Abstract
Objective:
We performed genome-wide expression profiling to develop an exosomal miRNA panel for predicting recurrence following surgery in patients with PDAC.
Summary Background Data:
Pre-treatment risk stratification is essential for offering individualized treatments to patients with pancreatic ductal adenocarcinoma (PDAC), but predicting recurrence following surgery remains clinically challenging.
Methods:
We analyzed 210 plasma and serum specimens from four cohorts of PDAC patients. Using a discovery cohort (n=25), we performed genome-wide sequencing to identify candidate exosomal miRNAs (exo-miRNAs). Subsequently, we trained and validated the predictive performance of the exo-miRNAs in two clinical cohorts (training cohort: n=82, validation cohort: n=57) without neoadjuvant therapy (NAT), followed by a post-NAT clinical cohort (n=46) as additional validation.
Results:
We performed exo-miRNA expression profiling in plasma specimens obtained before any treatment in a discovery cohort. Subsequently we optimized and trained a 6-exo-miRNA risk-prediction model, which robustly discriminated patients with recurrence (AUC: 0.81, 95% CI: 0.70–0.89) and relapse-free survival (RFS, P < 0.01) in the training cohort. The identified exo-miRNA panel was successfully validated in an independent validation cohort (AUC: 0.78, 95% CI: 0.65–0.88, RFS: P < 0.01), where it exhibited comparable performance in the post-NAT cohort (AUC: 0.72, 95% CI: 0.57–0.85, RFS: P < 0.01) and emerged as an independent predictor for RFS (HR: 2.84, 95% CI: 1.30–6.20).
Conclusions:
We identified a novel, non-invasive exosomal miRNA signature that robustly predicts recurrence following surgery in patients with PDAC; highlighting its potential clinical impact for optimized patient selection and improved individualized treatment strategies.
Keywords: Exosomal miRNAs, pancreatic ductal adenocarcinoma, recurrence prediction biomarkers, neoadjuvant therapy
MINI-ABSTRACT
Pre-treatment risk stratification is critical for offering individualized treatment in patients with PDAC. We analyzed a total of 210 PDAC patients from multiple independent cohorts. Genome-wide expression profiling followed by the validation effort led to identification of a 6-exosomal miRNA risk-prediction panel, which robustly discriminated patients with recurrence and relapse-free survival.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal cancers, and is projected to become the second most common cause of cancer-related deaths in the United States by 20301–3. Despite an improved understanding of the molecular and genetic basis of PDAC and recent advancements in treatments, including newly introduced chemotherapy and radiotherapy regimens, the 5-year survival rates have remained low—well under 10%3, 4. Surgical resection (i.e. pancreatectomy) for localized tumors is an invasive procedure with high complication and mortality rates; nonetheless, it is still considered the only treatment option for a potential cure or long-term survival in patients with PDAC. However, even for the most favorable cohort of patients with resectable PDAC, up to 80% of patients experience a recurrence following surgery, with a short recurrence-free interval1, 5, 6. These high recurrence rates are primarily attributed to the presence of occult micrometastatic disease at the time of resection7, 8. In hindsight, these patients should perhaps have been spared an ineffective invasive surgery. Unfortunately, this problem is exacerbated by a lack of clinically useful biomarkers that can predict the risk of post-surgical recurrence prior to PDAC patients receive such treatments.
Given that overall, surgery alone offers only minimal survival benefits, multidisciplinary treatment strategies, including neoadjuvant therapy (NAT), are being aggressively investigated and becoming increasingly more common, especially in western countries9–15. However, because there are no established criteria to implement specific NAT regimens, and lack of clinical tools for risk assessment following NAT, physicians and patients often have to make difficult decisions on whether to continue chemotherapy or to proceed with surgery. Currently, as per the National Comprehensive Cancer Network (NCCN) guidelines, such pre-treatment risk predictions are often estimated based on clinicopathological factors, including CA19-9 levels, tumor size, co-morbidities, nutritional status, and others features1, 5, 14, 16–22. Unfortunately, however, all of these prognostic tools remain clinically challenging and inadequate for predicting recurrence in PDAC patients following surgery, due to their poor sensitivity and specificity; highlighting the need to develop novel, preferably noninvasive molecular biomarkers, that can accurately predict cancer recurrence prior to any treatment. Translational research efforts to develop such risk-stratification biomarkers have been limited. Although a handful of studies have reported that preoperative levels of serum duke pancreatic monoclonal antigen type 2, gamma-glutamyltransferase-to-albumin ratio, or KRAS-mutated circulating tumor DNA could help predict recurrence in PDAC, the accuracy of these biomarkers remains insufficient for an effective clinical decision-making16, 23, 24.
Recent technological advances have enabled innovative genomic and epigenomic profiling in various malignancies and have facilitated identification of previously unrecognized molecular biomarkers25–33. MicroRNAs (miRNAs) are small noncoding RNAs that play a pivotal role in gene regulation34, 35 and are frequently deregulated in human cancers. The expression patterns of miRNAs have been shown to have diagnostic, prognostic, and therapeutic potential, in various cancers, including PDAC26, 31, 32, 34, 36. However, although miRNAs offer high detection sensitivity, the heterogeneity associated with sources of circulating, cell-free miRNAs appear to limit their overall detection accuracy. It is well-recognized that cf-miRNAs may be produced from multiple sources, including apoptotic bodies, dying cells and immune cells. One type of small (40–200 nm) membranous microvesicles are exosomes, which inherit molecular cargo, including DNA, RNA, and protein, secreted by living cancer cells37–43. The stark contrast between how cell-free nucleic acid and exosomes are released suggests that exosomes reveal information about living tumor cells and raises intriguing prospects for cancer biomarkers43. Accordingly, cancer-specific exosomal miRNA (exo-miRNA) signatures44–46, which may result in greater representation of cancer-derived miRNAs compared to cf-miRNAs, hence providing more specific biomarkers in systemic circulation.
Herein, for the first time, we performed comprehensive biomarker discovery to identify a pre-operative, blood-based, exo-miRNA panel (EMP) for risk prediction of recurrence following surgery in patients with PDAC. This panel was initially developed using genome-wide sequencing, followed by rigorous validation and performance evaluation in a total of three large, independent clinical cohorts including a post-NAT cohort, highlighting its potential clinical significance for the management of patients with PDAC.
METHODS
Study design and patient cohorts
The entire workflow for the discovery of candidate miRNAs and the development of a miRNA signature is shown in Supplementary figure 1. For the identification, clinical training, and validation of the EMP, biospecimens were analyzed from four independent cohorts of patients with PDAC. A total of 210 plasma or serum specimens were examined, which included a biomarker discovery cohort that was subjected to small RNA sequencing (n = 25; 16 with recurrence within 6 months after surgery and 9 without recurrence for more than 3 years). The patients within this cohort were enrolled at the Asan Medical Center, Korea between 2012 and 2015. Likewise, the training cohort (n = 82; 68 recurrence and 14 non-recurrence) of patients were enrolled at the Samsung Medical Center, Korea between 2008 and 2017; and a validation cohort (n = 57; 25 recurrence and 32 non-recurrence) of patients enrolled at the Nagoya University, Japan and Medical College of Wisconsin between 2012 and 2017. None of these patients received preoperative cancer treatment, all blood specimens were obtained prior to initiation of any treatment, and all patients were diagnosed as having PDAC by pathological examination following surgery. Post-NAT blood samples were collected for an additional validation cohort (n = 46; 25 recurrence and 21 non-recurrence) of patients who underwent NAT followed by surgery at Nagoya University, Japan and Medical College of Wisconsin between 2012 and 2018. In this additional validation cohort, all blood specimens were obtained during the waiting period for surgery following NAT. Tumors were classified according to the TNM staging system of the International Union Against Cancer (UICC) version 7 or 8 by pathological examination. Patients who had positive peritoneal washing cytology or para-aortic lymph node metastases-without other distant metastases were included in this study. Exclusion criteria included macroscopically incomplete resection, a tumor histology other than diagnosis of PDAC, or insufficient survival information (follow-up periods ≤ 6 months after surgery). The study was conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all patients, and the study was approved by the institutional review boards of all participating institutions.
Exosomal RNA extraction
For small RNA sequencing, total exosomal RNA was isolated from 400 μL plasma, using an exoRNeasy Midi Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. For real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR), total exosomal RNA was extracted from 200 μL plasma or serum using a Total Exosome Isolation Kit (Cat no: 4484450, Invitrogen) and an miRNeasy serum/plasma Kit (Qiagen, Valencia, CA).
Small RNA sequencing and biomarker discovery
A small RNA sequencing library was prepared from extracted exosomal RNA, using a NEXTflex™ Small RNA-Seq Kit v3 (PerkinElmer). Following the size selection and quality check, all libraries were pooled together and sequenced on an Illumina NovoSeq using paired-end sequencing. For data analysis, cutadapt (v2.2) was used to move adapters and low-quality bases in reads. Thereafter the reads were aligned to human rRNA precursor sequences from NCBI using Bowtie2 (v2.3.5) and matching reads were discarded from further analysis. A sRNAnalyzer pipeline was used to align the remaining reads and quantify miRNA expression. The quantified small RNA sequencing data was analyzed to identify differentially expressed miRNAs between patients in the discovery cohort who had early PDAC recurrence (within 6 months after surgery, n = 16) and those who had long-term (more than 3 years) non-recurrence (n = 9) after curative surgery. Differential expression analysis was conducted using DESeq2 (v1.26.0). A Benjamini-Hochberg false discovery rate (FDR) of 5% was used to account for multiple testing corrections47. Finally, differentially expressed miRNAs were used to build classifiers that distinguished early recurrence patients from non-recurrent patients. To evaluate the recurrence predictive potential of the discovered exosomal miRNAs, a multivariate cox regression model was first established using selected biomarkers. Thereafter, the resulting risk scores were used to determine the area under the curve (AUC) values for each of the receiver operator characteristic (ROC) plots33, 48–50.
Real-time quantitative reverse transcription polymerase chain reaction
Synthesis of complementary DNA from total exosomal RNA was performed using a miRCURY LNA RT Kit (Qiagen, Valencia, CA). RT-qPCR analysis was performed using a SensiFAST™ sybr Lo-ROX Kit (Bioline, London, UK) on the Quantstudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA), and expression levels were evaluated using Applied Biosystems QuantStudio 6 Flex Real-Time PCR System Software. The relative abundance of target transcripts was evaluated and normalized to the expression of miR-16-5p as an internal control using the 2−ΔDCt method. Normalized values were further log2 transformed49, 50. All of the primers for miRNAs used in this study were purchased from Qiagen (miRCURY LNA miRNA PCR Assays). Catalog numbers of each miRNA are as follows: has-miR-16-5p: YP00205702, hsa-miR-130b-5p: YP00204456, hsa-miR-133a-3p: YP00204788, hsa-miR-184-3p: YP00204601, hsa-miR-195-5p: YP00205869, hsa-miR-320c: YP00205706, hsa-miR-432-5p: YP00204776, hsa-miR-548q: YP02103735, hsa-miR-766-5p: YP02113546, hsa-miR-1229-3p: YP00206036, hsa-miR-1261: YP00205716, hsa-miR-1273f: YP02102122, and hsa-miR-3177-3p: YP02103017.
MicroRNA regulatory network analysis
The miRNA:mRNA regulatory network was constructed using the validated miRNAs to elucidate perturbed pathways, through data analysis using miRTarBase version 7.051–54. miRNA–mRNA pairs with weak evidence in the miRTarBase database were excluded from the analysis. Pathway enrichment analysis for selected target genes was performed using KEGG pathways (http://www.kegg.jp/ or http://www.genome.jp/kegg/).
Statistical analysis
Data pre-processing and handling were performed using R/Bioconductor. Univariate and multivariate cox regression analyses were employed to evaluate various preoperative clinicopathological variables (age, gender, carbohydrate antigen 19-9 [CA19-9], tumor location, tumor size evaluated by computed tomography) and the EMP for predicting a patient’s cancer prognosis. Relapse-free survival (RFS) times were calculated from the date of surgery to the date of death from any cause or recurrence, or last follow-up date. RFS was estimated using the Kaplan-Meier method. The primary endpoint was analyzed using a stratified log-rank test12, 55. Median follow-up was calculated using the reverse Kaplan-Meier method12, 56. A multivariate Cox proportional hazard regression model was established and a P value <0.05 was considered statistically significant. Statistical analyses were performed using Medcalc statistical software, GraphPad Prism V8.0 (GraphPad Software, San Diego, CA), and R (3.5.0, R Development Core Team, https://cran.r-project.org/).
RESULTS
Genome-wide miRNA expression profiling identifies a novel exosomal miRNA panel for predicting recurrence following surgery in patients with PDAC
To identify exo-miRNAs specifically dysregulated in patients with PDAC recurrence, we performed miRNA expression profiling in exosomes harvested from plasma before any treatment in a discovery cohort of 25 patients. This cohort included 16 patients with early recurrence within 6 months after surgery and 9 PDAC patients without recurrence for more than 3 years following curative surgery (Table 1). Among 2265 expressed miRNAs, candidate exo-miRNAs were selected if they were significantly and differentially expressed between early recurrence and non-recurrent patients (upregulated miRNAs in recurrent patients, absolute log2 foldchange > 1.0, P < 0.05) and were expressed in at least half of the samples; immature miRNAs were excluded (Supplementary Fig. 1). Subsequently, we identified 12 candidate miRNAs: hsa-miR-130b-5p, hsa-miR-133a-3p, hsa-miR-184-3p, hsa-miR-195-5p, hsa-miR-320c, hsa-miR-432-5p, hsa-miR-548q, hsa-miR-766-5p, hsa-miR-1229-3p, hsa-miR-1261, hsa-miR-1273f, and hsa-miR-3177-3p, which were upregulated in the exosomes of patients with early PDAC recurrence (Fig. 1A, 1B).
Table 1:
Clinicopathological characteristics of the preoperative clinical cohorts
| Characteristics | Discovery cohort (n = 25) | Training cohort (n = 82) | Validation cohort (n = 57) |
|---|---|---|---|
| Age | |||
| < 65 years | 15 (60.0) | 31 (37.8) | 16 (28.1) |
| ≥ 65 years | 10 (40.0) | 51 (62.2) | 41 (71.9) |
| Gender | |||
| Male | 16 (64.0) | 48 (58.5) | 41 (71.9) |
| Female | 9 (36.0) | 34 (41.5) | 16 (28.1) |
| Pre-treatment CA19-9 | |||
| ≤ 37 U/mL | 5 (20.0) | 25 (30.5) | 24 (42.1) |
| > 37 U/mL | 20 (80.0) | 57 (69.5) | 33 (57.9) |
| Tumor location | |||
| Ph | 19 (76.0) | 48 (58.5) | 37 (64.9) |
| Pbt | 6 (24.0) | 34 (41.5) | 20 (45.1) |
| Pre-treatment tumor size (CT) | |||
| ≤ 4 cm | 15 (60.0) | 41 (50.0) | 52 (91.2) |
| > 4 cm | 10 (40.0) | 41 (50.0) | 5 (8.8) |
| Resectability status | |||
| Resectable | NA | 47 (57.3) | NA |
| Borderline-resectable | NA | 33 (40.2) | NA |
| Unresectable | NA | 2 (2.4) | NA |
| Nodal metastasis | |||
| Present | 14 (56.0) | 59 (72.0) | 32 (56.1) |
| Absent | 11 (44.0) | 22 (26.8) | 25 (43.9) |
| Not Available | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| UICC pStage (ver.7) | |||
| IA | 0 (0.0) | NA | 3 (5.3) |
| IB | 3 (12.0) | NA | 0 (0.0) |
| IIA | 8 (32.0) | NA | 22 (38.6) |
| IIB | 14 (56.0) | NA | 31 (54.4) |
| III | 0 (0.0) | NA | 1 (1.8) |
| IV | 0 (0.0) | NA | 0 (0.0) |
| UICC pStage (ver.8) | |||
| IA | NA | 4 (4.9) | NA |
| IB | NA | 5 (6.1) | NA |
| IIA | NA | 12 (14.6) | NA |
| IIB | NA | 33 (40.2) | NA |
| III | NA | 26 (31.7) | NA |
| IV | NA | 2 (2.4) | NA |
| Adjuvant therapy | |||
| Yes | 19 (76.0) | 55 (67.1) | 43 (75.4) |
| No | 5 (20.0) | 26 (31.7) | 13 (22.8) |
| Not Available | 1 (4.0) | 1 (1.2) | 1 (1.8) |
| Recurrence | |||
| Present | 16 (64.0) | 68 (82.9) | 25 (43.9) |
| Absent | 9 (36.0) | 14 (17.1) | 32 (56.1) |
UICC, International Union Against Cancer
Figure 1. Small RNA sequencing identifies a blood-based EMP for predicting recurrence after surgery in patients with PDAC.
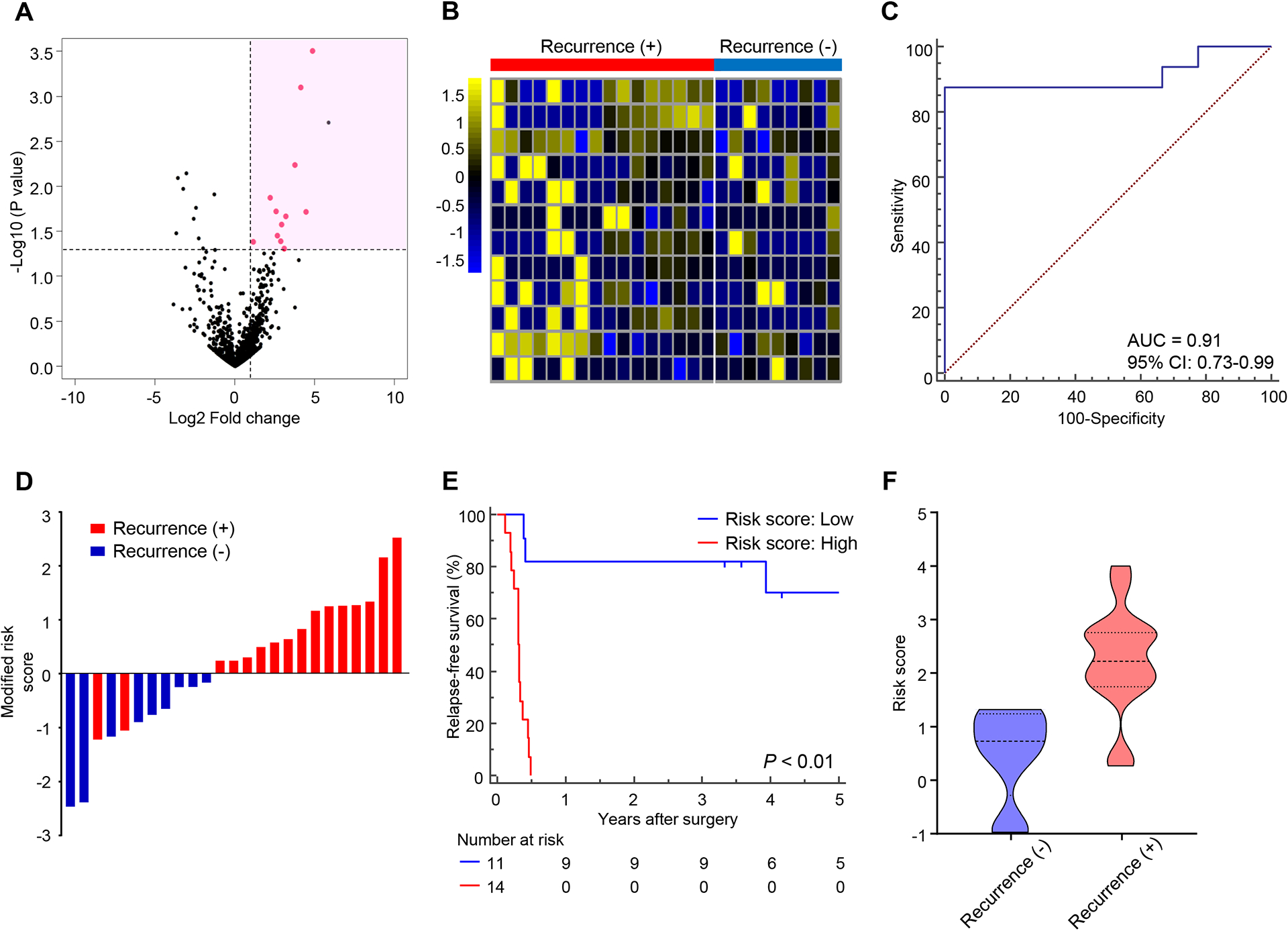
(A) Volcano plot showing significant and differentially upregulated exo-miRNAs selected from sequence data. miRNAs selected for further study are depicted in pink. (B) Heatmap representing the 12 significantly differentiated exo-miRNAs in pre-treatment plasma specimens from a discovery cohort of patients who had recurrence after surgery (n = 16) compared to non-recurrent patients (n = 9). (C) ROC curve of the 12-exo-miRNA EMP for predicting recurrence in the discovery cohort (AUC = 0.91). (D) Risk score distribution plot in the discovery cohort. Modified risk score was obtained by subtracting individual risk score from Youden’s index value of risk model. (E) Comparison of RFS between high- and low-risk groups estimated by 12-exo-miRNA EMP. (F) Distribution of risk scores according to recurrence status (P < 0.01, Mann Whitney test).
Finally, we constructed a cox regression model with these 12 miRNAs in the discovery cohort, which demonstrated excellent performance for recurrence prediction and RFS following surgery (Fig. 1C–E). Furthermore, when we assessed the distribution of risk scores and recurrence status, we observed that patients with recurrence had a significantly higher risk score than non-recurrent patients (P < 0.01, Fig. 1F); highlighting the performance of this model for the recurrence prediction following surgery in patients with PDAC.
Clinical training establishes an EMP for predicting recurrence and cancer prognosis in patients with PDAC
To evaluate the predictive robustness of our discovered exosomal miRNAs, we next performed training and validation of selected exo-miRNAs using RT-qPCR assays in blood specimens from two large, independent clinical cohorts (Table 1). Because eliminating redundancy and minimizing the number of candidate miRNAs would allow an easier translation of our EMP into clinical practice, we first optimized the model using cox regression with backward elimination for feature selection in the training cohort cases (n = 82; 68 recurrence and 14 non-recurrence). As a result, we successfully established a final pre-treatment recurrence prediction EMP model which comprised of a reduced 6 miRNA panel: miR-130b-5p, miR-133a-3p, miR-195-5p, miR-432-5p, miR-1229-3p, and miR-1273f. Subsequently, we developed a risk score based on the coefficients derived from individual exo-miRNAs and the constant derived from this analysis as follows: logit (−0.3305*miR-130b-5p) + (−0.2089*miR-133a-3p) + (1.1546*miR-195-5p) + (0.2297*miR-432-5p) + (0.4848*miR-1229-3p) + (−0.3591*miR-1273f). In order to confirm the expression patterns of these 6 miRNAs between tissues and exosomes, we investigated their correlation between exosomes and matched tissue specimens by small RNA sequencing in the discovery cohort (n = 25). Interestingly, for all 6 miRNAs, we could confirm positive correlation for the miRNA expression between tissues and exosomes, and 3 of these demonstrated statistically significant results (Supplementary figure 2). The risk-assessment EMP demonstrated excellent performance for predicting recurrence, with an AUC value of 0.81 (95% confidence interval [CI] = 0.70–0.89; Fig. 2A), and a corresponding specificity of 0.85 and a sensitivity of 0.72. Based on the distribution of risk scores and recurrence status, patients with recurrence after surgery demonstrated significantly higher risk scores vs. non-recurrent patients (P < 0.01; Fig. 2B). Subsequently, we dichotomized patients into low- and high-risk groups based on EMP risk scores obtained from Youden’s index-derived cutoff thresholds (Supplementary Table 1)48, 57. Importantly, high-risk patients exhibited a significantly worse RFS compared to low-risk patients, with a hazard ratio (HR) of 2.20 (95% CI = 1.36–3.55; Fig. 3A).
Figure 2. Clinical training and validation of establishes an EMP for predicting recurrence following surgery in patients with PDAC.
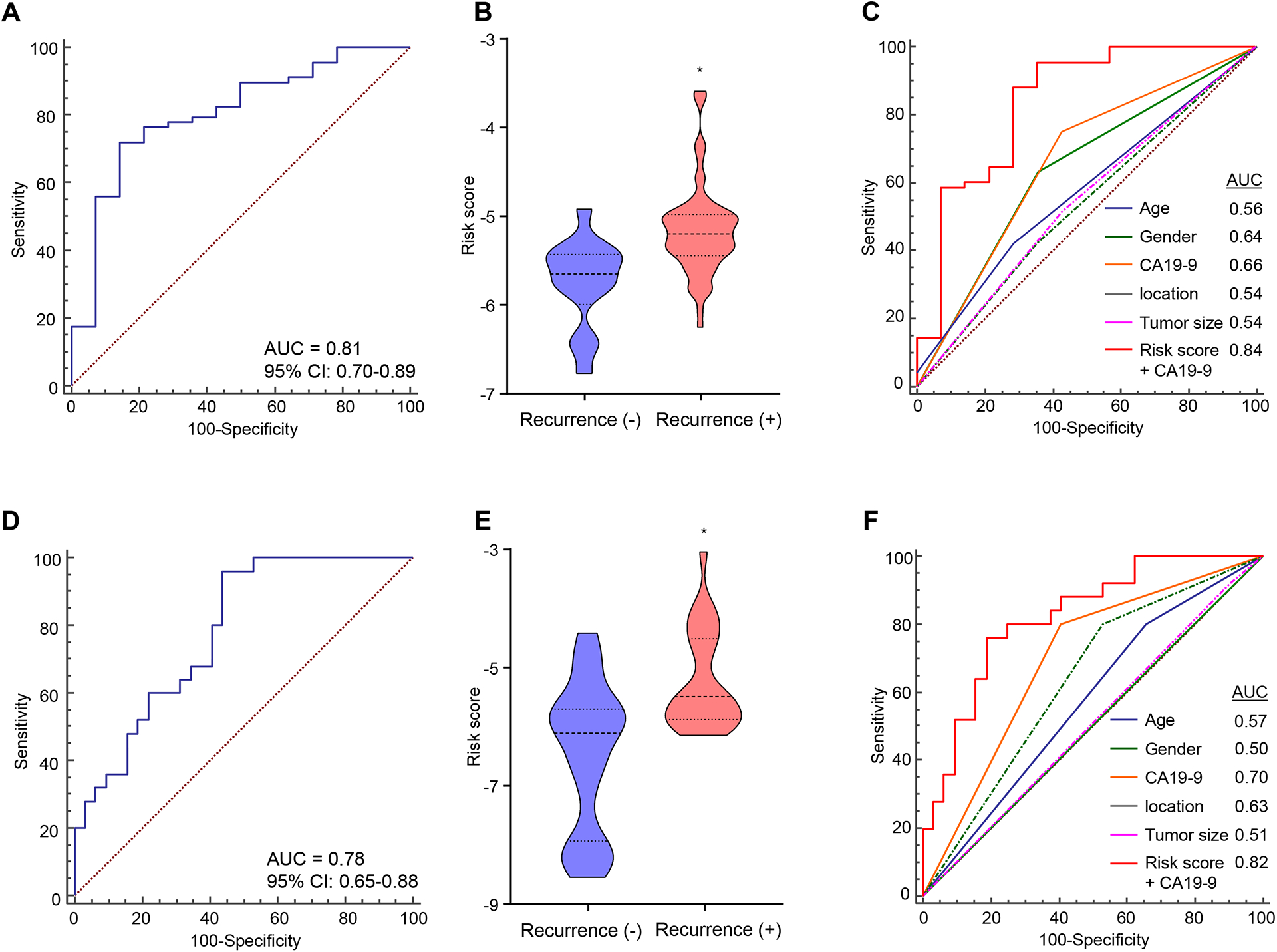
(A) ROC curve of the pre-treatment 6-exo-miRNA EMP recurrence prediction model constructed using cox regression with backward elimination for feature selection using the training cohort (AUC = 0.81). (B) Distribution of risk scores according to recurrence status (P < 0.01, Mann Whitney test). (C) The new combination model, EMP and CA19-9, outperformed the prediction accuracy of other variables in the training cohort (AUC = 0.84). (D) ROC curves of the 6-exo-miRNA EMP derived from the training cohort for recurrence prediction in an independent validation cohort (AUC = 0.78). (E) Distribution of risk scores according to recurrence status (P < 0.01, Mann Whitney test). (F) ROC curves showing the recurrence predictive performance of the combination model, EMP and CA19-9, compared to conventional clinical factors in the validation cohort (AUC = 0.82).
Figure 3. The EMP stratifies cancer prognosis in multiple independent clinical cohorts in patients with PDAC.
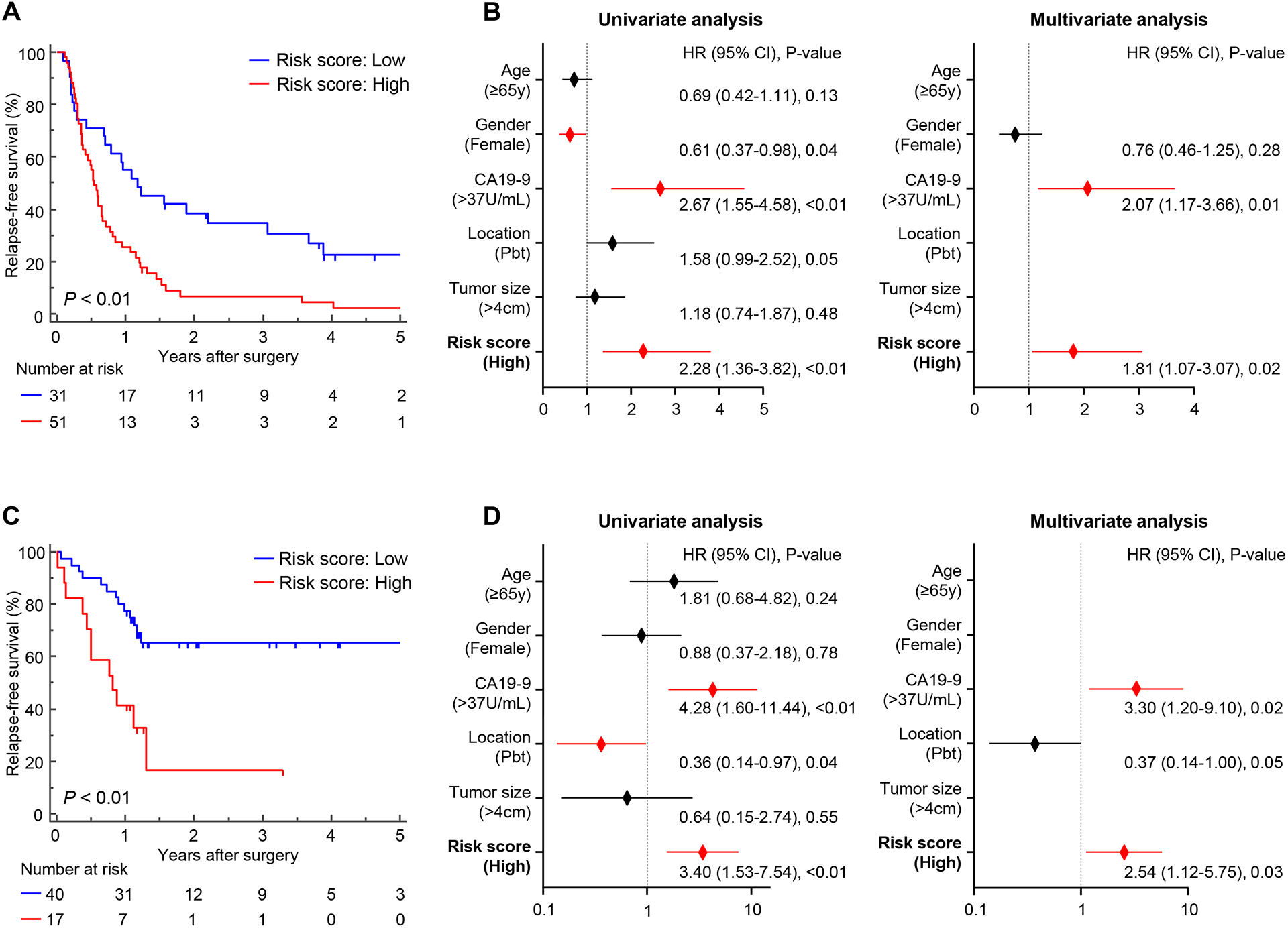
(A, C) Kaplan-Meier plot showing RFS between high- and low-risk groups estimated by the 6-exo-miRNA EMP in the training (A) and (C) validation cohorts. (B, D) Forest plots showing hazard ratios of clinicopathological variables and panel risk score status in uni- and multivariate cox proportional analyses of RFS in the training (B) and (D) validation cohorts.
Next, we performed univariate and multivariate cox regression analyses to evaluate the efficacy of various preoperative clinicopathological variables (age, gender, CA19-9, tumor location, tumor size) and the EMP for its ability to predict patient’s cancer prognosis. In multivariate analysis, high-risk patients defined by our EMP emerged as an independent predictor for worse RFS in patients with PDAC (HR = 1.81; 95% CI = 1.07–3.07; P = 0.02; Fig. 3B). These results demonstrate that we successfully developed the EMP as a blood-based, pre-treatment recurrence prediction assay, which has significant recurrence prediction potential for cancer prognosis in patients with PDAC.
A combined signature including EMP and CA19-9 levels demonstrates significantly superior accuracy for predicting recurrence in PDAC patients
The glycoprotein CA19-9 is a widely established and important biomarker in PDAC. In addition, our multivariate cox regression model demonstrated that both EMP and CA19-9 were selected as independent risk factors for predicting RFS in the training cohort. Accordingly, we next examined whether a model combining our EMP with CA19-9 expression levels might further improve the predictive accuracy for cancer prognosis in patients with PDAC. It was quite reassuring to observe that indeed the new combination signature demonstrated an improved predictive performance for patient prognosis (AUC= 0.84; Fig. 2C). Furthermore, the new combination signature also demonstrated significantly superior recurrence predictive accuracy compared to other classic preoperative clinicopathological features, including tumor location and size (Fig. 2C); highlighting potentially utility of this signature for more accurate prediction of recurrence following surgery in PDAC.
Successful validation of the EMP in an independent clinical cohort highlights its translational potential
To evaluate the translational potential of our EMP in identifying high-risk patients with PDAC, we evaluated its performance in an independent clinical validation cohort (n = 57; 25 recurrence and 32 non-recurrence; Supplementary Fig. 1). To this end, we applied the EMP using the same statistical model, coefficients, and cutoff values derived from the training cohort to the validation cohort, which once again confirmed the robustness of our risk-assessment model in predicting PDAC recurrence, with an AUC value of 0.78 (95% CI = 0.65–0.88; Fig. 2D). Moreover, our EMP demonstrated consistent results in the same statistical analysis with the training cohort, including in multivariate analysis for RFS (Fig. 2E, F and 3C, D), underscoring the clinical significance of our EMP in predicting recurrence and cancer prognosis in patients with PDAC.
Additional validation of the EMP predicts PDAC recurrence in patients after NAT
For patient with PDAC, multidisciplinary treatment strategies, including NAT, are being actively explored and becoming increasingly common treatment options, especially in western countries. Therefore, we hypothesized that if our EMP can also discriminate which patients will have worse prognosis after NAT, it could potentially facilitate a more informed decision-making by physicians and patients, to determine which patients should continue with chemo-/chemoradiotherapy and who should proceed to surgery following NAT. Therefore, to further advance the translation of our noninvasive miRNA marker into clinical practice, we collected post-NAT blood samples from an additional validation cohort (n = 46; 25 recurrence and 21 non-recurrence) of patients with PDAC who underwent NAT followed by curative surgery (Table 2), and evaluated the performance of EMP for predicting recurrence and cancer prognosis after surgery. Even in this instance, we successfully applied the same statistical model and coefficients to this post-NAT cohort, which once again confirmed the robustness of our risk-assessment model in predicting recurrence with an AUC value of 0.72 (95% CI = 0.57–0.85, Fig. 4A). Consistent with our previous results using pre-treatment specimens, when we assessed the distribution of risk scores and recurrence status, we observed that patients with recurrence had significantly higher risk scores than patients without recurrence (P < 0.01; Fig. 4B). Moreover, patients who were classified as high-risk for recurrence by EMP showed significantly worse RFS than the low-risk patients (Fig. 4C). Most importantly, in a multivariate cox regression analysis including EMP and other conventional post-NAT clinicopathological variables, our EMP emerged as an independent feature for RFS (HR = 2.84; 95% CI = 1.30–6.20; P < 0.01, Fig. 4D). In addition, a combination signature constructed from the EMP and CA19-9 demonstrated significantly superior performance compared to other clinicopathological factors (Fig. 4E). These results demonstrate that our EMP can be applied to post-NAT patients, which indicates physicians could re-evaluate potential risk for recurrence and poor prognosis in patients with PDAC even after NAT.
Table 2:
Clinicopathological characteristics of the post-NAT validation cohort
| Characteristics | Additional validation cohort (Post-NAT) (n = 46) |
|---|---|
| Age | |
| < 65 years | 18 (39.1) |
| ≥ 65 years | 28 (60.9) |
| Gender | |
| Male | 25 (54.3) |
| Female | 21 (45.7) |
| Post-NAT CA19-9 | |
| ≤ 37 U/mL | 22 (47.8) |
| > 37 U/mL | 24 (52.2) |
| Tumor location | |
| Ph | 36 (78.3) |
| Pbt | 10 (21.7) |
| Post-NAT tumor size (CT) | |
| ≤ 4 cm | 42 (91.3) |
| > 4 cm | 4 (8.7) |
| Nodal metastasis | |
| Present | 14 (30.4) |
| Absent | 32 (69.6) |
| UICC ypStage (ver.7) | |
| 0 | 2 (4.3) |
| IA | 3 (6.5) |
| IB | 5 (10.9) |
| IIA | 21 (45.7) |
| IIB | 13 (28.3) |
| III | 2 (4.3) |
| IV | 0 (0.0) |
| Neoadjuvant therapy | |
| Neoadjuvant chemotherapy | 17 (37.0) |
| Neoadjuvant chemoradiotherapy | 29 (63.0) |
| Adjuvant therapy | |
| Yes | 33 (71.7) |
| No | 8 (17.4) |
| Data not available | 5 (10.9) |
| Recurrence | |
| Present | 25 (54.3) |
| Absent | 21 (45.7) |
NAT, Neoadjuvant therapy
UICC, International Union Against Cancer
Figure 4. Additional clinical validation of the EMP predicts PDAC recurrence in patients after NAT.
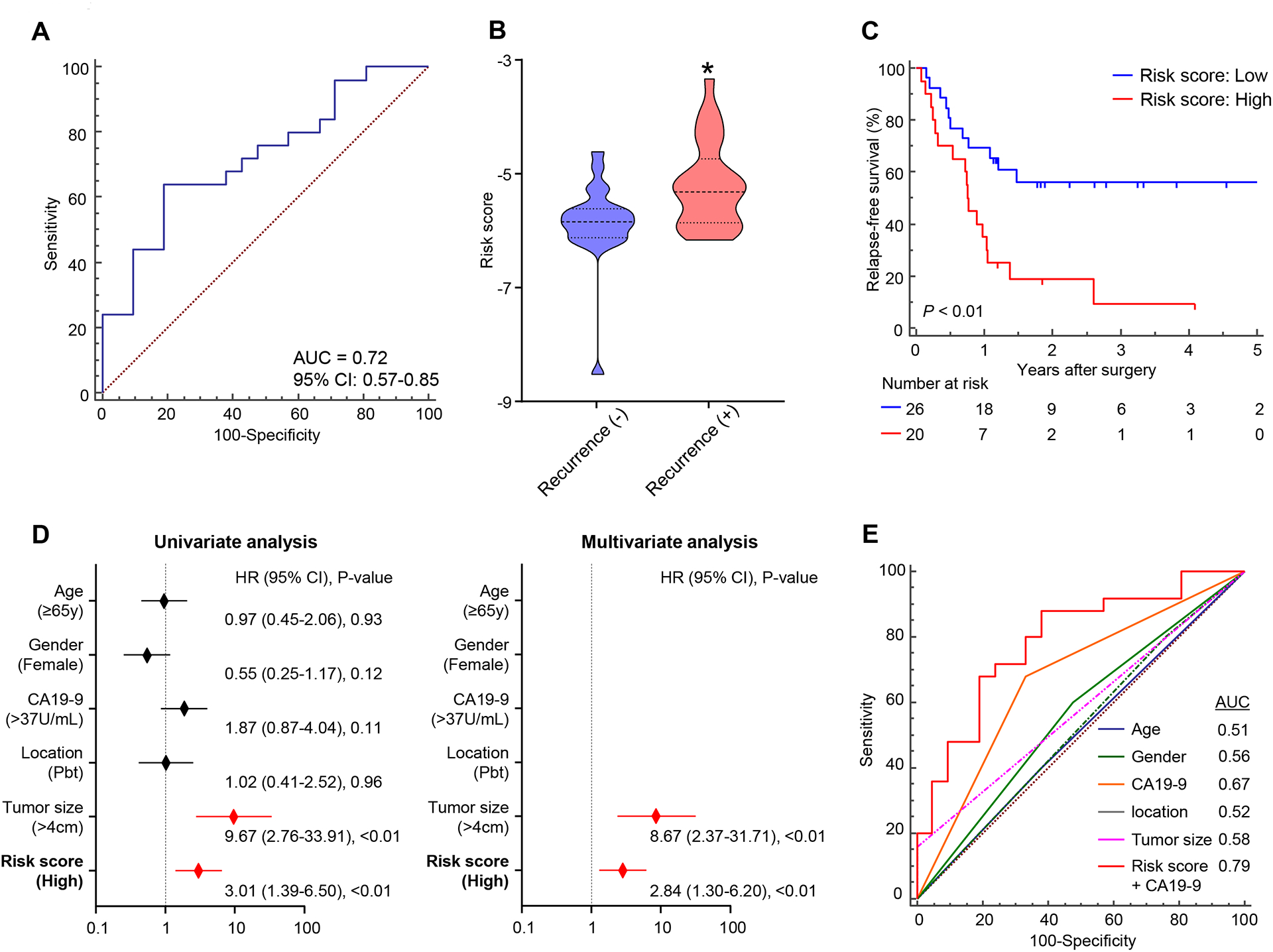
(A) ROC curve of the 6-exo-miRNA EMP derived from the training cohort for recurrence prediction in an additional validation cohort, comprised of post NAT blood samples from patients with PDAC who underwent NAT followed by curative surgery (AUC = 0.72). (B) Distribution of risk scores according to recurrence status (P < 0.01, Mann Whitney test). (C) Kaplan Meier plot showing RFS between high and low risk groups estimated by the EMP. (D) Forest plots showing hazard ratios of clinicopathological variables and panel risk score status in uni and multivariate cox proportional analyses of RFS in the post NAT validation cohort. (E) ROC curves showing the recurrence predictive performance of the combination model, EMP and CA19-9, compared to conventional clinical factors in the post-NAT validation cohort (AUC = 0.79).
The miRNA–mRNA regulatory network analysis identifies that candidate miRNAs are involved in key cancer-related signaling pathways
To determine the downstream gene targets of our discovered miRNAs, we conducted a miRNA–mRNA regulatory network analysis and identified 96 gene targets with predicted mechanistic involvement in gene regulatory pathways (Supplementary Fig. 2 and Supplementary Table 2). We then used KEGG analysis to perform pathway analysis of the validated downstream gene targets, which revealed biologically meaningful pathways, including several specific signaling pathways related to PDAC, such as PI3K-Akt signaling, pancreatic cancer, and miRNAs in cancer (Supplementary Fig. 2 and Supplementary Table 3). These results further enhance the credibility of our EMP and its biological significance in the pathogenesis of PDAC.
DISCUSSION
Recent innovations in medical technologies have led cancer treatment into a new era of precision medicine and development of individualized treatment strategies for cancer patients. However, especially in PDAC, which remains one of the most lethal malignancies worldwide, further advances including medical technologies, treatment strategies and basic/translational researches are essential3. In our quest to develop a robust pre-treatment, noninvasive risk-stratification model in PDAC, we used a systemic and comprehensive biomarker discovery approach to successfully develop a blood-based, 6-exo-miRNA panel that performed excellently in predicting tumor recurrence in patients with PDAC, which was subsequently validated in two independent clinical cohorts. Furthermore, we confirmed the predictive potential of our EMP even in post-NAT blood specimens, which was comparable to the performance of these biomarkers in blood from pre-treatment cohorts. Considering the current clinical strategies, in which NAT is becoming a common therapeutic option, this highlights the potential significance of our EMP for clinical translation for improving risk assessment and survival in patients with PDAC. Moreover, when compared with other conventional clinicopathological risk factors, our EMP remained a significant prognostic indicator in all independent clinical cohorts, including post-NAT specimens. Furthermore, a model combining our EMP with CA19-9, the most commonly used tumor marker in PDAC, demonstrated an even further improved predictive accuracy. To further highlight the clinical significance of our findings, although the NCCN guidelines are insufficient to identify high-risk patients with PDAC, our EMP robustly stratified patients from all clinical cohorts into significantly distinct high- and low-risk subgroups.
From a clinical perspective, our EMP has the potential to enable several significant improvements upon translation into clinical practice: 1) it could facilitate a reduction of ineffective and invasive surgeries currently being performed in patients with PDAC (e.g. presence of occult metastasis), 2) in patients with resectable status, our exosomal miRNA panel could provide an optimal indication for NAT which is still controversial in clinical settings, and (3) it could potentially help physicians and patients in making decisions to continue chemotherapy or proceed with surgery after NAT, especially in advanced cases. Further, our EMP panel could be applicable for evaluating the risk status at multiple timepoints in a patient’s clinical course. However, in order to realize its clinical application, the performance of our EMP should be further explored, especially for the post-NAT group. In this regard, in future, several options can be explored including; 1) For post-NAT group, since variations in exosomal miRNA expression could occur after preoperative treatment, establishing a miRNA panel using an independent NAT formula by utilizing these same miRNAs can be a potential option. Although we had only one post-NAT cohort in this study, it would be useful to utilize this formula and validate it in multiple post-NAT cohorts. 2) A combination model of two or more types of liquid biopsy analysis, such as cf-miRNA, ct-DNA and/or cf-DNA, can be synergistic to improve the clinical performance43. While we currently have no access to additional blood samples and patient cohorts, integrative biomarkers using these molecules should be considered in future. Although further basic and clinical studies, including a prospective study, are required, our study provides a framework for developing individualized treatment strategies and designing future genome-guided clinical trials for patients with PDAC.
We acknowledge a few potential limitations to our present study. First, we used a retrospective design and analyzed our EMP in moderately sized clinical cohorts. Since the management of the sample processing and storage conditions at each institution were not similar, such a bias could potentially be relevant. To minimize this bias, we evaluated the performance of our EMP using multiple independent clinical cohorts, but prospective studies using larger patient cohorts will be required before consideration of these biomarkers in clinical settings. Second, we successfully confirmed concordance of the molecular expression profiles between tissue and blood specimens in the discovery cohort; however, we did not have access to matched tissue specimens in other cohorts. Since our study had a limited sample size, further studies for validating the tumor-exosome correlation in larger cohorts is needed. Lastly, we did not have access to matched, pre- and post-NAT blood plasma specimens from the same patients, which would have permitted evaluation of the performance of our EMP with regards to NAT. Since it has been reported that real-time evaluation of exosomes may enable diagnosis of potential cancers and monitoring of cancer progression43, it could be promising to evaluate exosome expression pre- and post-treatments. Nonetheless, our present study provides promising evidence for the clinical significance of our EMP in predicting recurrence in patients with PDAC and is an important step toward the application of robust molecular biomarkers for risk assessment and management of a lethal malignancy such as PDAC.
In conclusion, using genome-wide expression profiling, we identified and developed a novel noninvasive EMP that was successfully validated in independent pre-treatment and post-NAT blood samples for predicting recurrence in patients with PDAC, which will have clinical significance for the selection of optimized treatment strategies. Moreover, our study provides a framework for developing individualized treatment strategies and designing future genome-guided prospective clinical trials for patients with PDAC.
Supplementary Material
Supplementary Figure 1. Overview of the study
Supplementary Figure 2. Pearson’s correlation analyses of miRNA expression between the exosome and matched tumor tissue specimens. In all 6 miRNAs, the expression were positively correlated between exosome and surgically resected tumor tissue samples, and the expression levels of miR130b-5p, miR133a-3p, and miR195-5p demonstrated significantly correlated profiles (P = 0.02, P < 0.01, and P = 0.04, respectively).
Supplementary Figure 3. miRNA–mRNA regulatory network analysis and pathway enrichment analysis of candidate miRNAs reveals biologically meaningful pathways. A miRNA–mRNA regulatory network analysis to determine the downstream gene targets of our discovered miRNAs. Subsequently we used KEGG analysis to perform pathway analysis of the identified downstream 96 gene targets from network analysis.
ACKNOWLEDGEMENTS
We thank Tatsuhiko Kakisaka, Raju Kandimalla, Souvik Ghatak, Priyanka Sharma, In-Seob Lee, Yuma Wada, Huanlin Wang, Yasuyuki Okada, Naoya Ikeda, Kenji Nakagawa, Minako Nagai, and Tadataka Takagi for discussing the experiments and analysis.
Financial support:
The present work was supported by the CA72851, CA187956, CA202797 and CA214254 grants from the National Cancer Institute, National Institute of Health; In addition, this work was also supported by a pilot research award from the City of Hope Ludwig Cancer Research-Hilton Foundation Partnership award.
Footnotes
Conflict of Interest: None of the authors have any potential conflicts of interest to disclose.
REFERENCES
- 1.Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Puleo F, Nicolle R, Blum Y, et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018; 155(6):1999–2013 e3. [DOI] [PubMed] [Google Scholar]
- 5.Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018; 267(5):936–945. [DOI] [PubMed] [Google Scholar]
- 6.Parikh AA, Maiga A, Bentrem D, et al. Adjuvant Therapy in Pancreas Cancer: Does It Influence Patterns of Recurrence? J Am Coll Surg 2016; 222(4):448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Court CM, Ankeny JS, Sho S, et al. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann Surg Oncol 2018; 25(4):1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013; 63(5):318–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy T, Bachet J-B, Ayav A, et al. Current standards and new innovative approaches for treatment of pancreatic cancer. European Journal of Cancer 2016; 57:10–22. [DOI] [PubMed] [Google Scholar]
- 10.Hackert T Surgery for Pancreatic Cancer after neoadjuvant treatment. Ann Gastroenterol Surg 2018; 2(6):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018; 268(2):215–222. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. The Lancet 2017; 389(10073):1011–1024. [DOI] [PubMed] [Google Scholar]
- 13.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013; 310(14):1473–81. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Akita H, Tomokuni A, et al. Preoperative Gemcitabine-based Chemoradiation Therapy for Borderline Resectable Pancreatic Cancer: Impact of Venous and Arterial Involvement Status on Surgical Outcome and Pattern of Recurrence. Ann Surg 2016; 264(6):1091–1097. [DOI] [PubMed] [Google Scholar]
- 15.Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). The Lancet 2016; 388(10041):248–257. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Xu H, Wu C, et al. Prognostic value of gamma-glutamyltransferase-to-albumin ratio in patients with pancreatic ductal adenocarcinoma following radical surgery. Cancer Med 2019; 8(2):572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SH, Kim HY, Lee EJ, et al. Preoperative Clinical and Computed Tomography (CT)-Based Nomogram to Predict Oncologic Outcomes in Patients with Pancreatic Head Cancer Resected with Curative Intent: A Retrospective Study. J Clin Med 2019; 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai S, George B, Wittmann D, et al. Importance of Normalization of CA19-9 Levels Following Neoadjuvant Therapy in Patients With Localized Pancreatic Cancer. Ann Surg 2020; 271(4):740–747. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto I, Murakami Y, Shinzeki M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: A multi-center retrospective study. Pancreatology 2015; 15(6):674–80. [DOI] [PubMed] [Google Scholar]
- 20.Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Annals of Surgery 2019:1. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Ohigashi H, Ishikawa O, et al. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg 2012; 255(1):95–102. [DOI] [PubMed] [Google Scholar]
- 22.He C, Huang X, Zhang Y, et al. A Quantitative Clinicopathological Signature for Predicting Recurrence Risk of Pancreatic Ductal Adenocarcinoma After Radical Resection. Front Oncol 2019; 9:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurahara H, Maemura K, Mataki Y, et al. Clinical significance of serum carbohydrate antigen 19.9 and duke pancreatic monoclonal antigen type 2 for the prediction of hematogenous metastases in patients with pancreatic ducal adenocarcinoma. Pancreatology 2016; 16(6):1051–1056. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe F, Suzuki K, Tamaki S, et al. Longitudinal monitoring of KRAS-mutated circulating tumor DNA enables the prediction of prognosis and therapeutic responses in patients with pancreatic cancer. PLoS One 2019; 14(12):e0227366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem 2008; 54(10):1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daoud AZ, Mulholland EJ, Cole G, et al. MicroRNAs in Pancreatic Cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019; 19(1):1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung G, Hernandez-Illan E, Moreira L, et al. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandimalla R, Gao F, Matsuyama T, et al. Genome-wide Discovery and Identification of a Novel miRNA Signature for Recurrence Prediction in Stage II and III Colorectal Cancer. Clin Cancer Res 2018; 24(16):3867–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993; 75(5):855–62. [DOI] [PubMed] [Google Scholar]
- 30.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75(5):843–54. [DOI] [PubMed] [Google Scholar]
- 31.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 2007; 297(17):1901–8. [DOI] [PubMed] [Google Scholar]
- 32.Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA Biomarkers in Whole Blood for Detection of Pancreatic Cancer. Jama 2014; 311(4):392. [DOI] [PubMed] [Google Scholar]
- 33.Shimura T, Toden S, Kandimalla R, et al. Genomewide Expression Profiling Identifies a Novel miRNA-Based Signature for the Detection of Peritoneal Metastasis in Patients With Gastric Cancer. Ann Surg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berindan-Neagoe I, Calin GA. Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin Cancer Res 2014; 20(24):6247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 36.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther 2009; 9(6):703–711. [DOI] [PubMed] [Google Scholar]
- 37.Mathivanan S, Lim JW, Tauro BJ, et al. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics 2010; 9(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics 2008; 8(19):4083–99. [DOI] [PubMed] [Google Scholar]
- 39.Tao L, Zhang L, Peng Y, et al. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in patients with pancreatic ductal adenocarcinoma (PDAC). Oncotarget 2016; 7(45):74314–74324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauro BJ, Greening DW, Mathias RA, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics 2013; 12(3):587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008; 110(1):13–21. [DOI] [PubMed] [Google Scholar]
- 42.Wei JX, Lv LH, Wan YL, et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015; 61(4):1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu W, Hurley J, Roberts D, et al. Exosome-based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshikawa M, Iinuma H, Umemoto Y, et al. Exosome-encapsulated microRNA-223–3p as a minimally invasive biomarker for the early detection of invasive breast cancer. Oncol Lett 2018; 15(6):9584–9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallo A, Tandon M, Alevizos I, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012; 7(3):e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.San Lucas FA, Allenson K, Bernard V, et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol 2016; 27(4):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thissen D, Steinberg L, Kuang D. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. Journal of Educational and Behavioral Statistics 2002; 27(1):77–83. [Google Scholar]
- 48.Kandimalla R, Ozawa T, Gao F, et al. Gene Expression Signature in Surgical Tissues and Endoscopic Biopsies Identifies High-Risk T1 Colorectal Cancers. Gastroenterology 2019; 156(8):2338–2341 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 50.Ozawa T, Kandimalla R, Gao F, et al. A MicroRNA Signature Associated With Metastasis of T1 Colorectal Cancers to Lymph Nodes. Gastroenterology 2018; 154(4):844–848 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 2018; 46(D1):D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalhori MR, Arefian E, Fallah Atanaki F, et al. miR-548x and miR-4698 controlled cell proliferation by affecting the PI3K/AKT signaling pathway in Glioblastoma cell lines. Sci Rep 2020; 10(1):1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng W, Li J, Chen R, et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 2019; 38(1):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dweep H, Sticht C, Pandey P, et al. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 2011; 44(5):839–47. [DOI] [PubMed] [Google Scholar]
- 55.Peto RPJ. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc 1972; 135:185–207. [Google Scholar]
- 56.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17(4):343–6. [DOI] [PubMed] [Google Scholar]
- 57.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3(1):32–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Overview of the study
Supplementary Figure 2. Pearson’s correlation analyses of miRNA expression between the exosome and matched tumor tissue specimens. In all 6 miRNAs, the expression were positively correlated between exosome and surgically resected tumor tissue samples, and the expression levels of miR130b-5p, miR133a-3p, and miR195-5p demonstrated significantly correlated profiles (P = 0.02, P < 0.01, and P = 0.04, respectively).
Supplementary Figure 3. miRNA–mRNA regulatory network analysis and pathway enrichment analysis of candidate miRNAs reveals biologically meaningful pathways. A miRNA–mRNA regulatory network analysis to determine the downstream gene targets of our discovered miRNAs. Subsequently we used KEGG analysis to perform pathway analysis of the identified downstream 96 gene targets from network analysis.


