Abstract
Macrophages play a central role in lung physiology and pathology. Herein, we show in mice that alveolar macrophages (AMs), unlike other macrophage types [interstitial macrophages (IMs), and peritoneal and splenic macrophages], constitutively express PD-L1, thereby possessing a superior phagocytic ability and the capacity to repress cytotoxic T lymphocytes (CTLs) by cis- and trans-interacting with CD80 and PD-1, respectively. This extraordinary ability of AMs assures optimal protective immunity and tolerance within the lung. These findings uncover a unique characteristic of AMs and an innate immune function of PD-L1 and CD80, therefore help understand lung physiology, diseases and PD-L1/PD-1-based immunotherapy.
Keywords: Alveolar macrophage, CD80, cis-PD-L1/CD80 interaction, immune homeostasis, immune tolerance, innate immunity, lung, PD-1, PD-L1, phagocytosis
Introduction
Macrophages are the most abundant immune cells in the lung and serve as key sentinels of the lung, warding off pathogens and maintaining immune and tissue homeostasis (1-3). They are also main culprits of lung diseases (4, 5). Yet, it remains largely unknown how lung macrophages maintain immune tolerance within the lung while clearing potential pathogens.
Lung macrophages are highly plastic and heterogeneous, consisting of two main subtypes: alveolar macrophages (AMs) and interstitial macrophages (IMs) in the steady state, healthy lung (1-4). AMs are long-lived, self-renewing cells derived embryonically from hematopoietic stem cells (6), and make up the majority of macrophages in the lung (and are often simply referred to as lung macrophages). They are located in the air space of the alveoli and express CD11c on the surface. IMs are resided within the parenchymal space (interstitium) between adjacent alveoli and express CD11b but not CD11c. Under certain conditions such as lung injury and tumorigenesis, blood monocytes, which express surface markers most similar to IMs, may be recruited into the lung and differentiate into macrophages (7, 8).
To understand the mechanisms by which lung macrophages maintain immune homeostasis within the lung, we examined the expression and function of PD-L1 [programmed death-1 (PD-1) ligand 1, also known as B7-H1 or CD274] in lung macrophages. By binding to the immune checkpoint PD-1 (CD279) on immune effector cells and activated cytotoxic T lymphocytes (CTLs) in particular, PD-L1 suppresses CTL activity and thereby plays important roles in immune tolerance and immune response termination (9). Recent studies have shown that PD-L1 can also physically interact with the co-stimulatory molecule CD80 (B7-1) on antigen presenting cells (APCs) in cis, and this PD-L1/CD80 cis-interaction can stimulate the costimulatory CD28 signaling in T cells while repressing the inhibitory signaling of PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, also known as CD152) for optimal T-cell response (10-14). Thus far, however, studies on these immune co-stimulatory or -inhibitory molecules have been focusing mainly on their roles in adaptive immunity, and in particular, CTL regulation (12).
Here, we report that different from IMs, peritoneal macrophages (PMs) and splenic macrophages (SMs), AMs constitutively express PD-L1 to increase their phagocytosis and repress CTLs via cis-interacting with CD80 and trans-interacting with PD-1, respectively. PD-1 and CD80 also compete for PD-L1 engagement to refine alveolar macrophage phagocytosis. These findings reveal the direct role of PD-L1 and CD80 in innate immunity and provide mechanistic insights into how the lung maintains immune and tissue homeostasis for its physiological function.
Materials and Methods
Animals
We have complied with all relevant ethical regulations for animal testing and research. The animal experiments were performed in accordance with the US National Institutes of Health (NIH) Guidelines on the Use of Laboratory Animals. All animals were maintained under pathogen-free conditions and used according to protocols approved by Institutional Animal Care and Use Committee (IACAUC) of the University of Pittsburgh. CD80 deficient (CD80KO) mice, PD-1 deficient (PD-1KO) mice, FVB/N mice, C57BL/6 mice and BALB/c mice were purchased from The Jackson Laboratory. CD80KO mice and PD-1KO mice were on a C57BL/6 background.
Bronchioalveolar lavage (BAL)
Upon sacrifice, mice lungs were lavaged with ice-cold phosphate buffered saline (PBS) as described (15). The recovered BAL fluids (BALF) were centrifuged. Cells pelleted from BALF were used for immunofluorescence (IF), or in vitro T cell suppression analysis as described (16-21). For lipopolysaccharide (LPS) treatment studies, cells were seeded on cover glass in 24-well plate and stimulated with 100 ng/ml of LPS (Sigma-Aldrich, St. Louis, MO, USA. O55:B5) for 2 hours, followed by IF staining.
IF confocal microscopic analysis
Cells were fixed with 4% paraformaldehyde at room temperature for 10 minutes, and subsequently incubated with the indicated primary antibodies, followed by fluorescein isothiocyanate (FITC)- and rhodamine-conjugated secondary antibodies or Alexa Fluor 488 (AF488)- and Alexa Fluor 568 (AF568)- conjugated secondary antibodies. Cells were also counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining. Stained proteins and their subcellular localizations were detected using an Olympus Fluoview 1000 confocal microscope (Melville, NY) (100 × 11.4 NA oil objective) (22, 23).
FACS analysis
The cells were incubated with the antibodies against cell surface antigens after blocking with anti-CD16/CD32. The cells were then fixed with paraformaldehyde (2%), permeabilized with saponin (0.5%), and incubated with antibodies against intracellular antigens if needed. For interferon-gamma (IFNγ) and granzyme B staining, cells were treated with brefeldin A (BFA, 3 μg/ml) and monensin (2 μM) for 4 h before they were stained for FACS analysis. Data were acquired and analyzed by Accuri C6 or BD LSRFortessa I (BD Biosciences, Bedford, MA, USA) and the FlowJo software (24, 25).
Peritoneal cell preparation
Ice-cold PBS was injected into mouse peritoneal cavity and then recovered from peritoneal cavity after peritoneum was gently and completely massaged (26). Peritoneal cells obtained were used for FACS analysis. Peritoneal exudate cells were also seeded on cover glass in 24-well plate and stimulated with 20 ng/ml of LPS for 24 hours, followed by IF staining.
In vitro CTL suppression by AMs
Splenic CD3+ T cells from WT mice and AMs from WT or PD-L1 deficient mice (1:0.05 ratio) were seeded together with CD28 antibody (2 μg/ml) and IL-2 (8.8 ng/ml) in the plates pre-coated with CD3 antibody (10 μg/ml). Two days later, PD-L1 antibody (20 μg/ml) or PD-1 antibody (20 μg/ml) or control IgG (20 μg/ml) were added. After one additional day of co-culture, cells were collected for FACS analysis to detect IFNγ+ and granzyme B+ CD8+ T cells and CD8+ T-cell numbers as described (26, 27).
Ex vivo phagocytosis assays
Fresh lung tissues of the indicated mice were minced into small pieces, gently pressed with syringe plunger top, and filtered with 40 μm cell strainers. Cells passed through the strainer were seeded in 24-well ultra-low attachment plate (Corning Inc. Corning, NY, USA) with 400 μl culture medium containing the indicated antibody for 20 minutes. Latex Beads-Rabbit IgG-FITC Complexes (Cayman Chemical, Ann Arbor, MI, USA) were then added into the cell culture with a volume ratio of 1:100. Or, the cells were spun down at 1800 rpm for 5 min and the supernatant was replaced with 400 μl pHrodo Green S. aureus Bioparticles Conjugate (Thermo Fisher Scientific, Waltham, MA, USA. 1 mg/ml) or Zymosan A (S. cerevisiae) Bioparticles, Alexa Fluor 488 conjugate (Thermo Fisher Scientific, Waltham, MA, USA 0.25 mg/ml) in Live Cell Imaging Solution (Thermo Fisher Scientific, Waltham, MA, USA). Two hours later, the phagocytic abilities of AMs and IMs were determined by FACS.
Statistical analysis
Student’s t test (two tailed) was used to assess significance of differences between two groups. All bars in the figures represent means ± standard error of the mean (SEM). The P values < 0.05 and 0.01 are considered statistically significant and highly statistically significant, respectively (28-30).
Antibodies
Information of antibodies used, including the company names, catalogue numbers and dilutions, is listed in Supplemental Table 1.
Results
PD-L1 is constitutively and selectively expressed on AMs
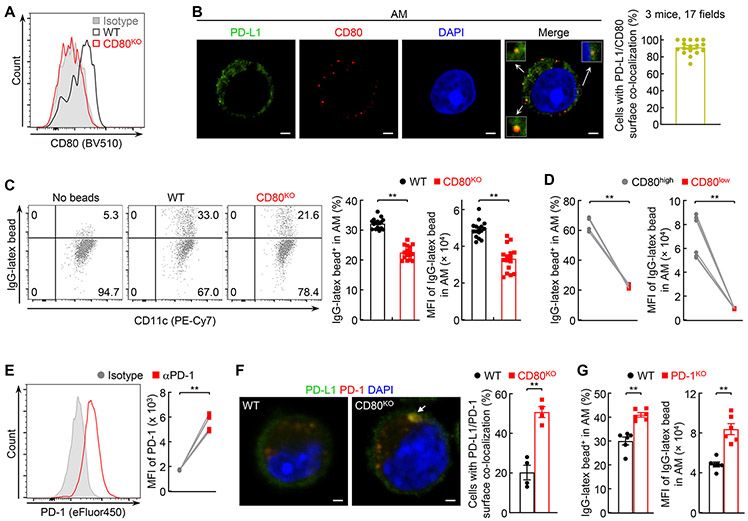
To define the mechanisms by which AMs contribute to the immune tolerance and homeostasis in the lung, we examined the expression levels of PD-L1 and PD-L2 (B7-DC, CD273), another key immune inhibitory molecule (9, 10), on the surface of AMs. Both, and in particular PD-L1, play important roles in immune tolerance and immune response termination by physically interacting with their receptor PD-1 on immune effector cells and activated CTLs in particular (9, 10). We included other macrophage populations, such as IMs, PMs and SMs, in our assays. PD-L1 was detected on all AMs, but not on IMs, PMs or SMs, in mice on different strain backgrounds (Fig. 1; Supplemental Fig. 1, A-D), indicating PD-L1 expression as a characteristic that distinguishes AMs from other macrophages and IMs in particular. On the other hand, neither AMs nor IMs expressed PD-L2 on the surface (Supplemental Fig. 1E).
Figure 1.
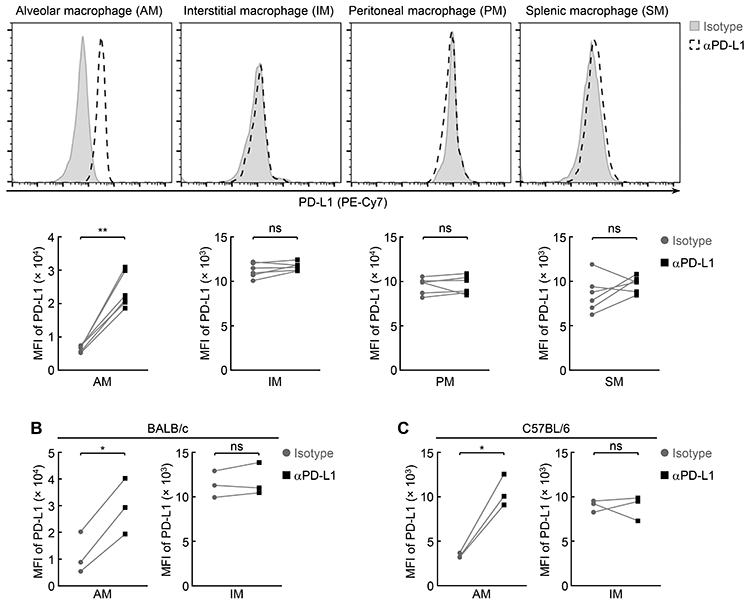
AMs inherently express PD-L1. (A) Specific expression of PD-L1 on the AMs but not IMs, PMs or SMs of FVB/N mice (n = 6). (B) Specific expression of PD-L1 on the AMs but not IMs of BALB/c mice (n = 3). (C) Specific expression of PD-L1 on the AMs but not IMs of C57BL/6 mice (n = 3). Student’s t test was performed (two tailed, paired) and data represent means ± SEM in (A-C). *P < 0.05; **P < 0.01; ns, not statistically significant.
PD-L1 provides AMs a superior ability to suppress CTLs
Given the prototypical role of PD-L1 in CTL suppression via trans-interacting with PD-1 on CTLs (9, 10), we examined whether the high expression of PD-L1 renders AMs capable of repressing CTLs. Indeed, AMs strongly repressed both the activity and number of activated CTLs when they were co-cultured, and this repression could be significantly, although partially, blocked by PD-L1 or PD-1 blocking antibody (Fig. 2). Consistently, AMs selectively defective in PD-L1 lost the suppressive ability significantly. Thus, PD-L1 expression arms AMs with a superior ability to repress CTLs.
Figure 2.
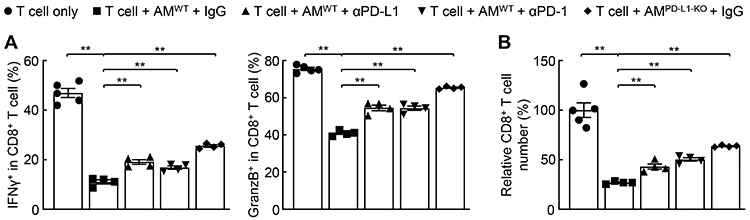
PD-L1 grants AMs high CTL suppression ability. (A) PD-L1-dependent repression of CD8+ T-cell activity by AMs (n ≥ 4). (B) PD-L1-dependent decrease of CD8+ T cells by AMs (n ≥ 4). Student’s t test was performed (two tailed, unpaired) and data represent means ± SEM. **P < 0.01.
PD-L1 renders AMs highly phagocytic
In line with the fact that AMs are the primary phagocytes in the lung, we found that AMs displayed much higher phagocytic activity compared to IMs (Fig. 3A). This drove us to examine the role of cell-intrinsic PD-L1 in the superior phagocytic ability of AMs, particularly given its different expression pattern on AMs and IMs. Remarkably, genetic deletion of PD-L1 significantly decreased AM phagocytosis (Fig. 3B, 3C). These data suggested that PD-L1 offers AMs a superior phagocytic ability.
Figure 3.
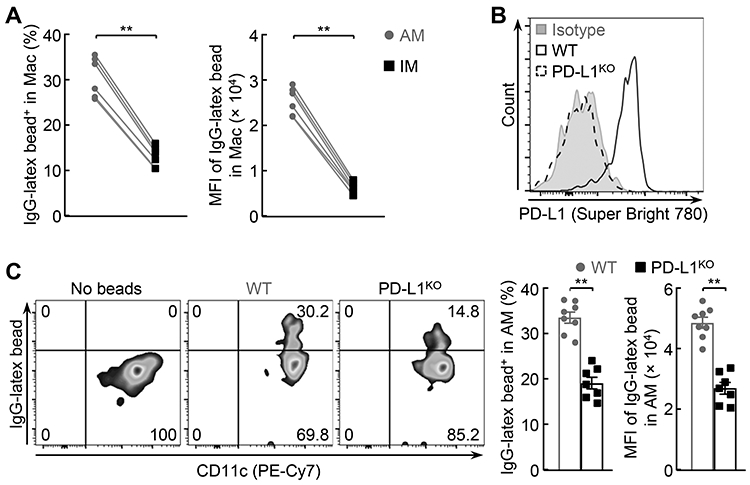
PD-L1 optimizes AM phagocytosis. (A) Superior phagocytic ability of AMs in comparison to IMs (n = 6). (B) No PD-L1 expression on the AMs of PD-L1 deficient (PD-L1KO) mice. (C) Decreased phagocytic ability of PD-L1KO AMs (n >= 7). Student’s t test was performed (two tailed, unpaired) and data represent means ± SEM in (A, C). **P < 0.01.
PD-L1 increases AM phagocytosis by cis-interacting with CD80
CD80 is the only known PD-L1 receptor other than PD-1 (10). Of note, PD-L1 binds to CD80 only in cis on the same cell surface (11). Thus, we tried to examine whether PD-L1 increases AM phagocytosis through cis-interacting with CD80. To this end, we first examined CD80 expression on AMs. CD80 was expressed on AMs, although at relatively low levels (Fig. 4A). Our confocal microscopic analysis showed around 90% of AMs with PD-L1/CD80 co-localization on the same cell surface, indicating a cis-PD-L1/CD80 interaction on AMs as well (Fig. 4B). Of note, PD-L1/CD80 co-localization on PMs was detected only after LPS treatment, which was consistent with that PD-L1 was not expressed on PMs but could be induced by LPS (Supplemental Fig. 2A). Importantly, selective deletion of CD80 significantly decreased the phagocytic ability of AMs (Fig. 4C; Supplemental Fig. 2B). Consistently, AMs expressing high levels of CD80 showed a higher phagocytic ability than those with low CD80 expression (Fig. 4D). These data suggested that PD-L1/CD80 cis-interaction contributes to the high phagocytic ability of AMs.
FIGURE 4.
PD-L1 interacts with CD80 in cis to boost AM phagocytosis. (A) CD80 expression on the AMs of wild type (WT) but not CD80 deficient (CD80KO) mice. (B) Co-localization of PD-L1 and CD80 on AMs. (C) Decreased phagocytic ability of CD80KO AMs (n = 16). (D) High phagocytic ability of AMs expressing high CD80 on the surface (n = 6). (E) PD-1 expression on AMs (n = 6). (F) Increased co-localization of PD-L1 and PD-1 on CD80KO AMs. (G) Enhanced phagocytic ability of AMs by PD-1 deletion (n = 6). Scale bar: 1 μm. Student’s t test was performed (two tailed, unpaired for C, F, G and paired for D, E) and data represent means ± SEM. **P < 0.01.
Most AMs also expressed PD-1 on the surface (Fig. 4E). Co-localization of PD-L1/PD-1 was detected on the surface of about 20% of AMs (Fig. 4F). Notably, the co-localization was found on over 50% of CD80 deficient AMs. Given that genetic deletion of PD-1 from AMs dramatically enhanced their phagocytic ability (Fig. 4G), these data together suggested that one important function of the cis-PD-L1/CD80 interaction on AMs is to restrict PD-L1 from binding to PD-1 to release the brake on phagocytosis.
Discussion
AMs are the most important guardians that patrol the lung around the clock, and in health, instruct immune tolerance to innocuous inhaled substances but initiate rapid and efficient immune responses to invading pathogens and terminate them after pathogens are cleared to prevent unnecessary inflammation and maintain immune and tissue homeostasis within this essential organ (1-5). Here, we find that AMs, but not IMs, PMs or SMs, inherently express PD-L1 on the surface and thereby are bestowed simultaneously with high phagocytic and CTL-suppressive activities to exert those pathophysiologically important roles. PD-L1 increases AM phagocytosis through cis-interacting with CD80 while terminating CTL activation via binding to PD-1 on activated CTLs.
Until now, studies on PD-L1, CD80 and other immune co-stimulatory or -inhibitory molecules have been focusing mainly on their roles in adaptive immunity, in particular CTL regulation (12). For example, PD-L1 on APCs and tumor cells binds to PD-1 on CTLs, leading to the suppression of those most important adaptive immune cells. On the other hand, engagement of CD80 on APCs with its receptor CD28 or CTLA4 on T cells, respectively, increases or decreases T-cell activation. Intriguingly, very recent studies suggest that cis-PD-L1/CD80 interaction on APCs restricts PD-L1 from binding to PD-1 and CTLA4 on CTLs for optimal T-cell response (13, 14). Our studies provide the first evidence showing a direct and important role of cis-PD-L1/CD80 interaction in innate immunity, AM phagocytosis. One important function of the cis-PD-L1/CD80 interaction in AM phagocytosis seems to restrict PD-L1 from binding to PD-1, suggesting CD80 and PD-1 competition for PD-L1 engagement as a common mechanism determining innate and adaptive immunity. Our studies also reveal that PD-L1 and PD-1 can bind to each other in cis on AMs. Interestingly, LPS induces PD-1 cis-interaction with PD-L1 on AMs (Supplemental Fig. 2C), suggesting a new mechanism underlying LPS pathogenic action.
The innate immune function of cis-PD-L1/CD80 interaction may be applicable to other immune and non-immune cells, particularly those with phagocytotic ability. Although constitutive PD-L1 expression is specific for AMs (compared to IMs, PMs and SMs), of note, this important immune regulatory molecule is induced on many macrophage types and other immune cells under pathogenic conditions, such as during tumorigenesis and infection (Supplemental Fig. 2A). In fact, PD-L1 is expressed on certain populations of several types of immune cells in the lung even under physiological conditions, such as non-classical monocytes, dendritic cells (DCs), eosinophils, basophils and mast cells (Supplemental Fig. 3).
In summary, the presented data provide innovative mechanistic insights into lung immunology and PD-L1/PD-1-targeted immunotherapies. Unlike their counterparts in other tissues, AMs have both superior phagocytic activity and inherent CTL-suppressive capability by constitutively expressing PD-L1 on the surface to respectively interact with AM CD80 and CTL PD-1, thereby assuring optimal protective immunity and tolerance within the lung. We believe that these findings have general significance, because although PD-L1 is not expressed on macrophages located at many tissues and organs other than the lung, up-regulation of PD-L1 and CD80 on these cells is common in response to pathophysiological stimuli.
Supplementary Material
Key Points.
Alveolar macrophages (AMs) inherently express PD-L1.
PD-L1/CD80 cis-interaction on AMs promotes AM phagocytosis.
Trans-interaction of PD-L1 on AMs with PD-1 on CTLs represses CTL activity.
Acknowledgments
The authors thank Y. X. Fu (University of Texas Southwestern Medical Center) and M. Morita and C. Bergmann (Cleveland Clinic Lerner College of Medicine) and H. Dong (Mayo Clinic College of Medicine) for providing the lung tissues of C57BL/6 PD-L1 deficient (PD-L1KO) mice.
This study was financially supported in part by the NIH National Cancer Institute (NCI) grant R01 CA172090 (to G. X.), and the American Cancer Society (ACS) Research Scholar grant RSG-19-166-01-TBG (to Z. Q.).
Abbreviations used in this article:
- AM
alveolar macrophage
- APC
antigen-presenting cells
- BAL
Bronchioalveolar lavage
- BALF
BAL fluid
- CTL
cytotoxic T lymphocytes
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- IF
immunofluorescence
- IM
interstitial macrophage
- PD-1
programmed death-1
- PD-L1
programmed death-1 ligand 1
- PM
peritoneal macrophage
- SM
splenic macrophage
Footnotes
The online version of this article contains supplemental material.
References
- 1.Kopf M, Schneider C, and Nobs SP. 2015. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol 16: 36–44. [DOI] [PubMed] [Google Scholar]
- 2.Garbi N, and Lambrecht BN. 2017. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch. 469: 561–572. [DOI] [PubMed] [Google Scholar]
- 3.Hussell T, and Bell TJ 2014. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol 14: 81–93. [DOI] [PubMed] [Google Scholar]
- 4.Byrne AJ, Mathie SA, Gregory LG, and Lloyd CM. 2015. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 70:1189–1196. [DOI] [PubMed] [Google Scholar]
- 5.Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, and Bennewith KL. 2016. Macrophages, inflammation, and lung cancer. Am. J. Respir. Crit. Care Med 193: 116–130. [DOI] [PubMed] [Google Scholar]
- 6.Sheng J, Ruedl C, and Karjalainen K. 2015. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. 43: 382–393. [DOI] [PubMed] [Google Scholar]
- 7.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, and Henson PM. 2011. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med 184: 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Sun F, Han L, Liu X, Xiao Y, Gregory AD, Shapiro SD, Xiao G, and Qu Z. 2021. PDLIM2 repression by ROS in alveolar macrophages promotes lung tumorigenesis. JCI Insight. 26: 144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, and Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, and Freeman GJ. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 27: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhri A, Xiao Y, Klee AN, Wang X, Zhu B, and Freeman GJ. 2018. PD-L1 binds to B7-1 only in cis on the same cell surface. Cancer Immunol. Res 6: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildberg FA, Klein SR, Freeman GJ, and Sharpe AH. 2016. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 44: 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, and Okazaki T. 2019. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 364: 558–566. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, Xiao C, Bonorino C, Lu LF, D Bui J, and Hui E. 2019. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 51: 1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun F, Xiao G, and Qu Z. 2017. Murine bronchoalveolar lavage. Bio. Protoc 7:e2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Qu Z, Yan S, Sun F, Whitsett JA, Shapiro SD, and Xiao G. 2015. Differential roles of STAT3 in the initiation and growth of lung cancer. Oncogene. 34: 3804–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qing G, Z. Qu, and G. Xiao. 2007. Endoproteolytic processing of C-terminally truncated NF-B2 precursors at κB-containing promoters. Proc. Natl. Acad. Sci. USA 104: 5324–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Sun F, Han L, and Qu Z. 2016. Kaposi's sarcoma herpesvirus (KSHV) microRNA K12-1 functions as an oncogene by activating NF-κB/IL-6/STAT3 signaling. Oncotarget. 7: 33363–33373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu Z, Sun F, Zhou J, Li L, Shapiro SD, and Xiao G. 2015. Interleukin-6 prevents the initiation but enhances the progression of lung cancer. Cancer Res. 75: 3209–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun F, Qu Z, Xiao Y, Zhou J, Burns TF, Stabile LP, Siegfried JM and Xiao G. 2016. NF-κB1 p105 suppresses lung tumorigenesis through the Tpl2 kinase but independently of its NF-κB function. Oncogene. 35: 2299–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu Z, Fu J, Ma H, Zhou J, Jin M, Mapara MY. Grusby MJ, and Xiao G. 2012. PDLIM2 restricts Th1 and Th17 differentiation and prevents autoimmune disease. Cell Biosci. 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan P, Fu J, Qu Z, Li S, Tanaka T, Grusby MJ, and Xiao G. 2009. PDLIM2 suppresses human T-cell leukemia virus type I Tax-mediated tumorigenesis by targeting Tax into the nuclear matrix for proteasomal degradation. Blood. 113: 4370–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F, Xiao Y, and Qu Z. 2015. Oncovirus Kaposi sarcoma herpesvirus (KSHV) represses tumor suppressor PDLIM2 to persistently activate nuclear factor kB (NF-κB) and STAT3 transcription factors for tumorigenesis and tumor maintenance. J. Biol. Chem 290: 7362–7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun F, Li L, Yan P, Zhou J, Shapiro SD, Xiao G, and Qu Z. 2019. Causative role of PDLIM2 epigenetic repression in lung cancer and therapeutic resistance. Nat. Commun 10: 5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun F, Guo ZS, Gregory AD, Shapiro SD, Xiao G, and Qu Z. 2020. Dual but not single PD-1 or TIM-3 blockade enhances oncolytic virotherapy in refractory lung cancer. J. Immunother. Cancer 8: e000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Han L, Sun F, Zhou J, Ohaegbulam KC, Tang X, Zang X, Steinbrecher KA, Qu Z, and Xiao G. 2018. NF-kB RelA renders tumor-associated macrophages resistant to and capable of directly suppressing CD8+ T cells for tumor promotion. Oncoimmunology. 7: e1435250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Qu Z, Sun F, Han L, Li L, Yan S, P Stabile L, Chen LF, Siegfried JM, and Xiao G. 2017. Myeloid STAT3 promotes lung tumorigenesis by transforming tumor immunosurveillance into tumor-promoting inflammation. Cancer Immunol. Res 5: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, and Xiao G. 2011. The tumor suppressor gene WWOX links the canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated tumorigenesis. Blood. 117: 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Z, Fu J, Yan P, Hu J, Cheng SY, and Xiao G. 2010. Epigenetic repression of PDZ-LIM domain-containing protein 2: implications for the biology and treatment of breast cancer. J. Bio.l Chem 285: 11786–11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu Z, Yan P, Fu J, Jiang J, Grusby MJ, Smithgall TE, and Xiao G. 2010. DNA methylation-dependent repression of PDZ-LIM domain-containing protein 2 in colon cancer and its role as a potential therapeutic target. Cancer Res. 70: 1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.