 (MIG)
- CXCL10
C-X-C Ligand 10 (IP-10)
- CXCR3
C-X-C chemokine receptor 3
- HDM
House Dust Mite
- ICS
Intracellular Staining
- IFN-γ
Interferon-gamma
- MMA
Mild to Moderate Asthma
- SA
Severe Asthma
- WT
Wild Type
Footnotes
The Authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005–2009. In: Services UDoHaH, ed. National Health Statistics Reports. Hyattsville, MD: National Center for Health Statistics, 2011:1–15. [PubMed] [Google Scholar]
- 2.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc 2018; 15:348–56. [DOI] [PubMed] [Google Scholar]
- 3.McGregor MC, Krings JG, Nair P, Castro M. Role of Biologics in Asthma. Am J Respir Crit Care Med 2019; 199:433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest 2015; 125:3037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier M, Chakraborty K, Oriss TB, Raundhal M, Das S, Chen J, et al. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI Insight 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev 2014; 13:272–80. [DOI] [PubMed] [Google Scholar]
- 8.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, et al. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol 2009; 183:4378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, et al. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol 2000; 165:1548–56. [DOI] [PubMed] [Google Scholar]
- 10.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 2005; 171:1103–8. [DOI] [PubMed] [Google Scholar]
- 11.Antonelli A, Ferri C, Ferrari SM, Colaci M, Fallahi P. Immunopathogenesis of HCV-related endocrine manifestations in chronic hepatitis and mixed cryoglobulinemia. Autoimmun Rev 2008; 8:18–23. [DOI] [PubMed] [Google Scholar]
- 12.Oriss TB, Raundhal M, Morse C, Huff RE, Das S, Hannum R, et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell D, Bouazza B, Kokalari B, Amrani Y, Khatib A, Ganther JD, et al. IFN-gamma-induced JAK/STAT, but not NF-kappaB, signaling pathway is insensitive to glucocorticoid in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2015; 309:L348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. The Journal of clinical investigation 1998; 101:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol 2001; 166:2842–8. [DOI] [PubMed] [Google Scholar]
- 16.Balzar S, Chu HW, Silkoff P, Cundall M, Trudeau JB, Strand M, et al. Increased TGF-β2 in severe asthma with eosinophilia. Journal of Allergy and Clinical Immunology 2005; 115:110–7. [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. Th1 Signatures Are Present in the Lower Airways of Children with Severe Asthma, Regardless of Allergic Status. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modena BD, Bleecker ER, Busse WW, Erzurum SC, Gaston BM, Jarjour NN, et al. Gene Expression Correlated with Severe Asthma Characteristics Reveals Heterogeneous Mechanisms of Severe Disease. Am J Respir Crit Care Med 2017; 195:1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Martmez L, Ochoa-Callejero L, Rubio-Mediavilla S, Narro J, Bernardo I, Oteo J-A, et al. Maraviroc improves hepatic triglyceride content but not inflammation in a murine nonalcoholic fatty liver disease model induced by a chronic exposure to high-fat diet. Translational Research 2018; 196:17–30. [DOI] [PubMed] [Google Scholar]
- 20.CRIMI E, SPANEVELLO A, NERI M, IND PW, ROSSI GA, BRUSASCO V. Dissociation between Airway Inflammation and Airway Hyperresponsiveness in Allergic Asthma. American Journal of Respiratory and Critical Care Medicine 1998; 157:4–9. [DOI] [PubMed] [Google Scholar]
- 21.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, et al. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol 2002; 168:5278–86. [DOI] [PubMed] [Google Scholar]
- 22.Muehling LM, Heymann PW, Wright PW, Eccles JD, Agrawal R, Carper HT, et al. Human TH1 and TH2 cells targeting rhinovirus and allergen coordinately promote allergic asthma. Journal of Allergy and Clinical Immunology 2020; 146:555–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus SC, Krishnan JA, King TS, Lang JE, Blake KV, Covar R, et al. Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level. N Engl J Med 2019; 380:2009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Yan H, Xiao Y, Piao H, Xiang R, Jiang L, et al. Attenuation of antigen- induced airway hyperresponsiveness and inflammation in CXCR3 knockout mice. Respir Res 2011; 12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Chen H, Chen X, Gao J, Guo Z. Characteristics of Allergic Pulmonary Inflammation in CXCR3Knockout Mice Sensitized and Challenged with House Dust Mite Protein. PLoS One 2016; 11:e0162905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaku Y, Nakagome K, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. IFN-gamma-inducible protein of 10 kDa upregulates the effector functions of eosinophils through beta2 integrin and CXCR3. Respir Res 2011; 12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, et al. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J Clin Invest 2011; 121:3133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchimoto Y, Kanehiro A, Miyahara N, Koga H, Ikeda G, Waseda K, et al. Requirement for chemokine receptor 5 in the development of allergen-induced airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol 2011; 45:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato Y, Pawankar R, Kimura Y, Kawana S. Increased expression of RANTES, CCR3 and CCR5 in the lesional skin of patients with atopic eczema. Int Arch Allergy Immunol 2006; 139:245–57. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Konno Y, Kanda A, Yamada Y, Yasuba H, Sakata Y, et al. Critical role of CCL4 in eosinophil recruitment into the airway. Clinical & Experimental Allergy 2019; 49:853–60. [DOI] [PubMed] [Google Scholar]
- 31.Camiolo MJ, Zhou X, Oriss TB, Yan Q, Gorry M, Horne W, et al. High Dimensional Profiling Clusters Asthma Severity by Lymphoid and Non-lymphoid Status. Cell Reports 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camiolo MJ, Kale SL, Oriss TB, Gauthier M, Ray A. Immune responses and exacerbations in severe asthma. Current Opinion in Immunology 2021; 72:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salomonsson M, Dahlin JS, Ungerstedt J, Hallgren J. Localization-Specific Expression of CCR1 and CCR5 by Mast Cell Progenitors. Frontiers in Immunology 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 1999; 162:3549–58. [PubMed] [Google Scholar]
- 35.Pejler G The emerging role of mast cell proteases in asthma. European Respiratory Journal 2019; 54:1900685. [DOI] [PubMed] [Google Scholar]
- 36.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? Journal of Allergy and Clinical Immunology 2004; 114:58–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.