Abstract
To date Borrelia lusitaniae is the only genospecies of Borrelia burgdorferi sensu lato isolated from Ixodes ricinus ticks collected in Portugal and Tunisia. This suggests that the genospecies diversity of B. burgdorferi sensu lato decreases toward the southwestern margin of its Old World subtropical range. In order to further explore the genetic diversity of B. burgdorferi sensu lato from this region, 55 I. ricinus and 27 Hyalomma marginatum questing adults, collected during the spring of 1998 from a sylvatic habitat south of Lisbon, Portugal, were analyzed. Infection prevalences of 75% in I. ricinus ticks and 7% in H. marginatum ticks were detected by a nested PCR that targets the rrf (5S)-rrl (23S) spacer of B. burgdorferi sensu lato. Restriction fragment length polymorphism (RFLP) analysis of the I. ricinus-derived amplicons showed that the sequences in the majority of samples were similar to those of B. lusitaniae type strains (76% for strain PotiB1, 5% for strain PotiB3). Two novel RFLP patterns were obtained from 12% of the samples. The remaining 7% of samples gave mixed RFLP patterns. Phylogenetic analysis of rrf-rrl spacer sequences revealed a diverse population of B. lusitaniae in questing adult I. ricinus ticks (the sequences did not cluster with those of any other genospecies). This population consisted of 10 distinct sequence types, suggesting that multiple strains of B. lusitaniae were present in the local I. ricinus population. We hypothesize that B. lusitaniae has a narrow ecological niche that involves host species restricted to the Mediterranean Basin.
In 1982 the etiological agent of Lyme disease was identified as a spirochete (2) which was later named Borrelia burgdorferi (13). Since then numerous strains related to this bacterium have been isolated. It is now widely accepted that these strains form a complex, B. burgdorferi sensu lato, which consists of 10 named genospecies and several yet to be named genomic groups. The genospecies are B. burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, Borrelia valaisiana, Borrelia lusitaniae, Borrelia andersonii, Borrelia bissettii, Borrelia japonica, Borrelia turdii, and Borrelia tanukii (4, 18, 25). B. garinii, B. afzelii, and B. burgdorferi sensu stricto are associated with disease in humans (32), while the pathogenic potential of the remaining genospecies is unknown. B. burgdorferi sensu lato is maintained in nature by zoonotic transmission cycles, in which hard ticks are the vectors and vertebrates are the reservoir hosts. Ixodes ricinus is the principal vector in western Europe (15, 16, 21).
To better understand the ecology and epidemiology of tick-borne spirochetes, knowledge of their genetic diversity in nature is required. Most genetic studies of B. burgdorferi sensu lato are based on data derived from isolated spirochetes. Isolation of these bacteria can be fastidious (24) and may select for genotypes (19, 22). Advances in PCR technology have made it possible to detect and genotype microorganisms directly from clinical and environmental samples, without the need for isolation (3, 27). By minimizing in vitro selection, PCR-based typing tools provide more accurate methods for assessing the natural genetic diversity of B. burgdorferi sensu lato populations.
Only a few tick isolates of B. burgdorferi sensu lato have ever been obtained from mainland Portugal (23) and Tunisia (34). These were identified as Borrelia lusitaniae sp. nov. (18). This suggests that the genospecies diversity of B. burgdorferi sensu lato is low near the southern margin of its European range. In contrast, a PCR-based study found B. afzelii, B. garinii, and B. burgdorferi sensu stricto in ticks from the Portuguese Island of Madeira (21).
In this study the genetic diversity of B. burgdorferi sensu lato in local tick populations from a sylvatic habitat in mainland Portugal was analyzed. Rigorous phylogenetic analysis of PCR-derived sequences revealed a diverse population of B. lusitaniae in questing adult I. ricinus ticks.
MATERIALS AND METHODS
Study site and tick collection.
During March, April, and May of 1998 ticks were collected by blanket dragging (16) from a sylvatic habitat south of Lisbon, Portugal (8°33′W, 38°05′N). Each tick was assigned the letters GT and a number. Fifty-five I. ricinus and 27 Hyalomma marginatum questing adult ticks were analyzed. Ticks were preserved in 70% ethanol at ambient temperature.
Borrelia strains and nucleotide sequences.
The cultured B. burgdorferi sensu lato strains used in this study are given in Table 1. All nucleotide sequences that were downloaded from GenBank (24) and then used in the phylogenetic analysis are also given in Table 1.
TABLE 1.
Strains and GenBank rrf-rrl nucleotide sequences used in this study
| Species | Strain or isolate | Origin | rrf-rrl accession no. |
|---|---|---|---|
| B. afzelii | ACA 1a | Human, Denmark | AF200659 |
| VS461 | I. ricinus, Switzerland | L30135 | |
| J 1 | Unknown | L30129 | |
| B. burgdorferi sensu stricto | ZS 7ab | I. ricinus, Germany | NAc |
| 212b | I. ricinus, France | L30121 | |
| B31 | Ixodes scapularis, United States | L30127 | |
| B. garinii | ZQ 1a | I. ricinus, Germany | AF200660 |
| 20047 | I. ricinus, France | L30119 | |
| NT29 | Ixodes persulcatus, Japan | L30130 | |
| B. lusitaniae | PotiB1ad | I. ricinus, Portugal | NA |
| PotiB2 | I. ricinus, Portugal | L30131 | |
| PotiB3 | I. ricinus, Portugal | L30132 | |
| B. valaisiana | UKa | I. ricinus, England | L30133 |
| VS116 | I. ricinus, Switzerland | L30134 | |
| B. bissettii | DN127 | Ixodes pacificus, United States | L30126 |
| CA55 | Ixodes neotomae, United States | L30124 | |
| 25015 | I. scapularis, United States | L30122 | |
| B. andersonii | 21123 | Ixodes dentatus, United States | L30120 |
| 19952 | I. dentatus, United States | L30118 | |
| B. japonica | HO14 | I. ovatus, Japan | L30125 |
| Cow611C | I. ovatus, Japan | L30128 | |
| Borrelia sp. | CA2 | Ixodes neotomae, United States | L30123 |
Strains cultured and used as positive controls.
Identical sequences.
NA, not applicable.
Sequence identical to that derived from GT058.
DNA preparation, rrf-rrl PCR, and reverse line blot.
Genomic DNA from ticks and cultured strains was prepared by alkaline hydrolysis in a final volume of 250 μl (8). A nested PCR that targeted the rrf (5S)-rrl (23S) intergenic spacer of B. burgdorferi sensu lato was performed with this prepared DNA (5 μl per reaction mixture) by using primers 23SN1, 23SC1, 23N2, and 5SCB as described previously (17, 27). All stages of the PCR were separated temporally and spatially (different laboratories) and were carried out under strictly aseptic conditions. Negative controls at a ratio of 2:3 were incorporated into the alkaline hydrolysis step and both the first and second rounds of PCR amplification. Prepared DNA of serial dilutions of cultured B. afzelii ranging between 2 × 107 and 2 spirochetes per reaction mixture (in log steps) was amplified repeatedly. Two dilutions that contained 2,000 and 2 spirochetes per reaction mixture were used as positive controls for each PCR amplification. All amplicons were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, and visualized with a UV transilluminator. Samples that tested positive were reamplified by nested PCR three times. DNA-DNA hybridization by the reverse line blot (RLB) assay was performed with samples that produced amplicons of approximately 380 and/or 230 bp as described previously (17, 27). DNA probes specific for B. burgdorferi sensu lato, B. burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana were used (17, 27). A probe specific for B. lusitaniae was not available at the time. Amplified DNAs derived from cultured B. burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana were used as positive controls. All samples that tested PCR positive were analyzed by the RLB assay twice.
Restriction fragment length polymorphism (RFLP) analysis of rrf-rrl PCR amplicons.
Intergenic spacer PCR products (10 μl) were digested with 5 U of MseI (New England Biolabs) in a total volume of 15 μl. Restriction products were separated by electrophoresis on a 20% polyacrylamide TBE (Tris-borate-EDTA) gel (NOVEX) for 4 h at 80 V. The gels were stained with ethidium bromide and were visualized with a UV transilluminator. A molecular size marker D-15 (NOVEX) was used for comparison.
DNA sequencing of PCR products.
rrf-rrl PCR products were reamplified with primers 23SN2 and 5SCB to which M13 (universal or reverse) tails had been added at the 5′ ends. These reamplified products were then cycle sequenced with M13 universal and reverse primers. A fifth of the rrf-rrl amplicons were cycle sequenced again with internally labeled primer 23N2 (35).
Sequence alignment and phylogenetic analysis.
The forward and reverse sequences of each PCR product which overlapped were aligned against one another and edited to produce a single sequence. These sequences were then aligned against each other and the reference sequences downloaded from GenBank by using Clustal W (31), followed by manual adjustment. Various rooted and unrooted phylogenetic trees were constructed with the PAUP package (29) by using both distance matrix (neighbor-joining, unweighted pair group method with arithmetic averages [UPGMA]) and discrete character (maximum likelihood, maximum parsimony) methods. In the maximum likelihood analysis the general reversible model of DNA substitution was used along with a gamma distribution of rate variation among sites. This substitution model was also used in the neighbor-joining analysis. Bootstrap analysis with 1,000 resamplings was performed to establish robustness for clusters in the neighbor-joining tree.
Nucleotide sequence accession numbers.
The rrf-rrl spacer sequences of B. burgdorferi sensu lato derived from I. ricinus and cultures have been deposited in GenBank and have been assigned accession nos. AF200649 (GT058), AF200650 (GT163), AF200651 (GT172), AF200652 (GT156), AF200653 (GT098), AF200654 (GT167), AF200655 (GT151), AF200656 (GT158), AF200657 (GT078), AF200658 (GT132), AF200659 (ACA1), and AF200660 (ZQ1).
RESULTS
Intergenic spacer PCR and RLB assay.
Forty-one of the I. ricinus ticks and two of the H. marginatum ticks tested positive for the rrf-rrl locus of B. burgdorferi sensu lato (infection prevalences, 75 and 7%, respectively). All positive tick-derived samples gave two bands of 380 and 230 bp. Amplification of DNA from 2 × 103 or more cultured spirochetes also gave two bands, whereas dilutions that contained DNA equivalent to <2 × 103 spirochetes generated only one band of 230 bp. This indicates that each (whole) tick was infected with at least 105 spirochetes. The DNA probes used for the RLB assay hybridized successfully with the corresponding positive control DNA. However, the 43 tick-derived PCR-positive amplicons hybridized only with the B. burgdorferi sensu lato probe. This indicates that the ticks were infected with B. burgdorferi sensu lato but not with B. burgdorferi sensu stricto, B. garinii, B. afzelii, or B. valaisiana. Repetition of PCR and the RLB assay with positive samples gave consistent results.
RFLP analysis of rrf-rrl PCR amplicons.
All five cultured strains used for reference were digested and gave distinct RFLP patterns which were assigned the letters A to E (Table 2). Forty-one of the I. ricinus-derived rrf-rrl PCR products were digested. Four different RFLP patterns were obtained from these samples (Table 2 and Fig. 1). Thirty-one (76%) gave pattern E, the same as that given by type strain PotiB1 (and type strain PotiB2, as deduced from the sequence). Two (5%) gave pattern F, which is similar to that given by PotiB3, as deduced by Postic et al. (24). Two patterns that were unlike any other pattern in our data set or previously published data sets were obtained from five (12%) samples and were assigned the letters G and H (10% gave pattern G and 2% gave pattern H). Two (5%) gave a mixed pattern of F and E, and one (2%) gave a mixed pattern of G and E.
TABLE 2.
MseI restriction pattern of the rrf-rrl spacer of B. burgdorferi sensu lato amplified by nested PCR directly from ticks or cultured strains
| Strain or tick-derived samplea | Amplicon size (bp) | RFLP pattern | MseI restriction fragment size (bp)b |
|---|---|---|---|
| B. burgdorferi ZS7 | 227 | A | 95, 37, 29, 28 |
| B. afzelii ACA 1 | 220 | B | 20, 37, 68, 95 |
| B. garinii ZQ 1 | 227 | C | 95, 95, 37 |
| B. valaisiana UK | 229 | D | 162, 37, 23, 7 |
| B. lusitaniae PotiB1 | 230 | E | 95, 67, 39, 29 |
| GT058c (I)d | 230 | E | 95, 67, 39, 29 |
| GT078 (V) | 230 | E | 96, 66, 39, 29 |
| GT151 (VI) | 231 | E | 96, 67, 39, 29 |
| GT172 (VII) | 230 | E | 95, 67, 39, 29 |
| GT132 (IV) | 229 | F | 95, 66, 52, 16 |
| GT098 (II) | 212 | G | 95, 78, 39 |
| GT167 (VIII) | 212 | G | 95, 78, 39 |
| GT156 (III) | 220 | H | 95, 66, 30, 29 |
| GT158 (IX) | 213 | G+E | 96, 78, 39 |
| GT163 (X) | 230 | F+E | 95, 67, 29, 39 |
Only representative samples are shown.
The exact sizes of the fragments were determined from the sequence of the nested PCR product.
Sequence identical to that of PotiB1.
Roman numerals in parentheses indicate sequence type.
FIG. 1.
MseI restriction patterns of B. burgdorferi sensu lato rrf-rrl spacer nested PCR products amplified directly from ticks or cultured strains. Lanes 1 and 10, D-15 marker; lanes 2 and 3, cultured B. burgdorferi sensu stricto (ZS 7) and B. lusitaniae (PotiB1), patterns A and E, respectively; lanes 4 and 5, tick-derived samples 58 and 151 (pattern E), respectively; Lane 6, tick-derived sample 132 (pattern F); lane 7, tick-derived sample 163 (patterns E and F); lane 8, tick-derived sample 156 (pattern H); lane 9, tick-derived sample 167 (pattern G).
Sequencing alignment and phylogenetic analysis of rrf-rrl locus.
Twenty-seven of the 43 tick-derived PCR positive amplicons for the rrf-rrl locus were sequenced successfully. The five cultured strains listed in Table 1 that were used as positive controls for the PCR were also sequenced successfully. These 32 sequences plus the 18 downloaded from GenBank (Table 1) were aligned and a tree was constructed by using UPGMA (data not shown). From this tree identical sequences and outgroups were identified. A representative sequence from each cluster of identical sequences was chosen. Sequences 19952 and 21133 were considered too divergent to be included in the analysis and so were removed. Thus, the final alignment contained 10 tick-derived and 15 reference sequences (Fig. 2). On the basis of this alignment, neighbor-joining (Fig. 3), maximum parsimony (data not shown), and maximum likelihood (Fig. 4) trees were constructed. In all trees the tick-derived sequences cluster with B. lusitaniae strains. The neighbor-joining tree is completely resolved and shows 10 distinct sequence types among the tick-derived samples. These sequence types are not resolved by the maximum likelihood tree.
FIG. 2.
Aligned rrf-rrl spacer DNA sequences of B. burgdorferi sensu lato amplified directly from ticks or cultured strains or downloaded from GenBank. Gaps were introduced to obtain maximum homology. Only the last three bases of the 3′ end of the rrf gene and the first three bases of the 5′ end of the rrl gene are shown (in boldface).
FIG. 3.
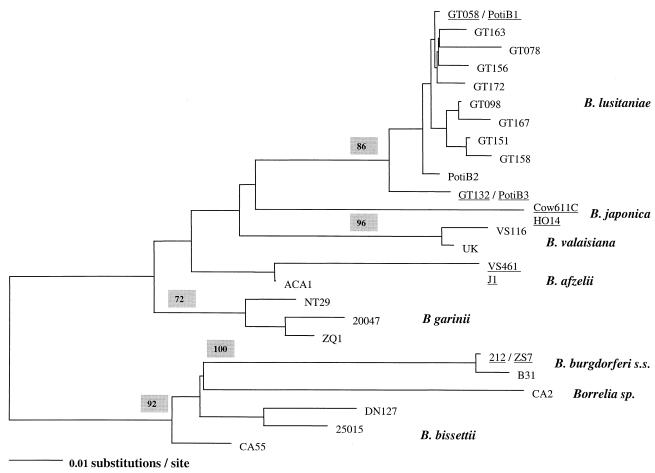
Neighbor-joining tree based on the comparison of rrf-rrl spacer sequences of B. burgdorferi sensu lato. The tree is drawn rooted at the midpoint for clarity. Sequences that are identical and that share the same branch are underlined. The numbers in the grey boxes are the results of 1,000 bootstrap resamplings (values of less than 70 are not shown). B. burgdorferi s.s., B. burgdorferi sensu stricto.
FIG. 4.
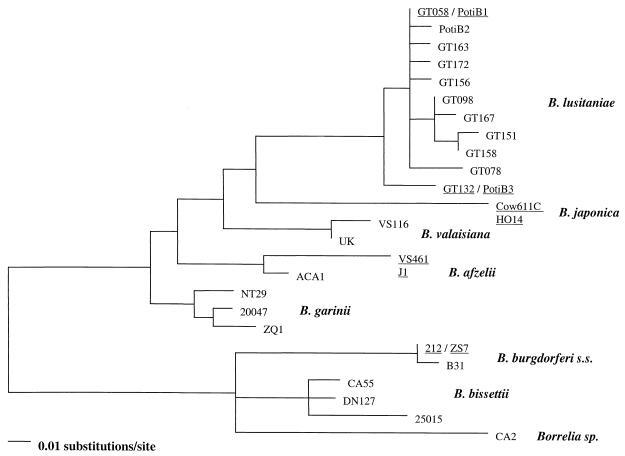
Maximum likelihood phylogenetic tree based on the comparison of rrf-rrl spacer sequences of B. burgdorferi sensu lato. The tree was constructed by using the general reversible model of DNA substitution and a gamma distribution of rate variation among sites (drawn rooted at the midpoint). Sequences that are identical and that share the same branch are underlined. B. burgdorferi s.s., B. burgdorferi sensu stricto.
Frequency distribution of sequence types.
Of the 27 tick-derived sequences analyzed, 10 sequence types were found (Table 2). The frequency of each type is as follows: 15 (56%) samples were of sequence type I (identical to that of PotiB1), 3 (11%) were of sequence type II, 2 (7%) were of sequence type III, and 1 (4%) each was of sequence type IV to X (type IV is identical to that of PotiB3).
DISCUSSION
B. lusitaniae was the only genospecies of B. burgdorferi sensu lato found in I. ricinus ticks from a sylvatic habitat in mainland Portugal. This result corroborates previous findings based on the isolation of spirochetes from Portugal (23) and Tunisia (34). It is, therefore, possible that the genospecies diversity of B. burgdorferi sensu lato decreases toward the southern margin of its European range. However, another study recorded B. burgdorferi sensu stricto, B. garinii, and B. afzelii in I. ricinus ticks collected from the Portuguese Island of Madeira, but not B. lusitaniae (21). As the fauna of Madeira differs from that of mainland Portugal (21), it may be that differences in the structures of the vertebrate host cenoses are part of the reason for these contrasting results.
Two distinct and novel RFLP patterns, G and H (Fig. 1, lanes 8 and 9, respectively), were obtained from 12% of the I. ricinus-derived PCR products, initially suggesting that novel genotypes of B. burgdorferi sensu lato may have been present in the ticks (S. De Michelis, H.-S. Sewell, M. Collares-Pereira, L. Vieira, M. Santos-Reis, L. Schouls, and K. Kurtenbach, Abstr. VIII Int. Conf. Lyme Borreliosis Other Emerg. Tick-Borne Dis., abstr. 08, p. 6, 1999). Upon phylogenetic analysis of sequences, these samples proved to be B. lusitaniae. For example, the PCR product from tick GT156, which gave RFLP pattern H (Fig. 1), clustered with type strains PotiB1 and PotiB2 (Fig. 3 and 4). Similarly, the samples from GT98 and GT167 gave RFLP pattern G but clustered with B. lusitaniae type strains. It should also be noted that the RFLP patterns of B. burgdorferi sensu stricto strain ZS 7 and B. lusitaniae type strain PotiB1 were so similar (Fig. 1, lanes 2 and 3, respectively) that it was not possible to unambiguously differentiate them. While PCR-RFLP analysis of the rrf-rrl locus correctly typed the majority of the tick-derived samples, the misleading novel patterns obtained and the similarity of certain genospecies patterns highlight the limitations of this commonly used typing method (5, 20, 24, 34).
Phylogenetic analysis of rrf-rrl spacer sequences has been used to delineate B. burgdorferi sensu lato genospecies and to assess their genetic diversity (9, 24, 25). In this study, the neighbor-joining (Fig. 3), maximum parsimony (data not shown), and maximum likelihood (Fig. 4) trees reveal that the rrf-rrl sequences derived from the Portuguese ticks form a cluster with B. lusitaniae type strains, thereby generally confirming the results obtained by RFLP analysis. The neighbor-joining tree is fully resolved, and within the B. lusitaniae cluster it discriminates 10 sequence types (Fig. 3). In contrast, the maximum likelihood tree is not fully resolved within the B. lusitaniae cluster (Fig. 4). In addition, some of the nodes in the neighbor-joining tree have relatively low bootstrap values at the genospecies level (bootstrap values of less than 70 are not shown). Both phylogenetic methods therefore indicate that the level of evolutionary information that can be gained from the rrf-rrl intergenic spacer is limited. Altogether our findings support previous suggestions that this locus is not suitable for analysis of the molecular phylogeny of B. burgdorferi sensu lato (24). Likely reasons for this are the fact that (i) the intergenic spacer is very short such that phylogenetic analysis is subject to large sampling errors, (ii) the intergenic spacer is composed of highly conserved and highly variable regions, (iii) and the alignment of sequences is ambiguous in places. In conclusion, while the rrf-rrl spacer of B. burgdorferi sensu lato is a suitable locus for use in the fingerprinting of genotypes and for the preliminary assessment of genetic diversity, it cannot be used to reliably infer evolutionary relationships between closely related Borrelia strains.
Recent studies on the population genetics of B. burgdorferi sensu lato in local tick populations from North America by PCR amplification of genes that encode outer surface proteins reported that numerous alleles of B. burgdorferi sensu stricto can be maintained simultaneously within local tick populations (7). The present study revealed 10 distinct sequence types (hereafter termed alleles) of B. lusitaniae, suggesting that the local tick population carried at least 10 different strains of this genospecies. The analysis of the frequency distribution of the 10 alleles revealed that allele I (identical to the sequence of PotiB1) was overrepresented (56%); i.e., half of the ticks were infected with the same genotype. The frequency distribution among the remaining nine alleles was much more even (4 to 11%). The biological significance of the frequency distribution of Borrelia alleles within this tick population from Portugal awaits determination. As recently proposed for B. burgdorferi sensu stricto (26), the population structure of B. lusitaniae is likely to be shaped by frequency-dependent selection. It remains to be analyzed whether the diversity of B. lusitaniae observed at a neutral locus (i.e., the rrf-rrl intergenic spacer) is mirrored at other loci, in particular, genes that encode outer surface proteins (e.g., OspA, OspB, and OspC).
Another ecologically interesting finding of this study was that 2 of 27 adult H. marginatum ticks contained DNA identical to that of B. lusitaniae strain PotiB1 (data not shown), suggesting that these ticks had been exposed to spirochetemic hosts. Subadult H. marginatum ticks mainly feed on birds and rodents (10), a behavior that may point to a possible role of avian or rodent species as reservoirs for B. lusitaniae.
The infection prevalence of B. burgdorferi sensu lato in questing adult I. ricinus ticks discovered in this study is significantly higher than that reported for most other regions of Europe, where values rarely exceed 40% in adult ticks (1, 12, 14–16, 27, 28, 30, 33). Apart from Portugal and Tunisia, B. lusitaniae has been found in the Czech Republic, Moldavia, Ukraine, and Belarus (18). In these Eastern European countries B. lusitaniae seems to be a rare genospecies of B. burgdorferi sensu lato. It has been reported that B. garinii, B. afzelii, and B. valaisiana account for the vast majority of infections in ticks from these areas (6, 11). We hypothesize that B. lusitaniae has a narrow ecological niche that involves vertebrate species that are geographically restricted to the Mediterranean Basin and that are highly competent reservoirs for this genospecies.
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust, London, United Kingdom (grants 050854/Z/97/Z and 054292/Z/98/Z).
We are grateful to Roy M. Anderson, Brian Spratt, and Patricia A. Nuttall for support, Stefanie M. Schäfer and Susanne Etti for useful comments, and Guy Baranton for supplying Borrelia cultures.
REFERENCES
- 1.Baumgarten B U, Röllinghoff M, Bogdan C. Prevalence of Borrelia burgdorferi and granulocytic and monocytic erlichiae in Ixodes ricinus ticks from southern Germany. J Clin Microbiol. 1999;37:3448–3451. doi: 10.1128/jcm.37.11.3448-3451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.Erlich H A, Gelfand D, Sninsky J J. Recent advances in the polymerase chain-reaction. Science. 1991;252:1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga M, Hamase A, Okada K, Nakao M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from Ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 5.Gern L, Hu C M, Kocianova E, Vyrostekova V, Rehacek J. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur J Epidemiol. 1999;15:665–669. doi: 10.1023/a:1007660430664. [DOI] [PubMed] [Google Scholar]
- 6.Gorelova N B, Korenberg E I, Kovalevskii Y V, Shcherbakov S V. Small mammals as reservoir hosts for Borrelia in Russia. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1995;282:315–322. doi: 10.1016/s0934-8840(11)80132-5. [DOI] [PubMed] [Google Scholar]
- 7.Guttman D S, Wang P W, Wang I N, Bosler E M, Luft B J, Dykhuizen D E. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J Clin Microbiol. 1996;34:652–656. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy E C, Stanek G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain-reaction. J Clin Pathol. 1991;44:610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gylfe A, Olsen B, Strasevicius D, Ras N M, Weihe P, Noppa L, Ostberg Y, Baranton G, Bergstrom S. Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J Clin Microbiol. 1999;37:890–896. doi: 10.1128/jcm.37.4.890-896.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillyard P D. Ticks of north-west Europe. Vol. 52. Shrewsbury, United Kingdom: Field Studies Council; 1996. [Google Scholar]
- 11.Hubalek Z, Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol. 1997;13:951–957. doi: 10.1023/a:1007426304900. [DOI] [PubMed] [Google Scholar]
- 12.Humair P F, Peter O, Wallich R, Gern L. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J Med Entomol. 1995;32:433–438. doi: 10.1093/jmedent/32.4.433. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R C, Schmid G P, Hyde F W, Steigerwalt A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 14.Junttila T, Peltomaa M, Soini H, Marjamäki M, Viljanen M K. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J Clin Microbiol. 1999;37:1361–1365. doi: 10.1128/jcm.37.5.1361-1365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahl O, Schmidt K, Schonberg A, Laukammjosten U, Knulle W, Bienzle U. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Berlin (West) Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1989;270:434–440. doi: 10.1016/s0176-6724(89)80013-6. [DOI] [PubMed] [Google Scholar]
- 16.Kurtenbach K, Kampen H, Dizij A, Arndt S, Seitz H M, Schaible U E, Simon M M. Infestation of rodents with larval Ixodes ricinus (Acari, Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. J Med Entomol. 1995;32:807–817. doi: 10.1093/jmedent/32.6.807. [DOI] [PubMed] [Google Scholar]
- 17.Kurtenbach K, Peacey M, Rijpkema S G T, Hoodless A N, Nuttall P A, Randolph S E. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol. 1998;64:1169–1174. doi: 10.1128/aem.64.4.1169-1174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Fleche A, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 19.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman R B, Wormser G P, Schwartz I. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–569. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuzawa T, Iwaki A, Sato Y, Miyamoto K, Korenberg E I, Yanagihara Y. Genetic diversity of Borrelia burgdorferi sensu lato isolated in near eastern Russia. Microbiol Immunol. 1997;41:595–600. doi: 10.1111/j.1348-0421.1997.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 21.Matuschka F-R, Klug B, Schinkel T W, Spielman A, Richter D. Diversity of European Lyme disease spirochetes at the southern margin of their range. Appl Environ Microbiol. 1998;64:1980–1982. doi: 10.1128/aem.64.5.1980-1982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris D E, Johnson B J B, Piesman J, Maupin G O, Clark J L, Black W C. Culturing selects for specific genotypes of Borrelia burgdorferi in an enzootic cycle in Colorado. J Clin Microbiol. 1997;35:2359–2364. doi: 10.1128/jcm.35.9.2359-2364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Núncio M S, Péter O, Alves M J, Bacellar F, Filipe A R. Isolamento e caracterização de Borrélias de Ixodes ricinus L. em Portugal. Rev Port Doenc Infec. 1993;16:175–179. [Google Scholar]
- 24.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 25.Postic D, Ras N M, Lane R S, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu W G, Bosler E M, Campbell J R, Ugine G D, Wang I N, Luft B J, Dykhuizen D E. A population genetic study of Borrelia burgdorferi sensu stricto from eastern Long Island, New York, suggested frequency-dependent selection, gene flow and host adaptation. Hereditas. 1997;127:203–216. doi: 10.1111/j.1601-5223.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.Rijpkema S G T, Molkenboer M J C H, Schouls L M, Jongejan F, Schellekens J F P. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–3095. doi: 10.1128/jcm.33.12.3091-3095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schouls L M, van de Pol I, Rijpkema S G T, Schot C S. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swofford D L. PAUP. Phylogenetic analysis using parsimony (and other methods) 4th ed. Sunderland, Mass: Sinauer Associates; 1998. [Google Scholar]
- 30.Tälleklint L, Jaenson T G T. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari, Ixodidae), in Sweden. J Med Entomol. 1994;31:880–886. doi: 10.1093/jmedent/31.6.880. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Dam A P, Kuiper H, Vos K, Widjojokusumo A, Dejongh B M, Spanjaard L, Ramselaar A C P, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 33.Wilske B. Prevalence of Borrelia burgdorferi in ticks. Hautarzt. 1986;37:415. [Google Scholar]
- 34.Zhioua E, Bouattour A, Hu C M, Gharbi M, Aeschliman A, Ginsberg H S, Gern L. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi sensu lato in North Africa. J Med Entomol. 1999;36:216–218. doi: 10.1093/jmedent/36.2.216. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann J, Voss H, Wiemann S, Erfle H, Rupp T, Dietrich T, Hewitt N, Schwager C, Stegemann J, Ansorge W. Direct sequencing of PCR products using magnetic beads and fluorescein-12-dUTP. Methods Mol Cell Biol. 1992;3:114–115. [Google Scholar]