Abstract
Background
The COVID-19 pandemic has created increased stress and anxiety for many; however, some individuals are particularly prone to heightened anxiety. It is unclear if and how prestress neurocognitive factors moderate risk for anxiety during high-stress situations. Enhanced error monitoring and a cognitive control strategy of more instantaneous (reactive) control have both been independently related to anxiety. We examined if a specific neurocognitive profile characterized by heightened error monitoring and a more reactive cognitive control strategy in adolescence predicts young adults’ anxiety trajectories across 3 early months of the COVID-19 pandemic.
Methods
As part of a longitudinal study (N = 291), data were acquired in adolescence (13 years) on error monitoring (n = 124) and cognitive control strategy (n = 119). In young adulthood (18 years), anxiety was assessed three times during the COVID-19 pandemic (n = 162).
Results
On average, participants experienced greater anxiety in the first COVID-19 pandemic assessment, then anxiety decreased in the following months. Error monitoring and cognitive control strategy interacted to predict anxiety trajectories, such that among adolescents with an increased reliance on reactive control, error monitoring predicted greater anxiety in the first assessment but greater decreases the following months as stay-at-home orders were lifted and families adapted to the restrictions.
Conclusions
Results suggest that neurocognitive profiles in adolescence predict young adults’ anxiety responses during a highly stressful period, such as the initial months of the COVID-19 pandemic. Our findings have implications for the early identification of individuals at greater risk for anxiety.
Keywords: Anxiety, Cognitive control, COVID-19 pandemic, Error monitoring, Neurocognitive
SEE COMMENTARY ON PAGE 127
The COVID-19 pandemic brought unprecedented changes to individuals’ lives, resulting in increased stress and anxiety for many, especially young adults (1,2). However, most young adults do not experience heightened anxiety (1), highlighting the importance of identifying risk factors that contribute to elevated symptoms of anxiety during the COVID-19 pandemic. Extant studies have examined psychosocial factors that influence youth’s reactivity to stress, but less is known about how prestress neurocognitive factors moderate risk for anxiety during stressful periods (e.g., the COVID-19 pandemic). Both enhanced error monitoring and a cognitive control strategy characterized by more instantaneous (reactive) control (as opposed to planful/proactive control) have been independently related to anxiety (3, 4, 5). In this study, we examine if a specific neurocognitive profile, characterized by heightened error monitoring and a more reactive cognitive control strategy in adolescence, predicts young adults’ anxiety trajectories across 3 early months of the COVID-19 pandemic in the United States.
A wealth of research relates cognitive control to anxiety (6). Cognitive control involves several processes, including detection and deployment (7). Detection processes involve registering the presence of salient information (e.g., detecting that an error has occurred), whereas deployment processes involve changes in attention and behavior in response to this information (e.g., planning ahead to prevent future errors) (7). Previous research relates both detection and control processes to anxiety.
For detection, extensive research links anxiety to exaggerated error monitoring (5,8), often reflected in the error-related negativity (ERN). The ERN is an event-related potential component that reaches maximal amplitude over frontocentral recording sites within 100 ms after errors (9,10). The ERN is not strictly implicated in cognitive control as it is thought to reflect the affective evaluation of errors (11, 12, 13). Converging evidence suggests that the ERN is generated, at least in part, within the anterior cingulate cortex, a brain region that integrates threat, pain, and negative feedback (e.g., punishment) to guide future behavior (14,15). The relation between heightened error monitoring, as indexed by a larger (i.e., more negative) ERN, and greater anxiety has been demonstrated both concurrently and longitudinally, such that a larger ERN predicts greater anxiety later in development (16,17). Although longitudinal data remain scarce and the effects are relatively weak, the available evidence suggests that individuals with an elevated ERN are prone to heightened anxiety following high levels of stress (18,19), such as natural disasters [e.g., Hurricane Sandy (19)]. Together, these findings suggest that heightened error monitoring may predict increased anxiety during the COVID-19 pandemic.
For deployment, previous models identify two cognitive control strategies with distinct chronometry: proactive and reactive control (20). Proactive control involves the early selection of goal-relevant information to prepare for future events, whereas reactive control deploys on an as-needed basis toward recently encountered events. Research connects anxiety to reduced proactive and increased reactive control (5,20,21). For instance, training highly anxious individuals to use a proactive control strategy lowers aspects of anxiety (3). Nevertheless, reactive tendencies may create risk when accompanied by increased detection (i.e., error monitoring). Indeed, we recently argued that increased detection confers risk for anxiety in a way that is moderated by strategy: relatively higher proactive control reduces, whereas relatively higher reactive control potentiates, this risk (7).
This prior work generates a relatively specific hypothesis. We propose that individuals with both heightened detection and heavy reliance on reactive control fixate their attention on the source of the detected information (e.g., an error). We view this cognitive profile as supporting maladaptive behaviors, such as avoidance and freezing, associated with anxiety. In contrast, enhanced detection is less problematic when accompanied by proactive control. Such strategies support adaptive changes in attention deployment and associated behaviors, aimed at avoiding future errors and maintaining original goal-directed behavior (7).
This study tests this hypothesis by examining whether anxiety trajectories during the COVID-19 pandemic differ depending on neurocognitive profiles in adolescence. Specifically, we used latent growth curve modeling to characterize anxiety trajectories during 3 consecutive months of the pandemic. We had three hypotheses: 1) anxiety levels would increase overall during the pandemic; 2) adolescents with an enhanced ERN would display a trajectory of high initial anxiety levels and increases in anxiety during the pandemic; and 3) the effect of the ERN on anxiety would be moderated by individuals’ cognitive control strategy, such that anxiety trajectories would differ based on individuals’ neurocognitive profiles. We hypothesized that a neurocognitive profile characterized by enhanced error monitoring and a heavier reliance on reactive control strategies in adolescence would predict higher initial anxiety levels and increases in anxiety during the pandemic.
Methods and Materials
Participants
Participants were involved in an ongoing longitudinal, multimethod study of temperament and socioemotional development conducted in a large metropolitan area in the Mid-Atlantic United States. A total of 291 4-month-old infants (156 female) were selected based on displays of positive and negative affect and motor reactivity to novel stimuli (22). Based on maternal report in infancy, mothers were 69.4% White, 16.5% African American, 7.2% Hispanic, 3.1% Asian, 3.4% other, and 0.3% missing. Mothers in the sample were highly educated: 35.7% graduate school graduates, 41.9% college graduates, 16.2% high school graduates, 5.5% with other forms of education, and 0.7% with missing information.
Of the original sample (N = 291), 124 participants successfully completed a flanker task while electroencephalography (EEG) data were collected to assess error monitoring in adolescence (meanage = 13.11, SD = 0.59 years). At the same assessment, 119 participants successfully completed an AX-Continuous Performance Test (AX-CPT) to assess cognitive control strategy (proactive vs. reactive). In young adulthood (meanage = 18.26, SD = 0.66 years), participants reported on their anxiety during 3 consecutive months of the COVID-19 pandemic. Overall, 155 participants completed their first assessment (month 1) of online questionnaires between April 20 and May 15 of 2020, which was approximately 1 month (mean = 29.67, SD = 6.01 days) after the stay-at-home order was implemented in Maryland, the state where most participants resided. Approximately a month later (mean = 26.48, SD = 7.31 days), 153 participants completed their second assessment (month 2) as gradual reopening started in Maryland, and approximately a month after that (mean = 28.86, SD = 5.83 days), 141 participants completed their third assessment after stay-at-home orders were lifted and nonessential businesses reopened in Maryland. The Institutional Review Board of the University of Maryland approved all study protocols. All participants were compensated for their time.
Examinations of the patterns of missing data revealed that mothers’ race and ethnicity (non-Hispanic White vs. other minority groups) was associated with missing data on the second (p = .027) and third (p = .022) assessments during the pandemic, ERN (p = .004), and cognitive control strategy (p = .013), such that children with data on these measures were more likely to have non-Hispanic White mothers. As such, maternal ethnicity was included as a covariate in the SEM analyses. Missing data on all other variables were not associated with maternal ethnicity or education, children’s gender, error monitoring, cognitive control strategy, or anxiety at any time point (p values > .06).
Measures
Generalized Anxiety (18 Years)
Generalized anxiety was measured using the Generalized Anxiety Disorder 7-Item (GAD-7) scale (23) during the three assessments of the COVID-19 pandemic when participants were, on average, 18 years old. The items consisted of various anxiety symptoms and were summed to create an overall score. Higher scores indicated greater anxiety, and scores ≥10 are considered to be in the clinical range. This scale has been shown to have high test-retest reliability and good convergent validity (23). The scale showed excellent internal consistency at all time points (α > 0.92) and good test-retest reliability (r > 0.65). Moreover, in this sample, the GAD-7 was significantly correlated to COVID-related worries at each assessment (r range = 0.48−0.59), suggesting that the GAD-7 is related to pandemic-induced distress (24).
Error Monitoring
At the 13-year assessment, adolescents completed a flanker task while continuous EEG data were acquired using a 128-channel HydroCel Geodesic Sensor Net, a NetAmps 300 Amplifier, and Net Station software (version 4.5.4; Electrical Geodesic, Inc., Eugene, OR). The task, data, and preprocessing pipeline have been previously described (25) and are described in detail in the Supplement. In brief, EEG activity surrounding erroneous behavior during the flanker task was isolated to measure error monitoring. Participants completed the flanker task twice, once under standard flanker conditions and once under a social pressure manipulation. Although there was a larger ERN in the social condition compared with the standard flanker (25), these manipulations were counterbalanced across individuals, and there was no evidence that manipulation order affected the amplitude of the standard ERN (t 122 = 0.21, p = .834), nor was there evidence of any significant interaction with manipulation order to predict anxiety trajectories (all p values > .496). In this study, we focus on the ERN data from the standard flanker task because extensive work has documented that it is related with anxiety (5,26,27), and focusing on the standard (i.e., nonsocial) ERN allows for comparison with a broader array of literature. A similar approach has been used by previous studies with this sample (28).
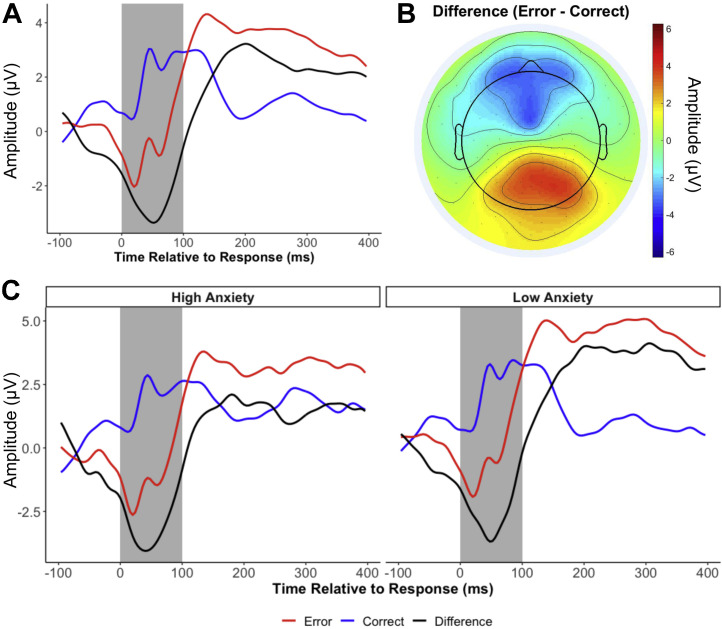
EEG data were preprocessed using MATLAB (version 2014b; The MathWorks, Inc., Natick, MA) scripts involving a combination of EEGLAB toolbox (29) and custom-made scripts (25,30) (see Supplement). Mean amplitudes of ERN and correct-related negativity were calculated from a cluster of frontocentral electrodes surrounding FCz for the first 100 ms following response (Figure 1 ). The correct-related negativity was then subtracted from the ERN to compute the delta-ERN to isolate error-specific processes, which was used for all subsequent analyses. More negative values indicate a larger delta-ERN and increased error monitoring. We determined the minimum number of trials to obtain a delta-ERN estimate with average acceptable reliability (0.6), using a Spearman-Brown split-half correlation procedure with multiple iterations (31,32). Results suggested that participants needed at least 10 trials for a reliable ERN and at least 15 trials for a reliable delta-ERN. Participants with at least 15 artifact-free trials were included. The delta-ERN showed good reliability (Spearman-Brown r = 0.84).
Figure 1.
The error-related negativity (delta-error-related negativity) and its relation to anxiety. (A) Grand average waveform for each condition and their difference. (B) Topographical plot of the mean amplitude of the difference between conditions (Error − Correct) during the shaded time window (0–100 ms). (C) Average waveforms for adolescents who reported high and low anxiety in the first assessments during the COVID-19 pandemic. For plotting purposes only, participants with an anxiety score 1 SD above or below the mean were plotted separately.
Cognitive Control Strategy
At the 13-year assessment, participants completed a standard behavioral AX-CPT to generate a measure of cognitive control strategy (i.e., proactive and reactive control) (20). The task, data, and cleaning of these data have been previously described (33) and are described in detail in the Supplement. In short, the AX-CPT is presented as a continuous series of letter pairs composed of 4 trial types (AX, AY, BX, and BY), which are presented at different rates. AX trials were the target trial type and required different response than the other three trial types. To obtain a measure of the sensitivity to the differences between target and nontarget trials while controlling for individual differences in response bias, d′ context was computed (see the Supplement for details). Higher d′ context scores indicate a more proactive style of cognitive control because the participant was sensitive to cue information and used it to inform future responses.
Analytic Strategy
First, to examine the average trajectory of anxiety across 3 months, a latent growth curve model was conducted with lavaan (34) in R (version 3.6) (35). The latent intercept factor, representing anxiety levels at the first COVID-19 pandemic assessment (month 1), was estimated by constraining the paths of each month to 1. The latent slope factor, representing the linear change in anxiety across the 3-monthly COVID-19 pandemic assessments, was estimated by constraining the paths for each month (month 1, month 2, and month 3) to 0, 1, and 2, respectively.
Second, to evaluate if the trajectories of anxiety varied as a function of error monitoring, the delta-ERN was modeled as a predictor of the intercept and slope latent factors. Third, to examine if the trajectories of anxiety varied as a function of different neurocognitive profiles, error monitoring (delta-ERN), cognitive control strategy (d′ context), and their interaction were modeled as predictors of the intercept and slope latent factors. The interaction between error monitoring and cognitive control strategy was created by first standardizing each variable and then computing their interaction term. To probe the interactions, we utilized the Johnson-Neyman procedure to examine the precise regions of the cognitive control strategy continuum in which the effect of the delta-ERN significantly predicted anxiety trajectories (i.e., intercept or slope factors) (36).
Based on the preliminary analyses described below with covariates and missing patterns and in line with previous studies with this sample (37), we controlled for maternal education, maternal ethnicity, gender, and participants’ average age during the COVID-19 pandemic assessments on the anxiety intercept and slope factors. In addition, we controlled for the date of the first assessment (month 1; in days) since the stay-at-home order on the intercept and the date of the last assessment (month 3; in days) since the stay-at-home order on the slope. Finally, to control for initial levels of anxiety, we controlled for anxiety levels at age 13 years, when error monitoring and cognitive control strategy were measured, using the Total Anxiety Score of the Screen for Child Anxiety Related Disorders (38). Missing data were handled using full information maximum likelihood estimation to reduce potential bias in the parameter estimates (39). Because of missing data and potential departures from multivariate normality, the model was estimated using a robust maximum likelihood estimator (40) and a scaled test χ2 statistic.
Results
Descriptive Analyses
Descriptive statistics and the correlations among all study variables are presented in Table 1 . Compared with males, females displayed a smaller delta-ERN (13 years) and higher levels of anxiety at month 1 and month 3 during the pandemic (18 years). Maternal education and maternal ethnicity were related to the delta-ERN, such that adolescents of non-Hispanic White mothers and of more educated mothers had a smaller delta-ERN. As such, gender, maternal education, and maternal ethnicity were included as covariates in the growth curve model examining predictors of the anxiety trajectories. Finally, the delta-ERN predicted anxiety levels at month 1 during the pandemic, such that a larger delta-ERN at age 13 was longitudinally related to greater anxiety at the first assessment during COVID-19 pandemic (Figure 1). Cognitive control strategy was not significantly related to any study variables.
Table 1.
Means, SDs, and Correlations
| Variable | n | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Gendera | 291 | – | – | – | – | – | – | – | – | – | – |
| 2 Maternal Ethnicityb | 290 | – | – | −0.05 | – | – | – | – | – | – | – |
| 3 Maternal Education | 273 | 1.21 | 0.72 | 0.01 | 0.19c | – | – | – | – | – | – |
| 4 Anxiety (13 Years) | 178 | 18.13 | 11.66 | −0.33c | −0.07 | −0.18c | – | – | – | – | – |
| 5 Delta-ERN (13 Years) | 124 | −2.53 | 2.93 | −0.24c | 0.23c | 0.24c | 0.14 | – | – | – | – |
| 6 AX-CPT d′ (13 Years) | 119 | 2.01 | 1.10 | −0.09 | 0.15 | −0.09 | −0.06 | −0.03 | – | – | – |
| 7 Anxiety T1 (18 Years) | 155 | 5.69 | 5.62 | −0.16c | 0.05 | 0.16 | 0.14 | −0.22c | 0.07 | – | – |
| 8 Anxiety T2 (18 Years) | 153 | 5.07 | 5.06 | −0.10 | 0.14 | 0.08 | 0.11 | −0.15 | 0.21 | 0.81c | – |
| 9 Anxiety T3 (18 Years) | 141 | 4.50 | 4.82 | −0.17c | 0.13 | 0.07 | 0.27c | 0.00 | 0.06 | 0.65c | 0.71c |
Maternal education was coded as high school graduate = 0, college graduate = 1, graduate school graduate = 2, and other = missing. Anxiety T1, Anxiety T2, and Anxiety T3 represent the first, second, and third anxiety assessments during the COVID-19 pandemic, respectively.
AX-CPT, AX-Continuous Performance Test; ERN, error-related negativity.
0 = females and 1 = males.
Non-Hispanic White = 1 and other = 0.
p < .05.
Growth Curve Analyses
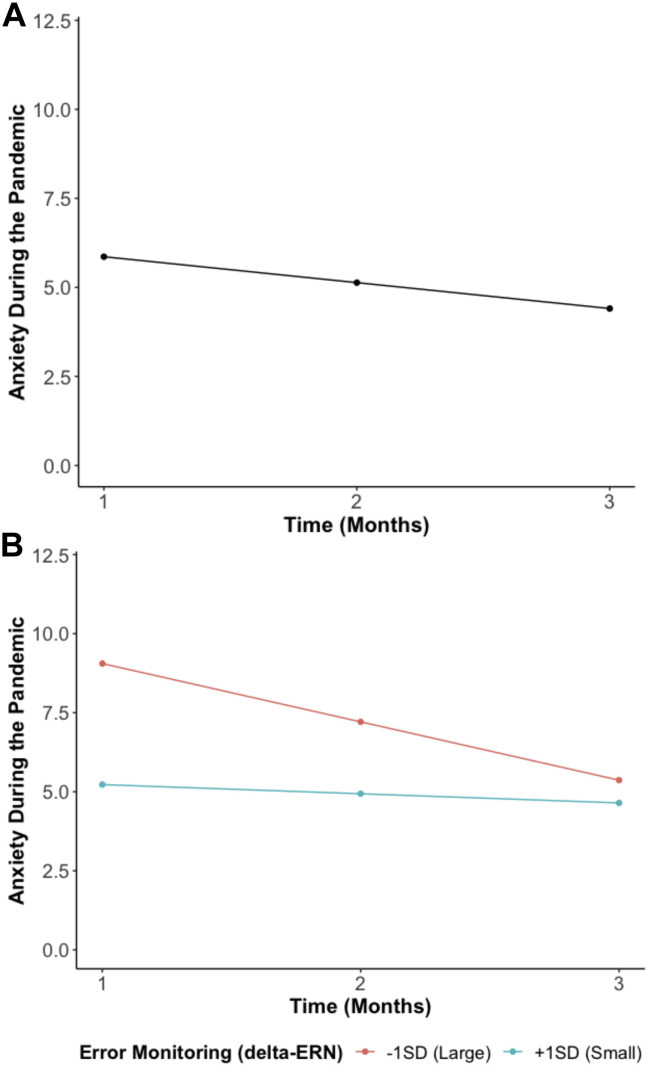
The growth curve model examining the trajectory of anxiety during the pandemic suggested that anxiety decreased across time (b = −0.73, p = .001) as the stay-at-home orders were lifted and reopening gradually occurred and/or families adapted to the pandemic-related restrictions (Figure 2A ). As a reference to clinical levels, at month 1, 20.0% of the participants reported anxiety symptoms considered to be in the clinical range. These frequencies declined to 18.3% at month 2 and 17.0% at month 3.
Figure 2.
(A) Average trajectory of anxiety (Generalized Anxiety Disorder 7-Item Scale) from T1 (during stay-at-home orders) to T3 (reopening). This model without predictors showed a good fit (χ21 = 0.01, p = .94, root mean square error of approximation = 0.00, standardized root mean square residual = 0.00, comparative fit index = 1.00). (B) Predicted anxiety trajectories at different levels of error monitoring (delta-error-related negativity [ERN]). More error monitoring (a larger delta-ERN) is indicated by a more negative value. This model with predictors showed a good fit (χ212 = 9.41, p = .67, root mean square error of approximation = 0.00, standardized root mean square residual = 0.02, comparative fit index = 1.00).
Error Monitoring Predicting Anxiety Trajectories
To examine our second hypothesis, we tested if error monitoring (delta-ERN) predicted anxiety trajectories. As shown in Figure 2B and in line with our hypothesis, we found that adolescents with an enhanced delta-ERN displayed a larger intercept (greater anxiety in month 1; b = −1.91, p = .008) but a more negative slope (i.e., greater decreases in anxiety across time; b = 0.78, p = .005).
Neurocognitive Profiles Predicting Anxiety Trajectories
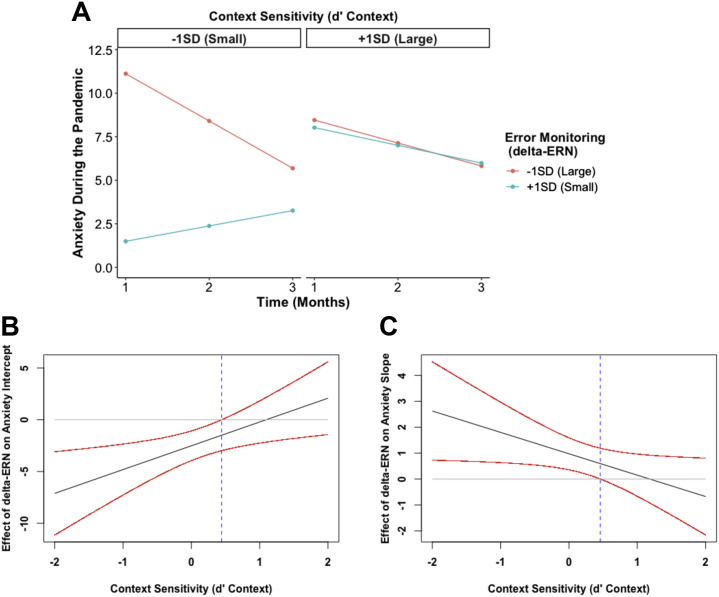
Our final model tested whether error monitoring, cognitive control strategy, and their interaction predicted anxiety trajectories. As shown in Table 2 , the interaction between error monitoring (delta-ERN) and cognitive control strategy (d′ context) predicted the intercept (b = 2.30, p = .005) and the slope (b = −0.83, p = .026) of the anxiety trajectory. As shown in Figure 3A, B , probing this interaction yielded a significant negative association between delta-ERN and anxiety at month 1 among individuals who showed a tendency to deploy relatively more reactive strategies (i.e., smaller d′ context scores, <0.44 SD from the mean). This relation was not significant among those who used a more proactive control strategy (i.e., larger d′ context scores). In contrast, Figure 3A, C, probing the interaction predicting the slope, revealed a positive association between the delta-ERN and the slope of anxiety, but only among individuals who showed relatively more reactive control (i.e., <0.46 SD from the mean; smaller d′ context scores), not among those with relatively more proactive control (i.e., larger d′ context scores).
Table 2.
Latent Growth Curve Analysis Results for Final Model Including Neurocognitive Predictors
| Predictors/Outcome | β | b | p Value | CI Lower | CI Upper |
|---|---|---|---|---|---|
| Anxiety Intercept | |||||
| Maternal educationa | 0.19 | 1.42 | .023 | 0.194 | 2.639 |
| Maternal ethnicity | 0.09 | 1.08 | .298 | −0.948 | 3.100 |
| Gender | −0.15 | −1.63 | .082 | −3.461 | 0.207 |
| Average age | 0.00 | −0.02 | .971 | −1.325 | 1.276 |
| Date of first assessment | −0.05 | −0.04 | .503 | −0.164 | 0.080 |
| Anxiety (13 years)a | 0.22 | 0.10 | .042 | 0.004 | 0.195 |
| Flanker task accuracy | −0.17 | −0.94 | .082 | −1.988 | 0.118 |
| Delta-ERNa | −0.47 | −2.52 | .000 | −3.828 | −1.202 |
| AX-CPT d′ | 0.18 | 0.97 | .143 | −0.326 | 2.260 |
| Delta-ERN × AX-CPT d′a | 0.43 | 2.30 | .005 | 0.702 | 3.897 |
| Anxiety Slope | |||||
| Maternal education | −0.14 | −0.30 | .204 | −0.774 | 0.165 |
| Maternal ethnicity | 0.09 | 0.30 | .514 | −0.595 | 1.189 |
| Gender | 0.15 | 0.47 | .255 | −0.338 | 1.274 |
| Average age | −0.01 | −0.01 | .966 | −0.590 | 0.564 |
| Date of third assessment | −0.08 | −0.02 | .383 | −0.075 | 0.029 |
| Anxiety (13 years) | 0.07 | 0.01 | .617 | −0.027 | 0.046 |
| Flanker task accuracy | 0.06 | 0.09 | .797 | −0.602 | 0.784 |
| Delta-ERNa | 0.61 | 0.97 | .001 | 0.410 | 1.538 |
| AX-CPT d′ | −0.08 | −0.13 | .657 | −0.685 | 0.432 |
| Delta-ERN × AX-CPT d′a | −0.52 | −0.83 | .026 | −1.550 | −0.100 |
Gender is coded as 0 = females and 1 = males. Maternal ethnicity is coded as non-Hispanic White = 1 and other = 0. Date of first assessment and date of third assessment were measured in days since the stay-at-home orders. This model fit the data well (χ214 = 16.56, p = .28, root mean square error of approximation = 0.03, standardized root mean square residual = 0.02, comparative fit index = 0.99).
AX-CPT, AX-Continuous Performance Test; ERN, error-related negativity.
p < .05.
Figure 3.
The impact of error monitoring and cognitive control strategy on anxiety trajectories during the COVID-19 pandemic. More error monitoring (a larger delta-error-related negativity [ERN]) is indicated by a more negative value, and more proactive control is indicated by higher d′ context values. (A) Predicted anxiety trajectories at different levels of error monitoring (delta-ERN) and cognitive control strategy. (B) Johnson-Neyman plot showing that the negative effect of error monitoring on anxiety intercept is greater as children exhibit more a reactive (less proactive) cognitive control strategy. (C) Johnson-Neyman plot showing that the effect of error monitoring on anxiety slope increases as children exhibit more reactive (less proactive) cognitive control.
In sum and as shown in Figure 3A, adolescents with both enhanced error monitoring (more negative delta-ERN) and an increased reliance on reactive control strategies (as opposed to planful/proactive control) displayed a larger intercept (i.e., greater anxiety at month 1) but a more negative slope (i.e., greater decreases in anxiety across time) compared with individuals with diminished error monitoring and more reactive control strategy use.
Sensitivity analyses demonstrated that modeling the times of assessment as continuous time based on participants’ assessment dates via multilevel modeling yielded the same conclusions as the SEM approach that treated time of assessment as ordinal. Importantly, the multilevel modeling approach used maximum likelihood with list-wise deletion on the covariates and predictors rather than full information maximum likelihood, suggesting that different ways of handing missing data also did not significantly impact the results. Moreover, sensitivity analyses suggest that the results were driven by the ERN rather than the correct-related negativity (see Supplement). Finally, exploratory analyses indicate that the effects of the delta-ERN or the interaction between error monitoring and cognitive control strategy were not moderated by gender (not shown).
Discussion
The COVID-19 pandemic has brought increased anxiety for many individuals, especially young adults (1,2). At the same time, not all young adults have experienced heightened anxiety during the pandemic, raising the question of which young adults are most at risk. This study utilizes neurocognitive factors previously linked to risk for elevated anxiety, specifically error monitoring and a more reactive cognitive control strategy, as predictors of anxiety during the COVID-19 pandemic. Our results suggest that error monitoring and a more reactive cognitive control strategy interact with each other to predict anxiety trajectories during the COVID-19 pandemic. Our results have implications for the identification of individuals at the highest risk for anxiety and can inform prevention and intervention efforts by providing potential malleable neurocognitive processes that may serve as resilience factors during highly stressful situations, such as the COVID-19 pandemic.
Results revealed that on average, anxiety decreased between the first and third months of the COVID-19 pandemic (Figure 1). This result was unexpected, but it is in line with emerging evidence indicating that some individuals, such as those who have a higher socioeconomic status, are not as impacted by the pandemic, especially when compared with individuals from low socioeconomic status backgrounds (2,41, 42, 43). For example, a weekly survey of a representative sample of the U.S. showed, on average, a similar decrease in anxiety for caregivers and youths; however, low-income households did not show decreases in anxiety, experiencing more anxiety than average-income households (41,42). It is important to note that participants of this study were largely from moderate-to-high socioeconomic status households, and none of them were impacted directly by the pandemic during the time sampled (e.g., family members getting seriously sick or hospitalized). In a similar sample of youth in New York State, anxiety increased through April, peaking around late April/early May, and then decreased rapidly through July (44). Likewise, for our sample of young adults, the first assessment, when stay-at-home orders were implemented and the uncertainty about the virus and its implications for individuals’ lives were highest, reflected the highest anxiety levels. As the restrictions were lifted and families adapted to the restrictions in the subsequent months, anxiety, on average, decreased.
In addition to the average response, we also observed important individual differences that varied as a function of previously measured neurocognitive factors. First, we observed that a larger delta-ERN in adolescence predicted a trajectory of increased intercept but a steeper slope compared with individuals with a small delta-ERN in adolescence. However, as expected and in line with our theoretical model (7), error monitoring and cognitive control strategy interacted to predict anxiety trajectories, such that the predictive effects of the delta-ERN were pronounced among individuals with increased reactive control strategies. In other words, individuals with a profile characterized by enhanced error monitoring and an increased reliance on instantaneous (reactive) control (as opposed to planful/proactive control) displayed greater anxiety in the first assessment (i.e., larger intercept). However, this profile also predicted greater anxiety decreases in subsequent months (i.e., a more negative slope). These results suggest that a neurocognitive profile of increased error monitoring and a more reactive control strategy predicted increased anxiety approximately 5 years later during the stay-at-home orders, the most anxiety-producing times of the pandemic. This difference was not observed during the last assessment when stay-at-home orders were no longer in place. This suggests that the risk associated with some neurocognitive factors may only be evident during acute periods of elevated stress or at higher levels of anxiety (e.g., clinical levels). In our sample, this was during the initial assessment during the pandemic; however, as our relatively advantaged sample adapted to the pandemic and reopening occurred, the effects of this neurocognitive profile or the delta-ERN were no longer observable.
Additionally, the effects of enhanced error monitoring on anxiety were not evident in individuals relying on a more proactive, rather than reactive, control strategy. This opens the possibility of intervention or prevention efforts that could target cognitive control strategy to promote planful and proactive strategies while reducing automatic and reactive control strategies (3). Our findings also highlight the importance of distinguishing detection from control processes, rather than considering cognitive control a unitary construct (7,21). This distinction has important clinical consequences because intervention or prevention strategies will likely differ whether detection or control is targeted. For example, in contrast to control processes, detection processes (e.g., error monitoring) are not impacted by explicit, cognitive, and behavioral strategies, such as cognitive behavioral therapy (45, 46, 47), but are modified by implicit interventions, such as attention-bias modification training (48, 49, 50) and a computerized intervention that was designed to directly reduce sensitivity to errors (51). Future studies should continue to develop and evaluate personalized intervention strategies for modifying specific components of cognitive control that may place individuals at higher risk for anxiety during adverse situations.
This study’s limitations should be considered when interpreting the results. Although our study is one of the first studies with multiple repeated assessments of anxiety symptoms during the COVID-19 pandemic, not having an assessment right before the COVID-19 pandemic outbreak did not allow us to capture the purported increases in anxiety. However, by leveraging our repeated assessments, we were able to detect anxiety levels potentially returning to pre-pandemic levels. As a longitudinal study, our study had missing data. To mitigate the impacts of missing data, we utilized statistical approaches to use all available data and reduce the biases associated with missing data. Moreover, sensitivity analyses suggest that different ways of handing missing data do not significantly impact the results (see the Supplement). Because of the urgency of data collection in response to the pandemic and the longitudinal design, our sample size was not determined by an a priori power analysis; we collected data on as many participants as possible. When interpreting the results of this study, it is also important to consider the nature of our sample—a community sample composed of relatively educated White families that were oversampled for temperamental patterns in infancy. Thus, caution is warranted when interpreting and generalizing our results and in particular the prevalence of anxiety during the COVID-19 pandemic. Moreover, because our results captured variability in anxiety as a continuum and the majority of participants experienced an adaptive response (anxiety increases followed by decreases), our findings need to be replicated in clinical and more diverse samples to better understand their implications for identifying individuals who may be at increased risk for experiencing elevated anxiety during high-stress situations as well as to inform the design of preventive or therapeutic interventions.
Overall, our findings provide evidence that neurocognitive profiles in adolescence predict young adults’ anxiety responses during a highly stressful situation, such as the initial phase of the COVID-19 pandemic. Specifically, among adolescents with an increased reliance on more instantaneous (reactive) control strategies (as opposed to planful/proactive control), error monitoring predicted increased anxiety during the initial phase of the pandemic (stay-at-home orders) but greater decreases in the following months. These findings highlight the importance of considering multiple components of cognitive control. A better characterization of these neurocognitive processes has implications for the early identification of individuals at greater risk for anxiety and can ultimately inform prevention and intervention efforts because these neurocognitive processes may serve as risk and resilience factors for anxiety.
Acknowledgments and Disclosures
This research was supported by grants from the National Institutes of Health (Grant Nos. MH093349 and HD017899 [to NAF]).
We thank the many research assistants involved in collecting and coding the data presented in this manuscript. We also thank the participating families without whom the study would not have been possible.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2021.06.004.
Supplementary Material
References
- 1.Czeisler M.É., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGinty E.E., Presskreischer R., Anderson K.E., Han H., Barry C.L. Psychological distress and COVID-19-related stressors reported in a longitudinal cohort of US adults in April and July 2020. JAMA. 2020;324:2555–2557. doi: 10.1001/jama.2020.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birk J.L., Rogers A.H., Shahane A.D., Urry H.L. The heart of control: Proactive cognitive control training limits anxious cardiac arousal under stress. Motiv Emot. 2018;42:64–78. [Google Scholar]
- 4.Buzzell G.A., Morales S., Bowers M.E., Troller-Renfree S.V., Chronis-Tuscano A., Pine D.S., et al. Inhibitory control and set shifting describe different pathways from behavioral inhibition to socially anxious behavior. Dev Sci. 2021;24 doi: 10.1111/desc.13040. [DOI] [PubMed] [Google Scholar]
- 5.Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Front Hum Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 7.Fox N.A., Buzzell G.A., Morales S., Valadez E.A., Wilson M., Henderson H.A. Understanding the emergence of social anxiety in children with behavioral inhibition. Biol Psychiatry. 2021;89:681–689. doi: 10.1016/j.biopsych.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olvet D.M., Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clin Psychol Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 10.Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 11.Dignath D., Eder A.B., Steinhauser M., Kiesel A. Conflict monitoring and the affective-signaling hypothesis-An integrative review. Psychon Bull Rev. 2020;27:193–216. doi: 10.3758/s13423-019-01668-9. [DOI] [PubMed] [Google Scholar]
- 12.Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg A., Meyer A., Hale-Rude E., Perlman G., Kotov R., Klein D.N., Hajcak G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53:372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzzell G.A., Richards J.E., White L.K., Barker T.V., Pine D.S., Fox N.A. Development of the error-monitoring system from ages 9-35: Unique insight provided by MRI-constrained source localization of EEG. Neuroimage. 2017;157:13–26. doi: 10.1016/j.neuroimage.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A., Hajcak G., Torpey-Newman D.C., Kujawa A., Klein D.N. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol. 2015;124:266–274. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer A., Nelson B., Perlman G., Klein D.N., Kotov R. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J Child Psychol Psychiatry. 2018;59:1162–1170. doi: 10.1111/jcpp.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banica I., Sandre A., Shields G.S., Slavich G.M., Weinberg A. The error-related negativity (ERN) moderates the association between interpersonal stress and anxiety symptoms six months later. Int J Psychophysiol. 2020;153:27–36. doi: 10.1016/j.ijpsycho.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer A., Danielson C.K., Danzig A.P., Bhatia V., Black S.R., Bromet E., et al. Neural biomarker and early temperament predict increased internalizing symptoms after a natural disaster. J Am Acad Child Adolesc Psychiatry. 2017;56:410–416. doi: 10.1016/j.jaac.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braver T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzzell G.A., Troller-Renfree S.V., Morales S., Fox N.A. In: Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives. Pérez-Edgar K., Fox N.A., editors. Springer International Publishing; Cham: 2018. Relations between behavioral inhibition, cognitive control, and anxiety: Novel insights provided by parsing subdomains of cognitive control; pp. 213–235. [Google Scholar]
- 22.Hane A.A., Fox N.A., Henderson H.A., Marshall P.J. Behavioral reactivity and approach-withdrawal bias in infancy. Dev Psychol. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 24.Morales S., Zeytinoglu S., Lorenzo N., Chronis-Tuscano A., Degnan K.A., Almas A.N., et al. Which anxious adolescents are most impacted by the COVID-19 pandemic? PsyArXiv. 2021 doi: 10.31234/osf.io/27sgp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzzell G.A., Troller-Renfree S.V., Barker T.V., Bowman L.C., Chronis-Tuscano A., Henderson H.A., et al. A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. J Am Acad Child Adolesc Psychiatry. 2017;56:1097–1105. doi: 10.1016/j.jaac.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajcak G., McDonald N., Simons R.F. Anxiety and error-related brain activity. Biol Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer A. A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Dev Cogn Neurosci. 2017;27:58–68. doi: 10.1016/j.dcn.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippi C.A., Subar A.R., Sachs J.F., Kircanski K., Buzzell G., Pagliaccio D., et al. Developmental pathways to social anxiety and irritability: The role of the ERN. Dev Psychopathol. 2020;32:897–907. doi: 10.1017/S0954579419001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Debnath R., Buzzell G.A., Morales S., Bowers M.E., Leach S.C., Fox N.A. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57 doi: 10.1111/psyp.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leach S.C., Morales S., Bowers M.E., Buzzell G.A., Debnath R., Beall D., Fox N.A. Adjusting ADJUST: Optimizing the ADJUST algorithm for pediatric data using geodesic nets. Psychophysiology. 2020;57 doi: 10.1111/psyp.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales S., Bowers M., Leach S., Buzzell G., Fifer W., Elliott A., Fox N.A. Time-frequency dynamics of error monitoring in childhood: An EEG study. PsyArXiv. 2021 doi: 10.31234/osf.io/ag9s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troller-Renfree S.V., Buzzell G.A., Pine D.S., Henderson H.A., Fox N.A. Consequences of not planning ahead: Reduced proactive control moderates longitudinal relations between behavioral inhibition and anxiety. J Am Acad Child Adolesc Psychiatry. 2019;58:768–775.e1. doi: 10.1016/j.jaac.2018.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosseel Y. Lavaan: An R Package for structural equation modeling and more. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 35.The R Project for Statistical Computing. R: A language and environment for statistical computing. http://www.R-project.org Available at:
- 36.Preacher K.J., Curran P.J., Bauer D.J. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 37.Morales S., Miller N.V., Troller-Renfree S.V., White L.K., Degnan K.A., Henderson H.A., Fox N.A. Attention bias to reward predicts behavioral problems and moderates early risk to externalizing and attention problems. Dev Psychopathol. 2020;32:397–409. doi: 10.1017/S0954579419000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Enders C.K., Bandalos D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary J. 2001;8:430–457. [Google Scholar]
- 40.Yuan K.H., Bentler P.M. 5. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Methodol. 2000;30:165–200. [Google Scholar]
- 41.Center for Translational Neuroscience Flattening the other curve: Trends for young children’s mental health are good for some but concerning for others. https://medium.com/rapid-ec-project/flattening-the-other-curve-7be1e574b340 Available at:
- 42.Center for Translational Neuroscience Flattening the other curve, part 2: Trends for parental well-being are improving overall, but not for everyone. https://medium.com/rapid-ec-project/flattening-the-other-curve-part-2-5661a2d36a82 Available at:
- 43.Fancourt D., Steptoe A., Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: A longitudinal observational study. Lancet Psychiatry. 2021;8:141–149. doi: 10.1016/S2215-0366(20)30482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawes M.T., Szenczy A.K., Olino T.M., Nelson B.D., Klein D.N. Trajectories of depression, anxiety and pandemic experiences; A longitudinal study of youth in New York during the spring-summer of 2020. Psychiatry Res. 2021;298:113778. doi: 10.1016/j.psychres.2021.113778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorka S.M., Burkhouse K.L., Klumpp H., Kennedy A.E., Afshar K., Francis J., et al. Error-related brain activity as a treatment moderator and index of symptom change during cognitive-behavioral therapy or selective serotonin reuptake inhibitors. Neuropsychopharmacology. 2018;43:1355–1363. doi: 10.1038/npp.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajcak G., Franklin M.E., Foa E.B., Simons R.F. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- 47.Riesel A., Endrass T., Auerbach L.A., Kathmann N. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: Evidence from a treatment study. Am J Psychiatry. 2015;172:665–673. doi: 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- 48.Nelson B.D., Jackson F., Amir N., Hajcak G. Single-session attention bias modification and error-related brain activity. Cogn Affect Behav Neurosci. 2015;15:776–786. doi: 10.3758/s13415-015-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson B.D., Jackson F., Amir N., Hajcak G. Attention bias modification reduces neural correlates of response monitoring. Biol Psychol. 2017;129:103–110. doi: 10.1016/j.biopsycho.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klawohn J., Hajcak G., Amir N., Kathmann N., Riesel A. Application of attentional bias modification training to modulate hyperactive error-monitoring in OCD. Int J Psychophysiol. 2020;156:79–86. doi: 10.1016/j.ijpsycho.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Meyer A., Gibby B., Wissemann K., Klawohn J., Hajcak G., Schmidt N.B. A brief, computerized intervention targeting error sensitivity reduces the error-related negativity. Cogn Affect Behav Neurosci. 2020;20:172–180. doi: 10.3758/s13415-019-00760-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.